Introduction
Endometrial cancer is a common gynecologic malignant
tumor and the fourth malignant tumor for women in developed
countries (1). A large number of
studies showed that obesity, diabetes and insulin resistance are
the high risk factors of endometrial cancer (2,3). The
incidence of endometrial cancer in patients with diabetes was two
times higher than that of non-diabetics (4). Diabetes also increased the mortality
of endometrial cancer (5,6).
The risks for anti-diabetes drugs with malignant
tumor are also of intensive concerned in recent years. Research
shows that metformin could reduce the risk of certain cancers
(7), and the mechanism was that
metformin as an AMP-activated protein kinase (AMPK) agonist
inhibits mTOR phosphorylation (8,9).
Moreover, insulin may increase the risk of some cancers through
insulin growth factor-1 receptor (IGF-1R)/PI3K/Akt/mTOR signaling
pathway (10,11). The relationship between diabetes
medication and tumor has attracted considerable attention. The
question of whether the new anti-diabetes drug glucagon-like
peptide-1 receptor (GLP-1R) agonist, such as exenatide (exendin-4),
exerts some effects on the tumor is of interest to researchers.
Clinical trials showed that exenatide could
significantly reduce fasting blood glucose and glycosylated
hemoglobin in patients with diabetes, also promoted β cell
proliferation and inhibited β cell apoptosis (12,13).
Studies have shown that GLP-1 could inhibit the apoptosis of
myocardial cells and neuronal cells, and its mechanism might be the
inhibition of apoptotic pathways including PI3K-Akt-mTOR pathway
and MAPK pathway (14,15). Based on the above, whether exenatide
promotes tumorigenesis or tumor growth has also been concidered.
Recent studies showed that exendin-4 did not enhance proliferation
of pancreatic adenocarcinoma cells (16), but inhibited the growth of tumor
cells of breast and colon cancer (17,18).
Previous studies revealed that exendin-4 could
inhibit lipid synthesis of hepatic cells by upregulation of AMPK
phosphorylation (19,20). In addition, AMPK has been confirmed
as the key of a series of complex molecular events, far more than
the cell energy metabolism in the body. AMPK is in the pivotal site
of PI3K-AKT-mTOR and other signal pathways, and mTOR signaling
pathway can promote apoptosis, which affects the occurrence and
development of tumors. The above findings will bring new
opportunities for cancer treatment (21).
Whether GLP-1R agonist exenatide also have effects
is not known. Therefore, we designed experiments in vivo and
in vitro to investigate the role of GLP-1R agonist on
endometrial cancer.
Materials and methods
Human tissues
Human normal endometrium tissues were obtained from
10 patients (42–48 years old) who received hysterectomy due to
uterine leiomyoma without endometrial disease, and endometrial
cancer tissues were obtained from 10 patients (45–55 years old) who
received hysterectomy due to endometrial cancer (type 1) at the
Third Affiliated Hospital of Sun Yat-sen University from January
2014 to July 2014. The tissues were paraffin-embedded,
formalin-fixed and cut into 4 µm sections for
immunohistochemistry staining. All the patients provided written
informed consent for participation in the present study. The study
protocol was approved by the Ethics Committees of the Third
Affiliated Hospital of Sun Yat-sen University.
Animal studies
Female 4-week-old BALB/c mice were obtained from
Beijing Laboratory Animal Research Center (Beijing, China) and
maintained in specific pathogen free facilities approved by the
Chinese Association for Accreditation of Laboratory Animal Care
with an approved protocol by the Institutional Animal Care and Use
Committee. Experiments were performed under institutional
guidelines established for Biomedical Research Center, The Third
Affiliated Hospital, Sun Yat-sen University. After 1 week of
acclimation, Ishikawa cells were injected subcutaneously into the
right flank of mice respectively (6×106 cells/mouse).
One week later, the mice were randomly divided into two groups (5
mice per group), given intraperitoneal injection of exenatide (Eli
Lilly and Company, Indianapolis, IN, USA) (24 nmol/kg/d) to the
mice in exenatide group and physiological saline to control group,
6 day/week, for 4 weeks. Tumor size was measured with a linear
digital caliper every 3–4 days. Tumor volume was estimated using
the equation V = (a×b2) × 0.5236, where 'a' is the
larger imension and 'b' is the perpendicular diameter. Weights of
BALB/c mice were measured using electronic balance every 3–4 days
during the medical treatment. Observations of BALB/c mouse diet,
activities and mental state in general were also recorded.
Endometrial cancer cell line
Human endometrial carcer cell line Ishikawa was
provided by the American Type Culture Collection (ATCC; Manassas,
VA, USA). The cells were routinely cultured in Dulbecco's modified
Eagle's medium (DMEM; Gibco, Carlsbad, CA, USA) supplied with 10%
fetal calf serum (FBS; HyClone Laboratories, Inc., South Logan, UT,
USA), at 37°C in the humidified atmosphere of a 5% CO2
incubation.
Random blood glucose
Before treatment and every 7 days during
intervention, random blood glucose of the mice was monitored
through the tail veins by superior blood glucose meter (Roche,
Basel, Switzerland).
Harvesting samples
At the end of the experiment, the mice were fasted
for 8 h, anesthetized and sacrificed for blood and tissue
collection. Blood samples were collected through the periorbital
venous of the mice and centrifuged for serum for the detection of
GLP-1, insulin and IGF-1 value. In addition, the harvested tumors
were weighed and separated for preservation in 4% neutral
formaldehyde solution at −80°C. Then, the tumor tissues preserved
in formaldehyde solution were paraffin-embedded, formalin-fixed and
cut into 4 µm sections for immunohistochemistry staining,
and the samples preserved at −80°C were used for western blot
analysis.
Enzyme-linked immunosorbent assay
(ELISA)
ELISA was performed with the serum samples of the
mice for the detection of GLP-1, insulin and IGF-1 value according
to the manufacturer's instructions, respectively of Glucagon-like
peptide-1, total ELISA kit (Merck Millipore, Darmstadt, Germany),
insulin (Abcam, Cambridge, UK) and mouse/rat IGF-1 immunoassay
(Merck Millipore).
Immunohistochemistry analysis and
evaluatoin
The human and animal tissue slides were
deparaffinized in xylene, rehydrated through graded alcohol,
immersed in 3% hydrogen peroxide for 10 min to block endogenous
peroxidase activity, and antigen retrieved by pressure cooking for
3 min in Tris/EDTA (pH 8.0). The slides were then incubated with
the primary antibody of GLP-1R antibody (ab39072, 1:50 dilution;
Abcam), rabbit anti-Ki-67 (1:100 dilution; Merck Millipore), DNA
fragmentation detection kit, fluorescent-TdT enzyme (1:1,000
dilution; Merck Millipore) for 1 h at room temperature according to
the manufacturer's instructions. After being incubated with the
secondary antibody for 30 min, specimens were stained with DAB
(3,3-diami-nobenzidine). Finally, the sections were counterstained
with hematoxylin, dehydrated and mounted. Slides were examined with
a Leica microscope (Leica Microsystems GmbH, Wetzlar, Germany).
Cell viability assay
Cell viability were measured using a 3-(4,
5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
(MP Biomedicals LLC, Santa Ana, CA, USA). Ishikawa cells
(2–3×103) were seeded in 96-well plates at varied
density (2–3×103/well), cultured in the appropriate
media and grown to 70% confluence. Exendin-4 (Sigma-Aldrich, St.
Louis, MO, USA) was added to culture medium at the indicated
concentrations for different times. Twenty microliters of MTT (5
mg/ml) was added to each well, and cells were subsequently
incubated at 37°C for an additional 4 h. Crystals were dissolved in
150 ml of DMSO. Absorbance was measured at a wavelength of 490 nm
using a microplate reader (ELx800; Bio-Tek Instruments, Inc.,
Winooski, VT, USA).
Colony assays
Fifty cells/well were plated on 96-well plates.
Twenty-four hours later, medium was replaced, and cells were
incubated with exendin-4 at indicate concentration. Medium was
replaced every day, and at day 14, the cells were fixed and stained
using 0.5% crystal violet (Sigma-Aldrich).
Apoptosis analysis
Cells (7×105) were plated in the
appropriate culture media containing 10% FCS in 12-well plates.
Cells were then serum starved for 24 h, and treated with exendin-4,
AICAR (Sigma-Aldrich) or compound C (Sigma-Aldrich) at indicated
concentration. After 48 h, cells and medium were collected and
stained with PI and Annexin V, using the Annexin V-PE apoptosis
detection kit I (Becton-Dickinson, Franklin Lakes, NJ, USA)
according to the manufacturer's protocol. Then flow cytometry
evaluation was performed using flow cytometry
(Becton-Dickinson).
Western blot analysis
Tumor tissue and cells were harvested, lysed and
total protein was extracted with RIPA buffer (50 mM Tris-Cl pH 7.4,
150 mM NaCl, 1% NP-40, 0.25% Na-deoxycholate, 1 mM EDTA, 1 mM NaF)
together with a protease inhibitor cocktail (Sigma-Aldrich).
Lysates were resolved on 10% SDS-PAGE and immunoblotted with the
indicated antibodies as GLP-1R antibody (ab39072; Abcam), AMPKa
antibody (Cell Signaling Technology, Beverly, MA, USA),
Phospho-AMPKa (Thr172) antibody (Cell Signaling Technology), mTOR
antibody (Cell Signaling Technology, Beverly), Phospho-mTOR
(Ser2448) antibody (Cell Signaling Technology), caspase-3 (Cell
Signaling Technology), GAPDH antibody (Cell Signaling Technology)
and β-actin (Cell Signaling Technology). The membranes were
incubated with secondary antibodies (1:10,000, DyLight 800; Thermo
Fisher Scientific, Waltham, MA, USA) at room temperature for 1 h.
The membranes were imaged with the Odyssey Infrared Imaging System
(LI-COR Biosciences, Lincoln, NE, USA).
Statistical analysis
The data are expressed as mean ± SD. The study
variables were compared between the study groups using t-test for
normal distribution, grouped Wilcoxon rank sum test for skewed
distribution. All the calculations were two tailed. P<0.05 was
considered as statistically significant.
Results
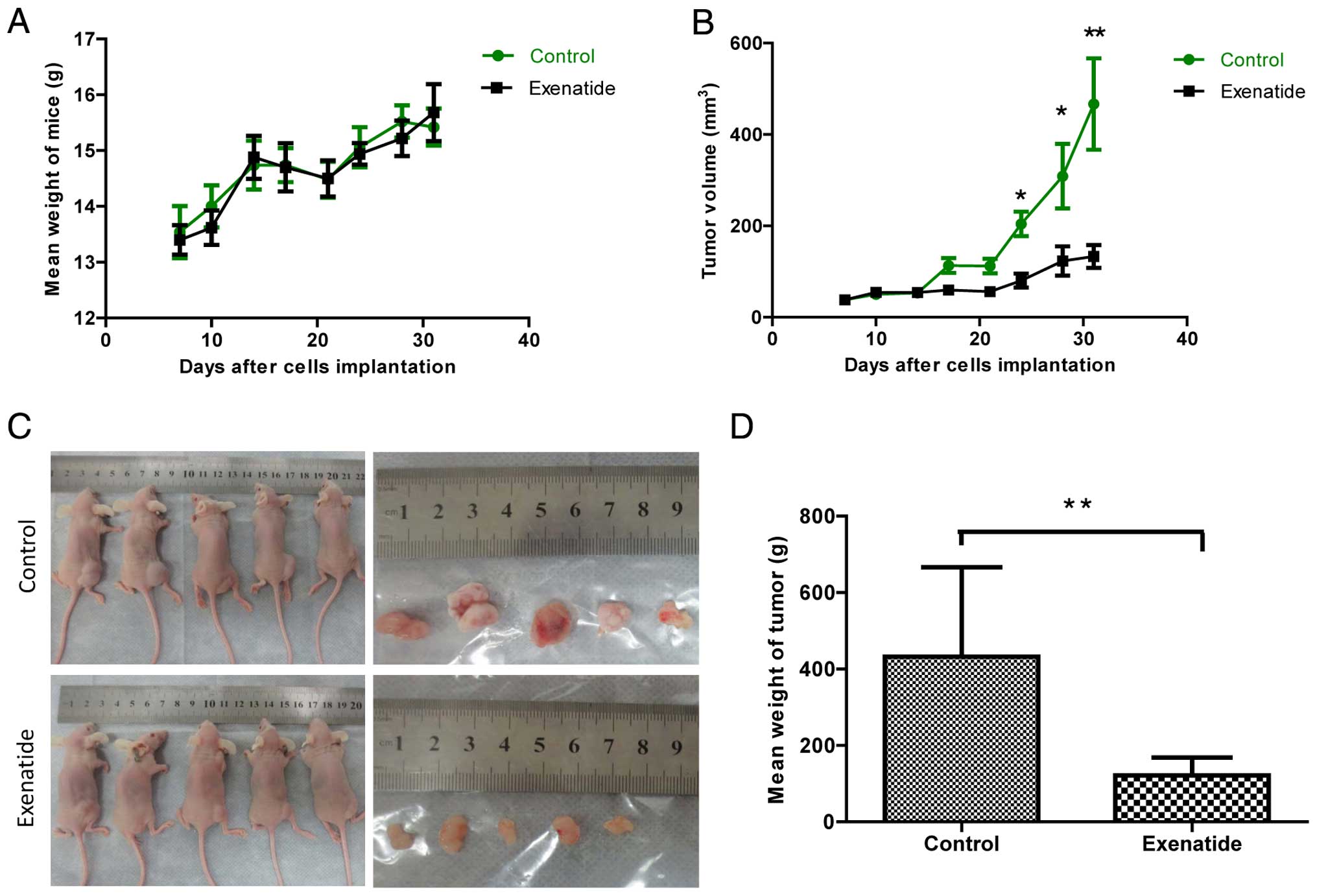
Exenatide inhibits the growth of
endometrial cancer Ishikawa xenografts in nude mice
At the 7th day after Ishikawa cell implantation, the
mean tumor volume (V) of subcutaneous tumor in nude mice was
38.4±9.92 mm3, and the mice were randomly divided into
control group [N=5, V=(38.4±9.1) mm3] and exenatide
group [N=5, V=(38.4±11.7) mm3]. During the intervention,
diet, activities and mental state of the nude mice were normal, and
all were alive until the end of the intervention. The mouse body
weight of two groups increased stably during the experiment period
without significant differences (P>0.05; Fig. 1A). According to the tumor growth
curves, the tumor growth rate of exenatide group was slower than
that in control group, especially at days 24, 28 and 31 (P<0.05,
P<0.01; Fig. 1B). At the end of
the intervention, the mean tumor volume of exenatide group and
control group was 125.9±20.4 and 440.2±117.1 mm3,
respectively (P<0.01; Fig. 1B),
and the mean tumor weight of exenatide group (116.9±47.1 mg) was
significantly lower than that in control group (440.2±142.5 mg)
(P<0.01; Fig. 1C and D).
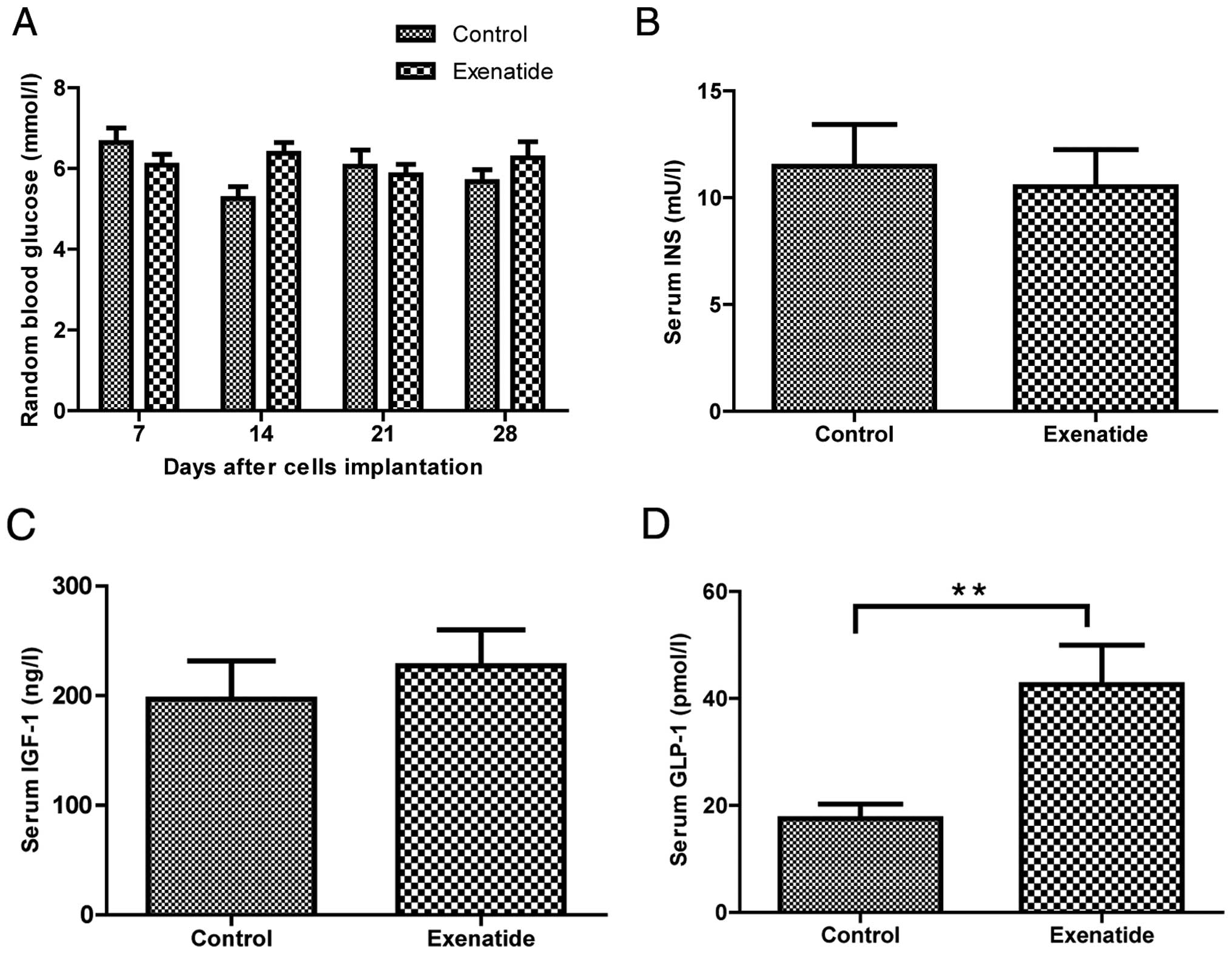
Higher serum GLP-1 level in exenatide
group
Random blood glucose of the mice was monitored every
7 days during the experiment, and it was in the normal range of
5–7.4 mmol/l in exenatide group and 4.9–7.7 mmol/l in control
group, respectively (P>0.05; Fig.
2A). At the end of the intervention, serum insulin value was
11.09±1.60 mU/l in exenatide group and 9.05±0.85 mU/l in control
group without significant difference (P>0.05; Fig. 2B). Serum IGF-1 value was 265.6±8.4
ng/ml in exenatide group and 276±8.1 ng/ml in control group without
significant difference (P>0.05; Fig.
2C). However, serum GLP-1 value of the nude mice was 41.01±4.78
pmol/l in exenatide group and 15.70±0.73 pmol/l in control group
with significant difference (P<0.01; Fig. 2D). Thus, exenatide did not change
blood glucose, serum insulin or IGF-1 level in the non-diabetes
mice. In addition, higher serum GLP-1 level in exenatide group was
observed.
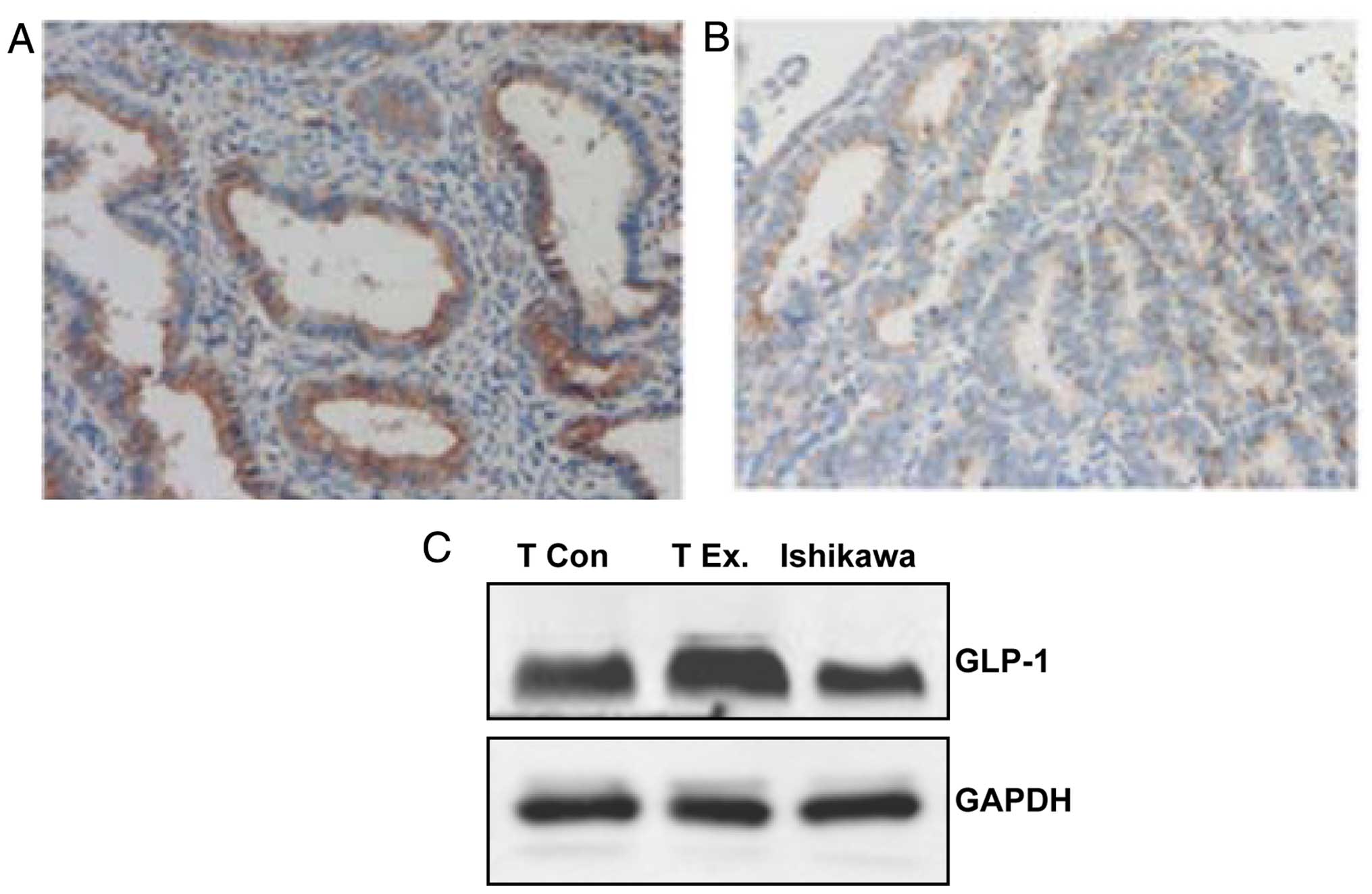
GLP-1 receptor is expressed in
endometrial cancer tissue and cells
We attempted to detect GLP-1R in the endometrial
cancer tissue and cells. Immunohistochemical analysis of GLP-1R was
performed in human normal endometrium tissues (Fig. 3A) and endometrial cancer tissues
(Fig. 3B). GLP-1R was expressed in
all of the 20 cases. GLP-1R was also detected by western blot
analysis in Ishikawa cell line and the tumor tissue of the Ishikawa
cell xenografts in nude mice, and the results were positive
(Fig. 3C). Thus, higher level of
GLP-1 might bind to GLP-1R to make function directly.
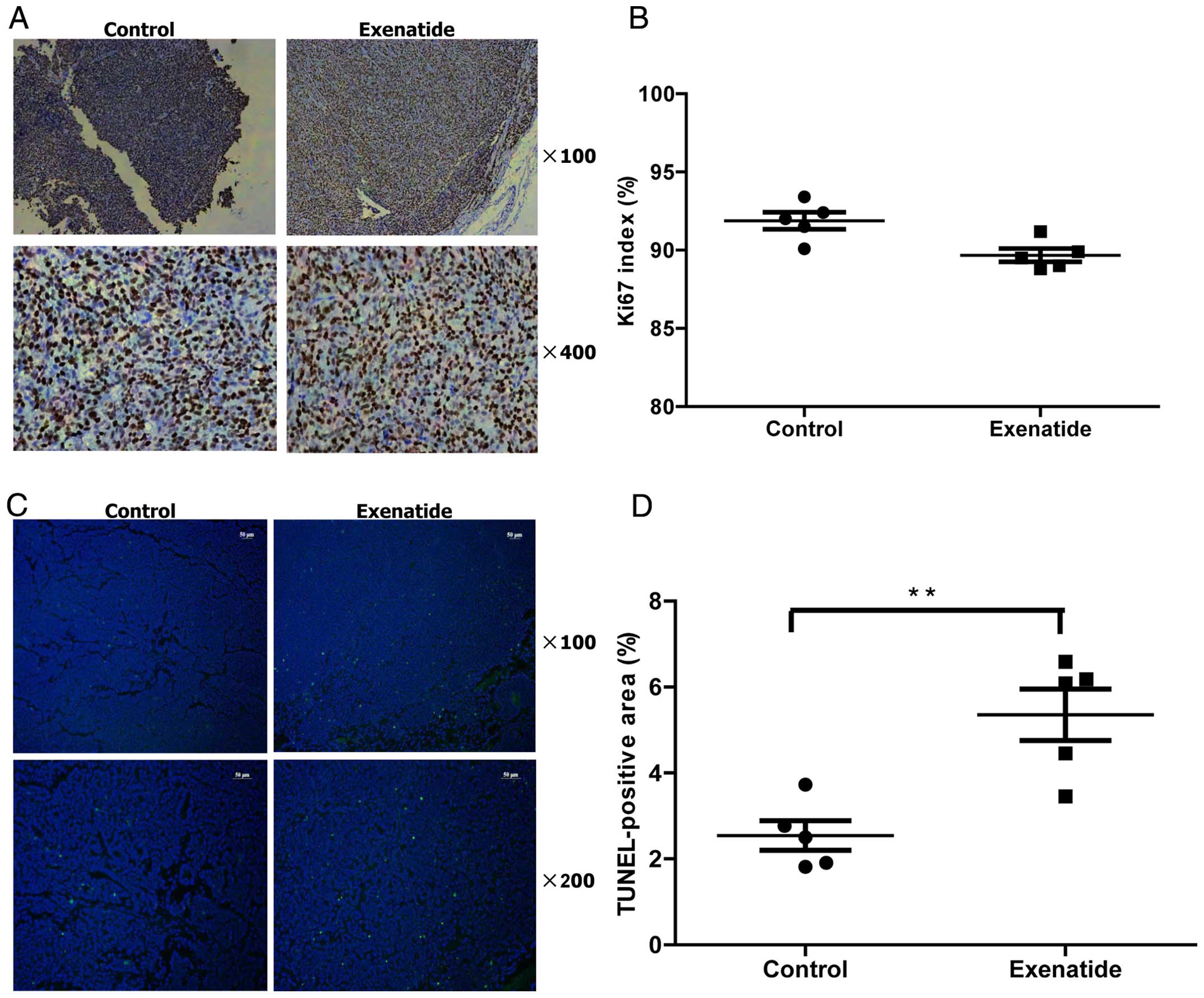
Exenatide promotes apoptosis to attenuate
tumor growth
The proportion of Ki-67 positive cells of the entire
tumor cells stands for the tumor proliferation index, and it was
(89.6±0.9%) in exenatide group and (91.1±1.35%) in control group,
respectively, without significant difference (P>0.05; Fig. 4A). Immunofluorescence TUNEL
detection showed that the apoptosis rate was significantly higher
in exenatide group (5.4±1.3%) than that in control group (2.5±0.7%)
(P<0.01; Fig. 4B). Effect of
exendin-4 on endometrial cancer cell line Ishikawa growth was
determined by MTT assay and colony formation assay. The results
showed that exendin-4 did attenuate the viability of Ishikawa cells
(P<0.05, P<0.01; Fig. 5A and
B). Based on the result of the MTT assay, apoptosis rate was
detected when Ishikawa cells were treated with 0 or 10 nM of
exendin-4 for 48 h, and the apoptosis rate was significantly higher
in the group treated with 10 nM of exendin-4 (P<0.01; Fig. 5C and D).
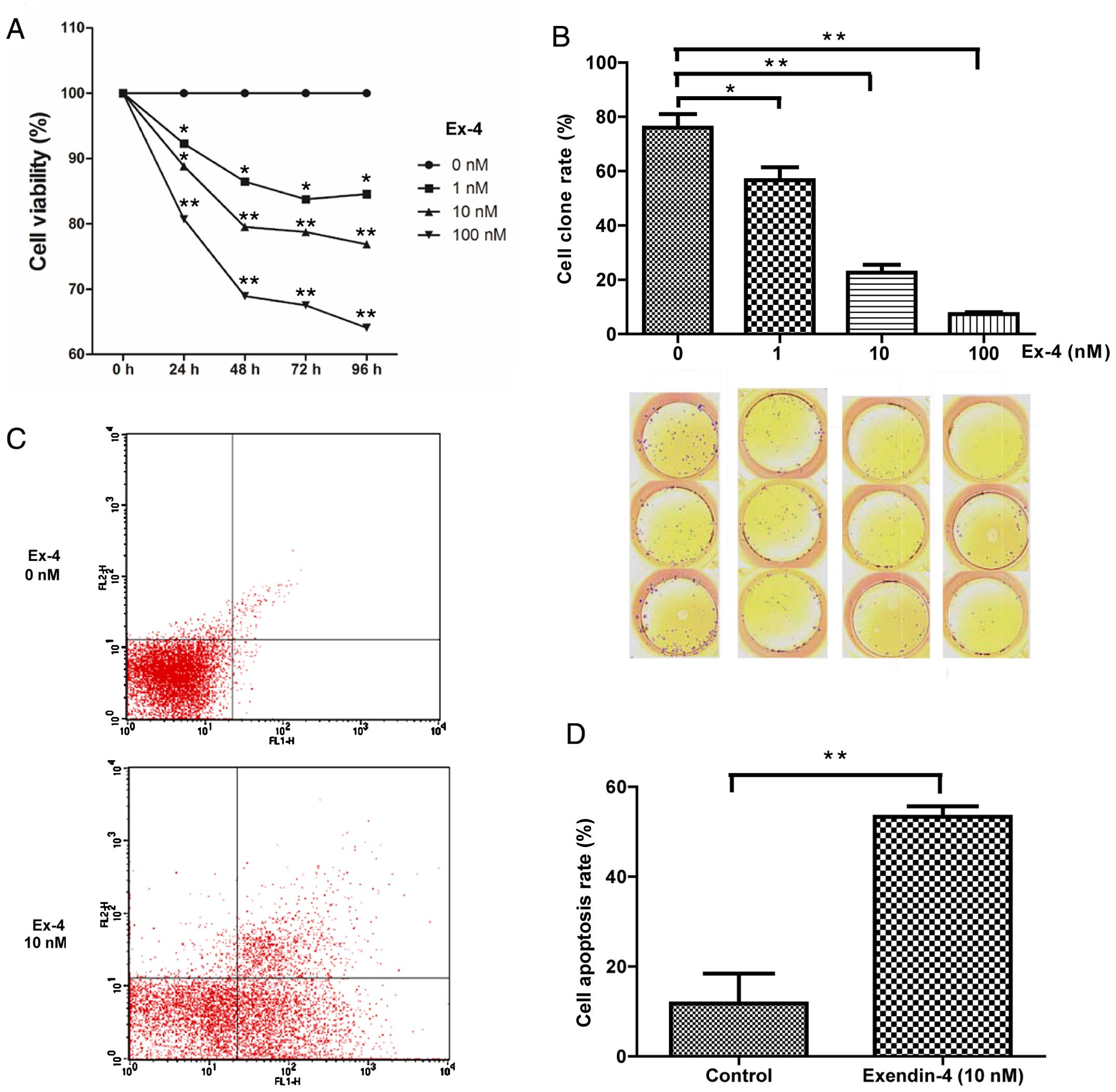 | Figure 5Exendin-4 inhibits endometrial cancer
Ishikawa cell line viability. (A) MTT assay result, comparing to 0
nM, Ishikawa cell viability with 1, 10 and 100 nM of exendin-4
(Ex-4) were significantly lower at 24, 48, 72 and 96 h
(*P<0.05, **P<0.01). (B) Ishikawa cells
were treated with Ex-4 (0, 1, 10 and 100 nM). The whole pictures of
clones are shown with bar graphs (*P<0.05,
**P<0.01). (C and D) Apoptosis analysis, Ishikawa
cells were treated with Ex-4 (0 or 10 nM) for 48 h, harvested and
stained as described in Materials and methods. Representative
results are shown (C). Results of three independent experiments are
shown in bars (D) (**P<0.01). |
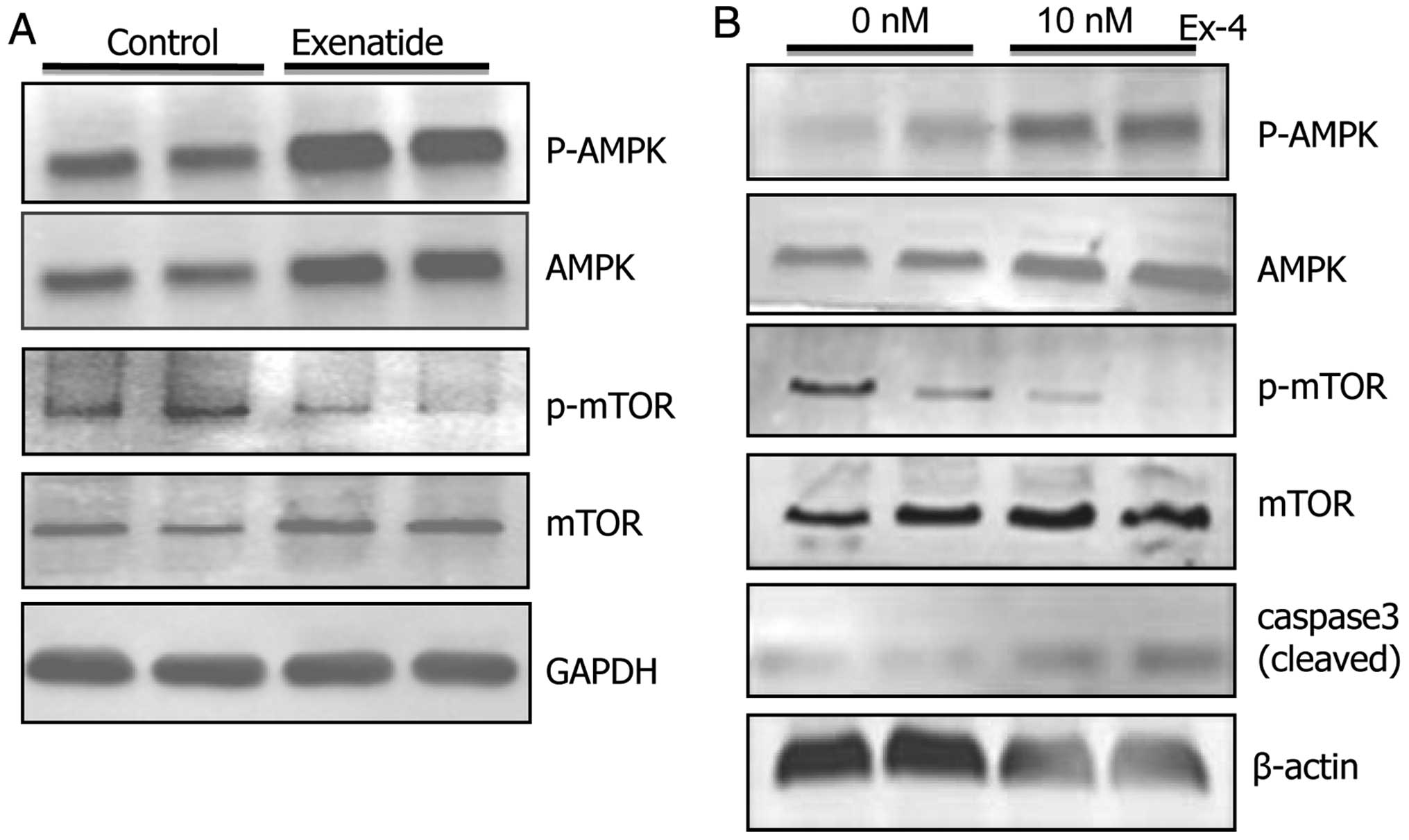
Exenatide regulates AMPK-mTOR signaling
to promote apoptosis
The role of AMPK has been proved as the key of a
series of complex molecular events, and AMPK-mTOR signaling pathway
regulates apoptosis (21). To test
whether the apoptosis action of exenatide is mediated by AMPK-mTOR
signaling, protein level of AMPK and mTOR were examined in Ishikawa
xenografts. The results showed that phosphorylated-AMPK protein
increased and phosphorylated-mTOR protein decreased (Fig. 6A). As confirmation, we examined
AMPK-mTOR signaling in vitro. Again, we observed that
phosphorylated-AMPK protein increased, phosphorylated-mTOR protein
decreased and cleaved caspase-3 increased in exendin-4 group (10
nM) (Fig. 6B). Moreover, when
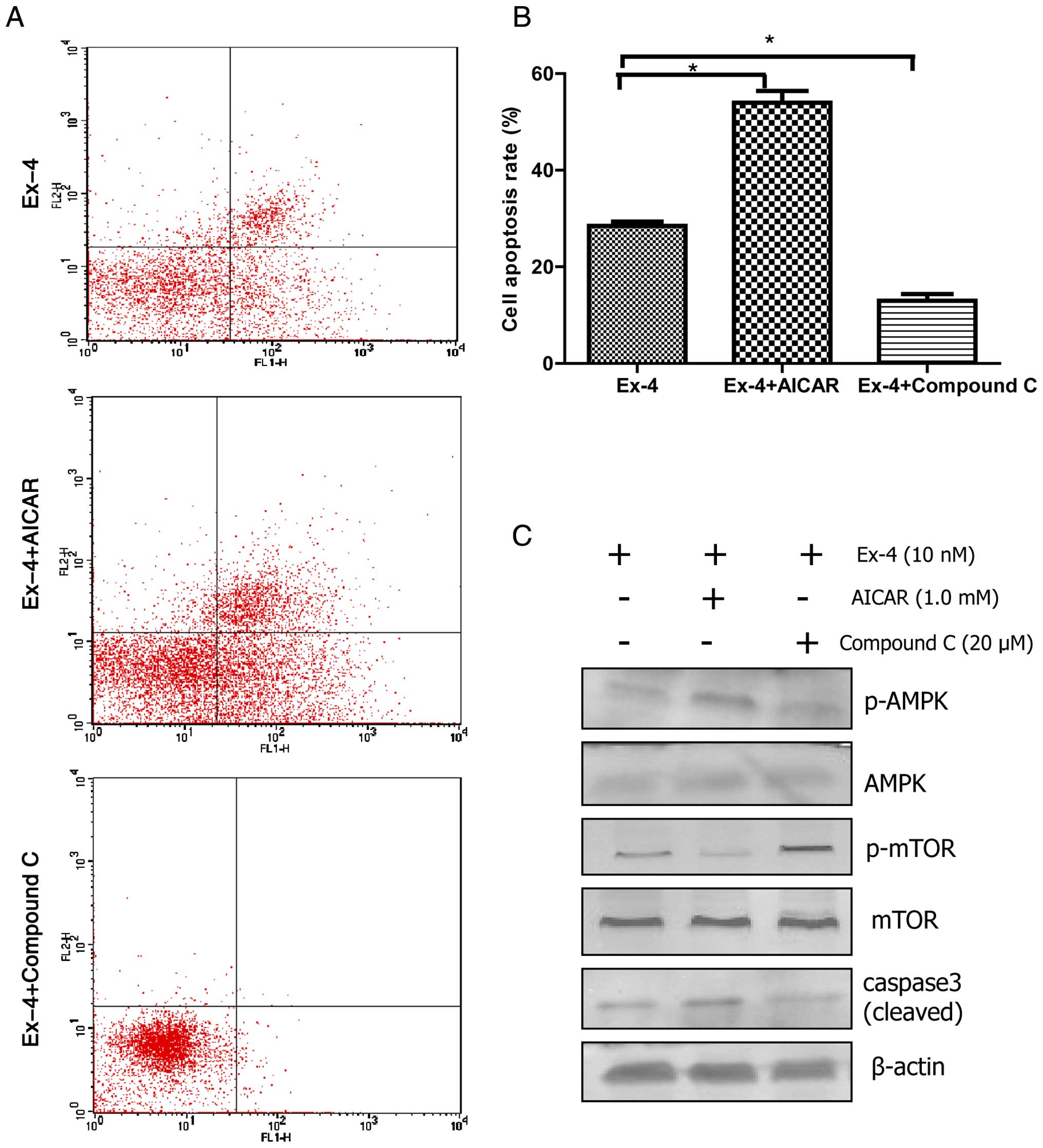
Ishikawa cells were treated with exendin-4 plus AICAR, an AMPK
activator, cell apoptosis increased with higher ratio of
phosphorylated-AMPK/AMPK, lower ratio of phosphorylated-mTOR/mTOR
and higher expression of cleaved caspase-3 than those in exendin-4
group, and the results were the opposite when cells were treated
with exendin-4 plus compound C, and AMPK inhibitor (Fig. 7). Thus, exendin-4 might
phosphorylate AMPK which results in the reduced phosphorylation of
mTOR and promoted apoptosis.
Discussion
Exenatide, as an early listed GLP-1 receptor
agonist, has become a widely-used anti-diabetic treatment
throughout the world with many benefits. However, the current
considerable interest in incretin therapy has raised the issue of
its long-term safety including the risk of carcinogenesis. We
report in the present study that exenatide could attenuate
endometrial cancer Ishikawa cell xenografts growth, which is
consistent with the results of the studies reported recently. Chen
et al (22) reported that
exendin-4 enhances the effect of chemotherapy in bile duct
carcinoma in vivo. Another study (23) revealed that a GLP-1 analogue
liraglutide inhibits the growth of pancreatic cancer in an animal
model. In addition, Honors et al (24) found that the application of
exendin-4 in animal models of malignant ascites tumor inhibits the
tumor growth and improve cachexia, and Nomiyama et al
(25) showed exendin-4 attenuates
prostate cancer growth.
Obesity and T2DM have been found to be associated
with increased endometrial cancer risk and adverse prognosis among
endometrial cancer patients (2–6). The
mechanisms involved in this interaction are not fully elucidated
and might be related with the increased blood glucose, serum
insulin levels, activation of the IGF-1 pathway, and production of
sex hormones by the adipose tissues (26). In this study, we found that
exenatide elevated the serum GLP-1 level but had no significant
effect on blood glucose, insulin and IGF-1 levels (Fig. 2) in the healthy young nude mice. The
higher serum GLP-1 in exenatide-treated group might be one of the
factors of inhibiting the human endometrial cancer Ishikawa cell
xenograft in nude mice. We detected GLP-1R in human endometrial
cancer cells, and the results suggested that GLP-1R is abundantly
expressed both in cancerous and non-cancerous endometrial cells
(Fig. 3), which is consistent with
the results of classic or functional GLP-1R existing in breast
cancer, colon cancer and prostate cancer cells (17,18,25).
Thus, exenatide-attenuated endometrial cancer growth might be
mediated by GLP-1R signaling.
Endometrial cancer is strongly associated with
obesity and diabetes (2,3). The IGF system has also been linked
with obesity, diabetes, hyperinsulinemia, and several human
malignancies including endometrial cancer (27). The signal pathway of
IGF-1R/PI3K/Akt/mTOR had been revealed to be upregulated in
endometrial cancer in our previous study and other studies
(28–30). Moreover, mTOR is upregulated in many
cancers as a result of genetic alterations or aberrant activation
of components of the PI3K/AKT pathway, which contributes to the
dysregulation of cell proliferation, growth, differentiation and
survival (31–33). The PI3K/AKT/mTOR pathway was
inhibited by phospholipids and phosphatases, such as PTEN, and
resulted in tumor suppression (34,35).
AMPK plays an important role among a series of complex molecular
events, including lipid metabolism, carbohydrate metabolism,
protein synthesis, cell apoptosis, angiogenesis, anti-inflammatory,
and regulation on cell senescence (36,37).
AMPK is at the pivotal site of PI3K-AKT, mTOR and other signal
pathways (21). AMPK activation
triggers the regulation of multiple downstream pathways, including
mTOR. AMPK mediates its effect on cell growth through inhibition of
mTOR (38), and mTOR signaling
pathway promoted the apoptosis-related protein caspase-3 expression
to affect the occurrence and development of tumor, which has
brought new opportunities for the treatment of metabolic diseases
and cancer (21). In our in
vivo study, the mechanism of exenatide inhibiting the growth of
endometrial cancer cell Ishikawa xenografts, maybe at least partly
includes activating AMPK phosphorylation and results in inhibiting
mTOR phosphorylation to promote apoptosis (Fig. 6A).
Further verification was conducted in vitro.
From the results of MTT and cloning analysis with exendin-4 of
different concentrations, exendin-4 inhibited the growth of
endometrial cancer Ishikawa cells at a dose- and time-dependent
manner (Fig. 5A and B). Exendin-4
promoted Ishikawa cell apoptosis (Fig.
5C and D), and the expression ratios of
phosphorylated-AMPK/AMPK and phosphorylated-mTOR/mTOR were
consistent with the result in vivo, which resulted in
increasing the expression level of apoptosis protein caspase-3
(Fig. 6B).
The in vitro study (17) on breast cancer cells suggests that
exendin-4 can increase intracellular cyclic adenosine monophosphate
(cAMP) value which promotes cAMP related apoptosis factor p38
expression through functional GLP-1R. The study (18) on colon cancer cells shows that
exendin-4 also increases the expression of apoptosis protein
caspase-3 by GLP-1R signaling. The study (25) on prostate cancer suggested that
exendin-4 attenuates prostate cancer growth through GLP-1R
signaling of inhibition of ERK-MAPK activation. Exendin-4 was able
to upregulate AMPK phosphorylation to inhibit lipid synthesis
(19,20), reduce glomerular mesangial cell
proliferation and fibronectin in high glucose induced rats partly
through upregulation of AMPK phosphorylation (39). Thus, we preliminarily conclude that
exenatide (exendin-4) inhibits endometrial cancer Ishikawa growth
through phosphorylating AMPK through GLP-1R signaling, which is
similar to the mechanism of metformin on endometrial cancer cells
(7–9). We conducted an experiment to confirm
the results showing AMPK agonist AICAR enhanced Ishikawa cell
apoptosis by upregulating AMPK phosphorylation, while the AMPK
inhibitor compound C effect was the opposite (Fig. 7).
The present study suggested that exenatide did not
enhance endometrial cancer growth, but even promote apoptosis to
slow down the growth of endometrial cancer both in vivo and
in vitro. However, studies on this class of medications on
malignant diseases is limited. More endometrial carcer cell
xenograft models, with different dose groups and positive control
group with diabetes need to be conducted to confirm the results
conclusively. The mechanistic and epidemiological investigations on
whether this class of medication affects the biological behavior or
risk of endometrial cancer, or other malignant disease development
are important.
In conclusion, our results suggest that exenatide
could attenuate the growth of endometrial cancer Ishikawa
xenografts in nude mice, and AMPK may be the target of the
underlying mechanism.
Acknowledgments
The present study was supported by grants from the
NSFC-CIHR (81261120565 to J.W.), the PCSIRT (82000-18811100 to
J.W.), and the National Natural Science Foundation of China Grant
Award (81300705 to F.X.).
References
|
1
|
Barakat RR, Grisby PW and Sabbatini:
Corpus: epithelial tumor. Principles and Practice of Gynecologic
Oncology. Hoskin WJ, Perez CA and Young RC: 2nd edition. Lippincott
Williams & Wilkins; Philadelphia, PA: pp. 919–959. 2007
|
|
2
|
Fader AN, Arriba LN, Frasure HE and von
Gruenigen VE: Endometrial cancer and obesity: Epidemiology,
biomarkers, prevention and survivorship. Gynecol Oncol.
114:121–127. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
von Gruenigen VE, Gil KM, Frasure HE,
Jenison EL and Hopkins MP: The impact of obesity and age on quality
of life in gynecologic surgery. Am J Obstet Gynecol. 193:1369–1375.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Giovannucci E, Harlan DM, Archer MC,
Bergenstal RM, Gapstur SM, Habel LA, Pollak M, Regensteiner JG and
Yee D: Diabetes and cancer: A consensus report. Diabetes Care.
33:1674–1685. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chia VM, Newcomb PA, Trentham-Dietz A and
Hampton JM: Obesity, diabetes, and other factors in relation to
survival after endometrial cancer diagnosis. Int J Gynecol Cancer.
17:441–446. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Calle EE, Rodriguez C, Walker-Thurmond K
and Thun MJ: Overweight, obesity, and mortality from cancer in a
prospectively studied cohort of U.S. adults. N Engl J Med.
348:1625–1638. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pollak M: Metformin and other biguanides
in oncology: Advancing the research agenda. Cancer Prev Res
(Phila). 3:1060–1065. 2010. View Article : Google Scholar
|
|
8
|
Xie Y, Wang YL, Yu L, Hu Q, Ji L, Zhang Y
and Liao QP: Metformin promotes progesterone receptor expression
via inhibition of mammalian target of rapamycin (mTOR) in
endometrial cancer cells. J Steroid Biochem Mol Biol. 126:113–120.
2011. View Article : Google Scholar
|
|
9
|
Emami Riedmaier A, Fisel P, Nies AT,
Schaeffeler E and Schwab M: Metformin and cancer: From the old
medicine cabinet to pharmacological pitfalls and prospects. Trends
Pharmacol Sci. 34:126–135. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Smith U and Gale EA: Does diabetes therapy
influence the risk of cancer? Diabetologia. 52:1699–1708. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gallagher EJ and LeRoith D: The
proliferating role of insulin and insulin-like growth factors in
cancer. Trends Endocrinol Metab. 21:610–618. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Amori RE, Lau J and Pittas AG: Efficacy
and safety of incretin therapy in type 2 diabetes: Systematic
review and meta-analysis. JAMA. 298:194–206. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bastien-Dionne PO, Valenti L, Kon N, Gu W
and Buteau J: Glucagon-like peptide 1 inhibits the sirtuin
deacetylase SirT1 to stimulate pancreatic β-cell mass expansion.
Diabetes. 60:3217–3222. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bose AK, Mocanu MM, Carr RD, Brand CL and
Yellon DM: Glucagon-like peptide 1 can directly protect the heart
against ischemia/reperfusion injury. Diabetes. 54:146–151. 2005.
View Article : Google Scholar
|
|
15
|
Kimura R, Okouchi M, Fujioka H, Ichiyanagi
A, Ryuge F, Mizuno T, Imaeda K, Okayama N, Kamiya Y, Asai K, et al:
Glucagon-like peptide-1 (GLP-1) protects against
methylglyoxal-induced PC12 cell apoptosis through the
PI3K/Akt/mTOR/GCLc/redox signaling pathway. Neuroscience.
162:1212–1219. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Elashoff M, Matveyenko AV, Gier B,
Elashoff R and Butler PC: Pancreatitis, pancreatic, and thyroid
cancer with glucagon-like peptide-1-based therapies.
Gastroenterology. 141:150–156. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ligumsky H, Wolf I, Israeli S, Haimsohn M,
Ferber S, Karasik A, Kaufman B and Rubinek T: The peptide-hormone
glucagon-like peptide-1 activates cAMP and inhibits growth of
breast cancer cells. Breast Cancer Res Treat. 132:449–461. 2012.
View Article : Google Scholar
|
|
18
|
Koehler JA, Kain T and Drucker DJ:
Glucagon-like peptide-1 receptor activation inhibits growth and
augments apoptosis in murine CT26 colon cancer cells.
Endocrinology. 152:3362–3372. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Samson SL and Bajaj M: Direct actions of
GLP-1 analogues on AMP-activated protein kinase activity are
distinct from cyclic AMP accumulation. J Hepatol. 58:634–635. 2013.
View Article : Google Scholar
|
|
20
|
Xu F, Li Z, Zheng X, Liu H, Liang H, Xu H,
Chen Z, Zeng K and Weng J: SIRT1 mediates the effect of GLP-1
receptor agonist exenatide on ameliorating hepatic steatosis.
Diabetes. 63:3637–3646. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang BB, Zhou G and Li C: AMPK: An
emerging drug target for diabetes and the metabolic syndrome. Cell
Metab. 9:407–416. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen BD, Zhao WC, Jia QA, Zhou WY, Bu Y,
Wang ZZ, Wang F, Wu WJ and Wang Q: Effect of the GLP-1 analog
exendin-4 and oxaliplatin on intrahepatic cholangiocarcinoma cell
line and mouse model. Int J Mol Sci. 14:24293–24304. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao H, Wang L, Wei R, Xiu D, Tao M, Ke J,
Liu Y, Yang J, Hong T, Yang J, et al: Activation of glucagon-like
peptide-1 receptor inhibits tumourigenicity and metastasis of human
pancreatic cancer cells via PI3K/Akt pathway. Diabetes Obes Metab.
16:850–860. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Honors MA and Kinzig KP: Chronic exendin-4
treatment prevents the development of cancer cachexia symptoms in
male rats bearing the Yoshida sarcoma. Horm Cancer. 5:33–41. 2014.
View Article : Google Scholar :
|
|
25
|
Nomiyama T, Kawanami T, Irie S, Hamaguchi
Y, Terawaki Y, Murase K, Tsutsumi Y, Nagaishi R, Tanabe M, Morinaga
H, et al: Exendin-4, a GLP-1 receptor agonist, attenuates prostate
cancer growth. Diabetes. 63:3891–3905. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Samani AA, Chevet E, Fallavollita L,
Galipeau J and Brodt P: Loss of tumorigenicity and metastatic
potential in carcinoma cells expressing the extracellular domain of
the type 1 insulin-like growth factor receptor. Cancer Res.
64:3380–3385. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Augustin LS, Dal Maso L, Franceschi S,
Talamini R, Polesel J, Kendall CW, Jenkins DJ and Vidgen E:
Association between components of the insulin-like growth factor
system and endometrial cancer risk. Oncology. 67:54–59. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hirano S, Ito N, Takahashi S and Tamaya T:
Clinical implications of insulin-like growth factors through the
presence of their binding proteins and receptors expressed in
gynecological cancers. Eur J Gynaecol Oncol. 25:187–191.
2004.PubMed/NCBI
|
|
29
|
Pavelić J, Radaković B and Pavelić K:
Insulin-like growth factor 2 and its receptors (IGF 1R and IGF
2R/mannose 6-phosphate) in endometrial adenocarcinoma. Gynecol
Oncol. 105:727–735. 2007. View Article : Google Scholar
|
|
30
|
Shu S, Li X, Yang Y, Zhang Y, Li T, Liang
C and Wan J: Inhibitory effect of siRNA targeting IGF-1R on
endometrial carcinoma. Int Immunopharmacol. 11:244–249. 2011.
View Article : Google Scholar
|
|
31
|
Navarro M and Baserga R: Limited
redundancy of survival signals from the type 1 insulin-like growth
factor receptor. Endocrinology. 142:1073–1081. 2001.PubMed/NCBI
|
|
32
|
Schmelzle T and Hall MN: TOR, a central
controller of cell growth. Cell. 103:253–262. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mita MM, Mita A and Rowinsky EK: Mammalian
target of rapamycin: A new molecular target for breast cancer. Clin
Breast Cancer. 4:126–137. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Feng Z, Zhang H, Levine AJ and Jin S: The
coordinate regulation of the p53 and mTOR pathways in cells. Proc
Natl Acad Sci USA. 102:8204–8209. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cantley LC and Neel BG: New insights into
tumor suppression: PTEN suppresses tumor formation by restraining
the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA.
96:4240–4245. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hardie DG: AMPK: A key regulator of energy
balance in the single cell and the whole organism. Int J Obes.
32(Suppl 4): S7–S12. 2008. View Article : Google Scholar
|
|
37
|
Kahn BB, Alquier T, Carling D and Hardie
DG: AMP-activated protein kinase: Ancient energy gauge provides
clues to modern understanding of metabolism. Cell Metab. 1:15–25.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Inoki K, Zhu T and Guan KL: TSC2 mediates
cellular energy response to control cell growth and survival. Cell.
115:577–590. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xu WW, Guan MP, Zheng ZJ, Gao F, Zeng YM,
Qin Y and Xue YM: Exendin-4 alleviates high glucose-induced rat
mesangial cell dysfunction through the AMPK pathway. Cell Physiol
Biochem. 33:423–432. 2014. View Article : Google Scholar : PubMed/NCBI
|