Introduction
A complete pathologic response of breast cancer
after neoadjuvant chemotherapy (NAC) appears to be particularly
favorable at 5 years in patients with the least evidence of a tumor
in the breast or lymph nodes after therapy (1,2). The
National Surgical Adjuvant Breast and Bowel Project trials
distinguished between the absence and presence of residual invasive
carcinoma in the breast and noted that long-term outcome was also
dependent on the extent of lymph node involvement (1). Axillary lymph node metastasis after
NAC in breast cancer is a poor prognostic factor (2). Residual micro-metastatic disease in
the axillary lymph nodes after NAC is a worse prognostic factor
than negative nodes in breast cancer (3). Therefore, prediction of lymph node
metastasis is important for prognosis and choosing an optimal
therapeutic strategy for the treatment of breast cancer after
NAC.
Previous reports have described predictive factors
of complete pathological response in primary breast tumors after
NAC (4). Judgement of pathological
therapeutic effects of breast cancer after NAC vary among studies
(5–12). However, no article has described
residual carcinoma patterns of the primary breast tumor to predict
the presence or absence of lymph node metastasis. Therefore, we
devised a classification system of three residual carcinoma
patterns using a simple method based on cell density: dense,
focal/nested, and sporadic/in-situ. In this study, we
examined 50 cases of breast cancer and associated dissected lymph
nodes after NAC and divided the cases into an eradicated lymph node
group (14 cases), a residual lymph node group (26 cases), and a no
change lymph node group (10 cases) to compare differences in the
three residual carcinoma patterns in primary breast tumor between
the eradicated lymph node and residual lymph node groups.
Furthermore, we analyzed differences in clinicopathological factors
in residual carcinoma patterns of the primary breast tumors.
Materials and methods
Patient samples
We retrospectively evaluated computed tomography
(CT) or positron emission tomography-computed tomography (PET-CT)
scans of 50 surgically resected breast cancer lesions taken before
and after NAC between 2006 and 2015 at Hirosaki University Hospital
(Hirosaki, Aomori, Japan). Informed consent was obtained from each
patient regarding the use of clinical records and pathological
specimens. Twenty-nine patients underwent total mastectomy, and 21
underwent partial mastectomy. Sentinel node biopsy was performed
for 2 cases, level I lymph node dissection for 48 cases, level II
lymph node dissection for 23 cases, level III lymph node dissection
for 3 cases, and Rotter dissection for 2 cases. Chemotherapy
regimens are shown in Table I. The
chemotherapy regimens were performed as follows: AC (doxorubicin +
cyclophosphamide), 4 cases (8%); AC→T (doxorubicin +
cyclophosphamide followed by taxane), 24 cases (48%); AC→T + HER
(doxorubicin + cyclophosphamide followed by taxane + trastuzumab),
12 cases (24%); EC (epirubicin hydrochloride + cyclophosphamide
hydrate), 2 cases (4%); EC→T (epirubicin hydrochloride +
cyclophosphamide hydrate followed by taxane), 7 cases (14%); and TC
(docetaxel + cyclophosphamide hydrate), 1 case (2%). The mean
number of cycles per regimen was 7.66 (range, 4–8). Union for
International Cancer Control (UICC) stages of before and after NAC
are shown in Table II. UICC
clinical stages (cStage) before NAC were as follows: cStage 0, 0
cases (0%); cStage I, 2 cases (4%); cStage II, 26 cases (52%); and
cStage III, 22 cases (44%). UICC cStages after NAC were as follows:
cStage 0, 0 cases (0%); cStage I, 16 cases (32%); cStage II, 24
cases (48%); and cStage III, 9 cases (18%). UICC pathological
stages (pStage) after NAC were as follows: pStage 0, 4 cases (8%);
pStage I, 13 cases (26%); pStage II, 18 (36%); and pStage III, 15
cases (30%).
 | Table IChemotherapy regimens and cycles. |
Table I
Chemotherapy regimens and cycles.
| Chemotherapy
regimen | n (%) |
|---|
| AC | 4 (8) |
| AC→T | 24 (48) |
| AC→T + HER | 12 (24) |
| EC | 2 (4) |
| EC→T | 7 (14) |
| TC | 1 (2) |
| Mean cycles
(range) | 7.66 (4–8) |
 | Table IIUICC stage before and after NAC. |
Table II
UICC stage before and after NAC.
| UICC cStage before
NAC n (%) | UICC cStage after NAC
n (%) | UICC pStage after NAC
n (%) |
|---|
| Stage 0 | 0 (0) | 0 (0) | 4 (8) |
| Stage I | 2 (4) | 16 (32) | 13 (26) |
| Stage II | 26 (52) | 24 (48) | 18 (36) |
| Stage III | 22 (44) | 9 (18) | 15 (30) |
Pathological examinations of the primary
tumors
Partial mastectomy specimens were sliced into 5 mm
sections and total mastectomy specimens, which included the maximal
tumor sectioned surface, were sliced as much as possible to
distinguish the lesion. For histopathological examination, breast
cancer specimens were routinely formalin-fixed, paraffin-embedded,
thinly sectioned, and stained with hematoxylin and eosin. Carcinoma
lesions were histologically graded according to the
Bloom-Richardson system (13).
Tumor maximal invasion diameters were measured and the extent of
lymphatic invasion, venous invasion and intraductal component was
evaluated. The status of the estrogen receptor (ER), progesterone
receptor (PgR), and HER2 was immunohistochemically detected. ER/PR
expression was defined as positive when ≥10% of nuclei in the total
tumor cells were stained. As to HER2: 1, negative, 2, uncertain,
and 3, positive. Cancers with an HER2 score of 2+ were additionally
evaluated using dual-color in situ hybridization. In this
study, breast cancer was classified into four groups as follows:
luminal A (ER and/PR-positive/HER2-negative/low Ki-67), luminal B
(ER- and/or PR-positive/HER2-negative/high Ki-67), luminal B (ER-
and/or PR-positive/HER2 overexpression/any Ki-67), HER2 (ER and PR
absent/HER2 overexpression), and triple-negative (ER and PR
absent/HER2-negative) (14).
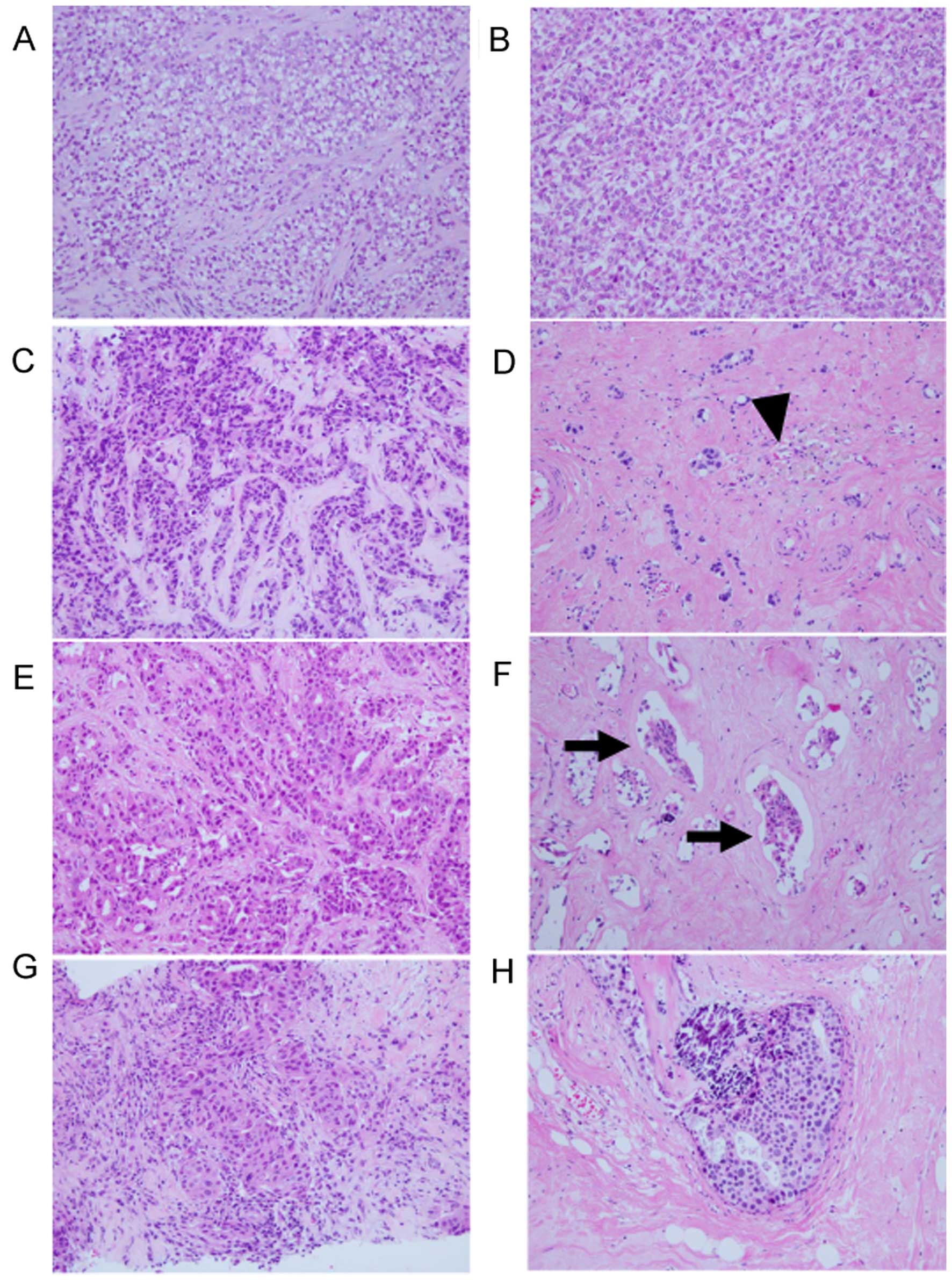
Residual carcinoma patterns
Whole sections examined in this study were divided
into three residual carcinoma patterns: dense (tumor cell density
remained in a very high state), focal/nested (tumor cells
disappeared focally with fibrosis or granulation tissue or
macrophage infiltration, or elastic fiber within the tumor area),
and sporadic/in situ (a few cancer nests remained or in
situ lesion only remained). If a biopsy was performed before
chemotherapy, we compared the surgically resected specimen with its
biopsy results as much as possible (Fig. 1). Forty biopsies of primary tumors
before NAC were evaluated in this study. Focal/nested and
sporadic/in-situ patterns were included in the non-dense
group when we analyzed residual carcinoma patterns and
clinicopathological factors.
Pathological examination of lymph
nodes
We chose lymph nodes from 14 cases that were
examined by fine needle aspiration biopsy cytology or core needle
biopsy before NAC. Five cases that were positive by lymph node
biopsy before NAC and in which lymph node metastasis disappeared
after NAC were histologically examined in detail. Lymph nodes that
were more than 5% fibrotic were defined as eradicated lymph nodes.
We did not consider fibrosis of the lymph node capsule. We chose
lymph node specimens with irregular fibrosis. The sections were
scanned using light microscopy at a low magnification (×12.5 or
×40). The composition of the image was carried out in Adobe
Photoshop (Adobe® Photoshop® CS2
Windows® USA). The extent of fibrosis within the lymph
nodes was measured using ImageJ software (15). Three cases that were negative by
lymph node biopsy before NAC and negative for lymph node metastasis
after NAC were also histologically examined in detail. 'No change'
lymph nodes included those with few hemosiderin laden macrophages
and no fibrosis.
Division of the eradicated and residual
lymph node groups
Fifty breast cancer cases after NAC were divided
into an eradiated lymph node group, a residual lymph node group,
and a no change lymph node group. The no change lymph node cases
were excluded in this study. Differences in the three residual
carcinoma patterns (dense, focal/nested, and
sporadic/in-situ) between the eradiated lymph node and
residual lymph node groups were examined.
Residual carcinoma patterns and
clinicopathological factors
Residual carcinoma patterns were divided into two
groups, a dense group and a non-dense group. Focal/nested and
sporadic/in-situ patterns were defined as the non-dense
group. Differences in the following clinicopathological factors
between the dense and non-dense groups were examined: trastuzumab
administration, reduced ratio on CT, primary tumor area
before/after NAC on CT, intrinsic subtype, ER status, PgR status,
HER2 status, Ki-67 labeling index, primary tumor pathological
diameter, lymphatic invasion, venous invasion, histological grade,
nuclear atypia, mitotic count, tubular formation, and extent of
intraductal components. Primary breast tumor areas before and after
NAC were calculated using DICOM data on CT images. The primary
tumor area was calculated three times for each case and the mean
value was used for analysis. The reduced ratio was calculated using
the mean area of the primary breast tumor before and after NAC as
follows: Reduced ratio = (1 - mean area after NAC/mean area before
NAC) × 100.
Statistical analyses
Statistical comparisons between two groups were
analyzed using the Pearson's Chi-square test for categorical data
and the Wilcoxon rank sum test for continuous data. Differences
were considered to be statistically significant at a p-value of
<0.05. An adjusted residue of ±2 or more was considered to be
significant. All statistical evaluations were performed using R
(http://www.r-project.org) and IBM®
SPSS® Statistics version 22 (IBM Corporation, Armonk,
NY, USA) software.
Results
Tumor characteristics
Tumor characteristics are shown in Table III. Intrinsic subtype included:
luminal A, 13 cases (26%); luminal B, 28 cases (56%); HER2 type, 4
cases (8%) and triple-negative, 5 cases (10%). Histology included:
ductal carcinoma in situ, 4 cases (4%); invasive ductal
carcinoma, 38 cases (76%); invasive lobular carcinoma, 4 cases
(8%); invasive micropapillary carcinoma, 1 case (2%); mucinous
carcinoma, 2 cases (4%) and intracystic papillary carcinoma, 1 case
(2%). Lymph node status included: negative, 26 cases (52%) and
positive, 24 cases (48%). Lymphatic invasion included: negative, 31
cases (62%) and positive, 19 cases (38%). Venous invasion included:
negative, 46 cases (92%) and positive, 4 cases (8%). Histological
grade included: I, 15 cases (30%); II, 27 cases (54%) and III, 8
cases (16%). Nuclear atypia included: 1, 3 cases (6%); 2, 27 cases
(54%) and 3, 20 cases (40%). Mitotic count included: 1, 32 cases
(64%); 2, 14 cases (28%) and 3, 4 cases (8%). Tubular formation
included: 1, 1 case (2%); 2, 23 cases (46%) and 3, 26 cases (52%).
Extensive intraductal components included: negative, 38 cases (76%)
and positive, 12 cases (24%).
 | Table IIIHistological findings of the surgical
resection specimens after neoadjuvant chemotherapy. |
Table III
Histological findings of the surgical
resection specimens after neoadjuvant chemotherapy.
| Features | n=50 (100%) |
|---|
| Intrinsic
subtype |
| Luminal A | 13 (26) |
| Luminal B | 28 (56) |
| HER2 | 4 (8) |
| Triple-negative | 5 (10) |
| Histology |
| Ductal carcinoma
in situ | 4 (4) |
| Invasive ductal
carcinoma | 38 (76) |
| Invasive lobular
carcinoma | 4 (8) |
| Invasive
micropapillary carcinoma | 1 (2) |
| Mucinous
carcinoma | 2 (4) |
| Intracystic
papillary carcinoma | 1 (2) |
| Lymph node
status |
| Negative | 26 (52) |
| Positive | 24 (48) |
| Lymphatic
invasion |
| Negative | 31 (62) |
| Positive | 19 (38) |
| Venous invasion |
| Negative | 46 (92) |
| Positive | 4 (8) |
| Histological
grade |
| I | 15 (30) |
| II | 27 (54) |
| III | 8 (16) |
| Nuclear atypia |
| 1 | 3 (6) |
| 2 | 27 (54) |
| 3 | 20 (40) |
| Mitotic count |
| 1 | 32 (64) |
| 2 | 14 (28) |
| 3 | 4 (8) |
| Tubular
formation |
| 1 | 1 (2) |
| 2 | 23 (46) |
| 3 | 26 (52) |
| Extensive intraductal
component |
| Negative | 38 (76) |
| Positive | 12 (24) |
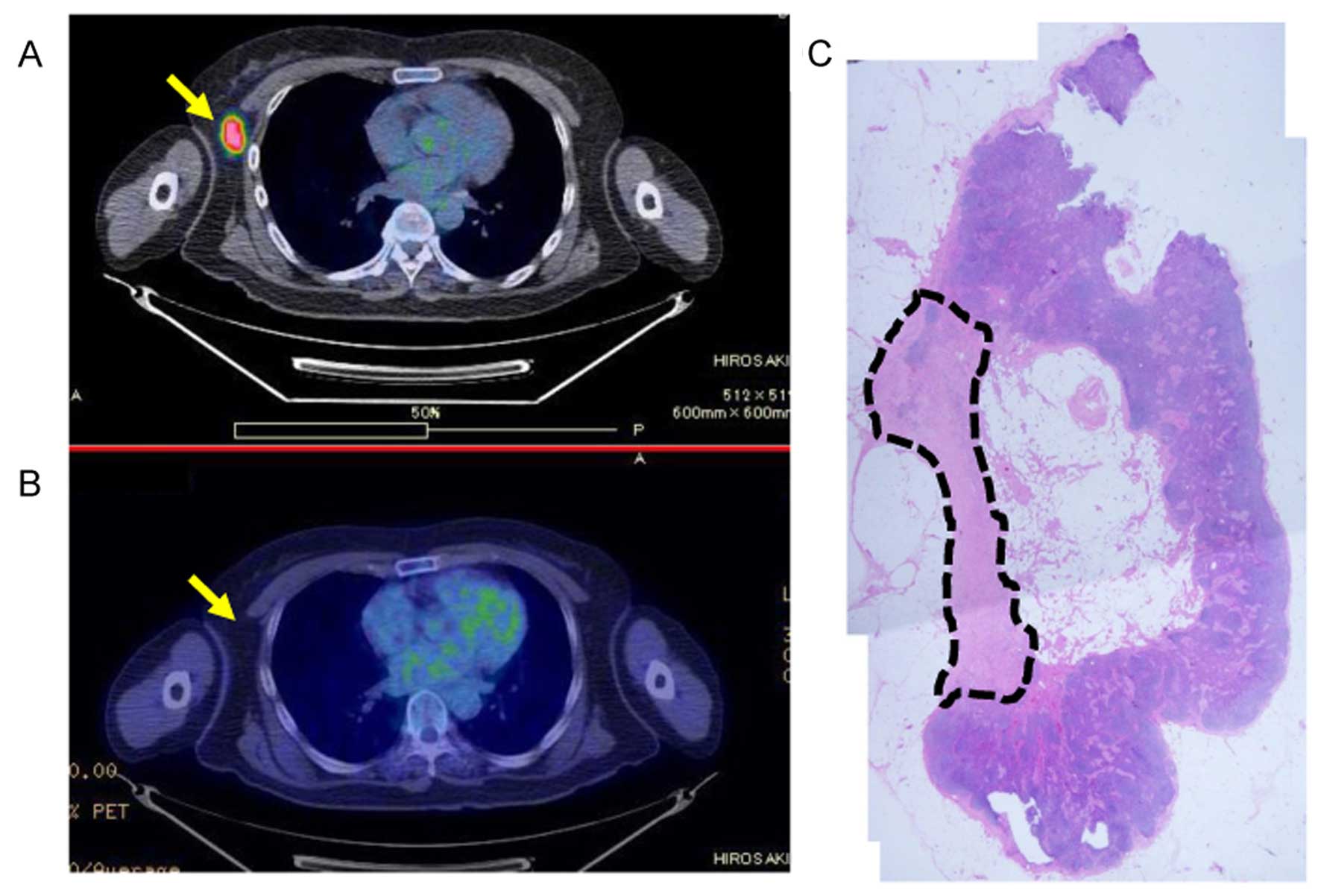
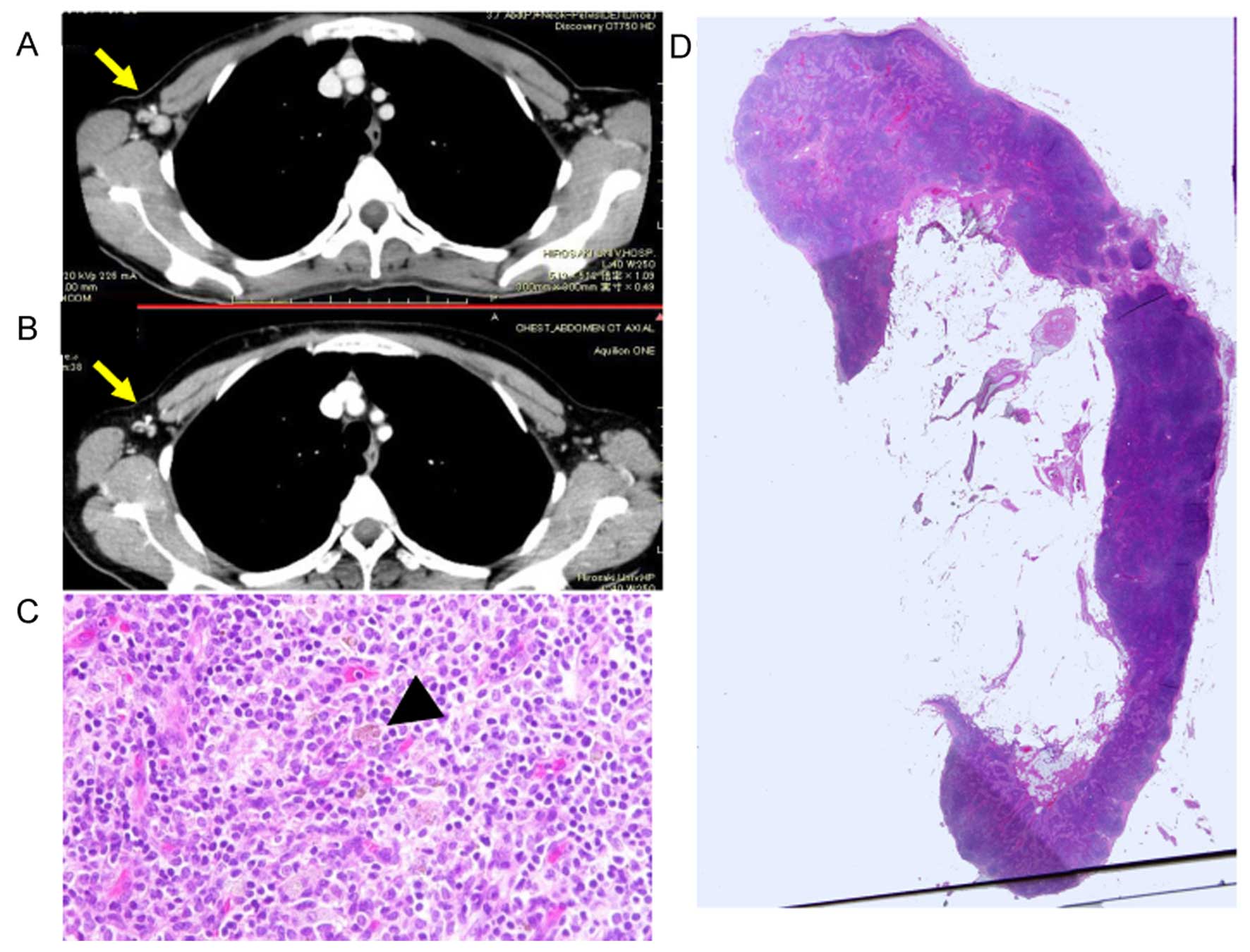
Eradicated lymph node
Fine needle aspiration biopsy cytology or core
needle biopsy of five eradicated lymph node cases performed before
NAC showed fibrosis within the lymph nodes. The percentage of
fibrosis of the five lymph nodes was 34.6, 19.8, 14.0, 9.6, and
5.3%, respectively. A representative specimen of an eradicated
lymph node with 19.8% fibrosis within the lymph node is shown in
Fig. 2. Three cases with lymph
nodes negative before NAC and no lymph node metastasis after NAC
had only a few hemosiderin laden macrophages and no fibrosis
(Fig. 3). These lymph nodes were
classified as no change lymph nodes.
Residual carcinoma patterns and lymph
node metastasis of the eradicated and residual lymph node
groups
There were significant differences in the residual
patterns (P=0.0034) between the eradicated and residual lymph node
groups as many of the residual lymph nodes had dense patterns
(adjusted residue +2.3) and many of the eradicated lymph nodes had
sporadic/in-situ patterns (adjusted residue +2.9) (Table IV).
 | Table IVResidual carcinoma patterns in primary
breast tumors and lymph node metastasis. |
Table IV
Residual carcinoma patterns in primary
breast tumors and lymph node metastasis.
| Residual carcinoma
pattern | Residual lymph node
group (n=26) (100%) n (%) | Eradicated lymph node
group (n=14) (100%) n (%) | P-value |
|---|
| Dense | 11 (42.3)a | 1 (7.1)b | 0.003 |
| Focal/nested | 15 (57.7) | 9 (64.3) | |
|
Sporadic/in-situ | 0 (0)b | 4 (28.6)a | |
Residual carcinoma patterns and
clinicopathological factors
Clinicopathological factors of the non-dense and
dense group are shown in Table V.
The dense group had a higher reduced ratio (P<0.001), larger
area before NAC (P=0.003), and larger area after NAC
(P<0.00001), as compared with the non-dense group. There were
significant differences in the intrinsic subtype (P=0.004) between
groups. Particularly, there were a larger number of triple-negative
cases in the dense group (adjusted residue +3.6). The Ki-67
labeling index was higher in the dense group (P=0.006) than that in
the non-dense group. Pathological tumor diameter was larger in the
dense group (P=0.010) than that in the non-dense group. The
histological grade (P=0.016) and mitotic count (P=0.004) were
higher, and tubular formation (P=0.027) was less frequent in the
dense group than that in the non-dense group. There were no
significant differences in age (P=0.567), trastuzumab
administration (P=0.304), ER (P=0.105), PgR (P=0.266), HER2 status
(P=0.098), lymphatic invasion (P=0.143), venous invasion (P=0.075),
nuclear atypia (P=0.616), or extensive intraductal component
(P=1.00) between the dense and non-dense groups.
 | Table VClinicopathological factors of the
non-dense and dense group. |
Table V
Clinicopathological factors of the
non-dense and dense group.
| Non-dense (n=35)
(100%) n (%) | Dense (n=15) (100%)
n (%) | P-value |
|---|
| Age (mean,
years) | 50.6 | 49.0 | 0.567 |
| Trastuzumab |
| On | 10 (28.6) | 2 (13.3) | 0.304 |
| Off | 25 (71.4) | 13 (86.7) | |
| Reduced ratio | 0.089 | 0.496 | <0.001a |
| Area before
NAC | 336.5
mm2 | 1014.0
mm2 | 0.003a |
| Area after | 33.6
mm2 | 213.7
mm2 | <0.00001a |
| Intrinsic
subtype |
| Luminal A | 10 (28.6) | 3 (20.0) | 0.004a |
| Luminal B | 21 (60.0) | 7 (46.7) | |
| HER2 | 4 (11.4) | 0 (0) | |
|
Triple-negative | 0 (0)c | 5 (33.3)b | |
| ER status |
| Positive | 31 (88.6) | 10 (66.7) | 0.105 |
| Negative | 4 (11.4) | 5 (33.3) | |
| PgR status |
| Positive | 20 (57.1) | 6 (40.0) | 0.266 |
| Negative | 15 (42.9) | 9 (60.0) | |
| HER2 status |
| Positive | 14 (40.0) | 2 (13.3) | 0.098 |
| Negative | 21 (60.0) | 13 (86.7) | |
| Ki-67 labeling
index | 5 | 50 | 0.006a |
| Tumor diameter
(mm) | 11 | 30 | 0.010a |
| Lymphatic
invasion |
| Positive | 11 (31.4) | 8 (53.3) | 0.143 |
| Negative | 24 (68.6) | 7 (46.7) | |
| Venous
invasion |
| Positive | 1 (2.9) | 3 (20.0) | 0.075 |
| Negative | 34 (97.1) | 12 (80.0) | |
| Histological
grade |
| I | 14 (40.0)b | 1 (6.7)c | 0.016a |
| II | 18 (51.4) | 9 (60.0) | |
| III | 3 (8.6)c | 5 (33.3)b | |
| Nuclear atypia |
| 1 | 3 (8.6) | 0 (0) | 0.616 |
| 2 | 19 (54.3) | 8 (53.3) | |
| 3 | 13 (37.1) | 7 (46.7) | |
| Mitotic count |
| 1 | 26 (74.3)b | 6 (40.0)c | 0.004a |
| 2 | 9 (25.7) | 5 (33.3) | |
| 3 | 0 (0)c | 4 (26.7)b | |
| Tubular
formation |
| 1 | 1 (2.9) | 0 (0) | 0.027a |
| 2 | 20 (57.1)b | 3 (20.0)c | |
| 3 | 14 (40.0)c | 12 (80.0)b | |
| EIC |
| Positive | 9 (25.7%) | 3 (20.0%) | 1.00 |
| Negative | 26 (74.3%) | 12 (80.0%) | |
Discussion
The results of this study suggest that residual
carcinoma patterns (dense, focal/nested, and
sporadic/in-situ) in primary breast cancer after NAC are
correlated with lymph node metastasis status. There was a high
incidence of dense patterns in the residual lymph node group and a
high incidence of sporadic/in-situ patterns in the
eradicated lymph node group. The dense group had malignant
potential of breast cancer after NAC when compared with the
non-dense group. The tumor areas before and after NAC were larger
in the dense group than that in the non-dense group. The Ki-67
labeling index, histological grade, mitotic count, and extent of
tubular formation were higher in the dense group than these
parameters in the non-dense group. Interestingly, the tumor reduced
ratio after NAC was larger in the dense group than that in the
non-dense group.
The 'dense' residual carcinoma pattern was
associated with the potential for residual carcinoma in the lymph
nodes. No previous report has predicted lymph node metastasis in
breast cancer after NAC using residual carcinoma patterns. The
Miller and Payne system is used to assess the response to
chemotherapy based on a 5-point histological grading system of
fundamental features that include reduction in tumor cellularity
and comparisons with pre-treatment core biopsies. This grading
system is correlated with overall survival and disease-free
survival (16). The Millar and
Payne system and our proposed histological classification system
suggest that tumor cellularity is the most important characteristic
to assess the response to NAC in breast cancer. Rajan et al
also reported that cellularity was useful to assess the pathologic
responses of breast cancer to chemotherapy (17). A 'dense' residual carcinoma pattern
has malignant potential after NAC in breast cancer. Resistance of
tumor cells to chemotherapy is suggested by a high Ki-67 labeling
index, high mitotic count, and large tumor area. A dense pattern is
associated with a high histological grade due to a high mitotic
count and less tubular formation.
A residual carcinoma pattern is based on a very
simple criteria of tumor cell density and change in histological
degeneration but is not dependent on tumor area before NAC.
Therefore, it is easy to predict the therapeutic effect using
histological diagnosis without image analysis. It is possible to
recognize the presence of a dense pattern using core needle biopsy
after NAC; therefore, it may be possible to predict lymph node
metastasis before surgery by core needle biopsy using our proposed
residual carcinoma patterns.
This new classification system considers only three
patterns. Although a relatively small number of cases were
analyzed, the focal/nested pattern demonstrated wide range
chemotherapy responses. By analyzing a larger number of cases in
the future, focal/nested cases can be divided into two groups,
i.e., focal/nested low and high. This study was a small-scale
analysis carried out at a single institution, and there was
difficulty with the unified chemotherapy regimen. Before NAC, the
prediction of the presence or absence of lymph node metastasis was
dependent only on imaging and sometimes after starting treatment.
The findings of this study were derived from detailed
histopathological analyses of primary breast tumors and associated
dissected lymph nodes after NAC. Therefore, these results were
realistic and may be useful to predict lymph node metastasis after
NAC. Further identification of lymph node metastasis predictors is
expected by the additional accumulation of cases in the future.
The results of this study suggest that a residual
carcinoma pattern may be predictive of lymph node metastasis after
NAC in breast cancer and a dense residual carcinoma pattern of a
primary breast tumor after NAC may be an indicator of therapeutic
resistance to NAC.
Acknowledgments
This study was supported by Grants-in-Aid for
Science from the Ministry of Education, Culture, Sports, Science,
and Technology of Japan; a Grant for Hirosaki University
Institutional Research; and the Fund for the Promotion of
International Scientific Research.
Abbreviations:
|
NAC
|
neoadjuvant chemotherapy
|
References
|
1
|
Fisher ER, Wang J, Bryant J, Fisher B,
Mamounas E and Wolmark N: Pathobiology of preoperative
chemotherapy: Findings from the National Surgical Adjuvant Breast
and Bowel (NSABP) protocol B-18. Cancer. 95:681–695. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kuerer HM, Sahin AA, Hunt KK, Newman LA,
Breslin TM, Ames FC, Ross MI, Buzdar AU, Hortobagyi GN and
Singletary SE: Incidence and impact of documented eradication of
breast cancer axillary lymph node metastases before surgery in
patients treated with neoadjuvant chemotherapy. Ann Surg.
230:72–78. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Klauber-DeMore N, Ollila DW, Moore DT,
Livasy C, Calvo BF, Kim HJ, Dees EC, Sartor CI, Sawyer LR, Graham M
II, et al: Size of residual lymph node metastasis after neoadjuvant
chemotherapy in locally advanced breast cancer patients is
prognostic. Ann Surg Oncol. 13:685–691. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kuerer HM, Newman LA, Smith TL, Ames FC,
Hunt KK, Dhingra K, Theriault RL, Singh G, Binkley SM, Sneige N, et
al: Clinical course of breast cancer patients with complete
pathologic primary tumor and axillary lymph node response to
doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol.
17:460–469. 1999.PubMed/NCBI
|
|
5
|
Mamounas EP, Anderson SJ, Dignam JJ, Bear
HD, Julian TB, Geyer CE Jr, Taghian A, Wickerham DL and Wolmark N:
Predictors of locoregional recurrence after neoadjuvant
chemotherapy: Results from combined analysis of National Surgical
Adjuvant Breast and Bowel Project B-18 and B-27. J Clin Oncol.
30:3960–3966. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang EH, Strom EA, Perkins GH, Oh JL,
Chen AM, Meric-Bernstam F, Hunt KK, Sahin AA, Hortobagyi GN and
Buchholz TA: Comparison of risk of local-regional recurrence after
mastectomy or breast conservation therapy for patients treated with
neoadjuvant chemotherapy and radiation stratified according to a
prognostic index score. Int J Radiat Oncol Biol Phys. 66:352–357.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Swain SM, Sorace RA, Bagley CS, Danforth
DN Jr, Bader J, Wesley MN, Steinberg SM and Lippman ME: Neoadjuvant
chemotherapy in the combined modality approach of locally advanced
nonmetastatic breast cancer. Cancer Res. 47:3889–3894.
1987.PubMed/NCBI
|
|
8
|
Mauri D, Pavlidis N and Ioannidis JP:
Neoadjuvant versus adjuvant systemic treatment in breast cancer: A
meta-analysis. J Natl Cancer Inst. 97:188–194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fisher B, Brown A, Mamounas E, Wieand S,
Robidoux A, Margolese RG, Cruz AB Jr, Fisher ER, Wickerham DL,
Wolmark N, et al: Effect of preoperative chemotherapy on
local-regional disease in women with operable breast cancer:
Findings from National Surgical Adjuvant Breast and Bowel Project
B-18. J Clin Oncol. 15:2483–2493. 1997.PubMed/NCBI
|
|
10
|
Julian TB, Patel N, Dusi D, Olson P,
Nathan G, Jasnosz K, Isaacs G and Wolmark N: Sentinel lymph node
biopsy after neoadjuvant chemotherapy for breast cancer. Am J Surg.
182:407–410. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
van der Hage JA, van de Velde CJ, Julien
JP, Tubiana-Hulin M, Vandervelden C and Duchateau L: Preoperative
chemotherapy in primary operable breast cancer: Results from the
European Organization for Research and Treatment of Cancer trial
10902. J Clin Oncol. 19:4224–4237. 2001.PubMed/NCBI
|
|
12
|
Mieog JS, van der Hage JA and van de Velde
CJ: Neoadjuvant chemotherapy for operable breast cancer. Br J Surg.
94:1189–1200. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bloom HJ and Richardson WW: Histological
grading and prognosis in breast cancer; a study of 1409 cases of
which 359 have been followed for 15 years. Br J Cancer. 11:359–377.
1957. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goldhirsch A, Winer EP, Coates AS, Gelber
RD, Piccart-Gebhart M, Thürlimann B, Senn HJ, Albain KS, Andre F,
Bergh J, et al: Panel members: Personalizing the treatment of women
with early breast cancer: Highlights of the St Gallen International
Expert Consensus on the Primary Therapy of Early Breast Cancer
2013. Ann Oncol. 24:2206–2223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abramoff MD, Magalhães PJ and Ram SJ:
Image processing with ImageJ. Biophotonics Int. 11:36–42. 2004.
|
|
16
|
Ogston KN, Miller ID, Payne S, Hutcheon
AW, Sarkar TK, Smith I, Schofield A and Heys SD: A new histological
grading system to assess response of breast cancers to primary
chemotherapy: Prognostic significance and survival. Breast.
12:320–327. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rajan R, Poniecka A, Smith TL, Yang Y,
Frye D, Pusztai L, Fiterman DJ, Gal-Gombos E, Whitman G, Rouzier R,
et al: Change in tumor cellularity of breast carcinoma after
neoadjuvant chemotherapy as a variable in the pathologic assessment
of response. Cancer. 100:1365–1373. 2004. View Article : Google Scholar : PubMed/NCBI
|