Introduction
Prostate cancer (PCa) is the most common solid-organ
malignancy and the second leading cause of cancer-related death in
males (1). This heterogeneous
neoplasm has been reported to be primarily regulated by androgenic
hormones and influenced by dietary habits (2). Chronic inflammation causes 20% of
human cancers and increases the risk of cancer, including gastric,
pancreatic, colon and lung cancer (3,4).
Increasing epidemiological and histopathological evidence also
suggests that the incidence of PCa is correlated with inflammation,
yet the concrete mechanism involved, particularly in human studies,
is still not fully understood (4,5).
Basically, chronic inflammation creates a milieu rich in
pro-inflammatory cytokines and growth factors that may lead to an
uncontrolled proliferative response and genetic mutations of
rapidly dividing cells (6,7). Thus, PCa is closely associated with
inflammation, particularly with the role of inflammatory
factors.
Interleukin-6 (IL-6) is a multifunctional
pro-inflammatory cytokine whose expression and function are altered
in a variety of human cancers. IL-6 has also been found to be
widely expressed in clinical specimens obtained from PCa patients
and in several PCa cell lines (2).
G protein-coupled receptor (GPCR) 48, also known as leucine-rich
repeat-containing GPCR (LGR) 4, is a glycoprotein hormone receptor
belonging to the GPCR superfamily (8) that plays an important role in the
development of multiple organs (9).
In general, LGR4 encodes one of the GPCRs for R-spondins (10). A recent study found that IL-6
enhanced the expression of LGR4 protein in osteosarcoma cells,
suggesting that LGR4 may be a novel responsive gene of IL-6 in
cancer progression (11).
Furthermore, previous preclinical and clinical studies have
confirmed that GPCR is overexpressed in PCa tissues (12). LGR4 contributes greatly to the
formation of various types of cancers, including lung, breast,
prostate and gastric cancer, as well as hepatoma (13,14).
The effect of LGR4 on cancer progression has been recognized, yet
the regulatory mechanism on LGR4 expression is still unclear.
microRNAs (miRNAs) are a set of endogenous small
non-coding RNAs (19–22 bases in length) and are used to regulate
gene expression by inhibiting translation or cleaving RNA
transcripts in a sequence-specific manner (15). Cumulative evidence indicates that
miRNAs regulate diverse biological processes, including cell
proliferation, invasion, migration and apoptosis (16). miRNAs downregulate multiple target
genes, including oncogenes and tumor-suppressor genes, while some
miRNAs function as tumor suppressors and others act as oncogenes
(17). In particular, miR-218, a
tumor-suppressing miRNA, has been extensively studied in several
types of cancers (18–21) and is highly downregulated in PCa
(22,23). In the present study, we demonstrated
that LGR4 is a downstream target of miR-218 and that miR-218
inhibited PCa cell proliferation and invasion through suppressing
LGR4 expression.
Materials and methods
Tissue samples and cell culture
Clinical specimens, including 51 tumor-adjacent
normal prostate (NP), 56 benign prostatic hyperplasia (BPH) and 58
PCa tissue samples from prostatectomy, were originally obtained
from patients at the First Affiliated Hospital of Zhengzhou
University. The specimens were maintained at −80°C and then
prepared for hematoxylin and eosin (H&E) staining, quantitative
real-time PCR (qRT-PCR), and western blot analyses following
institutional review board approval and the patient consent.
Human LNCaP and HEK293 cell lines were purchased
from the American Type Culture Collection (ATCC; Manassas, VA,
USA). LNCaP cells were maintained in RPMI-1640 medium supplemented
with 10% heat-inactivated fetal bovine serum (FBS) (both from
Gibco, Rockville, MD, USA), 100 U/ml penicillin and 100
μg/ml streptomycin (Life Technologies, Rockville, MD, USA)
in a humidified atmosphere of 5% CO2–95% air at 37°C.
LNCaP cells were passaged once weekly in medium containing FBS and
5 ng/ml rhIL-6 (human recombinant IL-6; R&D Systems,
Minneapolis, MN, USA). After 20 passages, the new LNCaP subline was
established and named 'LNCaP-IL-6+', and these cells
were characterized until passage 73 (24). During treatment with 25 ng/ml
exogenous IL-6, LNCaP-IL-6+ cells were cultured for 24 h
without supplementation with 5 ng/ml IL-6 during routine culture.
HEK293T cells were grown in Dulbecco's modified Eagle's medium
(DMEM) (Gibco) supplemented with high glucose, L-glutamine, sodium
pyruvate (Life Technologies) and 10% FBS.
Bromodeoxyuridine (BrdU) assay
Cultured cells (5×103) after transfection
or not were seeded in 96-well plates (Corning Inc., Corning, NY,
USA) with 0.1 nM synthetic androgen methyltrienolone (R1881;
17β-hydroxy-17α-methylestra-4,9,11-trien-3-one; New England
Nuclear, Dreieichenhain, Germany) treatment in the absence or
presence of 25 ng/ml IL-6 for 24 h. A cell proliferation
enzyme-linked immunosorbent assay (ELISA; BrdU kit; Beyotime,
Shanghai, China) was used to analyze the incorporation of BrdU
during DNA synthesis according to the manufacturer's instructions.
All of the experiments were performed in triplicate, and the
absorbance was measured at 450 nm by a microplate reader (Model
450; Bio-Rad, Hercules, CA, USA).
Cell cycle analysis
Cultured cells (4×105) in 6-well plates
(Corning Inc.) after transfection or not were treated with 0.1 nM
R1881 in the absence or presence of 25 ng/ml IL-6. After 24 h of
incubation, the cells were collected, fixed in 70% ethanol
overnight at 4°C, stained with propidium iodide (PI; 50
μg/ml) and DNase-free RNase A (100 μg/ml) (both from
Sigma, St. Louis, MO, USA), and then incubated at 37°C for 30 min
in the dark. Quantitative analysis of the DNA content was performed
on a fluorescence-activated cell sorting (FACS)Calibur flow
cytometer (Becton-Dickinson Immunocytometry Systems, San Jose, CA,
USA), and the fraction of the cell population in each phase of the
cell cycle was determined by flow cytometric analysis.
Transwell assay
An invasion assay was performed using
Matrigel-coated 24-well Transwell chambers with 8.0-μm
polycarbonate filters (Corning Inc.). The cultured cells
(1×105) after transfection or not were seeded in
serum-free RPMI-1640 medium in the upper chamber of each well,
while the bottom chamber was filled with normal culture medium
supplemented with 15% FBS. The drug was added to both the upper and
the lower chambers. After 24 h of treatment with 0.1 nM R1881 in
the absence or presence of 25 ng/ml IL-6, non-invading cells on the
upper surface were carefully removed with a cotton-tipped swab and
invading cells on the bottom surface of the filter were stained
with crystal violet (Sigma). Invasion ability was quantified by
counting the stained cells and statistically analyzing them.
qRT-PCR assay
Total RNA of the prostate tissues or cells,
including miRNAs was extracted using the miRNA isolation kit
(Ambion, USA) according to the manufacturer's instructions. The
purity and concentration of the RNA samples were determined using a
dual-beam ultraviolet spectrophotometer (Eppendorf, Hamburg,
Germany). The expression levels of mature miR-218 were analyzed
using TaqMan miRNA assay (Applied Biosystems, Foster City, CA,
USA), and were normalized to the expression of RNU48. The
2−ΔΔCt method was used to determine the relative
quantification of miR-218 levels. The above experiment was
performed in triplicate, and each assay included the negative
control that lacked cDNA (25).
IL-6 determination
Frozen prostate tissue samples were sectioned into
small pieces, homogenized and dissolved in RIPA lysis buffer
(Beyotime). The cells were lysed and IL-6 determination was
performed in triplicate via an hIL-6 ELISA kit (R&D Systems)
according to the manufacturer's instructions. The absorbance at 450
nm was determined using a microplate reader.
Cell transfection
hsa-miR-218 (5′-UUGUGCUUGAUCUAA CCAUGU-3′),
anti-miR-218 mimics (5′-ACAUGGUUAGAU CAAGCACAA-3′) and control
mimics (5′-CAGUACUUUUGUGUAGUACAA-3′) were synthesized by GenePharma
Technology (Shanghai, China). The cultured cells were seeded and
transfected with corresponding miRNAs at working concentrations
using Lipofectamime 2000 reagent (Invitrogen, Carlsbad, CA, USA)
according to the manufacturer's instructions. Transfection
efficiency was evaluated by qRT-PCR.
Luciferase reporter assay
The possible binding site between LGR4 and miR-218
was searched for in the microRNA database TargetScan 5.1
(http://www.targetscan.org/). The
3′-untranslated region (3′-UTR) of human LGR4 containing the
miR-218 targeting sequence was inserted into the pMIR-REPORT™ miRNA
expression reporter vector system (Ambion). The reporter vector
plasmid containing either the LGR4-wt 3′-UTR or LGR4-mut 3′-UTR
sequence was subsequently co-transfected with corresponding miRNAs
into HEK293T cells using Lipofectamine 2000 reagent. The cells were
harvested for luciferase assays 48 h after transfection. The
luciferase assay kit (Promega, Madison, WI, USA) was used to
measure the reporter activity according to the manufacturer's
protocol.
H&E staining
The prostate tissue samples from patients were fixed
in 10% formalin solution for at least 48 h, dehydrated in alcohol,
cleared in xylene and embedded in paraffin. Histological sections
(4-μm) were stained routinely with H&E (hematoxylin;
Fluka AG, Buchs SG, Switzerland; eosin Y, alcohol and water
soluble; Winlap, UK) and then subjected to microscopic
analysis.
Western blot analysis
Cell lysates were collected and protein
concentrations were determined using the Pierce BCA protein assay
kit (Thermo Scientific, Rockford, IL, USA). Equal amounts of
protein were processed for western blotting following standard
protocols. The primary antibodies used were rabbit anti-phospho-Akt
polyclonal antibody (#9271), rabbit anti-Akt polyclonal antibody
(#9272) and rabbit anti-β-catenin polyclonal antibody (#9562)
(dilution, 1:1,000; Cell Signaling Technology, Beverly, MA, USA);
rabbit anti-LGR4 polyclonal antibody (#SAB4502317) (dilution,
1:1,000; Sigma); mouse anti-human cyclin A1 monoclonal antibody
(#556600) (dilution, 1:250; BD Biosciences Pharmingen, San Diego,
CA, USA); mouse anti-human matrix metalloproteinase-9 (MMP-9)
monoclonal antibody (sc21733) and rabbit anti-β-actin polyclonal
antibody (sc130657) (dilution, 1:1,000; Santa Cruz Biotechnology,
Santa Cruz, CA, USA). The resultant protein bands after incubation
with a proper secondary antibody were visualized by enhanced
chemiluminescence (ECL) reagent (Beyotime). The absorbance values
of the target proteins were obtained through Gel-Pro Analyzer
version 4.0 software (Media Cybernetics, Silver Spring, MD,
USA).
Statistical analysis
Results expressed as mean ± SD were derived from
three independent experiments performed in triplicate. Statistical
analysis was performed by the Student's t-test or ANOVA. P<0.05
was considered to indicate a statistically significant result when
compared to the respective control.
Results
miR-218 expression is downregulated and
IL-6 expression is upregulated in the progression of prostate
cancer
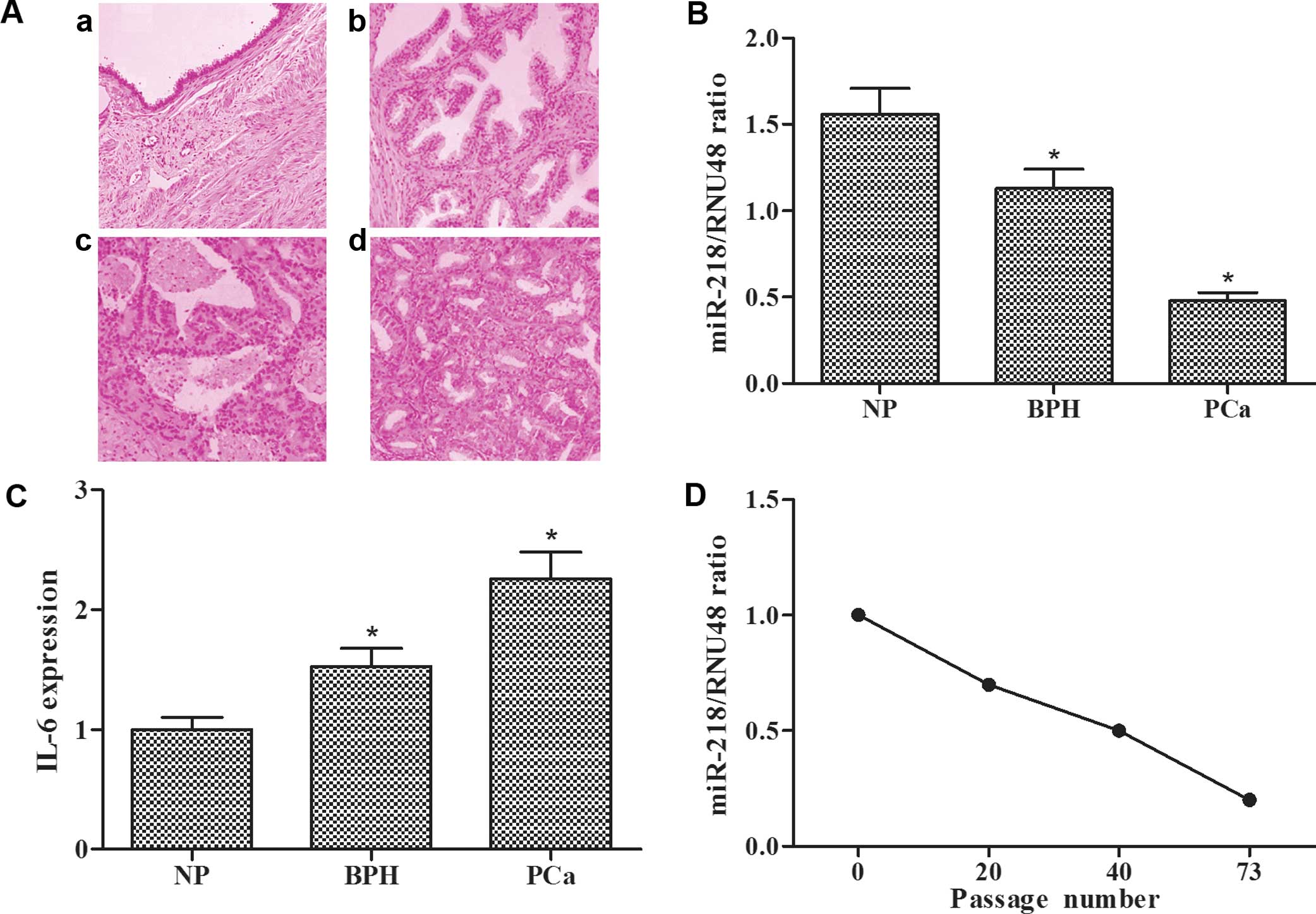
H&E stainings for human histological specimens
were examined, and representative images of NP, BPH and PCa tissue
samples are shown (Fig. 1A), in
which the probable pathological progression of prostate cancer can
be observed. qRT-PCR assay showed that miR-218 expression in the
BPH tissues was lower than that in the NP tissues and miR-218
expression in the PCa tissues was lower when compared with that in
the BPH tissues (Fig. 1B). However,
ELISA assay showed that IL-6 levels in the NP, BPH and PCa tissues
exhibited an opposite trend (Fig.
1C). The expression levels of miR-218 in the long-term
IL-6-stimulated LNCaP cells decreased with increasing passage
(Fig. 1D). These results revealed
that miR-218 expression was gradually decreased and IL-6 expression
was gradually increased in the process of prostate cancer
progression from NP, BPH to PCa and from LNCaP to
LNCaP-IL-6+ cells.
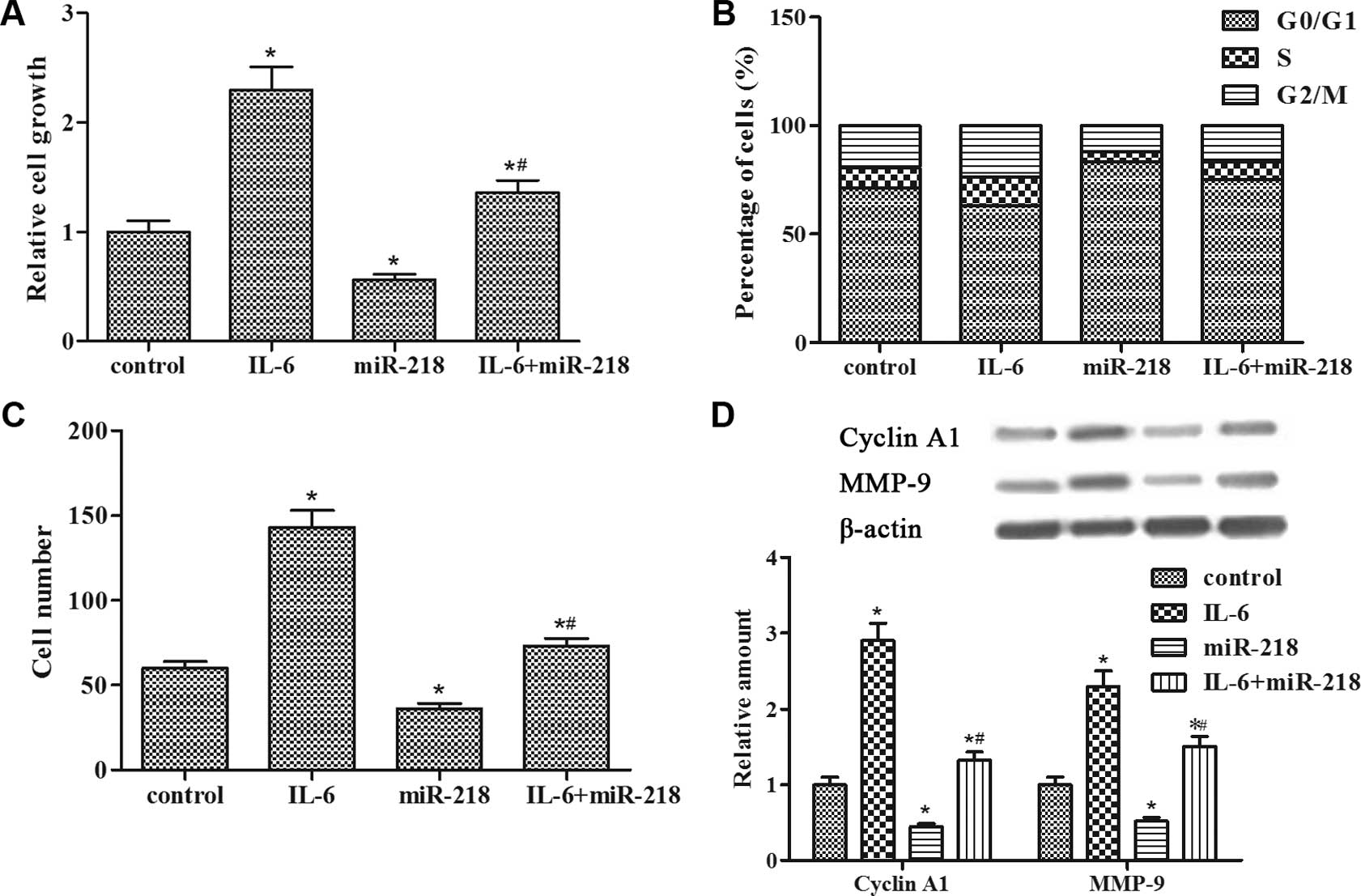
miR-218 impedes IL-6-induced
LNCaP-IL-6+ cell proliferation and invasion
BrdU assay revealed that IL-6 promoted
LNCaP-IL-6+ cell proliferation, whereas miR-218
transfection inhibited the enhanced cell growth (Fig. 2A). The data from FACS analysis
showed that IL-6 led to a reduction in the percentage of cells in
the G0/G1 phase of the cell cycle as compared to the control group;
conversely, miR-218 transfection promoted an accumulation of cells
in the G0/G1 phase (Fig. 2B).
Transwell assay showed that the invasive ability of the
LNCaP-IL-6+ cells was significantly promoted by IL-6 and
markedly inhibited by miR-218 transfection (Fig. 2C). In addition, miR-218 transfection
suppressed the increased expression of cyclin A1 and MMP-9 proteins
induced by IL-6 (Fig. 2D). Taken
together, these findings indicated that miR-218 impeded
IL-6-induced LNCaP-IL-6+ cell proliferation and
invasion.
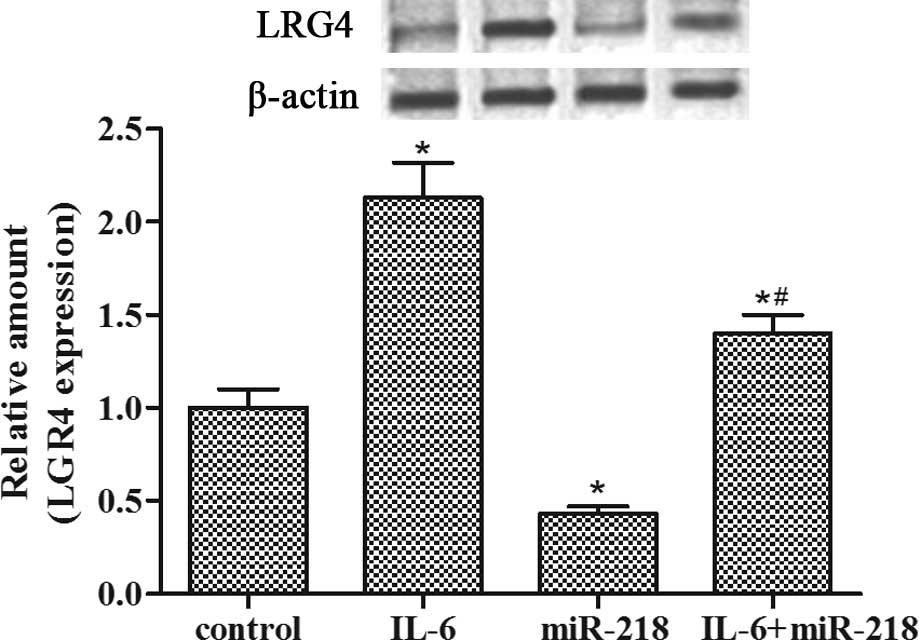
miR-218 inhibits IL-6-induced LGR4
expression in LNCaP-IL-6+ cells
Western blotting showed that LGR4 expression was
upregulated by IL-6 pretreatment while miR-218 transfection
abolished the enhanced expression of LGR4 protein induced by IL-6
incubation in the LNCaP-IL-6+ cells (Fig. 3). These data suggest that miR-218
may be a promising candidate for directly targeting LGR4 in
LNCaP-IL-6+ cells.
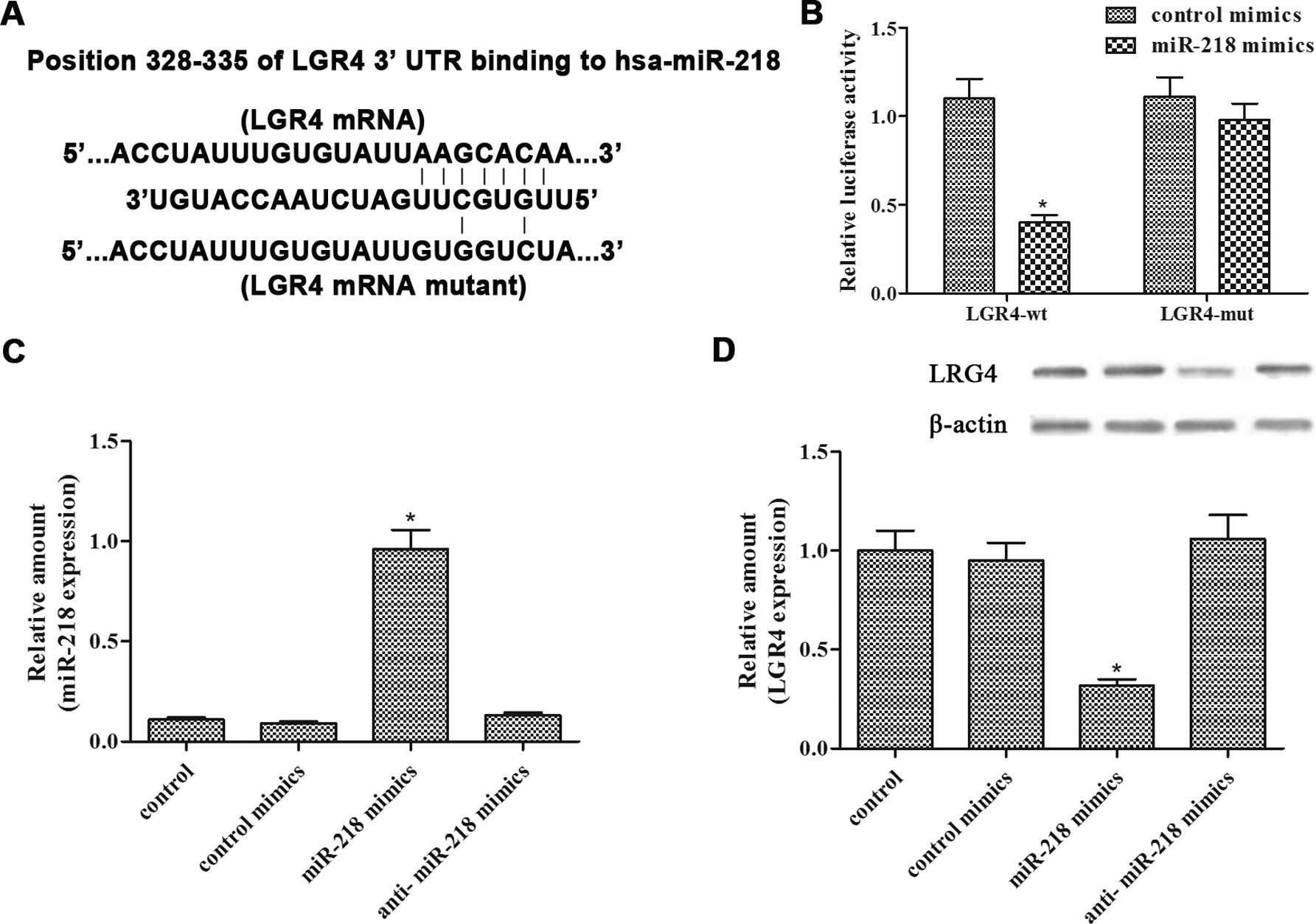
miR-218 targets LGR4 by binding to its
3′-UTR
Bioinformatic prediction showed that there was one
putative binding site between miR-218 and the 3′-UTR of LGR4
(Fig. 4A). To confirm the binding,
a luciferase reporter assay was performed by evaluating the
luciferase activity of HEK293T cells transfected with the pMIR-LGR4
3′-UTR plasmids and comparing this activity with that transfected
with control plasmids. The results showed that miR-218
significantly suppressed luciferase expression of LGR4-wt, whereas
LGR4-mut induced no suppressive effect (Fig. 4B). In addition, qRT-PCR analysis
confirmed that miR-218 transfection resulted in an increase in
mature miR-218 in the LNCaP-IL-6+ cells (Fig. 4C). In addition, the LGR4 protein
level was suppressed following miR-218 transfection in the
LNCaP-IL-6+ cells (Fig.
4D). In summary, these results indicated that miR-218 directly
targeted LGR4 in the LNCaP-IL-6+ cells.
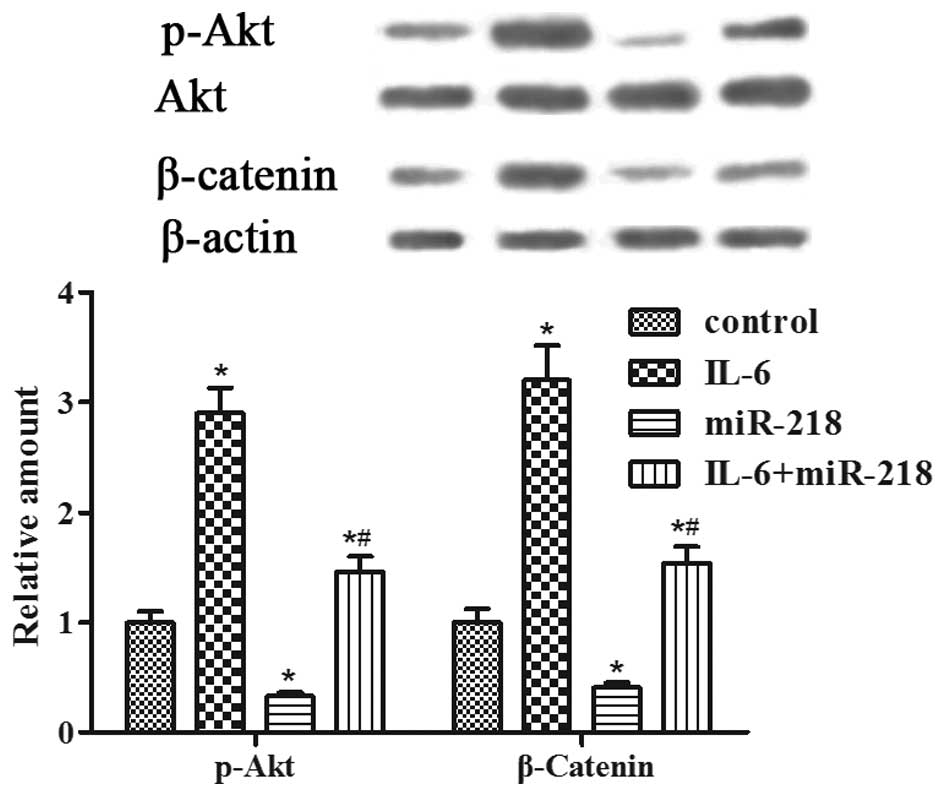
miR-218 inhibits IL-6-induced LGR4
expression via the PI3K/Akt and Wnt/β-catenin pathways
Furthermore, we determined whether the PI3K/Akt and
Wnt/β-catenin pathways are involved in miR-218-induced
LNCaP-IL-6+ cell proliferation and invasion. Western
blot analysis revealed that the levels of phospho-Akt and β-catenin
expression were enhanced by IL-6 pretreatment but were inhibited by
miR-218 transfection (Fig. 5). The
results indicated that miR-218 robustly suppressed the activated
PI3K/Akt and Wnt/β-catenin pathways induced by IL-6 in the
LNCaP-IL-6+ cells.
Discussion
Prostate cancer (PCa) is the most frequently
diagnosed cancer in males, and inflammation has been associated
with several diseases of the prostate, including benign enlargement
and cancer (26). The expression
and function of IL-6 and its receptor in PCa have also been the
subject of numerous recent studies and have been investigated in
human PCa cells as well as human PCa and BPH tissues obtained
directly from patients (26,27).
miRNAs have the ability to specifically regulate multiple
protein-coding genes, and bioinformatic predictions indicate that
miRNAs regulate more than 30–60% of the protein-coding genes in the
human genome (28). Hence,
identification of aberrantly expressed IL-6 and miRNAs is an
important first step towards elucidating IL-6-mediated or
miRNA-mediated oncogenic pathways.
Previous research has shown that miR-218 is
significantly lower in PCa specimens and loss of tumor-suppressive
miR-218 was found to enhance PCa cell invasion and migration
(22,23). IL-6 expression was significantly
higher in PCa than in NP tissues and in patients with BPH; IL-6
expression was also elevated in patients with prostatitis compared
with those without (29).
Consistently, our results showed that miR-218 expression was
gradually decreased and IL-6 expression was gradually increased in
the process of PCa progression from NP, BPH to PCa and from LNCaP
to LNCaP-IL-6+ cells.
miR-218 functions as a tumor-suppressing miRNA and
has been extensively studied in several types of cancers (18,20,21).
For instance, miR-218 inhibits PCa cell proliferation and induces
cell apoptosis, thus playing a tumor-suppressor role in PC3 cells
(22). The effect of IL-6 on the
proliferation of hormone-insensitive PCa and hormone-sensitive
LNCaP cells varies under different conditions (30), yet research has shown that long-term
treatment of human PCa LNCaP cells with IL-6 leads to abolishment
of inhibitory growth response (24)
and increased invasiveness (31).
In the present study, we established the LNCaP-IL-6+
cell line by long-term incubation with a low concentration of IL-6
and confirmed that IL-6 promoted LNCaP-IL-6+ cell
proliferation and invasion, whereas miR-218 reversed the
accelerative effect of IL-6 on cell proliferation and invasion in
the LNCaP-IL-6+ cells.
LGR4 exists widely in multiple tissues and acts as a
key regulator playing an important role in the process of prostate
development and prostate stem cell differentiation (14,32).
Convincing evidence confirms that abnormal expression and
activation of GPCR are intimately related to increased cancer cell
proliferation, tumor growth and metastasis (12). For instance, LGR4 promotes PCa cell
and tumor growth via the PI3K/Akt pathway (32), and LGR4 expression is essential for
the nuclear accumulation of β-catenin in osteosarcoma cells
(11). Additionally, IL-6 plays a
specific role in the induction of LGR4 (11). However, miR-218 was found to inhibit
AKT phosphorylation in oral cancer (33) and the downregulation of miR-218 was
found to lead to stabilization and nuclear accumulation of
β-catenin (34). Our results
demonstrated that miR-218 targeted LGR4 by binding to its 3′-UTR,
resulting in decreased LGR4 expression induced by IL-6 in the
LNCaP-IL-6+ cells and downregulated the phosphorylation
of PI3K/Akt and the accumulation of β-catenin.
Furthermore, cyclin A1, an important downstream
target of PI3K/Akt transduced survival signals in response to IL-6
stimulation. Indeed, IL-6 promoted cell survival by activating the
PI3K/Akt pathway and increasing the expression of cyclin A1 protein
in LNCaP and LNCaP-IL-6+ cells (35). In addition, IL-6 was found to
upregulate MMP-9 expression to facilitate PCa cell invasion through
the PI3K/Akt pathway (36,37). In the present study, the results
revealed that miR-218 robustly suppressed the expression of cyclin
A1 and MMP-9 via the downregulation of the PI3K/Akt and
Wnt/β-catenin pathways facilitated by IL-6 in the
LNCaP-IL-6+ cells.
In conclusion, our results clearly showed the
involvement of the miR-218/LGR4 regulatory pathway in IL-6-induced
cell proliferation and invasion in the LNCaP-IL-6+ cells
via PI3K/Akt and Wnt/β-catenin signaling. The present study
elucidated the anticancer effects of miR-218 in PCa and present a
novel target for PCa therapy.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (nos. 81100464 and 81200883), the
General Financial Grant from the China Postdoctoral Science
Foundation (no. 2012M521410), the Foundation of Henan Educational
Committee, the Scientific and Technological Innovation Project of
Zhengzhou City, the Henan Natural Science Foundation, the Henan
Postdoctoral Science Foundation of China, and the Youth Foundation
for Medical Doctor of the First Affiliated Hospital of Zhengzhou
University.
Abbreviations:
|
GPCR
|
G protein-coupled receptor
|
|
LGR4
|
leucine-rich repeat-containing G
protein-coupled receptor 4
|
|
IL-6
|
interleukin-6
|
|
NP
|
normal prostate
|
|
BPH
|
benign prostatic hyperplasia
|
|
PCa
|
prostate cancer
|
|
MMP-9
|
matrix metalloproteinase-9
|
|
miRNAs
|
microRNAs
|
|
H&E
|
hematoxylin and eosin
|
|
FACS
|
fluorescence-activated cell
sorting
|
|
qRT-PCR
|
quantitative real-time PCR
|
|
3′-UTR
|
3′-untranslated region
|
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Culig Z: Proinflammatory cytokine
interleukin-6 in prostate carcinogenesis. Am J Clin Exp Urol.
2:231–238. 2014.PubMed/NCBI
|
|
3
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
De Marzo AM, Platz EA, Sutcliffe S, Xu J,
Grönberg H, Drake CG, Nakai Y, Isaacs WB and Nelson WG:
Inflammation in prostate carcinogenesis. Nat Rev Cancer. 7:256–269.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cheng I, Witte JS, Jacobsen SJ, Haque R,
Quinn VP, Quesenberry CP, Caan BJ and Van Den Eeden SK:
Prostatitis, sexually transmitted diseases, and prostate cancer:
The California Men's Health Study. PLoS One. 5:e87362010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Caruso C, Balistreri CR, Candore G,
Carruba G, Colonna-Romano G, Di Bona D, Forte GI, Lio D, Listì F,
Scola L, et al: Polymorphisms of pro-inflammatory genes and
prostate cancer risk: A pharmacogenomic approach. Cancer Immunol
Immunother. 58:1919–1933. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Klein EA and Silverman R: Inflammation,
infection, and prostate cancer. Curr Opin Urol. 18:315–319. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hsu SY, Liang SG and Hsueh AJ:
Characterization of two LGR genes homologous to gonadotropin and
thyrotropin receptors with extracellular leucine-rich repeats and a
G protein-coupled, seven-transmembrane region. Mol Endocrinol.
12:1830–1845. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
O'Neill PR, Giri L, Karunarathne WK, Patel
AK, Venkatesh KV and Gautam N: The structure of dynamic GPCR
signaling networks. Wiley Interdiscip Rev Syst Biol Med. 6:115–123.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
de Lau W, Barker N, Low TY, Koo BK, Li VS,
Teunissen H, Kujala P, Haegebarth A, Peters PJ, van de Wetering M,
et al: Lgr5 homologues associate with Wnt receptors and mediate
R-spondin signalling. Nature. 476:293–297. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu J, Wei W, Guo CA, Han N, Pan JF, Fei T
and Yan ZQ: Stat3 upregulates leucine-rich repeat-containing G
protein-coupled receptor 4 expression in osteosarcoma cells. Biomed
Res Int. 2013:3106912013.
|
|
12
|
Dorsam RT and Gutkind JS:
G-protein-coupled receptors and cancer. Nat Rev Cancer. 7:79–94.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu YB, Xu L, Chen M, Ma HN and Lou F:
GPR48 promotes multiple cancer cell proliferation via activation of
Wnt signaling. Asian Pac J Cancer Prev. 14:4775–4778. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luo W, Rodriguez M, Valdez JM, Zhu X, Tan
K, Li D, Siwko S, Xin L and Liu M: Lgr4 is a key regulator of
prostate development and prostate stem cell differentiation. Stem
Cells. 31:2492–2505. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hwang HW and Mendell JT: MicroRNAs in cell
proliferation, cell death, and tumorigenesis. Br J Cancer.
94:776–780. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stahlhut Espinosa CE and Slack FJ: The
role of microRNAs in cancer. Yale J Biol Med. 79:131–140. 2006.
|
|
18
|
Yang L, Li Q, Wang Q, Jiang Z and Zhang L:
Silencing of miRNA-218 promotes migration and invasion of breast
cancer via Slit2-Robo1 pathway. Biomed Pharmacother. 66:535–540.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu DW, Cheng YW, Wang J, Chen CY and Lee
H: Paxillin predicts survival and relapse in non-small cell lung
cancer by microRNA-218 targeting. Cancer Res. 70:10392–10401. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tie J, Pan Y, Zhao L, Wu K, Liu J, Sun S,
Guo X, Wang B, Gang Y, Zhang Y, et al: MiR-218 inhibits invasion
and metastasis of gastric cancer by targeting the Robo1 receptor.
PLoS Genet. 6:e10008792010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
He X, Xiao X, Dong L, Wan N, Zhou Z, Deng
H and Zhang X: MiR-218 regulates cisplatin chemosensitivity in
breast cancer by targeting BRCA1. Tumour Biol. 36:2065–2075. 2015.
View Article : Google Scholar
|
|
22
|
Han G, Fan M and Zhang X: microRNA-218
inhibits prostate cancer cell growth and promotes apoptosis by
repressing TPD52 expression. Biochem Biophys Res Commun.
456:804–809. 2015. View Article : Google Scholar
|
|
23
|
Nishikawa R, Goto Y, Sakamoto S, Chiyomaru
T, Enokida H, Kojima S, Kinoshita T, Yamamoto N, Nakagawa M, Naya
Y, et al: Tumor-suppressive microRNA-218 inhibits cancer cell
migration and invasion via targeting of LASP1 in prostate cancer.
Cancer Sci. 105:802–811. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hobisch A, Ramoner R, Fuchs D,
Godoy-Tundidor S, Bartsch G, Klocker H and Culig Z: Prostate cancer
cells (LNCaP) generated after long-term interleukin 6 (IL-6)
treatment express IL-6 and acquire an IL-6 partially resistant
phenotype. Clin Cancer Res. 7:2941–2948. 2001.PubMed/NCBI
|
|
25
|
Gu CH, Tian FY, Pu JR, Zheng LD, Mei H,
Zeng FQ, Yang JJ, Kan QC and Tong QS: Over-expression of
testis-specific expressed gene 1 attenuates the proliferation and
induces apoptosis of GC-1spg cells. J Huazhong Univ Sci Technolog
Med Sci. 34:535–541. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ben Jemaa A, Sallami S, Ramarli D,
Colombatti M and Oueslati R: The proinflammatory cytokine, IL-6,
and its interference with bFGF signaling and PSMA in prostate
cancer cells. Inflammation. 36:643–650. 2013. View Article : Google Scholar
|
|
27
|
Hobisch A, Rogatsch H, Hittmair A, Fuchs
D, Bartsch G Jr, Klocker H, Bartsch G and Culig Z:
Immunohistochemical localization of interleukin-6 and its receptor
in benign, premalignant and malignant prostate tissue. J Pathol.
191:239–244. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Friedman RC, Farh KK, Burge CB and Bartel
DP: Most mammalian mRNAs are conserved targets of microRNAs. Genome
Res. 19:92–105. 2009. View Article : Google Scholar :
|
|
29
|
Engelhardt PF, Seklehner S, Brustmann H,
Lusuardi L and Riedl CR: Immunohistochemical expression of
interleukin-2 receptor and interleukin-6 in patients with prostate
cancer and benign prostatic hyperplasia: Association with
asymptomatic inflammatory prostatitis NIH category IV. Scand J
Urol. 49:120–126. 2015. View Article : Google Scholar
|
|
30
|
Nguyen DP, Li J and Tewari AK:
Inflammation and prostate cancer: The role of interleukin 6 (IL-6).
BJU Int. 113:986–992. 2014. View Article : Google Scholar
|
|
31
|
Shariat SF, Chromecki TF, Hoefer J,
Barbieri CE, Scherr DS, Karakiewicz PI, Roehrborn CG, Montorsi F,
Culig Z and Cavarretta IT: Soluble gp130 regulates prostate cancer
invasion and progression in an interleukin-6 dependent and
independent manner. J Urol. 186:2107–2114. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liang F, Yue J, Wang J, Zhang L, Fan R,
Zhang H and Zhang Q: GPCR48/LGR4 promotes tumorigenesis of prostate
cancer via PI3K/Akt signaling pathway. Med Oncol. 32:492015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Uesugi A, Kozaki K-I, Tsuruta T, Furuta M,
Morita K, Imoto I, Omura K and Inazawa J: The tumor suppressive
microRNA miR-218 targets the mTOR component Rictor and inhibits AKT
phosphorylation in oral cancer. Cancer Res. 71:5765–5778. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lerner C, Wemmert S and Schick B:
Preliminary analysis of different microRNA expression levels in
juvenile angiofibromas. Biomed Rep. 2:835–838. 2014.PubMed/NCBI
|
|
35
|
Wegiel B, Bjartell A, Culig Z and Persson
JL: Interleukin-6 activates PI3K/Akt pathway and regulates cyclin
A1 to promote prostate cancer cell survival. Int J Cancer.
122:1521–1529. 2008. View Article : Google Scholar
|
|
36
|
Ahmad A, Sarkar SH, Aboukameel A, Ali S,
Biersack B, Seibt S, Li Y, Bao B, Kong D, Banerjee S, et al:
Anticancer action of garcinol in vitro and in vivo is in part
mediated through inhibition of STAT-3 signaling. Carcinogenesis.
33:2450–2456. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shukla S, Maclennan GT, Hartman DJ, Fu P,
Resnick MI and Gupta S: Activation of PI3K-Akt signaling pathway
promotes prostate cancer cell invasion. Int J Cancer.
121:1424–1432. 2007. View Article : Google Scholar : PubMed/NCBI
|