Introduction
The fifth most common cancer globally is human
hepatocellular carcinoma (HCC), and in the context of
cancer-related mortality HCC ranks third as one of the leading
causes (1,2). Moreover, traditional therapy remains
disappointing. It is believed that HCC is sustained by liver cancer
stem cells (LCSCs), which constitute a relatively small frequency
of cells, and they do so through their ability to propagate highly
heterogeneous progeny, multi-drug resistance to chemotherapeutic
agents and a high capacity for cellular proliferation (3). A number of studies have suggested that
LCSCs can be identified by several cell surface antigens including
CD133, CD44, and ALDH1 (4–6). Furthermore, CD133+,
CD44+ and ALDH1+ cells that are isolated from
HCC cells display an enhanced capacity for malignant transformation
in vivo. These studies indicate LCSCs as the basis of HCC.
However, emerging evidence shows that cancer onset and progression
was not only determined by tumor cells but was also affected by the
local microenvironment (7,8).
It has been reported that almost 80% of
hepatocellular carcinoma (HCC) resulted from chronic hepatitis and
cirrhosis caused by inflammation and fibrosis (9). Hepatic stellate cells (HSCs), which
are the main liver stromal cells, can transform into a
myofibroblast-like phenotype from a quiescent state during chronic
liver injury (10,11). An important cellular source of
hepatic-derived cytokines (e.g., TGF-β, PDGF, HGF, FGF, and VEGF)
is secreted by HSCs that constitute the main component of the local
liver cancer microenvironment. Additionally, HSC activation might
play an important role in inflammation and fibrosis, even during
tumor metastasis (9). It has been
shown recently that malignant transformation in HSC is critical in
reprogramming cells that transform the physiologically normal
vitamin-A storage capacity of HSC to a remodeled extracellular
matrix phenotype (11), which then
provides a tumorigenic environment that is compatible for HCC.
Additionally, studies have shown that cross-talk of hepatocytes and
HSC can generate a permissive inflammatory microenvironment to
promote HCC development (12), and
the interaction of hepatocytes and HSCs leads to tumor metastasis,
proliferation and chemotherapy resistance (13). However, whether LCSCs activate HSC
in a way that promotes HCC progression remains to be fully
explored.
Signal transducer and activator of transcription-3
(STAT3), a transcription factor for cytokine signaling, is
constitutively activated in numerous cancer types, including
prostate cancer, breast cancer, leukemia, brain tumors, and lung
cancer (14–18). Niu et al reported that STAT3
mutations induced cellular transformation and tumor formation in
vivo and that activation of STAT3 signaling further inhibited
p53 transcriptional activity, fulfilling the definition of an
oncogene (19). In addition, it is
reported that activated STAT3 could promote LCSCs (20). Moreover, activation of STAT3 in
hepatic stellate cells promoted their survival and proliferation,
thereby contributing to liver fibrogenesis (21,22).
However, the mechanism of the STAT3 signaling pathways to interrupt
cross-talk of LCSLCs and HSC and the possible therapeutic targets
involved in HCC need further investigation.
Cucurbitacin I (JSI-124) is a cell permeable,
triterpenoid compound that was shown to specifically suppress
tyrosine phosphorylation of STAT3 (23). In the current study, we hypothesized
that JSI-124 would be useful to block cross-talk of LCSCs and HSC
by inhibiting activation of STAT3.
Chrysin (5,7-dihydroxyflavone, ChR), a natural
widely distributed flavonoid, has been shown to possess promising
effects on the inhibition of proliferation and induction of
apoptosis in a variety of cancer cells (24,25).
By using a STAT3-specific inhibitor, JSI-124, Lirdprapamongkol
et al showed that ChR overcame TRAIL resistance of cancer
cells through inhibiting STAT3 phosphorylation (26). Furthermore, Lin et al
reported that ChR suppresses IL-6-induced angiogenesis through
modulation of the STAT3 signaling pathway (27). In a previous study, we succeeded in
synthesizing 8-bromo-7-methoxychrysin (BrMC) based of the lead
compound ChR (28). Compared to
ChR, BrMC has stronger effects of inhibition of proliferation and
induction of apoptosis on colon cancer cell line HT-29 and gastric
cancer cell line SGC-7901 (28,29).
As previously described, BrMC can inhibit the proliferative
activity of glioma stem-like cells, and target to inhibit the
characteristic of LCSCs (30,31).
Thus, our aim was to analyze if BrMC can affect cross-talk of liver
cancer stem cells and HSC to reduce the activation of HSC and
inhibit the properties of LCSLCs by suppressing STAT3.
Materials and methods
Reagents
Dulbecco's modified Eagle's medium (DMEM) and
DMEM/F12 medium, Trypsin-EDTA, FBS and Penicillin-streptomycin were
from Gibco (Grand Island, NY, USA). All cell culture ware was from
Corning Life Sciences (New York, NY, USA). Monoclonal
anti-α-SMA-Cy3 antibody was obtained from Sigma-Aldrich (St. Louis,
Mo, USA). Polyclonal anti-FAP-α, polyclonal anti-E-cadherin,
polyclonal anti-N-cadherin, monoclonal anti-CD33, monoclonal
anti-CD44, monoclonal anti-stat3, and polyclonal anti-ALDH1
antibody were obtained from Abcam (Hong Kong, China). The STAT3
inhibitor, JSI-124, was obtained from Sigma-Aldrich, and was
prepared by dissolving in DMSO to a stock concentration of 100
µmol/l. JSI-124 was additionally diluted in culture medium
to required final concentrations immediately prior to use. BrMC was
synthesized as previously described (28). All other experimental reagents used
in this study were obtained from Sigma-Aldrich, unless indicated
otherwise in the text.
Cell culture and sphere formation
assay
The Chinese Academy of Sciences (Shanghai, China)
provided the human liver cancer SMMC-7721 cell line. The human
immortalized HSCs (LX-2) was obtained from Bogu Biotechnology Co.,
Ltd. (Shanghai, China). Cells were routinely passaged in complete
DMEM that was supplemented with 10% fetal bovine serum (FBS), and
antibiotics. Cell cultures were incubated at 37°C in an atmosphere
of 5% Co2 in air.
Suspensions of single-cells were seeded into ultra
low attachment 6-well plates (Corning Life Sciences) at a density
of 3,000 cells/ml in stem cell-conditioned medium. Culture
suspensions were passaged every five days when spheroid diameters
were at least 50 µm. Colonies were then scored under ten
independent fields of view by light microscopy (olympus, Tokyo,
Japan). The efficiency of sphere formation was calculated by
dividing the total sphere number that formed by the number of the
total viable cells that were seeded multiplied by one hundred.
Preparation of conditioned medium from
LCSLCs and LCAHSCs
To prepare LCSLC-CM, suspension culture and stem
cell-conditioned medium amplification of LCSLCs was performed using
ultra low-adhesion 6-well plates. Spent culture media was removed
24 h later, and this was filtered (0.22 µm) and stored at
−80°C until further use.
LX-2 cells were cultured with LCSLC-CM for 24 h,
washed with PBS and serum-free DMEM, respectively, and incubated
with serum-free DMEM for 24 h. Then the culture media was collected
and centrifuged at 3000 rpm for 5 min, followed by filtering (0.22
µm) as LCAHSC-CM and stored at −80°C for further use.
Immunofluorescence
LX-2 cells were cultured on coverslips, washed with
PBS, fixed in 4% paraformaldehyde, following which, 0.1% triton
X-100 for 4 min was used to permeabilize the cells. Next,
monoclonal anti-α-SMA-Cy3 or polyclonal anti-FAP-α antibody was
added for 45 min or 1 h at 37°C in the dark. Later, Alexa Fluor 488
anti-rabbit (Molecular Probes, 1:500, 1 h, RT) and DAPI [1:100, 10
min, room temperature (RT)] were incubated with the coverslips,
which were then mounted for visualization and quantification.
Images were collected by fluorescence microscopy (Olympus).
Western immunoblot analysis
Previously described procedures were used for
preparing whole cell lysates and the western immunoblot procedure
(32). The primary antibodies used
in this procedure were monoclonal anti-α-SMA antibody, polyclonal
anti-FAP-α, polyclonal anti-E-cadherin, polyclonal anti-n-cadherin,
monoclonal anti-CD33, monoclonal anti-CD44, monoclonal anti-stat3,
and polyclonal anti-ALDH1. In addition, internal loading of protein
was controlled by detecting the expression of β-actin. Immunoblots
were then visualized by chemiluminescent substrate (ECL; Amersham,
Arlington Heights, IL, USA). The autoradiographed images were
scanned to permit semi-quantitative densitometric analysis using
UN-SCAN-IT software program (Silk Scientific).
Statistical analyses
Representative data described in this report are the
product of at least three independent observations, unless
otherwise indicated. The data were analyzed using analysis of
variance followed by Dunnett's test for pairwise comparison. An
asterisk indicates that the experimental values were significantly
different from values at an α value of P<0.05.
Results
Characteristics of liver cancer stem-like
cells derived from SMMC-7721 cells
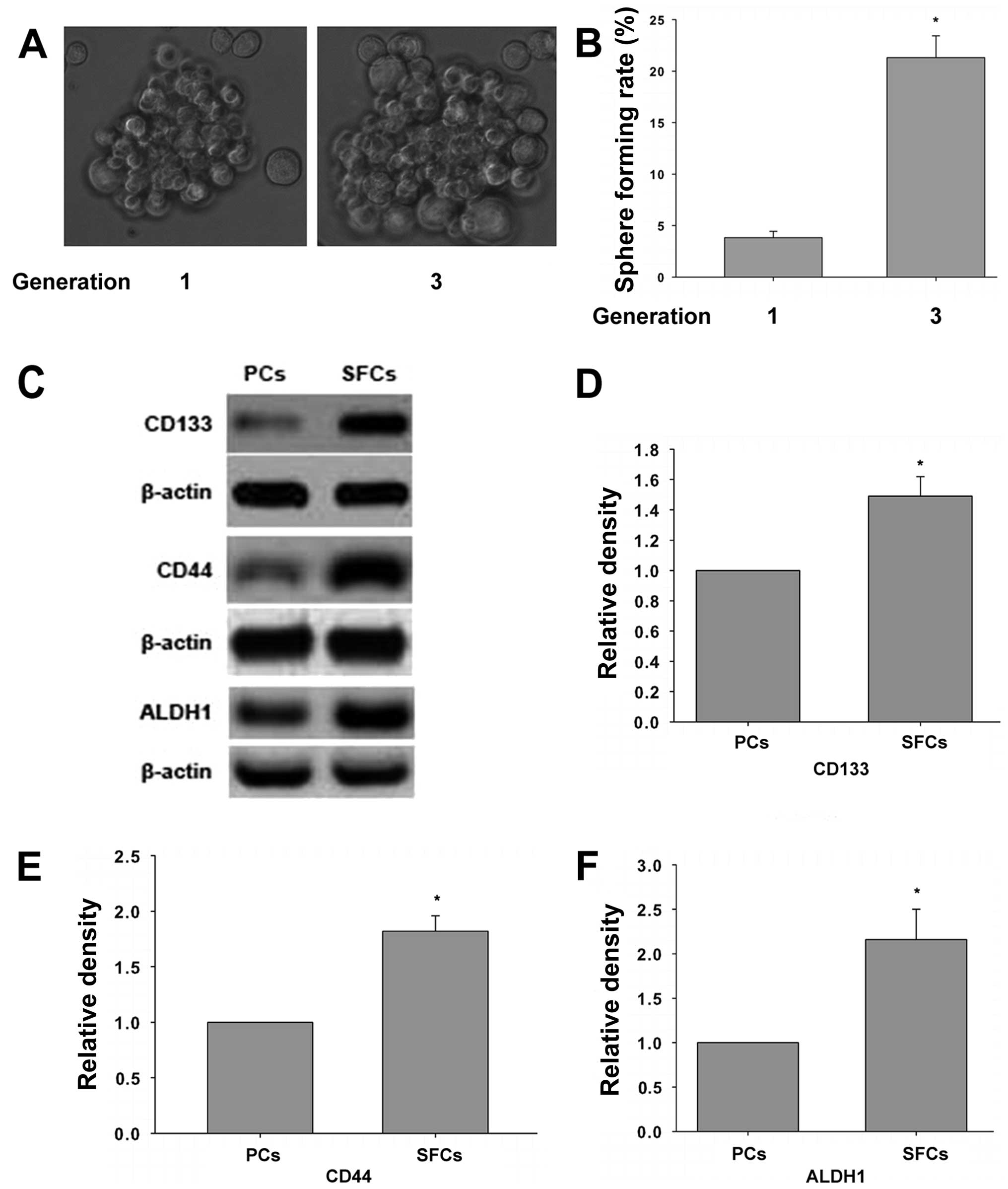
To investigate whether the third generation sphere
forming cells (SFCs) of SMMC-7721 cell line possess properties of
liver cancer stem-like cells (LCSLCs), sphere formation assay was
performed. Furthermore, the level of CD133, CD44, ALDH1 expression,
which are know as markers of stem cells, were determined.
Consistent with a previous study (30,31),
the third generation of SMMC-7721 SFCs have stronger capability of
proliferation and its sphere forming rate increases compared to
parental cells (Fig. 1A and B). In
addition, the expression of CD133, CD44, and ALDH1 is elevated in
the third generation of SFCs compared to parental cells (Fig. 1C). These results indicated that the
third generation SMMC-7721 SFCs have properties of LCSCs.
Generation of liver cancer
associated-stellate cells derived from human hepatic stellate cell
line LX-2
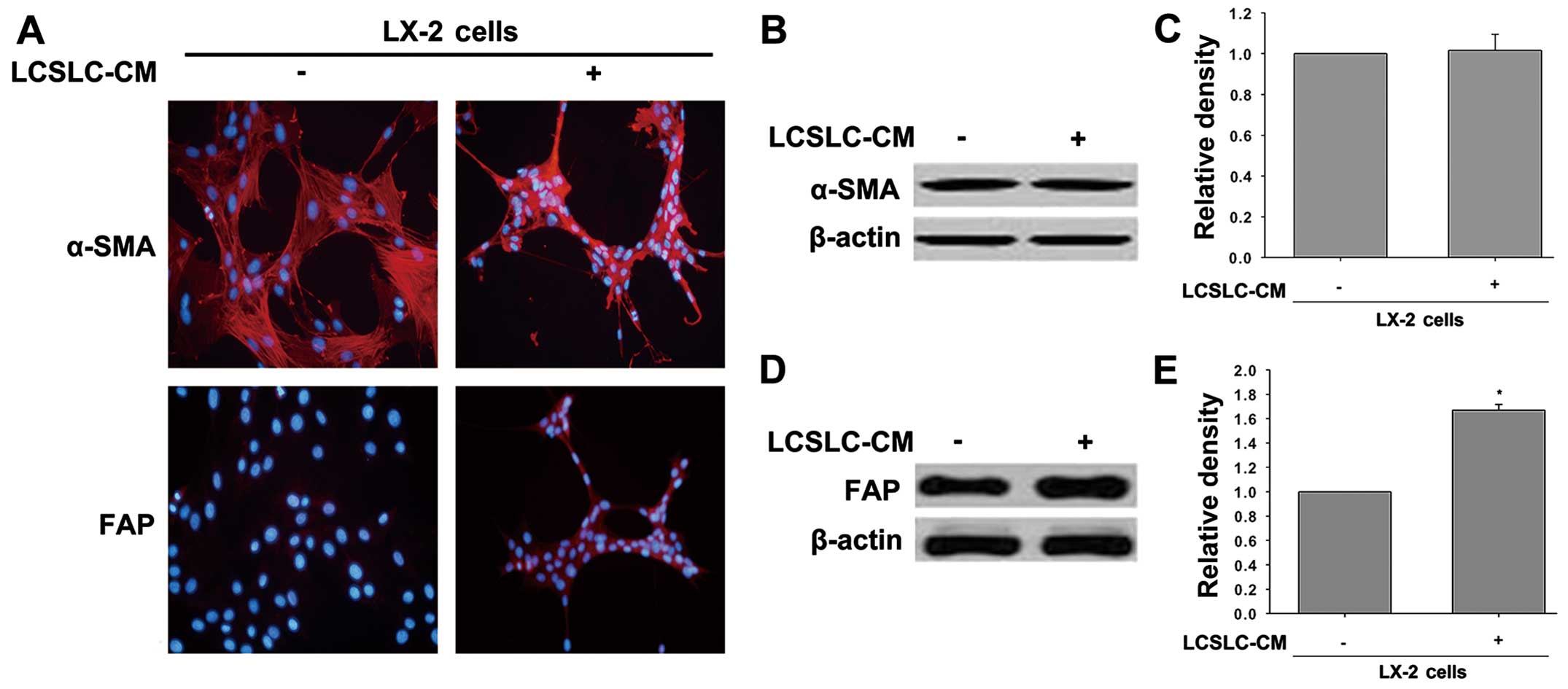
Human hepatic stellate cell line LX-2 was exposed to
conditioned medium from liver cancer stem-like cells (LCSLC-CM) for
24 h to generate liver cancer associated-hepatic stellate cells
(LCAHSCs), and verify the capacity of LCSLC-CM-induce LX-2 cell
pathologic activation. As shown in Fig.
2A, LX-2 cells treated with LCSLC-CM induced significant
changes in fibroblast activation protein (FAP) expression, but no
visible changes in α-smooth muscle actin (α-SMA) expression. This
result is consistent with western blot analysis.
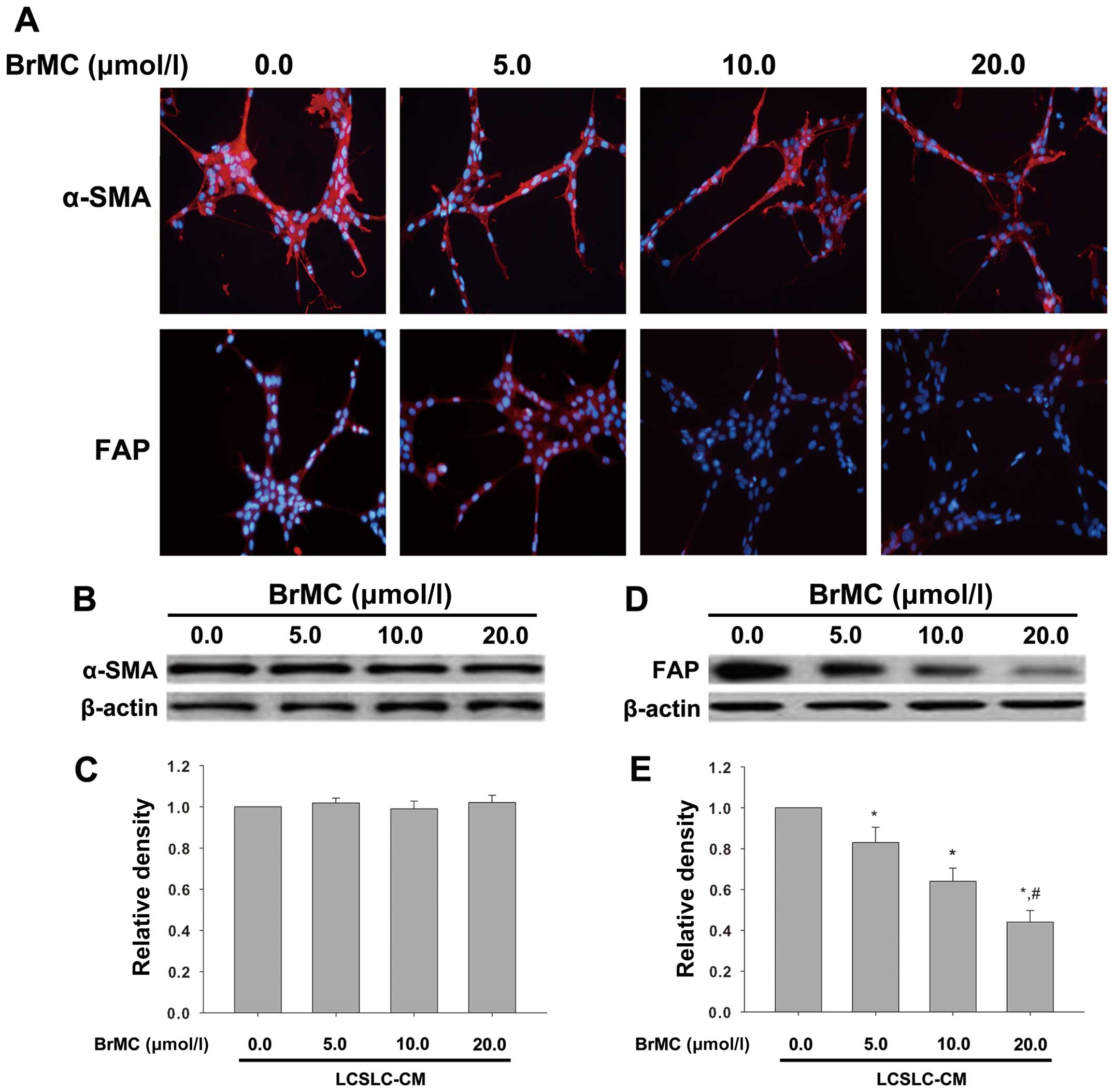
Effects of 8-bromo-7-methoxychrysin on
activation of LX-2 cells by LCSLC-CM
We previously described that BrMC can inhibit
several forms of cancer (31,33).
In the current study, to analyze if BrMC can affect cross-talk of
LCSLCs and HSCs to block the activation of HSC, we measured the
expression of α-SMA and FAP by immunofluorescence and western
blotting in LX-2 cells after being cultured with LCSLC-CM
containing various concentrations of BrMC (0.0, 5.0, 10.0, 20.0
µmol/l). As shown in Fig.
3A, immunofluorescence showed that BrMC had no effect on the
expression of α-SMA, but it reduced the level of FAP expression in
a dose-dependent manner. Consistent with the results of
immunofluorescence, western blot analysis indicated downregulation
of FAP (Fig. 3D and E), but not
α-SMA (Fig. 3B and C).
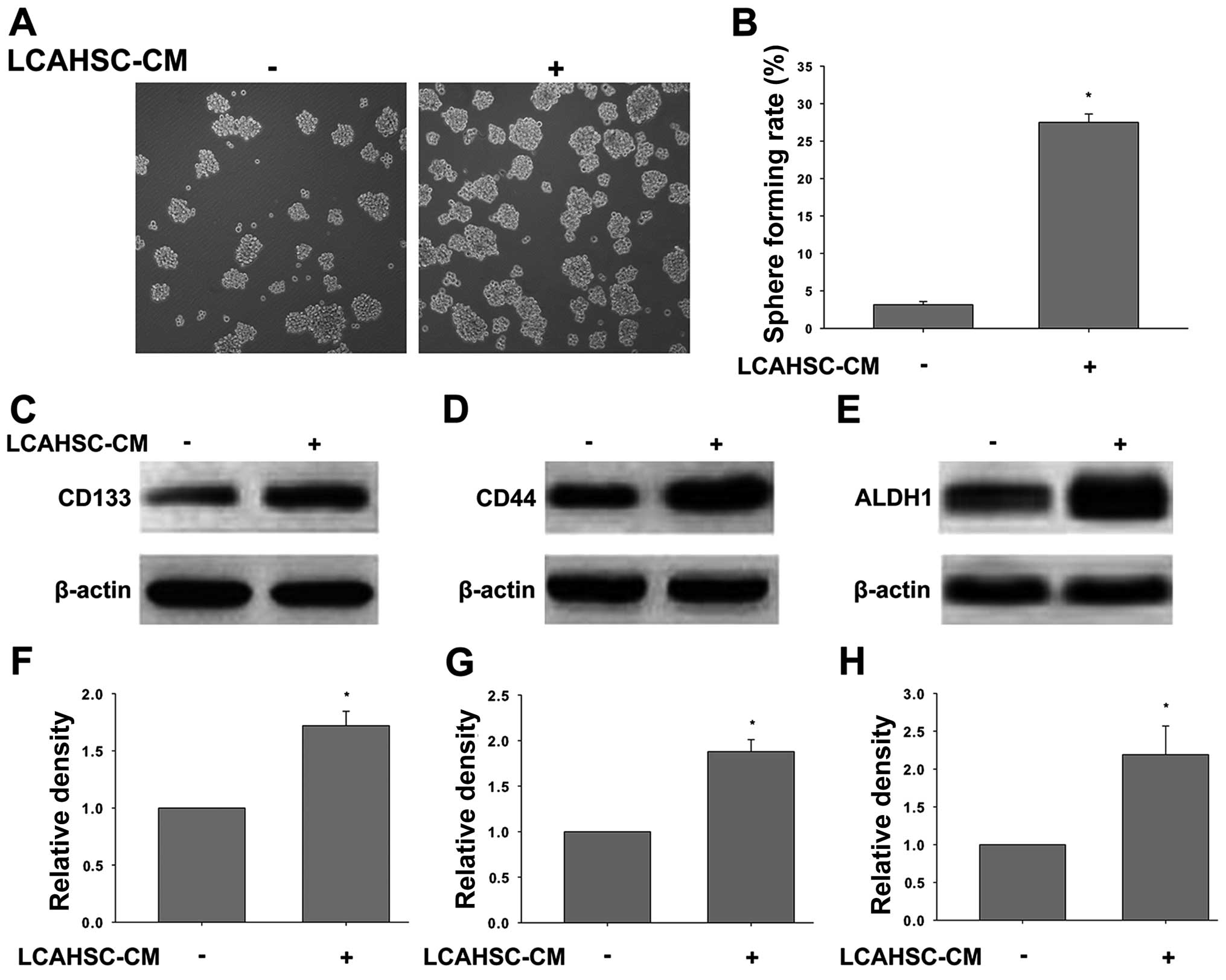
Conditioned medium from liver cancer
associated-stellate cells contributes to characteristics of LCSLCs
derived from SMMC-7721 cells
To evaluate the effects of LCAHSCs on self-renewal
capability and cancer stem cell marker expression of SMMC-7721
cells and LCSLCs derived from SMMC-7721 cells, respectively; we
collected conditioned medium from LCAHSCs (LCAHSC-CM), and the
cells were exposed to LCAHSC-CM for 24 h. Then sphere formation
assay and western blotting were performed. As expected, treatment
with LCASC-CM enhanced the sphere forming ability of SMMC-7721
cells (Fig. 4A and B). The level of
CD133 (Fig. 4C and F), CD44
(Fig. 4D and G) and ALDH1 (Fig. 4E and H) in the cells treated with
LCAHSC-CM were significantly increased compared to untreated
cells.
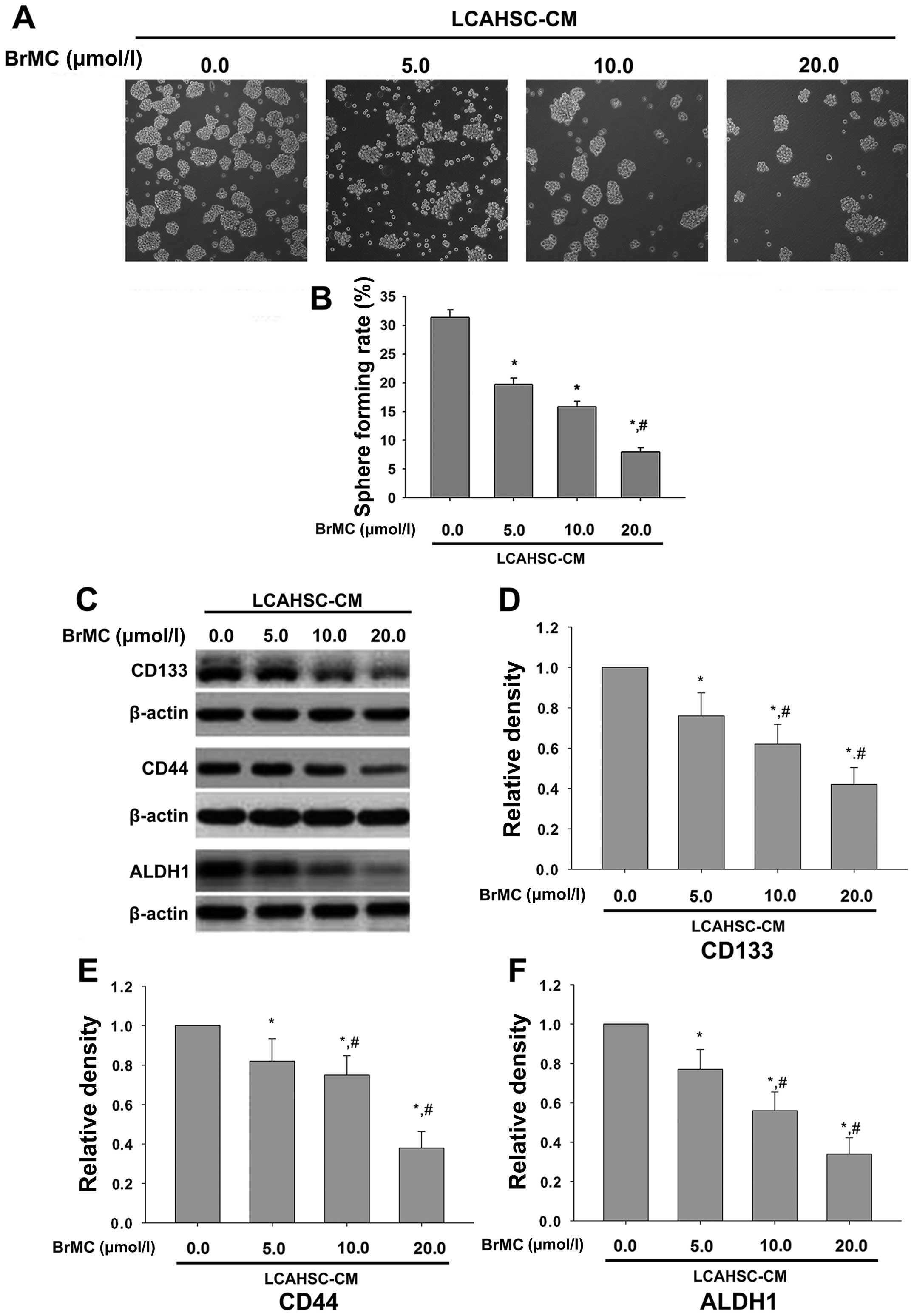
BrMC reverses the characteristics of
LCSLCs induced by LCAHSC-CM
Our findings showed that LCAHSC-CM can promote the
characteristics of LCSLCs and BrMC can inhibit activation of LX-2
cells. Thus, we investigated whether BrMC inhibits the
characteristics of LCSLCs induced by LCAHSC-CM. Sphere forming
assay indicated that BrMC inhibits self-renewal capability of
SMMC-7721 cells in a dose-dependent manner, and the sphere forming
rate was significant reduced when treatment with 20 µmol/l
BrMC (Fig. 5A and B). The markers
of cancer stem cells (including CD133, CD44 and ALDH1) were also
reduced by BrMC in a dose-dependent manner (Fig. 5C).
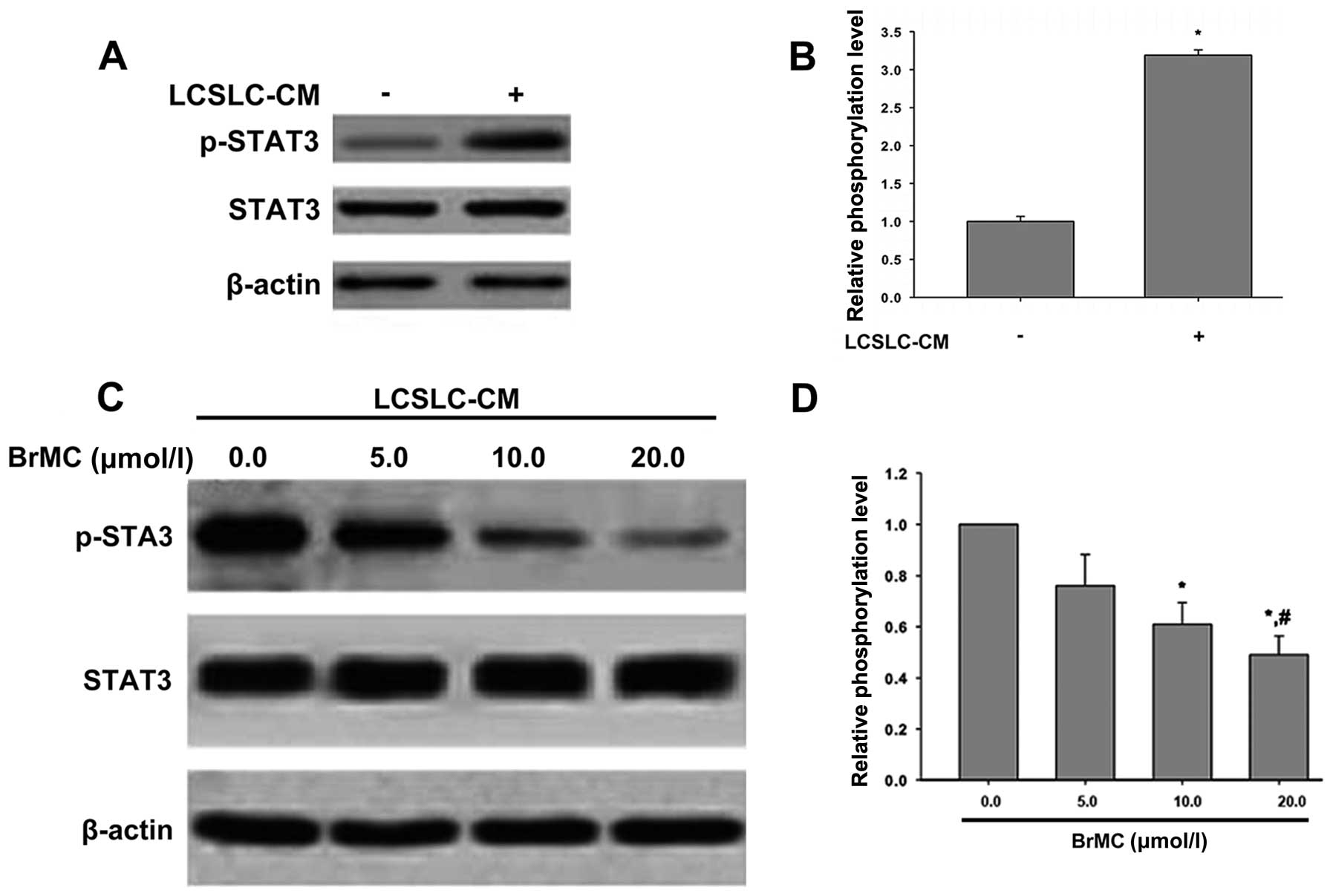
BrMC inhibits the phosphorylation of
STAT3 in LX-2 cells induced by LCSLC-CM
It was reported that IL6/STAT3 axis activated LX-2
cells (34). Thus, we studied
whether STAT3 was responsible for LX-2 cells activation induced by
LCSLC-CM, and if BrMC can decrease the phosphorylation of STAT3 to
inhibit LX-2 cells activation. The results in Fig. 6A show that compared to serum-free
DMEM/F12 medium, LCSLC-CM induced phosphorylation of STAT3 of LX-2
cells, but not total STAT3 protein expression. LCSLC-CM from LCSLCs
treated with different concentrations of BrMC (0.0, 5.0, 10.0, 20.0
µmol/l), showed phosphorylation of STAT3 decreased in a
dose-dependent manner, and no effect on the level of STAT3
expression (Fig. 6B).
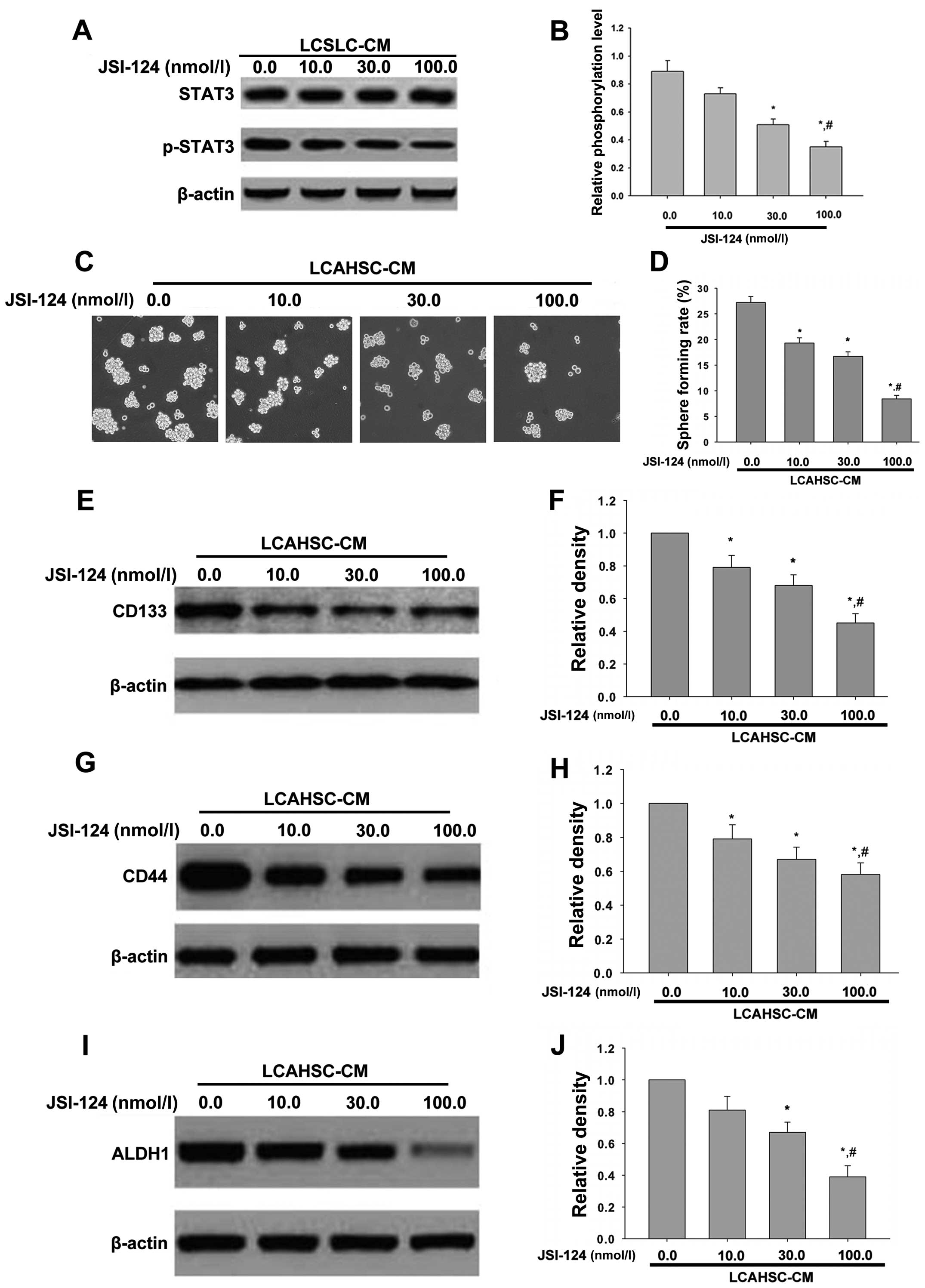
JSI-124 treatment reverses the
characteristics of LCSLCs induced by LCAHSC-CM
The above studies showed that LCSLC-CM induced the
phosphorylation of STAT3 in LX-2 cells and LCAHSC-CM contributed to
the characteristics of LCSLCs. To explain the mechanism that
activated the LX-2 cell promoted features of LCSLCs derived from
SMMC-7721 cells, STAT3 inhibitor JSI-124 was used. Our findings
showed that when added to LCSLC-CM treated with JSI-124, the
phosphorylation of STAT3 in LX-2 cells was significant reduced, but
it had no effect on the expression of STAT3 compared with
LCSLC-CM-treated LX-2 cells (Fig. 7A
and B). Then we treated LCSLCs and SMMC-7721 cells with
LCAHSC-CM containing different concentration of JSI-124 (0.0, 10.0,
30.0, 100.0 nmol/l) to determine their stem cell marker expression
and sphere formation, respectively. Sphere formation induced by
LCAHSC-CM was inhibited by JSI-124 in a dose-dependent manner
(Fig. 7C). Western blotting showed
that the level of cancer stem cell markers (CD133 (Fig. 7E), CD44 (Fig. 7G) and ALDH1 (Fig. 7I) were decreased after treated with
JSI-124. In conclusion, JSI-124 inhibited the characteristics of
LCSLCs induced by LCAHSC-CM.
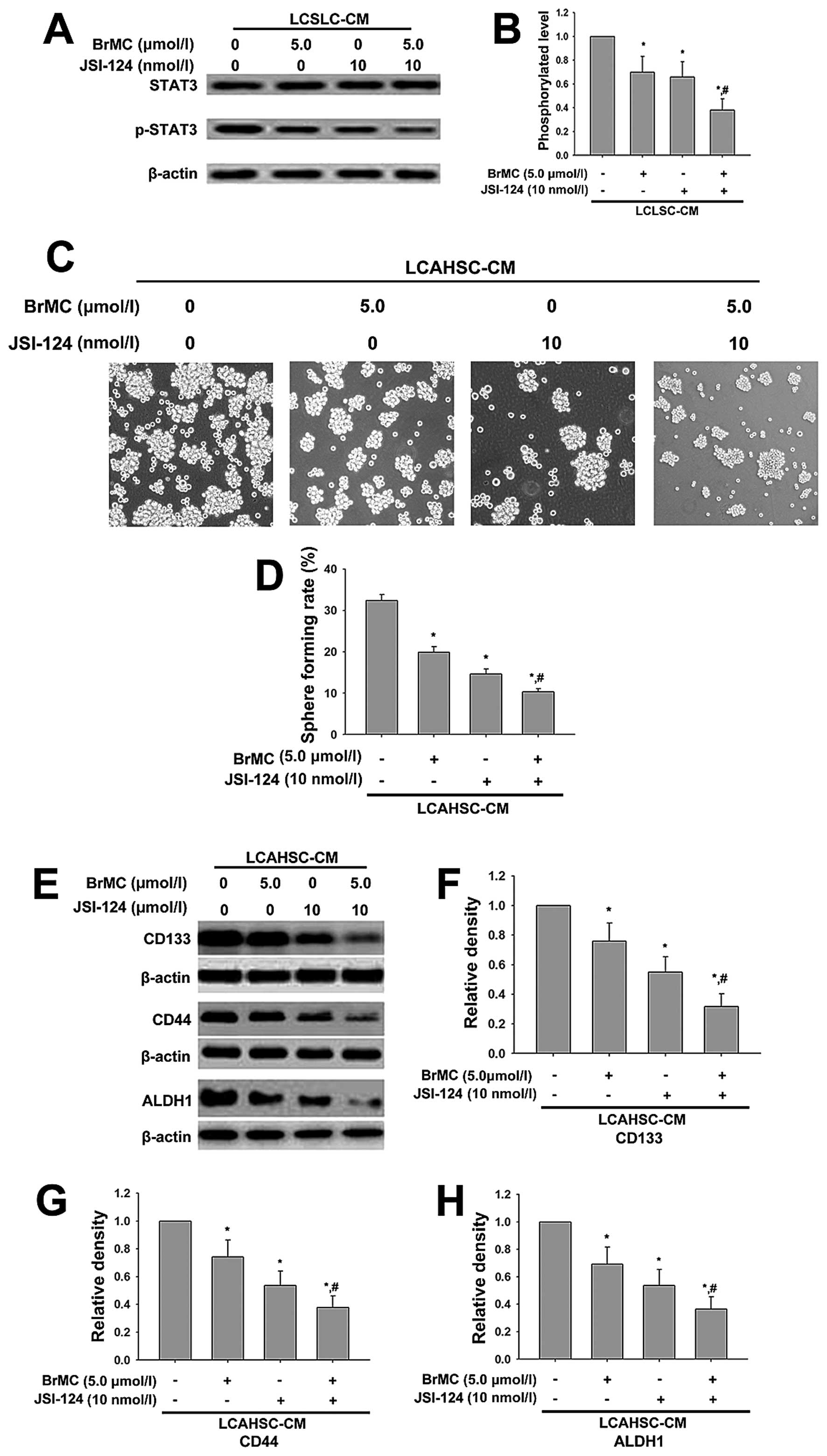
BrMC and JSI-124 synergistically inhibit
properties of LCSLCs induced by LCAHSC-CM
The above studies showed that BrMC reversed the
characteristic of LCSLCs through inhibiting STAT3. Thus, we used
STAT3 inhibitor JSI-124 (10 nmol/l) and BrMC (5.0 µmol/l)
alone or combined to administer LCSLCs and to obtain LCSLC-CM
containing BrMC or JSI-124 or both, which was used to culture LX-2
cells for 24 h and collect LCAHSC-CM. Next, the STAT3 in LX-2 was
determined by western blotting. We validated the role of BrMC and
JSI-124 in the inhibition of the characteristics of LCSLCs. Our
data showed that the presence of BrMC and JSI-124 was sufficient to
reduce phosphorylation of STAT3, which indicated that BrMC and
JSI-124 synergistically inhibited LX-2, which permitted them to be
activated by LCSLC-CM (Fig. 8A).
Then we collected LCAHSC-CM that contained BrMC and JSI-124 alone
or combined to measure properties of LCSLCs. The sphere forming
assay showed that combined BrMC (5.0 µmol/l) and JSI-124 (10
nmol/l) significantly inhibited the ability of sphere forming
compared to treating with BrMC and JSI-124 alone (Fig. 8C). Then, western blotting was
carried out to detect the expression of stem-cell markers (CD133,
CD44 and ALDH1). The results indicated that combination of BrMC
(5.0 µmol/l) and JSI-124 (10 nmol/l) had a significant
impact on the levels of CD133, CD44 and ALDH1 expression compared
to BrMC or JSI-124 alone (Fig.
8E).
Discussion
LCSCs are considered the key factors of HCC
progress. In addition, the tumor microenvironment plays an
important role during carcinogenesis. Carcinoma-associated
fibroblasts (CAFs) are one of the most crucial components of the
HCC microenvironment. In this study, we put forward that human
hepatic stellate cell line LX-2 can be activated to a
myofibro-blast-like phenotype through STAT3 pathway, and activated
LX-2 cells in turn promote the characteristics of LCSLCs. Moreover,
BrMC affected cross-talk of LCSLCs and LX-2 cells to reduce the
activation of HSC and then reversed the characteristics of
LCSLCs.
CAFs are thought to be activated, which is
characterized by the expression of α-SMA and FAP (35–37).
Moreover, activated fibroblasts in tumor tissues are considered as
CAFs. In our present study, we proved that LCSLCs derived from
SMMC-7721 cells had interaction with LX-2 cells, and made LX-2
cells pathologically activated with a myofibroblast-like phenotype,
named LCAHSC. Our results are consistent with the identification of
activated CAFs as these cells expressed α-SMA and FAP, whereas
cells treated without LCSLC-CM did not express FAP (Fig. 2). Noteworthy, the LX-2 cells treated
without LCSLC-CM also express α-SMA, which suggests that these
cells exist in an activated state under cell culture conditions. We
found that the conditioned medium from pathologically activated
LX-2 cells significantly promoted the characteristics of LCSLCs of
the SMMC-7721 cell line (Fig.
3).
STAT3, a transcription factor mediating various
cellular processes and participating in cellular transformation, is
aberrantly activated in numerous cancer types, including HCC. Since
the persistent activation of STAT3 promotes tumor cell
proliferation and survival, contributing to tumor progression,
abrogation of STAT3 signaling is emerging as a potential cancer
therapy strategy (38). We found
that the level of p-STAT3 was greater in LX-2 cell treated with
LCSLC-CM than control (Fig. 6A).
JSI-124, an inhibitor of the JAK/STAT pathway, could effectively
block STAT3 signaling in a dose-dependent manner, and further
inhibited the characteristics of liver cancer stem-like cells
induced by LCAHSC-CM (Fig. 7). To
our knowledge, this is the first study to demonstrate that the
STAT3 plays an important role in the interaction of LCSLCs and LX-2
cells.
8-bromo-7-methoxychrysin (BrMC) was synthesized
previously based of the lead compound ChR (28). In addition, BrMC has strong effects
of inhibition of proliferation and induction of apoptosis on
various types of cancer (39,40).
In the current study, we demonstrated that BrMC significantly
reduced the activation of LX-2 induced by LCSLC-CM, and inhibited
the properties of LCSLCs induced by LCAHSC-CM (Fig. 4). Of note, the level of p-STAT3 in
LCAHSC was greatly decreased after treated with BrMC (Fig. 6C), suggesting BrMC could inhibit the
activation of LX-2 induced by LCSLC-CM through attenuating STAT3
activation, and then reversed the characteristics of LCSLCs induced
by LCAHSC-CM.
Our data also showed that BrMC (5.0 µmol/l)
and JSI-124 (10 nmol/l) synergistically inhibited the expression of
p-STAT3 in LX-2 cells, and the expression levels of α-SMA and FAP
decreased in response to BrMC (5.0 µmol/l) and JSI-124 (10
nmol/l), which support the possibility that inhibition of the STAT3
pathway may significantly block transformation of LX-2 cells from a
quiescent state into a myofibroblast-like phenotype. Then,
combination of BrMC (5.0 µmol/l) and JSI-124 (10 nmol/l)
significantly decreased the sphere formation and the expression
levels of stem cell markers (CD133, CD44 and ALDH1) (Fig. 8). Taken together, our findings
indicated that combined BrMC and JSI-124 significantly inhibited
cross-talk of LX-2 cells and LCSLCs probably through suppressing
the activation of STAT3.
Compared with LCSCs, the relevance between LCSCs,
hepatic stellate cells, and STAT3 have been less clearly
identified. A recent report demonstrated that IL6/STAT3 axis was
sufficient for transdifferentiation of quiescent fibroblasts to
CAFs (34). It was reported that
cross-talk between hepatoma cells and activated HSCs engendered a
permissive inflammatory microenvironment that drives HCC
progression (12). In this study,
we discovered that human hepatic stellate cell line LX-2 could be
activated by LCSLC-CM through STAT3 signaling pathway, which was
inhibited by BrMC. However, the establishment of co-culture model
may be required to further demonstrate the physiological
significance of cross-talk of LCSLCs and LX-2 cells. Additionally,
LCSLCs-HSC crosstalk may result in extracellular matrix remodeling,
which can be associated with STAT3 signaling pathway. Studies might
also be necessary to explain the change of cytokine composing
extracellular matrix.
In conclusion, we demonstrated that the STAT3
signaling may be responsible for the interaction of LX-2 cells and
LCSLCs. BrMC and JSI-124 synergistically attenuate the CSC-like
properties induced by LCAHSC-CM through inhibiting STAT3 activation
and may present a potential clinical benefit for the treatment of
HCC. Thus, there is a great need to unravel the underlying
mechanisms of the STAT3 pathway in cross-talk of LX-2 cells and
LCSLCs and to further evaluate the therapeutic possibilities of
combination with JSI-124 and BrMC.
Acknowledgments
This study was supported by a Project of the NSFC
(grant nos. 30760248, 31400311 and 81172375), the Project of
Scientific Research Fund of Hunan Provincial Education Department
(grant no. 14C0707), the Project of Hunan Provincial natural
Science Foundation (grant no. 13JJ3061) and the Scientific Research
Fund of Hunan normal university (grant nos. 140668 and 140666).
References
|
1
|
Dudeck O and Ricke J: Advances in regional
chemotherapy of the liver. Expert Opin Drug Deliv. 8:1057–1069.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oishi N and Wang XW: Novel therapeutic
strategies for targeting liver cancer stem cells. Int J Biol Sci.
7:517–535. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ma S, Chan KW, Lee TK, Tang KH, Wo JY,
Zheng BJ and Guan XY: Aldehyde dehydrogenase discriminates the
CD133 liver cancer stem cell populations. Mol Cancer Res.
6:1146–1153. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang X, Sheng Y and Guan M: Co-expression
of stem cell genes CD133 and CD44 in colorectal cancers with early
liver metastasis. Surg Oncol. 21:103–107. 2012. View Article : Google Scholar
|
|
6
|
Zhang H, Chang WJ, Li XY, Zhang N, Kong JJ
and Wang YF: Liver cancer stem cells are selectively enriched by
low-dose cisplatin. Braz J Med Biol Res. 47:478–482. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Faurobert E, Bouin AP and Albiges-Rizo C:
Microenvironment, tumor cell plasticity, and cancer. Curr Opin
Oncol. 27:64–70. 2015. View Article : Google Scholar
|
|
8
|
Ye J, Wu D, Wu P, Chen Z and Huang J: The
cancer stem cell niche: Cross talk between cancer stem cells and
their microenvironment. Tumour Biol. 35:3945–3951. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang BB, Cheng JY, Gao HH, Zhang Y, Chen
ZN and Bian H: Hepatic stellate cells in
inflammation-fibrosis-carcinoma axis. Anat Rec (Hoboken).
293:1492–1496. 2010. View
Article : Google Scholar
|
|
10
|
Friedman SL: Hepatic stellate cells:
Protean, multifunctional, and enigmatic cells of the liver. Physiol
Rev. 88:125–172. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Friedman SL, Sheppard D, Duffield JS and
Violette S: Therapy for fibrotic diseases: Nearing the starting
line. Sci Transl Med. 5:167sr12013.PubMed/NCBI
|
|
12
|
Coulouarn C, Corlu A, Glaise D, Guénon I,
Thorgeirsson SS and Clément B: Hepatocyte-stellate cell cross-talk
in the liver engenders a permissive inflammatory microenvironment
that drives progression in hepatocellular carcinoma. Cancer Res.
72:2533–2542. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jia CC, Wang TT, Liu W, Fu BS, Hua X, Wang
GY, Li TJ, Li X, Wu XY, Tai Y, et al: Cancer-associated fibroblasts
from hepatocellular carcinoma promote malignant cell proliferation
by HGF secretion. PLoS One. 8:e632432013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Han Z, Wang X, Ma L, Chen L, Xiao M, Huang
L, Cao Y, Bai J, Ma D, Zhou J, et al: Inhibition of STAT3 signaling
targets both tumor-initiating and differentiated cell populations
in prostate cancer. Oncotarget. 5:8416–8428. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bharadwaj U, Eckols TK, Kolosov M,
Kasembeli MM, Adam A, Torres D, Zhang X, Dobrolecki LE, Wei W,
Lewis MT, et al: Drug-repositioning screening identified
piperlongumine as a direct STAT3 inhibitor with potent activity
against breast cancer. Oncogene. 34:1341–1353. 2015. View Article : Google Scholar
|
|
16
|
Liu C, Zeng Y, Dai LH, Cai TY, Zhu YM, Dou
DQ, Ma LQ and Sun YX: Mogrol represents a novel leukemia
therapeutic, via ERK and STAT3 inhibition. Am J Cancer Res.
5:1308–1318. 2015.PubMed/NCBI
|
|
17
|
Stechishin OD, Luchman HA, Ruan Y, Blough
MD, Nguyen SA, Kelly JJ, Cairncross JG and Weiss S: On-target
JAK2/STAT3 inhibition slows disease progression in orthotopic
xenografts of human glioblastoma brain tumor stem cells. Neuro
Oncol. 15:198–207. 2013. View Article : Google Scholar :
|
|
18
|
Song L, Rawal B, Nemeth JA and Haura EB:
JAK1 activates STAT3 activity in non-small-cell lung cancer cells
and IL-6 neutralizing antibodies can suppress JAK1-STAT3 signaling.
Mol Cancer Ther. 10:481–494. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Niu G, Wright KL, Ma Y, Wright GM, Huang
M, Irby R, Briggs J, Karras J, Cress WD, Pardoll D, et al: Role of
Stat3 in regulating p53 expression and function. Mol Cell Biol.
25:7432–7440. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wan S, Zhao E, Kryczek I, Vatan L,
Sadovskaya A, Ludema G, Simeone DM, Zou W and Welling TH:
Tumor-associated macrophages produce interleukin 6 and signal via
STAT3 to promote expansion of human hepatocellular carcinoma stem
cells. Gastroenterology. 147:1393–1404. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nieto N: Oxidative-stress and IL-6 mediate
the fibrogenic effects of [corrected] Kupffer cells on stellate
cells. Hepatology. 44:1487–1501. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Handy JA, Saxena NK, Fu P, Lin S, Mells
JE, Gupta NA and Anania FA: Adiponectin activation of AMPK disrupts
leptin-mediated hepatic fibrosis via suppressors of cytokine
signaling (SOCS-3). J Cell Biochem. 110:1195–1207. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qi J, Xia G, Huang CR, Wang JX and Zhang
J: JSI-124 (Cucurbitacin I) inhibits tumor angiogenesis of human
breast cancer through reduction of STAT3 phosphorylation. Am J Chin
Med. 43:337–347. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brechbuhl HM, Kachadourian R, Min E, Chan
D and Day BJ: Chrysin enhances doxorubicin-induced cytotoxicity in
human lung epithelial cancer cell lines: The role of glutathione.
Toxicol Appl Pharmacol. 258:1–9. 2012. View Article : Google Scholar :
|
|
25
|
Sun X, Huo X, Luo T, Li M, Yin Y and Jiang
Y: The anticancer flavonoid chrysin induces the unfolded protein
response in hepatoma cells. J Cell Mol Med. 15:2389–2398. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lirdprapamongkol K, Sakurai H, Abdelhamed
S, Yokoyama S, Athikomkulchai S, Viriyaroj A, Awale S, Ruchirawat
S, Svasti J and Saiki I: Chrysin overcomes TRAIL resistance of
cancer cells through Mcl-1 downregulation by inhibiting STAT3
phosphorylation. Int J Oncol. 43:329–337. 2013.PubMed/NCBI
|
|
27
|
Lin CM, Shyu KG, Wang BW, Chang H, Chen YH
and Chiu JH: Chrysin suppresses IL-6-induced angiogenesis via
downregulation of JAK1/STAT3 and VEGF: An in vitro and in ovo
approach. J Agric Food Chem. 58:7082–7087. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zheng X, Meng WD, Xu YY, Cao JG and Qing
FL: Synthesis and anticancer effect of chrysin derivatives. Bioorg
Med Chem Lett. 13:881–884. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ai XH, Zheng X, Tang XQ, Sun L, Zhang YQ,
Qin Y, Liu HQ, Xia H and Cao JG: Induction of apoptosis of human
gastric carcinoma SGC-7901 cell line by 5,
7-dihydroxy-8-nitrochrysin in vitro. World J Gastroenterol.
13:3824–3828. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ren KQ, Cao XZ, Liu ZH, Guo H, Quan MF,
Liu F, Jiang L, Xiang HL, Deng XY and Cao JG:
8-bromo-5-hydroxy-7-methoxychrysin targeting for inhibition of the
properties of liver cancer stem cells by modulation of Twist
signaling. Int J Oncol. 43:1719–1729. 2013.PubMed/NCBI
|
|
31
|
Quan MF, Xiao LH, Liu ZH, Guo H, Ren KQ,
Liu F, Cao JG and Deng XY: 8-bromo-7-methoxychrysin inhibits
properties of liver cancer stem cells via downregulation of
β-catenin. World J Gastroenterol. 19:7680–7695. 2013. View Article : Google Scholar
|
|
32
|
Ning Y, Li Q, Xiang H, Liu F and Cao J:
Apoptosis induced by 7-difluoromethoxyl-5,4′-di-n-octyl genistein
via the inactivation of FoxM1 in ovarian cancer cells. Oncol Rep.
27:1857–1864. 2012.PubMed/NCBI
|
|
33
|
Cao XZ, Xiang HL, Quan MF and He LH:
Inhibition of cell growth by BrMC through inactivation of Akt in
HER-2/neu-overexpressing breast cancer cells. Oncol Lett.
7:1632–1638. 2014.PubMed/NCBI
|
|
34
|
Lee KW, Yeo SY, Sung CO and Kim SH: Twist1
is a key regulator of cancer-associated fibroblasts. Cancer Res.
75:73–85. 2015. View Article : Google Scholar
|
|
35
|
Kalluri R and Zeisberg M: Fibroblasts in
cancer. Nat Rev Cancer. 6:392–401. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pietras K and Ostman A: Hallmarks of
cancer: Interactions with the tumor stroma. Exp Cell Res.
316:1324–1331. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xing F, Saidou J and Watabe K: Cancer
associated fibroblasts (CAFs) in tumor microenvironment. Front
Biosci (Landmark Ed). 15:166–179. 2010. View Article : Google Scholar
|
|
38
|
Yu H and Jove R: The STATs of cancer - new
molecular targets come of age. Nat Rev Cancer. 4:97–105. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang XH, Zheng X, Cao JG, Xiang HL, Liu F
and Lv Y: 8-Bromo-7-methoxychrysin-induced apoptosis of
hepatocellular carcinoma cells involves ROS and JNK. World J
Gastroenterol. 16:3385–3393. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xiao G, Tang X, Yao C and Wang C:
Potentiation of arsenic trioxide-induced apoptosis by
8-bromo-7-methoxychrysin in human leukemia cells involves depletion
of intracellular reduced glutathione. Acta Biochim Biophys Sin
(Shanghai). 43:712–721. 2011. View Article : Google Scholar
|