Introduction
Fatty acid synthase (EC 2.3.1.85, ab. FAS) is a key
metabolic enzyme which catalyzing the de novo synthesis of
long chain saturated fatty acids from acetyl-CoA (Ac-CoA) and
malonyl-CoA (Mal-CoA) in the presence of the reducing substrate
nicotinamide adenine dinucleotide phosphate (NADPH) (1). Most human tissues, except liver and
adipose tissue, exhibit low expression of FAS. However, the
expression of FAS is especially high in a variety of common human
cancers (2–5). It has been reported that many FAS
inhibitors, such as cerulenin, C75, orlistat and epigallocatechin
gallate (EGCG), have joint weight-loss and antitumor effects
(6–9). Therefore, FAS may be a dual
therapeutic target for treating both obesity and cancer.
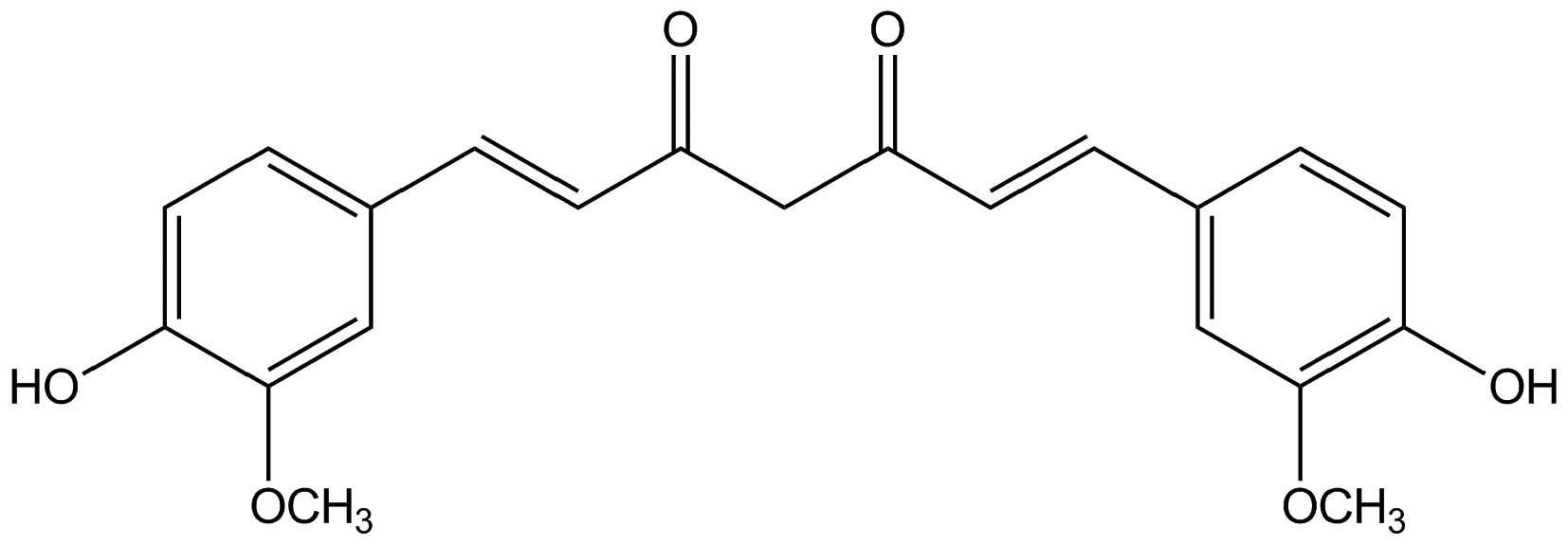
Curcumin (Fig. 1), a
hydrophobic polyphenol derived from the rhizome of Curcuma
longa, possesses wide pharmacological activities including
respiratory conditions, inflammation, breast disorders, diabetic
wounds, and certain tumors (10).
Curcumin induces cell death in some cancers, such as gastric and
colon cancers (11), human melanoma
(12), and lung cancer (13) without major cytotoxic effects on
healthy cells (14–16). However, the mechanism involved is
not fully understood.
In our previous study, we found curcumin to show
both fast-binding and slow-binding inhibition to FAS with a
half-inhibitory concentration (IC50) value of 10.5
µg/ml (17). Compared with
EGCG and cerulenin, two classical FAS inhibitors, curcumin showed
stronger inhibitory activity. In the present study, we investigated
the effect of curcumin on FAS overexpressed human breast cancer
cells. We demonstrated that curcumin inhibited FAS activity and
expression in MDA-MB-231 cells. Curcumin also regulated
pro-apoptotic and anti-apoptotic proteins such as Bax, B-cell
lymphoma 2 (Bcl-2), Akt, phosphorylate-Akt (p-Akt) expression in a
dose-dependent manner.
Materials and methods
Reagents
Ac-CoA, Mal-CoA, NADPH, DMSO, Hoechst 33258 and
curcumin were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum
(FBS) were purchased from Gibco-BRL (Beijing, China). FAS antibody
for immunoblotting was obtained from BD Biosciences Pharmingen
(Shanghai, China). GAPDH antibody was purchased from Cell Signaling
Technology, Inc. (Shanghai, China).
3-4,5-dimethylthiazol-2-yl-2,3-diphenyltetrazolium bromide (MTT),
phosphate-buffered saline (PBS) and the TRIzol reagent were
purchased from Invitrogen (Beijing, China). Annexin V-FITC/PI
apoptosis detection kit was purchased from Mbchem (Shanghai,
China). The PCR primers for human β-actin and FAS were synthesized
by SBS Genetech Co., Ltd. (Beijing, China). M-MLV, Rnasin and Oligo
(dT) were purchased from Promega (Beijing, China). ECL and PVDF
membrane were obtained from Millipore (Beijing, China).
Cell viability assay
Tests were performed in 96-well plates. MDA-MB-231
cells were cultured in the plates until confluence, cells were
incubated with either DMSO (1:1,000) or increasing concentrations
of curcumin for 24 h (37°C, 5% CO2). The medium was then
changed to a fresh one with 0.5 mg/ml MTT. After 4 h of incubation
at 37°C, the plates were again decanted, and 150 µl of DMSO
was added to solubilize the formazan crystals present in viable
cells. The plate was analyzed by spectrometry at the wavelength of
492 nm by a microplate spectrophotometer (Multiskan, MK3). Data
were obtained from the average of five wells, and the assay was
repeated three times.
Immunoblot analysis
Cells were washed three times with ice-cold PBS and
harvested in RIPA lysis buffer with 1 mM PMSF, and then lysed on
ice for 5 min. The homogenate was centrifuged at 12,000 rpm for 30
min at 4°C and supernatant was collected for FAS analysis. Equal
protein extracts were separated by SDS-PAGE, then
electrophoretically transferred to PVDF membranes. Incubation with
primary and secondary antibodies was performed in Tris-buffered
saline containing 5% non-fat dry milk for 2 h or more. After
incubation, membranes were washed in Tris-buffered saline
containing 0.1% Tween-20. ECL was used for detection. Blots were
reprobed with an antibody against GAPDH as a loading control of
protein loading and transfer.
RNA isolation and RT-PCR analysis
Total RNA was extracted with TRIzol reagent
according to the manufacturer's instructions and
reverse-transcribed by RiboClone M-MLV(H−) cDNA
technology. The synthesized single-stranded cDNA was used for
amplification of a specific target. The β-actin gene was amplified
as a loading control. The PCR conditions were denatured at 95°C for
5 min and followed by 30 cycles (95°C, 15 sec, 55°C, 15 sec, 72°C,
30 sec). The primer sequences were as follows: FAS forward,
5′-AGATCCTGGAACGAGAACACGAT-3′ and reverse,
5′-GAGACGTGTCACTCCTGGACTTG-3′; and β-actin forward,
5′-GTGGGCCGCTCTAGGCACCAA-3′ and reverse,
5′-CTCTTTGATGTCACGACGATTTC-3′.
The amplified products were visualized on 1% agarose
gels. Quantification was performed in duplicate and the experiments
were repeated independently three times. We used ImageJ software to
analyze the results of gels.
Cell FAS activity assay
FAS activity in cells was assessed in a routine
manner with some modifications. In brief, after cells were
harvested, pelleted by centrifugation, resuspended in cold assay
buffer (100 mM potassium phosphate buffer, 1 mM EDTA, 1 mM PMSF and
1 mM dithiolthreitol, pH 7.0), ultrasonically disrupted and
centrifuged at 12,000 rpm for 30 min at 4°C, the supernatant was
collected for the overall reaction assay. Fifty microliters of
supernatant was added to the reaction mix contained 25 mM
KH2PO4-K2HPO4 buffer,
0.25 mM EDTA, 0.25 mM dithiothreitol, 30 µM Ac-CoA, 100
µM Mal-CoA, 350 µM NADPH (pH 7.0) in a total volume
of 200 µl. Protein content in the supernatant was determined
using a bicinchoninic acid (BCA) assay (Pierce) and results were
expressed as the specific activity of FAS (U/mg).
Hoechst 33258 staining
MDA-MB-231 cells were seeded on 24-well culture
dishes and cultured in the plates until confluence. Cells were
treated with indicated dose of curcumin. After 24 h incubated in
37°C, 5% CO2 incubator, the medium was changed to a
fresh one with 0.5 µg/ml Hoechst 33258 and the dish was
incubated in an incubator for 30 min, then the cells were washed
three times with PBS. Nuclear staining was examined under the
fluorescence microscope and images were captured using ImagePro
Plus software (Media Cybernetics, Silver Spring, MD, USA).
Annexin V/propidium iodide (PI) dual
staining
MDA-MB-231 cells were treated with indicated
concentrations of curcumin. Then cells were harvested, and the
percentage of cells undergoing apoptosis was measured by
fluorescence microscopy after staining with fluorescein-conjugated
Annexin V and PI staining 5 min in the dark.
RNA interference
MDA-MB-231 cells were transiently transfected with a
small interfering RNA (siRNA) that silences expression of FAS. The
FAS-targeted siRNAs (sense, CCCUGAGAUCCCAGCGCUGdTdT and antisense,
CAGCGCUGGGAUCUCAGGGdTdT) were synthesized by Invitrogen. For
transient expression, cell lines were transfected by using
Lipofectamine 2000 reagent (Invitrogen) according to the
manufacturer's instructions. After incubating the cells for 6 h,
the lipid and siRNA complex was removed, and fresh growth medium
was added. Cells were lysed 24 h after transfection, and specific
protein levels were determined by western blot analysis with
specific antibodies against the targeted proteins and GAPDH as
control.
Results
Effect of curcumin on the viability of
MDA-MB-231 cells
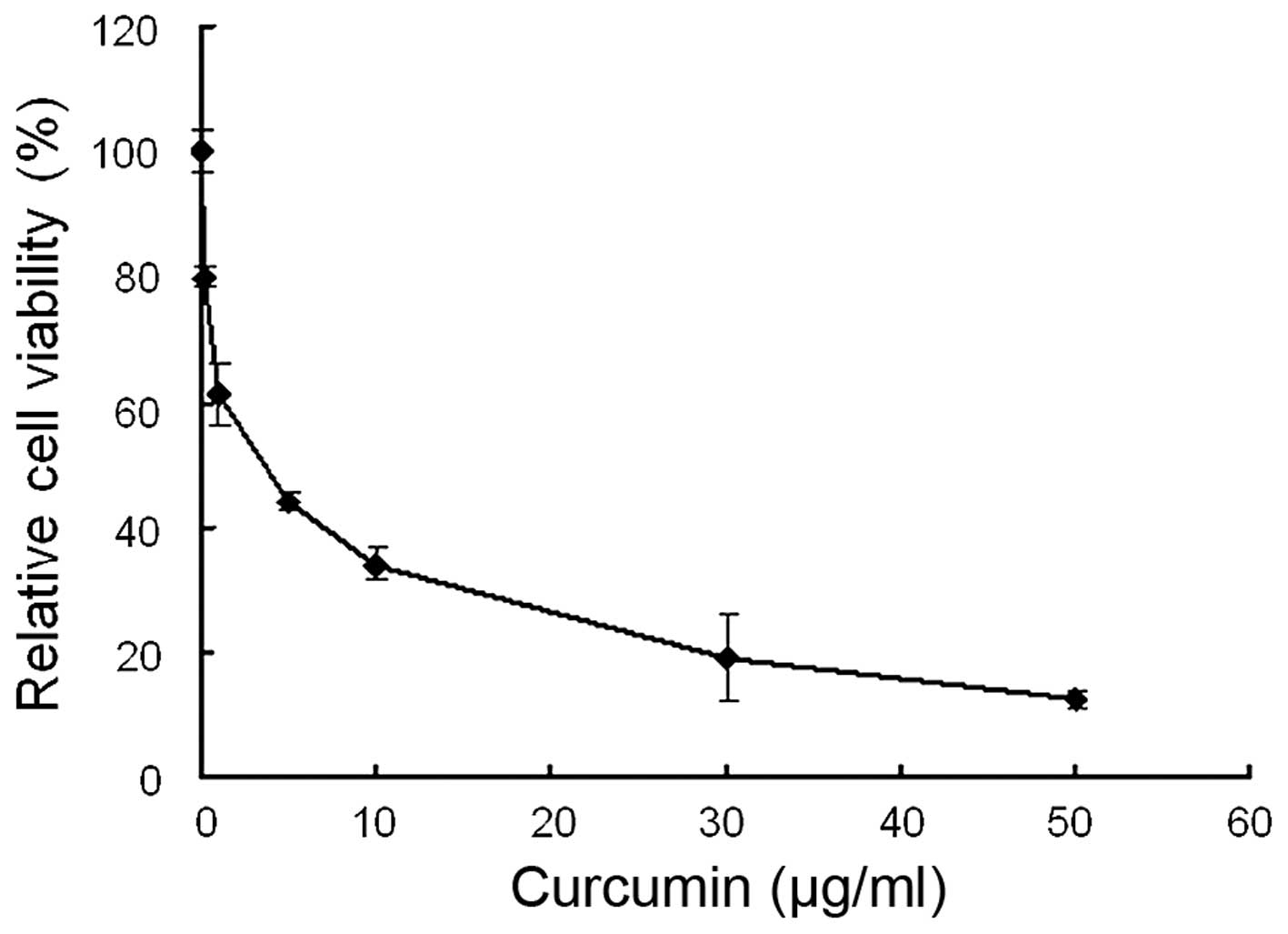
The anti-viability effect of curcumin on MDA-MB-231
cells was examined by MTT assay. Following treatment with 0–50
µg/ml curcumin, cell viability was significantly decreased.
As shown in Fig. 2, curcumin
reduced cell viability in a dose-dependent manner with an
IC50 value of 3.63±0.26 µg/ml.
Curcumin induces MDA-MB-231 cells
apoptosis
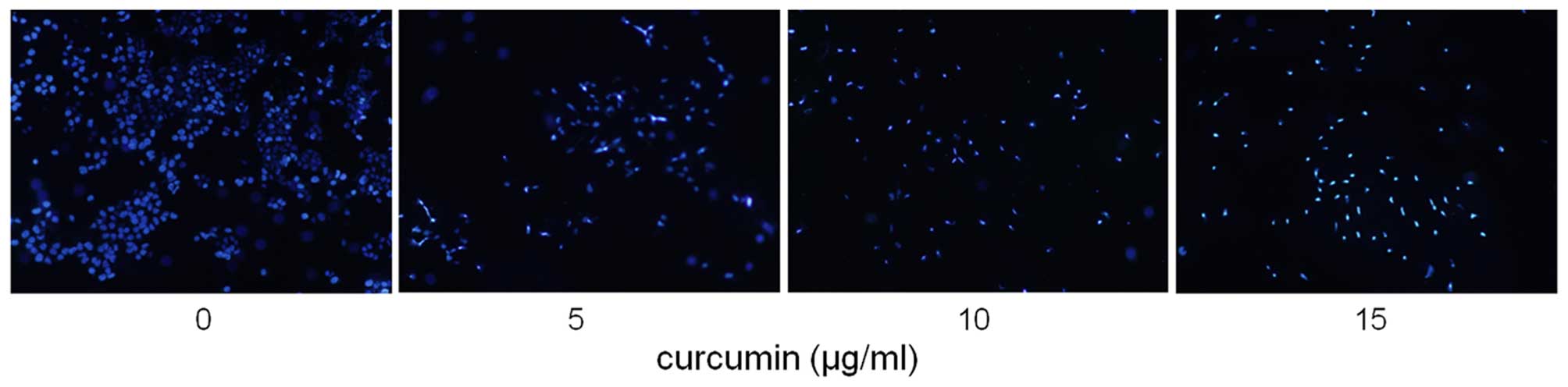
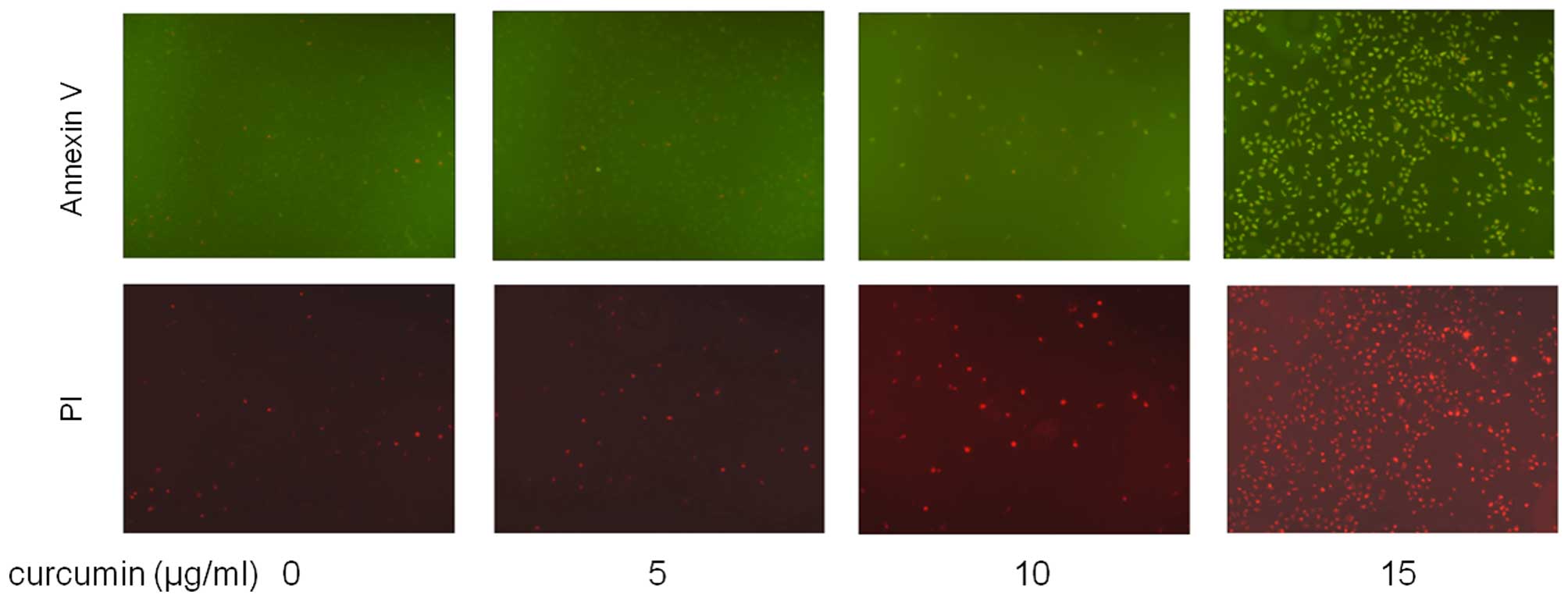
According to the results of MTT assay, the apoptotic
effect of curcumin (0, 5, 10 and 15 µg/ml) on MDA-MB-231
cells was examined by using Hoechst 33258 staining and the Annexin
V/PI dual staining. After exposure to four concentrations of
curcumin for 24 h, apoptotic MDA-MB-231 cells were demonstrated by
the result of Hoechst 33258 staining, which revealed a cell
membrane permeability increase and nuclear condensation (Fig. 3), and by Annexin V/PI dual staining,
where cells were observed to be Annexin V-FITC- and PI-positive,
indicating that they were in end-stage apoptosis or already dead
(Fig. 4).
Curcumin downregulated FAS expression and
inhibited FAS activity in MDA-MB-231 cells
Curcumin has been reported to be a highly active FAS
inhibitor, however, no previous studies focused on the activity of
curcumin on intracellular FAS activity in breast cancer cells.
Therefore, we measured the effects of curcumin on the expression
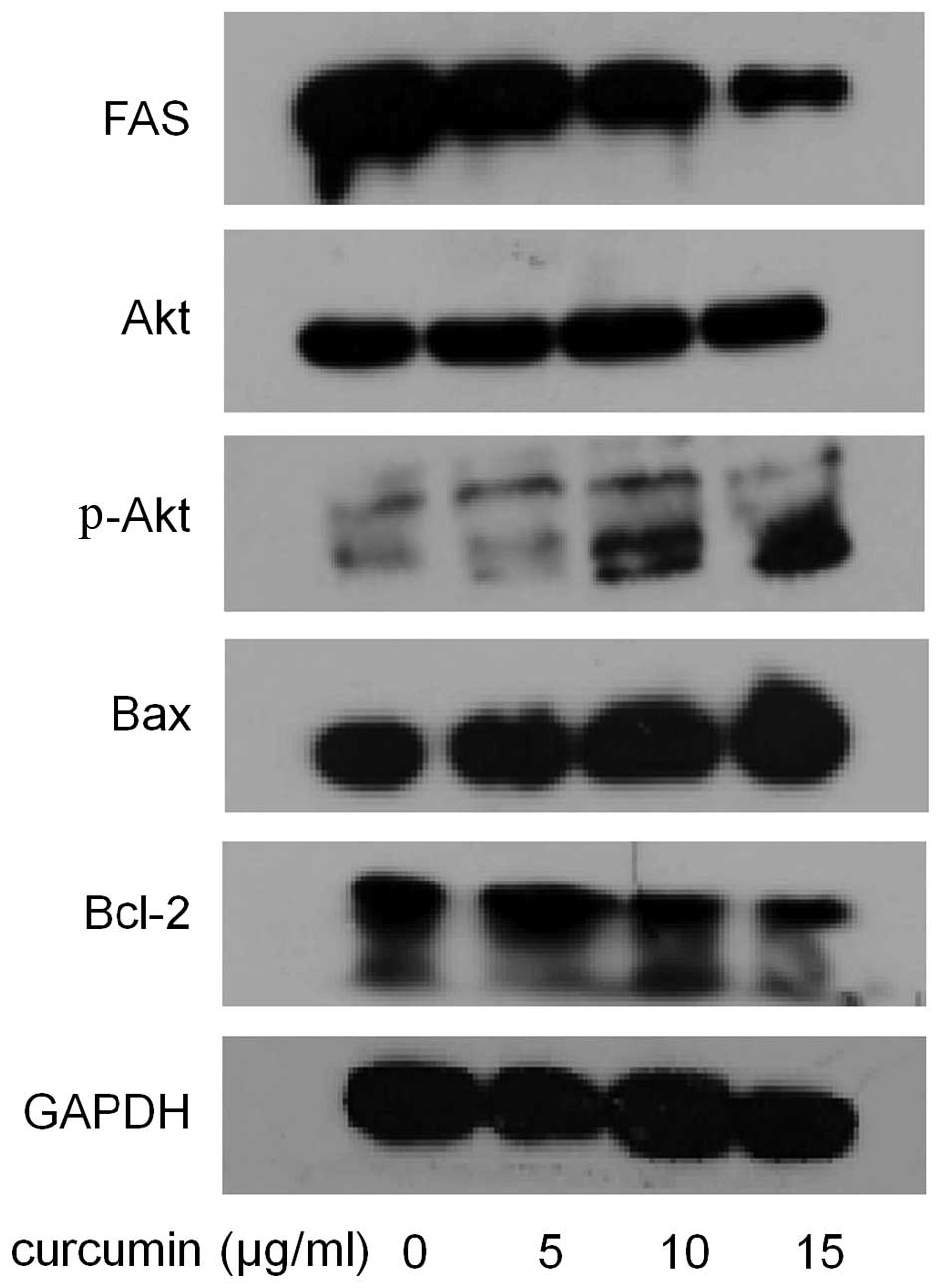
and activity of FAS in MDA-MB-231 cells. As shown in Fig. 5, compared with control, the cells
treated with curcumin showed much lower expression level of FAS.
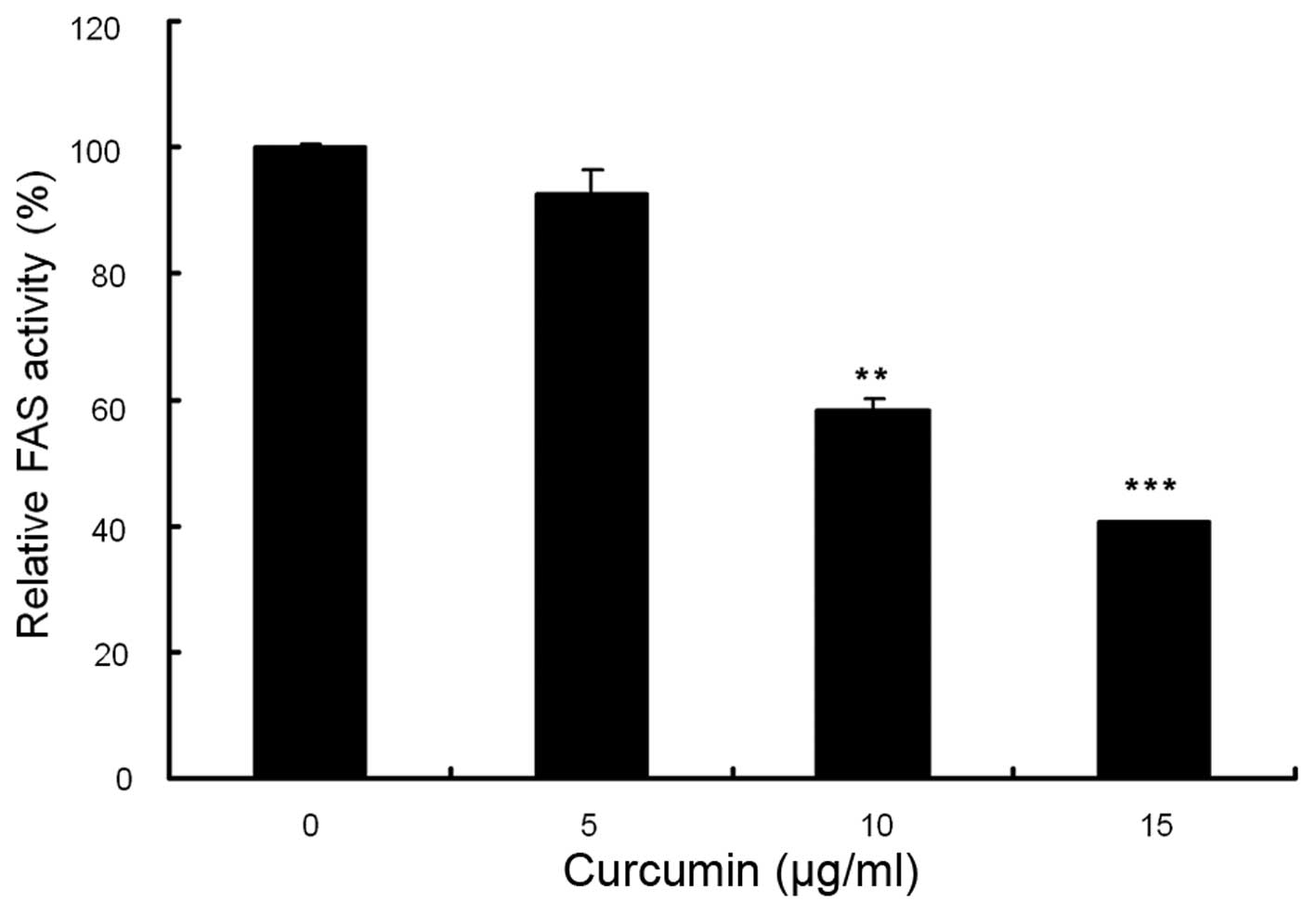
Compared with control, curcumin showed a dose-dependent inhibition
on FAS activity. As shown in Fig.
6, the relative activities of FAS were reduced by 6.8% at 5
µg/ml, 41.1% at 10 µg/ml and 59.7% at 15
µg/ml. These results indicated that curcumin potently
inhibits FAS activity in this breast cancer cell line.
Effects of curcumin on FAS mRNA levels in
MDA-MB-231 cells
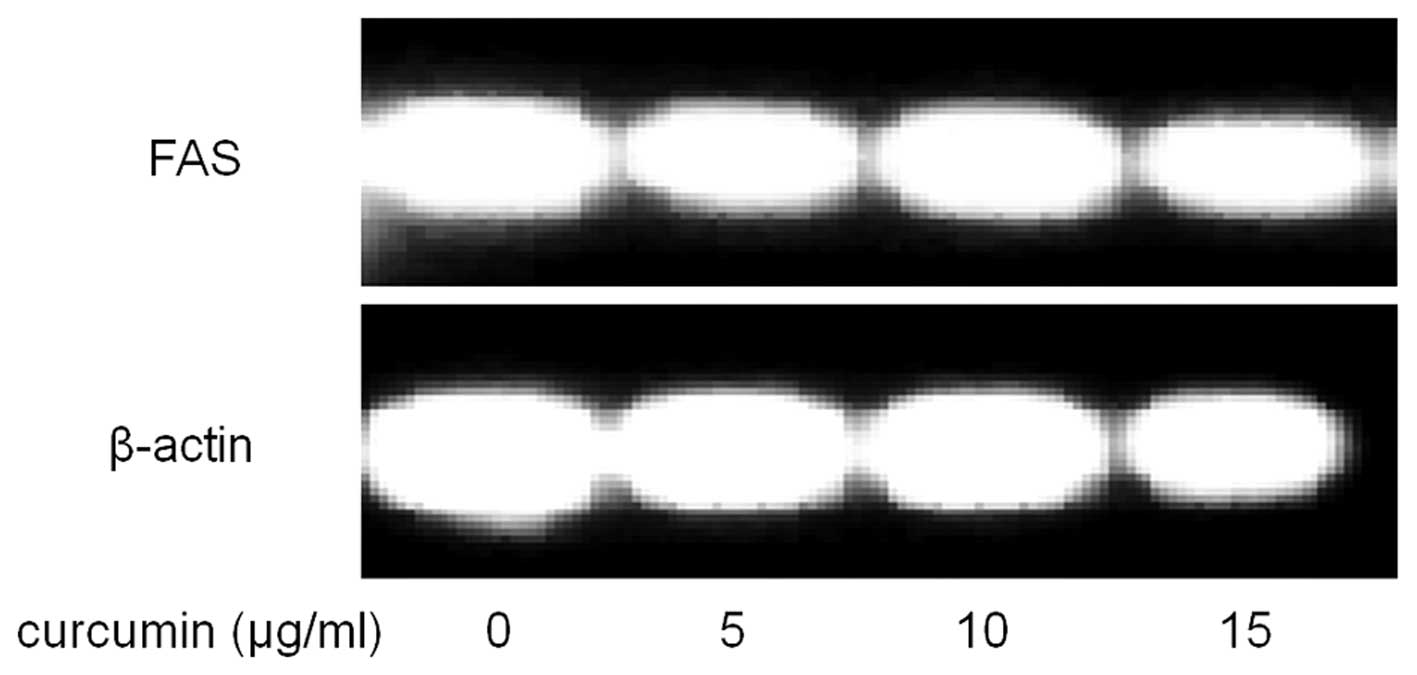
To further explore the mechanism underlying the
suppression of cancer cells by curcumin, the mRNA level of FAS was
also examined. As shown in Fig. 7,
compared with control, curcumin treated cells showed significantly
lower mRNA levels of FAS.
Effects of curcumin on Bax and Bcl-2
proteins
Bax is a pro-apoptotic protein and plays a key role
in mitochondrial stress-induced cell apoptosis. Increased levels of
Bax could promote cells apoptosis. Bcl-2 is an anti-apoptotic
protein that can inhibit apoptosis induced by cytochrome c
release. Decreased Bcl-2 protein levels may lead to cell apoptosis.
As shown in Fig. 5, after curcumin
administration, the expression level of Bax showed a dose-dependent
increase. In contrast, curcumin decreased Bcl-2 expression in a
dose-dependent manner.
Curcumin upregulated Akt phosphorylation
in MDA-MB-231 cells
The activity of curcumin on Akt was also determined.
After treated with 0, 5, 10 and 15 µg/ml curcumin for 24 h,
MDA-MB-231 cells were collected and cell lysates were subjected to
western blot analysis for phospho-Akt. The result in Fig. 5 showed that curcumin increased the
phosphorylation of Akt at Ser473 in a dose-dependent manner,
without affecting total Akt expression.
Effect of siRNA mediated FAS silencing on
MDA-MB-231 cells
Because overexpression of FAS protected immortalized
cancer cells from apoptosis, we wanted to assess the effect of
short interfering RNA to FAS on apoptosis in MDA-MB-231 cells. To
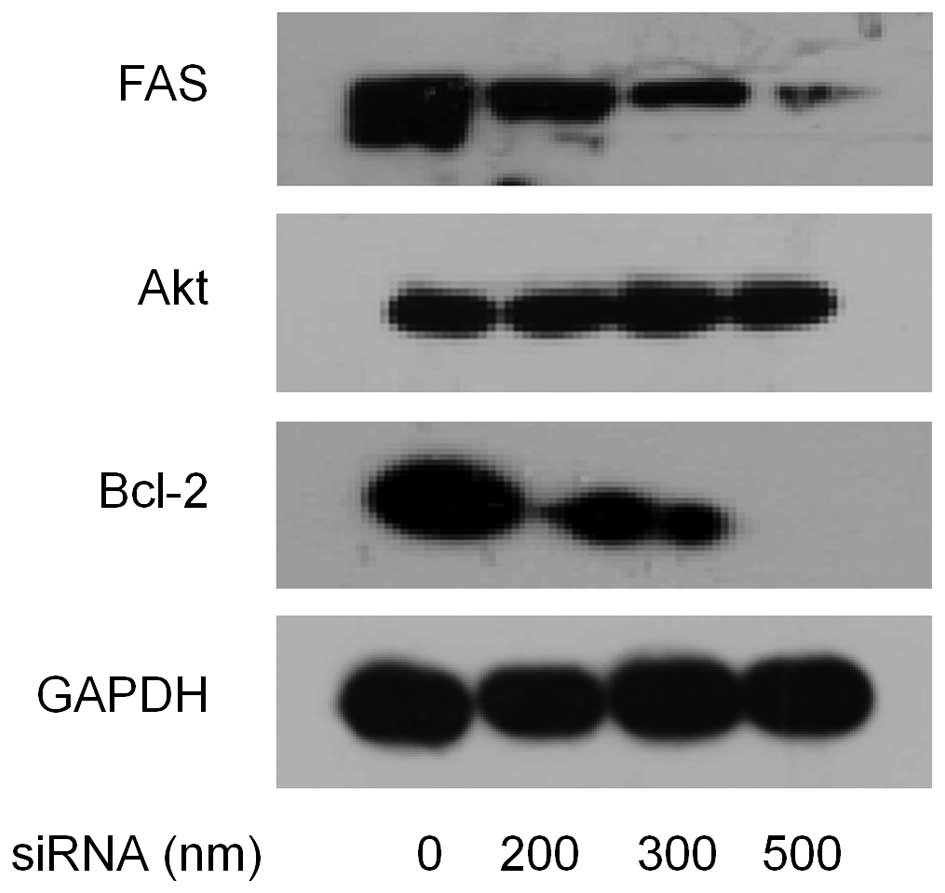
accomplish this, MDA-MB-231 cells were transfected with FAS siRNA
(0, 200, 300 and 500 nM), then the expression levels of FAS, Akt,
Bcl-2 were measured 48 h after transfection by western blotting. As
shown in Fig. 8, compared with the
control, the cells treated with siRNA showed much lower level of
FAS. Reduction of Bcl-2 expression showed a dose-dependent manner
with increase of siRNA, but total Akt expression had no change.
Discussion
FAS is a key enzyme catalyzing the synthesis of
long-chain fatty acids from small molecule carbon unit in
vivo. According to the biochemical and pharmacological studies
in recent years, FAS is believed to be a dual target of treating
both obesity and cancer (18,19).
Fatty acid biosynthesis is dependent on FAS to satisfy the needs of
cell division and proliferation. FAS expression level in cancer
cells is much higher than that of normal tissue cells (20). There is a correlation between
expression and prognosis in malignant tumors (21). RNAi knockdown experiments have shown
that multiple cancer cell lines depend on FAS for proliferation and
survival (22). FAS appears to play
a key role in tumor initiation and propagation for many
malignancies and represents an attractive target for cancer
treatment. Inhibition of FAS has emerged as a promising therapeutic
target in cancer, and numerous inhibitors have been investigated
(19,21). However, severe pharmacological
limitations have challenged their clinical testing.
Curcumin is a phenolic pigment extracted from
Curcuma longa, which is an effective anti-mutagenic and
anticancer agent. In Chinese medicine, Curcuma longa has
long been used as a medicinal plant (23). Numerous studies have suggested that
curcumin can inhibit the proliferation of cancer cells and promote
cancer cells apoptosis (24,25).
Yet, little is known about its mechanism that inhibits the growth
of cancer cells.
Since curcumin is a highly active FAS inhibitor,
this study is focus on the relationship between the apoptotic
effect and FAS inhibitory effect of curcumin. We found that
treatment with curcumin produced a dose-dependent decrease in the
viability of MDA-MB-231 cells. Hoechst 33258 staining, and the
Annexin V/PI dual staining results indicated that curcumin induced
apoptosis dose-dependently (Figs. 3
and 4). After treating with
curcumin, the mRNA, expression level, as well as activity of FAS
were also decreased in a dose-dependent manner (Figs. 5Figure 6–7). These results indicated that curcumin
potently blocked FAS in MDA-MB-231 cells.
Cell death signaling frequently converges on
mitochondria, which is controlled by the activities of pro- and
anti-apoptotic Bcl-2 family proteins (26–31).
In the present study, we found that curcumin treatment decreased
anti-apoptotic Bcl-2 and increased pro-apoptotic Bax proteins,
thereby causing a significant increase in the Bax/Bcl-2 ratio in
MDA-MB-231 cells. In addition, FAS siRNA also downregulated the
expression level of Bcl-2. These results indicated that bcl-2
family was involved in curcumin-induced apoptosis.
The activation of Akt is one of the most frequent
alterations observed in human cancer cells (32–34).
Inhibition of FAS activity preferentially inhibits cell growth and
induces apoptosis in a number of cancel cells (35). Considerable evidence demonstrates
that phosphatidylinositol 3-kinase (PI3K)/Akt signaling plays an
important role in cancer progression and has been linked with FAS
expression in cancer cells (36,37).
In the present study, to assess whether the activity of Akt was
affected by curcumin, we explored the phosphorylation of Akt in
MDA-MB-231 cells after treatment with curcumin and FAS siRNA. The
results showed that p-Akt was noticeably increased when treated
with curcumin in MDA-MB-231 cells. However, expression levels of
Akt phosphorylation were not detected in MDA-MB-231 cells exposed
to increasing concentrations of FAS siRNA. Moreover, the total
levels of the corresponding Akt were not altered in either curcumin
or siRNA treated breast cancer cells. These results demonstrated
that PI3K/AKT signaling pathway may be involved in curcumin-induced
apoptosis, but the detailed mechanisms involved are not fully
understood.
In conclusion, curcumin was able to induce
MDA-MB-231 cell apoptosis via inhibiting intracellular FAS activity
and downregulating FAS expression and mRNA level. FAS inhibition
plays a great role in curcumin regulated signal proteins including
Bax, Bcl-2, Akt and p-Akt. FAS knockdown by siRNA also illustrated
that FAS inhibition closely related to cell apoptosis. These
results support the important role of FAS in MDA-MB-231 cells and
suggest that FAS is the target that curcumin act on. Our findings
combined with its well-documented pharmacological safety profile
make curcumin a promising drug candidate for the treatment of
breast cancer.
Abbreviations:
|
Ac-CoA
|
acetyl CoA
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
DMSO
|
dimethyl sulfoxide
|
|
EGCG
|
epigallocatechin gallate
|
|
FAS
|
fatty acid synthase
|
|
FBS
|
fetal bovine serum
|
|
IC50
|
half-inhibitory concentration
|
|
Mal-CoA
|
malonyl-CoA
|
|
MAT
|
malonyl/acetyltransferase
|
|
MTT
|
3-4,5-dimethylthiazol-2-yl-2,3-diphenyltetrazolium bromide
|
|
NADPH
|
nicotinamide adenine dinucleotide
phosphate
|
|
PBS
|
phosphate-buffered saline
|
Acknowledgments
The present study was supported by the Fusion of
Science and Education Special Fund, College of Life Sciences,
University of Chinese Academy of Sciences (KJRH2015-012);
Application Basic Research Project of Qinghai Province
(2015-ZJ-728); Youth Innovation Promotion Association, CAS; 2014
Youth National Natural Science Foundation of China (no. 31300292);
The Key Program of 'The Dawn of West China' Talent Foundation of
CAS (2012); 2014 Youth National Natural Science Foundation of China
(no. 31300292); The Key Program of 'The Dawn of West China' Talent
Foundation of CAS (2012), as well as High-Tech Research and
Development Program of Xinjiang (no. 201315108) and China
Postdoctoral Science Foundation (no. 2013M540785).
References
|
1
|
Menendez JA and Lupu R: Fatty acid
synthase and the lipogenic phenotype in cancer pathogenesis. Nat
Rev Cancer. 7:763–777. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alò PL, Visca P, Trombetta G, Mangoni A,
Lenti L, Monaco S, Botti C, Serpieri DE and Di Tondo U: Fatty acid
synthase (FAS) predictive strength in poorly differentiated early
breast carcinomas. Tumori. 85:35–40. 1999.PubMed/NCBI
|
|
3
|
Shurbaji MS, Kalbfleisch JH and Thurmond
TS: Immunohistochemical detection of a fatty acid synthase (OA-519)
as a predictor of progression of prostate cancer. Hum Pathol.
27:917–921. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gansler TS, Hardman W III, Hunt DA,
Schaffel S and Hennigar RA: Increased expression of fatty acid
synthase (OA-519) in ovarian neoplasms predicts shorter survival.
Hum Pathol. 28:686–692. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Visca P, Sebastiani V, Botti C, Diodoro
MG, Lasagni RP, Romagnoli F, Brenna A, De Joannon BC, Donnorso RP,
Lombardi G, et al: Fatty acid synthase (FAS) is a marker of
increased risk of recurrence in lung carcinoma. Anticancer Res.
24:4169–4173. 2004.
|
|
6
|
Mashima T, Seimiya H and Tsuruo T: De novo
fatty-acid synthesis and related pathways as molecular targets for
cancer therapy. Br J Cancer. 100:1369–1372. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Knowles LM, Yang C, Osterman A and Smith
JW: Inhibition of fatty-acid synthase induces caspase-8-mediated
tumor cell apoptosis by up-regulating DDIT4. J Biol Chem.
283:31378–31384. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brusselmans K, De Schrijver E, Heyns W,
Verhoeven G and Swinnen JV: Epigallocatechin-3-gallate is a potent
natural inhibitor of fatty acid synthase in intact cells and
selectively induces apoptosis in prostate cancer cells. Int J
Cancer. 106:856–862. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Puig T, Vázquez-Martín A, Relat J, Pétriz
J, Menéndez JA, Porta R, Casals G, Marrero PF, Haro D, Brunet J, et
al: Fatty acid metabolism in breast cancer cells: Differential
inhibitory effects of epigallocatechin gallate (EGCG) and C75.
Breast Cancer Res Treat. 109:471–479. 2008. View Article : Google Scholar
|
|
10
|
Ruby AJ, Kuttan G, Babu KD, Rajasekharan
KN and Kuttan R: Anti-tumour and antioxidant activity of natural
curcuminoids. Cancer Lett. 94:79–83. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moragoda L, Jaszewski R and Majumdar AP:
Curcumin induced modulation of cell cycle and apoptosis in gastric
and colon cancer cells. Anticancer Res. 21:873–878. 2001.PubMed/NCBI
|
|
12
|
Bush JA, Cheung KJ Jr and Li G: Curcumin
induces apoptosis in human melanoma cells through a Fas
receptor/caspase-8 pathway independent of p53. Exp Cell Res.
271:305–314. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Radhakrishna Pillai G, Srivastava AS,
Hassanein TI, Chauhan DP and Carrier E: Induction of apoptosis in
human lung cancer cells by curcumin. Cancer Lett. 208:163–170.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chainani-Wu N: Safety and
anti-inflammatory activity of curcumin: A component of tumeric
(Curcuma longa). J Altern Complement Med. 9:161–168. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Syng-Ai C, Kumari AL and Khar A: Effect of
curcumin on normal and tumor cells: Role of glutathione and bcl-2.
Mol Cancer Ther. 3:1101–1108. 2004.PubMed/NCBI
|
|
16
|
Anand P, Sundaram C, Jhurani S,
Kunnumakkara AB and Aggarwal BB: Curcumin and cancer: An 'old-age'
disease with an 'age-old' solution. Cancer Lett. 267:133–164. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao J, Sun XB, Ye F and Tian WX:
Suppression of fatty acid synthase, differentiation and lipid
accumulation in adipocytes by curcumin. Mol Cell Biochem.
351:19–28. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Milgraum LZ, Witters LA, Pasternack GR and
Kuhajda FP: Enzymes of the fatty acid synthesis pathway are highly
expressed in in situ breast carcinoma. Clin Cancer Res.
3:2115–2120. 1997.
|
|
19
|
Kuhajda FP: Fatty acid synthase and
cancer: New application of an old pathway. Cancer Res.
66:5977–5980. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pizer ES, Wood FD, Heine HS, Romantsev FE,
Pasternack GR and Kuhajda FP: Inhibition of fatty acid synthesis
delays disease progression in a xenograft model of ovarian cancer.
Cancer Res. 56:1189–1193. 1996.PubMed/NCBI
|
|
21
|
Kuhajda FP: Fatty-acid synthase and human
cancer: New perspectives on its role in tumor biology. Nutrition.
16:202–208. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Flavin R, Peluso S, Nguyen PL and Loda M:
Fatty acid synthase as a potential therapeutic target in cancer.
Future Oncol. 6:551–562. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hatcher H, Planalp R, Cho J, Torti FM and
Torti SV: Curcumin: From ancient medicine to current clinical
trials. Cell Mol Life Sci. 65:1631–1652. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hermine O, Haioun C, Lepage E, d'Agay MF,
Briere J, Lavignac C, Fillet G, Salles G, Marolleau JP, Diebold J,
et al: Prognostic significance of bcl-2 protein expression in
aggressive non-Hodgkin's lymphoma. Groupe d'Etude des Lymphomes de
l'Adulte (GELA). Blood. 87:265–272. 1996.PubMed/NCBI
|
|
25
|
Anto RJ, Maliekal TT and Karunagaran D:
L-929 cells harboring ectopically expressed RelA resist
curcumin-induced apoptosis. J Biol Chem. 275:15601–15604. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Doglioni C, Dei Tos AP, Laurino L,
Chiarelli C, Barbareschi M and Viale G: The prevalence of BCL-2
immunoreactivity in breast carcinomas and its clinicopathological
correlates, with particular reference to oestrogen receptor status.
Virchows Arch. 424:47–51. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Onodera J, Nakamura S, Nagano I, Tobita M,
Yoshioka M, Takeda A, Oouchi M and Itoyama Y: Upregulation of Bcl-2
protein in the myasthenic thymus. Ann Neurol. 39:521–528. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Salakou S, Tsamandas AC, Bonikos DS,
Papapetropoulos T and Dougenis D: The potential role of bcl-2, bax,
and Ki67 expression in thymus of patients with myasthenia gravis,
and their correlation with clinicopathologic parameters. Eur J
Cardiothorac Surg. 20:712–721. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Oltvai ZN, Milliman CL and Korsmeyer SJ:
Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that
accelerates programmed cell death. Cell. 74:609–619. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang E and Korsmeyer SJ: Molecular
thanatopsis: A discourse on the BCL2 family and cell death. Blood.
88:386–401. 1996.PubMed/NCBI
|
|
31
|
Korsmeyer SJ: BCL-2 gene family and the
regulation of programmed cell death. Cancer Res. 59(Suppl 7):
1693s–1700s. 1999.PubMed/NCBI
|
|
32
|
Testa JR and Bellacosa A: AKT plays a
central role in tumorigenesis. Proc Natl Acad Sci USA.
98:10983–10985. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li S, Zhou Y, Wang R, Zhang H, Dong Y and
Ip C: Selenium sensitizes MCF-7 breast cancer cells to
doxorubicin-induced apoptosis through modulation of phospho-Akt and
its downstream substrates. Mol Cancer Ther. 6:1031–1038. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pizer ES, Chrest FJ, DiGiuseppe JA and Han
WF: Pharmacological inhibitors of mammalian fatty acid synthase
suppress DNA replication and induce apoptosis in tumor cell lines.
Cancer Res. 58:4611–4615. 1998.PubMed/NCBI
|
|
36
|
De Schrijver E, Brusselmans K, Heyns W,
Verhoeven G and Swinnen JV: RNA interference-mediated silencing of
the fatty acid synthase gene attenuates growth and induces
morphological changes and apoptosis of LNCaP prostate cancer cells.
Cancer Res. 63:3799–3804. 2003.PubMed/NCBI
|
|
37
|
Wang HQ, Altomare DA, Skele KL, Poulikakos
PI, Kuhajda FP, Di Cristofano A and Testa JR: Positive feedback
regulation between AKT activation and fatty acid synthase
expression in ovarian carcinoma cells. Oncogene. 24:3574–3582.
2005. View Article : Google Scholar : PubMed/NCBI
|