Introduction
Alternative splicing is the process by which splice
sites in precursor messenger RNA (pre-mRNA) are differentially
selected and paired to produce multiple mature mRNAs and protein
isoforms with distinct structural and functional properties.
Alternative splicing is a very accurate, efficient, and
extraordinarily flexible process that regulates many major aspects
of eukaryotic cell biology. Approximately 95% of human genes with
multiple exons undergo alternative splicing during pre-mRNA
maturation (1–3). In addition to its role in human
proteome diversity, alternative splicing is now accepted to play
important roles in human diseases, including diabetes,
neurodegenerative diseases, and cancer (4–6).
Aberrant alternative splicing has two major roles in
cancer by promoting the emergence of a cancer-specific isoform or
disturbing the balanced expression of normally expressed isoforms
in cancer cells (7). During tumor
growth and development, and during oncogenesis, cells progress
through various stages as they acquire additional oncogenic
properties. These stages were described by Hanahan and Weinberg
(8) in 2000 and in papers
describing the features of cancer, which were updated in 2011 to
include ten processes required for tumor development and
progression to metastases. These processes were growth factor
self-sufficiency; insensitivity to growth inhibitory signals;
limitless replicative potential; the ability to evade apoptosis;
the ability to sustain angiogenesis; the ability to invade tissues
and metastasize; the ability to evade the immune system; the
presence of inflammation; the tendency towards genomic instability;
and deregulated metabolism. The results of recent studies indicate
that alternative splicing regulates many of these processes
involved in tumorigenesis and development (9–17).
Accordingly, deregulated alternative splicing is considered to be a
key feature of cancer and an opportunity to identify cancer
biomarkers (18).
Because of its frequently occurring and important
role in cancer, alternative splicing has emerged as an important
target for molecular therapies. Therefore, many molecules have been
designed and developed in order to inhibit cancer-specific isoforms
or isoforms highly expressed in cancer cells, or switch the
expression of specific isoforms as cancer cell-specific treatments
(19,20). Splice-switching oligonucleotides
(SSOs) are antisense oligonucleotides that modify alternative
splicing by hybridizing to pre-mRNA sequences, which undergo
splicing, and blocking access to the transcript by splicing
factors, thereby redirecting the splicing machinery to the
alternative pathway. The efficacy of SSOs has been established in
various disease models, including β-thalassemia, Duchenne muscular
dystrophy, spinal muscular atrophy, inflammatory diseases, and
cancer (21,22).
In cancer, the first and most frequently cited
demonstration of the antitumor effects of SSOs were of SSOs
targeting Bcl-x pre-mRNA. Bcl-x is a member of the Bcl-2 family of
proteins, which regulate the permeability of the outer membrane of
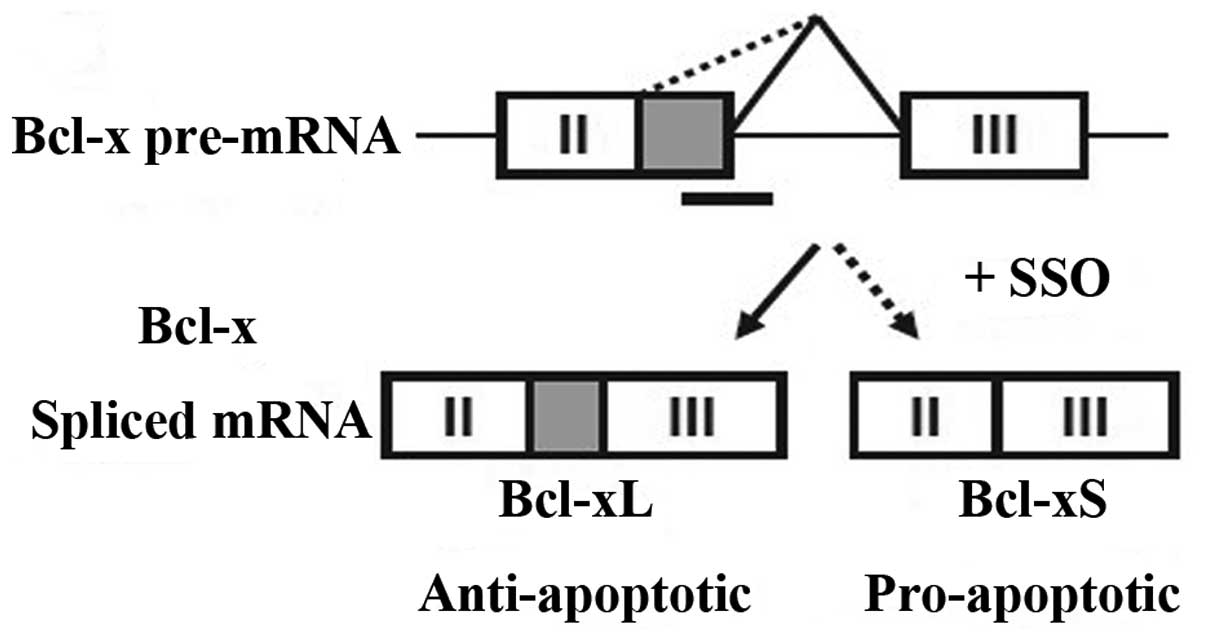
mitochondria. Bcl-x undergoes splicing at two alternative 5′ splice
sites in exon 2, yielding two distinct proteins, Bcl-xL and Bcl-xS,
with antagonistic properties (Fig.
1). Bcl-xL exerts anti-apoptotic effects by antagonizing and
inhibiting the pro-apoptotic Bax and Bak proteins. Bcl-xL
overexpression has been detected in several types of cancer. High
Bcl-xL expression is correlated with decreased cellular sensitivity
to chemotherapeutic reagents. Bcl-xS has pro-apoptotic effects by
directly binding to and inhibiting the pro-apoptotic Bcl-xL and
Bcl-2 proteins. High Bcl-xS expression was reported to induce
apoptosis in cancer cells from patients with colon or stomach
cancers (23,24).
Redirecting the pre-mRNA splicing of Bcl-x from
Bcl-xL to Bcl-xS with an SSO had pro-apoptotic and chemosensitizing
effects in various cancer cell lines. An SSO targeting Bcl-x
pre-mRNA (termed Bcl-x SSO) that blocked the downsteam 5′
alternative splice site in exon 2 of Bcl-x pre-mRNA redirected
Bcl-x pre-mRNA splicing from Bcl-xL to Bcl-xS. Accordingly, it
increased the expression of pro-apoptotic Bcl-xS and decreasing the
expression of anti-apoptotic Bcl-xL in various cancer cell lines.
Mercatante et al (25)
reported that Bcl-x SSO, which targeted the downstream alternative
5′-splice site in exon 2 of Bcl-x pre-mRNA, shifted splicing from
Bcl-xL to Bcl-xS in prostate and breast cancer cells in
vitro. They also found that Bcl-xS protein, induced by the SSO,
sensitized the cancer cells to treatment with ultraviolet- and
γ-irradiation and chemotherapeutic drugs. In other studies, the
same group showed that delivery of Bcl-x SSO using a lipid
nanoparticle redirected Bcl-x splicing and reduced the tumor burden
in melanoma lung metastases in vivo (26,27).
The effects of modifying a target gene's splicing
using an SSO vary depending on the expression profile of the target
cells. The differences in the cellular responses to Bcl-x
SSO-induced modification of Bcl-x pre-mRNA splicing were mainly
attributed to the endogenous Bcl-xL expression level. Tumor cells
containing higher levels of Bcl-xL were more susceptible to the
effects of Bcl-x SSO (9,25). To date, however, the effects of
Bcl-x SSO on glioma have not been reported. Previous studies have
reported that Bcl-xL is highly expressed in glioma, and confers
resistance to chemotherapies (28).
Therefore, we hypothesized that Bcl-x SSO can modulate alternative
splicing of Bcl-x pre-mRNA and inhibit the growth of glioma
cells.
In this study, we examined the effects of Bcl-x SSO
on glioma cell lines. First, we measured the endogenous mRNA and
protein expression of Bcl-xL in human glioma cell lines and a
normal human astrocyte cell line. Then, we determined the effects
of Bcl-x SSO on apoptosis and viability of these glioma cell lines.
Finally, we measured the shift in expression from Bcl-xL to Bcl-xS
in glioma cell lines treated with Bcl-x SSO.
Materials and methods
Ethics
The study was approved by the Ethics Committee of
the China-Japan Union Hospital of Jilin University (Changchun,
China).
Cell culture
Two human glioma cell lines (U87 and U251) and a
normal human astrocyte cell line (HA1800) were purchased from
Boster Biological Technology, Ltd. (Wuhan, China). The cell lines
were routinely cultured in Dulbecco's modified Eagle's medium
(DMEM) supplemented with 10% fetal bovine serum (FBS), penicillin
(100 U/ml), and streptomycin (100 µg/ml) in a 5%
CO2 atmosphere at 37°C. DMEM, FBS, and other tissue
culture reagents were purchased from Beijing Dingguo Changsheng
Biotechnology Co., Ltd. (Beijing, China).
SSO preparation and transfection
2′-O-methoxyethyl-phosphorothioate SSOs were
synthesized by Shanghai Sangon Biological Engineering Technology
and Services Co., Ltd. (Shanghai, China). The Bcl-x SSO
(5′-TGGTTCTTACCCAGCCGCCG-3′) targeted the downstream 5′ alternative
splice site of exon 2 of Bcl-x pre-mRNA. An oligonucleotide
(5′-GCTATTACCTTAACCCAG-3′) targeting human β-globin pre-mRNA was
used as negative control SSO.
The U87 and U251 cells were plated in 6-well culture
plates containing antibiotic-free DMEM at a density of
5.0×105 cells/ml. At >60% confluence, the cells were
transfected with SSO using Lipofectamine 2000 (Invitrogen,
Carlsbad, CA, USA) according to the manufacturer's
instructions.
RNA isolation and reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated from cells using TRIzol
reagent (Invitrogen Life Technologies) according to the
manufacturer's instructions. Complementary DNA (cDNA) was
synthesized using a HiFi-MMLV cDNA kit (Beijing Kang Century
Biotechnology Co., Ltd., Beijing, China) and random hexamer
primers. The primers were designed using Primer 5 software (Premier
Biosoft, Palo Alto, CA, USA) and synthesized by genScript Co., Ltd.
(Nanjing, China). The primers 5′-AGCGTAGACAAGGAGATGCAGG-3′
(forward) and 5′-GTGGATGGTCAGTGTCTGGTCA-3′ (reverse) were used to
amplify Bcl-xL, and the primers 5′-AGTAAAGCAAGCGCTGAGGGAG-3′
(forward) and 5′-ACTGAAGAGTGAGCCCAGCAGA-3′ (reverse) were used to
amplify both Bcl-xL and Bcl-xS. PCR was performed using GoldStar
Best DNA Polymerase (Beijing Kang Century Biotechnology Co., Ltd.).
The reaction conditions were as follows: 95°C for 10 min followed
by 40 cycles of degradation at 94°C for 30 sec, annealing at 58°C
for 30 sec, and extension at 72°C for 60 sec, and a final step of
72°C for 10 min.
The PCR products were separated on a 10%
non-denaturing polyacrylamide gel (Invitrogen Life Technologies)
and bands were visualized on a Typhoon 9400 imager (GE Healthcare,
Piscataway, NJ, USA). Images were quantified using imageQuant
analysis software (Molecular Dynamics, Sunnyvale, CA, USA). The
relative abundance of Bcl-xS in each lane was determined by
dividing the intensity of the 250-bp band (Bcl-xS) by the total
intensities of the 452-bp (Bcl-xL) and 250-bp (Bcl-xS) bands.
Western blot analysis
Cells in all experimental groups were collected
using a cell scraper. Total protein was extracted from cells using
protein extraction reagent (Boster Bioengineering, Wuhan, China)
containing 1 mM phenylmethanesulfonyl fluoride (PMSF; Roche
Molecular Biochemicals, indianapolis, IN, USA). The protein
concentration was determined using the BCA protein assay (Nanjing
Keygen Biotech Co., Ltd., Nanjing, China). Total protein was
electrophoresed on a 15% sodium dodecyl sulfate-polyacrylamide gel
and electro-transferred to polyvinylidene difluoride (PVDF)
membranes (Pall gelman Laboratory Corporation, Ann Arbor, Mi, USA).
The membranes were blocked overnight using 5% skimmed milk powder
at 4°C. The membranes were washed in Tris-buffered saline
containing Tween-20 (TBST) and incubated for 1 h at room
temperature with primary antibodies against target proteins,
followed by an additional TBST wash. The membranes were then
incubated with appropriate secondary antibodies for 1 h at room
temperature and washed again with TBST. The protein bands were
detected by enhanced chemiluminescence.
Cell viability assay
The effects of Bcl-x SSO on the viability of human
glioma cells were determined using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
method. Cells (2×104 cells/well) were cultured in
96-well culture plates for 1 day before SSO transfection. One day
later, after the cells had adhered to the culture plates, they were
transfected with Bcl-x SSO and control SSO. MTT solution (5 mg/ml,
20 µl; Sigma Chemical Co., St. Louis, MO, USA) was added to
each well and the cells were cultured in a CO2 incubator
for 4 h. Next, the culture solution was removed and 150 µl
of dimethyl sulfoxide was added to each well, and the plates were
agitated at room temperature for 10 min. The optical density of
each well was measured at 490 nm using a SpectraMax M3 microplate
reader (Molecular Devices, Sunnyvale, CA, USA). Each experimental
group was prepared in six duplicate wells. The mean values were
calculated and growth curves were constructed.
Flow cytometry analysis
Apoptosis was evaluated by flow cytometry analysis
(FCM). Cells from all experimental groups were digested in 0.25%
trypsin and re-suspended in phosphate-buffered saline to prepare
single-cell suspensions. The cell density was adjusted to
1×106 cells/ml. Next, 5 µl of Annexin V-FiTC and
5 µl of propidium iodide were added, and the cells were
incubated for 30 min at 4°C before flow cytometry.
Statistical analysis
Data are expressed as the mean ± standard deviation
of experiments performed in triplicate. Statistical analysis was
performed using one-way analysis of variance (ANOVA) for multiple
comparisons and unpaired t-tests for comparisons between pairs of
groups. P-values of <0.05 were considered statistically
significant.
Results
High expression of Bcl-xL in human glioma
cell lines
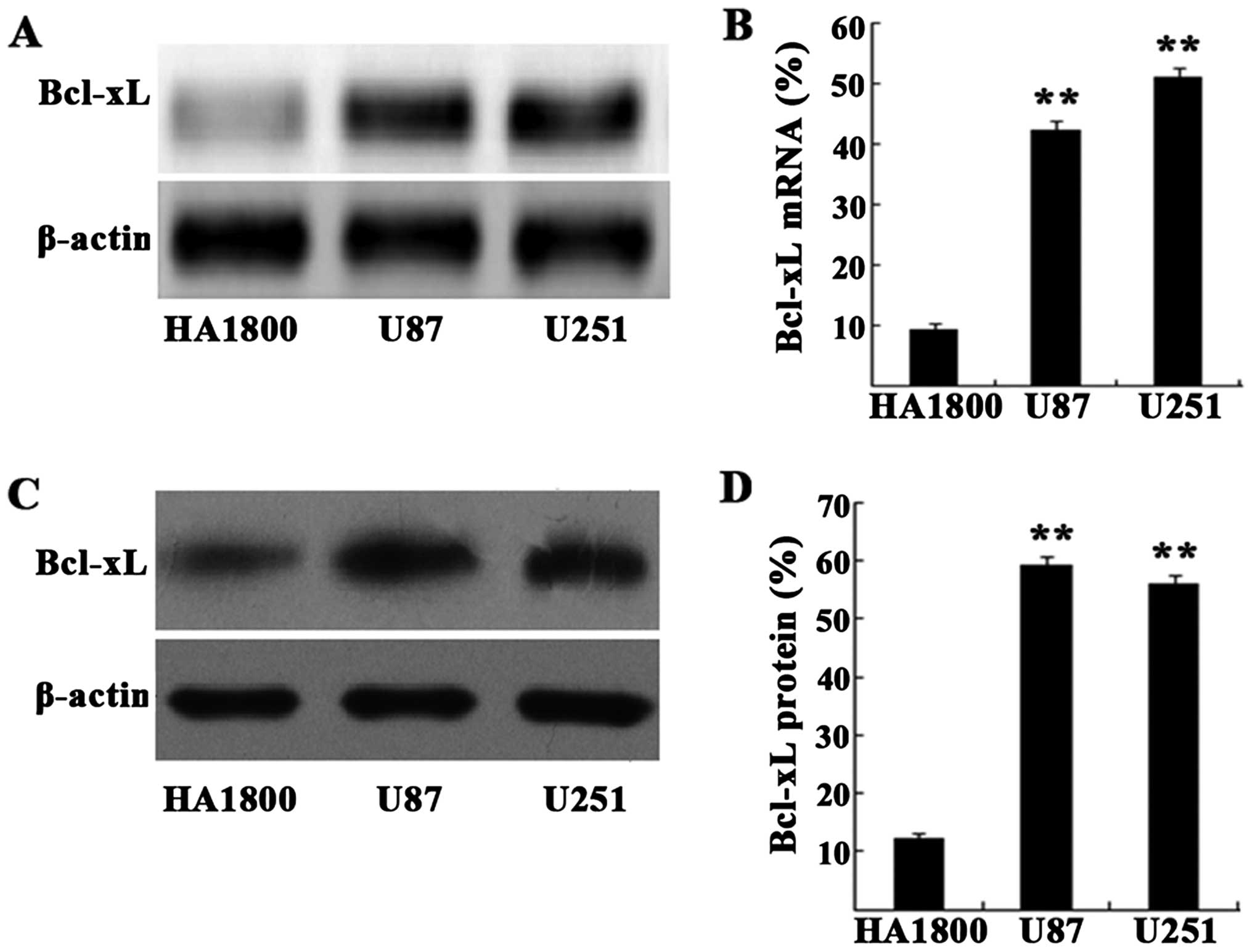
We first determined the expression profile of Bcl-xL
mRNA in two glioma cell lines (U87 and U251) and in a normal
astrocyte cell line (HA1800) by RT-PCR. Bcl-xL mRNA expression was
significant greater in U87 and U251 cells than in HA1800 (Fig. 2A and B). Likewise, western blot
analysis revealed that Bcl-xL protein expression was also increased
in U87 and U251 cells (Fig. 2C and
D). The results indicate that Bcl-xL mRNA and protein levels
are much greater in human glioma cell lines than in the control
astrocyte cell line.
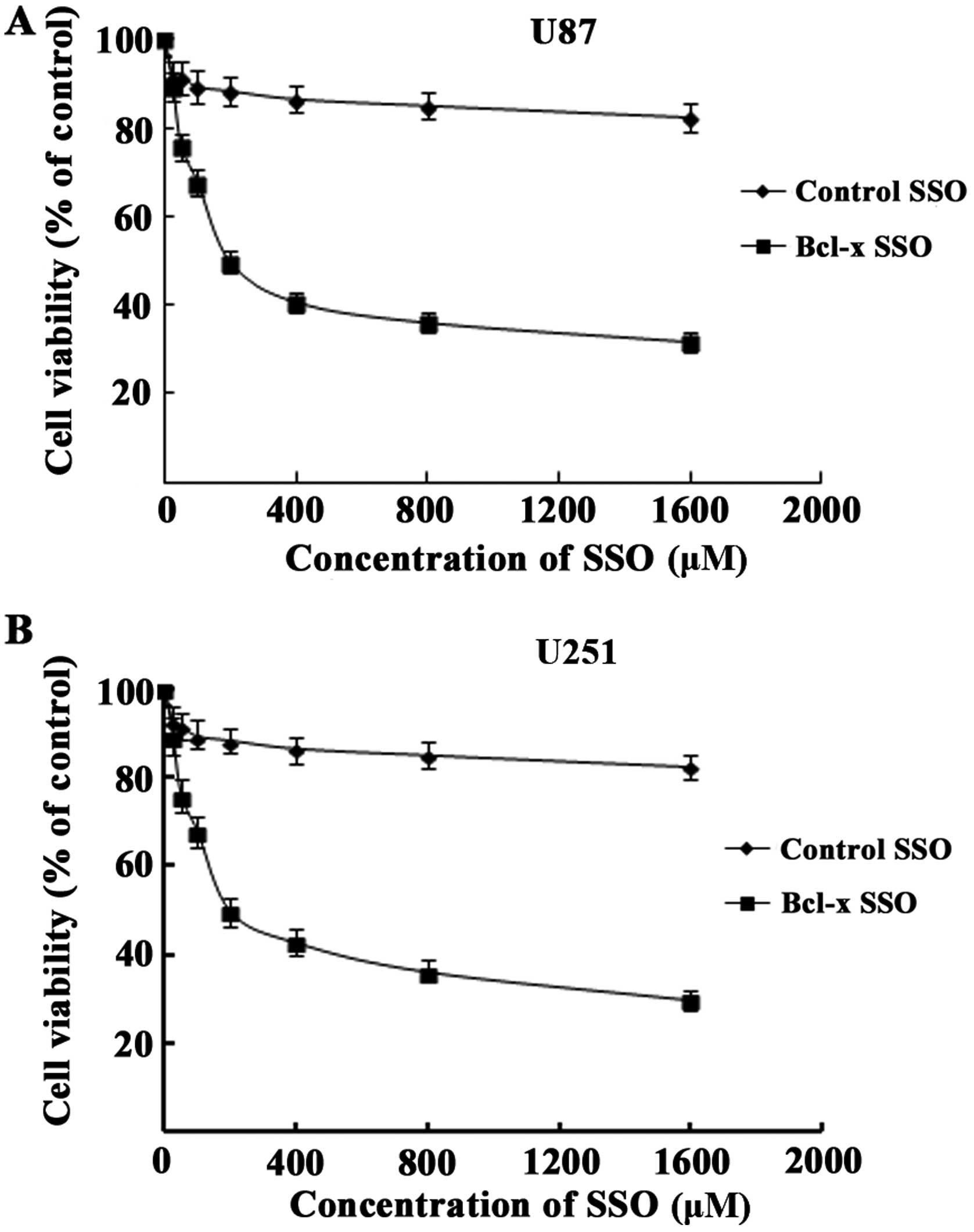
Bcl-x SSO inhibited the proliferation of
human glioma cell lines
To shift the alternative splicing pathway of Bcl-x
pre-mRNA from Bcl-xL to Bcl-xS, the U87 and U251 cells were
transfected with Bcl-x SSO, which targeted the downstream 5′
alternative splice site of exon 2, using the cationic lipid
Lipofectamine 2000. To investigate whether Bcl-x SSO-mediated
splice switching affected cell viability, MTT assays were performed
48 h after transfecting cells with different concentrations of SSO.
The results showed that transfection with Bcl-x SSO significantly
decreased the viability of U87 and U251 cells compared with
untreated cells and cells transfected with a control SSO (Fig. 3). These results indicate that Bcl-x
SSO inhibited the proliferation of human glioma cells.
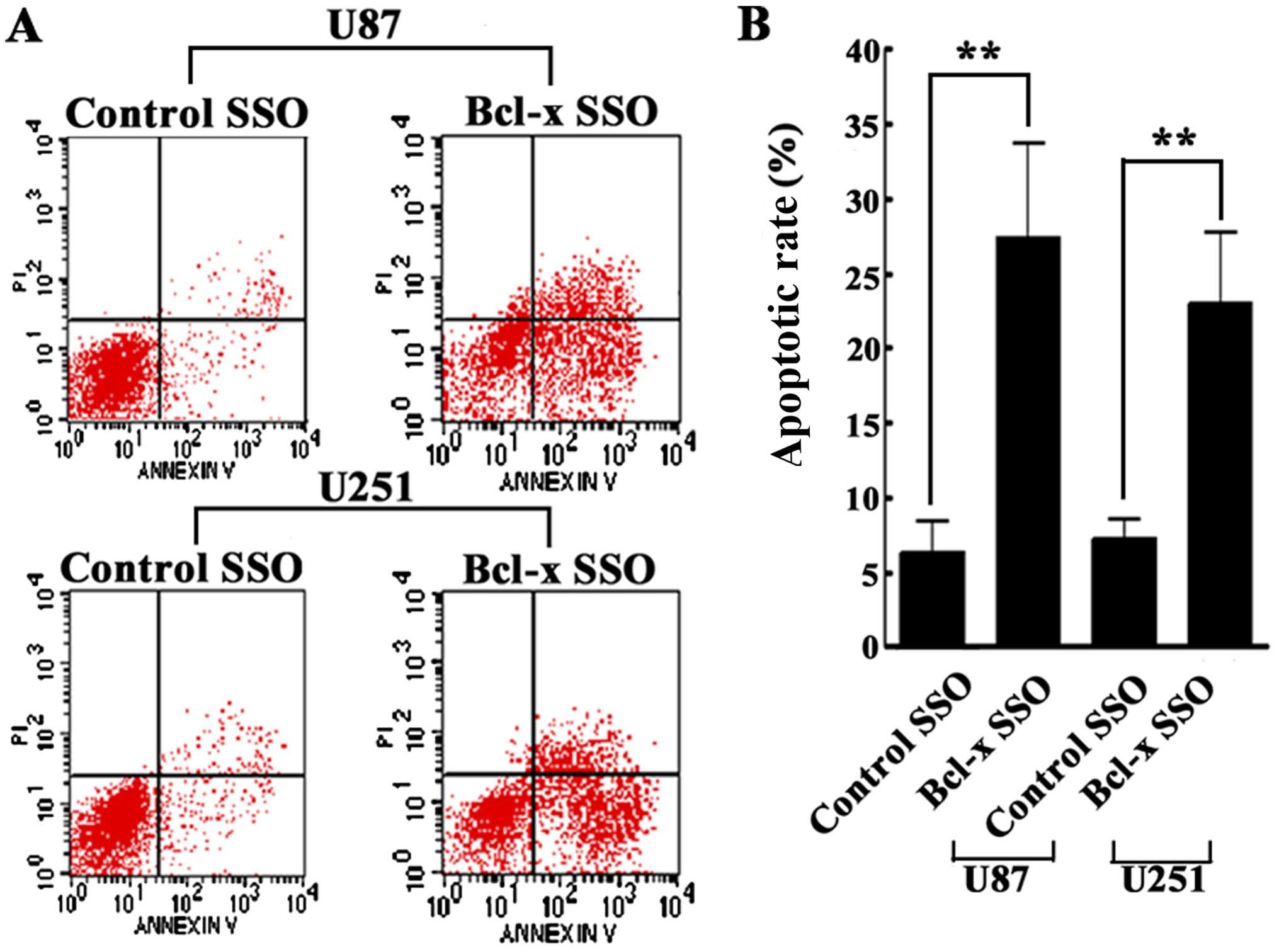
Bcl-x SSO has pro-apoptotic effects on
human glioma cell lines
To investigate whether Bcl-x SSO-mediated splice
switching has pro-apoptotic effects on human glioma cell lines,
flow cytometry was used to determine the rate of apoptosis.
Transfection with Bcl-x SSO (200 nM) for 48 h significantly
increased the apoptotic rate of U87 and U251 cells. By contrast,
the control SSO did not affect the apoptotic rate of either cell
line (Fig. 4). These results
indicate that Bcl-x SSO has pro-apoptotic effects on human glioma
cells.
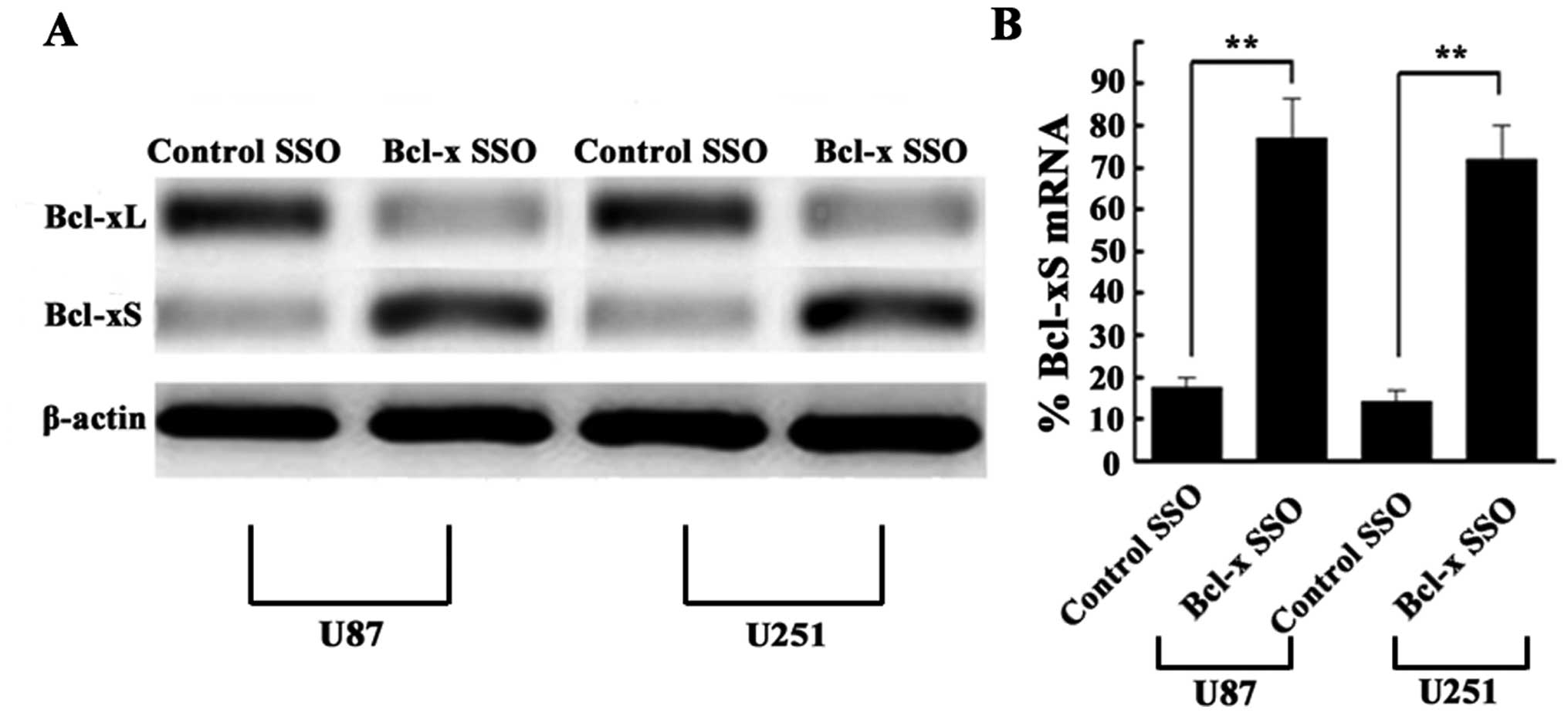
Bcl-x SSO shifts Bcl-x splicing from
Bcl-xL to Bcl-xS in human glioma cell lines
The shift in Bcl-x splicing in human glioma cell
lines transfected with Bcl-x SSO (200 nM) for 48 h was determined
by RT-PCR using primers to amplify both Bcl-xL and Bcl-xS. RT-PCR
analysis of total RNA from U87 and U251 cells at 48 h after
transfection showed that Bcl-x SSO caused a significant shift in
splicing from the Bcl-xL to Bcl-xS pathway, as indicated by a shift
in the ratio of these mRNAs. By contrast, the control SSO did not
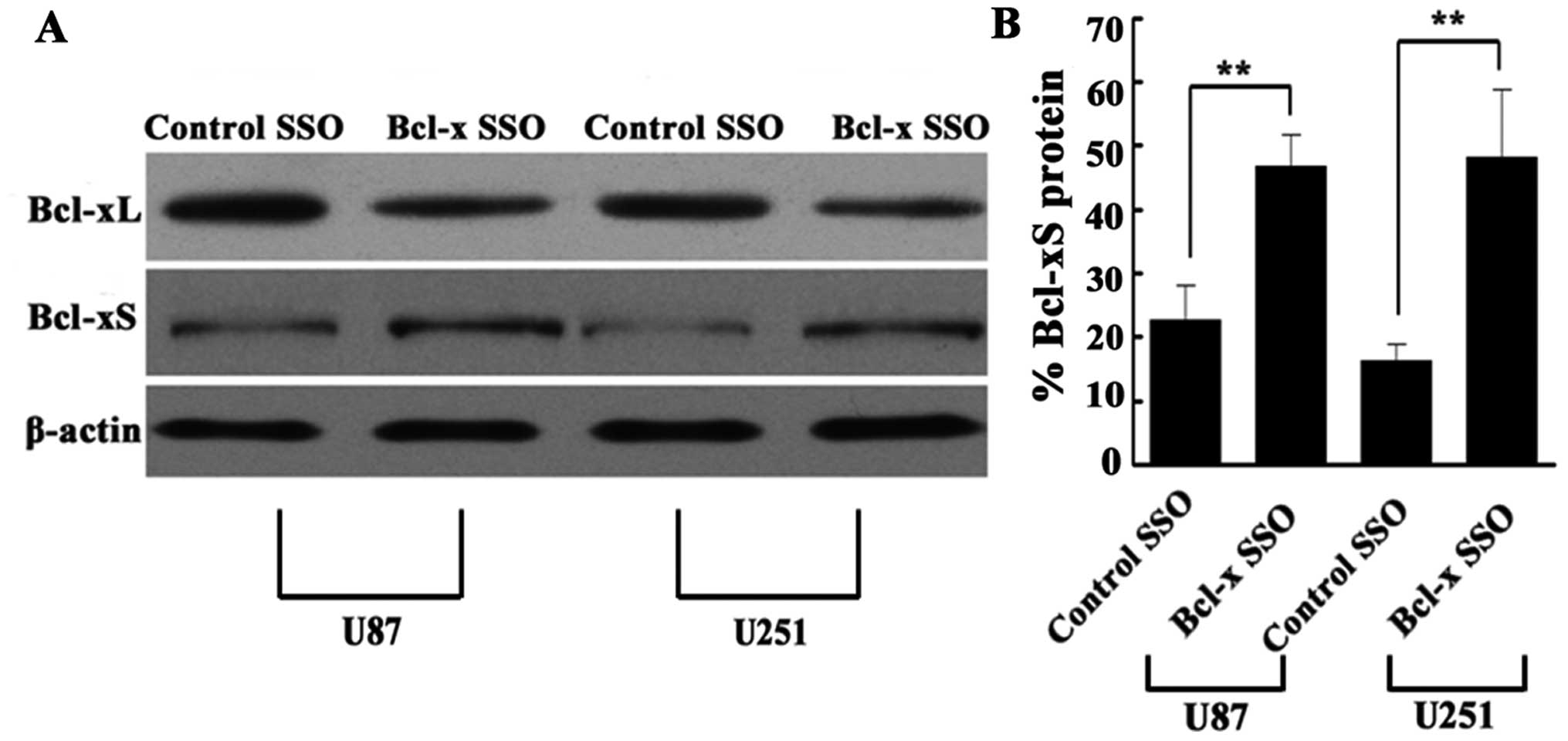
affect Bcl-x pre-mRNA splicing in either cell line (Fig. 5). Because a shift in the splicing
pattern of Bcl-x pre-mRNA from Bcl-xL to Bcl-xS should lead to a
change in Bcl-xL and Bcl-xS protein expression, we also conducted
western blotting to determine the Bcl-xL and Bcl-xS protein
expression levels. Consistent with the results of RT-PCR, the
western blot analysis using anti-Bcl-xL and anti-Bcl-xS antibodies
revealed a significant reduction in Bcl-xL protein expression and
an increase in Bcl-xS protein expression (Fig. 6). Thus, the SSO-induced shift in
Bcl-x splicing was confirmed in terms of the mRNA and protein
expression levels of Bcl-xL and Bcl-xS.
Discussion
Gliomas are the most aggressive and lethal tumors of
the central nervous system. In the past decades, the targeted
therapy of glioma has not gained significant breakthrough, mainly
due to the lack of an idea molecular target underlying gliomas
(29). Alternative splicing is a
key process involved in proteomic diversity and is essential for
normal cell growth and development. However, deregulation of
alternative splicing may occur and is implicated in various human
diseases, including cancer. Aberrant alternative splicing is now
considered an important event in cancer (1–6).
Recent studies show that alternative splicing also makes important
contributions to the genesis and development of gliomas (30,31).
Thus, aberrant alternative splicing has emerged as
an important target for molecular therapies, and methods to
manipulate alternative splicing are therapeutically valuable.
Accordingly, SSOs have been developed to regulate alternative
splicing by directing splice site selection. SSO are short
oligonucleotides designed to anneal to a specific target region on
pre-mRNA to interfere with pre-mRNA splicing. SSOs targeting an
exon-intron junction may sterically block access to the splicing
machinery, redirecting splicing reaction to an adjacent splicing
site. Numerous studies have demonstrated the potential anticancer
effects of SSO both in vitro and in vivo (20,32).
In cancer, the first and frequently quoted
demonstration of antitumor efficacy of SSO was Bcl-x SSO targeting
to Bcl-x pre-mRNA. Bcl-x SSOs block the downstream 5′ alternative
splice site in exon 2 of Bcl-x pre-mRNA and thereby redirect Bcl-x
pre-mRNA splicing from Bcl-xL to Bcl-xS. Redirection of Bcl-x
pre-mRNA splicing from Bcl-xL to Bcl-xS by SSOs had pro-apoptotic
and chemosensitizing effects in various cancer cell lines.
Mercatante et al were among the first to demonstrate the
antitumor effects of SSOs (9). They
reported that Bcl-x SSO initiated pro-apoptotic events and promoted
cell death by decreasing Bcl-xL expression and increasing Bcl-xS
expression in prostate cancer cells and breast cancer cells in
vitro. They also reported that Bcl-xS, which was upregulated by
SSO, sensitized the cancer cells to irradiation and
chemotherapeutic drugs. Soon after, Bauman et al (26,27)
demonstrated the in vivo antitumor efficacy of Bcl-x SSO.
Using lipid nanoparticles, they administered Bcl-x SSO to a mouse
model of metastatic melanoma. Bcl-x SSO efficiently redirected
Bcl-x pre-mRNA splicing from Bcl-xL to Bcl-xS, and significantly
reduced the tumor burden in mice with rapidly growing and highly
tumorigenic lung metastases. However, no studies have investigated
the antitumor effects of Bcl-x SSO on glioma cells, until now.
In another study by Mercatante et al
(25), they found that the
efficiency of splicing modulation and the corresponding antitumor
effects of Bcl-x SSO were highly dependent on the expression
profile of Bcl-xL. Tumor cells containing higher levels of Bcl-xL
were more susceptible to the effects of Bcl-x SSO, which suggests
that cancers with high Bcl-xL expression may show the greatest
responses to Bcl-x SSO. Previous studies have revealed that Bcl-xL
expression was elevated and contributed to chemotherapeutic
resistance in glioma (28). In this
study, in order to explore whether human gliomas are potential
candidates for Bcl-x SSO therapy, we analyzed the mRNA and protein
expression levels of Bcl-xL in two human glioma cell lines (U87 and
U251) and a normal astrocyte cell line (HA1800). We found that
Bcl-xL mRNA and protein expression levels were elevated in both
human glioma cell lines, and were significantly higher in these
cell lines than in HA1800 cells. Based on the results of previous
studies and our results, we speculate that human glioma cell lines
are good candidates for Bcl-x SSO therapy, which may modulate
alternative splicing of Bcl-x pre-mRNA and inhibit glioma cell
growth.
In our study, we designed the Bcl-x SSO to bind to
the 5′-splice site of exon 2 in Bcl-x pre-mRNA. We also used a
oligonucleotide targeting an aberrantly spliced human β-globin
intron as a negative control SSO. The Bcl-x SSO and control SSO
were modified using 2′-O-methoxyethyl-phosphorothioate and were
delivered into U87 and U251 cells using a cationic lipid. We next
examined the cellular effects of Bcl-x SSO on the human glioma
cells. In these experiments, administration of Bcl-x SSO
significantly reduced the replication rate of human glioma cells,
demonstrating that Bcl-x SSO can inhibit the proliferation of
glioma cells. We also examined the effects of Bcl-x SSO on cell
apoptosis by flow cytometry, and the results revealed that Bcl-x
SSO enhanced glioma cell apoptosis, whereas the control SSO had no
effects on apoptosis. Finally, we determined the effects of Bcl-x
SSO on switching the splicing from Bcl-xL to Bcl-xS in terms of the
RNA and protein expression levels of both isoforms. RT-PCR and
western blotting revealed that administration of Bcl-x SSO
significantly reduced the mRNA and protein expression levels of
Bcl-xL, and correspondingly increased the mRNA and protein
expression levels of Bcl-xS. In other words, by targeting the
alternative splicing of Bcl-x pre-mRNA, Bcl-x SSO reduced Bcl-xL
expression and increased Bcl-xS expression at the mRNA and protein
levels, and thereby promoted cancer cell apoptosis. Our data are
consistent with the predicted mechanism of action of SSOs.
Unlike siRNA and conventional antisense
oligonucleotides (ASOs), which degrade RNA via RNA-induced
silencing complex (RISC) and RNase H-mediated cleavage,
respectively, SSOs block sequences in pre-mRNA without causing RNA
degradation. To achieve this, the SSO forms a very stable duplex
with its pre-mRNA target sequence. This process can be encouraged
by chemically modifying the oligonucleotide sugar-phosphate
backbone to improve binding affinity and avoid RNase H cleavage,
for example. The oligonucleotide sugar-phosphate backbone can be
modified using 2′-O-methyl, 2′-O-methoxyethyl, phosphorodiamidate
morpholino oligomers, and peptide nucleic acids (33,34).
In our study, we modified the Bcl-xL SSO with
2′-O-methoxyethyl-phosphorothioate.
Two features of splicing modulation that distinguish
this approach from downregulation of anti-apoptotic genes using ASO
or siRNA are worth mentioning. First, Bcl-x SSO, which shifts the
splicing of Bcl-x pre-mRNA from Bcl-xL to Bcl-xS, should be
superior to Bcl-xL ASO and siRNA, which down-regulate Bcl-xL mRNA
expression. This is because Bcl-x SSO decreases Bcl-xL expression
and concomitantly increases the expression of the antagonistic
Bcl-xS, and thus amplifies the biological effects of the treatment.
Second, Bcl-x SSO shows good specificity because its splicing
modulation efficiency and antitumor effect are dependent on the
expression of Bcl-xL. These properties suggest that cells showing
higher Bcl-x expression are more susceptible to the effects of SSO,
by greater upregulation of anti-apoptotic Bcl-xS expression.
Accordingly, cells from aggressive cancers with higher Bcl-xL
expression levels are likely to be more susceptible to SSO-induced
apoptosis than healthy untransformed cells. This counterintuitive
mechanism should also help to reduce the undesirable side effects
associated with established chemotherapeutic drugs. Accordingly,
cancers displaying high Bcl-xL expression and those that depend on
Bcl-xL expression for survival represent good candidates for
treatment with an SSO targeting Bcl-x. By contrast, ASOs and siRNA
are less effective in cells showing high expression of the target
gene owing to incomplete inhibition of the target gene in such
cells (35,36). Here, we showed that Bcl-xL is highly
expressed in human glioma cells, and we proposed the hypothesis
that human glioma cells are susceptible to Bcl-xL SSO. We next
confirmed that Bcl-x SSO modulates Bcl-x pre-mRNA splicing and has
marked pro-apoptotic effects in human glioma cell lines. We also
found that Bcl-xL expression was significantly greater in the
glioma cell lines than in the normal human astrocyte cell line.
Accordingly, we may be able to find a suitable dosage of Bcl-x SSO
that has marked antitumor effects on glioma cells without causing
undesirable side effects on normal cells.
In conclusion, the present study was the first to
explore the pro-apoptotic effects of Bcl-x SSO on glioma cell
lines. The results showed that Bcl-x SSO modulated the alternative
splicing of Bcl-x pre-mRNA from Bcl-xL to Bcl-xS in glioma cell
lines. Bcl-x SSO had antitumor effects by inducing apoptosis and
cell death in human glioma cell lines in vitro. These
observations indicate that Bcl-x SSO may represent an efficient
gene therapy for gliomas.
Acknowledgments
The authors thank Professor Chunlei Fan for
technical assistance. The present study was supported by grants
from the National Science Foundation of China (no. 30672159) and
the Program for New Century Excellent Talents in Chinese
Universities (NCET-06-0306).
Abbreviations:
|
pre-mRNA
|
precursor messenger RNA
|
|
SSO
|
splice-switching oligonucleotides
|
|
PCR
|
polymerase chain reaction
|
|
RT-PCR
|
reverse transcription PCR
|
References
|
1
|
Nilsen TW and Graveley BR: Expansion of
the eukaryotic proteome by alternative splicing. Nature.
463:457–463. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gamazon ER and Stranger BE: Genomics of
alternative splicing: Evolution, development and pathophysiology.
Hum Genet. 133:679–687. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Beroukhim R, Mermel CH, Porter D, Wei G,
Raychaudhuri S, Donovan J, Barretina J, Boehm JS, Dobson J,
Urashima M, et al: The landscape of somatic copy-number alteration
across human cancers. Nature. 463:899–905. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kalsotra A and Cooper TA: Functional
consequences of developmentally regulated alternative splicing. Nat
Rev Genet. 12:715–729. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barta A and Schümperli D: Editorial on
alternative splicing and disease. RNA Biol. 7:388–389. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oltean S and Bates DO: Hallmarks of
alternative splicing in cancer. Oncogene. 33:5311–5318. 2014.
View Article : Google Scholar
|
|
7
|
Pal S, Gupta R and Davuluri RV:
Alternative transcription and alternative splicing in cancer.
Pharmacol Ther. 136:283–294. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mercatante DR, Bortner CD, Cidlowski JA
and Kole R: Modification of alternative splicing of Bcl-x pre-mRNA
in prostate and breast cancer cells. Analysis of apoptosis and cell
death. J Biol Chem. 276:16411–16417. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Palve V, Mallick S, Ghaisas G, Kannan S
and Teni T: Overexpression of Mcl-1L splice variant is associated
with poor prognosis and chemoresistance in oral cancers. PLoS One.
9:e1119272014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vegran F, Boidot R, Oudin C, Riedinger JM
and Lizard-Nacol S: Distinct expression of Survivin splice variants
in breast carcinomas. Int J Oncol. 27:1151–1157. 2005.PubMed/NCBI
|
|
12
|
Bojesen SE, Pooley KA, Johnatty SE,
Beesley J, Michailidou K, Tyrer JP, Edwards SL, Pickett HA, Shen
HC, Smart CE, et al Australian Cancer Study; Australian Ovarian
Cancer Study; Kathleen Cuningham Foundation Consortium for Research
into Familial Breast Cancer (kConFab); Gene Environment interaction
and Breast Cancer (GENICA); Swedish Breast Cancer Study (SWE-BRCA);
Hereditary Breast and Ovarian Cancer Research group Netherlands
(HEBON); Epidemiological study of BRCA1 and BRCA2 Mutation Carriers
(EMBRACE); Genetic Modifiers of Cancer Risk in BRCA1/2 Mutation
Carriers (GEMO): Multiple independent variants at the TERT locus
are associated with telomere length and risks of breast and ovarian
cancer. Nat Genet. 45:371–384. e1–e2. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Babic I, Anderson ES, Tanaka K, Guo D,
Masui K, Li B, Zhu S, Gu Y, Villa GR, Akhavan D, et al: EGFR
mutation-induced alternative splicing of Max contributes to growth
of glycolytic tumors in brain cancer. Cell Metab. 17:1000–1008.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Singh A, Karnoub AE, Palmby TR, Lengyel E,
Sondek J and Der CJ: Rac1b, a tumor associated, constitutively
active Rac1 splice variant, promotes cellular transformation.
Oncogene. 23:9369–9380. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mayer S, Hirschfeld M, Jaeger M, Pies S,
Iborra S, Erbes T and Stickeler E: RON alternative splicing
regulation in primary ovarian cancer. Oncol Rep. 34:423–430.
2015.PubMed/NCBI
|
|
16
|
Matsuyama M, Chijiwa T, Inoue Y, Abe Y,
Nishi M, Miyazaki N, Furukawa D, Mukai M, Suemizu H, Sekido Y, et
al: Alternative splicing variant of vascular endothelial growth
factor-A is a critical prognostic factor in non-small cell lung
cancer. Oncol Rep. 22:1407–1413. 2009.PubMed/NCBI
|
|
17
|
David CJ, Chen M, Assanah M, Canoll P and
Manley JL: HnRNP proteins controlled by c-Myc deregulate pyruvate
kinase mRNA splicing in cancer. Nature. 463:364–368. 2010.
View Article : Google Scholar :
|
|
18
|
Kim YJ and Kim HS: Alternative splicing
and its impact as a cancer diagnostic marker. Genomics Inform.
10:74–80. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schuster J, Lai RK, Recht LD, Reardon DA,
Paleologos NA, Groves MD, Mrugala MM, Jensen R, Baehring JM, Sloan
A, et al: A phase II, multicenter trial of rindopepimut (CDX-110)
in newly diagnosed glioblastoma: The ACT III study. Neuro Oncol.
17:854–861. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen J and Weiss WA: Alternative splicing
in cancer: Implications for biology and therapy. Oncogene. 34:1–14.
2015. View Article : Google Scholar
|
|
21
|
Bonomi S, Gallo S, Catillo M, Pignataro D,
Biamonti G and Ghigna C: Oncogenic alternative splicing switches:
Role in cancer progression and prospects for therapy. Int J Cell
Biol. 2013:9620382013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kinali M, Arechavala-Gomeza V, Feng L,
Cirak S, Hunt D, Adkin C, Guglieri M, Ashton E, Abbs S,
Nihoyannopoulos P, et al: Local restoration of dystrophin
expression with the morpholino oligomer AVi-4658 in Duchenne
muscular dystrophy: A single-blind, placebo-controlled,
dose-escalation, proof-of-concept study. Lancet Neurol. 8:918–928.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Moore MJ, Wang Q, Kennedy CJ and Silver
PA: An alternative splicing network links cell-cycle control to
apoptosis. Cell. 142:625–636. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miura K, Fujibuchi W and Unno M: Splice
variants in apoptotic pathway. Exp Oncol. 34:212–217.
2012.PubMed/NCBI
|
|
25
|
Mercatante DR, Mohler JL and Kole R:
Cellular response to an antisense-mediated shift of Bcl-x pre-mRNA
splicing and anti-neoplastic agents. J Biol Chem. 277:49374–49382.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bauman JA, Li SD, Yang A, Huang L and Kole
R: Anti-tumor activity of splice-switching oligonucleotides.
Nucleic Acids Res. 38:8348–8356. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bauman J, Jearawiriyapaisarn N and Kole R:
Therapeutic potential of splice-switching oligonucleotides.
Oligonucleotides. 19:1–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Weiler M, Bähr O, Hohlweg U, Naumann U,
Rieger J, Huang H, Tabatabai G, Krell HW, Ohgaki H, Weller M, et
al: BCL-xL: Time-dependent dissociation between modulation of
apoptosis and invasiveness in human malignant glioma cells. Cell
Death Differ. 13:1156–1169. 2006. View Article : Google Scholar
|
|
29
|
Alexandru-Abrams D, Jadus MR, Hsu FP,
Stathopoulos A and Bota DA: Therapeutic targeting of malignant
glioma. Anticancer Agents Med Chem. 14:1075–1084. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Z, Tian Y, Tian N, Zhao X, Du C, Han L
and Zhang H: Aberrant alternative splicing pattern of ADAR2
downregulates adenosine-to-inosine editing in glioma. Oncol Rep.
33:2845–2852. 2015.PubMed/NCBI
|
|
31
|
Fontana L, Rovina D, Novielli C, Maffioli
E, Tedeschi G, Magnani I and Larizza L: Suggestive evidence on the
involvement of polypyrimidine-tract binding protein in regulating
alternative splicing of MAP/microtubule affinity-regulating kinase
4 in glioma. Cancer Lett. 359:87–96. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Disterer P, Kryczka A, Liu Y, Badi YE,
Wong JJ, Owen JS and Khoo B: Development of therapeutic
splice-switching oligo-nucleotides. Hum Gene Ther. 25:587–598.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sazani P, Astriab-Fischer A and Kole R:
Effects of base modifications on antisense properties of
2′-O-methoxyethyl and PNA oligonucleotides. Antisense Nucleic Acid
Drug Dev. 13:119–128. 2003. View Article : Google Scholar
|
|
34
|
Adkin C, Fletcher S and Wilton SD:
Optimizing splice-switching oligomer sequences using 2′-O-methyl
phosphorothioate chemistry. Methods Mol Biol. 867:169–188. 2012.
View Article : Google Scholar
|
|
35
|
Wan J, Bauman JA, Graziewicz MA, Sazani P
and Kole R: Oligonucleotide therapeutics in cancer. Cancer Treat
Res. 158:213–233. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shchelkunova A, Ermolinsky B, Boyle M,
Mendez I, Lehker M, Martirosyan KS and Kazansky AV: Tuning of
alternative splicing - switch from proto-oncogene to tumor
suppressor. Int J Biol Sci. 9:45–54. 2013. View Article : Google Scholar :
|