Introduction
Photodynamic therapy (PDT) is an approved anticancer
treatment, which involves systemic or local administration of a
photosensitizer (PS) followed by illumination of the tumor with
visible light. In the presence of oxygen it generates reactive
oxygen species (ROS), which exert direct cytotoxic effects on tumor
cells, as well as on the tumor vasculature (1). PDT leads to a significant decrease or
elimination of the primary tumor. However, for long-term efficacy
of PDT, the intact immune response is essential (2). It was demonstrated that in
immunocompromised animals devoid of adaptive immunity, PDT
initially triggers tumor ablation, but permanent cures are not
observed (3). Specific depletion of
selected populations of leukocytes revealed that neutrophils and
CD8+ T cells play an indispensable role in achieving
maximum therapeutic effects of PDT (4). Further studies confirmed that PDT
generates damage-associated molecular pattern (DAMP) proteins and
that PDT-triggered cell death is associated with immunogenic
antigens (5,6). However, induction of the immune
response by PDT depends primarily on the treatment regimen as well
as on the tumor model. It was demonstrated that low-dose PDT
stimulates an inflammatory response better than high-dose PDT, both
in mice (7) and in clinical
settings (8). A number of
approaches to enhance the immune response triggered by PDT have
been proposed, including combinations with immunestimulating agents
(9,10).
On the other hand, the sub-lethal PDT doses, optimal
for immune stimulation, are usually insufficient to eradicate
primary tumor due to induction of cytoprotective responses. To
counterbalance PDT-triggered oxidative stress, tumor cells
upregulate ROS-scavenging enzymes, stress-related transcription
factors and chaperones. Moreover, the accumulation of misfolded
proteins in the endoplasmic reticulum (ER) triggers unfolded
protein response (UPR). It was shown that PDT induces expression of
the master regulator of the UPR, glucose regulated protein 78
(GRP78) (11,12). GRP78 is an endoplasmic reticulum
resident chaperone, which is highly expressed in tumor cells.
Increasing number of studies reveals that this protein is
upregulated in response to various therapies and contributes to
treatment resistance (13).
Therefore, attempts to inhibit GRP78 are considered as components
of anticancer therapy. In contrast to several small-molecule GRP78
inhibitors, which are very unspecific, a bacterial subtilase
cytotoxin (Sub) has been shown to specifically cleave and
inactivate GRP78 (14). The
holotoxin (SubAB5) produced by Shiga toxigenic strains of
Escherichia coli (STEC), is composed of a catalytic A
subunit (SubA) and a pentameric B subunit, enabling cell entry.
Previous research has revealed that the toxin is the major cause of
hemolytic uremic syndrome, the most common form of the disease
observed in humans infected with STEC. It is also lethal in mice,
as it triggers substantial systemic abnormalities due to
microvascular damage, thrombosis and hemorrhage in several organs,
including the kidneys, brain, and liver (15). Further study has shown that the
toxin also affects the immune system, which is manifested by spleen
atrophy and profound leukocyte redistribution (16). Moreover, sub-lethal doses of SubAB5
exert anti-inflammatory effects (17). To exploit the antitumor activity of
the toxin via GRP78 targeting, as well as to minimize severe toxic
effects, the fusion protein composed of epidermal growth factor
(EGF) and the catalytic A subunit of the toxin (EGF-SubA) was
generated for targeted toxin delivery. EGF-SubA selectively killed
EGF receptor (EGFR)-expressing tumor cells and had an antitumor
efficacy in a mouse model. Moreover, EGF-SubA synergized with
UPR-inducing agents such as tunicamycin or thapsigargin (18).
We recently found that the expression of GRP78 was
induced in vitro by Photofrin-PDT in human cancer cell lines
and that GRP78 plays a cytoprotective role. Moreover,
siRnA-mediated downregulation of GRP78 as well as EGF-SubA-mediated
specific cleavage of GRP78, sensitized tumor cells to in
vitro PDT (19). Here, we
investigated the combination of EGF-SubA with PDT in vivo in
mouse models.
Materials and methods
Reagents
Photofrin (Axcan Pharma Inc., Houdan, France) was
used as a photosensitizer. Photofrin was dissolved in 5% glucose,
aliquoted and stored at −20°C. EGF-SubA fusion protein was
purchased from SibTech Inc. (Brookfield, CT, USA). The 0.75
µM (in vitro studies) or 1 mg/ml (in vivo
studies) stock solutions were aliquoted and stored at −20°C.
Cell culture
Mouse colon carcinoma (CT26) and human prostate
cancer (DU-145) cell lines were purchased from the American Type
Culture Collection (ATCC; Rockville, MD, USA). Cells were cultured
in Roswell Park Memorial Institute (RPMI)-1640 medium (CT26) or
Dulbecco's modified Eagle's medium (DMEM) (DU-145) supplemented
with 20% (CT26) or 5% (DU-145) heat-inactivated fetal bovine serum
(Hyclone) and antibiotic/antimycotic solution (Sigma-Aldrich, St.
Louis, MO, USA). Cells were cultured under standard conditions in a
5% CO2 humidified incubator at 37°C.
Generation of CT26 cells stably
expressing human EGFR
A CT26 cell line stably expressing human EGFR
(CT26-EGFR) was generated using a retroviral system. HEK 293T cells
were co-transfected with the pBabe-EGFR-puro plasmid, packaging
pKAT and envelope pVSV-G vectors using a calcium chloride protocol.
The pBabe-EGFR-puro plasmid encoding WT human EGFR was a gift from
Matthew Meyerson (plasmid #11011; Addgene) (20). Twenty-four hours post-transfection,
medium from the HEK 293T cells was collected, centrifuged and
filtered through a 0.45-µm filter. Afterwards, the CT26
cells were added to retrovirus-containing medium and centrifuged
for 1 h (500 × g, RT) in the presence of 12 µg/ml Polybrene
(Sigma-Aldrich). Next, the cells were selected with 10 µg/ml
puromycin (Sigma-Aldrich) and sorted for high EGFR expression with
cell sorter FACSAria III (Becton Dickinson). The overexpression of
EGFR was confirmed by flow cytometry and western blotting.
Cell viability assay
In order to evaluate the cytostatic/cytotoxic effect
of EGF-SubA on cancer cells, 1.25×103 cells/well were
plated onto 96-well plates, incubated with increasing
concentrations of EGF-SubA for 48 h and stained with crystal violet
as described previously (21).
Flow cytometry and cell sorting
To confirm the presence of EGFR on the cell surface,
the cells were trypsinized, pelleted, washed with PBS and blocked
in 1% BSA for 15 min. Next, the cells were incubated with chimeric
anti-EGFR antibody (Erbitux) for 40 min (1:50) followed by
incubation with anti-human IgG-Alexa488 antibody (1:100, 30 min;
Jackson ImmunoResearch). The cells were analyzed by FACScan (Becton
Dickinson) using CellQuest Pro software version 5.2. To select the
EGFR-expressing population, the CT26 cells were stained for surface
EGFR as described and Alexa488-positive cells were sorted for using
FACSAria III (Becton Dickinson).
Splenocyte isolation and analysis of
leukocyte populations
Spleens were isolated from mice of each experimental
group. To obtain a single-cell suspension, the spleens were forced
through a 70-µm cell strainer. To lyse erythrocytes, the
cells were incubated for 5 min at 37°C in red blood cell lysis
buffer (150 mM NH4Cl, 1 mM NaHCO3, pH 7.4).
Next, the cells were washed with PBS and resuspended in RPMI-1640
medium containing 10% fetal bovine serum.
To determine the total splenocyte count, the cells
were stained with anti-CD45 antibody and the number of viable cells
in 10 µl was determined using the Accuri C6 flow cytometer
(Becton Dickinson). To evaluate leukocyte populations, the
splenocytes were stained with the following monoclonal antibodies
(mAbs): anti-B220-eFluor® 450 (REF. 48-0452-80),
anti-CD3-PE-Cy7 (REF. 25-0031-82),
anti-CD4-APC (REF. 17-0041),
anti-CD8-FITC (REF. 11-0081),
anti-CD11c-eFluor® 450 (REF. 48-0114) (all from
eBioscience) anti-CD45.2-BD HorizonBD Horizon™ V500 (REF. 562129;
BD Biosciences), anti-MHCII-PE-Cy7 (REF. 25-5321; eBioscience) and analyzed using
FACSAria III.
Western blotting
Cells were pelleted and lysed in a lysis buffer (50
mM HEPES pH 7.4, 1.0% Triton X-100, 150 mM NaCl, 10% glycerol, 5 mM
EDTA) supplemented with Complete protease inhibitors (Roche,
Mannheim, Germany) and phosphatase inhibitors (1 mM sodium
ortho-vanadate, 1 mM sodium fluoride, and 1 mM 2-glycerol
phosphate). The protein concentration was measured using the
Bio-Rad protein assay (Bio-Rad, Hercules, CA, USA). Equal amounts
of proteins were separated by SDS-PAGE and transferred to
Protran® nitrocellulose membranes (Schleicher and
Schuell BioScience, Dassel, Germany). The membranes were blocked
and incubated with the following primary antibodies (GRP78-3183;
Cell Signaling Technology, Beverly, MA, USA; β-actin-A3854;
Sigma-Aldrich) according to the manufacturer's protocols. After
extensive washing with TBST, the membranes were incubated for 40
min with HRP-linked secondary antibodies (Cell Signaling
Technology). The chemiluminescence reaction was developed using
self-made reagent (50 mM Tris-HCl pH 8.5, 0.2 mM cumaric acid, 1.25
mM luminol, 0.006% hydrogen peroxide) and visualized with Stella
8300 Bio-imager (Raytest, Straubenhardt, Germany).
Mice
For the in vivo experiments 8- to 14-week-old
female BALB/c or SCID mice were used. BALB/c mice were obtained
from the Animal House of the Polish Academy of Sciences, Medical
Research Center (Warsaw, Poland). SCID mice were obtained from
Charles River Laboratory (Erkrath, Germany). The experiments were
performed in accordance with the guidelines approved by the Ethics
Committee of the Medical University of Warsaw.
PDT in vivo
Before inoculation, the cells were harvested, washed
twice and resuspended with PBS (CT26-EGFR) or PBS with 25% Matrigel
(DU-145). The viability of the cells was determined by trypan blue
staining. A total of 3×105 CT26-EGFR cells (in 30
µl) or 2×106 of DU-145 cells (in 100 µl)
were injected subcutaneously into the right thigh of the
experimental mice (day 0). On days 6–8 (BALB/c) or 7–9, the (SCID)
mice were injected intraperitoneally (i.p.) with EGF-SubA (25 or 50
µg/kg) in PBS. Photofrin was administered i.p. at a dose of
10 mg/kg on day 6 (BALB/c) or day 7 (SCID). Twenty-four hours
later, the tumors were illuminated with 630 nm light delivered by a
He-ne ion laser (Laserinstruments, Warsaw, Poland) through optical
fibre. The power of the laser was 45–47 mW/cm2 and the
total fluence was 50 J/cm2 for CT26 and 43
J/cm2 for DU-145 cells. During the time of illumination,
the mice were anesthetized with ketamine and xylazine and
restrained. Tumor growth was monitored 3 times a week (BALB/c) or
once a week (SCID) with the use of a caliper, as described
previously (22). The mice were
sacrificed when any of the tumor diameters reached 15 mm.
Depletion
Mice were injected i.p. with 100 µg of
anti-CD8 (YTS169) monoclonal antibodies (mAbs) on days 5 and 11 of
the experiment. Control mice received 100 µg of isotype
control mAb. The level of depletion was evaluated 3 days after mAb
injection. Blood samples were collected from the jugular vein of
the experimental mice, stained with the anti-CD8 mAb and analyzed
by flow cytometry.
Histopathologic analysis
After animal sacrifice, livers, spleens and kidneys
were removed and fixed in buffered 4% formaldehyde for 48 h at RT.
Subsequently, the tissue samples were dehydrated through a series
of graded ethanol baths to displace the water, cleared in xylene,
infiltrated with paraffin wax and then embedded in paraffin blocks.
Paraffin sections were subsequently stained with hematoxylin and
eosin (H&E). All tissue sections were examined using light
microscopy.
Statistical analyses
Differences in tumor volumes were analyzed for
significance by the Mann-Whitney test with significance level set
at P<0.05. For ex vivo studies significance was
calculated by the Mann-Whitney test. The survival rate of the
animals was analyzed for significance by log-rank survival
analysis. Significance was defined as a two-sided P<0.05.
Results
EGF-SubA exerts cytostatic/cytotoxic
effects on CT26 cells expressing human EGFR
We previously observed that EGF-SubA potentiated the
cytotoxic effects of PDT in various human cancer cell lines in
vitro (19). In order to study
the effects of a combination of PDT and EGF-SubA in vivo, we
generated a murine cell line CT26 expressing human EGFR
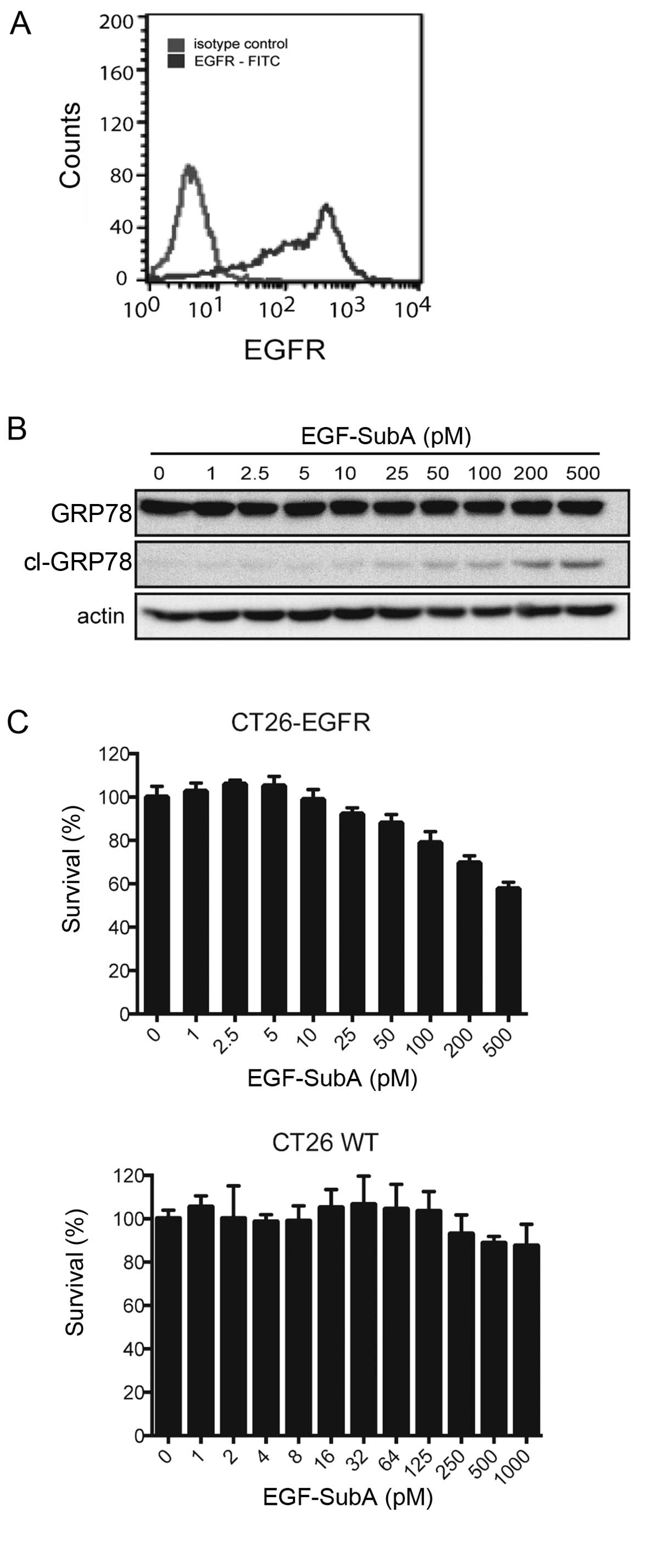
(CT26-EGFR). The presence of EGFR on the surface of the CT26 cells
was confirmed by flow cytometry (Fig.
1A). To determine the efficacy of EGF-SubA, CT26-EGFR cells
were incubated with increasing concentrations of the cytotoxin and
tested for its ability to cleave GRP78 and to exert
cytostatic/cytotoxic effects. Treatment with EGF-SubA resulted in
accumulation of the GRP78 cleavage product (Fig. 1B). Moreover, the expression of EGFR
sensitized CT26 cells to EGF-SubA as shown in the crystal violet
cell viability assay (Fig. 1C).
Effects of EGF-SubA on murine immune
cells and organs
Considering that an intact immune system is
essential for PDT efficacy and that previous studies have described
the impact of the SubAB5 holotoxin on immune cells, we investigated
whether similar effects could be observed in mice treated with
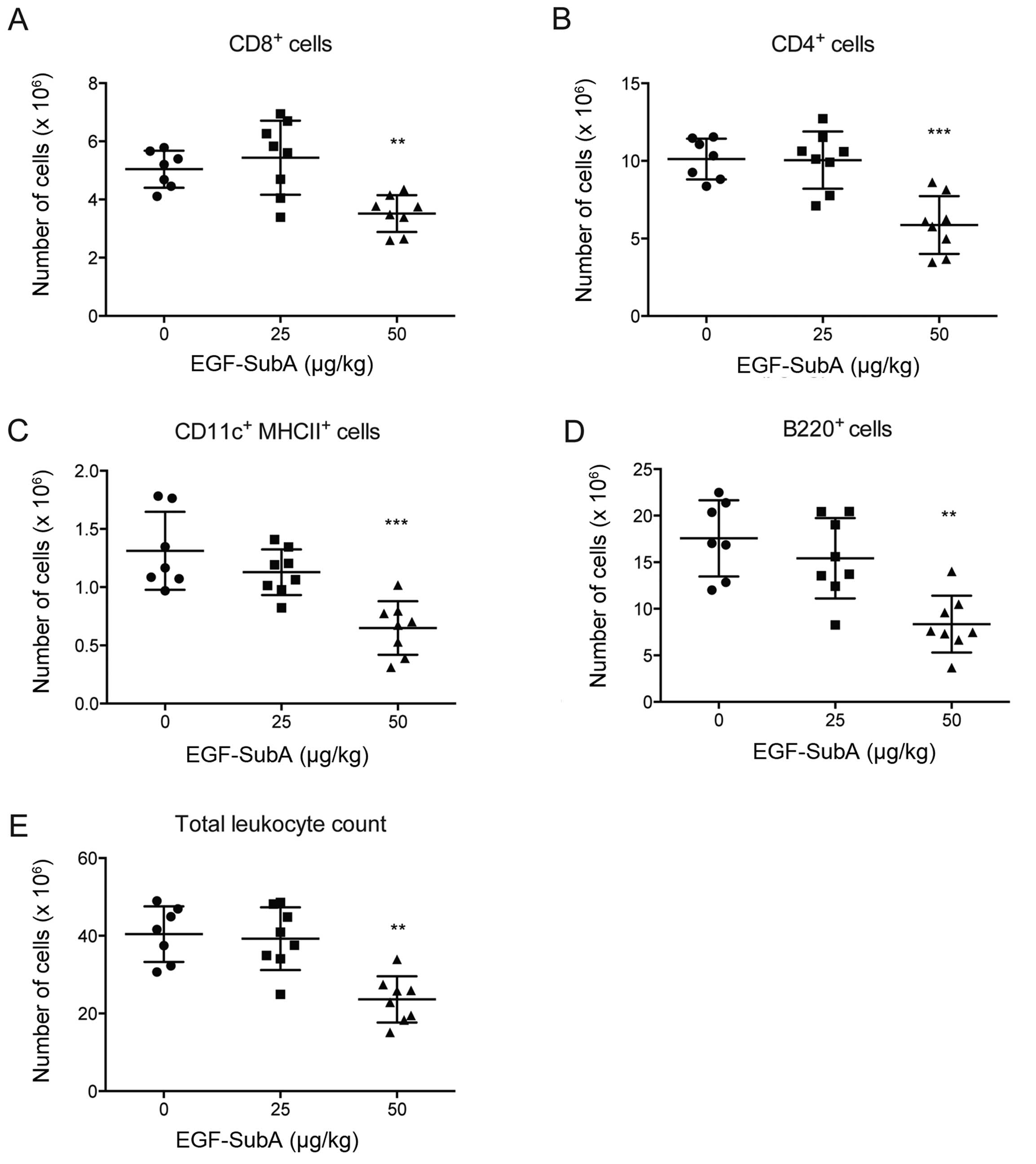
EGF-SubA. BALB/c mice were treated with 25 or 50 µg/kg of
EGF-SubA for three consecutive days. Twenty-four hours after the
last dose administration, spleens were collected, total numbers of
splenocytes were counted and cells were analyzed by flow cytometry.
We observed that a lower dose of EGF-SubA did not affect the immune
cells, whereas the higher dose significantly reduced the numbers of
B cells (B220+) and cytotoxic (CD8+) and
helper (CD4+) T cells, as well as dendritic cells
(MHCII+CD11c+) (Fig. 2).
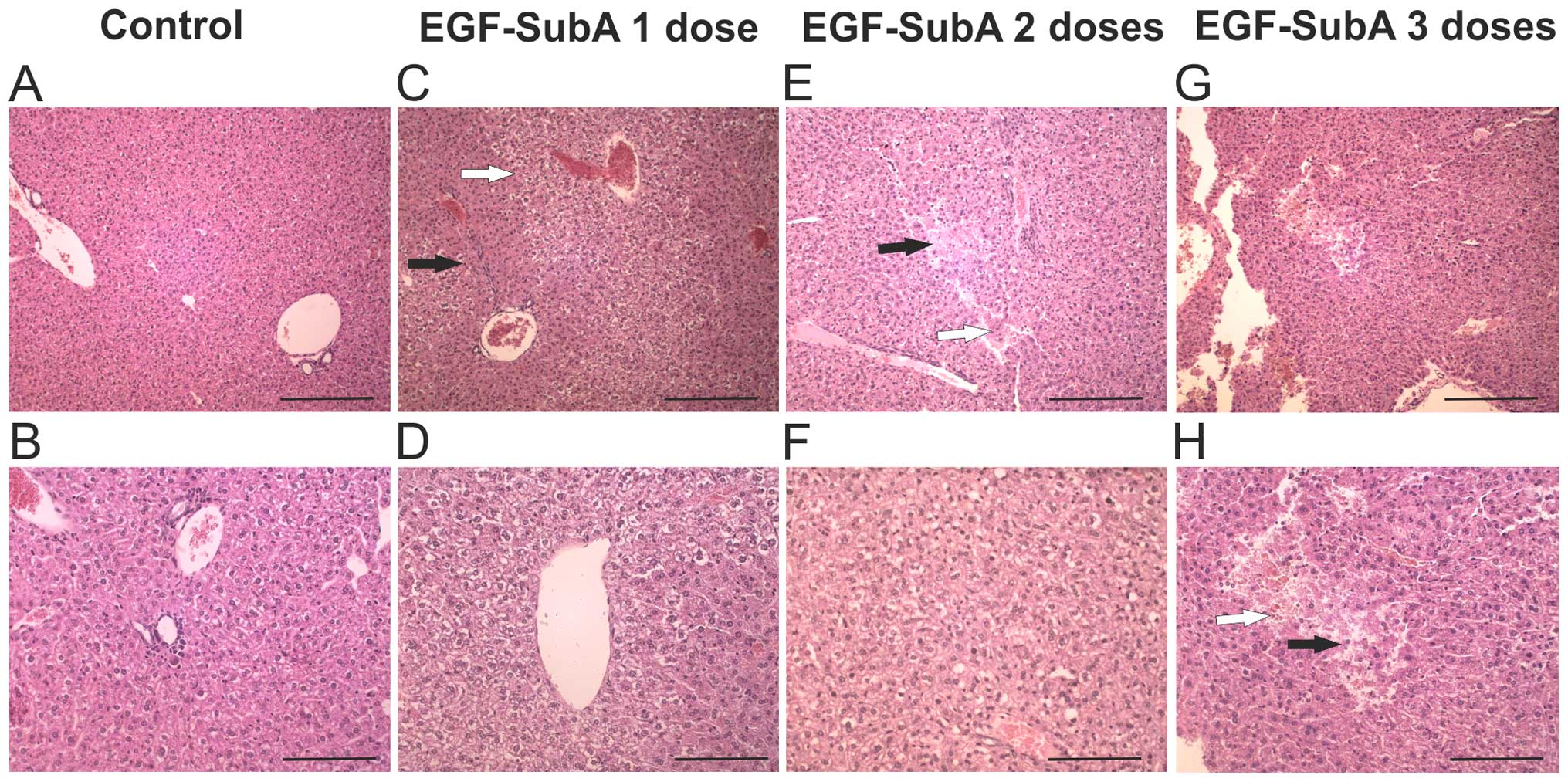
Moreover, livers, spleens and kidneys were removed
after 1, 2 and 3 doses of EGF-SubA and analyzed
histopathologically. In mice treated with 25 µg/kg EGF-SubA,
we occasionally observed hemorrhagic foci and hepatic necrosis as
well as very subtle changes in spleens and kidneys (data not
shown). More pronounced changes were observed in the mice treated
with 50 µg/kg of EGF-SubA. Splenic abnormalities included
small accumulations of reticular fibers in the white pulp; renal
abnormalities ranged from subtle edematous changes in deep cortical
layer tubules to more pronounced focal deposits of eosinophilic
material in some tubules, with obliteration of their lumina. The
most noticeable changes we observed in the livers of mice treated
with 50 µg/kg of EGF-SubA (Fig.
3). After one or two doses of the toxin we observed pronounced
hepatocyte anisocytosis and occasional hemorrhagic foci.
Hepatocytes located adjacent to the periportal areas were denser,
whereas those located around central veins were edematous with pale
cytoplasm. After three doses of EGF-SubA, vesicular fatty changes
predominated in the cytoplasm of hepatocytes located in
centrilobular areas. Moreover, isolated foci of necrosis and
hemorrhagic changes were also visible with occasional areas of
hepatocyte anisocytosis. Altogether, we observed that a 50
µg/kg dose of EGF-SubA negatively affected the immune cells
and triggered histopathological changes.
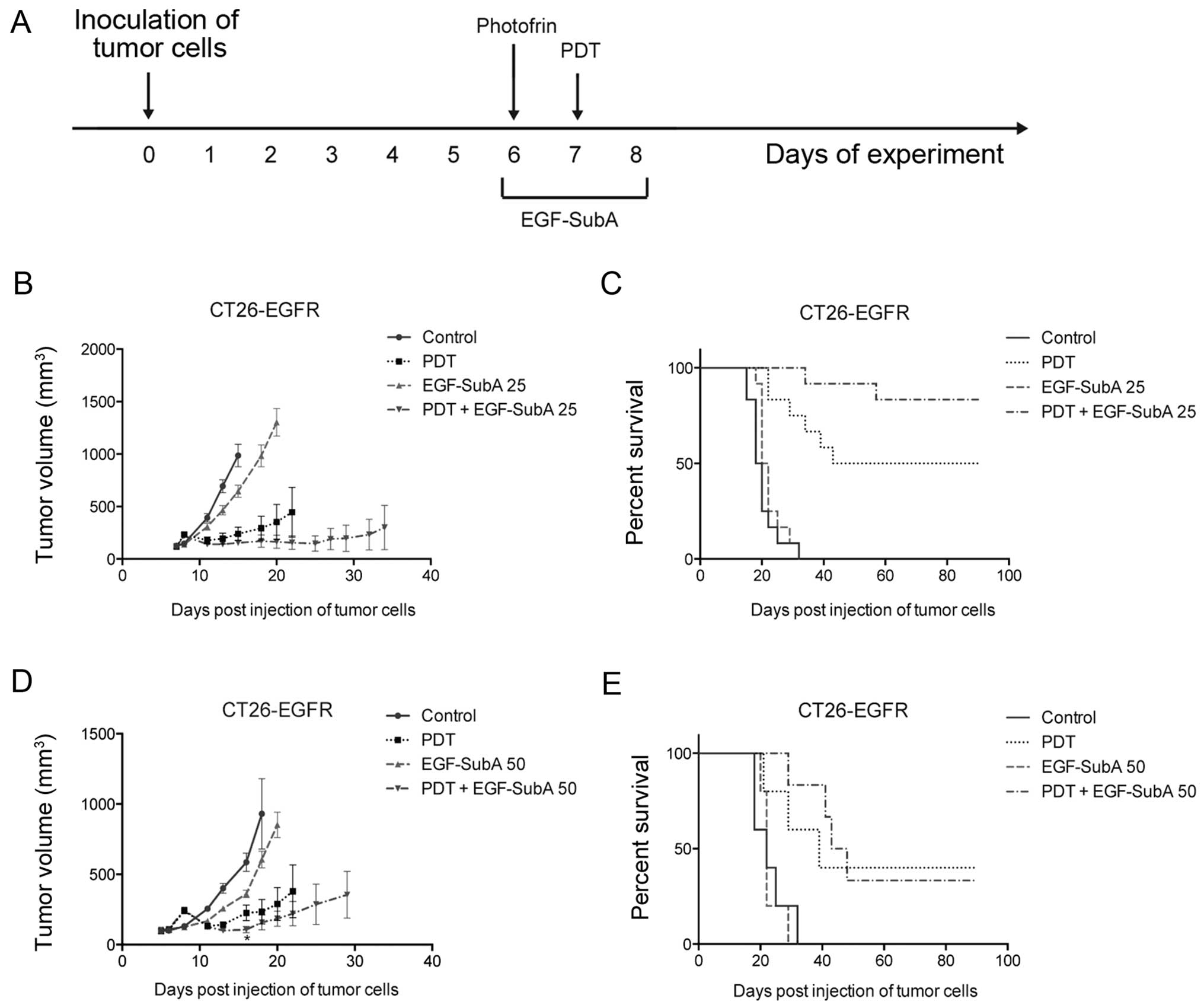
EGF-SubA improves PDT efficacy
To determine whether EGF-SubA is able to potentiate
the antitumor effects of PDT in vivo, we used the CT26-EGFR
cell line. BALB/c mice subcutaneously injected with the CT26-EGFR
cells were treated with PDT on day 7 after inoculation of tumor
cells and EGF-SubA was administered at a dose of 25 µg/kg
for three consecutive days (days 6–8) (Fig. 4A). We observed that the combination
treatment resulted in a trend towards decreased tumor size
(Fig. 4B) as well as prolonged
survival (Fig. 4C), as compared
with PDT alone. The difference in survival of mice treated with PDT
only and PDT in combination with EGF-SubA was noticeable, and close
to statistical significance (P=0.067, log-rank test). These effects
were not observed in the mice treated with a higher dose (50
µg/kg) of EGF-SubA (Fig. 4D and
E).
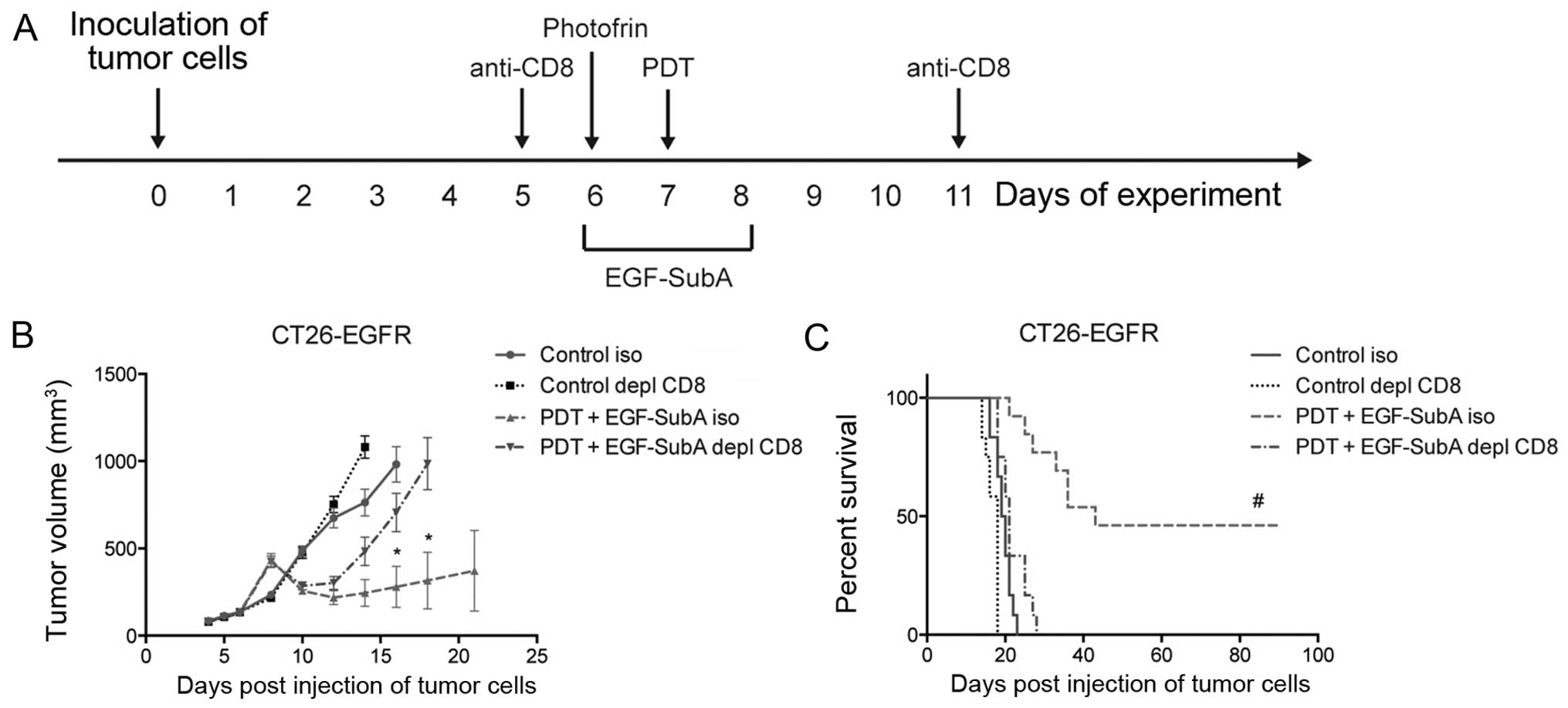
A functional immune system is necessary
for the effects of the combination treatment
All the above data suggest that a functional immune
response is essential for the final outcome of the combination
treatment. In order to confirm this hypothesis, we performed the
combination treatment in mice depleted of CD8+ cells,
which have been shown essential for immune effects of PDT (Fig. 5A) (4). Depletion of CD8+ cells in
the BALB/c mice completely abrogated the effects of the combination
treatment on tumor size (Fig. 5B).
Furthermore, no complete responses were observed in the
CD8+-depleted mice (Fig.
5C).
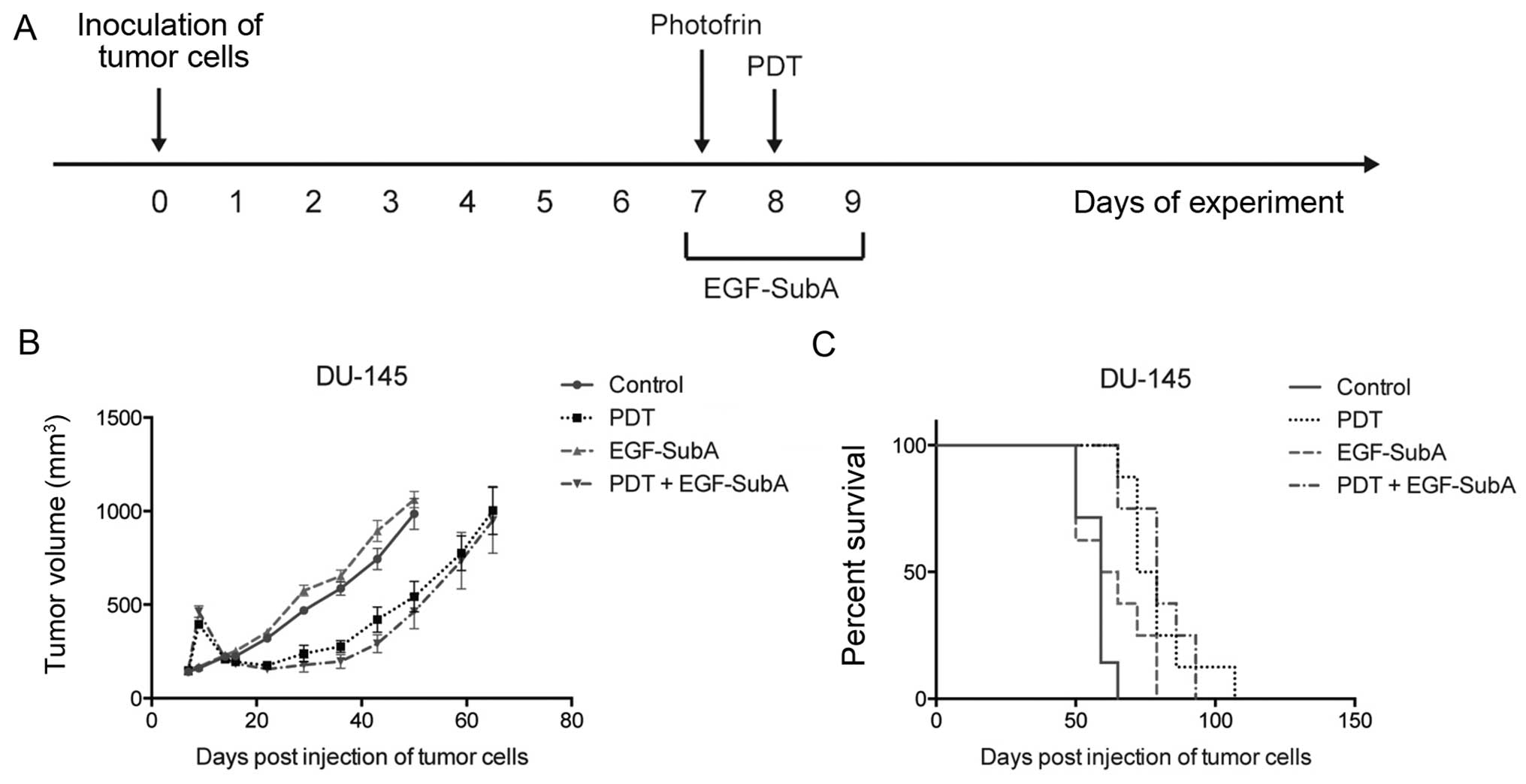
To further confirm that the functional immune system
is crucial for the efficacy of PDT + EGF-SubA treatment, we aimed
to determine whether the combination could be effective in
immunocompromised mice. Previously, we observed a strong
potentiation of in vitro cytostatic/cytotoxic effects of PDT
in the human prostate cancer cell line DU-145 (19). Therefore, SCID mice were
subcutaneously injected with DU-145 cells and treated with PDT on
day 8 after inoculation of tumor cells and with EGF-SubA at a dose
of 25 µg/kg for three consecutive days (days 7–9) (Fig. 6A). Treatment of DU-145 tumors with
PDT resulted in the prolonged survival of SCID mice, however, no
complete responses were observed. Despite the initial mild effects
on tumor growth (Fig. 6B), the
addition of EGF-SubA to the PDT protocol did not influence mouse
survival in this model (Fig.
6C).
Discussion
The clinical application of PDT in cancer treatment
is still limited to a small number of indications. However, the
undeniable feature of PDT is its potential to elicit a specific
antitumor immune response. Importantly, the ability of local PDT to
induce systemic antitumor immunity has been demonstrated in
patients with basal cell carcinoma (8). However, the degree of immune response
induction by PDT is variable, regimen-dependent and in the majority
of models insufficient to cure cancer. Paradoxically, the most
immune-stimulatory regimens are concomitantly sub-cytotoxic and the
overall PDT effect is insufficient to obtain long-term responses.
Therefore, the continuous search for treatment modalities combining
PDT with other antitumor agents is warranted. Notably, our previous
in vitro study in human cancer cell lines revealed a strong
synergism between PDT and EGF-SubA cytotoxin (19).
Here, we showed that a low dose of EGF-SubA, the
cytotoxin that cleaves and inactivates the master ER chaperone
GRP78, increased the efficacy of PDT in vivo in
immunocompetent mice. However, the antitumor effects were
critically dependent on a functional immune response. Firstly, the
antitumor effects were observed for the low (25 µg/kg), and
not for the high (50 µg/kg) EGF-SubA dose (Fig. 4). Administration of the latter
strongly depleted immune cells such as CD8+ and
CD4+ T cells, B cells and dendritic cells in the spleens
of mice. These effects were not observed in animals treated with
the low EGF-SubA dose (Fig. 2).
Secondly, the depletion of CD8+ T cells with monoclonal
antibodies entirely abolished the antitumor effects of the
combination treatment on both tumor growth rate and animal
survival, supporting the fundamental contribution of these cells to
the treatment outcome (Fig. 5B and
C). Finally, in immunocompromised mice devoid of adaptive
immunity, although the tumor-suppressive effect of both PDT alone
as well as PDT with EGF-SubA were evident in a few days following
PDT, none of the therapies were effective in the long run (Fig. 6B and C). All these results
demonstrated that the combination of PDT with EGF-SubA may be
effective providing an intact immune response.
Our results support previous findings that the
immune response is crucial for overall PDT efficacy (3,23). The
role of selected immune cell populations in the PDT-induced
antitumor immune response was extensively studied in various
experimental models over the last two decades. Our results, as the
majority of other reports (4,10,24),
substantiate the role of CD8+ T cells as critically
important players in the maintenance of durable PDT-induced
curative effects. Moreover, we observed that both the depletion of
CD8+ T cells (Fig. 5) as
well as the decrease in a number of CD8+ T cells and
other immune cells in the spleens of mice treated with a higher
dose of EGF-SubA (Fig. 2),
negatively affected PDT efficacy. These results are in line with
previous findings that a combination of a high dose of
cyclophosphamide, which decreases the number of splenocytes,
reduced the survival of mice and impaired PDT efficacy (25). Collectively these results imply that
a disturbance of the immune response may have detrimental impact on
the overall PDT effects and may have important implications for the
evaluation of novel agents in combination with PDT.
Another important finding was that EGF-SubA
cytotoxin at sub-lethal doses affects the immune system. EGF-SubA
is a fusion protein composed of EGF and a catalytic subunit of the
holotoxin SubAB5, and was designed to selectively target
EGFR-overexpressing tumor cells. Backer et al (18) demonstrated that the EGF-SubA fusion
protein selectively kills tumor cells expressing EGFR and
suppresses the growth of these tumors in vivo at a dose of
125 µg/kg, without observable toxic effects. In our experimental
settings, doses of EGF-SubA exceeding 100 µg/kg were already
toxic, leading to the deaths of approximately half of the treated
animals after two administrations of the fusion protein (data not
shown). The difference may result from various dosing schedules.
The lower EGF-SubA dose, 50 µg/kg, did not trigger any
lethal effects, yet it decreased the numbers of immune cells in the
spleens of treated animals (Fig.
2). Moreover, the histopathological examinations of the livers
of mice treated with 50 µg/kg EGF-SubA revealed the presence
of necrotic and hemorrhagic foci (Fig.
3). These observations are reminiscent of the effects reported
in mice treated with SubAB5 holotoxin (16). Several possible explanations exist
as to how the fusion protein affects cells not expressing EGFR. It
may be transmitted to immune cells via endocytosis together with
dying tumor cells. Alternatively, the EGF-SubA fusion protein
tropism may not be restricted to EGFR-expressing cells, especially
when administered at higher doses.
In summary, we showed that GRP78-targeting EGF-SubA
cytotoxin improves the efficacy of PDT in vivo, but only at
a lower dose which does not impair the immune system. Our results
further substantiate the indispensable role of the adaptive
immunity in achieving long-term curative PDT effects.
Acknowledgments
We thank Ewa Werner, Karolina Hajduk, Anna
Czerepinska and Elzbieta Gutowska for valuable technical
assistance. This study was supported by grants: DI2011 021141
(M.G.) and DI2013 006643 (A.D.) from Polish Ministry of Science and
Higher Education and FP7-REGPOT-2012-CT2012-316254-BASTION from the
European Commission 7th Framework Programme (J.G.).
References
|
1
|
Agostinis P, Berg K, Cengel KA, Foster TH,
Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel
D, et al: Photodynamic therapy of cancer: An update. CA Cancer J
Clin. 61:250–281. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Firczuk M, Nowis D and Gołąb J:
PDT-induced inflammatory and host responses. Photochem Photobiol
Sci. 10:653–663. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Korbelik M, Krosl G, Krosl J and Dougherty
GJ: The role of host lymphoid populations in the response of mouse
EMT6 tumor to photodynamic therapy. Cancer Res. 56:5647–5652.
1996.PubMed/NCBI
|
|
4
|
Korbelik M and Cecic I: Contribution of
myeloid and lymphoid host cells to the curative outcome of mouse
sarcoma treatment by photodynamic therapy. Cancer Lett. 137:91–98.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Garg AD, Krysko DV, Verfaillie T,
Kaczmarek A, Ferreira GB, Marysael T, Rubio N, Firczuk M, Mathieu
C, Roebroek AJ, et al: A novel pathway combining calreticulin
exposure and ATP secretion in immunogenic cancer cell death. EMBO
J. 31:1062–1079. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Korbelik M, Sun J and Cecic I:
Photodynamic therapy-induced cell surface expression and release of
heat shock proteins: Relevance for tumor response. Cancer Res.
65:1018–1026. 2005.PubMed/NCBI
|
|
7
|
Henderson BW, Gollnick SO, Snyder JW,
Busch TM, Kousis PC, Cheney RT and Morgan J: Choice of
oxygen-conserving treatment regimen determines the inflammatory
response and outcome of photodynamic therapy of tumors. Cancer Res.
64:2120–2126. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kabingu E, Oseroff AR, Wilding GE and
Gollnick SO: Enhanced systemic immune reactivity to a Basal cell
carcinoma associated antigen following photodynamic therapy. Clin
Cancer Res. 15:4460–4466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Korbelik M and Cecic I: Enhancement of
tumour response to photodynamic therapy by adjuvant mycobacterium
cell-wall treatment. J Photochem Photobiol B. 44:151–158. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wachowska M, Gabrysiak M, Muchowicz A,
Bednarek W, Barankiewicz J, Rygiel T, Boon L, Mroz P, Hamblin MR
and Golab J: 5-Aza-2′-deoxycytidine potentiates antitumour immune
response induced by photodynamic therapy. Eur J Cancer.
50:1370–1381. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jalili A, Makowski M, Switaj T, Nowis D,
Wilczynski GM, Wilczek E, Chorazy-Massalska M, Radzikowska A,
Maslinski W, Biały L, et al: Effective photoimmunotherapy of murine
colon carcinoma induced by the combination of photodynamic therapy
and dendritic cells. Clin Cancer Res. 10:4498–4508. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xue LY, Agarwal ML and Varnes ME:
Elevation of GRP-78 and loss of HSP-70 following photodynamic
treatment of V79 cells: Sensitization by nigericin. Photochem
Photobiol. 62:135–143. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luo B and Lee AS: The critical roles of
endoplasmic reticulum chaperones and unfolded protein response in
tumorigenesis and anticancer therapies. Oncogene. 32:805–818. 2013.
View Article : Google Scholar
|
|
14
|
Paton AW, Beddoe T, Thorpe CM, Whisstock
JC, Wilce MC, Rossjohn J, Talbot UM and Paton JC: AB5 subtilase
cytotoxin inactivates the endoplasmic reticulum chaperone BiP.
Nature. 443:548–552. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Paton AW, Srimanote P, Talbot UM, Wang H
and Paton JC: A new family of potent AB(5) cytotoxins produced by
Shiga toxigenic Escherichia coli. J Exp Med. 200:35–46. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang H, Paton JC and Paton AW: Pathologic
changes in mice induced by subtilase cytotoxin, a potent new
Escherichia coli AB5 toxin that targets the endoplasmic reticulum.
J Infect Dis. 196:1093–1101. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Harama D, Koyama K, Mukai M, Shimokawa N,
Miyata M, Nakamura Y, Ohnuma Y, Ogawa H, Matsuoka S, Paton AW, et
al: A subcytotoxic dose of subtilase cytotoxin prevents
lipopolysaccharide-induced inflammatory responses, depending on its
capacity to induce the unfolded protein response. J Immunol.
183:1368–1374. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Backer JM, Krivoshein AV, Hamby CV,
Pizzonia J, Gilbert KS, Ray YS, Brand H, Paton AW, Paton JC and
Backer MV: Chaperone-targeting cytotoxin and endoplasmic reticulum
stress-inducing drug synergize to kill cancer cells. Neoplasia.
11:1165–1173. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Firczuk M, Gabrysiak M, Barankiewicz J,
Domagala A, Nowis D, Kujawa M, Jankowska-Steifer E, Wachowska M,
Glodkowska-Mrowka E, Korsak B, et al: GRP78-targeting subtilase
cytotoxin sensitizes cancer cells to photodynamic therapy. Cell
Death Dis. 4:e7412013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Greulich H, Chen TH, Feng W, Jänne PA,
Alvarez JV, Zappaterra M, Bulmer SE, Frank DA, Hahn WC, Sellers WR,
et al: Oncogenic transformation by inhibitor-sensitive and
-resistant EGFR mutants. PLoS Med. 2:e3132005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Makowski M, Grzela T, Niderla J, ŁAzarczyk
M, Mróz P, Kopeé M, Legat M, Strusińska K, Koziak K, Nowis D, et
al: Inhibition of cyclooxygenase-2 indirectly potentiates antitumor
effects of photodynamic therapy in mice. Clin Cancer Res.
9:5417–5422. 2003.PubMed/NCBI
|
|
22
|
Szokalska A, Makowski M, Nowis D,
Wilczynski GM, Kujawa M, Wójcik C, Mlynarczuk-Bialy I, Salwa P, Bil
J, Janowska S, et al: Proteasome inhibition potentiates antitumor
effects of photodynamic therapy in mice through induction of
endoplasmic reticulum stress and unfolded protein response. Cancer
Res. 69:4235–4243. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Korbelik M: Induction of tumor immunity by
photodynamic therapy. J Clin Laser Med Surg. 14:329–334.
1996.PubMed/NCBI
|
|
24
|
Kabingu E, Vaughan L, Owczarczak B, Ramsey
KD and Gollnick SO: CD8+ T cell-mediated control of
distant tumours following local photodynamic therapy is independent
of CD4+ T cells and dependent on natural killer cells.
Br J Cancer. 96:1839–1848. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Castano AP, Mroz P, Wu MX and Hamblin MR:
Photodynamic therapy plus low-dose cyclophosphamide generates
antitumor immunity in a mouse model. Proc Natl Acad Sci USA.
105:5495–5500. 2008. View Article : Google Scholar : PubMed/NCBI
|