Introduction
Remarkable progress has been made in chemotherapy
for colorectal cancer during the last decade. Currently, standard
first-line treatments for unresectable advanced or recurrent
colorectal cancer include fluorouracil with irinotecan or
oxaliplatin, alone or in combination with molecular-targeted
agents, such as the monoclonal antibody against vascular
endothelial growth factor (VEGF) and epidermal growth factor
receptor (EGFR) (1,2). Combinations of drug therapies in
unresectable advanced or recurrent colorectal cancer patients has
prolonged the survival time to more than 30 months (3–5), but
the therapeutic effects vary depending on each case. Thus, it is
important to predict the chemotherapeutic effect and select
patients who will benefit from cancer chemotherapy. Several studies
have shown that various biomarkers predict the sensitivity to
chemotherapy or chemoradiation therapy. The presence of
microsatellite instability (MSI) and mutations of the KRAS
gene are reliable biomarkers for sensitivity to fluorouracil and
anti-EGFR monoclonal antibodies, respectively (5,6).
However, there is no reliable biomarker for oxaliplatin and
irinotecan.
DNA intrastrand cross-linking agents such as
oxaliplatin induce DNA double-strand breaks (DSBs) during the
process of DNA replication and repair (7). BRCA1, 2, the MRE11-RAD50-NBS1 (MRN)
complex and RAD51 play an important role in homologous
recombination during DSB repair. A previous study showed that DNA
damage repair competence varies among individual breast tumors, and
is closely correlated with chemosensitivity (8). The Fanconi anemia-BRCA pathway
plays an important role in restoring cytotoxic damage by anticancer
agents and radiation (9,10). Furthermore, previous studies have
shown that BRCA-associated cancer is particularly sensitive to DNA
interstrand cross-linking agents such as mitomycin C or
platinum-based drugs (11,12). Differences in the expression of DNA
DSB repair proteins (DDRPs) among individual colon cancer cases may
also be related to the sensitivity to treatment, as well as breast
cancer.
MRE11 forms the core of the MRN complex, which has
essential roles in detection, signaling, protection and repair of
DSBs (13,14). RAD51 is an important factor in
homologous recombination as well as MRE11, and is a predictive
factor for chemoradiotherapy response in a variety of human
cancers. Moreover, overexpression of RAD51 confers resistance to
DNA interstrand cross-linking agents such as cisplatin in non-small
cell lung cancer (15–17).
In the present study, we hypothesized that DNA
intrastrand cross-linking agents may significantly benefit
colorectal cancer patients with deficiencies in DSB repair. We
investigated the expression of MRE11 and RAD51 by
immunohistochemistry. Associations between expression and
therapeutic effect in colorectal cancer patients were also
explored.
Materials and methods
Patients
Seventy-eight patients with metastatic or recurrent
colorectal cancer who had measurable target lesions such as
hepatic, pulmonary, lymphatic and peritoneal metastases, underwent
resection for primary colon and rectal cancer at our institution
between April 2007 and March 2013. All patients underwent
combination chemotherapy including oxaliplatin.
Assessments of therapeutic effect
Descriptions of the therapeutic effects were
evaluated using the best overall response to first-line
chemotherapy using RECIST version 1.1. Changes in tumor size were
expressed as the relative change in the sum of the longest
diameters of the target lesions. Non-target lesions and newly
occurring lesions were not considered in the measurement of tumor
size changes (18). The endpoints
of the long-term outcome study were progression-free survival
(PFS). PFS was calculated by progression of target lesions as the
only events for survival analyses.
Immunohistochemistry
Five-micrometer sections were deparaffinized with
xylene and rehydrated with alcohol, and placed in 0.1 M NaOH
citrase buffer (pH 7.0) for RAD51 immunostaining or 0.01 M NaOH
citrate buffer (pH 6.0) for MRE11 immunostaining, and heated in an
autoclave at 121°C for 15 min. Sections were then preincubated with
3% H2O2 in methanol for 30 min at room
temperature to quench endogenous peroxidase activity. After
blocking with normal goat serum, the sections were incubated with
mouse anti-RAD51 3C10 monoclonal antibody (1:800; clone 51RAD01;
Thermo Scientific, Fremont, CA, USA) and mouse anti-MRE11 12D7
monoclonal antibody (1:1600; ab214; Abcam, Cambridge, UK) for 60
min at 4°C. Thereafter, the sections were incubated with a
secondary antibody (Vectastain Elite ABC kit; Vector Laboratories,
Burlingame, CA, USA) for 30 min, washed with phosphate-buffered
saline (PBS) and treated with peroxidase-conjugated streptavidin
for 30 min. The sections were visualized by incubation with
diaminobenzidine tetrahydrochloride (Liquid DAB+ Substrate
Chromogen System; Dako, Carpinteria, CA, USA) and counterstained
with hematoxylin.
Evaluation of immunohistochemical
staining
Immunohistochemistry (IHC) scores for <10% of
nuclear staining in cancer cells were negative, whereas those cases
with IHC scores for >10% stained cells were deemed positive.
Statistical analysis
Categorical analysis of variables was performed
using either the Chi-square or Fisher's exact test, as appropriate.
Continuous data were compared with the Mann-Whitney U test.
Survival curves were plotted according to the Kaplan-Meier method,
and any differences were analyzed using the log-rank test. A
multivariate analysis with Cox proportional hazards model was
adopted to clarify the independent prognostic factors. Differences
were considered to be significant if the P-value was <0.05. All
statistical analyses were carried out using the R software (version
3.1.1).
Results
Patient clinicopathological
characteristics
Patient clinical characteristics are detailed in
Table I. The median age was 64.5
years, and the cohort consisted of 49 males and 29 females. Most
patients received combination chemotherapy in addition to
bevacizumab (n=70, 90%). Fifty patients received mFOLFOX6 +
bevacizumab and 20 patients received CapeOX + bevacizumab. The
median PFS was 10.9 months.
 | Table IClinicopathological characteristics of
the patients (n=78). |
Table I
Clinicopathological characteristics of
the patients (n=78).
| Factors | Date |
|---|
| Median age
(years) | 64.5 |
| Gender, n |
| Men | 49 |
| Female | 29 |
| Location, n |
| Proximal | 20 |
| Distal | 58 |
| Median tumor size
(mm) | 50 |
| Histology, n |
| Well
differentiated | 25 |
| Moderately
differentiated | 44 |
| Poorly
differentiated | 4 |
| Others | 5 |
| Site of metastases,
n |
| Liver | 44 |
| Lung | 21 |
| Peritoneum | 11 |
| Lymph node | 8 |
| Bone | 1 |
| Regimen, n |
| mFOLFOX6 +
bevacizumab | 50 |
| CapeOX +
bevacizumab | 20 |
| mFOLFOX6 | 7 |
| CapeOX | 1 |
| Treatment cycles,
mean number | 10 |
| Median overall
survival (months) | 32.5 |
| Median
progression-free survival (months) | 10.9 |
MRE11 expression and clinical
outcome
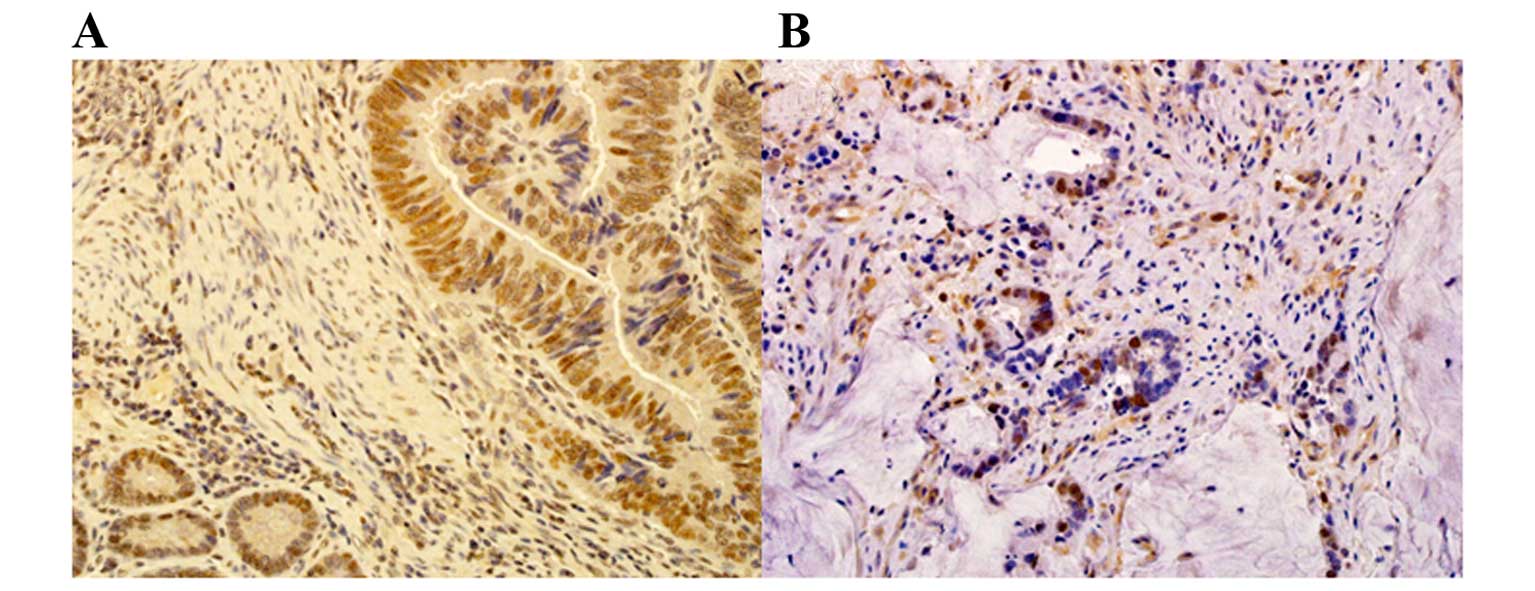
Positive nuclear staining of MRE11 was observed in
48 (61.5%) of the 78 cases (Fig.
1A). The association between MRE11 expression and
clinicopathological characteristics is shown in Table II. There was no significant
association between MRE11 expression and age, tumor location or
tumor size. Male gender and undifferentiated type tended to be
associated with MRE11 positivity. There was no significant
association between MRE11 expression and CEA reduction ratio.
MRE11-negative cases had significantly better relative change
compared with MRE1-positive cases. Thus, MRE11-negative cases
achieved better size reduction of the target lesion when compared
with MRE11-positive cases. The association between MRE11 expression
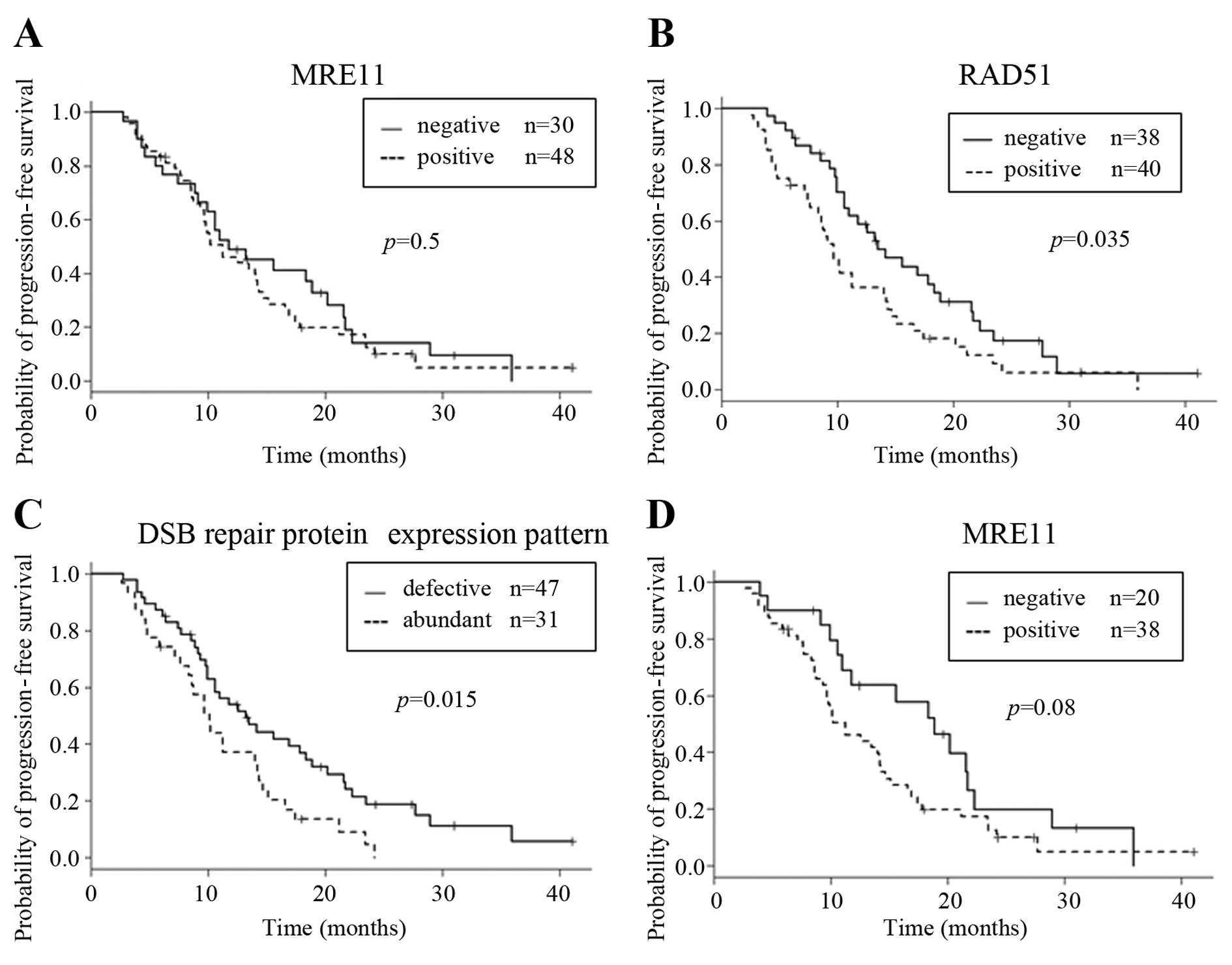
and prognosis is shown in Fig. 2A.
MRE11-positive cases exhibited poorer PFS when compared with
MRE11-negative cases, but no significant association was identified
between MRE11 expression and PFS.
 | Table IIRelationship between DDRP expression
and clinicopathological characteristics of the patients (n=90). |
Table II
Relationship between DDRP expression
and clinicopathological characteristics of the patients (n=90).
| Factors | MRE11
| P-value | RAD51
| P-value |
|---|
| Negative (n=30) | Positive (n=48) | Negative (n=38) | Positive (n=40) |
|---|
| Median age
(years) | 64 | 65.5 | 0.2 | 64 | 67 | 0.3 |
| Gender |
| Male | 15 | 34 | 0.064 | 26 | 23 | 0.3 |
| Female | 15 | 14 | | 12 | 17 | |
| Location |
| Proximal | 10 | 10 | 0.2 | 9 | 11 | 0.7 |
| Distal | 20 | 38 | | 29 | 29 | |
| Median tumor size
(mm) | 55 | 50 | 0.4 | 55 | 50 | 0.2 |
| Median treatment
cycles | 11.5 | 9.5 | 0.2 | 11.5 | 8 | 0.1 |
| Histological
type |
|
Differentiated | 29 | 40 | 0.080 | 35 | 34 | 0.3 |
|
Undifferentiated | 1 | 8 | | 3 | 6 | |
| Median serum CEA
level (ng/ml) |
| Before
treatment | 6.6 | 21.05 | 0.045 | 7.25 | 22.35 | 0.07 |
| 3 months
later | 5.25 | 10.1 | 0.047 | 4.45 | 12.65 | 0.003 |
| CEA reduction
ratio | 0.70 | 0.61 | 0.4 | 0.50 | 0.76 | 0.033 |
| Relative
change | 0.65 | 0.92 | 0.029 | 0.48 | 1.01 | <0.001 |
RAD51 expression and clinical
outcome
Positive nuclear staining of RAD51 was observed in
40 (51.2%) of the 78 cases (Fig.
1B). There was no significant association between RAD51
expression and age, gender, tumor location, tumor size or
histological type (Table II).
RAD51-negative cases had significantly better CEA reduction ratios
and relative change. RAD51-positive cases had significantly poorer
PFS when compared with RAD51-negative cases (Fig. 2B). However, for multivariate
analysis, RAD51 was not an independent prognostic factor (Table III).
 | Table IIIUnivariate and multivariate analyses
for progression-free survival. |
Table III
Univariate and multivariate analyses
for progression-free survival.
| Factor | Univariate
| Multivariate
|
|---|
| Median PFS | P-value | HR (95% CI) | P-value |
|---|
| Age (years) |
| >60 | 11.8 | 0.8 | | |
| ≤60 | 10.6 | | | |
| Gender |
| Male | 13.2 | 0.070 | | |
| Female | 10 | | | |
| Location |
| Proximal | 9.3 | 0.002 | 0.56
(0.31–1.00) | 0.051 |
| Distal | 13.5 | | | |
| Tumor size
(mm) |
| <50 | 11.3 | 0.5 | | |
| ≥50 | 11.3 | | | |
| Histology |
|
Undifferentiated | 11.8 | 0.1 | | |
|
Differentiated | 9.7 | | | |
| Treatment
cycles |
| <10 | 6.4 | 0.005 | 0.49
(0.30–0.80) | 0.005 |
| ≥10 | 14.2 | | | |
| CEA reduction
ratio |
| <0.6 | 11.3 | 0.4 | | |
| ≥0.6 | 11.3 | | | |
| Relative
change |
| <0.7 | 18.4 | <0.001 | 2.53
(1.39–4.62) | 0.002 |
| ≥0.7 | 9,0 | | | |
| MRE11 |
| Negative | 11.8 | 0.5 | | |
| Positive | 11.3 | | | |
| RAD51 |
| Negative | 13.5 | 0.035 | 0.80
(0.35–1.83) | 0.6 |
| Positive | 9.7 | | | |
| DSB repair protein
expression pattern |
| Defective | 13.2 | 0.015 | 1.39
(0.58–3.34) | 0.5 |
| Abundant | 10.1 | | | |
DSB repair protein expression
pattern
In the DSB repair pathway, the role of MRE11 and
RAD51 are sequential. When one of either is defect, it may be
impossible to recovery from DSBs. Therefore, we defined two groups.
The 'defective pattern' is described as the state when expression
of both MRE11 and RAD51 expression is negative or expression of
either one of these protein is negative. The 'abundant pattern' is
described as the state when expression of both proteins is
positive. In addition, we investigated the association between the
two groups and therapeutic effect. The association between
expression patterns and clinical characteristics or
chemotherapeutic effect is shown in Table IV. None of the examined
clinicopathological characteristics correlated with the expression
pattern. For the therapeutic effects, there was no significant
difference between expression pattern and CEA reduction ratio. The
defective pattern had significantly better relative change compared
with the abundant pattern. As shown in Fig. 2C, the median PFS for the defective
pattern and abundant pattern was 13.2 and 10.1 months,
respectively, and there was a significant difference between the
defective pattern and abundant pattern for PFS (Fig. 2C). Nonetheless, for the multivariate
analysis, the expression pattern or RAD51 expression alone were not
independent prognostic factors (Table
III).
 | Table IVRelationship between the DSB protein
expression pattern and clinicopathological characteristics. |
Table IV
Relationship between the DSB protein
expression pattern and clinicopathological characteristics.
| Factors | Defective
(n=47) | Abundant
(n=31) | P-value |
|---|
| Median age
(years) | 64 | 68 | 0.3 |
| Gender, n |
| Male | 30 | 19 | 0.8 |
| Female | 17 | 12 | |
| Location, n |
| Right | 13 | 7 | 0.8 |
| Left | 34 | 24 | |
| Median tumor size
(mm) | 55 | 50 | 0.6 |
| Median treatment
cycles | 11 | 8 | 0.1 |
| Histological type,
n |
|
Differentiated | 44 | 25 |
0.08a |
|
Undifferentiated | 3 | 6 | |
| Median serum CEA
level (ng/ml) |
| Before
treatment | 7 | 24 | 0.009 |
| 3 months
later | 4.7 | 12.8 | 0.001 |
| CEA reduction
ratio | 0.55 | 0.68 | 0.1 |
| Relative
change | 0.5 | 1.02 | <0.001 |
Distal colon cancer patients benefit more
from these ex vivo tests
MRE11 mutations occur in 83.7% of MMR-defective
primary colorectal cancers. MSI is displayed in ~15% of colorectal
cancer cases. We reviewed all subjects in the present study with
the exception of one case of tumor localization to a site proximal
to the splenic flexure due to the association of high-frequency MSI
with this tumor site. In these cases, MRE11-negative cases
exhibited longer PFS than the positive cases (P=0.077) (Fig. 2D). Moreover, by multivariate
analysis, DSB repair protein expression pattern was an independent
prognostic factor (P=0.036) (Table
V).
 | Table VUnivariate and multivariate analyses
for PFS in the distal colon. |
Table V
Univariate and multivariate analyses
for PFS in the distal colon.
| Factor | Univariate
| Multivariate
|
|---|
| Median | P-value | HR (95% CI) | P-value |
|---|
| Treatment
cycles |
| <10 | 8.8 | 0.009 | 0.46
(0.27–0.78) | 0.004 |
| ≥10 | 14.5 | | | |
| CEA reduction
ratio |
| <0.6 | 13.5 | 0.2 | | |
| ≥0.6 | 12.6 | | | |
| Relative
change |
| <0.7 | 18.9 | <0.001 | 2.23
(1.17–4.24) | 0.014 |
| ≥0.7 | 9.7 | | | |
| MRE11 |
| Negative | 18.9 | 0.077 | | |
| Positive | 11.3 | | | |
| RAD51 |
| Negative | 15.6 | 0.036 | 0.44
(0.15–1.37) | 0.2 |
| Positive | 10.1 | | | |
| DSB repair protein
expression pattern |
| Defective | 16.9 | 0.001 | 3.62
(1.09–11.99) | 0.036 |
| Abundant | 10.1 | | | |
Discussion
The recent development of chemotherapies such as
FOLFOX and FOLFIRI along with several molecular-targeting agents
has markedly improved the survival of unresectable colorectal
cancer patients. Previous studies have shown that the median
survival time was prolonged to 11–26 months in unresectable
advanced or recurrent colorectal cancer (19,20).
In the present study, we calculated the curative effect of
chemotherapy by determining the correlations between DSB repair
protein expression pattern and chemotherapeutic effect; those
patients exhibiting better relative changes in tumor size had
longer survival times. According to a previous study, the
chemotherapeutic effect by first-line treatment may be a prognostic
factor. Therefore, it is necessary to detect biomarkers that
predict the effect of first-line treatment to obtain further
chemotherapeutic effects.
Oxaliplatin is a DNA intrastrand cross-linking agent
and is frequently used to treat colorectal cancer that has spread.
The cytotoxic reaction of oxaliplatin is dependent on DSBs. In DSBs
defective cases, the chemotherapeutic effect of oxaliplatin may be
greater. MRE11 and RAD51 are important components of homologous
recombination, which functions in the repair of DSB. In the present
study, we examined the correlation between the expression of these
proteins and the chemotherapeutic effect in colorectal cancer
patients. RAD51 protein forms a helical nucleoprotein filament to
promote DNA strand exchange and stimulate DNA-pairing activity, the
basic steps of homologous recombination (21,22).
In the present study, patients negative for MRE11 or RAD51
expression obtained better size reduction of target lesions. We
showed that RAD51-negative cases achieved longer survival times
than positive cases. Several previous studies demonstrated that
RAD51 expression is correlated with resistance to chemotherapy and
survival in various types of cancer such as lung, breast and
esophageal cancer (23–25).
MRE11 is the core component of the MRN complex, the
primary sensor of DSBs (12,13).
In the present study, MRE11 expression was not an independent
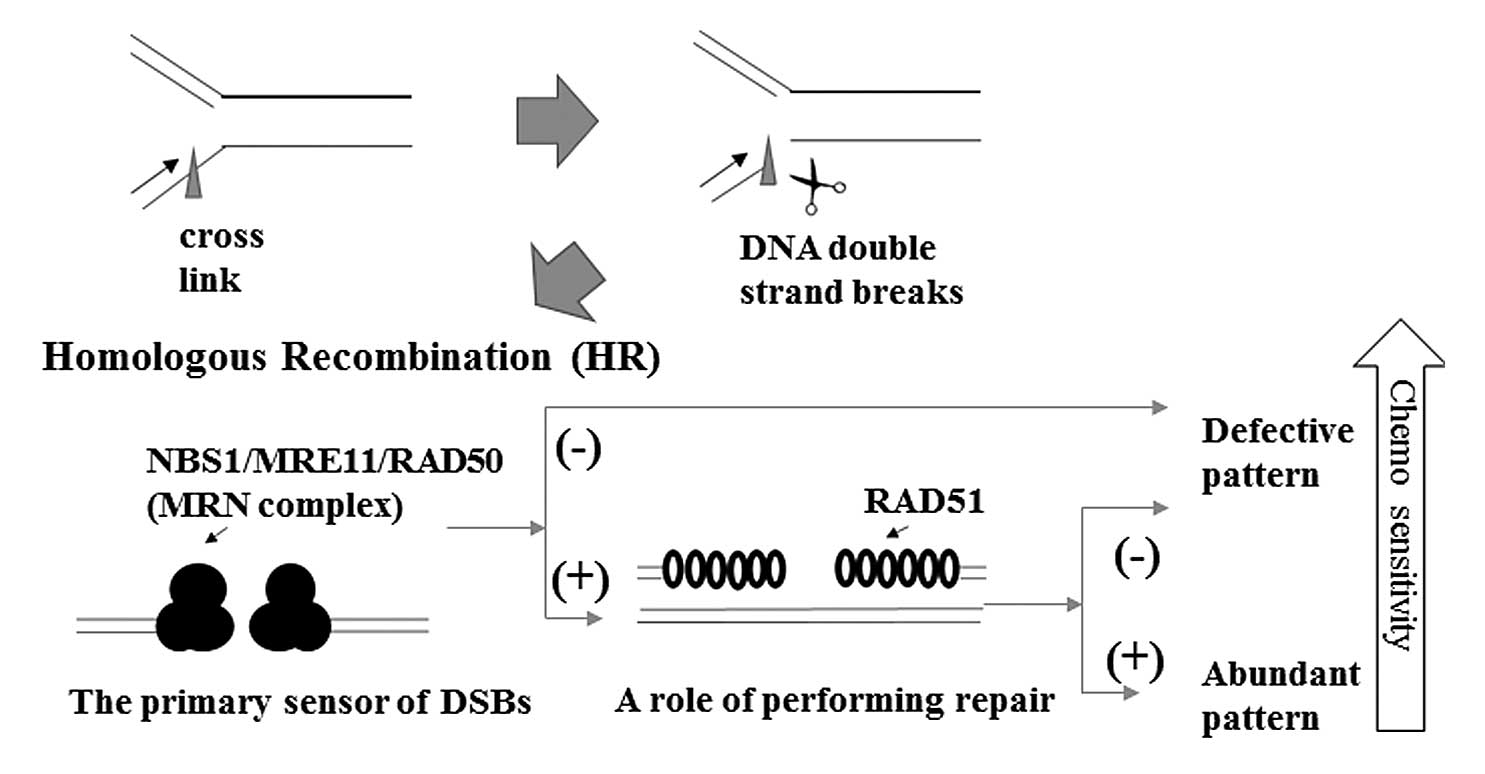
prognostic factor. The combined evaluation of MRE11, which acts as
a sensor, and RAD51, which functions in repair, may lead to a
better indication of the chemotherapeutic effect (Fig. 3). In fact, the relative change and
PFS were significantly different between the defective pattern and
abundant pattern, the expression pattern of DSB repair
proteins.
We investigated the reason for the difference
between MRE11 and RAD51 despite it also being a DSB repair protein,
and postulated that some factors that may affect MRE11 alone
intervened in the result. MRE11 mutations occur in 83.7% of
MMR-defective primary colorectal cancers (26,27).
Microsatellite instability (MSI) is displayed by ~15% of colorectal
cancer cases, and high levels of MSI may be a predictive marker for
lack of efficacy of fluorouracil-based therapy (28,29).
This raises the possibility that some MRE11-negative patients have
poor prognosis as a consequence of their MSI status. We reviewed
the subjects of our study with the exception of one case of tumor
localization to a site proximal to the splenic flexure due to the
association of high-frequency MSI with this tumor site (6). In this instance, MRE11 expression
tended to be related to PFS, and DSB repair protein expression
pattern is a factor that independently predicts PFS. In cases of
tumor development in the distal colon, DSB repair protein
expression may predict prognosis.
In the present study, we only examined cases with a
targeted lesion to clarify associations with chemotherapeutic
effect. Therefore, it is necessary to confirm expression in other
cases. Moreover, we only investigated cases receiving
oxaliplatin-based chemotherapy regimen as first-line treatment
since there were few cases undergoing other regimens as first-line
treatment. In future studies, we will investigate the association
between the expression of DSB repair proteins and the
chemotherapeutic effect for other regimens, and compare these
findings with the results of the present study.
In conclusion, cases with a defective pattern of DSB
repair protein expression may possess higher sensitivity to
chemotherapy for colorectal cancer. The expression pattern of DSB
repair proteins may be a useful prognostic indicator for colorectal
cancer patients.
Acknowledgments
The authors would like to thank H. Ozeki, Y. Ohashi,
and M. Asano for their secretarial assistance and N. Suzuki, a
member of the Research Support Center Division of Clinical Science,
for her technical assistance. The present study was supported in
part by JSPS KAKENHI grant no. 25462065.
References
|
1
|
Tournigand C, André T, Achille E, Lledo G,
Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, et
al: FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced
colorectal cancer: A randomized GERCOR study. J Clin Oncol.
22:229–237. 2004. View Article : Google Scholar
|
|
2
|
Yamaguchi S, Ogata H, Katsumata D,
Nakajima M, Fujii T, Tsutsumi S, Asao T, Sasaki K, Kuwano H and
Kato H: Early serum carcinoembryonic antigen reduction predicts
tumor shrinkage and overall survival in colorectal cancer patients
with distant metastasis, after primary surgery followed by mFOLFOX6
plus bevacizumab treatment. Hepatogastroenterology. In press.
|
|
3
|
Grothey A, Sargent D, Goldberg RM and
Schmoll HJ: Survival of patients with advanced colorectal cancer
improves with the availability of fluorouracil-leucovorin,
irinotecan, and oxaliplatin in the course of treatment. J Clin
Oncol. 22:1209–1214. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saltz LB, Clarke S, Díaz-Rubio E,
Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS,
Rivera F, et al: Bevacizumab in combination with oxaliplatin-based
chemotherapy as first-line therapy in metastatic colorectal cancer:
A randomized phase III study. J Clin Oncol. 26:2013–2019. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Van Cutsem E, Köhne CH, Hitre E, Zaluski
J, Chang Chien CR, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G,
et al: Cetuximab and chemotherapy as initial treatment for
metastatic colorectal cancer. N Engl J Med. 360:1408–1417. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ribic CM, Sargent DJ, Moore MJ, Thibodeau
SN, French AJ, Goldberg RM, Hamilton SR, Laurent-Puig P, Gryfe R,
Shepherd LE, et al: Tumor microsatellite-instability status as a
predictor of benefit from fluorouracil-based adjuvant chemotherapy
for colon cancer. N Engl J Med. 349:247–257. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chiu SJ, Lee YJ, Hsu TS and Chen WS:
Oxaliplatin-induced gamma-H2AX activation via both p53-dependent
and -independent pathways but is not associated with cell cycle
arrest in human colorectal cancer cells. Chem Biol Interact.
182:173–182. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Asakawa H, Koizumi H, Koike A, Takahashi
M, Wu W, Iwase H, Fukuda M and Ohta T: Prediction of breast cancer
sensitivity to neoadjuvant chemotherapy based on status of DNA
damage repair proteins. Breast Cancer Res. 12:R172010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Turner N, Tutt A and Ashworth A: Hallmarks
of 'BRCAness' in sporadic cancers. Nat Rev Cancer. 4:814–819. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chu G: Double strand break repair. J Biol
Chem. 272:24097–24100. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gallagher DJ, Konner JA, Bell-McGuinn KM,
Bhatia J, Sabbatini P, Aghajanian CA, Offit K, Barakat RR, Spriggs
DR and Kauff ND: Survival in epithelial ovarian cancer: A
multivariate analysis incorporating BRCA mutation status and
platinum sensitivity. Ann Oncol. 22:1127–1132. 2011. View Article : Google Scholar
|
|
12
|
Moynahan ME, Cui TY and Jasin M:
Homology-directed DNA repair, mitomycin-c resistance, and
chromosome stability is restored with correction of a Brca1
mutation. Cancer Res. 61:4842–4850. 2001.PubMed/NCBI
|
|
13
|
Stracker TH and Petrini JH: The MRE11
complex: Starting from the ends. Nat Rev Mol Cell Biol. 12:90–103.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Williams RS, Williams JS and Tainer JA:
Mre11-Rad50-Nbs1 is a keystone complex connecting DNA repair
machinery, double-strand break signaling, and the chromatin
template. Biochem Cell Biol. 85:509–520. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baumann P and West SC: Role of the human
RAD51 protein in homologous recombination and double-stranded-break
repair. Trends Biochem Sci. 23:247–251. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Slupianek A, Schmutte C, Tombline G,
Nieborowska-Skorska M, Hoser G, Nowicki MO, Pierce AJ, Fishel R and
Skorski T: BCR/ABL regulates mammalian RecA homologs, resulting in
drug resistance. Mol Cell. 8:795–806. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takenaka T, Yoshino I, Kouso H, Ohba T,
Yohena T, Osoegawa A, Shoji F and Maehara Y: Combined evaluation of
Rad51 and ERCC1 expressions for sensitivity to platinum agents in
non-small cell lung cancer. Int J Cancer. 121:895–900. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Piessevaux H, Buyse M, Schlichting M, Van
Cutsem E, Bokemeyer C, Heeger S and Tejpar S: Use of early tumor
shrinkage to predict long-term outcome in metastatic colorectal
cancer treated with cetuximab. J Clin Oncol. 31:3764–3775. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yoshida M, Goto M, Kii T, Nishitani H,
Kawabe S, Kuwakado S, Asaishi K, Miyamoto T and Higuchi K:
Retrospective study as first-line chemotherapy combined anti-VEGF
antibody with fluoropyrimidine for frail patients with unresectable
or metastatic colorectal cancer. Digestion. 87:59–64. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maeda K, Shibutani M, Otani H, Nagahara H,
Sugano K, Ikeya T, Kubo N, Amano R, Kimura K, Muguruma K, et al:
Low nutritional prognostic index correlates with poor survival in
patients with stage IV colorectal cancer following palliative
resection of the primary tumor. World J Surg. 38:1217–1222. 2014.
View Article : Google Scholar
|
|
21
|
Thompson LH and Schild D: The contribution
of homologous recombination in preserving genome integrity in
mammalian cells. Biochimie. 81:87–105. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sung P and Robberson DL: DNA strand
exchange mediated by a RAD51-ssDNA nucleoprotein filament with
polarity opposite to that of RecA. Cell. 82:453–461. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nakanoko T, Saeki H, Morita M, Nakashima
Y, Ando K, Oki E, Ohga T, Kakeji Y, Toh Y and Maehara Y: Rad51
expression is a useful predictive factor for the efficacy of
neoadjuvant chemoradiotherapy in squamous cell carcinoma of the
esophagus. Ann Surg Oncol. 21:597–604. 2014. View Article : Google Scholar :
|
|
24
|
Tsai MS, Kuo YH, Chiu YF, Su YC and Lin
YW: Down-regulation of Rad51 expression overcomes drug resistance
to gemcitabine in human non-small-cell lung cancer cells. J
Pharmacol Exp Ther. 335:830–840. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hannay JA, Liu J, Zhu QS, Bolshakov SV, Li
L, Pisters PW, Lazar AJ, Yu D, Pollock RE and Lev D: Rad51
overexpression contributes to chemoresistance in human soft tissue
sarcoma cells: A role for p53/activator protein 2 transcriptional
regulation. Mol Cancer Ther. 6:1650–1660. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Giannini G, Rinaldi C, Ristori E,
Ambrosini MI, Cerignoli F, Viel A, Bidoli E, Berni S, D'Amati G,
Scambia G, et al: Mutations of an intronic repeat induce impaired
MRE11 expression in primary human cancer with microsatellite
instability. Oncogene. 23:2640–2647. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vilar E, Bartnik CM, Stenzel SL, Raskin L,
Ahn J, Moreno V, Mukherjee B, Iniesta MD, Morgan MA, Rennert G, et
al: MRE11 deficiency increases sensitivity to poly(ADP-ribose)
polymerase inhibition in microsatellite unstable colorectal
cancers. Cancer Res. 71:2632–2642. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sargent DJ, Marsoni S, Monges G, Thibodeau
SN, Labianca R, Hamilton SR, French AJ, Kabat B, Foster NR, Torri
V, et al: Defective mismatch repair as a predictive marker for lack
of efficacy of fluorouracil-based adjuvant therapy in colon cancer.
J Clin Oncol. 28:3219–3226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yamaguchi S, Ogata H, Katsumata D,
Nakajima M, Fujii T, Tsutsumi S, Asao T, Sasaki K, Kuwano H and
Kato H: MUTYH- associated colorectal cancer and adenomatous
polyposis. Surg Today. 44:593–600. 2014. View Article : Google Scholar
|