Introduction
Lung cancer is the most common cause of
cancer-related death in both men and women (1), with ~1.5 million new cases diagnosed
annually worldwide. Lung cancer can be histologically divided into
two types: small cell lung carcinomas (SCLCs) and non-small cell
lung carcinomas (NSCLCs), with the latter accounting for more than
80% of all lung cancer cases (2).
Within NSCLCs, there are also heterogeneous subtypes of tumors,
including squamous, adenocarcinoma and large cell carcinomas
(1). Once diagnosed, patients with
NSCLC are often found to be in advanced stages (3), and are therefore prone to metastasis
and recurrence. As a result, despite recent progress, the
therapeutic outcome of NSCLC remains poor. Currently, a combination
of chemotherapy regimens with platinum-based drugs, particularly
cisplatin, is the most effective first-line therapy for the
treatment of NSCLC according to clinical guidelines. It has been
well established in the literature that the induction of DNA
lesions and cell apoptosis is the major mechanism of cisplatin
action (4). However, the efficacy
of chemotherapy is limited for NSCLC since chemoresistance commonly
develops and results in failure of chemotherapy. The development of
cisplatin resistance is mediated through multiple mechanisms,
including gene mutations or epigenetic changes that influence the
uptake and metabolism of drugs by cancer cells (5,6).
Recently, a series of studies suggest the
involvement of spindle and kinetochore-associated complex subunit 1
(SKA1) in the growth and proliferation of multiple cancer types,
including oral adenosquamous (7)
and hepatocellular carcinoma (8),
bladder (9), gastric (10), prostate (11) and thyroid cancer (12), and glioblastoma (13). SKA1, along with SKA2 and SKA3,
constitute the SKA complex. The SKA complex is localized to the
spindle microtubule and on the outer kinetochore interface during
mitosis. As it has been shown, this complex is essential for
stabilizing the attachment of spindle microtubules to kinetochores
and for maintaining the metaphase plate, and is therefore essential
for proper chromosome segregation during mitosis (14–16).
Efficient depletion of the SKA complex was found to lead to
unstable kinetochore microtubule structure formation and severe
chromosome alignment defects (14).
In addition, the SKA complex has been implicated in bypassing of
the spindle checkpoint (17) and in
the maintenance of sister chromatid cohesion (16).
Although SKA1 has been implicated in the development
of multiple cancers, its involvement in the recurrence, metastasis
and drug resistance in NSCLC has not been studied. In the present
study, we demonstrated that the expression levels of SKA1 were
elevated in NSCLC and were correlated with cancer progression and
malignancy. We also reported that SKA1 positively regulated the
proliferation and metastatic ability of NSCLC cells. Additionally,
we determined that SKA1 contributed to cisplatin resistance in
NSCLC cells by protecting these cells from cisplatin-induced cell
apoptosis. Finally, SKA1 appeared to regulate the ERK1/2 and Akt
signaling pathways in NSCLC cells.
Materials and methods
Reagents and antibodies
TRIzol reagent and Lipofectamine 2000 were purchased
from Invitrogen (Carlsbad, CA, USA). The First Strand cDNA
Synthesis kit and SYBR Premix Taq were from Takara (Dalian,
Liaoning, China).
3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide
(MTT), bromodeoxyuridine (BrdU) and the anti-BrdU antibody were
purchased from Sigma (St. Louis, MO, USA). DAPI, BCA protein assay
and ECL Plus kits were obtained from Beyotime Institute of
Biotechnology (Beijing, China). BD BioCoat Matrigel invasion
chambers were purchased from BD Biosciences (San Jose, CA,
USA).
The primary antibodies against human SKA1 and
cleaved caspase-3 were obtained from Abcam (Cambridge, MA, USA).
Anti-Bcl-2, anti-Bax, anti-p-ERK1/2, anti-ERK1/2, anti-p-Akt,
anti-Akt, anti-p21, anti-cyclin D1 and anti-GAPDH were purchased
from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The
horseradish peroxidase-conjugated anti-rabbit and anti-mouse IgG
secondary antibodies were purchased from Santa Cruz Biotechnology.
Biotinylated- and Cy3-conjugated anti-rabbit secondary antibodies
were purchased form Boster (Wuhan, Hubei, China).
Cell culture and transfection
The human non-small cell lung cancer (NSCLC) cell
lines (A549, H23, H520 and H1975) were purchased from the American
Type Culture Collection (ATCC; USA) and cultured in RPMI-1640
medium supplemented with 10% fetal calf serum (FBS), 100 U/ml
penicillin G, 100 mg/ml streptomycin sulfate, and 2 mmol/l
glutamine (all from Gibco, Rockville, MD, USA) at 37°C in a
humidified incubator under an atmosphere of 5% CO2 in
air.
Human SKA1 cDNA was amplified from A549 cells by PCR
and constructed into the pcDNA3 vector (Invitrogen). SKA1 siRNA and
control siRNA were purchased from Santa Cruz Biotechnology.
Transfections of the vector or siRNA to cells were performed using
Lipofectamine 2000 according to the manufacturer's protocol.
Quantitative real-time RT-PCR
(qRT-PCR)
Total RNA was extracted with TRIzol reagent from
NSCLC samples and cell lines according to the manufacturer's
instructions. Total RNA (5 μg) was reversely transcribed to
cDNA using the First Strand cDNA Synthesis kit. The expression of
mRNA was examined by qRT-PCR with SYBR Premix Taq and
Applied Biosystems 7500 Sequence Detection system. The relative
expression levels of mRNA were normalized to GAPDH expression and
the amplification results for qRT-PCR were calculated using the
2−ΔΔCt method. The PCR reaction was performed using
SKA1 primers: 5′-TGATGTGCCAGGAAGGTGAC-3′ (forward) and
5′-CAAAGGATACAGATGAACAACAGC-3′ (reverse); GAPDH primers:
5′-GTGGACATCCGCAAAGAC-3′ (forward) and 5′-AAAGGGTGTAACGCAACTA-3′
(reverse).
Immunohistochemistry
The paraffin-embedded tissue samples from
postoperative patients were sectioned into 5-μm slices and
embedded with paraffin after fixation with 10% formaldehyde. The
samples were then deparaffinized in xylene, rehydrated and the
endogenous peroxidase activity was quenched by 3% hydrogen
peroxide. Non-specific binding was blocked with 1% bovine serum
albumin. The sections were then incubated overnight at 4°C with the
SKA1 primary antibody, and the subsequent secondary antibody
followed by the DAB secondary antibody.
MTT and cell number counting assay
MTT assay was used to assess cell proliferation and
viability. Briefly, transfected NSCLC cells were seeded at a
density of 2.5×103 cells/well in a 100 μl volume
of medium in 96-well plates and allowed to attach overnight. MTT
(20 μl at 5 mg/ml) was added to the medium after treatment.
After incubation at 37°C for 4 h, the supernatant was aspirated,
and 100 μl dimethyl sulfoxide (DMSO) was added to each well
to dissolved the precipitate. The negative control well contained
medium only. Absorbance at 570 nm was measured by a 96-well
microplate reader. The survival ratio was calculated using the
following equation: Survival ratio (%) =
ODtreated/ODcontrol × 100. For cell counting,
NSCLC cells were plated onto 35-mm dishes at 2×103
cells/dish. On days 0–6, the cells were trypsinized and counted
with a hemocytometer under a microscope.
BrdU assay
BrdU assay was used to analyze cell proliferation
and was carried out according to the manufacturer's
recommendations. The NSCLC cells were seeded on a 4-well chamber
slide and cultured in growth medium overnight. BrdU-labeled medium
was then replaced and incubated at 37°C for 3 h. The cell medium
was removed and cells were fixed with ethanol for 20 min at −20°C.
The cells were permeablized with 0.1% Triton X-100 in
phosphate-buffered saline (PBS) and blocked with 3% FBS in PBS
solution. After washing the cells, anti-BrdU reagent was added for
30 min at 37°C. The nuclei were counterstained with DAPI. For
fluorescence detection, cell images of each treatment were captured
with an Olympus inverted microscope.
Wound healing assay
The wound healing assay was used to evaluate the
cancer cell motility capacity. Briefly, transfected NSCLC cells
were seeded in 6-well plates and cultured overnight. When the
culture had reached nearly 90% confluency, the cell layer was
wounded by dragging a 1,000-μl sterile pipette tip through
the monolayer and washed with PBS twice to remove cellular debris.
Cells were then cultured for up to 48 h with growing medium.
Photographic images of the plates were acquired under a microscope
and the relative surface traveled by the leading edge was
assessed.
Transwell assay
Cell invasion activity was assessed using 24-well BD
BioCoat Matrigel invasion chambers (8-μm pores) according to
the manufacturer's instructions. Transfected NSCLC cells
(5×104 cells/ml) were loaded into the top chamber with
serum-free media. In the lower chamber, 450 μl growing
medium with 10% FBS was added. After 24 h of cultivation, the
medium was removed and the Transwell chamber was gently wiped with
a cotton swab. The migrated cells were fixed in 4%
paraformaldehyde, stained with crystal violet solution and counted
under a microscope.
Western blotting
NSCLC cells were washed with PBS and incubated in
RIPA buffer (150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1%
SDS, 50 mM Tris pH 8.0, 5.0 mM EDTA pH 8.0, 0.5 mM dithiothreitol
and 1 mM phenylmethylsulfonyl fluoride) containing protease
inhibitor on ice for 30 min, and centrifuged at 12,000 × g for 15
min at 4°C. Protein concentration was determined with the BCA
protein assay kit and equal amounts of total protein were separated
by 10% SDS page, and the protein was transferred from the gel to
the nitrocellulose membrane. The nitrocellulose membrane was then
blocked with 5% BSA for 1 h. The membrane was then probed with a
specific primary antibody and gently shaken at 4°C overnight. The
next day, the membrane was washed three times with TBST buffer, and
incubated with secondary antibodies for 1 h at room temperature.
The image was acquired using the ECL plus kit.
Gelatin zymography
Gelatinolytic activity of MMP-2 (gelatinase A) and
MMP-9 (gelatinase B) was analyzed by gelatin gel zymography. NSCLC
cells were harvested using lysis buffer [0.5 M Tris-HCl, pH 6.8, 4%
sodium dodecyl sulfate (SDS), 0.005% bromophenol blue, 20%
glycerol] at room temperature for 10 min, and an equal amount of
total protein was separated on 10% SDS-polyacrylamide
electrophoresis gels containing 0.1% gelatin. The gels were
denatured in denaturing buffer (2.5% Triton X-100) for 30 min at
room temperature, and then incubated at 37°C overnight in
developing buffer (50 mM Tris, 0.2 M NaCl, 5 mM CaCl2,
0.02% Brij-35, pH 7.6) allowing the reactivated enzyme to degrade
the copolymerized substrate. After incubation, the gels were
stained with 0.25% Coomassie brilliant blue R-250 for 4 h at room
temperature and destained in distilled water containing 30%
methanol and 10% glacial acetic acid. The areas where the gelatin
substrate had been degraded by gelatinases developed into white
lines on a dark background.
Patient samples
A total of 65 samples were surgically obtained from
NSCLC patients at the Department of Thoracic Surgery, Fudan
University Shanghai Cancer Center. The clinical characteristics of
all of the patients were recorded (Table I). The stage of NSCLC was
categorized according to surgical and pathological findings, which
were based on the guidelines described by the 6th edition of
AJCC/UICC. Written informed consent was obtained from all patients.
The present study was approved by the Institutional Ethical Review
Boards of Fudan University Shanghai Cancer Center.
 | Table ICorrelation between SKA1 and the
clinicopathological characteristics of the 65 NSCLC patients. |
Table I
Correlation between SKA1 and the
clinicopathological characteristics of the 65 NSCLC patients.
| Features | No. of pts. | SKA1
| P-value |
|---|
| Low n (%) | High n (%) |
|---|
| All patients | 65 | 32 (49.2) | 33 (50.8) | |
| Age (years) | | | | 0.108 |
| <60 | 35 | 14 (40.0) | 21 (60.0) | |
| ≥60 | 30 | 18 (60.0) | 12 (40.0) | |
| Gender | | | | 0.302 |
| Male | 47 | 25 (53.2) | 22 (46.8) | |
| Female | 18 | 7 (38.9) | 11 (61.1) | |
| Smoking status | | | | 0.362 |
| Smokers | 26 | 11 (42.3) | 15 (57.7) | |
| Non-smokers | 39 | 21 (53.8) | 18 (46.2) | |
| Histology | | | | 0.034a |
| Squamous cell
carcinoma | 21 | 9 (42.9) | 12 (57.1) | |
| Adenocarcinoma | 32 | 18 (56.3) | 14 (43.7) | |
| Othersb | 12 | 5 (41.7) | 7 (58.3) | |
| Tumor size
(cm) | | | | 0.031 |
| <3 | 24 | 16 (66.7) | 8 (33.3) | |
| ≥3 | 41 | 16 (39.0) | 25 (61) | |
| TNM stage | | | | 0.054 |
| I–II | 39 | 23 (59.0) | 16 (41.0) | |
| III–IV | 26 | 9 (34.6) | 17 (65.4) | |
| Lymph node
metastasis | | | | 0.018 |
| Metastasis | 36 | 13 (36.1) | 23 (63.9) | |
|
Non-metastasis | 29 | 19 (65.5) | 10 (34.5) | |
|
Differentiation | | | | 0.009 |
| Well and
moderate | 28 | 19 (67.9) | 9 (32.1) | |
| Poor | 37 | 13 (35.1) | 24 (64.9) | |
Statistical analysis
Data were analyzed using SPSS software 19.0 for
Windows (SPSS, Inc., Chicago, IL, USA). Results represent the mean
values of at least three different experiments and are expressed as
the mean ± SD and were analyzed by t-test or one-way ANOVA.
P<0.05 was considered to indicate a statistically significant
result.
Results
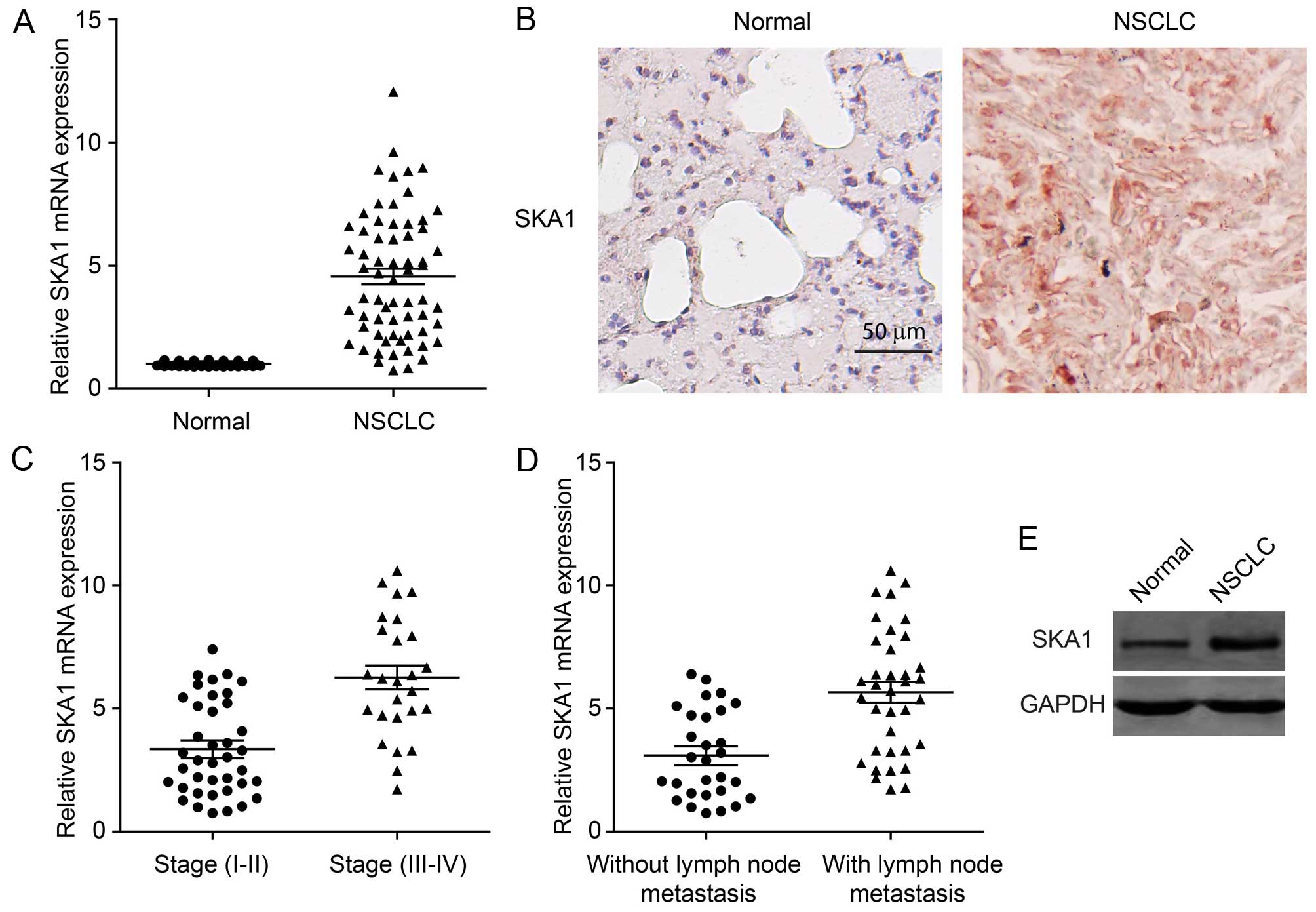
SKA1 expression is upregulated in
NSCLC
It has been reported that SKA1 may play a role in a
series of human cancers. However, its function in NSCLC is unknown.
In order to investigate the involvement of SKA1 in the development
of NSCLC, we first examined the levels of SKA1 expression in cancer
tissues from 64 NSCLC patients. We found that the expression of
SKA1 was significantly elevated in NSCLC tissues, compared to that
in the adjacent normal lung tissues (Fig. 1A). This result was also confirmed by
immunohistochemical staining of the NSCLC samples for SKA1
(Fig. 1B). We next sought to
determine whether the expression levels of SKA1 were associated
with the pathological progression of NSCLC. We found that the
levels of SKA1 were significantly increased in cancer samples from
stage III–IV patients, than the levels in samples from stage I–II
patients (Fig. 1C; Table I). Furthermore, we examined the
correlation between SKA1 expression levels and cancer metastasis of
NSCLC. The expression levels of SKA1 in 36 NSCLC samples with lymph
node metastasis were significantly higher than those in 29 samples
without lymph node metastasis (Fig.
1D; Table I). Elevated SKA1
expression was further confirmed by western blotting using NSCLC
patient tissue (Fig. 1E). Taken
together, these results revealed that the expression levels of SKA1
were upregulated in NSCLC and were correlated with cancer
progression and malignancy.
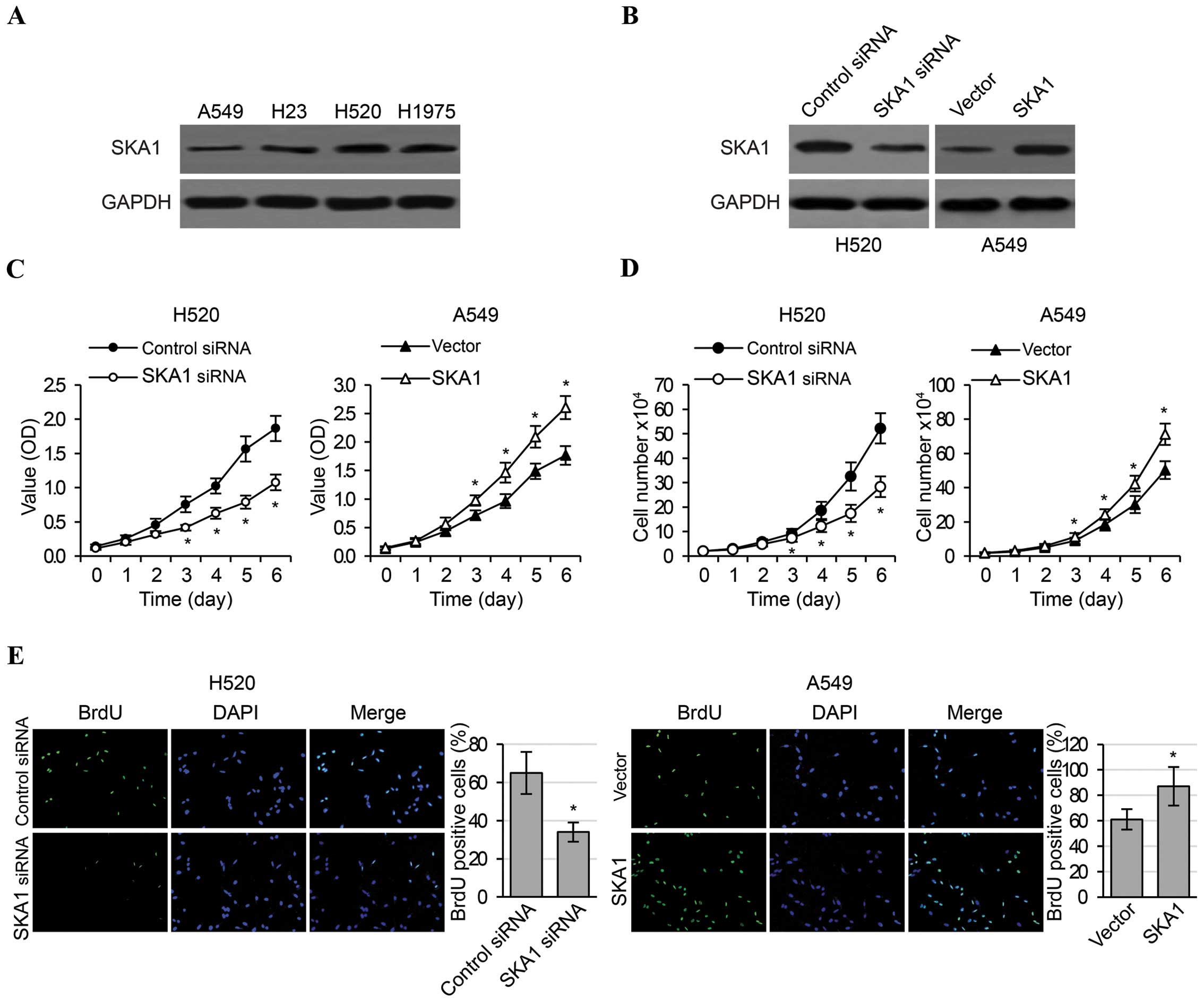
SKA1 regulates the proliferation,
migration and invasion of NSCLC cells
In order to investigate the functional roles of SKA1
in an in vitro setting, we first examined the levels of SKA1
expression in four NSCLC cell lines (A549, H23, H520 and H1975),
and determined that the levels of endogenous SKA1 expression were
highest in H520 cells and were lowest in A549 cells (Fig. 2A). Therefore, in order to obtain
most pronounced changes in SKA1 expression, these two cell lines
were respectively selected to perform in vitro loss- and
gain-of-function experiments. We effectively reduced SKA1
expression in the H520 cells by siRNA transfection, and increased
SKA1 expression in the A549 cells by transfection of a plasmid
overexpressing SKA1 (Fig. 2B). We
then carried out MTT, cell counting and BrdU assays to examine cell
proliferation in the H520 and A549 cells with modified SKA1
expression. We found that the knockdown of SKA1 expression in the
H520 cells significantly reduced cell proliferation, as evidenced
by cellular metabolic activities (Fig.
2C), cell numbers (Fig. 2D) and
percentage of cells in active division (Fig. 2E). In contrast, overexpression of
SKA1 in the A549 cells significantly increased cell proliferation
(Fig. 2C–E). Furthermore, we also
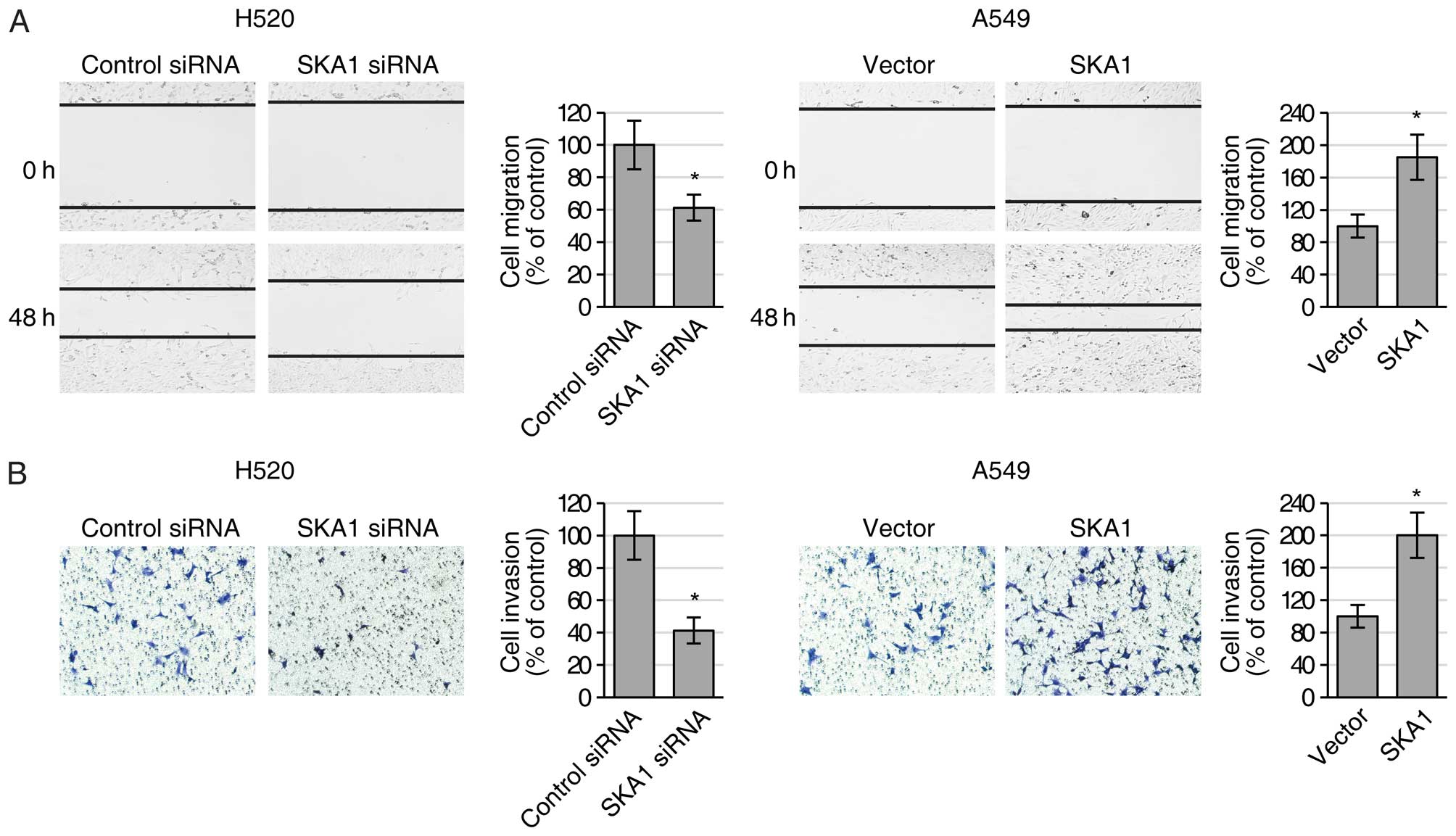
examined the migration and invasion activities of the H520 and A549
cells with modified SKA1 expression using both wound healing and
Transwell invasion assays. We found that reduced expression of SKA1
in the H520 cells led to significantly decreased ability of both
cell migration (Fig. 3A) and cell
invasion (Fig. 3B), while elevated
expression of SKA1 in the A549 cells resulted in significantly
increased ability of cell migration (Fig. 3A) and invasion (Fig. 3B). Collectively, these results
indicated that SKA1 positively regulated the proliferation and
metastatic ability of the NSCLC cells.
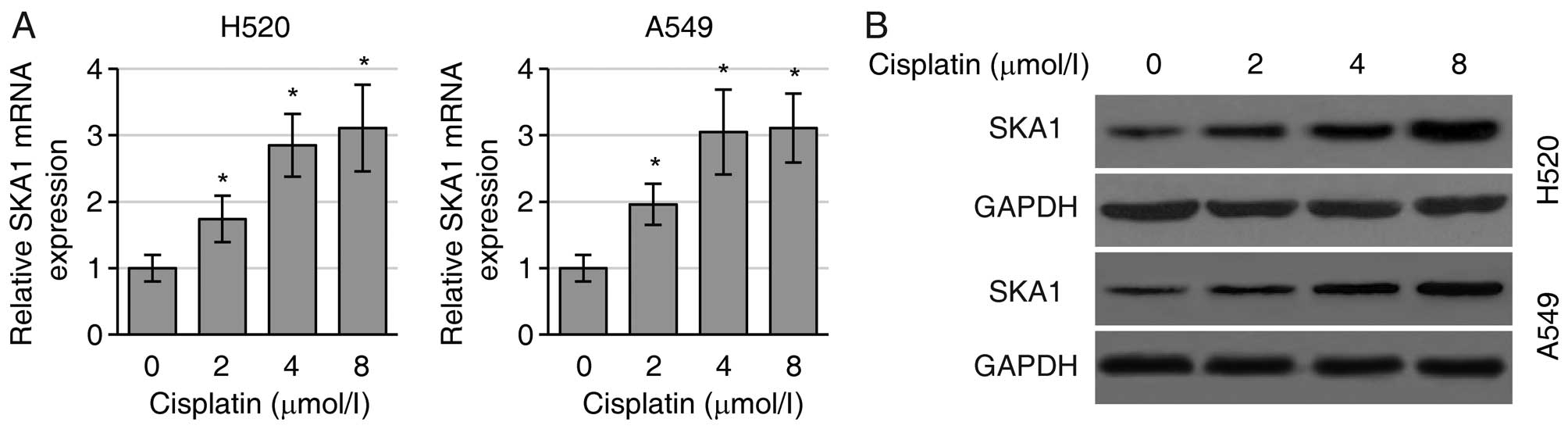
SKA1 mediates NSCLC cell sensitivity to
cisplatin
Since SKA1 plays roles in the regulation of NSCLC
cell proliferation and metastasis, we next sought to determine
whether SKA1 expression levels were associated with the sensitivity
of NSCLC cells to cisplatin treatment. We first found that
cisplatin treatment substantially increased the levels of SKA1 mRNA
(Fig. 4A) and protein (Fig. 4B) in both the H520 and A549 cells.
In order to assess the function of SKA1 in regulating NSCLC cell
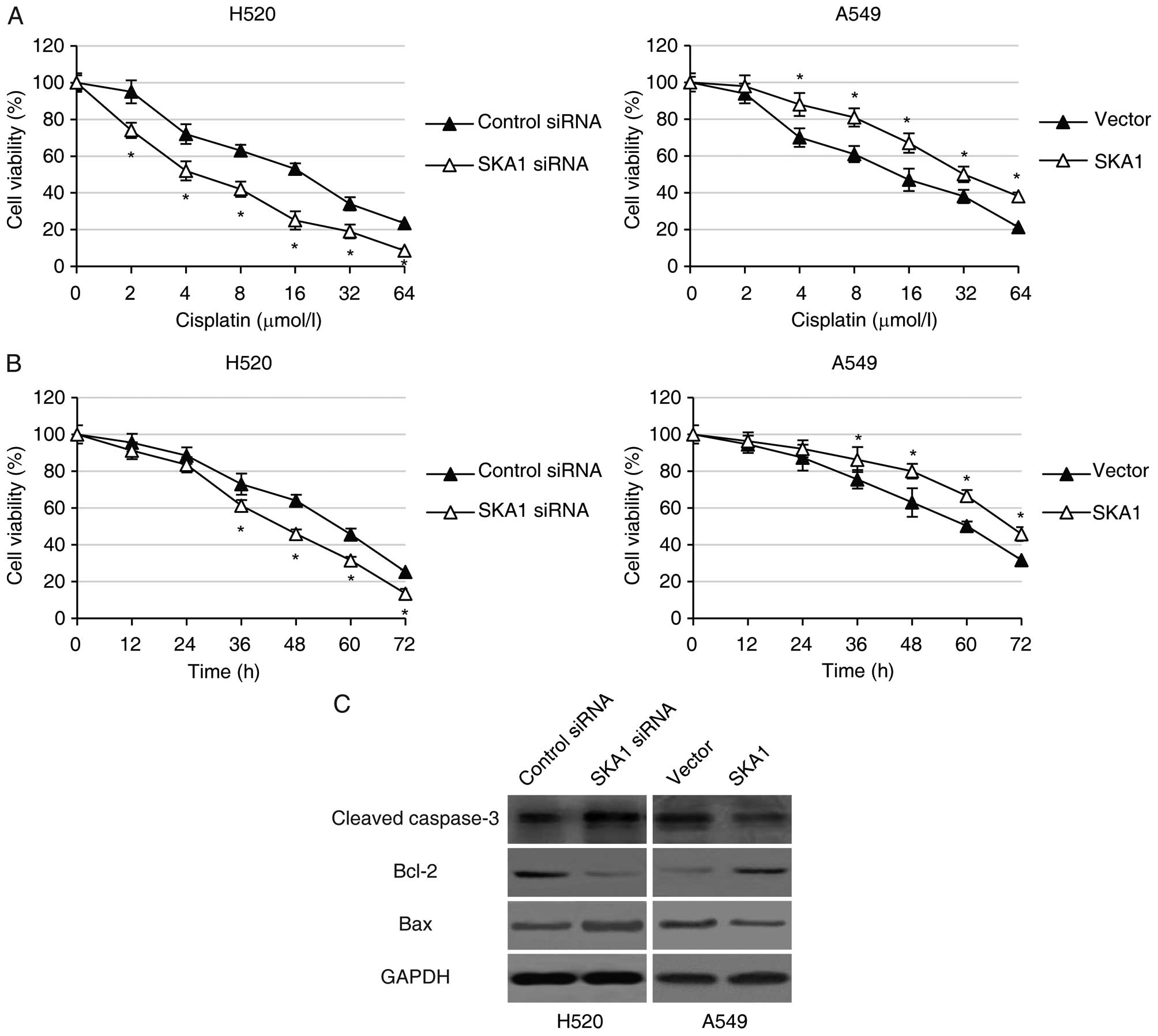
sensitivity to cisplatin, we performed MTT cell viability assay in
the H520 and A549 cells with modified SKA1 expression following
treatment with different concentrations (0, 2, 4, 8, 16, 32 or 64
μmol/l) of cisplatin for 48 h. We found that while
increasing concentrations of cisplatin led to decreased cell
viability in both the H520 and A549 cells, knockdown of SKA1 in the
H520 cells further reduced cell viability, and over-expression of
SKA1 significantly enhanced cell viability in the A549 cells
treated with cisplatin (Fig. 5A).
In addition, we also analyzed the time course of NSCLC cell
viability following treatment with 8 μmol/l cisplatin.
Consistent with previous findings, we found that downregulation of
SKA1 by siRNA in the H520 cells led to significantly decreased
levels of NSCLC cell viability, and that SKA1 overexpression in the
A549 cells resulted in significantly higher cell viability in the
NSCLC cell lines (Fig. 5B).
Furthermore, we examined the levels of several key proteins
involved in the apoptosis signaling pathway in NSCLC cells with
modified expression levels of SKA1. We determined by western blot
analysis that in the H520 cells with SKA1 knockdown, the levels of
pro-apoptotic proteins including cleaved caspase-3 and Bax were
significantly higher, and the levels of anti-apoptotic proteins
including Bcl-2 were significantly lower. The opposite results were
observed in the A549 cells with SKA1 overexpression (Fig. 5C). Taken together, these data
support the hypothesis that SKA1 protects NSCLC cells from
cisplatin-induced cell apoptosis.
SKA1 regulates the ERK1/2 and Akt
signaling pathways in NSCLC cells
It has been reported that SKA1 regulates the
activities of ERK1/2 and Akt signaling in other cancer types
(9). In order to determine whether
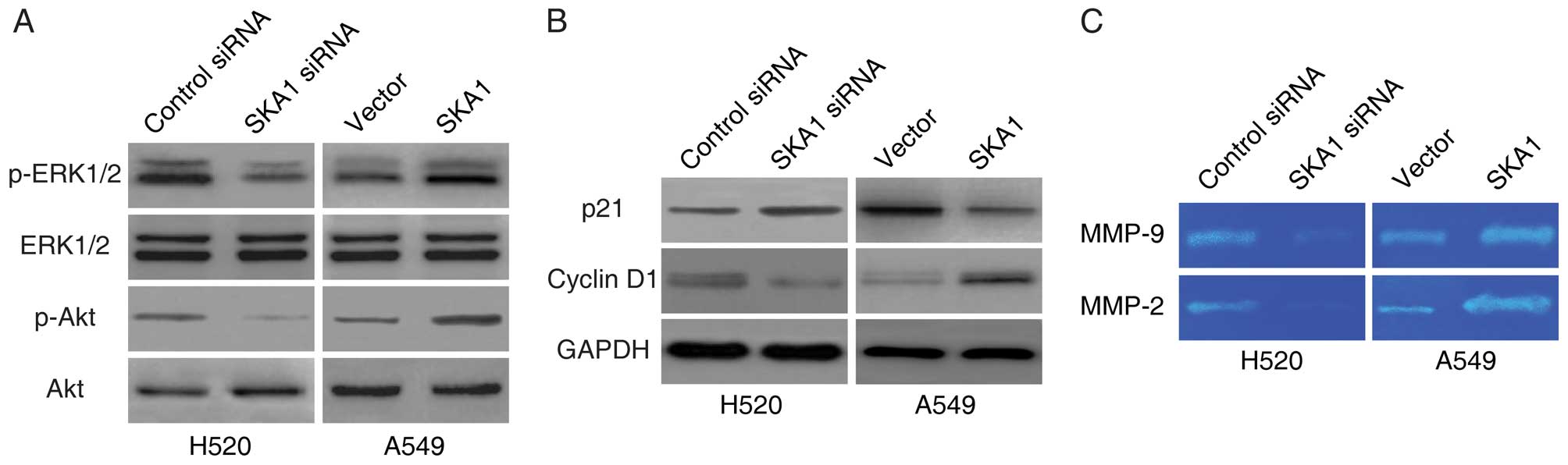
SKA1 also modulates these pathways in NSCLC, we examined the levels
of phosphorylated ERK1/2 and phosphorylated Akt in the H520 and
A549 cells with modified SKA1 expression. We found that the levels
of both phosphorylated ERK1/2 and phosphorylated Akt were
significantly lower in the H520 cells with SKA1 knockdown, and were
significantly higher in the A549 cells with SKA1 overexpression
(Fig. 6A). We next examined the
levels of downstream target proteins of the ERK1/2 and the Akt
signaling pathways. Consistent with the regulation patterns of
these pathways, we found that the levels of p21 were elevated, and
the levels of cyclin D1 were reduced with SKA1 knockdown, and that
the levels of p21 were reduced, and the levels of cyclin D1 were
elevated with SKA1 overexpression (Fig.
6B). In addition, as effectors of the ERK1/2 and the Akt
signaling pathways, we also found that the levels of MMP-2 and
MMP-9 were decreased by the knockdown of SKA1, and increased by the
overexpression of SKA1 (Fig. 6C) as
demonstrated in the gelatin zymography assay. In summary, these
data indicated that the activities of ERK1/2 and Akt signaling were
regulated by SKA1 in the NSCLC cells.
Discussion
In the present study, we investigated the functional
association between SKA1 and the development of NSCLC. We
demonstrated that the expression levels of SKA1 were elevated in
NSCLC and were correlated with cancer progression and metastasis.
We also found that SKA1 positively regulated the proliferation,
migration and invasion of NSCLC cells. Importantly, we found that
SKA1 contributed to cisplatin resistance in the NSCLC cells by
protecting these cells from apoptosis induced by cisplatin. The
ERK1/2 and Akt signaling pathways, which play essential roles in
cancer development, appeared to be regulated by SKA1 in the NSCLC
cells.
Elevated SKA1 expression has been shown by a series
of recent studies to be associated with aggressive progression and
poor outcome in several cancer types. In papillary thyroid
carcinoma, a significant correlation was noted between SKA1
expression and clinical stage and extrathyroid invasion, and
patients with high SKA1 expression were likely to relapse after
surgery (12). SKA1 has also been
shown to be significantly overexpressed in gastric cancer tissues,
and SKA1 silencing significantly inhibited gastric cancer cell
colony formation, and led to S phase cell cycle arrest (10). Consistent with these studies, the
present study confirmed the relationship between SKA1 expression
and the progression of NSCLC. Significantly, for the first time, we
established the relationship between SKA1 expression and resistance
to cisplatin.
We provided convincing evidence that modulation of
SKA1 expression affects cisplatin sensitivity in NSCLC cell lines.
It has been reported by previous studies that activation of the
ERK1/2 and Akt signaling pathways is associated with cisplatin
resistance in lung cancer (18,19).
In the present study, we found that the expression levels of SKA1
were positively correlated with the activities of the ERK1/2 and
Akt signaling pathways. We noted that the baseline levels of
p-ERK1/2 and p-Akt were higher in the H520 cells, which had higher
levels of SKA1 expression, compared to those in the A547 cells,
which had lower levels of SKA1 expression. Knockdown and
overexpression of SKA1 also resulted in consistent changes in the
activities of the ERK1/2 and Akt signaling pathways. The ERK1/2 and
Akt signaling pathways play essential roles in many aspects of
cancer development, including regulation of cell survival and
apoptosis, cell proliferation, cell cycle progression and cell
matrix interaction (20,21). Therefore, it is reasonable to
hypothesize that the mechanism of SKA1 in the regulation of NSCLC
progression and drug resistance could be through the modulation of
ERK1/2 and Akt signaling. We examined the effects of SKA1 on a
series of downstream targets of the ERK1/2 and Akt signaling
pathways and confirmed that these targets were also regulated by
SKA1. Most studies concerning SKA1 have focused on its functions
with kinetochore-microtubule structure during mitosis or meiosis.
However, the present study revealed that this protein clearly has
other functions.
Notably, in our investigation, we noted that
cisplatin treatment led to increased levels of SKA1 in NSCLC cell
lines. This result, combined with the findings that SKA1
overexpression protected cells from cisplatin-induced apoptosis,
suggest that upregulation of SKA1 may be part of an adaptive
response of NSCLC cells to chemotherapy drugs, and may therefore
serve as an intrinsic player in the cellular mechanism following
chemotherapy treatment. The detailed mechanism of SKA1 upregulation
by cisplatin is not currently known, nor do we have evidence that
the same response may occur in vivo. Although further
investigation is needed to answer these important questions, SKA1
could potentially be a therapeutic target in combating drug
resistance in the chemotherapy of NSCLC.
References
|
1
|
Cersosimo RJ: Lung cancer: A review. Am J
Health Syst Pharm. 59:611–642. 2002.PubMed/NCBI
|
|
2
|
Parsons A, Daley A, Begh R and Aveyard P:
Influence of smoking cessation after diagnosis of early stage lung
cancer on prognosis: Systematic review of observational studies
with meta-analysis. BMJ. 340:b55692010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sakashita S, Sakashita M and Sound Tsao M:
Genes and pathology of non-small cell lung carcinoma. Semin Oncol.
41:28–39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Galluzzi L, Senovilla L, Vitale I, Michels
J, Martins I, Kepp O, Castedo M and Kroemer G: Molecular mechanisms
of cisplatin resistance. Oncogene. 31:1869–1883. 2012. View Article : Google Scholar
|
|
5
|
Trédan O, Galmarini CM, Patel K and
Tannock IF: Drug resistance and the solid tumor microenvironment. J
Natl Cancer Inst. 99:1441–1454. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang B, Li KY, Chen HY, Pan SD, Jiang LC,
Wu YP and Liu SW: Spindle and kinetochore associated complex
subunit 1 regulates the proliferation of oral adenosquamous
carcinoma CAL-27 cells in vitro. Cancer Cell Int. 13:832013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qin X, Yuan B, Xu X, Huang H and Liu Y:
Effects of short interfering RNA-mediated gene silencing of SKA1 on
proliferation of hepatocellular carcinoma cells. Scand J
Gastroenterol. 48:1324–1332. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tian F, Xing X, Xu F, Cheng W, Zhang Z,
Gao J, Ge J and Xie H: Downregulation of SKA1 gene expression
inhibits cell growth in human bladder cancer. Cancer Biother
Radiopharm. 30:271–277. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun W, Yao L, Jiang B, Guo L and Wang Q:
Spindle and kinetochore-associated protein 1 is overexpressed in
gastric cancer and modulates cell growth. Mol Cell Biochem.
391:167–174. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li J, Xuan JW, Khatamianfar V, Valiyeva F,
Moussa M, Sadek A, Yang BB, Dong BJ, Huang YR and Gao WQ: SKA1
over-expression promotes centriole over-duplication, centrosome
amplification and prostate tumourigenesis. J Pathol. 234:178–189.
2014.PubMed/NCBI
|
|
12
|
Dong C, Wang XL and Ma BL: Expression of
spindle and kinetochore-associated protein 1 is associated with
poor prognosis in papillary thyroid carcinoma. Dis Markers.
2015:6165412015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shi X, Chen X, Peng H, Song E, Zhang T,
Zhang J, Li J, Swa H, Li Y, Kim S, et al: Lentivirus-mediated
silencing of spindle and kinetochore-associated protein 1 inhibits
the proliferation and invasion of neuronal glioblastoma cells. Mol
Med Rep. 11:3533–3538. 2015.PubMed/NCBI
|
|
14
|
Gaitanos TN, Santamaria A, Jeyaprakash AA,
Wang B, Conti E and Nigg EA: Stable kinetochore-microtubule
interactions depend on the Ska complex and its new component
Ska3/C13Orf3. EMBO J. 28:1442–1452. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Raaijmakers JA, Tanenbaum ME, Maia AF and
Medema RH: RAMA1 is a novel kinetochore protein involved in
kinetochore-microtubule attachment. J Cell Sci. 122:2436–2445.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Theis M, Slabicki M, Junqueira M,
Paszkowski-Rogacz M, Sontheimer J, Kittler R, Heninger AK, Glatter
T, Kruusmaa K, Poser I, et al: Comparative profiling identifies
C13orf3 as a component of the Ska complex required for mammalian
cell division. EMBO J. 28:1453–1465. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hanisch A, Silljé HH and Nigg EA: Timely
anaphase onset requires a novel spindle and kinetochore complex
comprising Ska1 and Ska2. EMBO J. 25:5504–5515. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang M, Liu ZM, Li XC, Yao YT and Yin ZX:
Activation of ERK1/2 and Akt is associated with cisplatin
resistance in human lung cancer cells. J Chemother. 25:162–169.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen TJ, Zhou YF, Ning JJ, Yang T, Ren H,
Li Y, Zhang S and Chen MW: NBM-T-BMX-OS01, an osthole derivative,
sensitizes human lung cancer A549 cells to cisplatin through
AMPK-dependent inhibition of ERK and Akt Pathway. Cell Physiol
Biochem. 36:893–906. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stinchcombe TE and Johnson GL: MEK
inhibition in non-small cell lung cancer. Lung Cancer. 86:121–125.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yip PY: Phosphatidylinositol
3-kinase-AKT-mammalian target of rapamycin (PI3K-Akt-mTOR)
signaling pathway in non-small cell lung cancer. Transl Lung Cancer
Res. 4:165–176. 2015.PubMed/NCBI
|