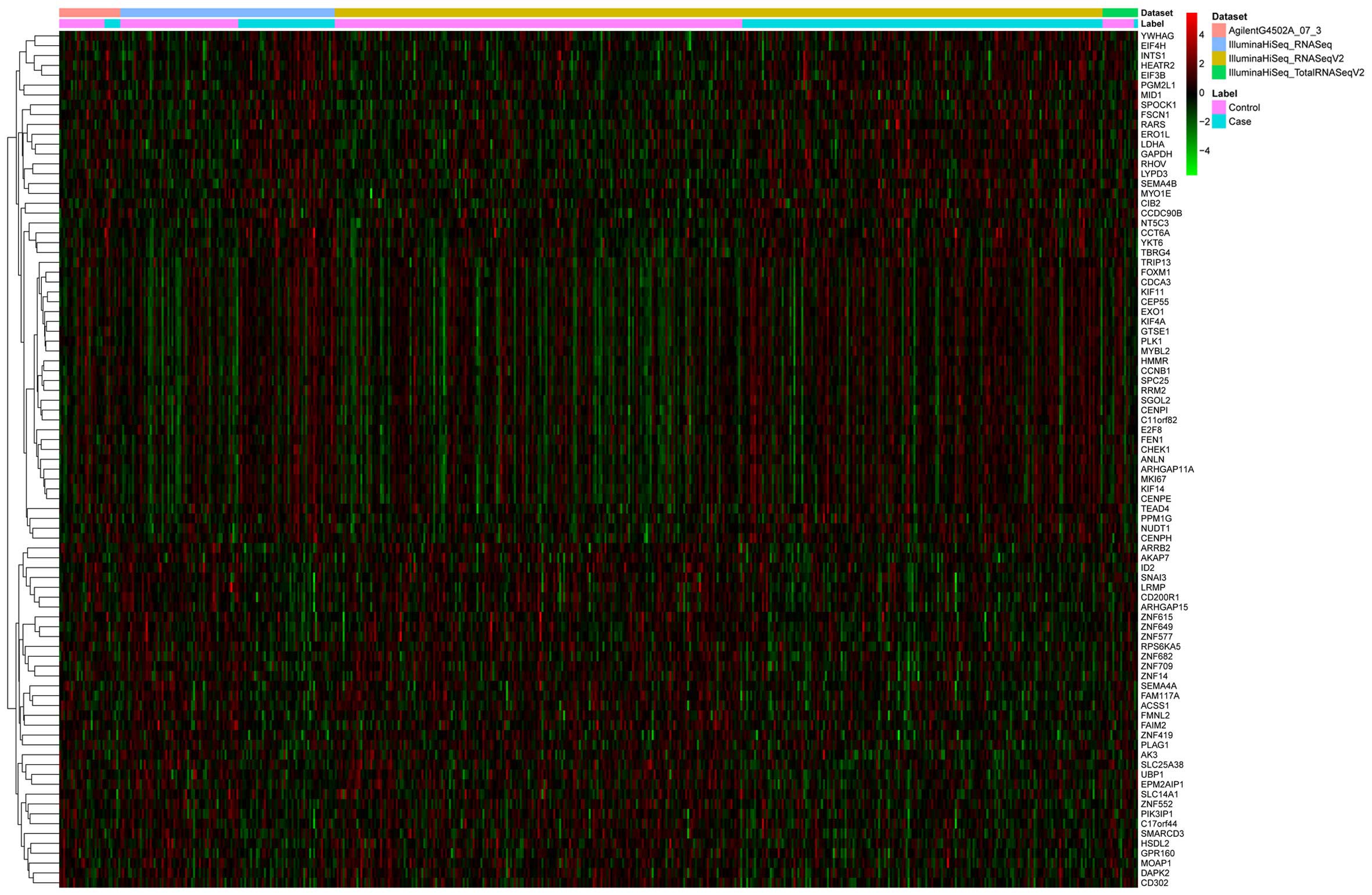
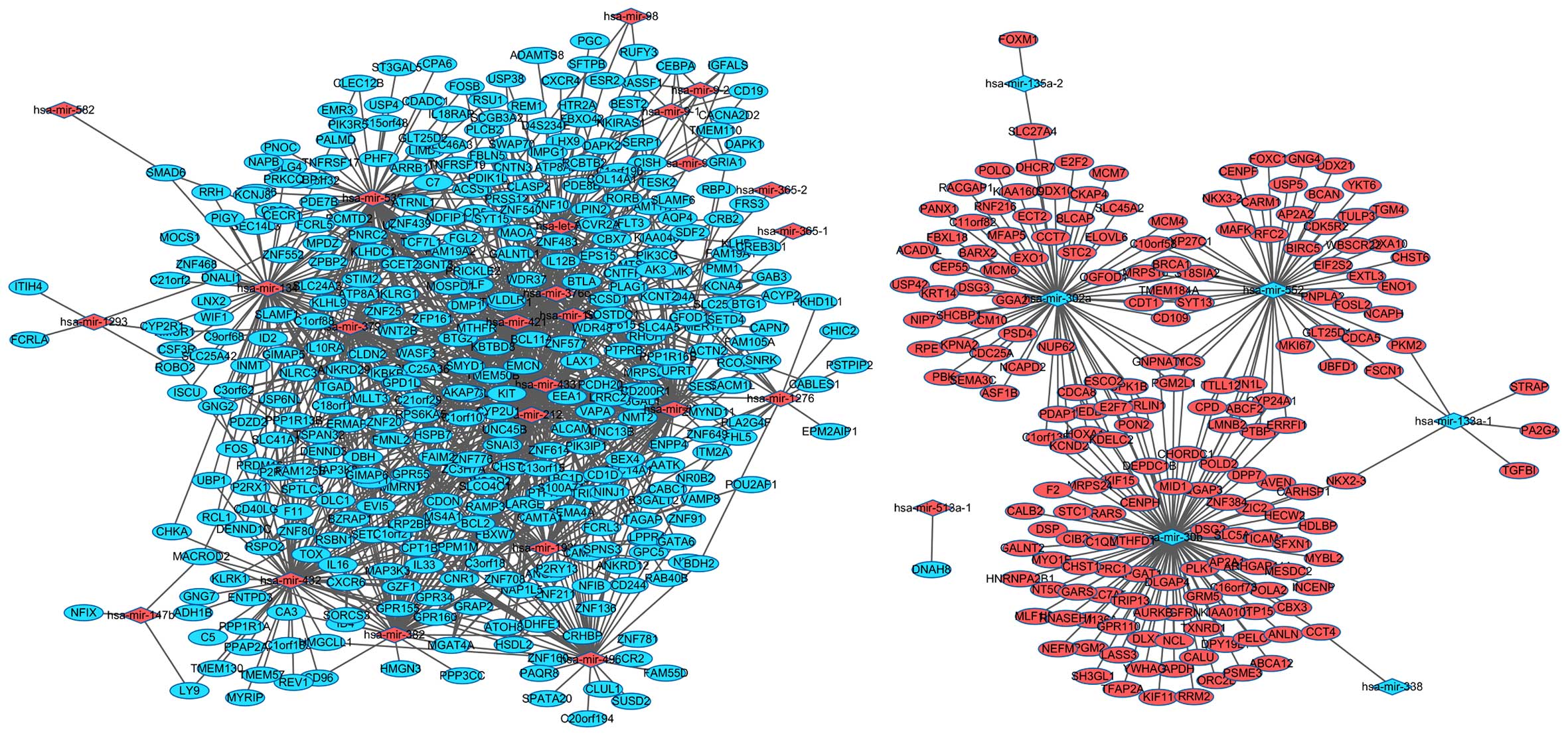
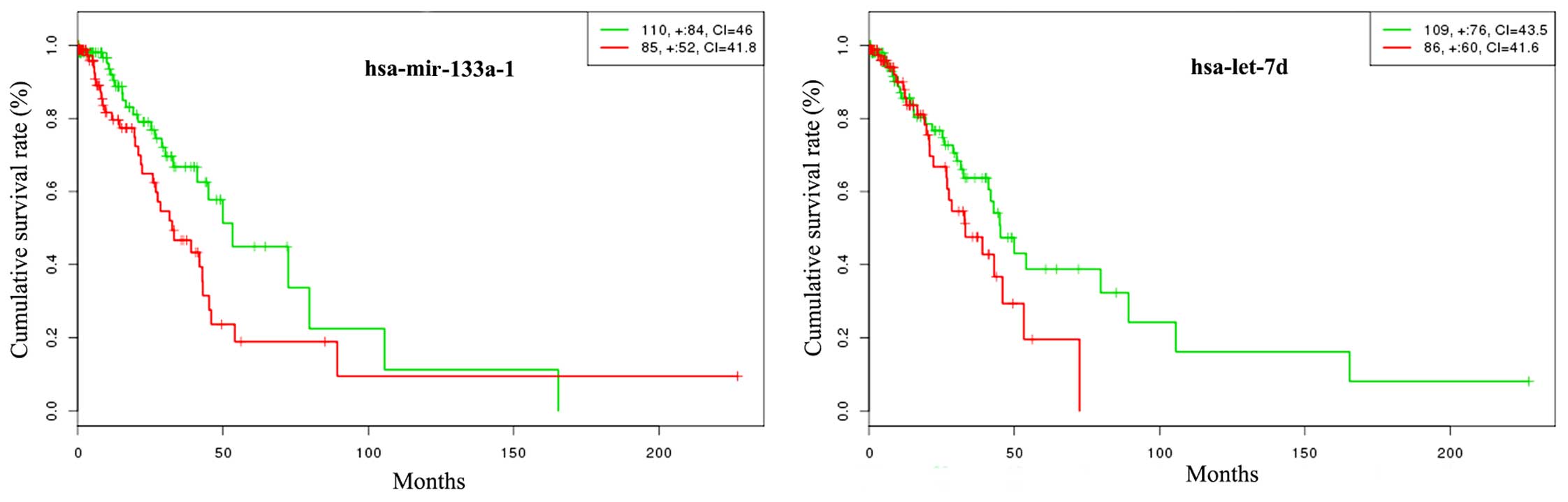
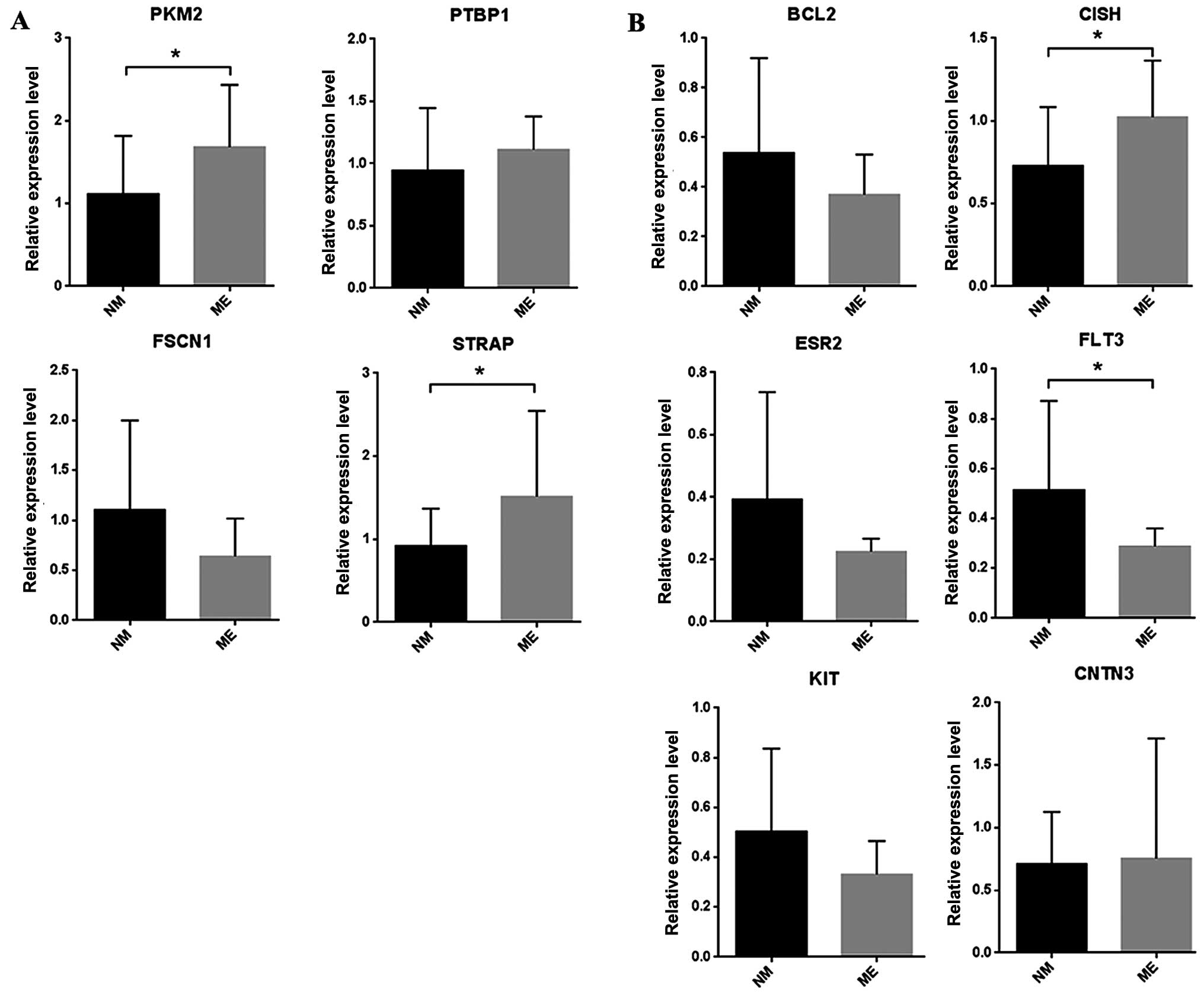
| hsa-mir-133a-1 | Downregulated | 7 | PTBP1, PKM2,
NKX2-3, STRAP, FSCN1, TGFBI, PA2G4 |
| hsa-mir-135a-2 | Downregulated | 2 | SLC27A4, FOXM1 |
| hsa-mir-302a | Downregulated | 67 | MCM7, SLC27A4,
ELOVL6, RPE, CKAP4, KPNA2, SEMA3C, POLQ, KCND2, SLC45A2, MCM10,
ASF1B, PBK, ST8SIA2, USP42, E2F7, CCT7, ERLIN1, BLCAP, PDAP1,
CD109, KDELC2, ESCO2, DDX10, DHCR7, DSG3, E2F2, TMEM184A, C11orf82,
GGA2, PSD4, NUP62, PANX1, PGM2L1, RACGAP1, HOXA11, CYP27C1, ACADVL,
KRT14, MCM4, MCM6, NEDD4, MRPS16, NIP7, CYCS, PON2, RNF216, CDCA8,
CEP55, OGFOD1, SYT13, KIAA1609, GNPNAT1, BRCA1, UPK1B, C1orf135,
SHCBP1, FBXL18, MFAP5, CDT1, C10orf58, BARX2, STC2, EXO1, NCAPD2,
CDC25A, ECT2 |
| hsa-mir-30b | Downregulated | 100 | MRPS24, DPP7,
NKX2-3, F2, TRIP13, AP2A1, MYO1E, GARS, PLK1, UTP15, ZNF384,
KDELC2, DSG2, DSP, AURKB, ABCF2, POLD2, TXNRD1, NCL, MYBL2,
CARHSP1, SFXN1, CHORDC1, NEDD4, PTBP1, RARS, GAPDH, PTGFRN, IQGAP3,
GNPNAT1, LMNB2, POLA2, NT5C3, DLGAP4, GALNT2, CYP24A1, LASS3,
MESDC2, DPY19L1, GRM5, INCENP, KIF11, PELO, ERRFI1, PON2, AVEN,
HECW2, RRM2, YWHAG, SLC7A5, ARHGAP11A, LPGAT1, CHST1, PSME3, CIB2,
CCT4, ERLIN1, C1QL1, PDAP1, CBX3, SLC5A11, CPD, TICAM1, ESCO2,
DLX1, TTLL12, RNASEH1, C16orf73, ABCA12, GPR110, PGM2L1, HDLBP,
HNRNPA2B1, HOXA11, KCND2, MID1, MTHFD1, NEFM, ORC2L, CYCS, ANLN,
CDCA8, PGM2, DEPDC1B, KIF15, SH3GL1, CENPH, STC1, TFAP2A, UPK1B,
ZIC2, C1orf135, CALB2, MLF1IP, CALU, FAM136A, PRC1, HN1L, KIAA0101,
E2F7 |
| hsa-mir-338 | Downregulated | 1 | CCT4 |
| hsa-mir-552 | Downregulated | 52 | CYP24A1, FOXC1,
CYCS, UBFD1, C10orf58, EIF2S2, ABCF2, CARM1, CENPF, YKT6, CDCA5,
WBSCR22, CD109, CPD, AP2A2, ENO1, TMEM184A, EXTL3, TTLL12, NCAPH,
FOSL2, GNG4, PGM2L1, HOXA10, BIRC5, CYP27C1, CHST6, MCM4, MKI67,
MRPS16, PKM2, ERRFI1, PNPLA2, PTBP1, SYT13, NKX3-2, RFC2, BCAN,
GNPNAT1, FSCN1, BRCA1, TGM4, TULP3, GLT25D1, MAFK, USP5, ST8SIA2,
CDT1, LMNB2, CDK5R2, HN1L, DDX21 |
| hsa-mir-1276 | Upregulated | 15 | CNOT6L, CHIC2,
PIK3CG, C1orf190, BDH2, BCL2, SLC14A1, HSDL2, PSTPIP2, CD5,
PKHD1L1, ITM2A, LPIN2, MTSS1, EPM2AIP1 |
| hsa-mir-1293 | Upregulated | 5 | NMUR1, ITIH4,
ROBO2, DNALI1, FCRLA |
| hsa-mir-134 | Upregulated | 74 | KIT, KLHDC1,
SEC14L3, IL16, PCMTD2, SLC14A1, FCRL5, RCL1, ATP8A1, RRH, CHKA,
WIF1, ERMAP, FMNL2, CYP2R1, CNR1, CSF3R, PRICKLE2, ZNF25, LNX2,
DENND3, MMRN1, P2RX2, PDZD2, GPD1L, ISCU, CBX7, CNOT6L, SLC41A1,
GCET2, PDE7B, ZNF776, ZNF615, SLC25A42, S100A7A, FAM19A2, ID2,
RSPO2, C3orf62, KCNJ8, CLEC12B, SMAD6, MOCS1, UNC13C, MTHFR,
GIMAP6, CDON, CECR1, PLCB2, GNG2, RSBN1, TNFRSF19, PRKCQ, MOSPD1,
SLC24A3, BCL2, SLAMF1, UBP1, C18orf1, ZFP161, C21orf2, ZNF20,
DNALI1, BTG2, ZNF552, C1orf21, PIGY, MPDZ, LIMD1, ZNF468, IL33,
RPS6KA5, TP53INP1, LPIN2 |
| hsa-mir-147b | Upregulated | 3 | MACROD2, LY9,
NFIX |
| hsa-mir-193b | Upregulated | 42 | BCL2, KIT, UPRT,
CAMTA1, CBX7, MMP19, UBP1, RAMP3, UNC13B, MGAT4A, UNC45B, PDIK1L,
PRICKLE2, TAPT1, FAIM2, CNOT6L, PPP1R16B, GATA6, CRB2, C3orf62,
RHOH, MAP3K3, CD244, PLAG1, C21orf29, POU2AF1, SNRK, TRIM68, NXF3,
WDR48, SLC4A5, C18orf1, EVI5, C1orf21, CAMKK1, KBTBD8, ATOH8,
VAMP8, NMT2, TP53INP1, AATK, SPNS3 |
| hsa-mir-196b | Upregulated | 53 | FLT3, BCL2, GATA6,
AQP4, ZMYND11, EPS15, WDR37, CAPN7, ATRNL1, PDE7B, SMAD6, MGAT4A,
CYP2U1, UNC45B, PRICKLE2, KLHDC8B, GPD1L, SLC41A1, PPP1R16B, SERP1,
HLF, SNAI3, RSPO2, C3orf62, MAOA, MTHFR, CDON, PLAG1, PLCB2,
BCL11A, BEST2, TNFRSF19, MRPS25, COL14A1, WNT2B, TMEM50B, SLC25A20,
EEA1, ZNF577, ATOH8, PRSS12, FAM125B, LIMD1, CREB3L1, ACVR2A, VAPA,
MS4A1, TP53INP1, FHL5, AATK, LPIN2, WSCD2, LPPR4 |
| hsa-mir-212 | Upregulated | 92 | RPS6KA5, REM1,
ANKRD29, PRICKLE2, SOSTDC1, PDE7B, SERP1, MAOA, SLC24A3, TMEM50B,
BTG2, SLC25A20, USP38, KLRG1, CXCR6, WASF3, FGL2, MGAT4A, CYP2U1,
PIK3IP1, FMNL2, CNR1, C1orf88, MAP3K8, CPT1B, UPRT, GAB3, GPR155,
BTLA, ZNF483, TAPT1, ENPP4, KLRK1, CLASP2, GLT25D2, TBC1D9, GPD1L,
CAMTA1, FOSB, CNOT6L, PPP1R16B, NAP1L5, GPR160, SESN1, ZNF615,
FAM19A2, IKBKB, IL12B, IL16, IMPG1, B3GNT8, MAP3K3, MLLT3, UNC13C,
AK3, CDON, EMCN, PLAG1, C21orf29, GFOD1, LAX1, PCMTD2, FBXW7,
PNRC2, MOSPD1, LHX9, GALNTL1, WDR48, STIM2, PTPN4, SLC4A5, RSU1,
SDF2, C18orf1, ZFP161, ZNF708, ZNF80, C1orf21, TCF7L1, SYT15,
HSDL2, KBTBD8, PDE8B, LIMD1, LARGE, VAPA, NMT2, TP53INP1, CHST10,
TOX, ELMO1, SEMA4A |
| hsa-mir-365-1 | Upregulated | 1 | BCL2 |
| hsa-mir-365-2 | Upregulated | 1 | BCL2 |
| hsa-mir-376c | Upregulated | 51 | PDIK1L, SMYD1,
ALCAM, GNG2, KLHL9, ARID4A, VLDLR, ACYP2, TESK2, FBLN5, RCBTB2,
SLAMF6, KLHDC1, UNC45B, ZNF483, NLRC3, DAPK2, PLA2G4F, C1orf101,
GCET2, D4S234E, SERP1, SNAI3, FAM19A2, FAM19A1, MAP3K3, GIMAP6,
AK3, C3orf18, PLAG1, P2RY13, RCOR3, MOSPD1, ST6GAL1, ZNF649,
C18orf1, ZFP161, ZNF10, ZNF708, ZNF614, NDFIP1, C1orf21, IL33,
CABLES1, GPR55, MS4A1, WSCD2, TOX, MTSS1, FLT3, RORB |
| hsa-mir-379 | Upregulated | 52 | ATP8A2, CYP2U1,
KLHL9, C7, KLRG1, NMUR1, UNC13B, ZNF211, INMT, SCGB3A2, C1orf88,
GAB3, ANKRD29, PRICKLE2, ALCAM, DENND3, FAIM2, SLC41A1, C1orf101,
HSPB7, ZNF776, C13orf15, GZMK, HLF, S100A7A, ID4, SLCO4C1, IL16,
KIT, ARRB1, UNC13C, GIMAP6, CDON, C9orf68, SLC25A36, SPTLC3, BEX4,
WNT2B, C18orf1, ZNF10, DNALI1, C1orf21, ACSS1, IL18RAP, CD5, LARGE,
VAPA, GPR55, MS4A1, CD40LG, WSCD2, LPPR4 |
| hsa-mir-381 | Upregulated | 102 | KIT, DLC1, WDR37,
MMRN1, ID2, CNTN3, SLC25A36, SLC24A3, PCDH20, ACVR2A, MERTK, FBLN5,
CXCR6, WASF3, MGAT4A, CYP2U1, FMNL2, KLHDC1, CNR1, CNTFR, C1orf88,
CD200R1, MAP3K8, UPRT, PDIK1L, SMYD1, BTLA, ZNF483, PRICKLE2,
SUSD3, F11, ENPP4, SACM1L, SORCS3, SWAP70, CLASP2, TBC1D9, GPD1L,
ANKRD12, CAMTA1, CAPN7, CNOT6L, SLC41A1, GCET2, NAP1L5, PDE7B,
SERP1, ZNF776, ZNF615, GPR34, GRIA1, C13orf15, ZC3H7A, GZMK, HTR2A,
ID4, RSPO2, KCNT2, RBPJ, SLCO4C1, IKBKB, IL12B, KCNA4, RHOH, MLLT3,
NINJ1, PLAG1, BCL11A, PMM1, P2RY13, SETD4, FAM105A, SNRK, LAX1,
TRIM68, PCMTD2, TNFRSF19, KLHL9, BEX4, WDR48, SLC4A5, ARID4A,
PRDM16, SLAMF1, BTG1, VLDLR, C18orf1, ZNF10, ZNF136, DNALI1,
LRRC27, FAM117A, EEA1, HSDL2, KBTBD8, CD1D, VAPA, RCSD1, TP53INP1,
KIAA0408, MTSS1, NFIB |
| hsa-mir-382 | Upregulated | 59 | DLC1, ATP8A1,
MAP3K8, CRHBP, CAPN7, GPR160, ZNF615, ID4, SLCO4C1, CABC1, ROBO2,
CD96, MGAT4A, CISH, CNR1, PPM1M, MACROD2, GPR155, BTLA, PRICKLE2,
TAPT1, EPS15, ALCAM, SORCS3, CAMTA1, CNOT6L, PPP1R16B, RSPO2,
IL10RA, IL12B, ITGAD, B3GNT8, MAOA, LRP2BP, KLHL9, MOSPD1, SLC24A3,
SLC4A5, PRDM16, PCDH20, C18orf1, ZNF708, TMEM50B, CA3, ZNF614,
C1orf21, SYT15, CLDN2, IL33, CD1D, RCSD1, HMGN3, GRAP2, ITM2A,
AKAP7, CHST10, TOX, NFIB, PPP3CC |
| hsa-mir-421 | Upregulated | 79 | CBX7, TESK2,
CLASP2, SOSTDC1, RHOH, SLC24A3, NDFIP1, KBTBD8, B3GALT2, VAPA,
TSPAN32, CXCR6, WASF3, FRS3, FGL2, MGAT4A, ERMAP, CNR1, GAB3,
PDIK1L, SMYD1, DBH, ZNF540, PRICKLE2, NLRC3, ZNF25, ENPP4, ANKRD12,
CAMTA1, CNOT6L, SLC41A1, PDE7B, SERP1, SLC46A3, NKIRAS1, ZC3H7A,
FAM19A2, RSPO2, SLCO4C1, IL10RA, IL12B, AQP4, C3orf62, NR3C2,
MTHFR, GIMAP6, CDON, BCL11A, FBXO42, RSBN1, LAX1, SLC25A36, PCMTD2,
LRP2BP, GALNTL1, WDR48, STIM2, PTPRB, RSU1, GZF1, MRPS25, C18orf1,
BTG2, ZNF614, LRRC27, FCRL5, SYT15, ZNF577, LIMD1, CLDN2, ACVR2A,
GPR55, NMT2, AKAP7, USP6NL, TOX, LPPR4, RORB, ACTN2 |
| hsa-mir-432 | Upregulated | 79 | CXCR6, SLCO4C1,
GNG2, CA3, C1orf21, HSDL2, PPAP2A, RCL1, CD96, DLC1, WASF3, CHKA,
MGAT4A, ADH1B, CNR1, MAP3K8, CPT1B, MACROD2, UNC45B, ANKRD29,
GPR155, NLRC3, TAPT1, F11, TMEM130, KLRK1, SORCS3, GPD1L, CAMTA1,
PPP1R13B, FOS, SLC41A1, MYRIP, SETBP1, NAP1L5, GPR160, HSPB7, GNG7,
SNAI3, ID4, RSPO2, IL16, LY9, MAP3K3, MLLT3, GIMAP6, P2RX1,
C3orf18, REV1, HMGCLL1, RSBN1, PPP1R1A, SLC25A36, TMEM57, FBXW7,
SPTLC3, GIMAP5, LRP2BP, C1orf183, BCL2, PRDM16, GZF1, C5, UBP1,
C18orf1, ZNF80, EVI5, DENND1C, ATOH8, FAM125B, BZRAP1, GPR55,
MS4A1, ENTPD3, CD40LG, WSCD2, USP6NL, TOX, PDZD2 |
| hsa-mir-433 | Upregulated | 55 | NR0B2, ZNF211,
RAB40B, TAGAP, NLRC3, ZNF615, NINJ1, RSBN1, PTPN4, PRDM16, MRPS25,
ZNF649, ZNF91, BTG2, ACVR2A, RAMP3, ATP8A1, UNC13B, FGL2, FCRL3,
CNR1, CD200R1, GAB3, PDIK1L, BTLA, DMP1, GPC5, SACM1L, SWAP70,
ANKRD12, CAMTA1, CNOT6L, NAP1L5, ZNF776, IL16, UNC13C, C9orf68,
TRIM68, FBXW7, TNFRSF19, LRP2BP, SLC4A5, PTPRB, BCL2, ZNF708, EVI5,
LRRC27, C1orf21, KBTBD8, ZNF439, VAPA, NMT2, AKAP7, KIAA0408,
LPPR4 |
| hsa-mir-496 | Upregulated | 49 | KIT, ANKRD12,
FAM55D, ZNF20, ZNF136, MGAT4A, CNR1, ADHFE1, CR2, CRHBP, GPR155,
ZNF781, P2RX2, TBC1D9, C20orf194, SETBP1, GATA6, CLUL1, HSPB7,
ANGPT1, ZNF776, GPR34, S100A7A, SLCO4C1, C3orf62, CDON, EMCN,
P2RY13, HMGCLL1, LAX1, SUSD2, BEX4, BDH2, BCL2, MRPS25, SPATA20,
SLC14A1, ZNF708, ZNF614, C1orf21, EEA1, ZNF160, IL33, VAPA, BZRAP1,
ITM2A, LPPR4, NFIB, PAQR8 |
| hsa-mir-513a-1 | Upregulated | 1 | DNAH8 |
| hsa-mir-539 | Upregulated | 101 | KIT, KLRG1, WASF3,
PIK3IP1, MACROD2, BTLA, CLASP2, PDE7B, ZNF776, ID4, RSPO2, PNRC2,
C7, NDFIP1, FCRL5, SYT15, PRSS12, ZNF266, RCBTB2, ZPBP2, CNR1,
CNTFR, MAP3K8, UNC45B, SMYD1, GPR155, ZNF483, DLG4, DMP1, FAIM2,
PDZD2, GLT25D2, ANKRD12, PPP1R13B, FOS, PIK3R5, FOSB, CCNDBP1,
SLC41A1, GCET2, ATRNL1, SEC14L3, ANGPT1, S100A7A, SLCO4C1, C3orf62,
CLEC12B, MAOA, NR3C2, MTHFR, GIMAP6, P2RX1, CNTN3, CDON, PHF7,
CECR1, PIK3CG, BCL11A, PNOC, C21orf29, C1orf190, GNG2, PALMD,
SLC25A36, SPTLC3, GIMAP5, TNFRSF19, MOSPD1, CPA6, TNFRSF17, RSU1,
NAPB, USP4, C18orf1, DNALI1, EVI5, BTG2, DENND1C, ZNF614, LRRC27,
C1orf21, CDADC1, EEA1, C15orf48, KBTBD8, EMR3, ST3GAL5, FAM125B,
LIMD1, ZNF439, CLDN2, CD1D, C22orf32, CD5, VAPA, MS4A1, NMT2,
TP53INP1, AKAP7, CD69, USP6NL |
| hsa-mir-582 | Upregulated | 1 | SMAD6 |
| hsa-mir-9-1 | Upregulated | 9 | CD19, CEBPA, BCL2,
RASSF1, CBX7, ATP8A2, CISH, GRIA1, IGFALS |
| hsa-mir-9-2 | Upregulated | 8 | ATP8A2, CD19,
RASSF1, BCL2, GRIA1, CEBPA, IGFALS, CISH |
| hsa-mir-9-3 | Upregulated | 7 | ATP8A2, CD19, BCL2,
IGFALS, GRIA1, CEBPA, CISH |
| hsa-mir-98 | Upregulated | 3 | ESR2, CISH,
CNTN3 |
| hsa-let-7d | Upregulated | 61 | ACVR2A, BCL2, FOSB,
FLT3, PGC, KIT, CNTN3, ESR2, ATP8A2, CXCR4, CEBPA, FBXW7, CACNA2D2,
CISH, MTSS1, RASSF1, MGAT4A, USP38, ADAMTS8, WDR37, RUFY3, RSPO2,
COL14A1, WASF3, CYP2U1, SLAMF6, CNR1, CD200R1, GAB3, GPR155, DAPK1,
ZNF540, NLRC3, KLHDC8B, EPS15, ENPP4, CLASP2, DAPK2, PPP1R16B,
HSPB7, CRB2, HLF, SNAI3, TMEM110, P2RX1, PLCB2, GFOD1, BEST2,
SFTPB, MRPS25, C18orf1, ZNF10, ZNF614, C1orf21, TCF7L1, SYT15,
EEA1, ZNF577, MPDZ, FAM125B, TP53INP1 |