Introduction
Pancreatic cancer, mainly pancreatic ductal adeno
carcinoma (PDAC), is one of the most deadly and aggressive cancers
(1). It is the world-wide seventh
(2), and the fourth in the United
States (3) of most common cause of
cancer-related death, with a poor prognosis and swift progression
before death. Surgical eradication is still the only potentially
curative treatment of this malignancy, but there are only 15–20%
cases indicative for surgery, because of the early occurrence of
local advancement or distal metastasis (4). Five-year overall survival rate ranges
from 1 to 6% (3,5,6). Even
after surgical eradication plus adjuvant chemotherapy, OS rates do
not exceed 30% (5,6).
Various chemotherapic agents have failed to improve
survival of pancreatic cancer patients. In recent years,
FOLFIRINOX, a cocktail of 5-fluorouracil (5′-FU), irinotecan and
oxaliplatin has significantly improved OS of pancreatic patients
with metastasis, compared with the singe treatment with gemcitabine
(7,8). Besides conventional chemotherapy,
accumulating understanding of the biological pathogenesis of
pancreatic cancer has provided a variety of targeted approaches.
However, except erlotinib, which is an inhibitor to epidermal
growth factor receptor (EGFR), no other targeted therapy has as yet
demonstrated significant effect against pancreatic cancer (9,10).
Insulin-like growth factor 1 (IGF-1) and its receptor,
PI3-kinase/Akt/mTOR and mitogen-activated protein
kinases/extracellular signal-regulated kinases (MEK/ERK) pathway
are upregulated in the majority of PDACs (11,12).
However, a phase II trial on a monoclonal antibody against IGF
receptor (IGFR) indicates no significant effect in OS and
progression-free survival (PFS) for PDAC patients (4). The chemical agents targeting the
PADC-overexpressed vascular endothelial growth factor (VEGF) which
promotes cancer angiogenesis and metastasis (13), also failed to improve PFS and OS
(14). Other treatments targeting
farnesyltransferase, the tumor stroma in pancreatic cancer or
autophagy are on the way.
Forkhead box L1 (FOXL1) belongs to a forkhead/winged
helix-box (FOX) family of transcription factors. All Fox members,
being classified as FOXA to FOXR (15), Fox molecules play critical roles in
a variety of physiologic (16) or
pathologic processes such as cancer, as tumor suppressors (17–19).
Particularly, FOXM1 was identified to be oncogenic in pancreatic
cancer, and is associated with poor prognosis and pathologic stage
of PADC (20,21), whereas, FOXL1 has recently been
recognized as a tumor suppressor in PADC (22). Therefore, FOXL1 might be another
target for the treatment of pancreatic cancers. Protein phosphatase
2A (PP2A) is a large collection of oligomeric protein
serine/threonine phosphatases and accounts for a large fraction of
phosphatase activity in eukaryotic cells. PP2A is a critical tumor
suppressor, via controlling a number of cellular processes,
including cell cycle progression (23,24).
Key signaling pathways that are negatively regulated by PP2A
include members of the MAPK/ERK pathways, NF-κB, and c-Myc
signaling (25). In particular,
PP2A suppresses the oncogenic activity (26) of c-Myc via specifically
dephosphorylating the key serine 62 (S62) in c-Myc (27), stimulating its ubiquitination
(28) and thus accelerating the
degradation of c-Myc. The overexpression of endogenous PP2A
inhibitors, such as SET (I2PP2A) and cancerous inhibitor of PP2A
(CIP2A) in head and neck squamous cell carcinoma, colon cancer,
gastric cancer, breast cancer, and most recently, pancreatic cancer
(29,30). Thus, the tumor suppressive PP2A
might also facilitate pancreatic cancer therapy.
In the present study, we constructed a recombinant
adenovirus, which carries the coding sequence both of FOXL1 and
PP2A, with a self-cleavage sequence. Then we evaluated the
regulation of the recombinant adenovirus on the proliferation of
pancreatic cancer cells, on the sensitivity of pancreatic cancer
cells to 5′-FU. In addition, we investigated the activation of
TNF-related apoptosis-inducing ligand (TRAIL) by the recombinant
virus. The present study provides a novel antitumor strategy
against PADC.
Materials and methods
Cell lines and culture conditions
Human pancreatic carcinoma Panc-1 cells were
purchased from American Type Culture Collection (ATCC, Manassas,
VA, USA). Cells were cultured in RPMI-1640 media (Gibco Life
Technologies, Rockville, MD, USA) supplemented with GlutaMAX-I
(Invitrogen, Carlsbad, CA, USA), 50 IU/m penicillin and 50 mg/ml
streptomycin (both from CSPC Zhongnuo Pharmaceutical Co., Ltd.,
Shijiazhuang, China) and 10% (v/v) fetal bovine serum (FBS)
(Sijiqing, Hangzhou, China). Cells were incubated at 37°C in a
humidified incubator with 5% CO2. For the treatment with
10 µM 5′-FU (Sigma-Aldrich, St. Louis, MO, USA), cells at
>85% confluence were inoculated with RPMI-1640 media
supplemented with 2% FBS, containing 10 µM 5′-FU.
Adenovirus-mediated coexpression of FOXL1
and PP2A
Human FOXL1 and PP2A coding sequences (both from
Sinobio, Beijing, China) were amplified, respectively, or were
overlapped with with 2A peptide coding sequence as a linker. Then
each coding sequence of FOXL1 or PP2A, the over-lapped
FOXL1-2A-PP2A was cloned into the the pShuttle-CMV vector to
generate the recombinant plasmid of pShuttle-CMV-FOXL1,
pShuttle-CMV-PP2A, or pShuttle-CMV-FOXL1-2A-PP2A, the control
pShuttle-CMV-con was generated with an insertion of the coding
sequence of enhanced green fluorescence protein (EGFP). The
recombinant adenovirus, Ad (FOXL1), Ad (PP2A), Ad (FOXL1 + PP2A) or
the Ad (con) virus was rescued under the guidance of the vector
manual. To over-express FOXL1, PP2A, or both molecules in Panc-1
cells, Panc-1 cells were infected with the Ad (FOXL1), Ad (PP2A),
Ad (FOXL1 + PP2A) or the Ad (con) virus with 1 or 3 multiplicities
of infection (MOI) for 2 h at 37°C, and then were updated with
fresh RPMI-1640 media supplemented with 2% FBS.
Quantitative assay for the mRNA level of
FOXL1 or PP2A
Total cellular mRNA in Panc-1 cells was isolated
with TRIzol reagent (Life Technologies, Grand Island, NY, USA) and
was supplemented with RNase inhibitor (Takara, Tokyo, Japan). mRNA
samples were directly quantified via real-time PCR, using the
SuperScript III Platinum One-Step qRT-PCR kit (Qiagen GmbH, Hilden,
Germany) on an ABI PRISM 7300 detection system. The housekeeping
β-actin gene was simultaneously quantified to standardize the
amount of target mRNA. Relative quantification of gene
transcription level was performed by the −ΔΔCt method (31), the relative target mRNA was
presented as relative level to the control group.
Western blot assay
Harvested Panc-1 cells post various treatment were
promptly homogenized in a Cell Lysis Buffer (Cell Signaling
Technology Inc., Danvers, MA, USA), then centrifuged at 13,000 × g
for 20 min at 4°C to collect the supernatant. Next, each sample was
quantified with a BCA protein assay reagent kit (Pierce, Rockford,
IL, USA) and was diluted to same concentration. Before being added
with loading buffer and being boiled, protein samples with equal
amount were separated with 10% sodium dodecyl
sulfate-polyacrylamide (SDS-PAGE) electrophoresis and then were
transferred to a PVDF membrane (Millipore, Bedford, MA, USA). After
the block with 2% bovine serum albumin (BSA) (Ameresco, Framingham,
MA, USA) overnight at 4°C, the membrane was incubated overnight
again at 4°C with the rabbit poly-clone antibody against FOXL1,
PP2A, β-actin, caspase-3, poly(ADP-ribose) polymerase (PARP),
TRAIL, MYC or phosphorylated MYC (Ser at 62). After triple washes
with Tris-buffered saline and Tween-20 (TBST), the membrane was
incubated with horseradish peroxidase-linked secondary anti-rabbit
antibody (Sigma-Aldrich) for an inoculation for 1 h at room
temperature. The specific bingding was scanned via a molecular
dynamics densitometer (Imaging Technology, Ontario, Canada). ImageJ
software was used to quantify band density.
Growth curve assay and colony formation
assay
The proliferation of Panc-1 cells was evaluated via
cell counting assay, briefly as follows. Panc-1 cells were
quantitatively seeded in 12-well plates with 104/ml,
post an inoculation for 8 h (cells closely attached), cells were
infected with 3 MOI of Ad (FOXL1), Ad (PP2A), Ad (FOXL1 + PP2A) or
Ad (con) respectively, and were incubated at 37°C for 1, 3, 5 or 7
days. Then cells in each well were trypsinized and were counted in
a hemocytometer with the use of trypan blue staining. Colony
formation assay was also utilized to determine the proliferation of
Panc-1 cells, 1,000 cells were seeded into a 6-well plate, and were
infected with 3 MOI of Ad (FOXL1), Ad (PP2A), Ad (FOXL1 + PP2A) or
Ad (con), respectively; then cells were incubated at 37°C for
another 5 days, and the cell colonies were stained by 0.5% crystal
violet (Sigma-Aldrich) and were counted.
MTT assay
Panc-1 cells were seeded in 96-well plates, at
>85% confluence, cells were treated with 10 µM 5′-FU, and
were infected with 3 MOI of Ad (FOXL1), Ad (PP2A), Ad (FOXL1 +
PP2A) or Ad (con) respectively, and were incubated for 24 or 48 h.
Then, the MTT solution was added and incubated for 4 h. After the
MTT solution was aspirated, 100 ml dimethylsulfoxide was added to
each well. The absorbance was measured at 570 and 650 nm using a
microplate reader (Bio-Rad Laboratories Inc., Hercules, CA,
USA).
Determination of apoptosis and the assay
of caspase-3 activity
Apoptosis induced by 5′-FU treatment and adenovirus
infection for 24 or 48 h was determined by Annexin V-FITC kit
(Roche Diagnostics, Indianapolis, IN, USA). Cells were incubated in
the dark with Annexin V-FITC and PI for 20 min. The apoptotic cells
were quantified using flow cytometry, and the percentage of Annexin
V-positive cells (early apoptosis) or Annexin V-plus-PI positive
cells (late apoptosis) were calculated and were presented as total
apoptotic cells. Caspase-3 activity was measured by Apo-ONE
homogeneous caspase-3 assay (Promega, Madison, WI, USA) and was
calculated and compared with control cells.
Statistical analysis
Quantitative data are presented as the mean ±
standard deviation that is calculated from three independent
results. Comparison between two groups was performed with a
Student's t-test. A two-way ANOVA test was used for multiple
comparisons between three or more groups. P<0.05 was considered
to indicate a statistically significant difference.
Results
Overexpression of FOXL1 and PP2A in
panc-1 cells with a FOXL1- and PP2A co-expressed adenovirus
To investigate the tumor suppressive role of FOXL1
and PP2A simultaneously in pancreatic cancer Panc-1 cells, we
adopted a strategy to clone the coding sequences of both genes into
one opening reading-frame (ORF), and to co-express both proteins
simultaneously. As shown in Fig.
1A, both FOXL1 and PP2A coding sequences were linked with a
self-cleaved '2A' peptide coding sequence (32) and were cloned into the multiple
cloning sites of the shuttle plasmid, with the promoter of human
cytomegalovirus (CMV) immediate early enhancer and promoter
(PCMV). The Ad (con) (over-expressing EGFP), Ad (FOXL1)
(overexpressing FOXL1), Ad (PP2A) (overexpressing PP2A) or Ad
(FOXL1 + PP2A) (overexpressing both FOXL1 and PP2A) was rescued
respectively via the co-transfection with the adenoviral genomic
plasmid and the shuttle plasmid. Fig.
1B indicates that the infection with the Ad (con) virus caused
EGFP expression in >85% of Panc-1 cells. The expression
efficiency by the adenovirus of both FOXL1 and PP2A was evaluated
in Panc-1 cells post-infection with Ad (FOXL1), Ad (PP2A) or Ad
(FOXL1 + PP2A). As shown in Fig. 1C and
D, the mRNA level of FOXL1 was significantly promoted by the
infection with Ad (FOXL1) or Ad (FOXL1 + PP2A) (either P<0.001
for 1 or 3 MOI), and there was no significant difference between Ad
(FOXL1) and Ad (FOXL1 + PP2A); Whereas the PP2A mRNA level was
significantly upregulated by the infection with Ad (PP2A) or Ad
(FOXL1 + PP2A) (P<0.001 or P<0.0001). Western blotting
indicated that the expression of FOXL1 or PP2A was also
significantly promoted at protein level in the Panc-1 cells by the
infection with Ad (FOXL1)/Ad (FOXL1 + PP2A) virus, or by Ad
(PP2A)/Ad (FOXL1 + PP2A) virus (Fig.
1D–F) (P<0.001 or P<0.0001). Therefore, the three
recombinant adenoviruses overexpressed FOXL1 or/and PP2A in Panc-1
cells.
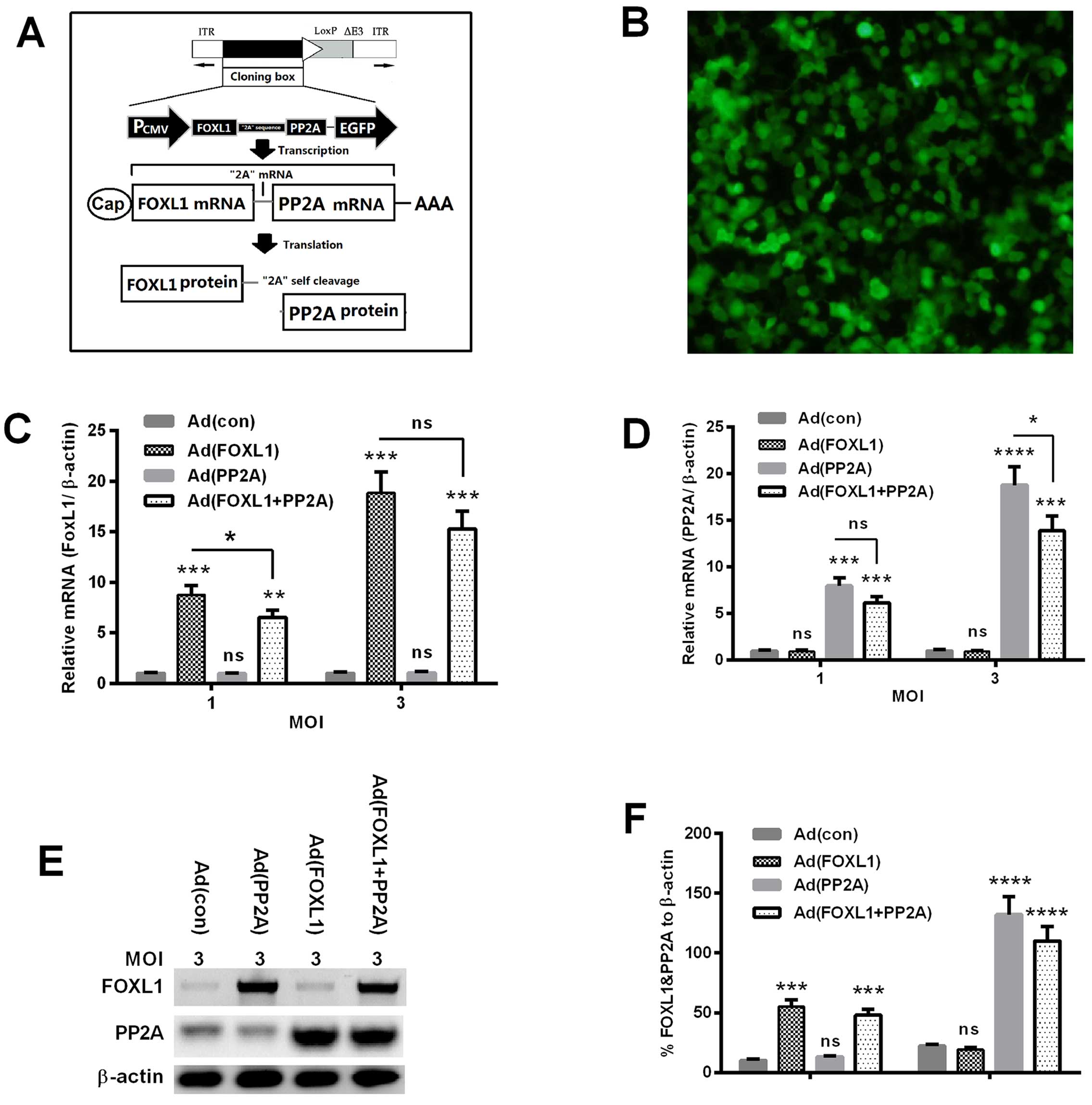 | Figure 1Construction of FOXL1- and
PP2A-co-expressed adenovirus. (A) The co-expression strategy of
FOXL1 and PP2A with a '2A' self-cleavage sequence in adenovirus.
(B) Expression of enhanced green fluorescence protein (EGFP) in
Panc-1 cells post-infection with 1 multiplicity of infection (MOI)
Ad (con). (C and D) mRNA levels of FOXL1 (C) and PP2A (D) in Panc-1
cells which were infected with 1 or 3 MOI Ad (con), Ad (FOXL1), Ad
(PP2A), or Ad (FOXL1 + PP2A) virus for 24 h. (E and F) Western blot
analysis of FOXL1 and PP2A in Panc-1 cells infected with 3 MOI Ad
(con), Ad (FOXL1), Ad (PP2A), or Ad (FOXL1 + PP2A) virus, with
β-actin as an internal reference protein. *P<0.05,
**P<0.01, or ***P<0.001; ns, no
significance. |
Synergistic inhibition by the
co-expression of FOXL1 and PP2A to the proliferation and migration
of Panc-1 cells
To investigate the regulatory role of FOXL1 or/and
PP2A co-expression on the proliferation of pancreatic cancer cells,
the in vitro proliferation of Panc-1 cells post the
infection with Ad (con), Ad (FOXL1), Ad (PP2A) or Ad (FOXL1 + PP2A)
virus was examined with the cell counting and colony formation
assays. As shown in Fig. 2A, the
growth curve of Panc-1 cells infected with Ad (FOXL1) or with Ad
(PP2A) was significantly retardant, compared with the Ad (con)
infection. Moreover, the infection with Ad (FOXL1 + PP2A) virus
caused a more retardant growth curve of Panc-1 cells (P<0.05,
P<0.001 or P<0.0001). In particular, the growth efficiency of
Panc-1 cells was significant inhibited by the Ad (FOXL1 + PP2A)
virus, even compared with the Ad (FOXL1) or Ad (PP2A) virus, at 5-
or 7-day post-infection (DPI) (P<0.05 for 5 DPI, or P<0.01
for 7 DPI).
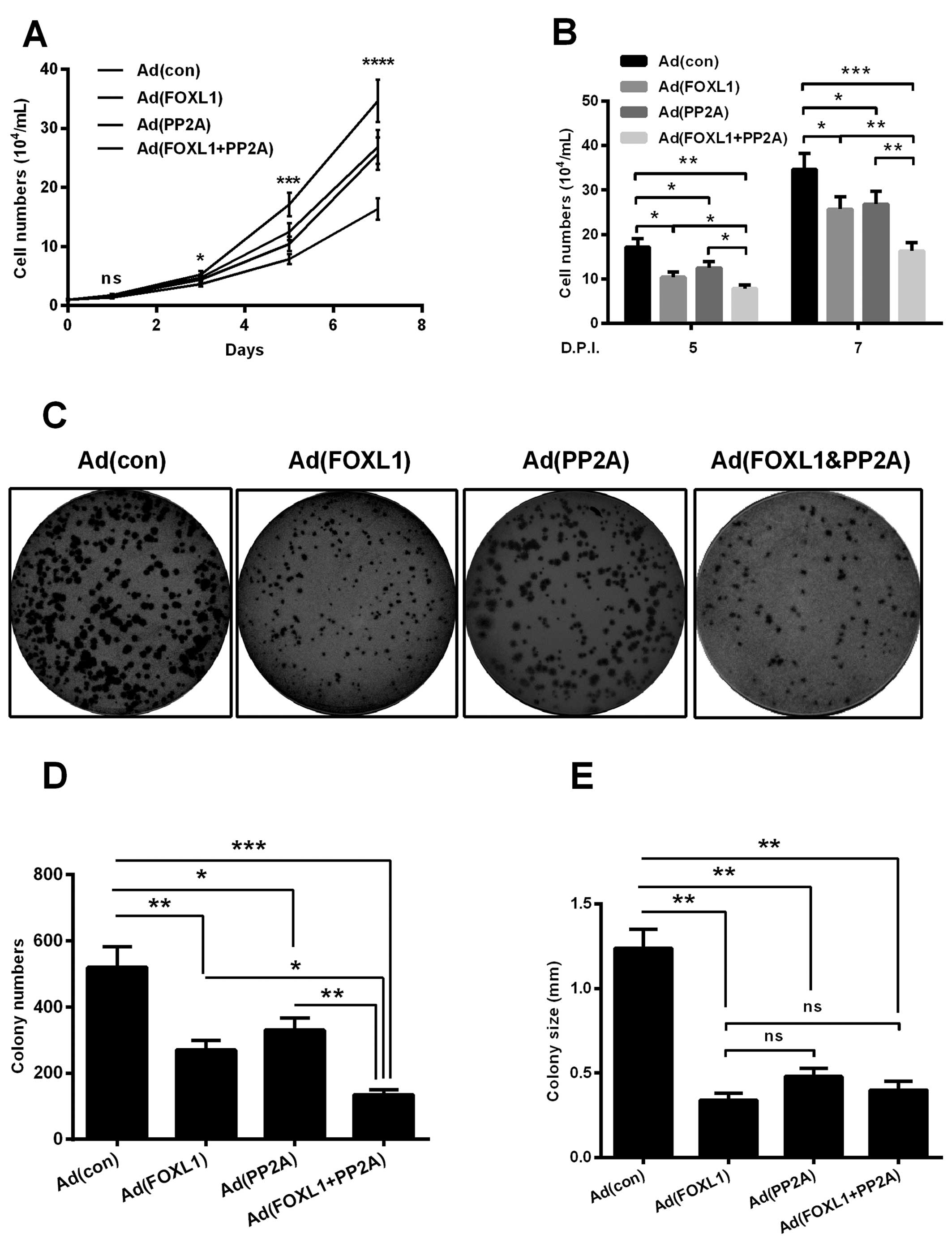 | Figure 2Proliferation of the Panc-1 cells
post the overexpression of FOXL1 or (and) PP2A. (A) Proliferation
of Panc-1 cells post-infection with 3 MOI Ad (con), Ad (FOXL1), Ad
(PP2A), or Ad (FOXL1 + PP2A) virus for 1, 3, 5 or 7 days, with an
initial 104 cells/ml seeded. (B) Difference in the
proliferation of Panc-1 cells infected at 3 MOI Ad (con), Ad
(FOXL1), Ad (PP2A), or Ad (FOXL1 + PP2A) virus for 5 or 7 days. (C)
Colony formation of Panc-1 cells post-infection at 3 MOI Ad (con),
Ad (FOXL1), Ad (PP2A), or Ad (FOXL1 + PP2A) virus for 5 days. (D
and E) Difference in the number (D) and the size (E) of colonies
formed by Panc-1 cells infected with the above-mentioned virus.
*P<0.05, **P<0.01, or
***P<0.001; ns, no significance. |
Then the regulation by the overexpression of FOXL1,
PP2A or both molecules on the proliferation of Panc-1 cells was
evaluated with the colony forming assay. As indicated in Fig. 2C and D, Panc-1 cells formed less
colonies post-infection with Ad (FOXL1), Ad (PP2A) or Ad (FOXL1 +
PP2A), compared with the infection with Ad-con (P<0.05,
P<0.01 or P<0.001). Interestingly, there was also a
significant difference in the colony size among the four groups.
Colonies in the group of Ad (FOXL1), Ad (PP2A) and Ad (FOXL1 +
PP2A) were significantly smaller than in the group of Ad (con)
(Fig. 2E) (P<0.01, respectively)
Thus, the co-expression of both FOXL1 and PP2A inhibited the
proliferation of Panc-1 cells.
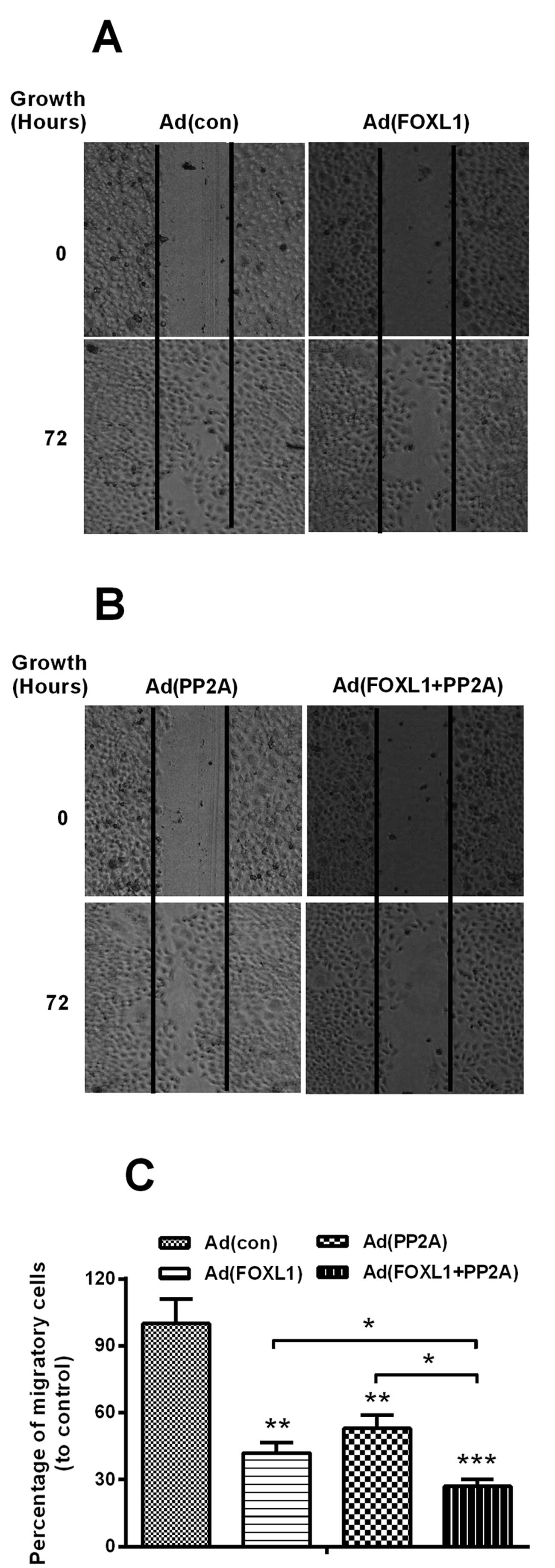
We then determined the regulation of the FOXL1-
or/and PP2A-overexpression on the migration of Panc-1 cells. The
migration assay of Panc-1 cells indicated that in contrast to the
Ad (con) virus infection, the infection with either Ad (FOXL1), Ad
(PP2A) or Ad (FOXL1 + PP2A) at 3 MOI significantly reduced the
migration of Panc-1 cells, there were less migratory cells in the
three groups (Fig. 3A-C) P<0.01
for Ad (FOXL1) or Ad (PP2A; P<0.001 for Ad (FOXL1 + PP2A)]. In
addition, the migratory cells in the Ad (FOXL1 + PP2A) group were
far less than in the Ad (FOXL1) or Ad (PP2A) group (P<0.05,
respectively). Thus, we confirmed the inhibition of the
overexpression of FOXL1 in the migration of pancreatic cancer
cells.
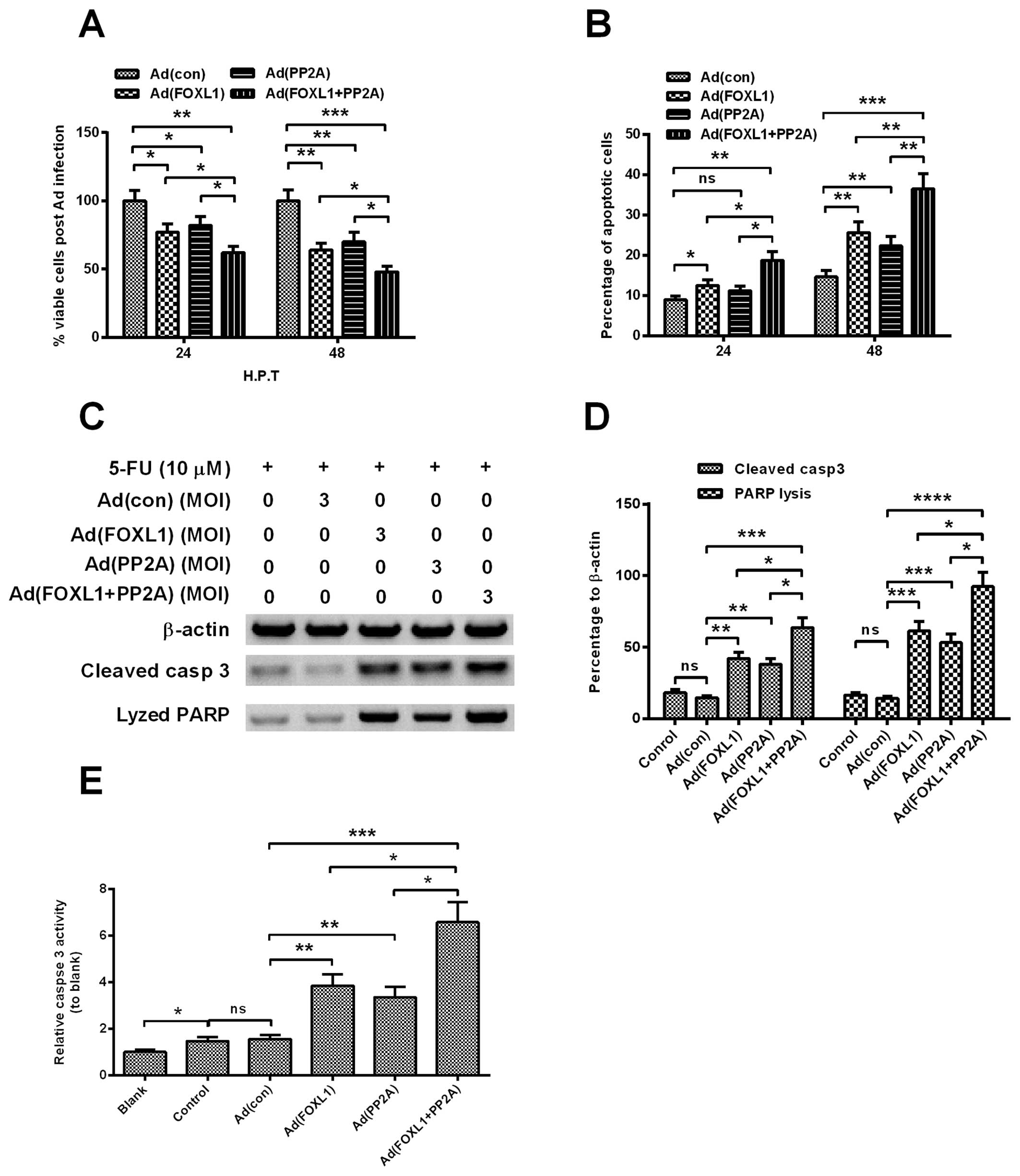
Co-expression of FOXL1 and PP2A
sensitized Panc-1 cells to chemotherapy via enhancing the apoptosis
induction
To evaluate the influence of the co-expression of
FOXL1 and PP2A on the chemosensitivity of pancreatic cancer cells,
we also examined the viability reduction and apoptosis induction of
Panc-1 cells by 10 µM 5′-FU, one of widely-used anticancer
agent, post-infection with Ad (con), Ad (FOXL1), Ad (PP2A) or Ad
(FOXL1 + PP2A) virus. Fig. 4A
indicated that the viability decreased more significantly in the
5′-FU-treated Panc-1 cells, post-infection at 3 MOI Ad (FOXL1), Ad
(PP2A) or Ad (FOXL1 + PP2A) virus [P<0.05 for Ad (FOXL1), Ad
(PP2A), P<0.01 for Ad (FOXL1 + PP2A)] and the viability
reduction was more significant by the infection with Ad (FOXL1 +
PP2A) than with Ad (FOXL1), Ad (PP2A) (P<0.05, respectively).
The deteriorated apoptosis induction was also confirmed by the
overexpression of FOXL1 or/and PP2A in Panc-1 cells. As shown in
Fig. 4B, compared with the Ad (con)
infection, there were more apoptotic cells induced by the infection
at 3 MOI Ad (FOXL1), Ad (PP2A) or Ad (FOXL1 + PP2A) at 24
(P<0.05 or P<0.01) or 48 h post-infection (HPI) (P<0.01 or
P<0.001), with markedly higher apoptosis in the Ad (FOXL1 +
PP2A) group (P<0.05 respectively at 24 HPI or P<0.01 at 48
HPI). In addition, we also analyzed the activation and activity of
caspase-3, which is the apoptosis-executor, in each groups of
cells. Fig. 4C–E demonstrated that
there was higher levels of activated caspase-3 (Fig. 4D) and caspase activity (Fig. 4E) induced by the Ad (FOXL1), Ad
(PP2A) or Ad (FOXL1 + PP2A) than Ad (con) (P<0.05, P<0.01 or
P<0.001, respectively), particularly higher by the Ad (FOXL1 +
PP2A) (P<0.05, respectively). Therefore, we confirmed that the
co-expression of FOXL1 and PP2A sensitized Panc-1 cells to 5′-FU
via enhancing apoptosis induction.
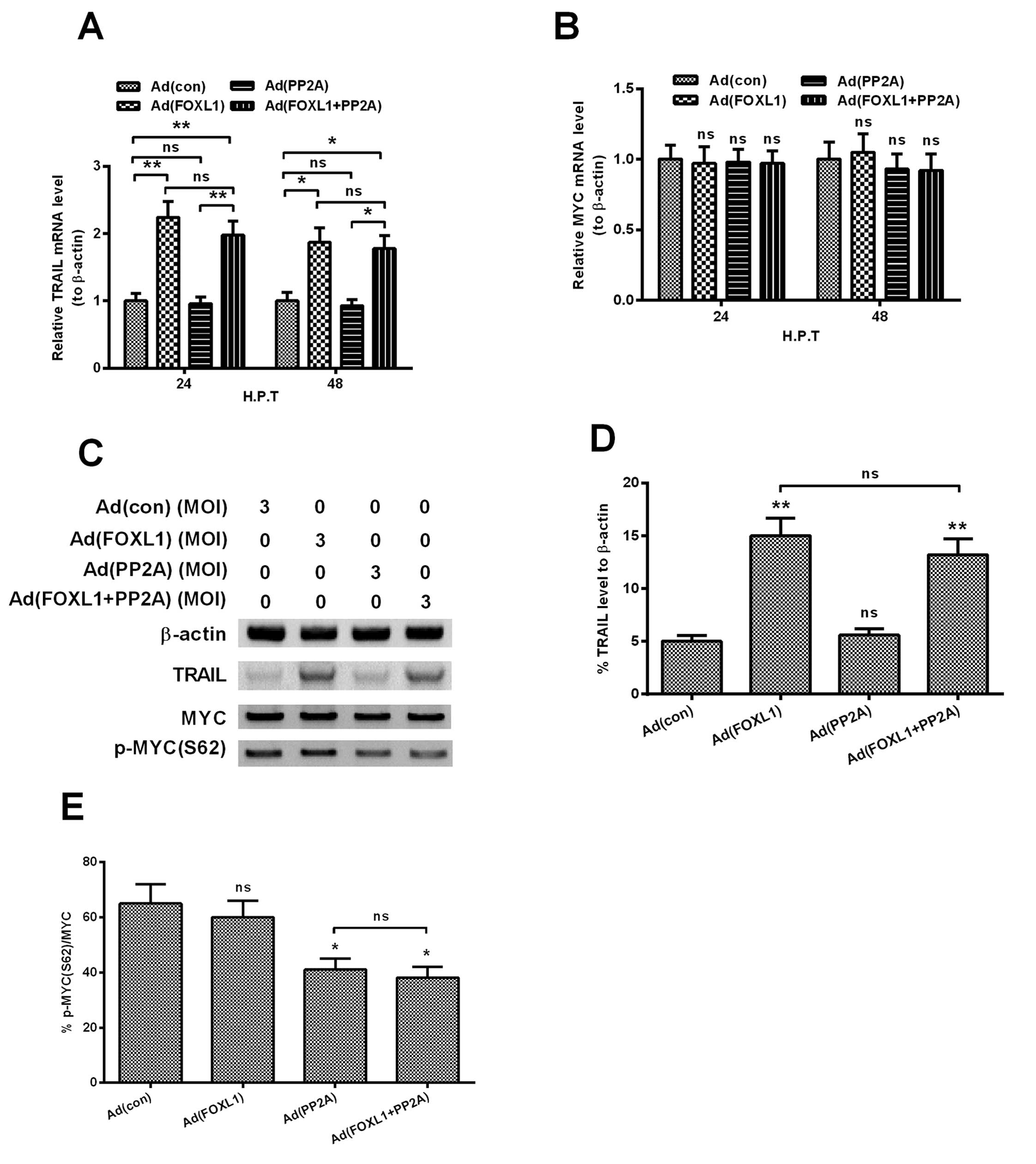
Co-expression of FOXL1 and PP2A promotes
TRAIL, whereas inhibits MYC phosphorylation
TRAIL is a member of the TNF superfamily and
triggers apoptosis by recruiting the initiator caspase-8 and by
directly activating downstream effector caspases (33), and FOXL1 has been indicated to
inhibit the tumor aggressiveness in human pancreatic cancer via
promoting TRAIL (22). The
oncogenic MYC (also c-MYC) has also been deregulated in pancreatic
cancers and has been confirmed to promote pancreatic cancers
(34), and the targeted inhibition
of MYC by antagonizing PP2A inhibitor has been indicated to inhibit
the growth of breast cancers (35).
To explore the possible mechanism in apoptosis induction by the
co-expression of FOXL1 and PP2A, we then determined the expression
of TRAIL and the phosphorylation of MYC, in Panc-1 cells
post-infection at 3 MOI Ad (con), Ad (FOXL1), Ad (PP2A) or Ad
(FOXL1 + PP2A) virus. Fig. 5A shows
that the mRNA level of TRAIL was significantly upregulated by the
infection with Ad (FOXL1) or Ad (FOXL1 + PP2A) for 24 h (either
P<0.01), rather than the infection with Ad (con) or Ad (PP2A)
(no significance). However, the mRNA level of MYC was not
significantly regulated by any infection with the above-mentioned
virus (Fig. 5B). The western
blotting results reconfirmed the regulation of TRAIL and MYC. The
protein level of TRAIL was significantly higher in the Panc-1 cells
infected with Ad (FOXL1) or Ad (FOXL1 + PP2A) at 3 MOI (P<0.01,
respectively), whereas no significantly differene of MYC was found
among the groups (Fig. 5C).
However, the level of phosphorylated MYC (S62), was significantly
downregulated by the infection with either Ad (PP2A) or Ad (FOXL1 +
PP2A) (Fig. 5E; P<0.05,
respectively). Taken together, the co-expression of FOXL1 and PP2A
promotes TRAIL, whereas inhibits MYC phosphorylation at S62.
Discussion
The pathogenesis and the incurable nature of
pancreatic cancer, and the rapid metastasis and the poor response
to chemo-drugs might contribute to the poor prognosis (1,36).
Several pathways have been recognized to regulate the pathogenesis
or progression of pancreatic cancers. Hedgehog (Hh) signaling and
the nuclear factor-κB (NF-κB) pathway have been implicated to
involve in the pathogenesis of the disease (1,37–40).
Many other pathways have also been found to be deregulated in
pancreatic cancers, and to promote the growth aggression of the
cancer (36). Such pathways as
epidermal growth factor receptor (EGFR) and cyclooxygenase-2
(COX-2) act with an orchestrated interaction each other, and play a
significant role in tumorgenesis (40). However, there is no chemo- or
immuno-therapeutic agent against these pathways indicating
significant effect for PDAC patients.
Previous studies have indicated the tumor
suppressive roles of FOXL1 (22)
and PP2A (41) in pancreatic
cancers. Thus, the tumor suppression mechanism of FOXL1 and PP2A
might facilitate to find novel strategy or target for pancreatic
cancer therapy. In the present study, we reconfirmed the tumor
suppressive role of either FOXL1 or PP2A in the pancreatic cancer
Panc-1 cell line. The Ad-mediated overexpression of either FOXL1 or
PP2A significantly inhibited the proliferation of Panc-1 cells via
multiple assays, and such overexpression sensitized Panc-1 cells to
the treatment with 5′-FU via enhancing apoptosis induction.
Moreover, we used a strategy of co-expression of FOXL1 and PP2A to
obtain an enhanced tumor suppressive effect on pancreatic cancers.
The Ad (FOXL1 + PP2A) virus not only more significantly inhibited
the proliferation of Panc-1 cells, but also deteriorated the
viability reduction, or enhanced the apoptosis induction in the
Panc-1 cells subjected to 5′-FU. 2A peptide is encoded by
foot-and-mouth disease virus (FMDV), with a 'self-cleavage'
characteristic (42). This
'self-cleavage' peptide composed of 2A and 2B, both of which are
translated from one mRNA molecule and function independently
(42). Therefore, the 2A peptide is
well used for the multiple expression of foreign proteins (43,44).
In the present study, we confirmed that the adenovirus encoding
both FOXL1 and PP2A with the '2A peptide' linker overexpressed both
tumor suppressors in pancreatic cancer cells, and exerted
synergistic growth inhibition of pancreatic cancer cells.
TRAIL is a member of the tumor necrosis factor
superfamily inducing apoptosis through interaction with the
TRAIL-R1 and TRAILR2 receptors (alternatively known as DR4 and DR5,
respectively) (45–47). TRAIL has emerged as a potential
therapeutic agent due to its selective induction of apoptosis in
cancer cells (48). Preliminary
clinical trials with TRAIL indicate promising outcomes without
obvious toxicity (49,50). The present study presents another
confirmation of the antitumor effect of TRAIL via upregulating the
upstream FOXL1. Significant promotion of TRAIL in both mRNA and
protein levels was confirmed by the infection with either Ad
(FOXL1) or Ad (FOXL1 + PP2A). On the contrary, the oncogenic MYC
(also namely C-MYC) has been found to be deregulated in pancreatic
cancers and has been confirmed to promote pancreatic cancers
(34,51). The targeted inhibition of MYC has
been indicated to inhibit the growth of breast cancers (35). In mammalian cells, Ser-62
phosphorylation of MYC is associated with the MYC stabilization,
and the dephosphorylation of the site by PP2A promotes its
polyubiquitination and degradation (52). Previous studies confirmed that
inhibited PP2A resulted in increased MYC half-life (53). The current study confirmed the
inhibition to Ser-62 phosphorylation of MYC by PP2A overexpression,
and it might be associated with the inhibiton of pancreatic cancer
cells.
In conclusion, the adenovirus-mediated co-expression
of FOXL1 and PP2A with the 2A peptide linker exterts synergistic
suppression of pancreatic cancer cells via inhibiting the growth
and promoting apoptosis of cancer cells. The coexpressed FOXL1 and
PP2A functions independently via upregulating TRAIL (by FOXL1) and
reducing the phosphorylation of MYC (by PP2A). Our findings
re-confirmed the tumor suppressive role of PP2A and FOXL1 in
pancreatic cancer cells, with an enhanced antitumor effect via
co-expression of both molecules.
Acknowledgments
The present study was supported by grants from the
First Hospital of China Medical University, Shengyang, China, the
Social Development Program from Shenyang and Technology Bureau,
China (no. F15-139-9-19), and the Grants-in-aid for Special-Term
Professor Scientific Research from the Educational Department of
Liaoning Province, China (Liao Zhi Jiao no. 2012-512).
References
|
1
|
Thayer SP, di Magliano MP, Heiser PW,
Nielsen CM, Roberts DJ, Lauwers GY, Qi YP, Gysin S, Fernández-del
Castillo C, Yajnik V, et al: Hedgehog is an early and late mediator
of pancreatic cancer tumorigenesis. Nature. 425:851–856. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bosetti C, Bertuccio P, Negri E, La
Vecchia C, Zeegers MP and Boffetta P: Pancreatic cancer: Overview
of descriptive epidemiology. Mol Carcinog. 51:3–13. 2012.
View Article : Google Scholar
|
|
3
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kleger A, Perkhofer L and Seufferlein T:
Smarter drugs emerging in pancreatic cancer therapy. Ann Oncol.
25:1260–1270. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Haberland J, Bertz J, Wolf U, Ziese T and
Kurth BM: German cancer statistics 2004. BMC Cancer. 10:522010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vaccaro V, Sperduti I and Milella M:
FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N
Engl J Med. 365:768–769. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Conroy T, Desseigne F, Ychou M, Bouché O,
Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de
la Fouchardière C, et al Groupe Tumeurs Digestives of Unicancer;
PRODIGE Intergroup: FOLFIRINOX versus gemcitabine for metastatic
pancreatic cancer. N Engl J Med. 364:1817–1825. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moore MJ, Goldstein D, Hamm J, Figer A,
Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D and Wolff RA;
National Cancer Institute of Canada Clinical Trials Group:
Erlotinib plus gemcitabine compared with gemcitabine alone in
patients with advanced pancreatic cancer: A phase III trial of the
National Cancer Institute of Canada Clinical Trials Group. J Clin
Oncol. 25:1960–1966. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kang SP and Saif MW: Optimal second line
treatment options for gemcitabine refractory advanced pancreatic
cancer patients. Can we establish standard of care with available
data? JOP. 9:83–90. 2008.PubMed/NCBI
|
|
11
|
Bergmann U, Funatomi H, Yokoyama M, Beger
HG and Korc M: Insulin-like growth factor I overexpression in human
pancreatic cancer: Evidence for autocrine and paracrine roles.
Cancer Res. 55:2007–2011. 1995.PubMed/NCBI
|
|
12
|
Rieder S, Michalski CW, Friess H and
Kleeff J: Insulin-like growth factor signaling as a therapeutic
target in pancreatic cancer. Anticancer Agents Med Chem.
11:427–433. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Seo Y, Baba H, Fukuda T, Takashima M and
Sugimachi K: High expression of vascular endothelial growth factor
is associated with liver metastasis and a poor prognosis for
patients with ductal pancreatic adenocarcinoma. Cancer.
88:2239–2245. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kindler HL, Niedzwiecki D, Hollis D,
Sutherland S, Schrag D, Hurwitz H, Innocenti F, Mulcahy MF,
O'Reilly E, Wozniak TF, et al: Gemcitabine plus bevacizumab
compared with gemcitabine plus placebo in patients with advanced
pancreatic cancer: Phase III trial of the Cancer and Leukemia Group
B (CALGB 80303). J Clin Oncol. 28:3617–3622. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hannenhalli S and Kaestner KH: The
evolution of Fox genes and their role in development and disease.
Nat Rev Genet. 10:233–240. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Carter ME and Brunet A: FOXO transcription
factors. Curr Biol. 17:R113–R114. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Paik JH, Kollipara R, Chu G, Ji H, Xiao Y,
Ding Z, Miao L, Tothova Z, Horner JW, Carrasco DR, et al: FoxOs are
lineage-restricted redundant tumor suppressors and regulate
endothelial cell homeostasis. Cell. 128:309–323. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tothova Z, Kollipara R, Huntly BJ, Lee BH,
Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams
IR, Sears C, et al: FoxOs are critical mediators of hematopoietic
stem cell resistance to physiologic oxidative stress. Cell.
128:325–339. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Z, Ahmad A, Li Y, Banerjee S, Kong D
and Sarkar FH: Forkhead box M1 transcription factor: A novel target
for cancer therapy. Cancer Treat Rev. 36:151–156. 2010. View Article : Google Scholar :
|
|
20
|
Xia JT, Wang H, Liang LJ, Peng BG, Wu ZF,
Chen LZ, Xue L, Li Z and Li W: Overexpression of FOXM1 is
associated with poor prognosis and clinicopathologic stage of
pancreatic ductal adenocarcinoma. Pancreas. 41:629–635. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Z, Banerjee S, Kong D, Li Y and
Sarkar FH: Down-regulation of Forkhead Box M1 transcription factor
leads to the inhibition of invasion and angiogenesis of pancreatic
cancer cells. Cancer Res. 67:8293–8300. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang G, He P, Gaedcke J, Ghadimi BM, Ried
T, Yfantis HG, Lee DH, Hanna N, Alexander HR and Hussain SP: FOXL1,
a novel candidate tumor suppressor, inhibits tumor aggressiveness
and predicts outcome in human pancreatic cancer. Cancer Res.
73:5416–5425. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Arnold HK and Sears RC: A tumor suppressor
role for PP2A-B56alpha through negative regulation of c-Myc and
other key oncoproteins. Cancer Metastasis Rev. 27:147–158. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Westermarck J and Hahn WC: Multiple
pathways regulated by the tumor suppressor PP2A in transformation.
Trends Mol Med. 14:152–160. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Eichhorn PJ, Creyghton MP and Bernards R:
Protein phosphatase 2A regulatory subunits and cancer. Biochim
Biophys Acta. 1795:1–15. 2009.
|
|
26
|
Farrell AS, Pelz C, Wang X, Daniel CJ,
Wang Z, Su Y, Janghorban M, Zhang X, Morgan C, Impey S, et al: Pin1
regulates the dynamics of c-Myc DNA binding to facilitate target
gene regulation and oncogenesis. Mol Cell Biol. 33:2930–2949. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sears RC: The life cycle of c-Myc: From
synthesis to degradation. Cell Cycle. 3:1133–1137. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Welcker M, Orian A, Grim JE, Eisenman RN
and Clurman BE: A nucleolar isoform of the Fb 7 ubiquitin ligase
regulates c-Myc and cell size. Curr Biol. 14:1852–1857. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Côme C, Laine A, Chanrion M, Edgren H,
Mattila E, Liu X, Jonkers J, Ivaska J, Isola J, Darbon JM, et al:
CIP2A is associated with human breast cancer aggressivity. Clin
Cancer Res. 15:5092–5100. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang L, Gu F, Ma N, Zhang L, Bian JM and
Cao HY: CIP2A expression is associated with altered expression of
epithelial-mesenchymal transition markers and predictive of poor
prognosis in pancreatic ductal adenocarcinoma. Tumour Biol.
34:2309–2313. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Szymczak AL, Workman CJ, Wang Y, Vignali
KM, Dilioglou S, Vanin EF and Vignali DA: Correction of multi-gene
deficiency in vivo using a single 'self-cleaving' 2A peptide-based
retroviral vector. Nat Biotechnol. 22:589–594. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ashkenazi A: Targeting death and decoy
receptors of the tumour-necrosis factor superfamily. Nat Rev
Cancer. 2:420–430. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
He C, Jiang H, Geng S, Sheng H, Shen X,
Zhang X, Zhu S, Chen X, Yang C and Gao H: Expression of c-Myc and
Fas correlates with perineural invasion of pancreatic cancer. Int J
Clin Exp Pathol. 5:339–346. 2012.PubMed/NCBI
|
|
35
|
Janghorban M, Farrell AS, Allen-Petersen
BL, Pelz C, Daniel CJ, Oddo J, Langer EM, Christensen DJ and Sears
RC: Targeting c-MYC by antagonizing PP2A inhibitors in breast
cancer. Proc Natl Acad Sci USA. 111:9157–9162. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sarkar FH, Banerjee S and Li Y: Pancreatic
cancer: Pathogenesis, prevention and treatment. Toxicol Appl
Pharmacol. 224:326–336. 2007. View Article : Google Scholar
|
|
37
|
Berman DM, Karhadkar SS, Maitra A, Montes
De Oca R, Gerstenblith MR, Briggs K, Parker AR, Shimada Y, Eshleman
JR, Watkins DN, et al: Widespread requirement for Hedgehog ligand
stimulation in growth of digestive tract tumours. Nature.
425:846–851. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liptay S, Weber CK, Ludwig L, Wagner M,
Adler G and Schmid RM: Mitogenic and antiapoptotic role of
constitutive NF-kappaB/Rel activity in pancreatic cancer. Int J
Cancer. 105:735–746. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Karin M: Nuclear factor-kappaB in cancer
development and progression. Nature. 441:431–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nakashima H, Nakamura M, Yamaguchi H,
Yamanaka N, Akiyoshi T, Koga K, Yamaguchi K, Tsuneyoshi M, Tanaka M
and Katano M: Nuclear factor-kappaB contributes to hedgehog
signaling pathway activation through sonic hedgehog induction in
pancreatic cancer. Cancer Res. 66:7041–7049. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Farrell AS, Allen-Petersen B, Daniel CJ,
Wang X, Wang Z, Rodriguez S, Impey S, Oddo J, Vitek MP, Lopez C, et
al: Targeting inhibitors of the tumor suppressor PP2A for the
treatment of pancreatic cancer. Mol Cancer Res. 12:924–939. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ryan MD, King AM and Thomas GP: Cleavage
of foot-and-mouth disease virus polyprotein is mediated by residues
located within a 19 amino acid sequence. J Gen Virol. 72:2727–2732.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sharma P, Yan F, Doronina VA,
Escuin-Ordinas H, Ryan MD and Brown JD: 2A peptides provide
distinct solutions to driving stop-carry on translational recoding.
Nucleic Acids Res. 40:3143–3151. 2012. View Article : Google Scholar :
|
|
44
|
Szymczak-Workman AL, Vignali KM and
Vignali DA: Design and construction of 2A peptide-linked
multicistronic vectors. Cold Spring Harb Protoc. 2012:199–204.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pan G, O'Rourke K, Chinnaiyan AM, Gentz R,
Ebner R, Ni J and Dixit VM: The receptor for the cytotoxic ligand
TRAIL. Science. 276:111–113. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Walczak H, Degli-Esposti MA, Johnson RS,
Smolak PJ, Waugh JY, Boiani N, Timour MS, Gerhart MJ, Schooley KA,
Smith CA, et al: TRAIL-R2: A novel apoptosis-mediating receptor for
TRAIL. EMBO J. 16:5386–5397. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sheridan JP, Marsters SA, Pitti RM, Gurney
A, Skubatch M, Baldwin D, Ramakrishnan L, Gray CL, Baker K, Wood
WI, et al: Control of TRAIL-induced apoptosis by a family of
signaling and decoy receptors. Science. 277:818–821. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Walczak H, Miller RE, Ariail K, Gliniak B,
Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, et al:
Tumoricidal activity of tumor necrosis factor-related
apoptosis-inducing ligand in vivo. Nat Med. 5:157–163. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Newsom-Davis T, Prieske S and Walczak H:
Is TRAIL the holy grail of cancer therapy? Apoptosis. 14:607–623.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mahalingam D, Szegezdi E, Keane M, de Jong
S and Samali A: TRAIL receptor signalling and modulation: Are we on
the right TRAIL? Cancer Treat Rev. 35:280–288. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Schild C, Wirth M, Reichert M, Schmid RM,
Saur D and Schneider G: PI3K signaling maintains c-myc expression
to regulate transcription of E2F1 in pancreatic cancer cells. Mol
Carcinog. 48:1149–1158. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Escamilla-Powers JR and Sears RC: A
conserved pathway that controls c-Myc protein stability through
opposing phosphorylation events occurs in yeast. J Biol Chem.
282:5432–5442. 2007. View Article : Google Scholar
|
|
53
|
Yeh E, Cunningham M, Arnold H, Chasse D,
Monteith T, Ivaldi G, Hahn WC, Stukenberg PT, Shenolikar S, Uchida
T, et al: A signalling pathway controlling c-Myc degradation that
impacts oncogenic transformation of human cells. Nat Cell Biol.
6:308–318. 2004. View Article : Google Scholar : PubMed/NCBI
|