Introduction
The WHO reported that breast cancer is one of the
leading causes of death and the most common cancer among women
worldwide (1). Important progress
has been made in regards to breast cancer treatment in recent
years, including novel targeted therapies (2). However, classical chemotherapy is
still most frequently used in the clinic and thus, chemoresistance
is a severe complication of breast cancer therapy (3–6).
Approximately 30% of early breast cancer patients face a relapse
involving increased resistance to chemotherapeutics and metastasis
(7,8). Moreover, in later stages of breast
cancer, the response rates of classical chemotherapeutics, e.g.
taxanes and anthracyclines, have declined from 70 to 20–30%.
Accordingly, research on drug resistance development and solutions
to overcome drug resistance are urgently needed to improve the
efficacy of chemotherapeutic drugs.
Salinomycin is an ionophore antibiotic, introduced
in 2009 as a cancer stem cell targeting drug (9). Salinomycin also inhibits cell
survival, growth, migration and invasion of human non-small lung
cancer cells (10). Studies have
shown that salinomycin induces cell death in breast (11), ovarian (12), as well as in head and neck cancer
cells (13). Moreover, salinomycin
was found to inhibit cell growth in prostate (14) and pancreatic cancer cells (15). Salinomycin treatment additionally
reduces metastasis formation by decreasing the migratory capability
of cancer cells (16).
Further studies of salinomycin on overcoming drug
resistance have also been conducted. Fuchs et al have shown
that salinomycin overcomes ABC transporter-mediated multi-drug- and
apoptosis-resistance in human cancer cells (17,18).
Salinomycin co-treatment was also found to increase doxorubicin and
etoposide cytotoxicity by enhancing DNA damage and downregulating
p21 (19), to increase doxorubicin
cytotoxicity in soft tissue sarcomas (20), to elevate apoptosis in
cisplatin-resistant colorectal cancer cells by accumulating
reactive oxygen species (21), and
to induce apoptosis in cisplatin-resistant human ovarian cancer
cells (22). A combination of
salinomycin and trail circumvents trail resistance in glioma cells
(23). We have previously shown
that a combination of salinomycin and trastuzumab efficiently
targets cancer stem cells and Her-2-positive cancer cells (24). Moreover, a recent study by Kim et
al have shown that combined treatment of salinomycin and
doxorubicin enhances the cytotoxicity in multidrug resistant
MCF-7/MDR human breast cancer cells by decreasing the efflux of
doxorubicin (25).
In the present study, we investigate whether
consecutive treatment of salinomycin, i.e. analogous to the
treatment regimen in the clinic, sensitizes cancer cells to
standard chemotherapeutic drug treatment. We demonstrate that
consecutive salinomycin treatment generates salinomycin-resistant
cells with increased susceptibility to doxorubicin. Furthermore,
salinomycin-resistant cells exhibit a downregulation of the
prominent multiple drug resistance (MDR) proteins MDR1 and BCRP1.
The reduced expression of these efflux pumps decreases the overall
pump activity, which leads to an intracellular accumulation of
drugs, i.e. doxorubicin and eventually increased cytotoxicity.
Therefore, consecutive salinomycin treatment sensitizes the cancer
cells to doxorubicin.
Materials and methods
Cell lines and culture
MCF-7, BT-474, MDA-MB 231 and MDA-MB 436 human
breast cancer cells were purchased from ATCC. MCF-7 cells were
grown in Dulbecco's modified Eagle's medium (DMEM) high glucose
supplemented with 20% fetal calf serum (FCS) (both from Gibco) and
2 mM L-glutamine at 37°C and 5% CO2. BT-474 cells were
grown in RPMI-1640 medium (supplemented with 10% FCS) and 2 mM
L-glutamine (both from Gibco) at 37°C and 5% CO2. MDA-MB
231 and MDA-MB 436 cells were grown in L-15 Leibovitz's medium
(Biochrom) supplemented with 10% FCS and 2 mM glutamine at 37°C
without CO2. Cells were routinely tested and confirmed
as mycoplasm-free.
Molecular evolution assay
Cells (80% of confluency) were treated with
salinomycin (Sigma-Aldrich, Germany) for 72 h as previously
described (26). The concentration
of salinomycin was 500 nM for the MCF-7 and BT-474 cells and 50 nM
for the MDA-MB 231 and MDA-MB 436 cells as mesenchymal cells
display increased sensitivity to salinomycin (16,27).
Thereafter, the treatment medium was replaced with fresh medium and
cells were grown until recovery (80% of confluency). As soon as
recovered, the cells were split for RNA analysis, as well as for
the next treatment, and also seeded for the cell viability assay.
R0 represents the untreated control cell line (parental cells),
whereas R1, R2, R3, R4 and R5 represent cells that were treated for
one, two, three, four and five times with salinomycin,
respectively.
Cell viability assay
For the cytotoxicity assays, 5×103 cells
were seeded into a 96-well plate (TPP) and incubated for 24 h.
Cells were then treated with various concentrations of salinomycin,
doxorubicin or verapamil (Sigma-Aldrich) for 72 h, followed by
CellTiter-Glo assay (Promega, Germany) according to the
manufacturer's instructions. Three replicas were analyzed.
The EC50 was determined by GraphPad Prism
software. The drug concentration was transformed into log scale,
and a nonlinear regression (curve fit) was made.
RNA extraction and quantitative
RT-PCR
Total RNA was extracted and isolated from cells
using the miRCURY™ RNA isolation kit (Exiqon, Denmark). RNA was
then reverse transcribed to cDNA from 1.0 µg of total RNA
using the Transcriptor First Strand cDNA synthesis kit (Roche).
Real-time PCR was then performed with the Light Cycler®
480 using the Master Probes kit and the Universal ProbeLibrary
(UPL) (all from Roche). All protocols were performed according to
the instructions of the manufacturer. RT-PCR was performed using
the following primers and UPLs: MDR1, UPL probe #7, left primer,
caagcatctgccaaaacctc and right primer, ctgggtttccccctgtaaat; BCRP1,
UPL probe #56, left primer, tggcttagactcaagcacagc and right primer,
tcgtccctgcttagacatcc; GAPDH, UPL probe #45, left primer,
tccactggcgtcttcacc and right primer, ggcagagatgatgaccctttt. GAPDH
was used as an internal control. The results were analyzed using
comparative threshold cycle (ΔΔCT method).
Internalization of doxorubicin
Cells were treated with 10 µM of doxorubicin
for 3 h. The medium was replaced with fresh medium 1 h prior to
imaging and FACS analysis. To perform FACS analysis, cells were
prepared by trypsinization and measured with a CyAn™ ADP flow
cytometer (DakoCytomation). Doxorubicin intracellular levels were
analyzed using FlowJo software (Tree Star).
Statistical analysis
All data are presented as mean ± SD, and were
analyzed using GraphPad Prism 5.0 software.
Results
Molecular evolution assays generate
salinomycin-resistant cancer cells
To mimic clinical applications of chemotherapeutics
in vitro, we performed repeated salinomycin treatment of the
epithelial breast cancer cell lines MCF-7 and BT-474, as well as of
the mesenchymal breast cancer cell lines MDA-MB 231 and MDA-MB 436.
We found that the consecutive treatment resulted in enhanced
formation of salinomycin-resistant cells. The IC50 value
of salinomycin increased during the treatment cycles, both in the
epithelial MCF-7 (Fig. 1A) and
BT-474 cells (Fig. 1B) as well as
in the mesenchymal MDA-MB 231 (Fig.
1C) and MDA-MB 436 (Fig. 1D)
cells. Furthermore, the resistant cells also displayed a decreased
proliferation compared to parental cells, as indicated by a longer
period of time to reach 80% confluency (Fig. 1A–D, right panel).
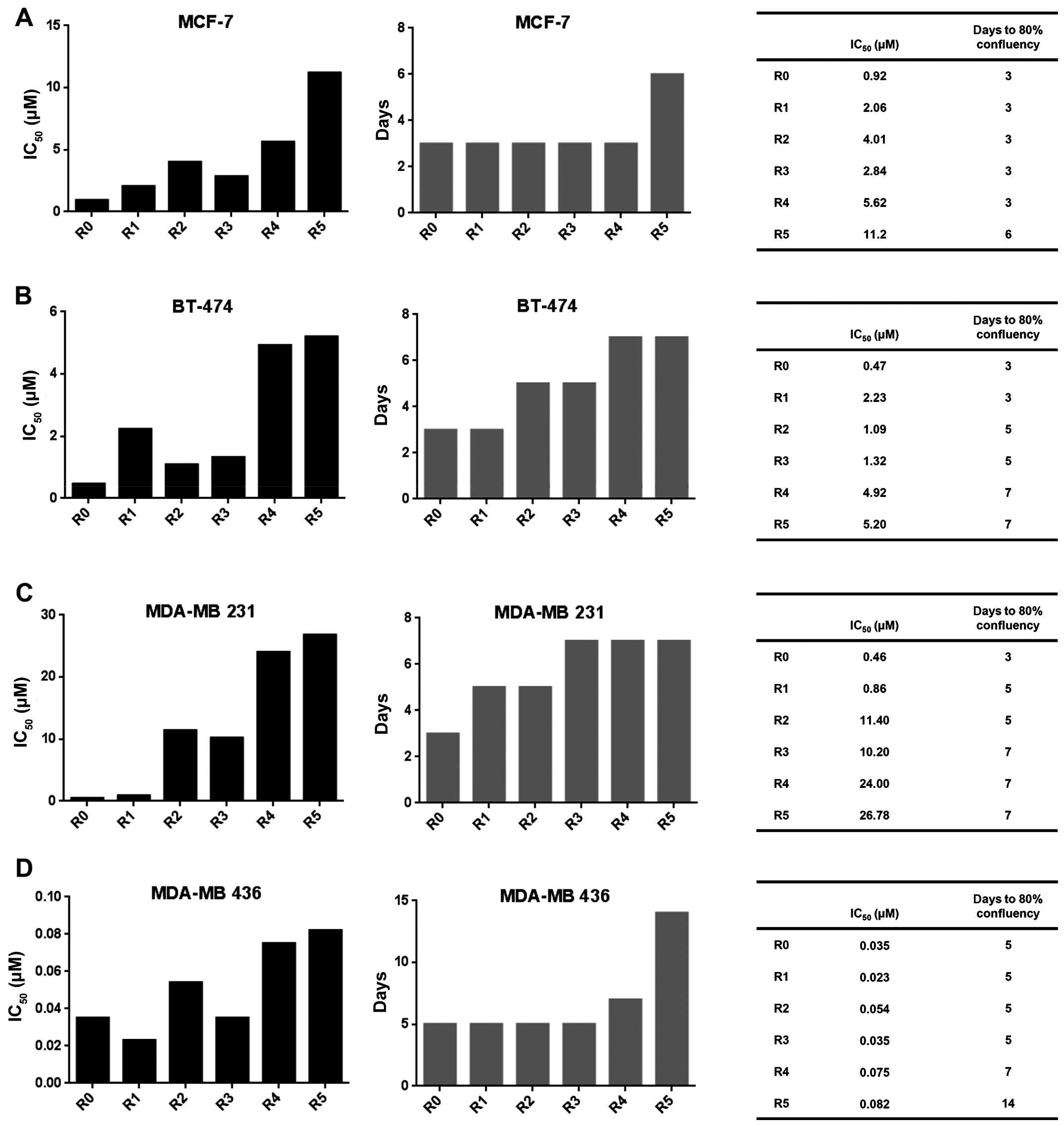 | Figure 1Molecular evolution assays generate
salinomycin-resistant cancer cells. IC50 values of
salinomycin during the molecular evolution assays (left panel) and
the number of days to reach 80% confluency after first cell
splitting (central panel). The right panel presents the
corresponding values in tabular form. (A) MCF-7, (B) BT-474, (C)
MDA-MB 231, (D) MDA-MB 436 cells. To determine the IC50
values, the cells were treated with various concentrations of
salinomycin for 72 h. Cell viability in comparison to untreated
cells was measured with CellTiter-Glo assay. IC50 value
was analyzed with GraphPad. R0 represents the parental cells,
whereas R1, R2, R3, R4 and R5 represent cells that were treated
with salinomycin for one, two, three, four and five times,
respectively. |
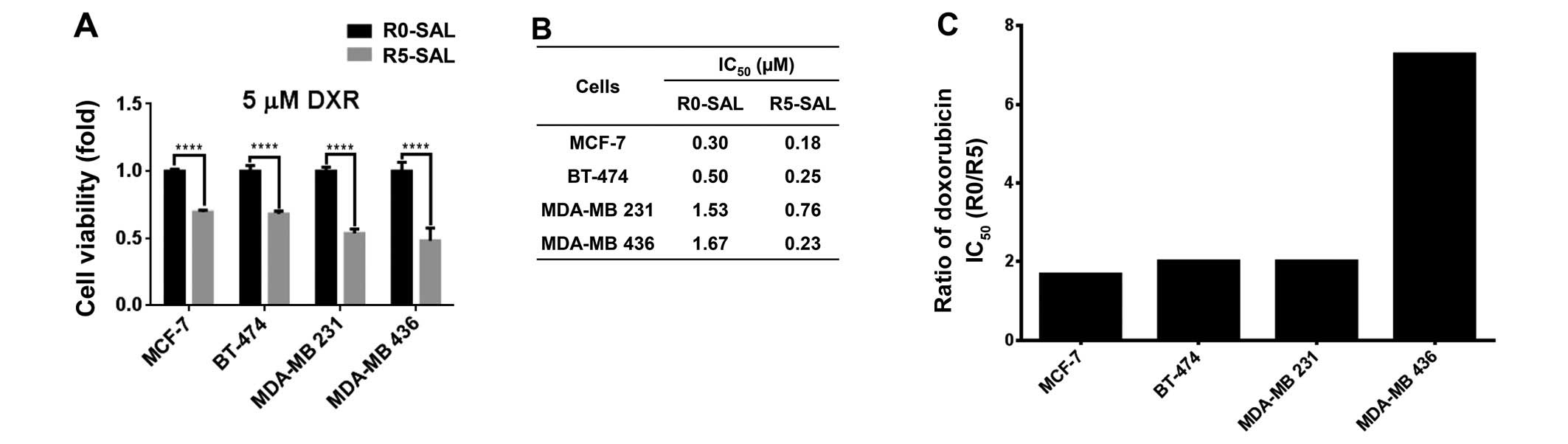
Salinomycin-resistant cells show enhanced
sensitivity for doxorubicin
Since salinomycin acts on different targets than
classical chemotherapeutics, we studied the cross-resistance to
doxorubicin (5 µM) by performing cytotoxicity assay on both
resistant (R5) and parental cells (R0). The results revealed that
all of the MCF-7, BT-474, MDA-MB 231 and MDA-MB 436
salinomycin-resistant cells (R5) became more sensitive to
doxorubicin compared to the parental cells (R0) (Fig. 2A). Accordingly, a similar effect was
observed when analyzing the IC50 values for doxorubicin
for the R0 and R5 cells (Fig. 2B).
Cells displayed a 2- to 7-fold increase in doxorubicin sensitivity
for all of the corresponding R5 cells (Fig. 2C).
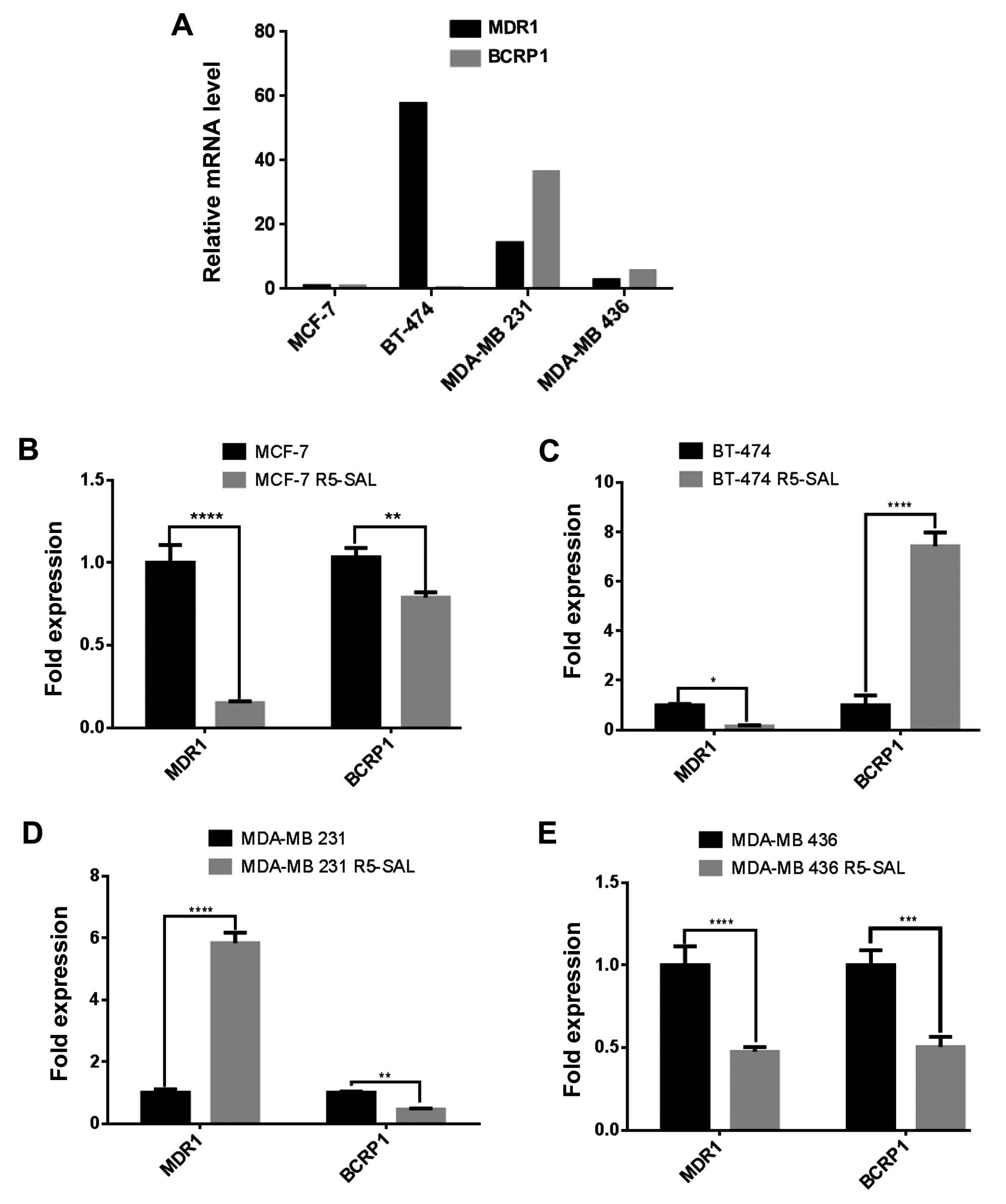
Drug efflux pumps are downregulated in
salinomycin-resistant cells
A common mechanism in drug resistance is the
upregulation and increased activity of efflux pumps, which play an
important role in absorbing, distributing and eliminating drugs
from the cells. We analyzed whether a decrease in multiple drug
resistance (MDR) is responsible for the increased doxorubicin
sensitivity in R5 cells. We therefore first determined the basal
expression of the prominent efflux pump genes MDR1 and BCRP1 in all
parental cells (R0) by quantitative RT-PCR. MDR1 was highly
expressed in the BT-474 cells, whereas BCRP1 was highly expressed
in the MDA-MB 231 cells (Fig. 3A).
Furthermore, the MCF-7 cells had the lowest efflux pump expression
among the analyzed cells. To ascertain whether the consecutive
treatment of salinomycin influences the expression of the drug
efflux pump, we measured MDR1 and BCRP1 expression in the
salinomycin-resistant cells (R5) and made a comparison to
respective parental cells (R0). Both MDR1 and BCRP1 were
downregulated in the MCF-7 and MDA-MB 436 resistant cells (Fig. 3B and E). In the BT-474
salinomycin-resistant cells, MDR1 was downregulated, whereas BCRP1
was upregulated (Fig. 3C) and in
the MDA-MB 231 salinomycin-resistant cells (Fig. 3D), MDR1 was upregulated and BCRP1
displayed decreased expression.
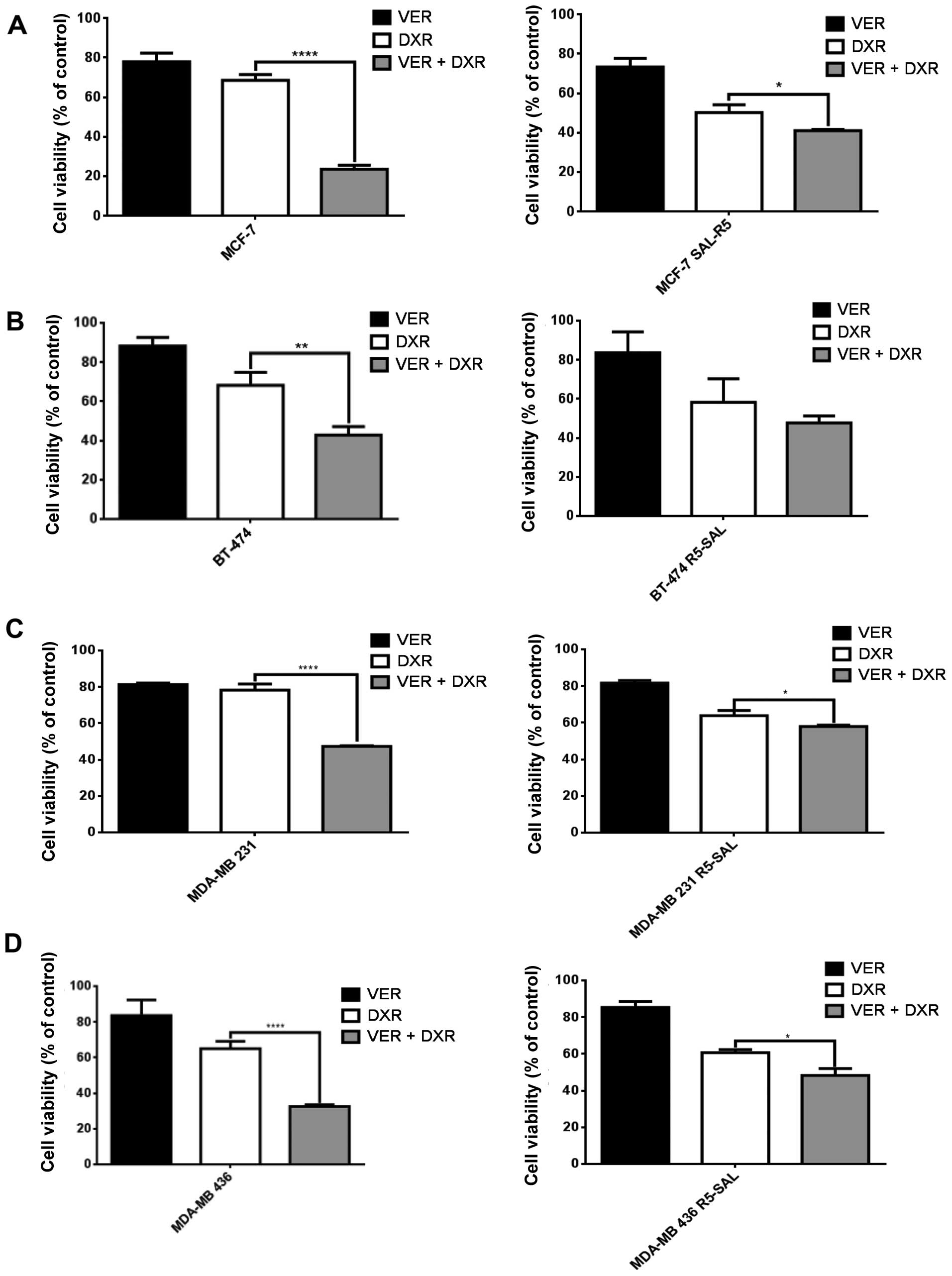
Blocking the efflux pump activity affects
chemoresistance mainly of parental cells
In order to analyze whether the decrease in efflux
pump expression affects the pump activity, we blocked these pumps
with the selective inhibitor verapamil, and measured cytotoxicity
upon doxorubicin treatment. In the MCF-7 parental cells, addition
of verapamil increased the doxorubicin-induced cytotoxicity,
whereas in the salinomycin-resistant cells addition of verapamil
had almost no effect (Fig. 4A).
Similar effects were also observed in the cell lines BT-474
(Fig. 4B), MDA-MB 231 (Fig. 4C) and MDA-MB 436 (Fig. 4D), and their corresponding
salinomycin-resistant descendants. Thus, the inhibition of the
efflux pumps increased the doxorubicin-induced cytotoxicity mainly
in the parental (R0) cells.
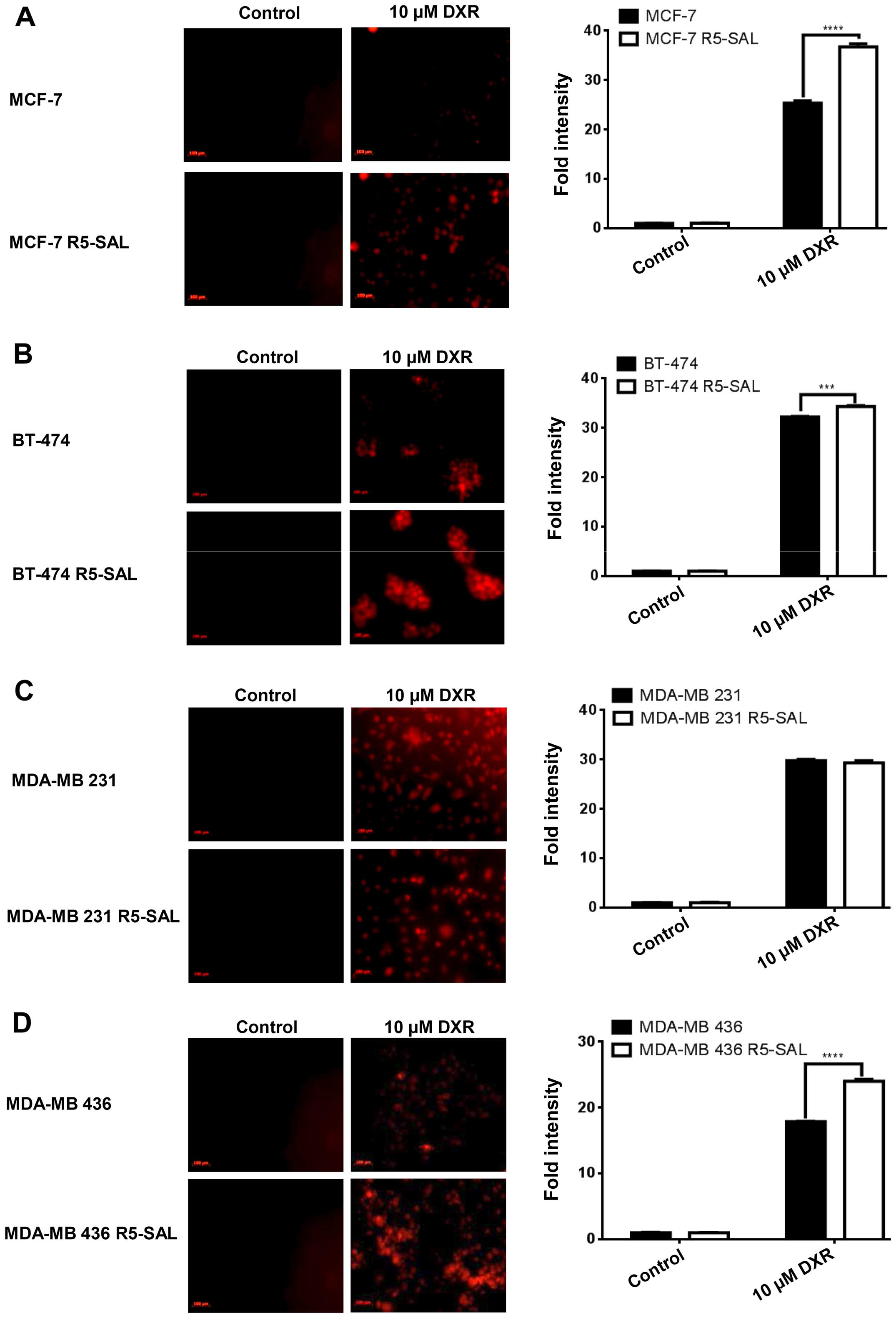
Salinomycin-resistant cells show
increased intracellular doxorubicin accumulation
As salinomycin-resistant cells downregulated the
gene expression of drug efflux pumps and thus their activity, we
also analyzed whether these cells show enhanced intracellular drug
accumulation. We treated both the parental (R0) and the
salinomycin-resistant (R5) cells with doxorubicin and studied the
internalization of doxorubicin by image analysis (Fig. 5A), and by measuring the
intracellular doxorubicin level with FACS. We observed an enhanced
accumulation of doxorubicin in the MCF-7, BT-474 and MDA-MB 436
salinomycin-resistant cells (R5) (Fig.
5A, B and D) compared to parental cells (R0). MDA-MB 231
salinomycin-resistant cells (Fig.
5C) showed less accumulation of doxorubicin than parental cells
(R0), which is in line with the enhanced MDR1 expression.
Discussion
Chemoresistance is still a major threat to
successful cancer therapy. To investigate novel mechanisms and
drugs circumventing therapy resistance, we consecutively treated
epithelial and mesenchymal breast cancer cells with salinomycin and
generated salinomycin-resistant cells. Numerous studies on cancer
drug resistance utilize continuous drug treatment with increasing
concentrations of the drugs (28–31).
Unlike previous studies on salinomycin and doxorubicin (25,32),
we consecutively treated breast cancer cells with the same
concentration of salinomycin and gave the cells time to recover
after each treatment round. This procedure was performed for five
treatment cycles. Our settings sought to mimic the application
process of chemotherapeutics in the clinic. There, the patient, and
thus the tumor or residual tumor cells, also undergo a recovery
phase during treatment cycles, as chemotherapy is only tolerated to
a certain extent. The resistance formation in our assay exhibited
no linear increase of the IC50 value for salinomycin.
We, therefore, concluded that the observed resistance formation is
based on the principle of clonal selection. According to the clonal
evolution theory, tumor cells display heterogeneity and genetic
instability. When treated with chemotherapy, selection pressure is
applied and some of the cells will survive the treatment. These
cells will form a new polyclonal cell population with various new
genetic predispositions (33).
After each round of the molecular evolution assay, different cell
population may have survived and thus no linear increase in the
IC50 value was detected in our assay. Additionally, many
different resistance mechanism possibly exist in parallel caused by
the clonal selection in the molecular evolution assay. For example,
we observed decreasing cell proliferation, indicating that
salinomycin treatment eradicates fast growing cell populations.
Unlike in several molecular evolution assays we performed with
doxorubicin, the growth retardation here was only modest. A recent
study by Kopp et al also showed that consecutive treatment
of mesenchymal breast cancer cells with salinomycin resulted in
resistance formation by mesenchymal to epithelial transition (MET)
(27).
Notably, we observed that the salinomycin-resistant
cells exhibited increased sensitivity to doxorubicin in both
epithelial and mesenchymal tumor cells. As it was reported that
combinational, i.e. simultaneous treatment of tumor cells with
salinomycin and doxorubicin, cisplatin or etoposide increased the
cytotoxicity (19,21), we hypothesized that in our assay the
sensitivity of the salinomycin-resistant cells to other drugs may
be increased as well.
The most important drug resistance mechanism is the
upregulation and increased activity of ABC-transporters (ATP
binding cassette transporter) (34). Among these efflux pumps in charge of
decreasing intracellular drug levels are p-glycoprotein [encoded by
multidrug resistance 1 (MDR1) gene], breast cancer resistance
protein (encoded by the BCRP1 gene), and multidrug
resistance-associated proteins (encoded by MRP genes) (28,35–37).
The ABC transporter family consists of membrane proteins that
translocate a variety of substrates across extracellular and
intracellular membranes (28). They
play an important role in absorbing, distributing and eliminating
drugs from the cells (38). Hence,
the upregulation of efflux pumps decreases intracellular drug
accumulation and increases drug resistance leading to multiple drug
resistance (MDR) (39).
Several MDR reversing agents modify cell membrane
fluidity and increase cell membrane permeability (40). Salinomycin is able to circumvent MDR
by acting as an ionophore. Salinomycin, a transmembrane
Na+/K+ ionophore, is embedded in biological
membranes (41,42), and thus by changing membrane
integrity it is able to inhibit the activity of drug efflux pumps.
Additionally, salinomycin disturbs the intracellular balance of
ions by increasing the sodium ion level (42–44).
In the present study, we showed that MDR1 and BCRP1 were
downregulated after repeated salinomycin treatment. The decrease in
the expression of MDR proteins could occur via a direct and
indirect mechanism. On the one hand, salinomycin was found to be an
anticancer stem cell drug. One of the hallmarks of cancer stem
cells is an increase in efflux pump activity (45). That is why cancer stem cells are
thought to be present in the so-called side-population (46). Salinomycin eradicates this
population, as seen by a decreased expression of stem cell markers
(data not shown) thereby increasing the non-cancer stem cell
fraction with low efflux pump expression. Due to the cyclic drug
treatment, the offspring of these efflux pump-positive cancer stem
cells, which usually accounts for less than 10% of the cell
population, may also die out over time. Whether the killing of
cancer stem cells and their offspring accounts for such a strong
indirect effect in gene expression of efflux pumps and eventually
doxorubicin sensitivity, remains to be elucidated. On the other
hand, efflux pumps may be important for the direct execution of the
salinomycin cytotoxicity, as they regulate the intracellular
balance of molecules.
In contrast to the breast cancer cell lines MCF7 and
MDA-MB 436, where MDR1 and BCRP1 expression was decreased upon
consecutive salinomycin treatment, we observed an upregulation of
some of these proteins in the BT-474 and MDA-MB 231 cells. BCRP1
for example was increased 7-fold in the BT-474 cells. However, the
BCRP1 level in the BT-474 parental cells was very low compared to
the other cell lines, thus the upregulation in
salinomycin-resistant cells did not substantially influence the
efflux pump activity, as shown by blocking the pump activity with
verapamil. Doxorubicin is a substrate of p-glycoprotein (28), while verapamil acts as competitive
inhibitor by directly binding to p-glycoprotein on a specific site
(47), thereby preventing
doxorubicin efflux across the cell membrane. In contrast to the
BT474 cell line, MDA-MB 231 salinomycin-resistant cells displayed a
6-times higher level of MDR1 which resulted in increased efflux
activity. Therefore, the increased doxorubicin sensitivity was due
to a different mechanism. Since ABC transporters are downregulated
in salinomycin-resistant cells, we observed an enhanced doxorubicin
accumulation. Doxorubicin induces DNA damage by generating
malondialdehyde-DNA adducts induced by free radicals (48). Hence, we suppose increased DNA
damage in the salinomycin-resistant cells that finally led to cell
death.
In conclusion, we demonstrated that consecutive
salinomycin treatment generates salinomycin-resistant tumor cells
and increases their susceptibility to doxorubicin by downregulating
MDR1 and BCRP1 gene expression (except for MDR1 in MDA-MB 231
cells). These findings suggest salinomycin pretreatment of
chemoresistant tumors in order to resensitize them to
chemotherapeutics and therefore provide the fundament for novel
approaches circumventing chemoresistance with salinomycin.
Acknowledgments
The authors want to thank Bianca Köhler for
excellent technical support. Adam Hermawan received a doctoral
fellowship from the Islamic Development Bank, IDB (28/IND/P32;
Student ID 600014391).
References
|
1
|
Stewart BW and Wild CP: World Cancer
Report 2014. International Agency for Research on Cancer; WHO,
Lyon: 2015
|
|
2
|
Hryniewicz-Jankowska A, Augoff K,
Biernatowska A, Podkalicka J and Sikorski AF: Membrane rafts as a
novel target in cancer therapy. Biochim Biophys Acta. 1845:155–165.
2014.PubMed/NCBI
|
|
3
|
Al Saleh S, Sharaf LH and Luqmani YA:
Signalling pathways involved in endocrine resistance in breast
cancer and associations with epithelial to mesenchymal transition
(Review). Int J Oncol. 38:1197–1217. 2011.PubMed/NCBI
|
|
4
|
Chuthapisith S, Eremin J, El-Sheemey M and
Eremin O: Breast cancer chemoresistance: Emerging importance of
cancer stem cells. Surg Oncol. 19:27–32. 2010. View Article : Google Scholar
|
|
5
|
Malenfant SJ, Eckmann KR and Barnett CM:
Pertuzumab: A new targeted therapy for HER2-positive metastatic
breast cancer. Pharmacotherapy. 34:60–71. 2014. View Article : Google Scholar
|
|
6
|
Piccart M: Circumventing de novo and
acquired resistance to trastuzumab: New hope for the care of
ErbB2-positive breast cancer. Clin Breast Cancer. 8(Suppl 3):
S100–S113. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
O'Driscoll L and Clynes M: Biomarkers and
multiple drug resistance in breast cancer. Curr Cancer Drug
Targets. 6:365–384. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gonzalez-Angulo AM, Morales-Vasquez F and
Hortobagyi GN: Overview of resistance to systemic therapy in
patients with breast cancer. Adv Exp Med Biol. 608:1–22. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gupta PB, Onder TT, Jiang G, Tao K,
Kuperwasser C, Weinberg RA and Lander ES: Identification of
selective inhibitors of cancer stem cells by high-throughput
screening. Cell. 138:645–659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arafat K, Iratni R, Takahashi T, Parekh K,
Al Dhaheri Y, Adrian TE and Attoub S: Inhibitory effects of
salinomycin on cell survival, colony growth, migration, and
invasion of human non-small cell lung cancer A549 and LNM35:
Involvement of NAG-1. PLoS One. 8:e669312013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Al Dhaheri Y, Attoub S, Arafat K, Abuqamar
S, Eid A, Al Faresi N and Iratni R: Salinomycin induces apoptosis
and senescence in breast cancer: Upregulation of p21,
downregulation of survivin and histone H3 and H4 hyperacetylation.
Biochim Biophys Acta. 1830:3121–3135. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Koo KH, Kim H, Bae YK, Kim K, Park BK, Lee
CH and Kim YN: Salinomycin induces cell death via inactivation of
Stat3 and downregulation of Skp2. Cell Death Dis. 4:e6932013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li T, Su L, Zhong N, Hao X, Zhong D,
Singhal S and Liu X: Salinomycin induces cell death with autophagy
through activation of endoplasmic reticulum stress in human cancer
cells. Autophagy. 9:1057–1068. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ketola K, Hilvo M, Hyötyläinen T, Vuoristo
A, Ruskeepää AL, Orešič M, Kallioniemi O and Iljin K: Salinomycin
inhibits prostate cancer growth and migration via induction of
oxidative stress. Br J Cancer. 106:99–106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schenk M, Aykut B, Teske C, Giese NA,
Weitz J and Welsch T: Salinomycin inhibits growth of pancreatic
cancer and cancer cell migration by disruption of actin stress
fiber integrity. Cancer Lett. 358:161–169. 2015. View Article : Google Scholar
|
|
16
|
Kopp F, Hermawan A, Oak PS, Herrmann A,
Wagner E and Roidl A: Salinomycin treatment reduces metastatic
tumor burden by hampering cancer cell migration. Mol Cancer.
13:162014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fuchs D, Daniel V, Sadeghi M, Opelz G and
Naujokat C: Salinomycin overcomes ABC transporter-mediated
multidrug and apoptosis resistance in human leukemia stem cell-like
KG-1a cells. Biochem Biophys Res Commun. 394:1098–1104. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fuchs D, Heinold A, Opelz G, Daniel V and
Naujokat C: Salinomycin induces apoptosis and overcomes apoptosis
resistance in human cancer cells. Biochem Biophys Res Commun.
390:743–749. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim JH, Chae M, Kim WK, Kim YJ, Kang HS,
Kim HS and Yoon S: Salinomycin sensitizes cancer cells to the
effects of doxorubicin and etoposide treatment by increasing DNA
damage and reducing p21 protein. Br J Pharmacol. 162:773–784. 2011.
View Article : Google Scholar :
|
|
20
|
Liffers ST, Tilkorn DJ, Stricker I, Junge
CG, Al-Benna S, Vogt M, Verdoodt B, Steinau HU, Tannapfel A,
Tischoff I, et al: Salinomycin increases chemosensitivity to the
effects of doxorubicin in soft tissue sarcomas. BMC Cancer.
13:4902013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou J, Li P, Xue X, He S, Kuang Y, Zhao
H, Chen S, Zhi Q and Guo X: Salinomycin induces apoptosis in
cisplatin-resistant colorectal cancer cells by accumulation of
reactive oxygen species. Toxicol Lett. 222:139–145. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang B, Wang X, Cai F, Chen W, Loesch U
and Zhong XY: Antitumor properties of salinomycin on
cisplatin-resistant human ovarian cancer cells in vitro and in
vivo: Involvement of p38 MAPK activation. Oncol Rep. 29:1371–1378.
2013.PubMed/NCBI
|
|
23
|
Calzolari A, Saulle E, De Angelis ML,
Pasquini L, Boe A, Pelacchi F, Ricci-Vitiani L, Baiocchi M and
Testa U: Salinomycin potentiates the cytotoxic effects of TRAIL on
glioblastoma cell lines. PLoS One. 9:e944382014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Oak PS, Kopp F, Thakur C, Ellwart JW, Rapp
UR, Ullrich A, Wagner E, Knyazev P and Roidl A: Combinatorial
treatment of mammospheres with trastuzumab and salinomycin
efficiently targets HER2-positive cancer cells and cancer stem
cells. Int J Cancer. 131:2808–2819. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim KY, Kim SH, Yu SN, Park SK, Choi HD,
Yu HS, Ji JH, Seo YK and Ahn SC: Salinomycin enhances
doxorubicin-induced cytotoxicity in multidrug resistant MCF-7/MDR
human breast cancer cells via decreased efflux of doxorubicin. Mol
Med Rep. 12:1898–1904. 2015.PubMed/NCBI
|
|
26
|
Kopp F, Oak PS, Wagner E and Roidl A:
miR-200c sensitizes breast cancer cells to doxorubicin treatment by
decreasing TrkB and Bmi1 expression. PLoS One. 7:e504692012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kopp F, Hermawan A, Oak PS, Ulaganathan
VK, Herrmann A, Elnikhely N, Thakur C, Xiao Z, Knyazev P, Ataseven
B, et al: Sequential salinomycin treatment results in resistance
formation through clonal selection of epithelial-like tumor cells.
Transl Oncol. 7:702–711. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dönmez Y, Akhmetova L, İşeri OD, Kars MD
and Gündüz U: Effect of MDR modulators verapamil and promethazine
on gene expression levels of MDR1 and MRP1 in doxorubicin-resistant
MCF-7 cells. Cancer Chemother Pharmacol. 67:823–828. 2011.
View Article : Google Scholar
|
|
29
|
Latorre E, Tebaldi T, Viero G, Spartà AM,
Quattrone A and Provenzani A: Downregulation of HuR as a new
mechanism of doxorubicin resistance in breast cancer cells. Mol
Cancer. 11:132012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin ST, Chou HC, Chang SJ, Chen YW, Lyu
PC, Wang WC, Chang MD and Chan HL: Proteomic analysis of proteins
responsible for the development of doxorubicin resistance in human
uterine cancer cells. J Proteomics. 75:5822–5847. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qinghong S, Shen G, Lina S, Yueming Z,
Xiaoou L, Jianlin W, Chengyan H, Hongjun L and Haifeng Z:
Comparative proteomics analysis of differential proteins in respond
to doxorubicin resistance in myelogenous leukemia cell lines.
Proteome Sci. 13:12015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou Y, Liang C, Xue F, Chen W, Zhi X,
Feng X, Bai X and Liang T: Salinomycin decreases doxorubicin
resistance in hepatocellular carcinoma cells by inhibiting the
β-catenin/TCF complex association via FOXO3a activation.
Oncotarget. 6:10350–10365. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cahill DP, Kinzler KW, Vogelstein B and
Lengauer C: Genetic instability and Darwinian selection in tumours.
Trends Cell Biol. 9:M57–M60. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Israeli D, Ziaei S, Gonin P and Garcia L:
A proposal for the physiological significance of mdr1 and
Bcrp1/Abcg2 gene expression in normal tissue regeneration and after
cancer therapy. J Theor Biol. 232:41–45. 2005. View Article : Google Scholar
|
|
35
|
Katayama K, Yoshioka S, Tsukahara S,
Mitsuhashi J and Sugimoto Y: Inhibition of the mitogen-activated
protein kinase pathway results in the down-regulation of
P-glycoprotein. Mol Cancer Ther. 6:2092–2102. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou S, Schuetz JD, Bunting KD, Colapietro
AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M,
Nakauchi H, et al: The ABC transporter Bcrp1/ABCG2 is expressed in
a wide variety of stem cells and is a molecular determinant of the
side-population phenotype. Nat Med. 7:1028–1034. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Doyle L and Ross DD: Multidrug resistance
mediated by the breast cancer resistance protein BCRP (ABCG2).
Oncogene. 22:7340–7358. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xiang W, Gao A, Liang H, Li C, Gao J, Wang
Q, Shuang B, Zhang J, Yan Y and Wang X: Reversal of
P-glycoprotein-mediated multidrug resistance in vitro by milbemycin
compounds in adriamycin-resistant human breast carcinoma
(MCF-7/adr) cells. Toxicol In Vitro. 24:1474–1481. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hoffmann EK and Lambert IH: Ion channels
and transporters in the development of drug resistance in cancer
cells. Philos Trans R Soc Lond B Biol Sci. 369:201301092014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Drori S, Eytan GD and Assaraf YG:
Potentiation of anticancer-drug cytotoxicity by
multidrug-resistance chemosensitizers involves alterations in
membrane fluidity leading to increased membrane permeability. Eur J
Biochem. 228:1020–1029. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bissinger R, Malik A, Jilani K and Lang F:
Triggering of erythrocyte cell membrane scrambling by salinomycin.
Basic Clin Pharmacol Toxicol. 115:396–402. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mitani M, Yamanishi T, Miyazaki Y and
Otake N: Salinomycin effects on mitochondrial ion translocation and
respiration. Antimicrob Agents Chemother. 9:655–660. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Matsumori N, Morooka A and Murata M:
Conformation and location of membrane-bound salinomycin-sodium
complex deduced from NMR in isotropic bicelles. J Am Chem Soc.
129:14989–14995. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Boehmerle W and Endres M: Salinomycin
induces calpain and cytochrome c-mediated neuronal cell death. Cell
Death Dis. 2:e1682011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Moitra K, Lou H and Dean M: Multidrug
efflux pumps and cancer stem cells: Insights into multidrug
resistance and therapeutic development. Clin Pharmacol Ther.
89:491–502. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bunting KD: ABC transporters as phenotypic
markers and functional regulators of stem cells. Stem Cells.
20:11–20. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yusa K and Tsuruo T: Reversal mechanism of
multidrug resistance by verapamil: Direct binding of verapamil to
P-glycoprotein on specific sites and transport of verapamil outward
across the plasma membrane of K562/ADM cells. Cancer Res.
49:5002–5006. 1989.PubMed/NCBI
|
|
48
|
Minotti G, Menna P, Salvatorelli E, Cairo
G and Gianni L: Anthracyclines: Molecular advances and
pharmacologic developments in antitumor activity and
cardiotoxicity. Pharmacol Rev. 56:185–229. 2004. View Article : Google Scholar : PubMed/NCBI
|