Introduction
Gastric cancer (GC) accounts for ~10% of all
invasive cancers worldwide (1,2) and is
the second leading cause of cancer-related death worldwide
(3), resulting in an urgent medical
need to develop more efficient diagnosis methods and effective
treatments. While the pathogenesis of the disease is not completely
understood, epidemiological studies suggest that chronic
inflammation plays a significant role in the development of gastric
malignancies (4). In superficial
gastritis (SG), inflammatory changes only affect the superficial
epithelium gastric pit region and related lamina propria, but the
changes occur in the atrophic gastric glands (5). Given that the recognition of bacterial
and/or viral products by toll-like receptor (TLR)-expressing cells
induces the activation of nuclear factor κ-light-chain-enhancer of
activated B cells (NF-κB) and triggers an inflammatory response
associated with tumor promotion (6), the targeted inhibition of specific TLR
pathways may provide an effective strategy for preventing the
development of selected gastric malignancies. To date, several
negative regulators of TLR signaling pathways have been identified
and characterized (7), among which
tumor necrosis factor (TNF)-α-induced protein 8-like-2 (TIPE2) and
interferon regulatory factor 4 (IRF4) exert their activity via
dissociation of TLR adaptor complexes (8).
TIPE2, a member of the tumor necrosis
factor-α-induced protein-8 (TNFAIP8) family, was recently
identified as a novel negative regulator of the immune system that
independently maintains immune homeostasis (9). In vitro experiments
demonstrated that TIPE2 knockout cells showed hyper-responsiveness
to TLR and T cell receptor (TCR) activation (9). In addition to lymphoid tissue, TIPE2
is expressed in the nervous, digestive, urinary, respiratory and
reproductive systems (10–14), suggestive of roles other than immune
regulation. Our previous results demonstrated that TIPE2 regulates
the proliferation of gastric cells (15). Colony-forming assays showed that
restoration of TIPE2 expression in gastric cells significantly
suppressed cell proliferation. Flow cytometric analysis showed that
the number of cells in the S phase of the cell cycle was reduced
concomitant with TIPE2 expression. In addition, TIPE2 selectively
upregulated p27 expression, which controls cell growth; however,
the molecular mechanism by which TIPE2 regulates p27 remains
unknown.
IRF4 also participates in the negative regulation of
TLR signaling as well as the regulation of inflammation and
carcinogenesis. Negishi et al (16) found that IRF4 forms a complex with
MyD88, functioning not only as a transcription factor in lymphocyte
differentiation but also as a negative regulator of TLR signaling.
IRF4 is also expressed in macrophages, lens cells and melanocytes,
suggestive of its involvement in the regulation of cells other than
lymphocytes. Studies by Pathak et al demonstrated that
reconstitution of IRF4 expression restored p27 in leukemic cells
and inhibited cell proliferation in vivo (17). Moreover, Fanzo et al
(18) showed that stable IRF4
expression in a human lymphoid cell line that normally lacks IRF4
significantly enhanced the apoptotic response in Fas receptor
engagement. However, the effects of IRF4 on p27 expression and
growth modulation as well as upstream molecules of IRF4 in
epithelial cells are yet to be explored.
In the present study, initial examination of TIPE2
expression patterns in superficial gastritis (SG, 22 patients),
atrophic gastritis (AG, 30 patients), atypical hyperplasia (AH, 24
patients) and gastric cancer (GC, 34 patients) tissues revealed a
negative correlation between TIPE2 expression and tumor development
and malignant progression. This negative correlation demonstrates
the role of TIPE2 in preventing the occurrence and development of
GC, and suggests that TIPE2 may be a potential biomarker for GC
progression. To elucidate downstream molecular targets, a
TIPE2-expressing plasmid was introduced into the AGS gastric
epithelial cell line. Microarray analysis of signal transduction
data revealed that TIPE2-induced IRF4 expression in AGS cells, and
in turn, enhanced p27 expression and suppressed cell proliferation.
To the best of our knowledge, this is the first study to
demonstrate that TIPE2 expression is mediated by another negative
TLR signaling regulator, IRF4, to modulate cell growth, and IRF4
functions as an inhibitor of epithelial cell proliferation.
Materials and methods
Patient samples
Newly obtained endoscopic biopsy specimens from 22
SG, 30 AG, 24 AH and 34 GC tissues were obtained from The Second
Hospital of Shandong university (Jinan, Shandong, China). All
studies were reviewed and approved by the Ethics Committee of
Shandong university. All the patients gave their informed consent
prior to their inclusion in the study. The tissues were kept at
−80°C and used for subsequent analysis.
Immunohistochemistry for TIPE2 protein
expression
Immunohistochemical analysis was performed according
to a previous study (12). Briefly,
sections were thawed and fixed in acetone for 10 min at 20°C, then
rehydrated in 0.1 M phosphate-buffered saline (PBS) for 5 min.
Next, the sections were treated by blocking goat serum for 10 min,
rabbit anti-TIPE2 polyclonal antibody (dilution 1:50; Boster Co.
Ltd., Wuhan, China) for 60 min, and then with peroxidase-labeled
goat anti-rabbit IgG antibody (Maixin Bio, Fuzhou, China) for 15
min, respectively, at room temperature. DAB peroxidase substrate
kit (Maixin Bio) was used to quantify the peroxidase. Finally the
sections were counter-stained with hematoxylin.
According to both staining intensity and percentage
of positive cells (H-score) (19),
all slides were scored as: staining intensity, 0, no staining; 1,
weak staining; 2, moderate staining; 3, strong staining; percentage
of positive cells, 0, <1%; 1, 1–10%; 2, 10–25%; 3, 25–50%; and
4, >50%. The staining intensity multiplied by the percentage of
positive cells in each slide produced a final score of TIPE2
expression and was graded as: 0, final score = 0; 1+, final score =
1-3; 2+, final score = 4-6; 3+, final score = 7-9; and 4+, final
score = 10-12.
Cell culture and transfection
Gastric adenocarcinoma cell line AGS and BGC-823
were maintained in our laboratory. AGS cells were cultured in Ham's
F-12 medium (HyClone, Logan, UT, USA) containing 10% fetal calf
serum (FCS) and 1% penicillin-streptomycin. BGC-823 cells were
cultured in RPMI-1640 medium (Life Technologies, Foster City, CA,
USA) supplemented with 10% FCS (Tianhang Co. Ltd., Hangzhou, China)
and 1% penicillin-streptomycin. The full-length human TIPE2 cDNA
expression plasmid pRK5-tipe2 and control plasmid pRK5-mock were
kindly provided by Professor Youhai Chen (university of
Pennsylvania, USA) and were previously described (12). FuGENEs HD Transfection Reagent
(Roche Applied Science, Basel, Switzerland) was used for
transfection. All transfections were performed according to the
manufacturer's instructions.
RNA extraction and quantitative real-time
PCR
Total cellular RNA was extracted with TRIzol (Life
Technologies) according to the protocol provided by the
manufacturer. First-strand cDNA was synthesized from 1 µg
total cellular or tissue RNA using the RevertAid™ First Strand cDNA
Synthesis kit (Thermo Fisher Scientific, Waltham, MA, USA) with
random primers. Then cDNA was amplified for quantitative real-time
PCR, the specific primers used were as follows: for TIPE2 forward
primer, 5′-CTGAGTAAGATGGCGGGTCG-3′ and reverse primer,
5′-TCTGGCGAAAGCGGGTAG-3′; for β-actin forward primer,
5′-AGTTGCGTTACACCCTTTCTTG-3′ and reverse primer,
5′-CACCTTCACCGTTCCAGTTTT-3′; for p27 forward primer,
5′-GGTTAGCGGAGCAATGCG-3′ and reverse primer,
5′-TCCACAGAACCGGCATTTG-3′; for human IRF4 forward primer,
5′-AAAGGAAAGTTCCGAGAAGG-3′ and reverse primer,
5′-CGAAGGGTAAGGCGTTGT-3′; for mouse IRF4 forward primer,
5′-CTCTTCAAGGCTTGGGCATT-3′ and reverse primer,
5′-TGCTCCTTTTTTGGCTCCCT-3′. The real-time PCR reactions were
performed at: 95°C, 10 sec (denaturation); 55°C, 30 sec
(annealing); 72°C, 30 sec (extension) for 35 cycles. The real-time
PCR reactions were performed on the ABI 7000 Fast Real-Time PCR
system with SYBR Premix Ex Taq™ according to the procedures.
Western blot analysis
Western blot analysis was performed as previously
described (20). Briefly, cell
lysates (20 µg/lane) were separated on 10% SDS
polyacrylamide gel and were then transferred to a poly(vinylidene
fluoride) membrane. TIPE2 and IRF4 protein was detected by a rabbit
polyclonal IgG (Boster Co. Ltd.) and visualized by the enhanced
chemiluminescence system (Amersham, Arlington Heights, IL, USA).
The density of the bands was quantitated using the NIH image
software package (version 1.61). The intensity of TIPE2 and IRF4
expression was judged by the ratio of their expression in
experiment groups to their corresponding expression in control
groups and a ratio of >1.0 was considered to be an indication of
overexpression.
Colony formation assay
Gastric cell line AGS cultured in a 6-well plate
(2×105/well) was transfected with pRK5-tipe2 and its
control plasmid pRK5-mock using the FuGENEs HD transfection reagent
(Roche Applied Science). After a certain time of growth the cells
were digested with trypsin and counted, 300 cells were transferred
to a new well of a 6-well plate and medium containing 10% fetal
bovine serum (FBS) serum was added to make up the volume of 3 ml.
After a week's growth at 37°C, the formation of cell clones could
be visually seen. After washing 3 times with PBS buffer, the cells
were fixed for 10 min with 1 ml of methanol in each well at room
temperature. Then, 1 ml diluted Giemsa dye was added to each well
and incubated at room temperature for ~20–25 min. Finally, the
wells were washed with PBS until no residual background Giemsa dye
was observed and the 6-well plate was scanned for colony counting
and analysis.
Microarray analysis
The microarray chip consisted of 27,326 probes for
different human cDNAs (Agilent Technologies, Wilmington, DE, USA),
in which house-keeping gene glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) served as internal control. The cDNAs from
pRK5-tipe2 transfected AGS cells were labeled with Cy3, and the
cDNAs from the control pRK5-mock transfected AGS cells were labeled
with Cy5. The labeled cDNAs were hybridized with microarray chip
under standard conditions according to the manufacturer's
instructions. The data were analyzed by Molecule annotation system
3.0.
siRNA interference
Chemical modified stealth siRNA targeting IRF4 and
control siRNA were from RiboBio Co., Ltd. (Guangzhou, Guangdong,
China). The sequence for IRF4 siRNA was 5′-UGGAGCGUGAGAGUCAAAG-3′.
Cells were transfected with siRNA by the Lipofectamine 2000 method
(Life Technologies).
Statistical and data analyses
Data are expressed as mean ± standard deviation
(SD). Differences between three groups were compared using the
Student's t-tests. All experiments were repeated at least 3 times
and p<0.05 was considered statistically significant.
Results
TIPE2 expression is negatively correlated
with the progression of gastritis to cancer
To examine the expression pattern of TIPE2 in serial
clinical tissue with the development of GC from gastritis,
immunohistochemical analysis of TIPE2 protein was performed in
gastric tissues collected from patients with SG (22 patients), AG
(30 patients), AH (24 patients) and GC (34 patients). Each of these
four diseases represents a stage of GC progression.
Immunohistochemistry scores were based on both staining intensity
and percentage of positive cells in all of the slides (H-score). As
shown in Fig. 1A and B, TIPE2
expression in SG tissues significantly differed from expression in
AH (p=0.0118) and GC (p<0.0001) tissues. AG and AH tissues also
had verified levels of TIPE2 compared to GC tissues (p<0.0001
and p=0.0203, respectively). The percentage of highly scored slides
(2+ and 3+) decreased in the following order: SG, AG, AH and GC,
whereas low scoring slides (total score=0) increased in this order.
These variations in TIPE2 expression patterns with disease
progression clearly demonstrate that its expression is suppressed
in deteriorating gastritis and cancer tissues. Our finding, that
TIPE2 expression is significantly negatively correlated with the
progression of gastritis to GC, further supports the theory that
TIPE2 is a molecular bridge from inflammation to cancer. Therefore,
TIPE2 plays a role in the prevention of GC progression and may be a
potential biomarker for GC progression.
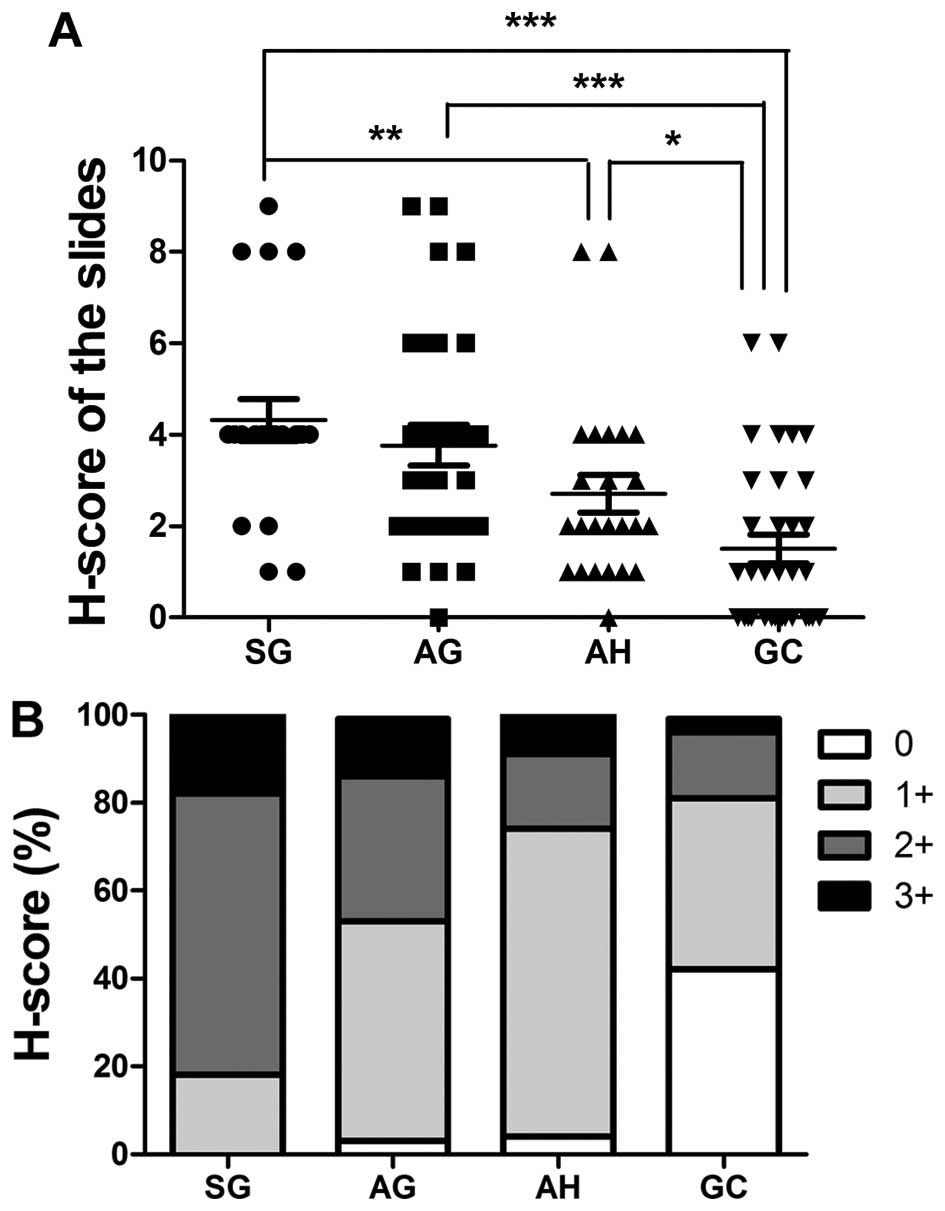 | Figure 1Expression levels of TIPE2 in gastric
cells. Immunohistochemical scores of TIPE2 were based on both
staining intensity and percentage of positive cells (H-score) in
all slides. Staining was scored as follows: staining intensity, 0,
no staining; 1, weak staining; 2, moderate staining; 3, strong
staining. The percentage of positive cells was scored as follows:
0, <1%; 1, 1–10%; 2, 10–25%; 3, 25–50%; and 4, >50%. Staining
intensity was multiplied by the percentage of positive cells for
each slide to produce a final H-score of TIPE2 expression (A). (B)
The H-scores of (A) were graded: 0, H-score = 0; 1+, H-score = 1-3;
2+, H-score = 4-6; 3+, H-score = 7-9; and 4+, H-score = 10-12.
Rabbit anti-TIPE2 polyclonal antibody (1:50 dilution) was used for
immunohistochemical analysis. |
TIPE2 upregulates IRF4 expression in
gastric epithelial cells
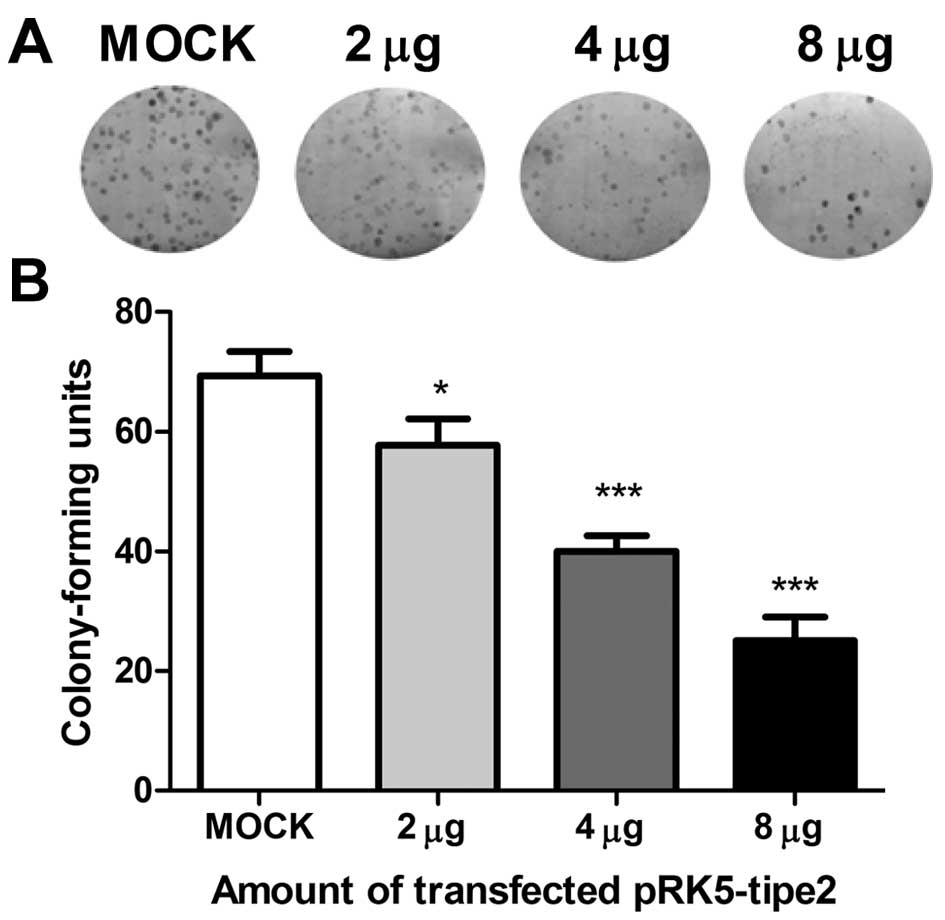
To simulate TIPE2 expression in gastric cell lines
and establish its function in carcinogenesis, we transfected AGS
cells with the TIPE2 expression plasmid, pRK5-tipe2; our results
proved that the transfection efficiency was high under transfection
with up to 8 µg plasmid. As shown in Fig. 2A and B, cells with restored TIPE2
expression had a significantly decreased colony forming capability;
thus, the number and colony size were reduced compared to control
cells transfected with mock plasmid. This growth inhibition effect
was also observed in the BGC-823 gastric cell line.
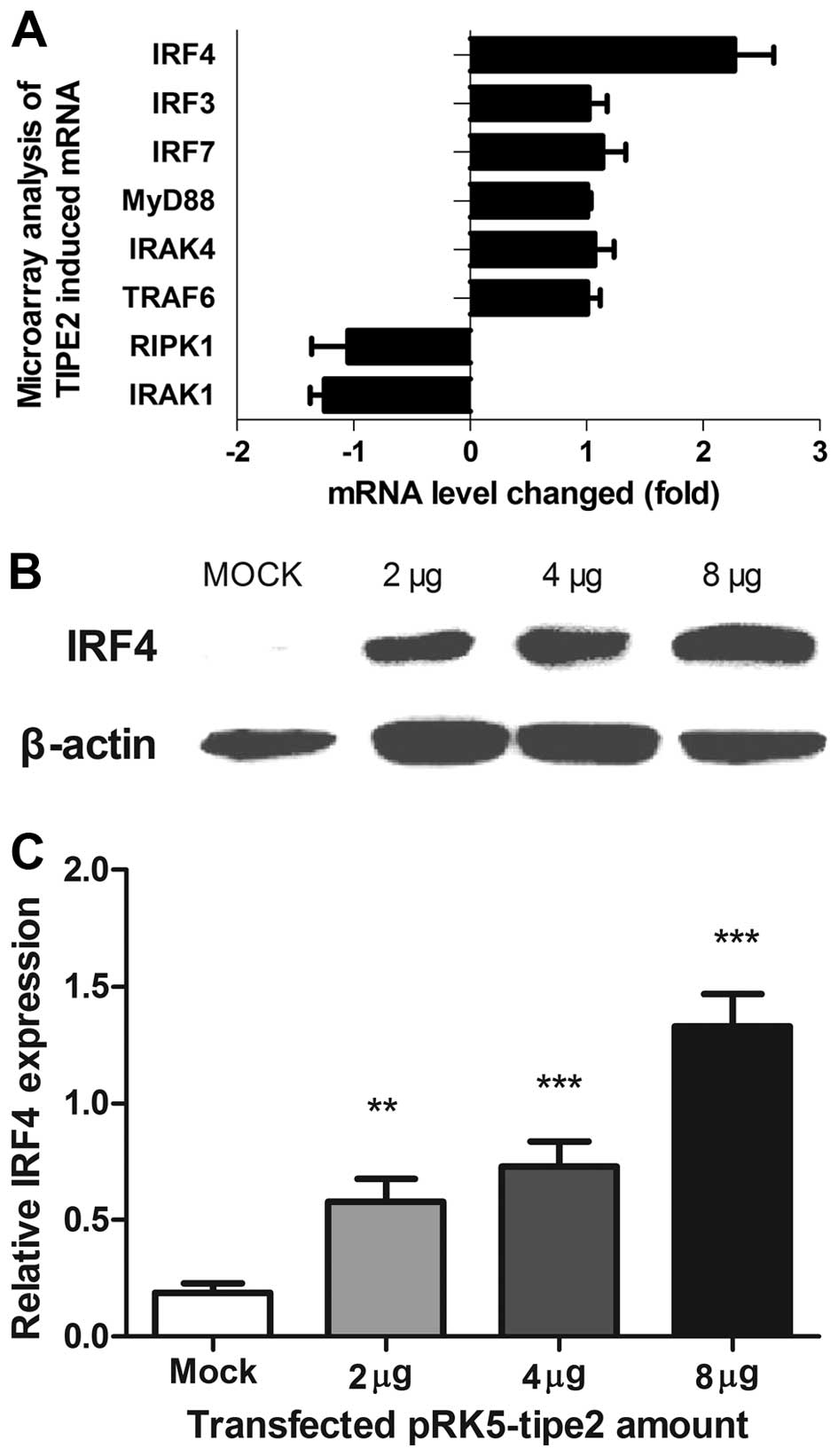
To determine the molecular agents involved in
TIPE2-induced growth inhibition, cDNA microarray assays were
conducted to analyze changes in gene levels upon TIPE2 expression.
IRF4 was among the top upregulated genes with a >2-fold increase
in expression, as confirmed by western blot analysis and
quantitative PCR (qPCR) (Fig.
3A–C). Moreover, IRF4 mRNA and protein levels increased in a
dose-dependent manner upon transfection of the pRK5-tipe2 plasmid
in AGS cells, consistent with the TIPE2 expression pattern.
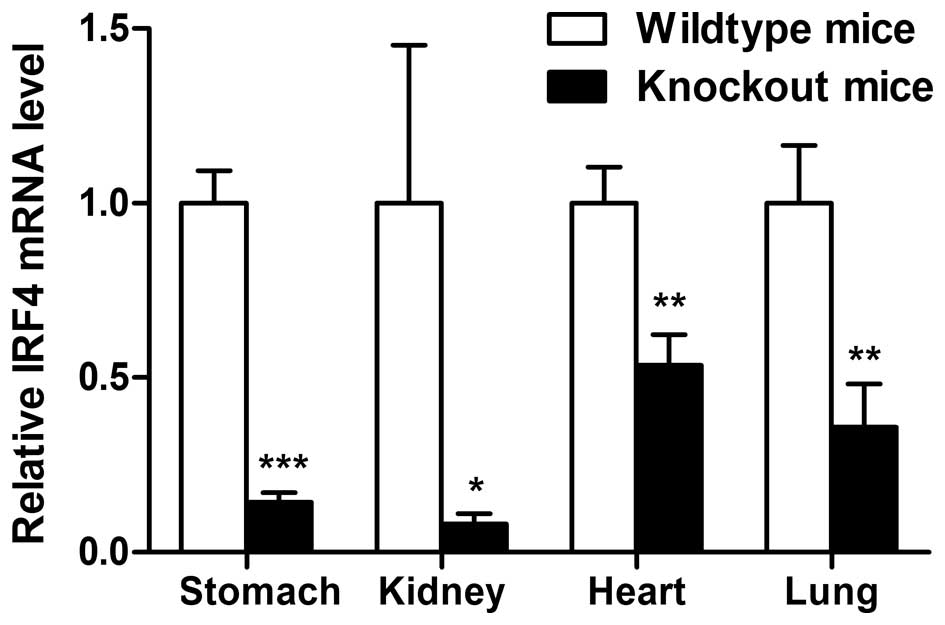
TIPE2-induced IRF4 expression in TIPE2 knockout mice led to
downregulation of IRF4 in the stomach (Fig. 4). Similar results were obtained from
other organs of TIPE2 knockout mice.
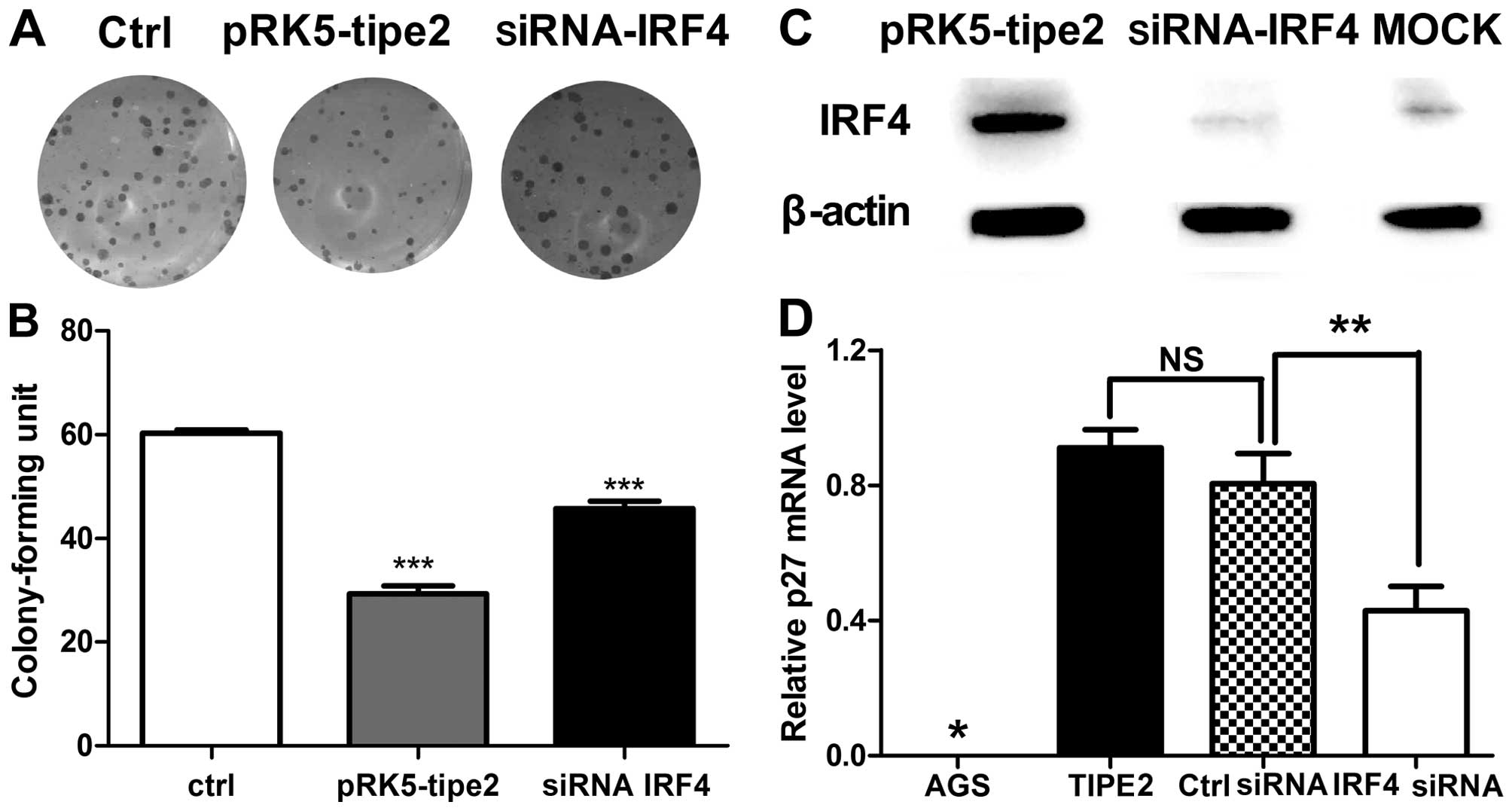
Next, we determined whether AGS cell growth and p27
expression level are modulated, concomitant with TIPE2
administration and IRF4 interference. As shown in Fig. 5A and B, cells transfected with the
TIPE2 expression plasmid and IRF4 siRNA showed restored colony
forming capacity and colony size. Knockdown of IRF4 protein was
verified in specific cells (Fig.
5C). Subsequently, expression of p27 that TIPE2 selectively
upregulated and takes control of cell growth was investigated. The
p27 mRNA level was clearly decreased upon transfection of IRF4
siRNA (Fig. 5D). These results
suggest that IRF4 plays a role in p27 regulation, and as such, is a
critical mediator of TIPE2-induced growth inhibition.
Pathways employed by TIPE2 to regulate
IRF4 expression
Next, we explored the signaling pathways used by
TIPE2 to regulate IRF4, which plays a pivotal role in cell
proliferation. The small molecule inhibitors SB203580 (p38
inhibitor, 10 µM), PD98059 (MEK inhibitor, 25 µM),
SP600125 (JNK inhibitor, 10 µM), LY294002 (PI3K inhibitor,
40 µM), curcumin (AP-1 and NF-κB inhibitors), BAY11-7082
(NF-κB inhibitor, 4 µM) and SB431542 (TGFβ inhibitor, 10
µM) were employed for intervention in potential
TIPE2-induced pathways involving IRF4 expression. Notably,
TIPE2-induced IRF4 expression in AGS cells was significantly
suppressed upon treatment with BAY11-7082 and curcumin, whereas no
obvious effects were observed with the other inhibitors (Fig. 6A). These results are consistent with
previous finding that IRF4 expression is positively regulated by
the NF-κB pathway. Variations in NF-κB-dependent transcription in
AGS cells were further assessed with the NF-κB luciferase reporter
assay (Fig. 6B). Our results showed
that NF-κB activity increased in AGS cells in response to changes
in TIPE2 expression.
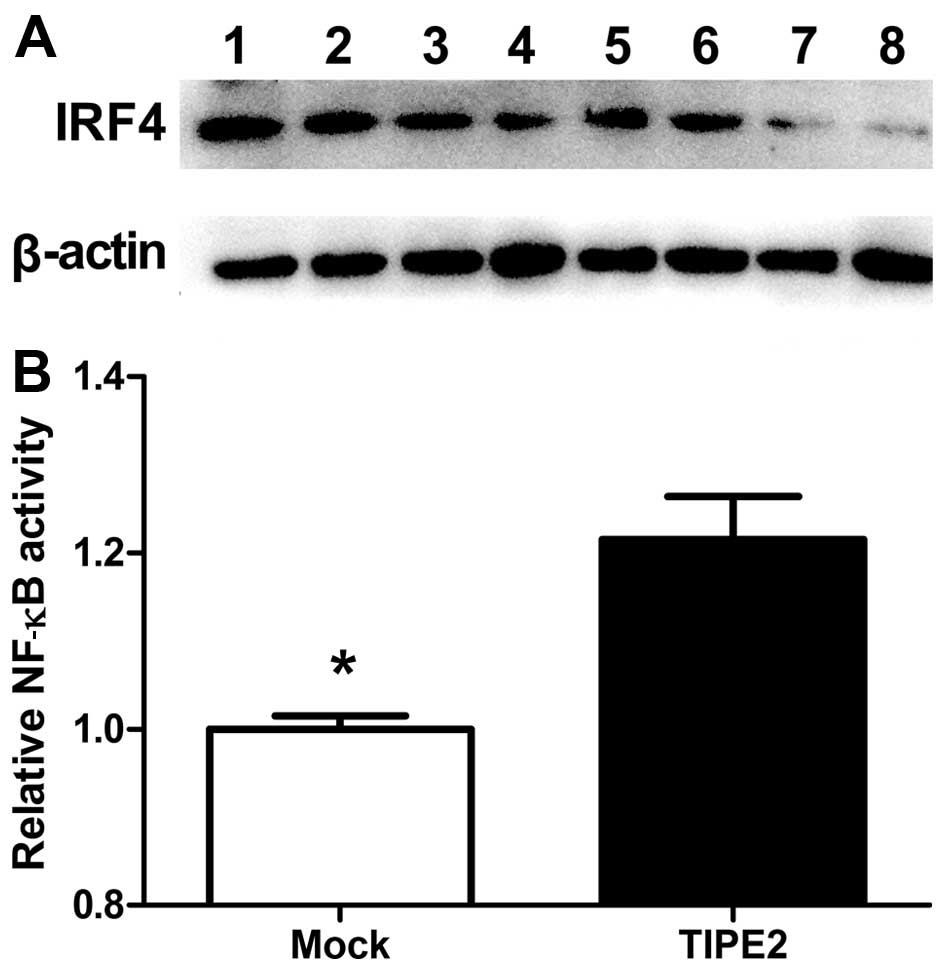 | Figure 6NF-κB pathway was employed by TIPE2 to
regulate IRF4 expression. (A) The small molecule inhibitors: 1,
control; 2, SB203580 (p38 inhibitor, 10 µM); 3, PD98059 (MEK
inhibitor, 25 µM); 4, SP600125 (JNK inhibitor, 10 µM); 5, LY294002
(PI3K inhibitor, 40 µM); 6, SB431542 (TGFβ inhibitor, 10 µM); 7,
curcumin (AP-1 and NF-κB inhibitor); and 8, BAY11-7082 (NF-κB
inhibitor, 4 µM) were employed for intervention in potential
TIPE2-induced pathways involving IRF4 expression. TIPE2-induced
IRF4 expression in AGS cells was significantly suppressed upon
treatment with the NF-κB signaling pathway inhibitors BAY11-7082
and curcumin. (B) Variations in NF-κB-dependent transcription in
AGS cells were assessed with the NF-κB luciferase reporter assay,
our results showed that NF-κB activity is increased in AGS
cells. |
These findings collectively indicate that in AGS
cells, activation of the NF-κB pathway is involved in TIPE2-induced
IRF4 expression. Although the precise pathway responsible for
maintaining NF-κB activity upon TIPE2 expression remains unknown,
we propose that restoration of TIPE2 activity may contribute, at
least in part, to the initiation of IRF4 expression.
Discussion
To reduce mortality and improve the effectiveness of
therapy, numerous studies have tried to find key biomarkers.
Biomarkers are important molecular signposts of the biologic state
of a cell at a specific condition, which play crucial roles in a
number of processes important for tumor progression such as cell
proliferation, motility, adhesion, invasion, survival and
angiogenesis (21–25). TIPE2 induces cell death and inhibits
tumor formation, providing a molecular bridge from inflammation to
cancer. Zhang et al initially reported TIPE2 expression in
several types of non-immune cells including the lung, stomach and
liver (12). Separate studies in
patients with systemic lupus erythematosus, chronic hepatitis B and
chronic hepatitis C have shown significantly reduced levels of
TIPE2 in peripheral blood mononuclear cells, compared to those in
healthy individuals (10,13,14,26).
Our previous RT-qPCR and western blot results showed that TIPE2
upregulated p27 protein expression in AGS cells, this variation was
verified in tumor tissues as well as by siRNA interference studies.
Our present study disclosed variations in TIPE2 levels at different
stages of gastritis progression and gastric cancer (GC)
development, with a trend towards decreasing expression in the
order of SG, AG, AH and GC. Based on these findings, we speculate
that TIPE2 may be useful as a potential biomarker for GC
progression, and therefore as a tool for the prevention of GC.
IRF4 is predominantly expressed in the immune system
and plays an important role in its development and function
(27,28). IRF4 can function either as a
transcriptional activator or repressor, depending upon its
interactions with different transcription factors or DNA-binding
domains on specific promoters (28). The enhanced understanding of the
functional roles of IRF4 is dependent on the identification of
genes that are uniquely regulated by the transcription factor. As
IRF4 mRNA is induced upon TLR activation, IRF4 appears to
participate in negative regulation of TLR signaling. IRF4 plays a
significant role in disease progression and pathology under
specific conditions. A recent study by Jo and Ren revealed that
IRF4 functions as a tumor suppressor in bone marrow cells deficient
in MyD88, an IRF4-interacting protein located in the cytoplasm
(29). The tumor suppressor
activity of IRF4 was lost in IRF association domain (IAD) deletion
mutants, demonstrating that IRF4 suppresses BCR/ABL transformation
through a novel cytoplasmic function involving its IAD domain
(29). Additionally, Pathak et
al (17) reported that
c-Myc-induced leukemia is greatly accelerated in IRF4 heterozygous
mutant mice, providing evidence that IRF4 functions as a classical
tumor suppressor gene to inhibit c-Myc-induced leukemogenesis. The
group further showed that deficiency of IRF4 accelerates loss of
p27kip in EuMyc mice. Reconstitution of IRF4 in leukemic cells
restored p27kip expression in leukemic cells and inhibited
proliferation in vivo. Our experiments demonstrated that
TIPE2 triggers a p27-associated signaling cascade that leads to
restoration of control of the cell cycle and cell division.
Association of IRF4 with p27 may therefore provide mechanistic
links between IRF4 and cell proliferation inhibition.
NF-κB plays a key role in regulation of IRF4
expression. Previous studies have established that NF-κB is an
upstream molecule of IRF4 that binds to its promoter and regulates
expression. Wang et al reported that the IRF4 expression is
initiated by TNFα/NF-κB signaling, suggesting that viral infection
is inhibited through modulation of this pathway (30). Grumont and Gerondakis reported a
novel mechanism in which Rel/NF-κB represses the transcription of
IFN-regulated genes in a cell type-specific manner (31). Moreover, Sharma et al
(32) observed that IRF4 expression
in HTLV-1-infected cells is driven through activation of NF-κB and
NF-AT pathways. In the present study, expression of IRF4 was
significantly decreased upon blockage of the NF-κB pathway.
Furthermore, the NF-κB luciferase reporter assay revealed that
NF-κB activity is slightly increased in AGS cells consistent with
the results of Sharma et al (32).
In conclusion, TIPE2 acts as an inhibitor of GC cell
growth and triggers an IRF4-associated signaling cascade that
restores control of cell proliferation. Our study revealed a novel
molecular mechanism by which TIPE2 regulates gastric cell
proliferation. To the best of our knowledge, this is the first
report of IRF4 as an inhibitor of epithelial cell proliferation and
mediator of another negative TLR signaling regulator, TIPE2 to
control cell growth.
Acknowledgments
We gratefully acknowledge the financial support from
the Ph.D. Programs Foundation of Ministry of Education of China
(20120131120046), The Fundamental Research Funds of Shandong
university (2015JC010), the Natural Science Foundation (General
programs 81272351), the Natural Science Foundation of Shandong
Province (ZR2012HM020) and the Open Research Fund of State Key
Laboratory of Environmental Chemistry and Ecotoxicology
(KF2014-08).
References
|
1
|
Yang L, Parkin DM, Li LD, Chen YD and Bray
F: Estimation and projection of the national profile of cancer
mortality in China: 1991–2005. Br J Cancer. 90:2157–2166.
2004.PubMed/NCBI
|
|
2
|
Yang L, Parkin DM, Ferlay J, Li L and Chen
Y: Estimates of cancer incidence in China for 2000 and projections
for 2005. Cancer Epidemiol Biomarkers Prev. 14:243–250.
2005.PubMed/NCBI
|
|
3
|
Roder DM: The epidemiology of gastric
cancer. Gastric Cancer. 5(Suppl 1): S5–S11. 2002. View Article : Google Scholar
|
|
4
|
Fukata M and Abreu MT: Role of Toll-like
receptors in gastrointestinal malignancies. Oncogene. 27:234–243.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Whitehead R, Truelove SC and Gear MW: The
histological diagnosis of chronic gastritis in fibreoptic
gastroscope biopsy specimens. J Clin Pathol. 25:1–11. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liew FY, Xu D, Brint EK and O'Neill LA:
Negative regulation of toll-like receptor-mediated immune
responses. Nat Rev Immunol. 5:446–458. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kondo T, Kawai T and Akira S: Dissecting
negative regulation of Toll-like receptor signaling. Trends
Immunol. 33:449–458. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun H, Gong S, Carmody RJ, Hilliard A, Li
L, Sun J, Kong L, Xu L, Hilliard B, Hu S, et al: TIPE2, a negative
regulator of innate and adaptive immunity that maintains immune
homeostasis. Cell. 133:415–426. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li D, Song L, Fan Y, Li X, Li Y, Chen J,
Zhu F, Guo C, Shi Y and Zhang L: Down-regulation of TIPE2 mRNA
expression in peripheral blood mononuclear cells from patients with
systemic lupus erythematosus. Clin Immunol. 133:422–427. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang G, Hao C, Lou Y, Xi W, Wang X, Wang
Y, Qu Z, Guo C, Chen Y and Zhang Y: Tissue-specific expression of
TIPE2 provides insights into its function. Mol Immunol.
47:2435–2442. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang L, Shi Y, Wang Y, Zhu F, Wang Q, Ma
C, Chen YH and Zhang L: The unique expression profile of human
TIPE2 suggests new functions beyond its role in immune regulation.
Mol Immunol. 48:1209–1215. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xi W, Hu Y, Liu Y, Zhang J, Wang L, Lou Y,
Qu Z, Cui J, Zhang G, Liang X, et al: Roles of TIPE2 in hepatitis B
virus-induced hepatic inflammation in humans and mice. Mol Immunol.
48:1203–1208. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kong L, Liu K, Zhang Y-Z, Jin M, Wu BR,
Wang WZ, Li W, Nan YM and Chen YH: Downregulation of TIPE2 mRNA
expression in peripheral blood mononuclear cells from patients with
chronic hepatitis C. Hepatol Int. 7:844–849. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao Q, Zhao M, Dong T, Zhou C, Peng Y,
Zhou X, Fan B, Ma W, Han M and Liu S: Tumor necrosis
factor-α-induced protein-8 like-2 (TIPE2) upregulates p27 to
decrease gastic cancer cell proliferation. J Cell Biochem.
116:1121–1129. 2015. View Article : Google Scholar
|
|
16
|
Negishi H, Ohba Y, Yanai H, Takaoka A,
Honma K, Yui K, Matsuyama T, Taniguchi T and Honda K: Negative
regulation of Toll-like-receptor signaling by IRF-4. Proc Natl Acad
Sci USA. 102:15989–15994. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pathak S, Ma S, Trinh L, Eudy J, Wagner
KU, Joshi SS and Lu R: IRF4 is a suppressor of c-Myc induced B cell
leukemia. PLoS One. 6:e226282011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fanzo JC, Hu CM, Jang SY and Pernis AB:
Regulation of lymphocyte apoptosis by interferon regulatory factor
4 (IRF-4). J Exp Med. 197:303–314. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Budwit-Novotny DA, McCarty KS, Cox EB,
Soper JT, Mutch DG, Creasman WT, Flowers JL and McCarty KS Jr:
Immunohistochemical analyses of estrogen receptor in endometrial
adenocarcinoma using a monoclonal antibody. Cancer Res.
46:5419–5425. 1986.PubMed/NCBI
|
|
20
|
Wang H, Sun Y, Liu S, Yu H, Li W, Zeng J,
Chen C and Jia J: Upregulation of progranulin by Helicobacter
pylori in human gastric epithelial cells via p38MAPK and MEK1/2
signaling pathway: Role in epithelial cell proliferation and
migration. FEMS Immunol Med Microbiol. 63:82–92. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hartwell LH and Kastan MB: Cell cycle
control and cancer. Science. 266:1821–1828. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Woodburn JR: The epidermal growth factor
receptor and its inhibition in cancer therapy. Pharmacol Ther.
82:241–250. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Coussens LM, Fingleton B and Matrisian LM:
Matrix metalloproteinase inhibitors and cancer: Trials and
tribulations. Science. 295:2387–2392. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ferrara N: Role of vascular endothelial
growth factor in physiologic and pathologic angiogenesis:
Therapeutic implications. Semin Oncol. 29(Suppl 16): S10–S14. 2002.
View Article : Google Scholar
|
|
25
|
Ghobrial IM, Witzig TE and Adjei AA:
Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin.
55:178–194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gus-Brautbar Y, Johnson D, Zhang L, Sun H,
Wang P, Zhang S, Zhang L and Chen YH: The anti-inflammatory TIPE2
is an inhibitor of the oncogenic Ras. Mol Cell. 45:610–618. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lu R: Interferon regulatory factor 4 and 8
in B-cell development. Trends Immunol. 29:487–492. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Marecki S and Fenton MJ: The role of IRF-4
in transcriptional regulation. J Interferon Cytokine Res.
22:121–133. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jo SH and Ren R: IRF-4 suppresses BCR/ABL
transformation of myeloid cells in a DNA binding-independent
manner. J Biol Chem. 287:1770–1778. 2012. View Article : Google Scholar :
|
|
30
|
Wang WL, Liu W, Gong HY, Hong JR, Lin CC
and Wu JL: Activation of cytokine expression occurs through the
TNFα/NF-κB-mediated pathway in birnavirus-infected cells. Fish
Shellfish Immunol. 31:10–21. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Grumont RJ and Gerondakis S: Rel induces
interferon regulatory factor 4 (IRF-4) expression in lymphocytes:
Modulation of interferon-regulated gene expression by rel/nuclear
factor kappaB. J Exp Med. 191:1281–1292. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sharma S, Mamane Y, Grandvaux N, Bartlett
J, Petropoulos L, Lin R and Hiscott J: Activation and regulation of
interferon regulatory factor 4 in HTLV type 1-infected T
lymphocytes. AIDS Res Hum Retroviruses. 16:1613–1622. 2000.
View Article : Google Scholar : PubMed/NCBI
|