Introduction
Thousands of women worldwide from all walks of life
are diagnosed every day with breast cancer. It is by far the most
common cancer among females worldwide and it is also the leading
cause of cancer-related mortality (1). Breast cancer is no longer viewed as a
single disease since it is heterogeneous consisting of a multitude
of subgroups at the molecular, histopathological and clinical level
with different prognostic and therapeutic implications. The
understanding of the biological mechanisms of breast cancer have
elucidated a number of potential therapeutic molecular targets
(2). In recent years, specific
drugs which modulate these targets in a way that interferes with
their ability to promote cancer cell growth or invasion of
carcinoma cells have become a part of the standard care of patients
with breast cancer (anti-HER2 agents, e.g. trastuzumab and
lapatinib). Numerous other agents have not been approved for
clinical practice, yet their potential utility is currently under
extensive investigation (3–5). In this context, one of the potential
candidates appears to be agents that target the actin cytoskeleton
of cancer cells or regulate actin cytoskeleton dynamics (6,7).
Numerous studies have indicated that F-actin
participates in the induction of cell death and apoptosis. It is
known that both cytoplasmic and nuclear actin pools and their
proper balance are necessary to maintain cellular homeostasis
(8). Translocation of actin between
the cytoplasm and the nucleus is not well understood, since this
protein does not have a classical nuclear localization signal
(9). The transport of actin is
possible in association with actin-binding proteins which contain a
functional nuclear localization sequence (NLS). Moreover, it has
been shown that the export of actin is mediated by exportin 6
(XPO6) and imported by importin-9 (IPO9). IPO9 was identified in
2012 by dopie et al (10) as
the nuclear import factor for actin. Using RNA interference (RNAi)
technique they demonstrated that the level of nuclear actin could
be regulated by altering the ratio of protein to promote both
export and import of actin. They also suggested that this area of
study requires further elucidation (10). It has also been shown that cofilin
(CFL) participates in the transport of actin between the cytoplasm
and the nucleus. CFL belongs to the actin-binding protein group and
promotes the depolymerization of actin filaments (11–13).
Three ADF/CFL isoforms, which fulfill a specific function in actin
filament reorganization and possess NLS have been identified in
mammalian cells (11,14). Although CFL participates in the
active transport of actin to the nucleus under physiological
conditions, the mechanism is still unclear (13).
Naturally occurring compounds, particularly those
from dietary sources, have recently gained increased scientific
attention as potential anticancer drugs and, in particular, as a
partner for conventional cytostatic drugs. Garlic, Allium
sativum L. is a member of the Alliaceae family, which may have
anticarcinogenic effects inter alia by the inhibition of
growth and invasive potential of cancer cells and induction of
apoptosis (15–18). Such antitumor actions of this
vegetable have been attributed to the presence of organosulfur
compounds, among which the most abundant in intact garlic is
S-allyl-L-cysteine sulfoxide (alliin) (19). Once garlic is processed by cutting
or crushing, alliin is rapidly converted to allicin and further
into hundreds of di-, tri-, and polysulfides (20). Importantly, it has been shown that
some of these metabolites retain anticancer activity comparable to
the parent compound or even may be more toxic toward tumor cells
(18,19).
Paclitaxel (PTX) is one of the most widely used and
effective anticancer agents derived from natural sources (21). Its anticancer activity is associated
with binding to tubulin and stabilization of microtubules,
resulting in the inhibition of cell division. Additionally, this
cytostatic drug induces apoptosis by the mitochondrial pathway and
inhibits the function of the apoptosis inhibitor protein B-cell
leukemia 2 (Bcl-2) (22). In the
present study, the apoptosis in MCF-7 cells was induced by PTX and
enhanced by the combined treatment with PTX and garlic-derived
alliin.
The aim of present study was to study the impact of
the altered actin transport between the cytoplasm and the nucleus
by the downregulation of IPO9 in breast adenocarcinoma MCF-7 cells
exposed to an apoptosis-inducing combination of PTX and
garlic-derived alliin. In the present study, we also investigated
whether IPO9 influences the post-translational expression of
cofilin-1 (CFL1) and confirmed that CFL1 itself does not influence
the nuclear F-actin function in the process of apoptosis.
Materials and methods
Cell culture and treatment
MCF-7, a human breast adenocarcinoma cell line, was
purchased from the American Type Culture Collection (ATCC;
Manassas, VA, USA). The cells were grown in tissue culture flasks
or 12-well plates (BD Biosciences, Franklin Lakes, NJ, USA) and
cultured as a monolayer in Eagle's Minimum Essential Medium (MEM;
Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% fetal
bovine serum (FBS; Gibco/Invitrogen Life Technologies, Carlsbad,
CA, USA) and 50 µg/ml gentamycin (Sigma-Aldrich). The MCF-7
cultures were maintained in 5% CO2 at 37°C.
MCF-7 cells were treated with 10 µM alliin,
0.1 or 1 µM PTX (both from Sigma-Aldrich) and the
combination of these agents (10 µM alliin/0.1 µM PTX
or 10 µM alliin/1 µM PTX) for 24 h. The control cells
were cultured under the same conditions but without exposure to
these agents.
Transfection by nucleofection
For the nucleofection of MCF-7 cells, the cells were
grown to 80–90% confluency in MEM with FBS, and 50 µg/ml
gentamycin. Following trypsinization, the cells (1×106)
were transfected using the SE Cell Line 4D-Nucleofector™ X kit and
3 pmol siRNA against human IPO9 (Hs_IPO9_7; Qiagen, Hilden,
germany) according to the manufacturer's instructions and as
previously described (23). For
determining the unspecific effects of siRNA transfection, the
non-targeting AllStars negative control siRNA (Qiagen) was used. At
72 h post transfection, the cells were used for the subsequent
experiments.
Western blot analysis
Semi-quantitative analysis of the post-translational
expression of IPO9 and CFL1 was performed using western blot
analysis. The cells were lysed with RIPA buffer (Sigma-Aldrich).
Following normalization of the protein concentration using the BCA
protein assay kit (Thermo Scientific Pierce Rockford, IL, USA),
equal amounts of protein (15 µg of total protein per lane)
were separated using 4–12% NuPAGE Bis-Tris Gel (Novex/Life
Technologies, Carlsbad, CA, USA) and transferred onto
nitrocellulose membranes using the iBlot dry western blotting
system (Invitrogen/Life Technologies). To determine the position of
the protein bands, pre-stained molecular weight markers (Novex/Life
Technologies) were used. Next, the membrane was processed using
WesternBreeze® chromogenic western blot immunodetection
kit by the BenchPro™ 4100 Card Processing Station (both from
Invitrogen/Life Technologies) according to the manufacturer's
instructions. After that, the membranes were blocked with
WesternBreeze® blocking solution for 30 min. The next
step was incubation with the primary rabbit monoclonal
anti-importin-9 (1:1,000; Abcam, Cambridge, MA, USA), rabbit
anti-cofilin-1 (1:1,000) or rabbit polyclonal anti-GADPH (1:2,000;
both from Sigma-Aldrich) antibodies for 2 h at room temperature
(RT) and the membrane washing. After that, membranes were incubated
for 1 h at RT with a ready-to-use solution of alkaline
phosphatase-conjugated anti-species IgG. The immunoreactive bands
were visualized using a ready-to-use solution of BCIP/NBT substrate
for alkaline phosphatase. After scanning, the densitometry of the
bands was quantified using Quantity One Basic software (ver. 3.6.5;
Bio-Rad, Hercules, CA, USA).
Cell death analysis
The analysis of cell death was carried out using a
Tali® image-based cytometer and Tali®
apoptosis kit (both from Invitrogen/Life Technologies) according to
the manufacturer's instructions and as previously described
(23). The analysis was performed
using Tali® image-based cytometer and FCS Express
Research Edition software (v.4.03; de Novo Software, glendale, CA,
USA) on the condition that early apoptotic cells stain with only
Annexin V-Alexa Fluor 488, late apoptotic cells stain with both
propidium iodide (PI) and green Annexin V-Alexa Fluor 488, necrotic
cells stain with PI, and live cells remain unstained.
Fluorescence staining of F-actin
MCF-7 cells transfected with siRNA-IPO9 and
non-targeting AllStars negative control siRNA were seeded on
sterile glass coverslips placed in 12-well plates (BD Biosciences).
After 24 h, PTX (at concentrations of 0.1 and 1 µM) or 10
µM alliin were added to the cells for the indicated times as
either single or combined agents. Next, the cells were fixed with
4% paraformaldehyde in PBS for 20 min at RT and stained with
phalloidin conjugated to Alexa Fluor 488 (1:40, Invitrogen, Life
Technologies, Carlsbad, CA, USA) as previously described (24). Nuclear staining was performed with
4′,6′-diamidino-2-phenylindole dihydrochloride (DAPI) (100 ng/ml;
Sigma-Aldrich) for 10 min. The cells were examined using an Eclipse
E800 fluorescence microscope equipped with a CDD camera (dS-5Mc-u1)
and NIS-Elements 3.30 image analysis system (all from Nikon, Tokyo,
Japan).
Measurement of F-actin fluorescence
intensity
The measurement of fluorescence intensity of F-actin
in MCF-7 cells was performed on fluorescent images captured at
equal camera settings using ImageJ 1.45s (NIH, Bethesda, MD, USA).
Corrected fluorescence intensity (CFI) of total, cortical or
nuclear/perinuclear F-actin was calculated using the following
formula: CFI = integrated density - (region of interest × mean
fluorescence of background), where integrated density was the area
region of interest multiplied by the mean fluorescence intensity of
F-actin.
Statistical analysis
Statistical analyses were performed by paired t-test
using graphPad Prism 5.0 (graphPad Software, La Jolla, CA, USA).
The results were considered as significant at P<0.05. The data
are presented as mean ± SD.
Results
Analysis of post-translational
downregulation of importin-9 expression and the associated
alteration in cofilin-1 expression
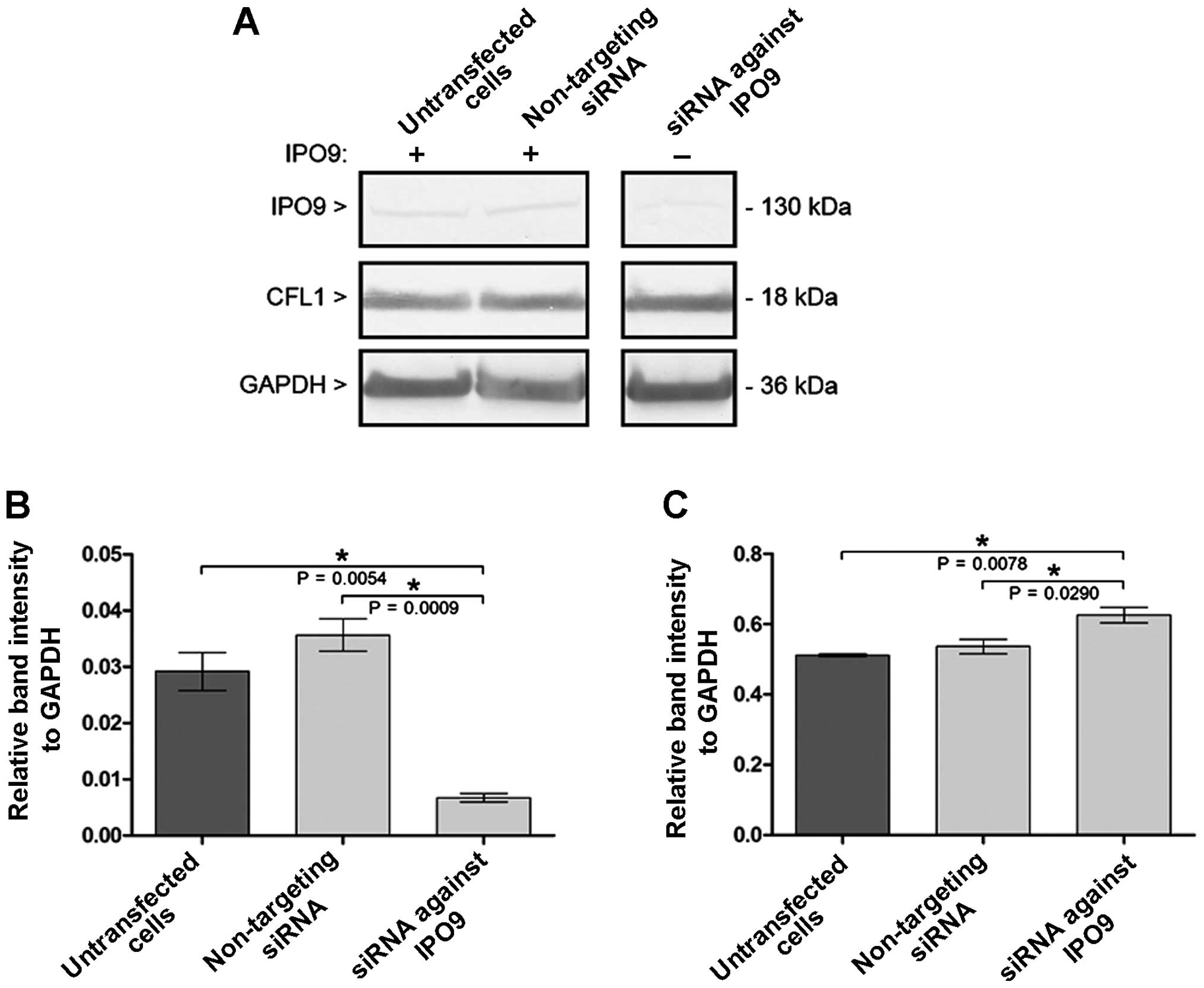
Western blot analysis confirmed that siRNA-IPO9
mediated the downregulation of IPO9 post-translational expression,
as compared with that in the untransfected cells or cells
transfected with the non-targeting siRNA (Fig. 1A). Densitometric analysis also
confirmed the decrease in initial IPO9 post-translational
expression in the cells transfected with siRNA-IPO9. Furthermore,
no statistically significant differences were observed in the
relative IPO9 to GAPDH band intensities between the untransfected
cells and cells transfected with non-targeting siRNA (Fig. 1B). As shown in Fig. 1B, the transfection of the MCF-7
cells with siRNA-IPO9 induced a statistically significant reduction
in IPO9 (23.10 and 18.91% of initial expression, as compared to
untransfected cells and cells transfected with non-targeting siRNA,
respectively) as normalized to GAPDH post-translational
expression.
Western blot analysis indicated that siRNA-mediated
downregulation of IPO9 increased the post-translational expression
of CFL1 (Fig. 1A). Moreover,
densitometric analysis also confirmed the increase of the initial
CFL1 post-translational expression in the cells transfected with
siRNA-IPO9. Furthermore, no statistically significant differences
were observed in CFL1 band intensities between the untransfected
cells and cells transfected with non-targeting siRNA relative to
GADPH (Fig. 1C). As shown in
Fig. 1C, the transfection of MCF-7
cells with siRNA-IPO9 resulted in a statistically significant
increase in the post-translational expression of CFL-1 (122.31 and
116.59% of the initial expression, as compared to the untransfected
cells and the cells transfected with non-targeting siRNA,
respectively) as normalized to GAPDH.
Downregulation of importin-9 induces
alterations in cofilin-1 protein levels and F-actin organization in
the MCF-7 cells
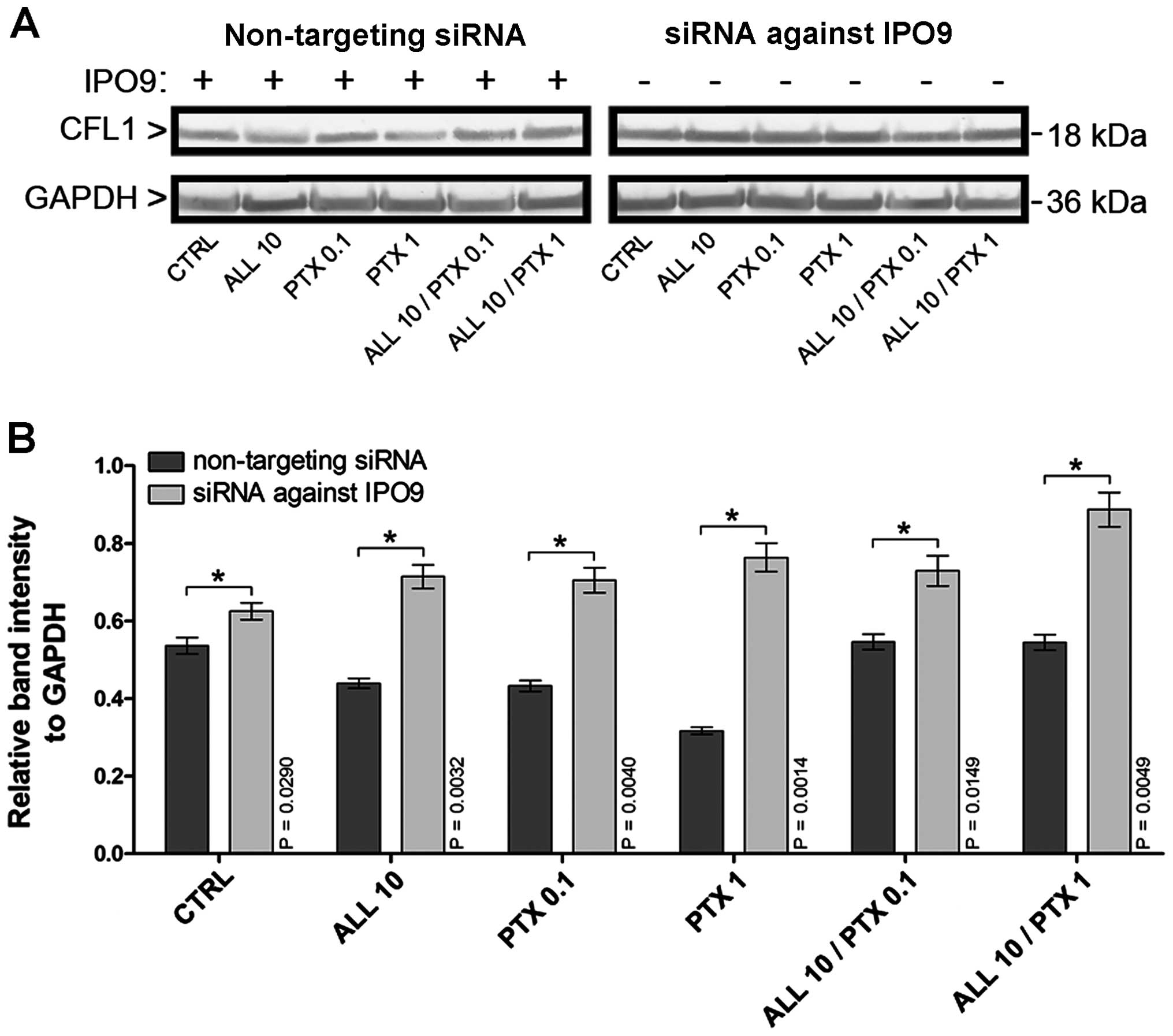
Western blot analysis showed that the downregulation
of IPO9 using siRNA-IPO9 induced alterations in the
post-translational CFL1 expression, even following the treatment of
MCF-7 cells with alliin and PTX (Fig.
2A). The densitometric analysis also confirmed the increase of
CFL1 post-translational expression in the cells transfected with
siRNA-IPO9, as compared to the cells transfected with non-targeting
siRNA (Fig. 2B). As depicted in
Fig. 2B, the downregulation of IPO9
in the control cells resulted in the increased CFL1
post-translational expression by 1.17-fold (P=0.0290), when
calculated relatively to GAPDH protein levels, respectively. In the
cell populations with downregulated IPO9 and incubated with 10
µM alliin, the post-translational CFL1 level was elevated by
1.63-fold (P=0.0032). The cells transfected with siRNA-IPO9 and
treated with 0.1 µM PTX were characterized by a similar
increase in the CFL1 protein level (1.63-fold; P=0.0040). The most
elevated CFL1 level was observed after the treatment of the IPO9
downregulated MCF-7 cells with 1 µM PTX (2.41-fold;
P=0.0014). Furthermore, after exposure of these cells to the
combination of 10 µM alliin and 0.1 µM PTX, the CFL1
protein level was increased by 1.34-fold (P=0.0149). After the
treatment of the IPO9-downregulated MCF-7 cells with the
combination of 10 µM alliin and 1 µM PTX, a 1.63-fold
(P=0.0049) increase in CFL1 protein content was observed.
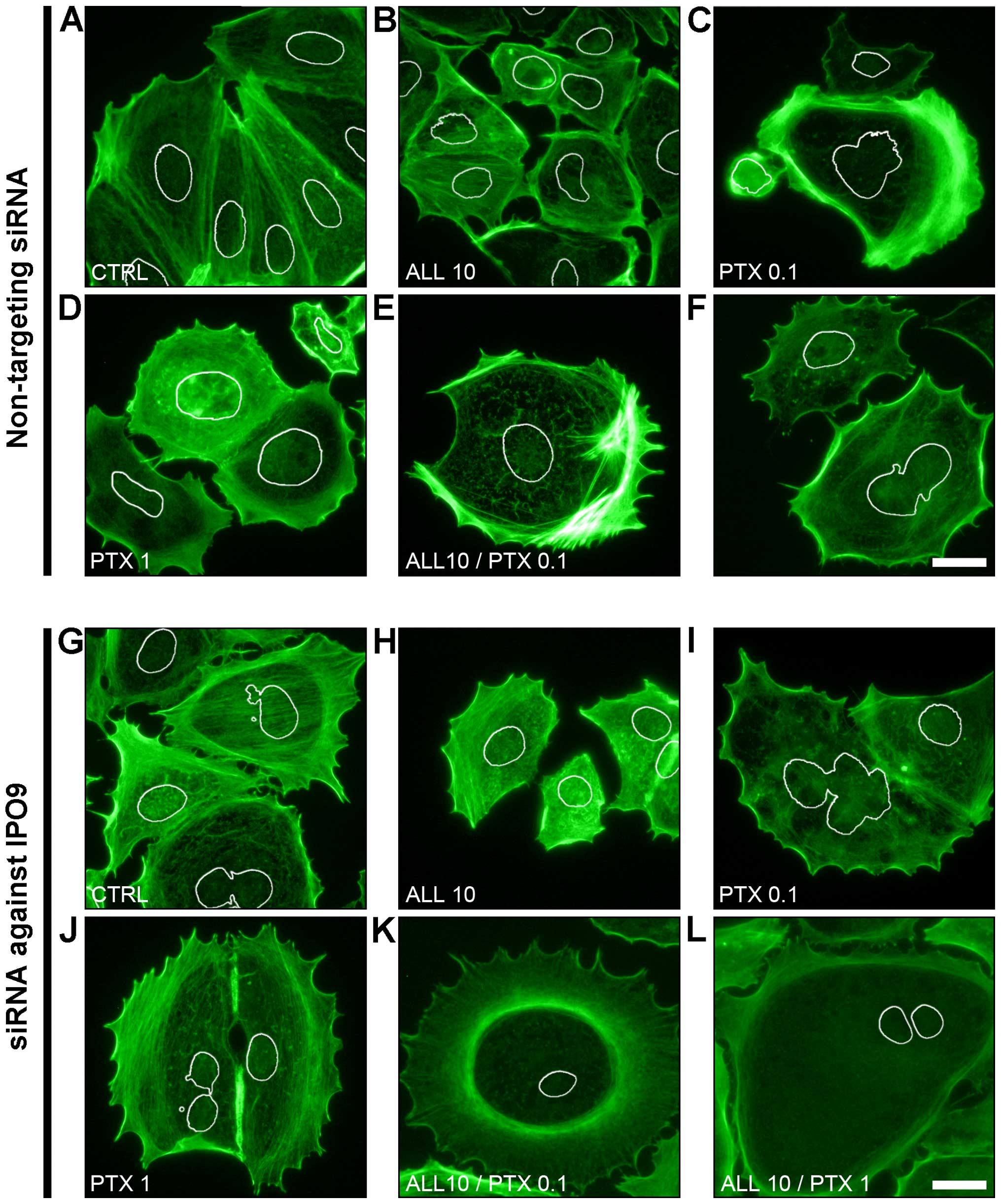
Fluorescence labeling of F-actin showed that the
downregulation of IPO9 using siRNA-IPO9 induced organizational
changes in F-actin in the MCF-7 cells, as compared to the cells
transfected with non-targeting siRNA. In the control cells
transfected with non-targeting siRNA and the cells treated with 10
µM alliin, a voluminous network of tension fibers of actin
was observed (Fig. 3A and B). In
contrast, the treatment of these cells with 0.1 µM PTX
resulted in the disorganization of F-actin, which was observed as a
diffused fluorescence signal in both the cortical and
nuclear/perinuclear areas of the cells (Fig. 3C). Furthermore, after exposure of
the cells to 1 µM PTX as well as to the combination of 10
µM alliin with 0.1 µM or 1 µM PTX, the
nuclear/perinuclear F-actin cytoskeleton was clearly seen as
disassembled or in the form of short fibers (Fig. 3d–F). However, the fluorescence
labeling of these organizational forms of actin was much higher, as
compared to the control cells and the cells treated with 10
µM alliin or 0.1 µM PTX.
Similar to the cells transfected with non-targeting
siRNA, after the induction of IPO9 downregulation, the F-actin of
the control cells was also organized in the form of tension fibers
(Fig. 3g). However, the
downregulation of IPO9 was accompanied by increased cortical actin
staining. Furthermore, the treatment of siRNA-IPO9-transfected
MCF-7 cells with 10 µM alliin or 0.1 µM PTX did not
reveal any significant differences in F-actin organization, as
compared to cells transfected with the non-targeting siRNA
(Fig. 3H and I). In contrast,
treatment of these cells with 1 µM PTX or the combination of
10 µM alliin with 0.1 µM or 1 µM PTX resulted
in a gradual disassembly of both the cortical and
nuclear/perinuclear F-actin cytoskeleton (Fig. 3J–L).
Downregulation of importin-9 reduces the
apoptotic response of MCF-7 cells to PTX and alliin which is
correlated with disassembly of nuclear/perinuclear F-actin
cytoskeleton
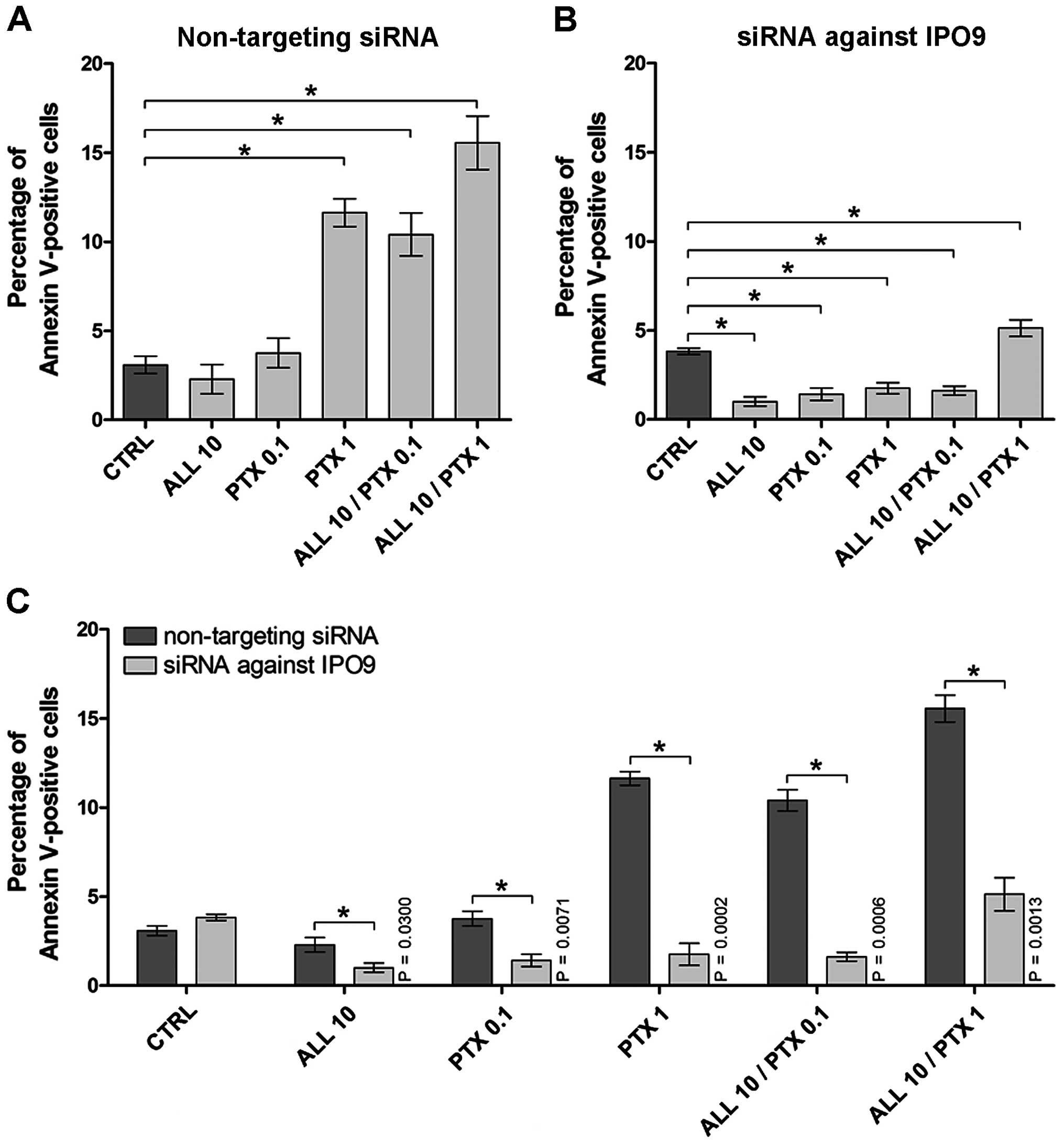
The quantitative analysis of cell death was
performed using Tali Image-based cytometer after Annexin V-Alexa
Fluor 488 and PI double staining. As shown in Fig. 4A and Table I, after the treatment of the MCF-7
cells transfected with non-targeting siRNA with 10 µM alliin
or 0.1 µM PTX, no statistically significant differences were
found in the percentage of Annexin V-positive cells. However, after
exposure of these cells to 1 µM PTX, the percentage of
Annexin V-positive cells increased by 3.78-fold (P=0.0016).
Furthermore, when the cells transfected with non-targeting siRNA
were treated with the combinations of alliin and PTX, the
percentage of Annexin V-positive cells was increased by 3.38-fold
(P=0.0005) and 5.05-fold (P=0.0014), respectively (Fig. 4A).
 | Table IP-values of changes in the percentage
of Annexin V-positive MCF-7 cells transfected with non-targeting
siRNA in response to garlic-derived alliin, paclitaxel or
combination of these agents. |
Table I
P-values of changes in the percentage
of Annexin V-positive MCF-7 cells transfected with non-targeting
siRNA in response to garlic-derived alliin, paclitaxel or
combination of these agents.
| CTRL | ALL 10 | PTX 0.1 | PTX 1 | ALL 10/PTX 0.1 | ALL 10/PTX 1 |
|---|
| CTRL | – | | | | | |
| ALL 10 | NS | – | | | | |
| PTX 0.1 | NS | P=0.0042 | – | | | |
| PTX 1 | P=0.0016 | P=0.0147 | P=0.0008 | – | | |
| ALL 10/PTX 0.1 | P=0.0005 | P=0.0006 | P=0.0013 | NS | – | |
| ALL 10/PTX 1 | P=0.0014 | P=0.0006 | P=0.0010 | P=0.0143 | P=0.0075 | – |
In contrast, after exposure of the
siRNA-IPO9-transfected cells to 10 µM alliin or 0.1
µM PTX, statistically significant differences in the
percentages of Annexin V-positive cells were noted, as compared to
the control. As shown in Fig. 4B
and Table II, a decrease in the
Annexin V-positive cell percentages after the treatment with 10
µM alliin or 0.1 µM PTX by 3.84-fold (P<0.0001) or
2.71-fold (P=0.0002), respectively, was noted. Similar, exposure of
the cells with downregulated IPO9 to 1 µM PTX resulted in a
2.18-fold (P=0.0067) reduction in Annexin V-positive cells. The
treatment of these cells with the combination of 10 µM
alliin and 0.1 µM PTX decreased the population of Annexin
V-positive cells by 2.36-fold (P<0.0001). However, a 1.34-fold
(P=0.0389) increase in the percentage of Annexin V-positive cells
was observed after treatment of the IPO9-downregulated cells with
the combination of 10 µM alliin and 1 µM PTX
(Fig. 4B). Moreover, comparison of
the percentages of Annexin V-positive cells transfected with
non-targeting siRNA and siRNA-IPO9 showed a statistically
significant reduction in apoptosis after downregulation of IPO9 and
the treatment of the cells with alliin, PTX or combination of these
agents. As shown in Fig. 4C, the
silencing of IPO9 decreased the percentage of Annexin V-positive
cells after treatment with 10 µM alliin (2.30-fold,
P=0.0300), 0.1 µM PTX (2.66-fold, P=0.0071), 1 µM PTX
(6.61-fold, P=0.0002), and after exposure of MCF-7 cells to the
combination of 10 µM alliin and 0.1 or 1 µM PTX
(6.41-fold, P=0.0006; or 3.03-fold, P=0.0013), as compared to the
cells expressing IPO9. Furthermore, a >8% reduction in Annexin
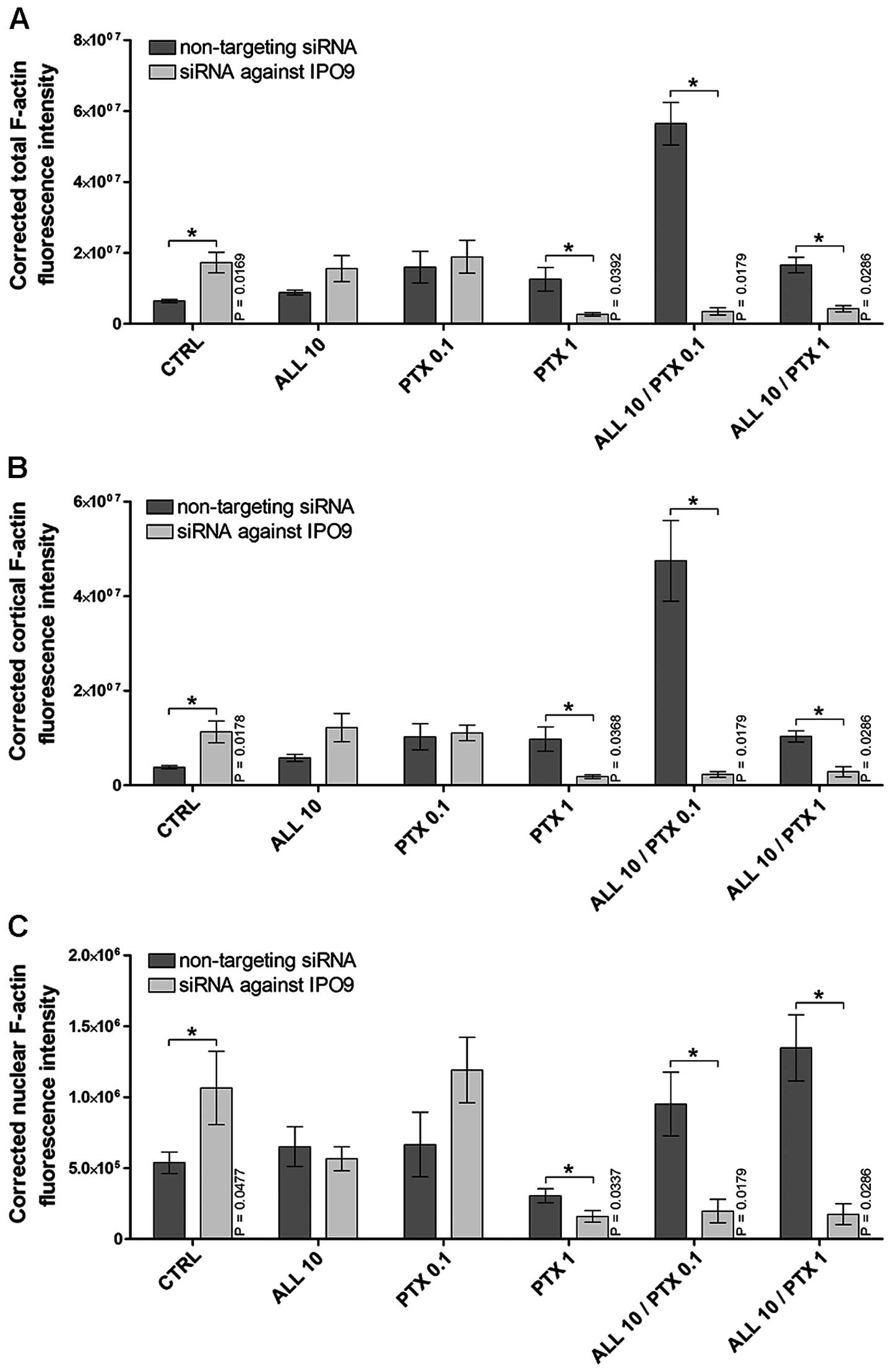
V-positive cells was correlated with a decrease in F-actin content
measured in whole cells, as well as in the cortical and
nuclear/perinuclear area of cells. This, was clearly noted after
the treatment of cells with 1 µM PTX (reduction of
4.69-fold, P=0.0392; 5.35-fold, P=0.0368; and 1.91-fold, P=0.0337,
respectively), the combination of 10 µM alliin and 0.1 PTX
(reduction of 16.02-fold; 20.46-fold; and 4.82-fold, P=0.0179,
respectively) or the combination of 10 µM alliin and 1
µM PTX (reduction of 3.89-fold, 2.61-fold and 7.71-fold,
P=0.0286, respectively) (Fig.
5A–C). The data presented here suggest that the altered import
of F-actin into the nucleus prevents apoptotic cell death.
 | Table IIP-values of changes in the percentage
of Annexin V-positive MCF-7 cells transfected with siRNA against
importin-9 in response to garlic-derived alliin, paclitaxel or
combination of these agents. |
Table II
P-values of changes in the percentage
of Annexin V-positive MCF-7 cells transfected with siRNA against
importin-9 in response to garlic-derived alliin, paclitaxel or
combination of these agents.
| CTRL | ALL 10 | PTX 0.1 | PTX 1 | ALL 10/PTX 0.1 | ALL 10/PTX 1 |
|---|
| CTRL | – | | | | | |
| ALL 10 | P<0.0001 | – | | | | |
| PTX 0.1 | P=0.0002 | P=0.0172 | – | | | |
| PTX 1 | P=0.0067 | P=0.0443 | NS | – | | |
| ALL 10/PTX 0.1 | P<0.0001 | P=0.0049 | NS | NS | – | |
| ALL 10/PTX 1 | P=0.0389 | P=0.0018 | P=0.0039 | P=0.0137 | P=0.0037 | – |
Discussion
Many authors have shown that the human breast
adenocarcinoma MCF-7 cell line may be useful for in vitro
breast cancer research (24–26).
It is known that breast cancer is one of the most common cancers
among females worldwide and its treatment is difficult (1). Therefore, new therapeutic strategies,
including the targeted silencing of gene expression using e.g. RNAi
technique have recently been developed to induce apoptosis directly
or inhibit intracellular pro-survival signaling pathways and
angiogenesis as well (27). The
list of promising targeted agents against breast cancer includes
tyrosine kinase inhibitors (TKIS), inhibitors of the PI3K/AKT/mTOR
pathway and agents that interfere with DNA repair (28). Furthermore, Foerster et al
(6) suggested that the actin
cytoskeleton can be used as a target for breast cancer therapy. The
actin cytoskeleton is a structure regulated by many proteins,
including actin binding proteins, Rho GTPases, kinase C-ε, which
offer unlimited potential for the development of anticancer
treatment strategies (6,29,30).
Research has shown that natural dietary agents,
either alone or in combination with chemotherapeutic agents, have
the potential to prevent the occurrence and/or spread of cancer
(31–33). The mechanisms leading to the
induction of apoptosis by garlic derivatives have been described in
a number of cancer cell lines, including the MCF-7 human breast
cancer cell line (15,34). Moreover, the potentiation of the
cytotoxic effects of traditional cytostatic drugs by
sulfur-containing compounds of garlic has also been documented
in vitro (35). In the
present study, we used alliin to enhance the apoptotic effect of
PTX. The study verified the alliin-mediated potentiation of
apoptosis induced by PTX. The results presented in the present
study support the potentiation of the cytotoxic effects of
traditional cytostatic drugs by sulfur-containing compounds of
garlic and indicate that alliin and PTX act synergistically to
promote apoptosis in MCF-7 cells.
A large body of literature has shown the nuclear
localization of actin (10,23,36–38).
In the nucleus, actin is involved indirectly in transcription,
since it is associated with all three RNA polymerases (Pol I, II
and III) (39,40). Percipalle et al (41) found that through the interaction
with RNA polymerase II nuclear actin is involved in transcription
processes of Balbiani rings. Actin has also been described as a
component of the BAF complex and ATP-dependent chromatin remodeling
complex SWR1 (42–45). Furthermore, Cisterna et al
(46) postulated that actin can
also be located in nucleoli where it participates in the transport
of small ribosomal subunits to the cytoplasm. Although significant
progress in this field has been made in recent years, research is
still investigating new nuclear functions of actin and the
molecular mechanisms by which actin functions within the nucleus
but also the tools that enable us to manipulate nuclear actin
concentrations without perturbing cytoplasmic actin (47). In addition, finally, we are
interested in whether such a manipulation may be employed as a
novel approach for the treatment of cancers, in particular, those
that are currently incurable.
Our previous studies demonstrated not only the
translocation of actin into the nucleus but also the involvement of
nuclear F-actin in chromatin remodeling during death processes of
different cell lines (23,38). Hofmann et al (9) suggested that nuclear actin may be
translocated from the cytoplasm into the nucleus, but it does not
have a classical nuclear localization signal itself. However, the
transport of actin is possible by its association with
actin-binding proteins which contain a functional NLS (13). As it has been shown, the export of
actin is mediated by XPO6 and the import by IPO9. However, the
mechanisms of actin translocation between the cytoplasm and nucleus
require further investigation. In the present study, we showed that
the downregulation of IPO9 using siRNA-IPO9 induced alterations in
post-translational CFL1 expression, even after the treatment of
MCF-7 cells with the combination of alliin and PTX. Pendleton et
al (48) showed that during
stress conditions actin is transported into the nucleus in a
complex with actin-binding proteins such as CFL which contains the
NLS. In contrast, the involvement of CFL in actin transport under
physiological conditions is not clear. Huet et al (49) revealed that IPO9 interacted both
with actin and CFL in a co-immunoprecipitation experiment, and
hypothesized that actin enters into the nucleus in a complex with
IPO9 and CFL. In the present study to ascertain whether
downregulation of IPO9 induces alterations in the
post-translational expression of NLS-containing proteins, the
post-translational level of CFL1 was investigated. In the present
study, we confirmed that downregulation of IPO9 increased the
post-translational expression of CFL1.
Our previous studies showed that CFL1 mediates
apoptosis in response to stress conditions (50). Similarly, Huot et al
(51) observed actin at the border
of the apoptotic blebs whereas Levee et al (52) revealed its accumulation in the area
of apoptotic body formation and suggested that the reorganization
of the F-actin network is essential for this process. Furthermore,
Chua et al (53)
demonstrated that CFL has an important function during the initial
phase of apoptosis. Moreover, oxidant-induced apoptosis and
mitochondrial integrity can be regulated through oxidation of CFL
(54). We also demonstrated that
doxorubicin treatment of CHO AA8 cells with the functional
expression of CFL1 induced both apoptosis and mitotic catastrophe
as the main types of cell death. In contrast, the downregulation of
CFL1 preferentially induced mitotic catastrophe as
doxorubicin-induced cell death (50). We also showed that the presence of
actin is important for induction of active cell death (23). Furthermore, we observed an increase
in nuclear F-actin labeling after the induction of CFL1 expression
in MCF-7 cells. Vartiainen (13)
suggested that such an effect was possibly associated with the
involvement of CFL1 in the transport of actin monomers to the cell
nucleus and their reassembly into short polymers. In the present
study, we showed a significant reduction in both cortical and
nuclear F-actin fluorescence in cells with downregulated expression
of IPO9 after treatment of cells with a high dose of PTX as well as
the combination of PTX and alliin. Similarly, the downregulation of
IPO9 resulted in significant reduction in apoptosis induced by PTX,
allin or a high apoptosis-inducing combination of PTX and alliin.
These data suggest that CFL1 itself does not translocate actin into
the cell nucleus but this transport requires the functional
expression of IPO9.
In conclusion, the results presented here showed
that alliin and PTX act synergistically to promote apoptosis in
MCF-7 cells and support the potentiation of the cytotoxic effects
of traditional cytostatic drugs by sulfur-containing compounds of
garlic. As it has been shown by many authors, actin may translocate
into the cell nucleus to function as a transcriptional modulator of
gene expression (47,55–58).
Our previous studies demonstrated not only the translocation of
F-actin into the nucleus but also its involvement in chromatin
remodeling during cell death processes (23,38,50,59,60).
In the present study, we revealed that the downregulation of IPO9
correlates with the significant reduction in the apoptotic cell
population as well as with the decrease in F-actin content in whole
cells, and in cortical and nuclear/perinuclear areas of MCF-7
cells. Simultaneously, the downregulation of IPO9 was also
accompanied by the increased post-translational expression of CFL1.
Therefore, the obtained data suggest that CFL1 itself does not
translocate actin into the cell nucleus but this transport requires
the functional expression of IPO9.
Acknowledgments
The present study was supported by grant no. MN-5/WL
from Nicolaus Copernicus university (NCU).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carey LA, Perou CM, Livasy CA, Dressler
LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S,
et al: Race, breast cancer subtypes, and survival in the Carolina
Breast Cancer Study. JAMA. 295:2492–2502. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Baselga J, Gelmon KA, Verma S, Wardley A,
Conte P, Miles D, Bianchi G, Cortes J, McNally VA, Ross GA, et al:
Phase II trial of pertuzumab and trastuzumab in patients with human
epidermal growth factor receptor 2-positive metastatic breast
cancer that progressed during prior trastuzumab therapy. J Clin
Oncol. 28:1138–1144. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tutt A, Robson M, Garber JE, Domchek SM,
Audeh MW, Weitzel JN, Friedlander M, Arun B, Loman N, Schmutzler
RK, et al: Oral poly(ADP-ribose) polymerase inhibitor olaparib in
patients with BRCA1 or BRCA2 mutations and advanced breast cancer:
A proof-of-concept trial. Lancet. 376:235–244. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Macaskill EJ, Bartlett JM, Sabine VS,
Faratian D, Renshaw L, White S, Campbell FM, Young O, Williams L,
Thomas JS, et al: The mammalian target of rapamycin inhibitor
everolimus (RAD001) in early breast cancer: Results of a
pre-operative study. Breast Cancer Res Treat. 128:725–734. 2011.
View Article : Google Scholar
|
|
6
|
Foerster F, Braig S, Moser C, Kubisch R,
Busse J, Wagner E, Schmoeckel E, Mayr D, Schmitt S, Huettel S, et
al: Targeting the actin cytoskeleton: Selective antitumor action
via trapping PKCε. Cell Death Dis. 5:e13982014. View Article : Google Scholar
|
|
7
|
Groth-Pedersen L, Aits S, Corcelle-Termeau
E, Petersen NH, Nylandsted J and Jäättelä M: Identification of
cytoskeleton-associated proteins essential for lysosomal stability
and survival of human cancer cells. PLoS One. 7:e453812012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dingová H, Fukalová J, Maninová M,
Philimonenko VV and Hozák P: Ultrastructural localization of actin
and actin-binding proteins in the nucleus. Histochem Cell Biol.
131:425–434. 2009. View Article : Google Scholar
|
|
9
|
Hofmann WA, Arduini A, Nicol SM, Camacho
CJ, Lessard JL, Fuller-Pace FV and de Lanerolle P: SUMOylation of
nuclear actin. J Cell Biol. 186:193–200. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dopie J, Skarp KP, Rajakylä EK, Tanhuanpää
K and Vartiainen MK: Active maintenance of nuclear actin by
importin 9 supports transcription. Proc Natl Acad Sci USA.
109:E544–E552. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bamburg JR and Wiggan OP: ADF/cofilin and
actin dynamics in disease. Trends Cell Biol. 12:598–605. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Theriot JA: Accelerating on a treadmill:
ADF/cofilin promotes rapid actin filament turnover in the dynamic
cytoskeleton. J Cell Biol. 136:1165–1168. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vartiainen MK: Nuclear actin dynamics-from
form to function. FEBS Lett. 582:2033–2040. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vartiainen MK, Mustonen T, Mattila PK,
Ojala PJ, Thesleff I, Partanen J and Lappalainen P: The three mouse
actin-depolymerizing factor/cofilins evolved to fulfill
cell-type-specific requirements for actin dynamics. Mol Biol Cell.
13:183–194. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oommen S, Anto RJ, Srinivas G and
Karunagaran D: Allicin (from garlic) induces caspase-mediated
apoptosis in cancer cells. Eur J Pharmacol. 485:97–103. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu B, Monsarrat B, Gairin JE and
Girbal-Neuhauser E: Effect of ajoene, a natural antitumor small
molecule, on human 20S proteasome activity in vitro and in human
leukemic HL60 cells. Fundam Clin Pharmacol. 18:171–180. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vinay K and Singh DK: Pharmacological
effects of garlic (Allium sativum L.). ARBS. 10:6–26. 2008.
|
|
18
|
Chu Q, Ling MT, Feng H, Cheung HW, Tsao
SW, Wang X and Wong YC: A novel anticancer effect of garlic
derivatives: Inhibition of cancer cell invasion through restoration
of E-cadherin expression. Carcinogenesis. 27:2180–2189. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Omar SH and Al-Wabel NA: Organosulfur
compounds and possible mechanism of garlic in cancer. Saudi Pharm
J. 18:51–58. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kelkel M, Cerella C, Mack F, Schneider T,
Jacob C, Schumacher M, Dicato M and Diederich M: ROS-independent
JNK activation and multisite phosphorylation of Bcl-2 link diallyl
tetrasulfide-induced mitotic arrest to apoptosis. Carcinogenesis.
33:2162–2171. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Surapaneni MS, Das SK and Das NG:
Designing paclitaxel drug delivery systems aimed at improved
patient outcomes: Current status and challenges. ISRN Pharmacol.
2012:6231392012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Barbuti AM and Chen ZS: Paclitaxel through
the ages of anticancer therapy: Exploring its role in
chemoresistance and radiation therapy. Cancers. 7:2360–2371. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Grzanka D, Izdebska M,
Klimaszewska-Wisniewska A and Gagat M: The alterations in SATB1 and
nuclear F-actin expression affect apoptotic response of the MCF-7
cells to geldanamycin. Folia Histochem Cytobiol. 53:79–87. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lamparska-Przybysz M, Gajkowska B and
Motyl T: BID-deficient breast cancer MCF-7 cells as a model for the
study of autophagy in cancer therapy. Autophagy. 2:47–48. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bullinger D, Neubauer H, Fehm T, Laufer S,
Gleiter CH and Kammerer B: Metabolic signature of breast cancer
cell line MCF-7: Profiling of modified nucleosides via LC-IT MS
coupling. BMC Biochem. 8:252007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Holliday DL and Speirs V: Choosing the
right cell line for breast cancer research. Breast Cancer Res.
13:2152011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schlotter CM, Vogt U, Allgayer H and
Brandt B: Molecular targeted therapies for breast cancer treatment.
Breast Cancer Res. 10:2112008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Higgins MJ and Baselga J: Targeted
therapies for breast cancer. J Clin Invest. 121:3797–3803. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
dos Remedios CG, Chhabra D, Kekic M,
Dedova IV, Tsubakihara M, Berry DA and Nosworthy NJ: Actin binding
proteins: Regulation of cytoskeletal microfilaments. Physiol Rev.
83:433–473. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kaibuchi K, Kuroda S and Amano M:
Regulation of the cytoskeleton and cell adhesion by the Rho family
GTPases in mammalian cells. Annu Rev Biochem. 68:459–486. 1999.
View Article : Google Scholar
|
|
31
|
Surh YJ: Cancer chemoprevention with
dietary phytochemicals. Nat Rev Cancer. 3:768–780. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Khan N, Afaq F and Mukhtar H: Cancer
chemoprevention through dietary antioxidants: Progress and promise.
Antioxid Redox Signal. 10:475–510. 2008. View Article : Google Scholar
|
|
33
|
Mukhtar E, Adhami VM, Khan N and Mukhtar
H: Apoptosis and autophagy induction as mechanism of cancer
prevention by naturally occurring dietary agents. Curr drug
Targets. 13:1831–1841. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Na HK, Kim EH, Choi MA, Park JM, Kim DH
and Surh YJ: Diallyl trisulfide induces apoptosis in human breast
cancer cells through ROS-mediated activation of JNK and AP-1.
Biochem Pharmacol. 84:1241–1250. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Howard EW, Lee DT, Chiu YT, Chua CW, Wang
X and Wong YC: Evidence of a novel docetaxel sensitizer,
garlic-derived S-allylmercaptocysteine, as a treatment option for
hormone refractory prostate cancer. Int J Cancer. 122:1941–1948.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Grosse R and Vartiainen MK: To be or not
to be assembled: Progressing into nuclear actin filaments. Nat Rev
Mol Cell Biol. 14:693–697. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kapoor P and Shen X: Mechanisms of nuclear
actin in chromatin-remodeling complexes. Trends Cell Biol.
24:238–246. 2014. View Article : Google Scholar :
|
|
38
|
Grzanka D, Gagat M and Izdebska M:
Involvement of the SATB1/F-actin complex in chromatin
reorganization during active cell death. Int J Mol Med.
33:1441–1450. 2014.PubMed/NCBI
|
|
39
|
Hu P, Wu S and Hernandez N: A role for
beta-actin in RNA polymerase III transcription. Genes Dev.
18:3010–3015. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fomproix N and Percipalle P: An
actin-myosin complex on actively transcribing genes. Exp Cell Res.
294:140–148. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Percipalle P, Zhao J, Pope B, Weeds A,
Lindberg U and Daneholt B: Actin bound to the heterogeneous nuclear
ribonucleoprotein hrp36 is associated with Balbiani ring mRNA from
the gene to polysomes. J Cell Biol. 153:229–236. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhao K, Wang W, Rando OJ, Xue Y, Swiderek
K, Kuo A and Crabtree GR: Rapid and phosphoinositol-dependent
binding of the SWI/SNF-like BAF complex to chromatin after T
lymphocyte receptor signaling. Cell. 95:625–636. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rando OJ, Zhao K, Janmey P and Crabtree
GR: Phosphatidylinositol-dependent actin filament binding by the
SWI/SNF-like BAF chromatin remodeling complex. Proc Natl Acad Sci
USA. 99:2824–2829. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Morrison AJ and Shen X: Chromatin
remodelling beyond transcription: The INO80 and SWR1 complexes. Nat
Rev Mol Cell Biol. 10:373–384. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bogolyubova I: F-actin distribution
pattern in the nuclei of early mouse embryos. Folia Histochem
Cytobiol. 47:461–463. 2009.
|
|
46
|
Cisterna B, Necchi D, Prosperi E and
Biggiogera M: Small ribosomal subunits associate with nuclear
myosin and actin in transit to the nuclear pores. FASEB J.
20:1901–1903. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Olave IA, Reck-Peterson SL and Crabtree
GR: Nuclear actin and actin-related proteins in chromatin
remodeling. Annu Rev Biochem. 71:755–781. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pendleton A, Pope B, Weeds A and Koffer A:
Latrunculin B or ATP depletion induces cofilin-dependent
translocation of actin into nuclei of mast cells. J Biol Chem.
278:14394–14400. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Huet G, Rajakylä EK, Viita T, Skarp KP,
Crivaro M, Dopie J and Vartiainen MK: Actin-regulated feedback loop
based on Phactr4, PP1 and cofilin maintains the actin monomer pool.
J Cell Sci. 126:497–507. 2013. View Article : Google Scholar
|
|
50
|
Grzanka D, Marszałek A, Gagat M, Izdebska
M, Gackowska L and Grzanka A: Doxorubicin-induced F-actin
reorganization in cofilin-1 (nonmuscle) down-regulated CHO AA8
cells. Folia Histochem Cytobiol. 48:377–386. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Huot J, Houle F, Rousseau S, Deschesnes
RG, Shah GM and Landry J: SAPk2/p38-dependent F-actin
reorganization regulates early membrane blebbing during
stress-induced apoptosis. J Cell Biol. 143:1361–1373. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Levee MG, Dabrowska MI, Lelli JL Jr and
Hinshaw DB: Actin polymerization and depolymerization during
apoptosis in HL-60 cells. Am J Physiol. 271:C1981–C1992.
1996.PubMed/NCBI
|
|
53
|
Chua BT, Volbracht C, Tan KO, Li R, Yu VC
and Li P: Mitochondrial translocation of cofilin is an early step
in apoptosis induction. Nat Cell Biol. 5:1083–1089. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Klamt F, Zdanov S, Levine RL, Pariser A,
Zhang Y, Zhang B, Yu LR, Veenstra TD and Shacter E: Oxidant-induced
apoptosis is mediated by oxidation of the actin-regulatory protein
cofilin. Nat Cell Biol. 11:1241–1246. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Gieni RS and Hendzel MJ: Actin dynamics
and functions in the interphase nucleus: Moving toward an
understanding of nuclear polymeric actin. Biochem Cell Biol.
87:283–306. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Louvet E and Percipalle P: Transcriptional
control of gene expression by actin and myosin. Int Rev Cell Mol
Biol. 272:107–147. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Qi T, Tang W, Wang L, Zhai L, Guo L and
Zeng X: G-actin participates in RNA polymerase II-dependent
transcription elongation by recruiting positive transcription
elongation factor b (P-TEFb). J Biol Chem. 286:15171–15181. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Aoyama K, Yuki R, Horiike Y, Kubota S,
Yamaguchi N, Morii M, Ishibashi K, Nakayama Y, Kuga T, Hashimoto Y,
et al: Formation of long and winding nuclear F-actin bundles by
nuclear c-Abl tyrosine kinase. Exp Cell Res. 319:3251–3268. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Izdebska M, Grzanka D, Gagat M, Gackowska
L and Grzanka A: The effect of G-CSF on F-actin reorganization in
HL-60 and K562 cell lines. Oncol Rep. 28:2138–2148. 2012.PubMed/NCBI
|
|
60
|
Izdebska M, Gagat M, Grzanka D and Grzanka
A: Ultrastructural localization of F-actin using phalloidin and
quantum dots in HL-60 promyelocytic leukemia cell line after cell
death induction by arsenic trioxide. Acta Histochem. 115:487–495.
2013. View Article : Google Scholar : PubMed/NCBI
|