Introduction
Glioma is one of the most common malignant tumors in
the brain, with a median patient survival of 12 months (1). Despite the rapid progress of new
insights and technology in therapy and nursing care, the poor
prognosis has persisted during the past few decades, in particular
in glioblastoma cases (1–5). Therefore, it is essential to
investigate the mechanisms involved in glioma development and
progression and to identify a cure.
Wnt/β-catenin signaling has been proven to be
associated with various disease pathologies, especially in
gliomagenesis. The pathway activates downstream targets and thereby
regulates many biological processes through a complex of β-catenin
and T cell factor/lymphoid-enhancer factor 1 (TCF/LEF-1) family.
Wnt stabilizes cytosolic β-catenin, which then binds to TCF/LEF-1
in the nucleus and recruits transcription factors Brg1 and
CREB-binding protein to initiate Wnt-targeted gene expression
(6). Wnt inhibitory factor (WIF)
inhibits Wnt signaling by direct binding to Wnt molecules, acting
as an important antagonist. Wnt inhibitory factor-1 (WIF-1)
silencing, due to promoter hypermethylation, has been observed in
glioma (7–9).
Norcantharidin (NCTD) is the demethylated analog of
cantharidin isolated from blister beetles. NCTD has been reported
to possess anticancer activity but less nephrotoxicity than
cantharidin (10). Investigators
have reported the anticancer effect of NCTD against human glioma
cells. Yet, the demethylation of NCTD has not been previously
studied. Herein, we hypothesized that NCTD may be used as an
effective and nontoxic demethylating agent of the WIF-1
promoter.
In the present study, we showed that NCTD suppressed
Wnt/β-catenin signaling via demethylation of the WIF-1 promoter in
glioma cell lines. The results indicated that NCTD blocks
Wnt/β-catenin signaling, with impaired nuclear translocation of
β-catenin. Our results establish an important role for NCTD in the
treatment of brain gliomas, since it can cross the blood-brain
barrier. Thus, this may enhance the potential use of NCTD to
relieve patients suffering from gliomas.
Materials and methods
Cell culture, culture conditions and
reagents
The human LN229 and U251 cell lines were purchased
from the Institute of Biochemistry and Cell Biology, the Chinese
Academy of Sciences. All cells were maintained at 37°C in a 5%
CO2 incubator in Dulbecco's modified Eagle's medium
(DMEM; Gibco-BRL, Grand Island, NY, USA) supplemented with 10%
fetal bovine serum (Invitrogen). NCTD was purchased from
Sigma-Aldrich (St. Louis, MO, USA).
Cell proliferation and MTS assays
Cells were plated in 96-well plates at a density of
5,000 cells/well. Cells were allowed to attach overnight in growth
medium. After 24 h, the cells were treated with NCTD. After
incubation for 72 h, cellular proliferation was measured using the
MTS assay and absorbance was measured at 490 nm. Proliferation data
are presented as mean ± SD.
Cell cycle assay
For cell cycle analysis by flow cytometry,
NCTD-treated and NC cells in the log phase of growth were
harvested, washed with phosphate-buffered saline (PBS), fixed with
90% ethanol overnight at 4°C, and then incubated with RNase at 37°C
for 30 min. Nuclei of the cells were stained with propidium iodide
for an additional 30 min. A total of 20,000 nuclei were examined in
a FACSCalibur flow cytometer (Becton-Dickinson), and DNA histograms
were analyzed using Modifit software. Three independent experiments
were performed, and the data are presented as the mean ± SD.
Apoptosis assay
Forty-eight hours after treatment with NCTD and PBS,
apoptosis in the cultured cells was evaluated using Annexin V
labeling. For the Annexin V assay, an Annexin V-FITC-labeled
Apoptosis Detection kit (Abcam) was used according to the
manufacturer's instructions. Three independent experiments were
performed, and the data are presented as the mean ± SD.
Transwell invasion and migration
assays
Transwell invasion assay filters (Costar) were
coated with Matrigel (3.9 mg/ml, 60–80 µl) on the upper
surface of a polycarbonate membrane (diameter 6.5 mm and pore size
8 µm). After incubating at 37°C for 30 min, the Matrigel had
solidified and served as the extracellular matrix for the tumor
cell invasion analysis. The Transwell migration assay was carried
out without the presentation of Matrigel on the upper surface of
the polycarbonate membrane. A total of 600 µl of conditioned
medium derived from the tumor cells was used as a source of
chemoattractant and was placed in the bottom compartment of the
chamber. Harvested cells (5×104) in 100 µl of
serum-free DMEM were added into the upper compartment of the
chamber. After 24 h of incubation at 37°C with 5% CO2,
the medium was removed from the upper chamber. The non-invaded
cells on the upper side of the chamber were scraped off with a
cotton swab. The cells that had invaded from Matrigel into the
pores of the inserted filter were fixed with 100% methanol, and
stained with crystal violet. The number of cells invading through
the Matrigel was counted using three randomly selected visual
fields from the central and peripheral portions of the filter by an
inverted microscope at x200 magnification. Each assay was repeated
three times.
RNA extraction and quantitative real-time
polymerase chain reaction
Total RNA was isolated using TRIzol reagent
(Invitrogen) and cDNA was synthesized using the High Capacity cDNA
Archive kit (Applied Biosystems) according to the manufacturer's
instructions.
WIF-1 and cyclin B1 mRNA were quantified by qRT-PCR
using SYBR Premix Ex Taq (Takara Bio, Inc.). The primers were:
WIF-1 forward, 5′-CCGAAATGGAGGCTTTTGTA-3′ and reverse,
5′-TGGTTGAGCAGTTTGCTTTG-3′; cyclin B1 forward,
5′-GCAACCTCCAAGCCCGGACTG-3′ and reverse,
5′-GCAACCTCCAAGCCCGGACTG-3′.
The PCR conditions included an initial denaturation
step of 95°C for 2 min, followed by 35 cycles of 95°C for 10 sec,
56°C for 20 sec and 72°C for 20 sec, and a final elongation step of
72°C for 10 min. All qRT-PCRs were performed in duplicate.
Quantitation relative to the endogenous control was carried out
using Applied Biosystems 7500 Fast System SDS software.
Methylation-specific PCR (MS-PCR)
Genomic DNA from the cell lines and normal lung
tissue was extracted using the DNeasy kit (Qiagen), according to
the manufacturer's instructions. Bisulfite modification of genomic
DNA was performed using a methylation kit (EZ DNA Methylation kit;
Zymo Research, Orange, CA, USA). Bisulfite-treated genomic DNA was
amplified using either a methylation-specific or an
unmethylation-specific primer set. HotStarTaq DNA polymerase
(Qiagen) was used in the experiments.
Sequences of the methylation-specific primers were
5′-GGGCGTTTTATTGGGCGTAT-3′ (forward) and 5′-AAACCAACAATCAACGAAC-3′
(reverse). Sequences of the unmethylation-specific primers were
5′-GGGTGTTTTATTGGGTGTAT-3′ (forward) and 5′-AAACCAACAATCAACAAAAC-3′
(reverse).
Western blotting
Extraction of proteins from the cultured cells was
followed by immunoblotting with the relevant antibodies. Each
experiment was repeated at least three times.
Immunofluorescence staining assay
Immunofluorescence staining was conducted with LN229
and U251 cells cultured on coverslips. The cells were fixed in 4%
paraformaldehyde and permeabilized for 10 min in buffer containing
0.1% Triton X-100. The relevant antibodies were then added at the
dilutions recommended by the manufacturers. DAPI reagent was used
to stain the glioma cell nuclei, and the cells were visualized
using an FV-1000 laser-scanning confocal microscope and analyzed
using IPP5.1 (Olympus, Tokyo, Japan).
Statistical analysis
Quantitative variables are expressed as mean ± SD
and analyzed by one-way analysis of variance (ANOVA) and the
Student's t-test. All differences were considered to be
statistically significant at the level of P<0.05. Statistics
were performed using the SPSS Graduate Pack version 11.0
statistical software (SPSS, Inc., Chicago, IL, USA).
Results
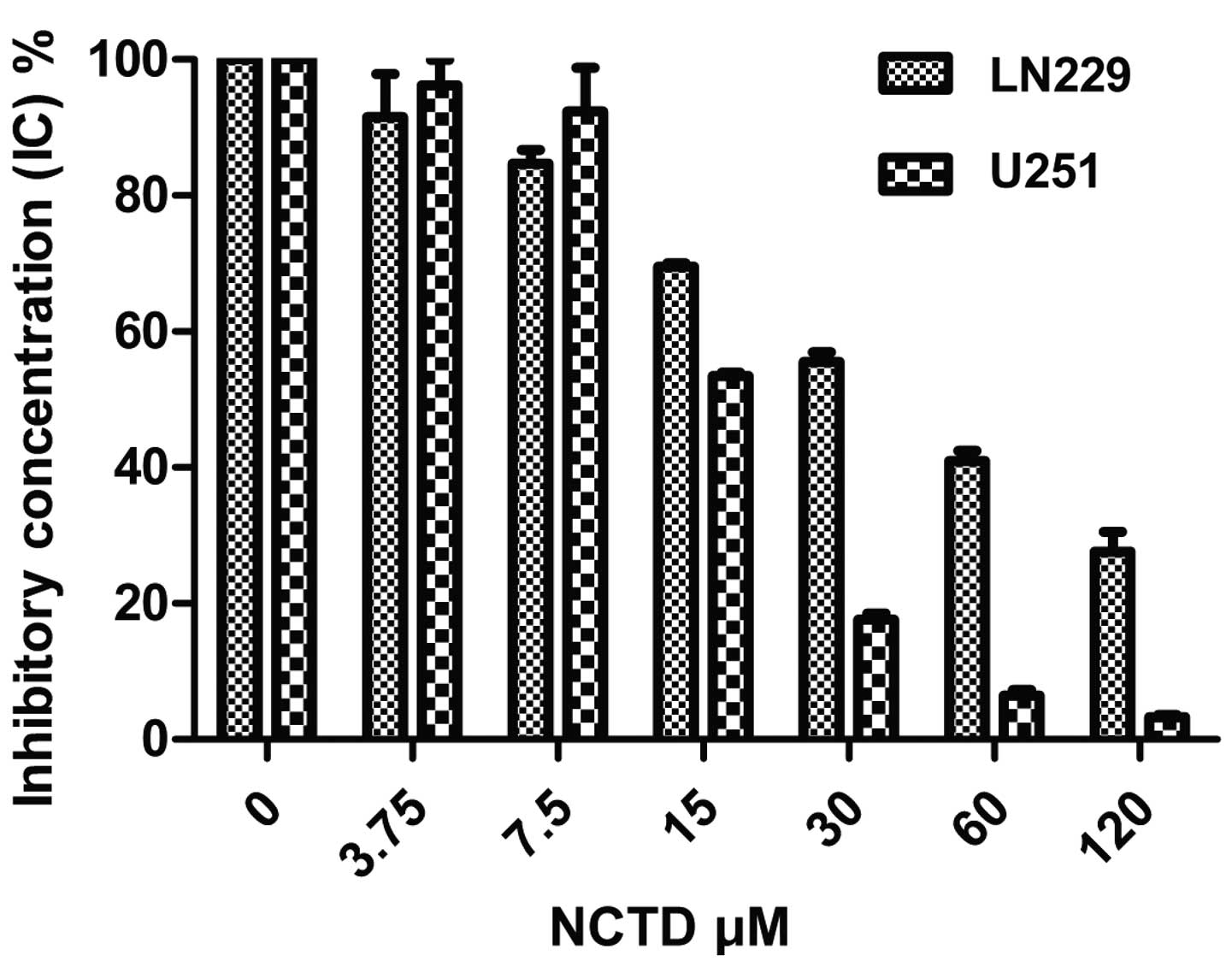
NCTD suppresses the growth of LN229 and
U251 cell lines
The LN229 and U251 cells were exposed to 0, 3.75,
7.5, 15, 30, 60 and 120 µM NCTD and PBS for 48 h. The
results indicated that the cellular viability decreased with
increasing NCTD concentrations (Fig.
1). The 50% growth inhibition (IC50) of NCTD was 44
µM for LN229 and 10 µM for U251 cells after 48 h,
respectively.
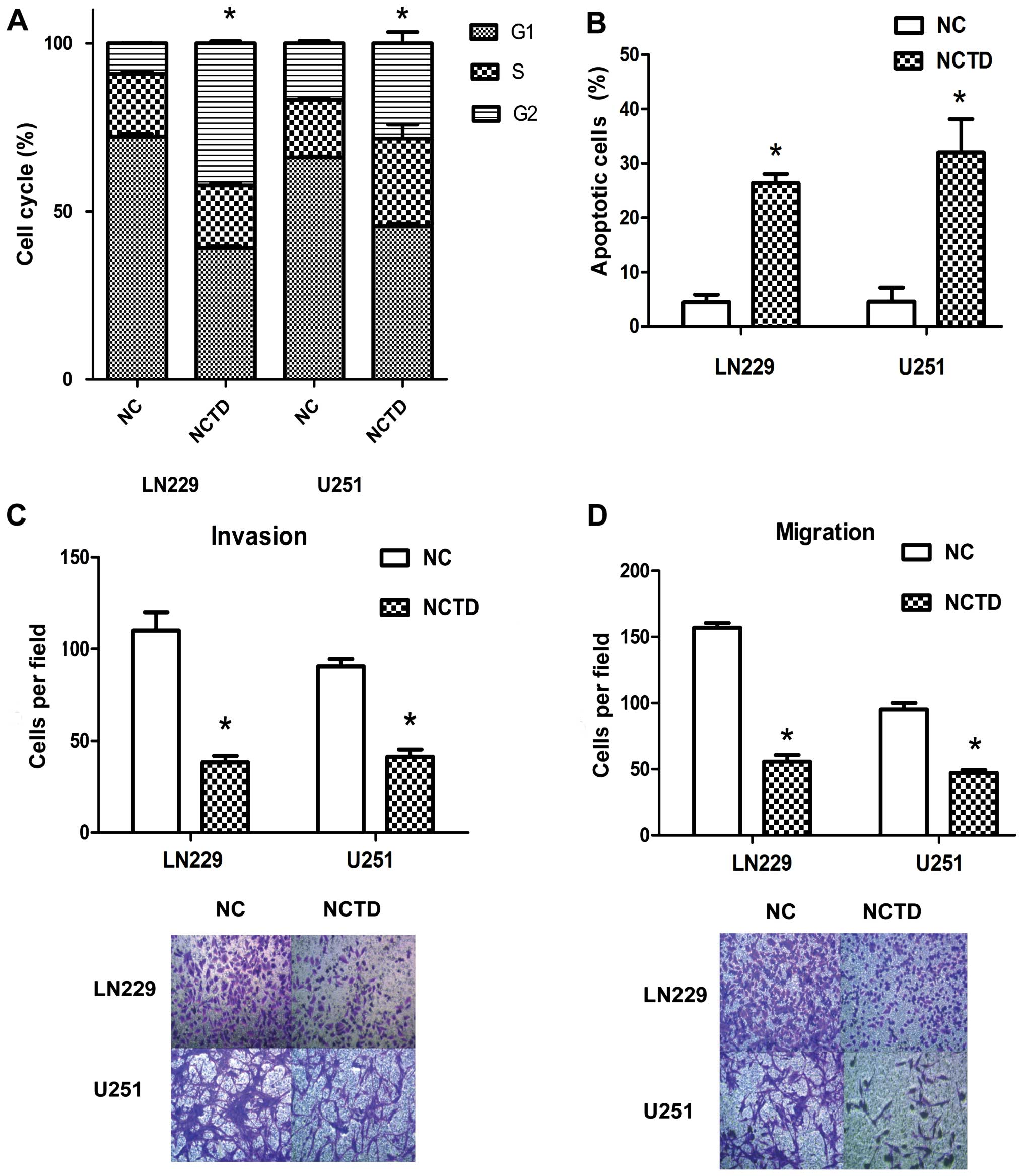
NCTD increases cell cycle arrest at the
G2 phase in the LN229 and U251 cell lines
To further characterize the growth arrest, LN229 and
U251 cell lines were treated with 44 and 10 µM NCTD, and
after 48 h they were subjected to propidium iodide-staining and
FACS analysis. Treatment with NCTD resulted in accumulation of
cells in the G2 phase, with ~42.33±0.514% of NCTD-treated LN229 and
28.28±2.74% of NCTD-treated U251 cells in the G2 phase compared
with 9.09±0.09% PBS-treated LN229 and 16.80±0.57% PBS-treated U251
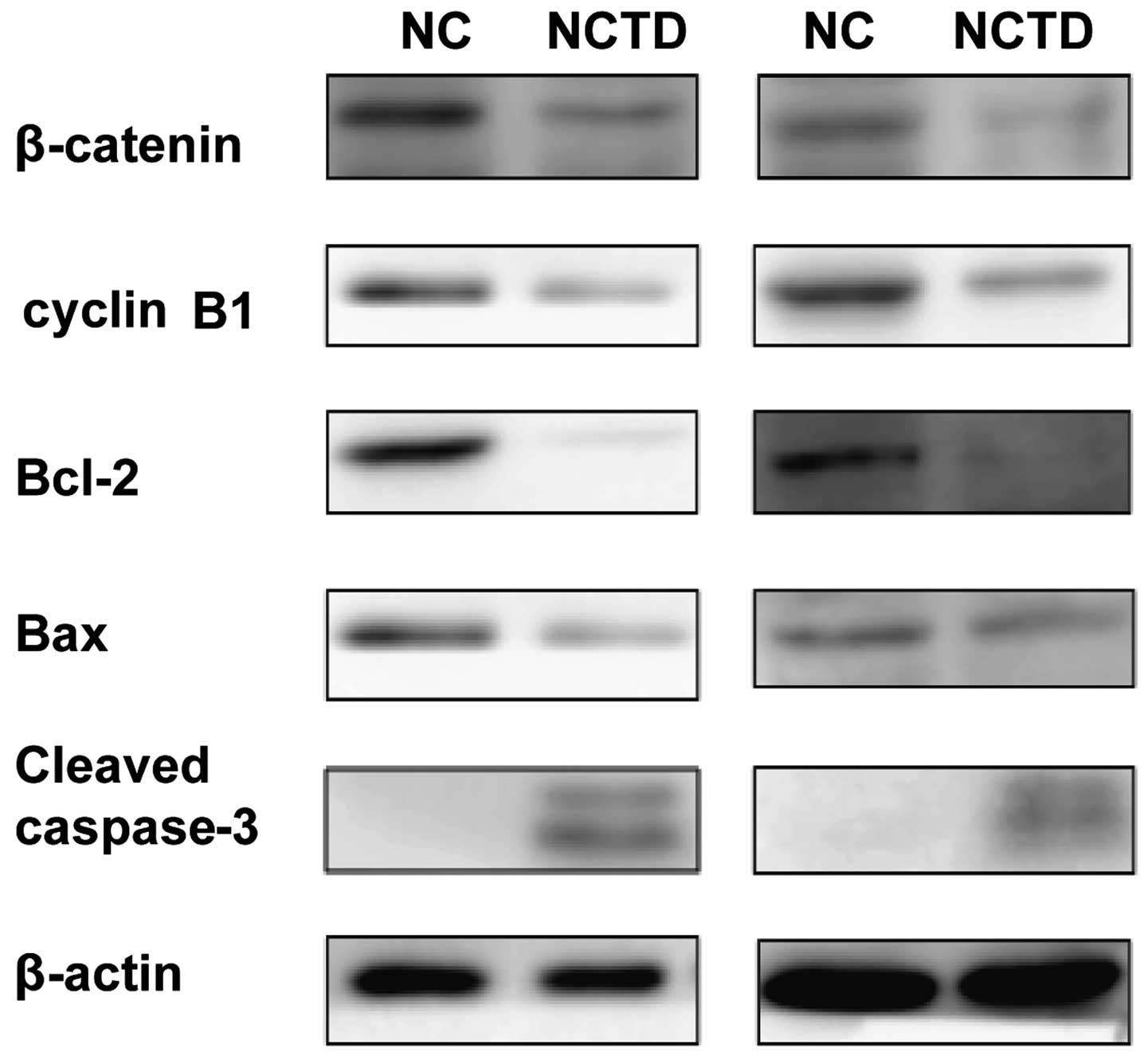
cells (Fig. 2A) (P<0.05). Cyclin
B1 level was decreased in the cell lines, as shown using western
blotting (Fig. 3). These analyses
indicate that NCTD induced cell cycle arrest at the G2 phase.
NCTD increases cell apoptosis in the
LN229 and U251 cell lines
NCTD also inhibited glioma cell survival. As shown
in Fig. 2B, compared with the NC
groups (4.47±1.13 and 4.55±2.11%) in the LN229 and U251 cells, the
treatment of NCTD caused a significant increase in apoptotic death
(26.40± and 32.00±5.00%) (P<0.05). The Bcl-2 protein level
decreased and the cleaved caspase-3 protein level increased in the
NCTD-treated cell lines, as shown using western blotting (Fig. 3).
The intrinsic pathway of apoptosis is controlled by
Bcl-2 family proteins, and cell death depends on the balance
between pro-apoptotic (Bax) and anti-apoptotic (Bcl-2, Bcl-xl and
Mcl-1) proteins. We also investigated the treatment effect on such
proteins. As shown in Fig. 3,
treatment with NCTD had some effect on Bax expression. However,
treatment with NCTD resulted in a significant downregulation of
Bcl-2. Cleaved caspase-3 release was detected by western blot
analysis after treatment of NCTD. The activity of pro-caspase-3
cleaved to caspase-3 increased after treatment of NCTD. Together,
this analysis demonstrated that NCTD induced downregulation of
Bcl-2 as previously reported but had little effect on Bax.
Activation of cleaved caspase-3 indicated that the compound induced
the intrinsic and extrinsic apoptotic pathways.
NCTD suppresses the invasion and
migration of the LN229 and U251 cell lines
To test whether NCTD regulates tumor cell invasion
and migration, Transwell assays were performed. The assays showed
that NCTD markedly inhibited Transwell invasion and migration of
glioma cells (Fig. 2C and D). In
regards to the LN229 cells, the number of cells invading through
the Matrigel in the NCTD group was 38.30±2.87 which was
significantly decreased compared with the number of invading cells
in the NC group (110.00±8.20) (P<0.05). Regarding the U251
cells, the invasive activity was also inhibited in the NCTD group
(41.3±3.30), when compared with that of the NC group (90.67±8.16)
(P<0.05). Regarding the LN229 cells, the number of cells
migrating through the polycarbonate membrane in the NCTD group was
55.67±4.19, significantly decreased when compared with the number
of invading cells in the NC group (157.00±2.94) (P<0.05).
Regarding the U251 cells, the invasive activity was also inhibited
in the NCTD group (47.33±1.70), compared with that of the NC group
(95.00±4.08) (P<0.05). The above data showed that NCTD
suppressed the invasion and migration of the human glioma
cells.
Expression of the mRNA transcript,
promoter methylation and protein expression of WIF-1 in the LN229
and U251 cell lines
qRT-PCR assay was also performed to analyze the
expression of WIF-1 at the transcription level. We found that the
WIF-1 transcript was downregulated in the LN229 and U251 cell lines
in the NC group. In contrast, it was upregulated in the
NCTD-treated cells (Fig. 4A). The
results showed that WIF-1 expression in the LN229 and U251 cell
lines in the NC group (10.00±0.00) was significantly lower compared
with the expression level in NCTD-treated cells (50.23±5.00 and
61.00±6.56) (P<0.05). To examine whether the methylation status
of the promoter correlates with the expression of WIF-1,
methylation-specific PCR was carried out (Fig. 4B). Promoter demethylation was
observed in the NCTD-treated cells. In contrast, aberrant
methylation was observed in the NC group. To detect the expression
level of WIF-1, western blotting was performed in the LN229 and
U251 cell lines (Fig. 4C). The
level of WIF-1 expression was significantly lower in the LN229 and
U251 cell lines in the NC group than those levels in the
NCTD-treated cells, but expression of DNA methyl-transferase-1
(DNMT1) was not influenced (Fig.
4C).
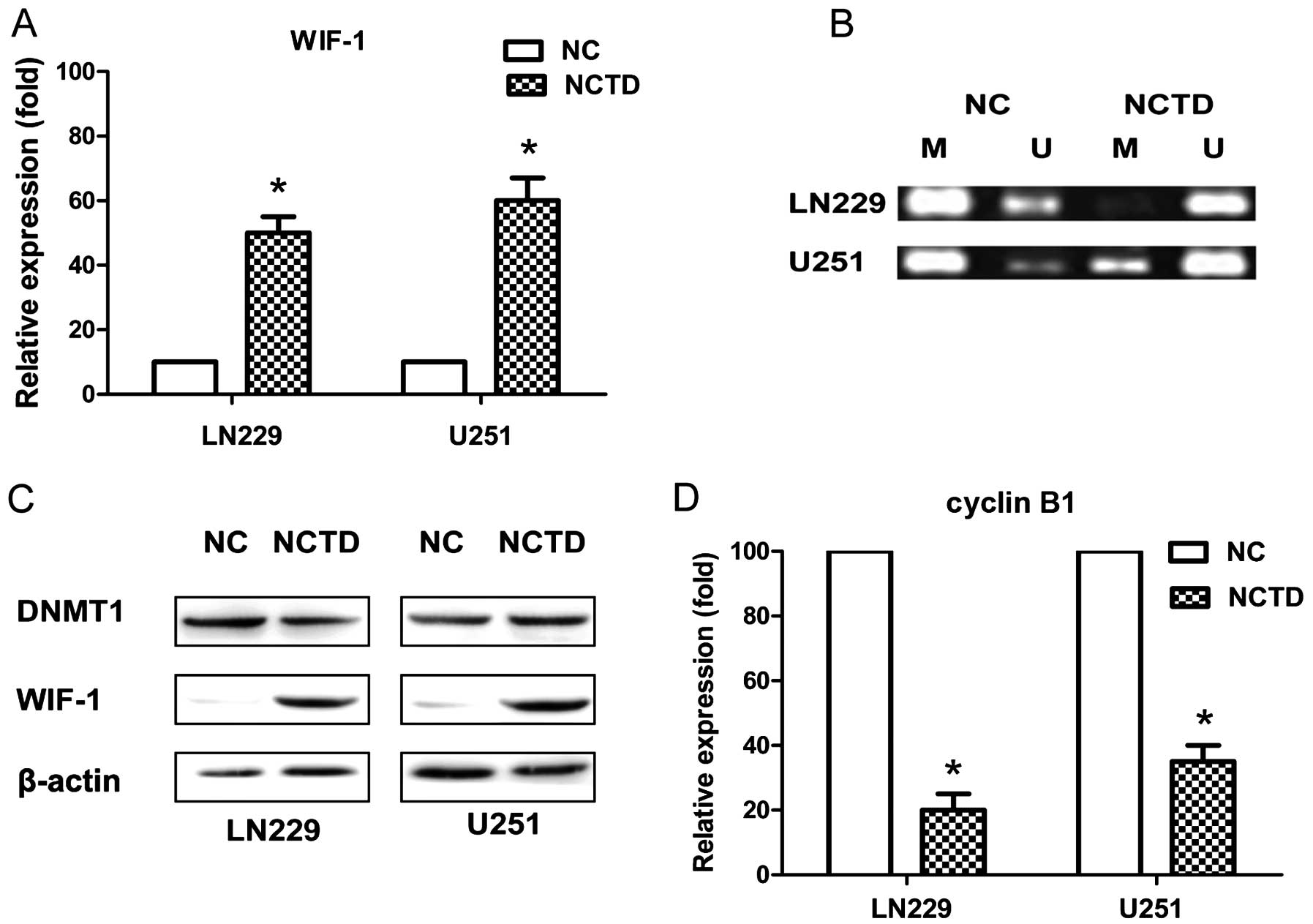 | Figure 4NCTD promotes the expression of WIF-1
and suppresses the expression of cyclin B1. (A) WIF-1 mRNA
expression was quantified by qRT-PCR analysis, and the expression
was significantly promoted after administration of NCTD in both the
LN229 and U251 cells (*P<0.05), relative to the NC
group. (B) Methylation-specific PCR (MS-PCR) analysis of the CpG
island in the NC and NCTD-treated groups. Bands in lane U indicate
the unmethylated DNA product with unmethylation-specific primers.
Bands in lane M indicate the methylated DNA product amplified with
methylation-specific primers. NCTD significantly promoted
unmethylation in both the LN229 and U251 cells, relative to the NC
group. (C) Expression of DNMT1 and WIF-1 protein was quantified by
western blot analysis. WIF-1 expression was significantly promoted
in both the LN229 and U251 cell lines, relative to the NC group.
(D) Expression of cyclin B1 mRNA was quantified by qRT-PCR
analysis, and the expression was significantly promoted in both the
LN229 and U251 cell lines (*P<0.05), relative to the
NC group. NCTD, norcantharidin; WIF-1, Wnt inhibitory factor-1;
DNMT1, DNA methyltransferase-1; NC, negative control. |
NCTD decreases cyclin B1 mRNA
expression
qRT-PCR results determined that the relative
expression level of cyclin B1 in the NCTD-treated LN229 cells was
20.40±5.43% (P<0.05), and 35.54±5.99% in the NCTD-treated U251
cells (P<0.05), compared with the NC groups, respectively
(Fig. 4D).
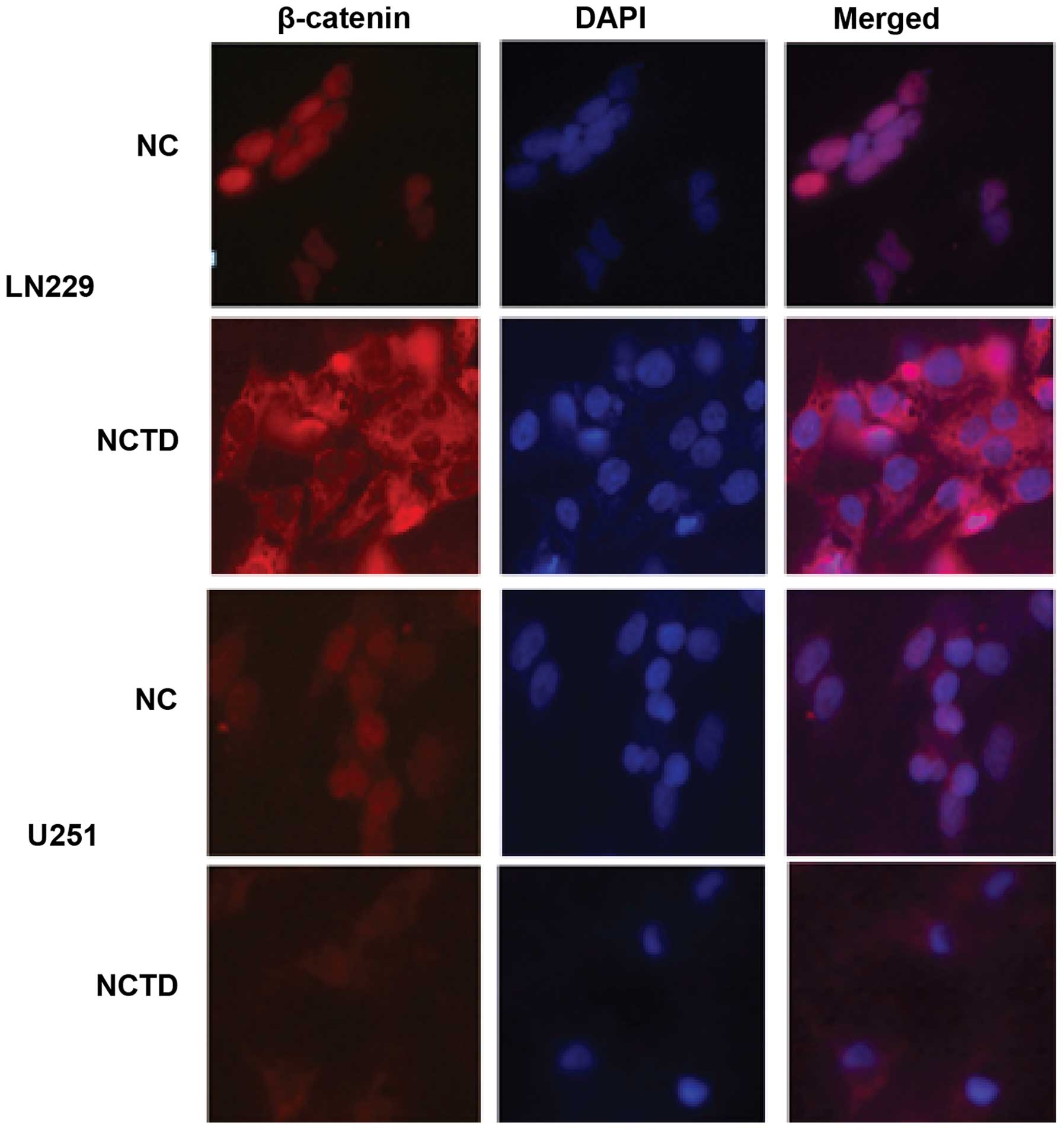
NCTD inhibits Wnt/β-catenin signaling
through loss of nuclear β-catenin
β-catenin, Wnt-target proteins and their expression
in many cell lines have been described. Wnt signaling has been
proven to be associated with various disease pathologies. NCTD was
reported to act as a negative regulator of Wnt signaling. Western
blotting of total protein extracts from the LN229 and U251 cells
revealed that the total β-catenin content was reduced after
treatment with NCTD (Fig. 3). The
level of protein cyclin B1, a known Wnt downstream target, was also
significantly reduced (Fig. 3).
Immunofluorescence assays in the LN229 and U251 cell lines revealed
altered nuclear location of β-catenin. After treatment of NCTD,
β-catenin was mainly located in the cytoplasm, while β-catenin was
mainly located in the nucleus in the NC cells (Fig. 5). This showed that the location of
β-catenin in cells shifted from the nucleus to the cytoplasm when
the cells were treated with NCTD.
Discussion
Glioma is one of the most frequent types of brain
tumor and it is invariably associated with a poor prognosis.
Standard treatments include surgery, radiotherapy and chemotherapy
(1–4). Prognosis is dismal with an average
survival of ~12 months despite the rapid progress of new insights
and technology in therapy and nursing care, and the poor prognosis
of patients with glioma has remained the same during the past few
decades (2–5).
The mechanisms involved in the development and
progression depend on many factors, and high-grade gliomas are
heterogeneous tumors in both cytology and genetic signatures
(5). One major signaling pathway
that has been linked to glioma is the Wnt (wingless-type mouse
mammary tumor virus integration site family) signaling pathway.
The Wnt signaling pathway activates Wnt-downstream
targets and thereby regulates many biological processes through a
complex of β-catenin and the TCF/LEF-1 family. Wnt stabilizes
cytosolic β-catenin, which then binds to TCF/LEF-1 in the nucleus
and recruits transcription factors Brg1 and CREB-binding protein to
initiate Wnt-targeted gene expression (6). WIF inhibits Wnt signaling by direct
binding to Wnt molecules and acts as an important antagonist
(7–9).
Wnt signaling has been proven to be associated with
embryonic development (6), tissue
renewal and regeneration (11), and
various cancer pathologies (12–14),
such as cell proliferation, cycle, death invasion and migration
(14).
The Wnt/β-catenin signaling pathway is constitutive
activated in glioma (15), and
aberrant expression of β-catenin in astrocytic gliomas and
glioblastoma is linked to a higher tumor grade (16). Some investigators indicate that
epigenetic events inactivate the inhibitory components of the Wnt
pathway in human cancers. As such, WIF1 in high-grade gliomas is
downregulated by promoter hypermethylation (7), and the SFRP1 promoter is commonly
hypermethylated in human cancers, including gliomas, among which
SFRP1 promoter hypermethylation is most commonly detected in
primary glioblastomas (17,18).
Aberrant methylation of promoter regions that
silences transcription of genes has been recognized as a mechanism
for inactivating tumor-suppressor genes. It occurs at cytosine
bases located 5′ to a guanosine and so-called CpG dinucleotide
short regions of CpG dinucleotides known as CpG islands are found
in the proximal promoter region of over half of human genes. WIF-1
silencing may be an early epigenetic carcinogenic event and plays a
role in tumor development and progression (7–9).
NCTD is the demethylated analog of cantharidin
isolated from blister beetles, and it is reported to possess
anticancer activity but less nephrotoxicity than cantharidin
(10). There is increasing evidence
that NCTD inhibits the proliferation, induces apoptosis (19,20,22–28,30),
blocks the cell cycle (20,21,26),
suppresses invasion, metastasis (27,29),
angio-genesis (27), and possess
anticancer activity via MAPK, TnR3, VEGFR2/MEK/ERK,
PI3-K/MMPs/Ln-5γ2 signaling, DNA replication, or ROS-mediated
mitochondrial dysfunction.
However, few studies have reported the anticancer
effect of NCTD against human glioma cells and the early study of
anti-glioma focused on Akt and MAPK signaling (31,32).
Similarly, NCTD could promote the loss of β-catenin activation and
inhibit the proliferation with dominant β-catenin signaling
(33–35). These data suggest that NCTD has
significant therapeutic potential in cancer via Wnt/β-catenin
pathways. DNMT1 could induce gene promoter methylation (36). The demethylation of NCTD has not
been previously studied. Herein, we hypothesized that NCTD may be
used as an effective and nontoxic demethylating agent of the WIF-1
promoter via suppressing the activity of DNMT1.
In this study, we demonstrated that the expression
of the WIF-1 gene was silenced in NC cells via methylation.
However, after exposure to NCTD, this epigenetic change was
reversed. We also showed that NCTD suppressed nuclear translocation
and expression of β-catenin using western blot analysis. These
results are consistent with our hypothesis that NCTD may recovery
the methylation of WIF-1 by demethylation, and downregulate the Wnt
canonical pathway in glioma cell lines.
We provide initial evidence that NCTD reactivates
WIF-1 from a methylation state, downregulates the canonical Wnt
pathway and establishes an important role for NCTD in the treatment
of gliomas, since it can cross the blood-brain barrier. This thus
enhances the potential use to relieve patients from suffering from
gliomas.
Abbreviations:
|
NC
|
negative control
|
|
WIF-1
|
Wnt inhibitory factor-1
|
|
NCTD
|
norcantharidin
|
|
DNMT1
|
DNA methyltransferase-1
|
Acknowledgments
The present study was supported by the Natural
Science Foundation of Zhejiang Province of China (nos. LY13H160003
and LY14H160017) and the National Science Foundation for Young
Scientists of China (no. 81402044).
References
|
1
|
Chen W, Wu Q, Mo L and Nassi M:
Intra-arterial chemotherapy is not superior to intravenous
chemotherapy for malignant gliomas: A systematic review and
meta-analysis. Eur Neurol. 70:124–132. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bregy A, Shah AH, Diaz MV, Pierce HE, Ames
PL, Diaz D and Komotar RJ: The role of Gliadel wafers in the
treatment of high-grade gliomas. Expert Rev Anticancer Ther.
13:1453–1461. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yin AA, Cai S, Dong Y, Zhang LH, Liu BL,
Cheng JX and Zhang X: A meta-analysis of temozolomide versus
radiotherapy in elderly glioblastoma patients. J Neurooncol.
116:315–324. 2014. View Article : Google Scholar
|
|
4
|
Yin AA, Zhang LH, Cheng JX, Dong Y, Liu
BL, Han N and Zhang X: Radiotherapy plus concurrent or sequential
temozolomide for glioblastoma in the elderly: A meta-analysis. PLoS
One. 8:e742422013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang Y and Jiang T: Understanding high
grade glioma: Molecular mechanism, therapy and comprehensive
management. Cancer Lett. 331:139–146. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schneider AJ, Branam AM and Peterson RE:
Intersection of AHR and Wnt signaling in development, health, and
disease. Int J Mol Sci. 15:17852–17885. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim SA, Kwak J, Nam HY, Chun SM, Lee BW,
Lee HJ, Khang SK and Kim SW: Promoter methylation of WNT inhibitory
factor-1 and expression pattern of WNT/β-catenin pathway in human
astrocytoma: Pathologic and prognostic correlations. Mod Pathol.
26:626–639. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu J, Fang J, Yang Z, Chen F, Liu J and
Wang Y: Wnt inhibitory factor-1 regulates glioblastoma cell cycle
and proliferation. J Clin Neurosci. 19:1428–1432. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang Z and Wang Y, Fang J, Chen F, Liu J,
Wu J and Wang Y: Expression and aberrant promoter methylation of
Wnt inhibitory factor-1 in human astrocytomas. J Exp Clin Cancer
Res. 29:262010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang YM, Meng ZZ, Yue GX and Chen JX:
Norcantharidin induces HL-60 cell apoptosis in vitro. Evid Based
Complement Alternat Med. 2012:1542712012. View Article : Google Scholar
|
|
11
|
Clevers H, Loh KM and Nusse R: Stem cell
signaling. An integral program for tissue renewal and regeneration:
Wnt signaling and stem cell control. Science. 346:12480122014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jamieson C, Sharma M and Henderson BR:
Targeting the β-catenin nuclear transport pathway in cancer. Semin
Cancer Biol. 27:20–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Le PN, McDermott JD and Jimeno A:
Targeting the Wnt pathway in human cancers: therapeutic targeting
with a focus on OMP-54F28. Pharmacol Ther. 146:1–11. 2015.
View Article : Google Scholar
|
|
14
|
Bilir B, Kucuk O and Moreno CS: Wnt
signaling blockage inhibits cell proliferation and migration, and
induces apoptosis in triple-negative breast cancer cells. J Transl
Med. 11:2802013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen X, Hu W, Xie B, Gao H, Xu C and Chen
J: Downregulation of SCAI enhances glioma cell invasion and stem
cell like phenotype by activating Wnt/β-catenin signaling. Biochem
Biophys Res Commun. 448:206–211. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schüle R, Dictus C, Campos B, Wan F,
Felsberg J, Ahmadi R, Centner FS, Grabe N, Reifenberger G, Bermejo
JL, et al: Potential canonical wnt pathway activation in high-grade
astrocytomas. Sci World J. 2012:6973132012. View Article : Google Scholar
|
|
17
|
Wang K, Wang X, Zou J, Zhang A, Wan Y, Pu
P, Song Z, Qian C, Chen Y, Yang S, et al: miR-92b controls glioma
proliferation and invasion through regulating Wnt/beta-catenin
signaling via Nemo-like kinase. Neuro-oncol. 15:578–588. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Delic S, Lottmann N, Stelzl A, Liesenberg
F, Wolter M, Götze S, Zapatka M, Shiio Y, Sabel MC, Felsberg J, et
al: MiR-328 promotes glioma cell invasion via SFRP1-dependent
Wnt-signaling activation. Neuro-oncol. 16:179–190. 2014. View Article : Google Scholar :
|
|
19
|
Liu D, Shi P, Yin X, Chen Z and Zhang X:
Effect of norcantharidin on the human breast cancer Bcap-37 cells.
Connect Tissue Res. 53:508–512. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee YC, Lee LM, Yang CH, Lin AM, Huang YC,
Hsu CC, Chen MS, Chi CW, Yin PH, Kuo CD, et al: Norcantharidin
suppresses cell growth and migration with enhanced anticancer
activity of gefitinib and cisplatin in human non-small cell lung
cancer cells. Oncol Rep. 29:237–243. 2013.
|
|
21
|
Liao HF, Chen YJ, Chou CH, Wang FW and Kuo
CD: Norcantharidin induces cell cycle arrest and inhibits
progression of human leukemic Jurkat T cells through
mitogen-activated protein kinase-mediated regulation of
interleukin-2 production. Toxicol In Vitro. 25:206–212. 2011.
View Article : Google Scholar
|
|
22
|
Liu S, Yu H, Kumar SM, Martin JS, Bing Z,
Sheng W, Bosenberg M and Xu X: Norcantharidin induces melanoma cell
apoptosis through activation of TR3 dependent pathway. Cancer Biol
Ther. 12:1005–1014. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shen B, He PJ and Shao CL: Norcantharidin
induced DU145 cell apoptosis through ROS-mediated mitochondrial
dysfunction and energy depletion. PLoS One. 8:e846102013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shou LM, Zhang QY, Li W, Xie X, Chen K,
Lian L, Li ZY, Gong FR, Dai KS, Mao YX, et al: Cantharidin and
norcantharidin inhibit the ability of MCF-7 cells to adhere to
platelets via protein kinase C pathway-dependent downregulation of
α2 integrin. Oncol Rep. 30:1059–1066. 2013.PubMed/NCBI
|
|
25
|
An WW, Gong XF, Wang MW, Tashiro S,
Onodera S and Ikejima T: Norcantharidin induces apoptosis in HeLa
cells through caspase, MAPK, and mitochondrial pathways. Acta
Pharmacol Sin. 25:1502–1508. 2004.PubMed/NCBI
|
|
26
|
Yu CC, Ko FY, Yu CS, Lin CC, Huang YP,
Yang JS, Lin JP and Chung JG: Norcantharidin triggers cell death
and DNA damage through S-phase arrest and ROS-modulated apoptotic
pathways in TSGH 8301 human urinary bladder carcinoma cells. Int J
Oncol. 41:1050–1060. 2012.PubMed/NCBI
|
|
27
|
Zhang L, Ji Q, Liu X, Chen X, Chen Z, Qiu
Y, Sun J, Cai J, Zhu H and Li Q: Norcantharidin inhibits tumor
angiogenesis via blocking VEGFR2/MEK/ERK signaling pathways. Cancer
Sci. 104:604–610. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang S, Li G, Ma X, Wang Y, Liu G, Feng
L, Zhao Y, Zhang G, Wu Y, Ye X, et al: Norcantharidin enhances
ABT-737-induced apoptosis in hepatocellular carcinoma cells by
transcriptional repression of Mcl-1. Cell Signal. 24:1803–1809.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang JT, Sun W, Zhang WZ, Ge CY, Liu ZY,
Zhao ZM, Lu XS and Fan YZ: Norcantharidin inhibits tumor growth and
vasculogenic mimicry of human gallbladder carcinomas by suppression
of the PI3-K/MMPs/Ln-5γ2 signaling pathway. BMC Cancer. 14:1932014.
View Article : Google Scholar
|
|
30
|
Chen S, Wan P, Ding W, Li F, He C, Chen P,
Li H, Hu Z, Tan W and Li J: Norcantharidin inhibits DNA replication
and induces mitotic catastrophe by degrading initiation protein
Cdc6. Int J Mol Med. 32:43–50. 2013.PubMed/NCBI
|
|
31
|
Pitre A, Davis N, Paul M, Orr AW and
Skalli O: Synemin promotes AKT-dependent glioblastoma cell
proliferation by antagonizing PP2A. Mol Biol Cell. 23:1243–1253.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zheng J, Du W, Song LJ, Zhang R, Sun LG,
Chen FG and Wei XT: Norcantharidin induces growth inhibition and
apoptosis of glioma cells by blocking the Raf/MEK/ERK pathway.
World J Surg Oncol. 12:2072014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu MY, Xie X, Xu ZK, Xie L, Chen Z, Shou
LM, Gong FR, Xie YF, Li W and Tao M: PP2A inhibitors suppress
migration and growth of PANC-1 pancreatic cancer cells through
inhibition on the Wnt/β-catenin pathway by phosphorylation and
degradation of β-catenin. Oncol Rep. 32:513–522. 2014.PubMed/NCBI
|
|
34
|
Chuang KA, Lieu CH, Tsai WJ, Wu MH, Chen
YC, Liao JF, Wang CC and Kuo YC: Evaluation of anti-Wnt/β-catenin
signaling agents by pGL4-TOP transfected stable cells with a
luciferase reporter system. Braz J Med Biol Res. 43:931–941. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hsieh CH, Chao KS, Liao HF and Chen YJ:
Norcantharidin, derivative of cantharidin, for cancer stem cells.
Evid Based Complement Alternat Med. 2013:8386512013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu YL, Yang HP, Zhou XD, Gong L, Tang CL
and Wang HJ: The hypomethylation agent bisdemethoxycurcumin acts on
the WIF-1 promoter, inhibits the canonical Wnt pathway and induces
apoptosis in human non-small-cell lung cancer. Curr Cancer Drug
Targets. 11:1098–1110. 2011. View Article : Google Scholar : PubMed/NCBI
|