Introduction
Glioblastoma multiforme (GBM), the most aggressive
glial tumor, is associated with a median survival rate of 9–12
months. Advances in the basic knowledge of cancer biology, as well
as surgical techniques, chemotherapy and radiotherapy, have led to
only a marginal improvement in the survival rate for GBM (1,2). Other
well-tolerated and effective therapeutic approaches are clearly
needed. In the present study, we aimed to identify a factor
associated with tumor cell migration and invasion, which may
provide a more effective treatment strategy.
Nogo or reticulon-4 (RTN4), also known as neurite
outgrowth inhibitor, is a member of the reticulon family of genes,
and is encoded by a single gene on human chromosome 2 (2p16) with
14 exons spanning a 75-kb stretch, and 10 known splice variants
(3). Nogo occurs in 3 major forms,
Nogo-A, Nogo-B and Nogo-C, which are generated from alternate
splicing (4). Nogo-A is the longest
isoform based on transcript and protein level analyses, and is
enriched in the central nervous system (CNS). However, the other
abundant splice isoform, Nogo-B, is expressed more ubiquitously
(5–7). Nogo-C is highly expressed in skeletal
muscle, but is weakly expressed in the liver and kidney (8). All 3 splice isoforms have an
N-terminal domain of varying length, and share an identical
C-terminal domain comprising a 66-amino acid loop (Nogo-66) flanked
by two hydrophobic segments, followed by a short C-terminal
hydrophilic stretch.
Nogo-A has been described as a myelin-associated
neuronal growth inhibitory molecule that interacts with the Nogo
receptor (NgR), and signaling appears to be related to the
activation of Rho A (9). Rho is a
member of the Ras superfamily of small GTP-binding proteins, and
the mammalian Rho GTPase family currently consists of 3 major
subfamilies: Rho (RhoA, RhoB and RhoC), Rac (Rac1, Rac2 and Rac3)
and Cdc42 (Cdc42Hs and G25K). These subfamilies play a central role
in diverse biological processes, such as actin cytoskeleton
organization, microtubule dynamics, gene transcription, oncogenic
transformation, cell cycle progression, adhesion and epithelial
wound repair (10).
In brain tumors, Nogo-A was found to be expressed in
oligodendroglioma and GBM in an immunohistochemical study (11) and was negatively related to the
malignancy of oligodendroglial tumors (12,13).
Furthermore, Nogo was found to inhibit neurite outgrowth molecules
(14). These previous studies
suggest the possibility that Nogo-A is a negative regulator of the
migration and invasion of glioma cells (15); nevertheless, the role of Nogo-A in
brain tumors remains unclear. Thus, in the present study, we
investigated whether Nogo-A can regulate the migration and invasion
of malignant glioma.
Materials and methods
Cell lines and tissues
Human glioblastoma cell line U87MG was obtained from
the Korean Cell Line Bank (Seoul, Korea). All cell lines were
maintained in Dulbecco's modified Eagle's medium (DMEM) (Gibco-BRL,
Grand Island, NY, USA) and supplemented with 10% fetal bovine serum
(FBS) at 37°C in a humidified 95% air/5% CO2
atmosphere.
Brain tumor tissues (38 GBMs, 30 oligodendrogliomas,
7 mixed gliomas, 3 pituitary adenomas and 3 schwannomas) were
obtained from the Chonnam National University Hwasun Hospital
National Biobank of Korea. The specimens were snap-frozen in liquid
nitrogen and stored at −70°C until use.
Immunohistochemistry of Nogo-A
Immunohistochemistry was performed using the
avidin-biotin complex method with a microprobe immuno/DNA stainer
(Fisher Scientific, Pittsburgh, PA, USA). Paraffin-embedded blocks
of formalin-fixed surgical specimens (20 GBM and 29
oligoden-drogliomas obtained from the Chonnam National University,
Hwasun Hospital National Biobank of Korea) were cut into
3-µm thick sections with a microtome and placed on
microscope slides. The sections were deparaffinized in xylene and
treated with 3% H2O2 in methanol for 20 min
to block endogenous peroxidase activity. After washing several
times with immuno/DNA buffer (Invitrogen, Carlsbad, CA, USA), the
sections were incubated with 2% normal serum to block any
non-specific binding, followed by incubation with a rabbit
anti-Nogo-A polyclonal antibody (1:100 dilution; Santa Cruz
Biotechnology, Santa Cruz, CA, USA) at 4°C overnight. A
streptavidin horseradish peroxidase (Dako Cytomation, Denmark)
detection system was applied to the capillary channels, followed by
a 20-min incubation at 37°C. After rinsing, the tissue sections
were ready for the chromogen reaction with 3-amino-9-ethyl
carbazole. The sections were counterstained with hematoxylin and
mounted on universal mounts (Shandon, Pittsburgh, PA, USA).
Finally, the coverslips were fixed to the slides with mounting
solution.
Preparation of the plasmid containing
human Nogo-A cDNA
Full-length Nogo-A cDNA was purchased from Open
Biosystems (Huntsville, AL, USA) (clone ID 40148984), and cloned
into the pcDNA3.1 (+) expression vector (Invitrogen) containing the
cytomegalovirus promoter and the neomycin-resistance gene. The
resulting pcDNA3.1 (+)-Nogo-A sequence completely matched the NCBI
accession (BC 150182) and was used to transfect the U87MG
cells.
Transfection procedure
U87MG cells were maintained under exponential growth
conditions in DMEM supplemented with 10% FBS, in the absence of
antibiotics. The optimum cell density for transfection is normally
between 50 and 80% confluency for adherent cells. U87MG cells were
transfected with the empty pcDNA3.1 (+) vector and pcDNA3.1
(+)-Nogo-A using Lipofectamine™ 2000 transfection reagent
(Invitrogen). These transfectants were designated as 'U87MG-E' and
'U87-Nogo-A', respectively. Plasmid DNA (6 µg) and 6
µl Lipofectamine 2000/serum-free media were added to the
cells growing in serum-free media, according to the manufacturer's
protocol. After a 5-h incubation at 37°C in 5% CO2, the
transfection mixture was replaced with DMEM supplemented with 10%
FBS. Forty-eight hours later, the medium was replaced with DMEM
containing 10% FBS and 800 µg/ml G418 and cultured in a
CO2 incubator. The G418-resistant clones were isolated,
and the level of expression of the Nogo-A protein was determined by
western blot analysis. The stable transfectants were maintained in
DMEM supplemented with 10% FBS and 400 µg/ml G418.
Western blotting
Cell lines and brain tissues were lysed in lysis
buffer [50 mM Tris (pH 8.0), 5 mM ethylene diamine tetraacetic
acid, 150 mM NaCl, 0.5% deoxycholic acid, 0.1% sodium dodecyl
sulphate, 1% NP-40, 1 mM phenylmethylsulphonyl fluoride and 1 mg/ml
protease inhibitor cocktail]. The protein concentrations were
determined using the bicinchoninic acid (BCA) method (16). Subsequently, 50 µg whole cell
lysate was separated by 8% sodium dodecyl sulphate-polyacrylamide
gel electrophoresis (SDS-PAGE) and transferred onto a
poly-vinylidene fluoride membrane (PALL Corp., Mexico). The
membrane was then incubated for 2 h at room temperature in a
solution of TBS-T [10 mM Tris-Cl (pH 8.0), 150 mM NaCl and 0.05%
Tween-20] supplemented with 5% non-fat dry milk, and probed
overnight at 4°C with primary antibodies against Nogo-A, cofilin
and phosphorylated cofilin (Santa Cruz Biotechnology). The bound
antibodies were visualized with an anti-rabbit secondary antibody
(Jackson ImmunoResearch Laboratories, West Grove, PA, USA)
conjugated to horseradish peroxidase using enhanced
chemiluminescence reagents (Amersham Biosciences, Pittsburg, PA,
USA). β-actin was used as an internal control and rat brain tissue
was used as a Nogo-A-positive control.
Migration assay
For the comparison of motility between the U87MG-E
and U87-Nogo-A cells, a simple scratch technique was performed.
Cells were grown to confluency on a 60-mm dish, and the medium was
replaced with medium containing 5 mM hydroxyurea to stop cell
proliferation. After 24 h, the cultures were scraped using a
single-edged razor blade. Cells were washed twice with
phosphate-buffered saline (PBS), placed in medium containing 5 mM
hydroxyurea, and incubated for 48 h. Following this, the cells were
washed twice with PBS, fixed with absolute alcohol, and stained
with 0.1% toluidine blue. Six microscopic fields were evaluated for
each wound injury. The number of cells migrating across the wound
edge and the maximum distance migrated were determined in each
field, and averaged for each injury. These experiments were
repeated 3 times.
Matrigel invasion assay
Matrigel (reconstituted basement membrane; 25 mg)
was dried on a polycarbonated filter (polyvinylpyrrolidone-free;
Nucleopore Whatmann, UK). The cells were harvested by brief
exposure to 1 mm/l EDTA, washed with DMEM containing 0.1% bovine
serum albumin, and added to a Boyden chamber (1×104
cells). Cells were incubated for 24 h at 37°C in a humidified
atmosphere of 95% air and 5% CO2. The cells that
transversed the Matrigel layer and attached to the filter were
stained with Hemacolor (Darmstadt, Germany) and counted in five
randomized fields. The results are expressed as the mean ± SE of 3
independent experiments.
Rho activity assay
According to the manufacturer's protocol included in
the Rho activity assay kit (Millipore, Billerica, MA, USA), U87MG-E
and U87-Nogo-A cells were cultured to ~85–90% confluency, the
culture medium was removed, and the cells were rinsed twice with
ice-cold Tris-buffered saline (TBS). Ice-cold Mg2+
lysis/wash buffer (MLB) [25 mM HEPES (pH 7.5), 150 mM NaCl, 1%
Igepal CA-630, 10 mM MgCl2, 1 mM EDTA and 2% glycerol
containing 1 mM phenylmeth-ylsulfonyl fluoride (PMSF)] was added to
the rinsed cells in plates on ice. The detached cells were
collected and lysed in microfuge tubes on ice, the tubes were
incubated for 20 min at 4°C with agitation, and then centrifuged at
14,000 × g for 5 min at 4°C. The supernatant was removed. The
minimum protein concentration was ~8 mg/ml. A 500 ml aliquot of
each cell extract was placed in a microfuge tube, 40 ml of the Rho
assay reagent slurry was added, and the reaction mixture was
incubated for 45 min at 4°C with gentle agitation. The agarose
beads were pelleted by brief centrifugation (10 sec at 14,000 × g
at 4°C), and the supernatant was removed and discarded. The beads
were washed 3 times with 0.5 ml 1X MLB. The beads were mixed
gently, pelleted and the supernatant was removed. The agarose beads
were resuspended in 25 ml 2X Laemmli reducing sample buffer and
boiled for 5 min. The beads were then pelleted by centrifugation.
The supernatant and the agarose pellets were mixed and then loaded
for Rho western blot analysis. In parallel, a sample that was not
treated with the Rho assay reagent slurry was loaded to determine
the total Rho amount.
Doubling time of stable
transfectants
The cells were seeded at 3×104 cells on
6-well culture dishes. After serum starvation for 48 h, the cells
were counted every 24 h. The cells were trypsinized and the number
of viable cells was counted with a hemocytometer. The doubling-time
was calculated from the cell growth curve over 4 days using the
following equation: Doubling time = (final time − initial time) ×
[log 2/log(final cell number) − log(initial cell number)].
Immunofluorescence confocal
microscopy
The cells were cultured on coverslips in 35-mm
dishes until subconfluency was reached, washed with PBS, and fixed
with 4% paraformaldehyde for 10 min. After washing (3–5 times) in
PBS, the cells were treated with 0.1% Triton X-100 for 5 min at
room temperature, and washed again 3–5 times with PBS. The cells
were incubated with anti-vimentin (BD Pharmingen, San Diego, CA,
USA) in a humid chamber for 1 h, and then with Alexa 488-conjugated
goat anti-mouse antibody (Molecular Probes, Eugene, CA, USA) for 40
min. For actin staining, Rhodamine-conjugated phalloidin (Molecular
Probes) was used. The coverslips were mounted on slides with Immuno
Mounts (Shandon). Confocal microscopy was performed using an
FV10-ASW, version 1.7 confocal laser scanning biological microscope
(Olympus, Tokyo, Japan) equipped with an UPlanSApo 60×/1.35 oil
objective lens. The confocal images were acquired using FluoView
FV1000 software.
Data analysis
The comparison of the nucleotide sequence of the
cDNA (purchased) with the registered sequence in GeneBank was
carried out using the BLAST algorithm. The statistical significance
of the cell distance and cell number was measured using the
Mann-Whitney U test, and the doubling time by repeated measures
ANOVA. P<0.05 was considered to indicate a statistically
significant result. Statistical analysis was performed using SPSS
(version 12.0 for Windows; SPSS, Inc., Chicago, IL, USA).
Results
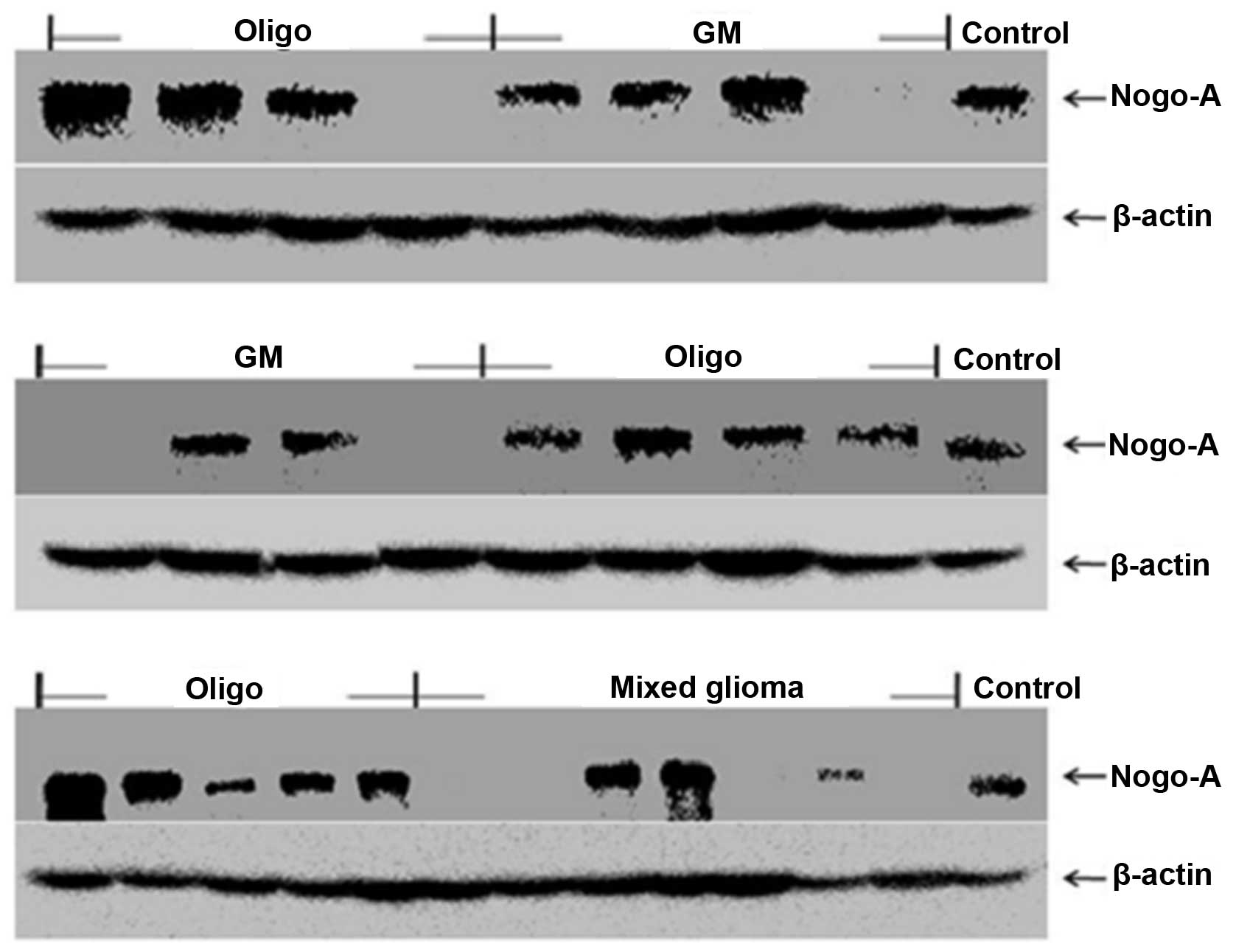
Expression of Nogo-A in glial tumors and
Nogo-A transfectants
The expression of Nogo-A was examined using
immunohistochemistry (IHC) and western blotting. The expression of
Nogo-A was exhibited in 90, 68.4 and 42.9% of oligodendrogliomas,
glioblastomas and mixed gliomas from patients, respectively.
However, schwannomas and pituitary adenomas did not express Nogo-A
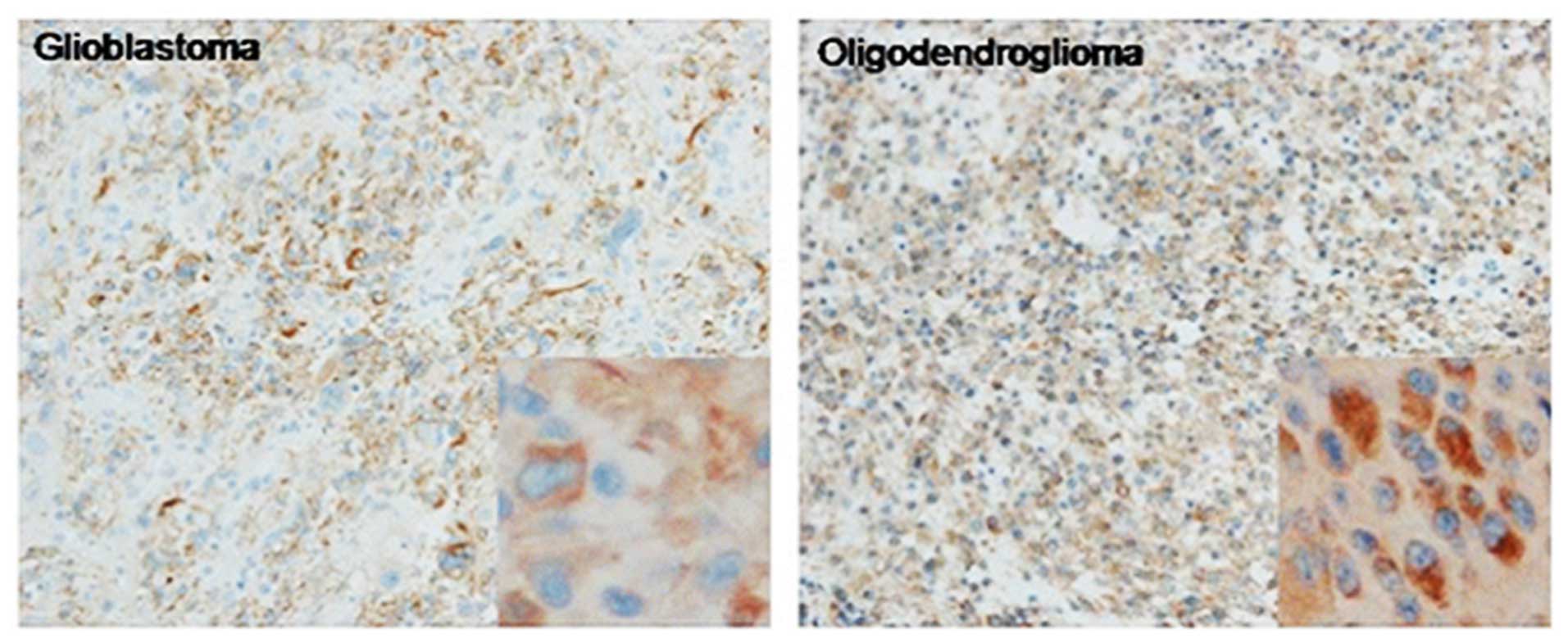
(Fig. 1). Similarly, Nogo-A was
expressed in a higher percentage of oligodendrogliomas (79.3%),
compared with the glioblastomas (35%) by IHC (Fig. 2). The brown-positive signals were
mainly distributed in the cytoplasm. These results are summarized
in Table I.
 | Table INogo-A expression in glial
tumors. |
Table I
Nogo-A expression in glial
tumors.
| Histology | Western blotting
n/total (%) |
Immunohistochemistry n/total (%) |
|---|
| Glioblastoma | 26/38 (68.4) | 7/20 (35.0) |
|
Oligodendroglioma | 27/30 (90.0) | 23/29 (79.3) |
| Mixed glioma | 3/7 (42.9) | |
| Pituitary
adenoma | 0/3 (0) | |
| Schwannoma | 0/3 (0) | |
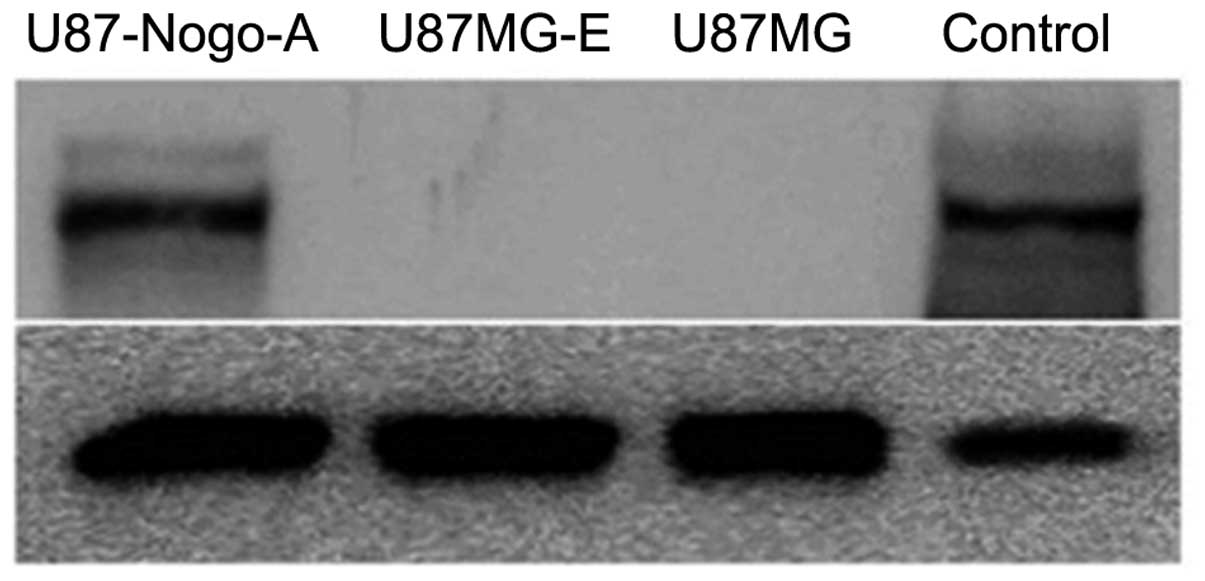
Endogenous Nogo-A content
To assess the effect of Nogo-A on proliferation,
migration and invasion, U87MG malignant glioma cells, not
expressing Nogo-A, were transfected with an empty vector
('U87MG-E') and a sense Nogo-A cDNA construct ('U87-Nogo-A'). The
best clone among the transfectants was selected using western
blotting. Nogo-A was highly expressed in the U87-Nogo-A cells,
compared with its level in the U87MG parental and U87MG-E cells
(Fig. 3).
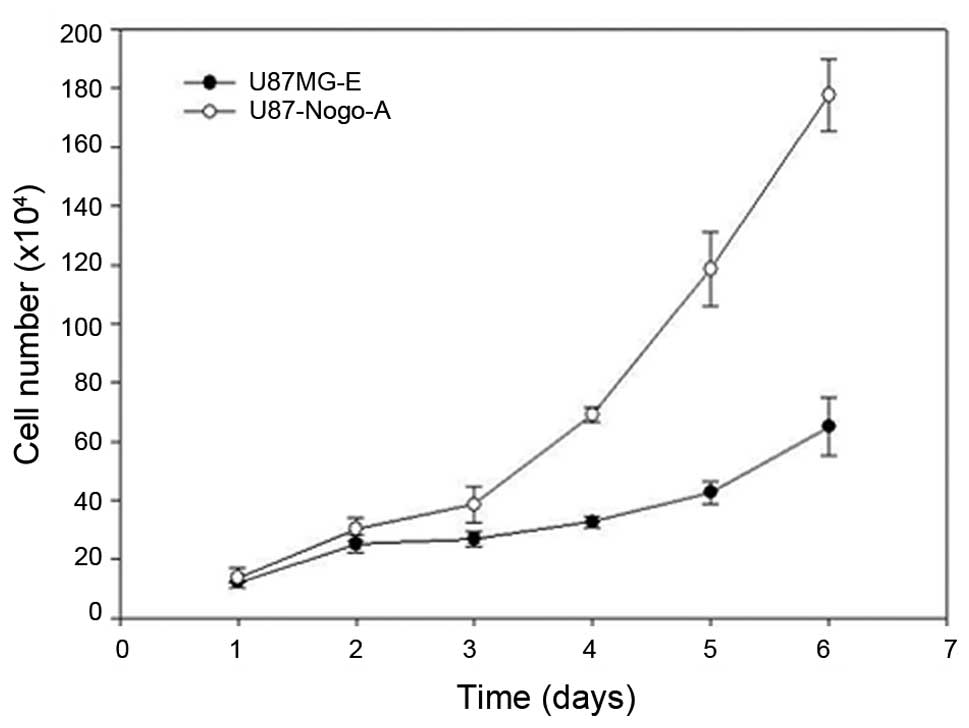
Proliferation rate
We examined the effect of Nogo-A on cell
proliferation. The doubling times in the U87MG-E and U87-Nogo-A
cells were 37.1 and 29.2 h, respectively, showing statistical
significance (P<0.001; Fig. 4
and Table II).
 | Table IIComparison of the growth rate between
the control and Nogo-A-overexpressing cells. |
Table II
Comparison of the growth rate between
the control and Nogo-A-overexpressing cells.
| Cell line | Doubling time
(h) |
|---|
| U87MG-E | 37.1 |
| U87-Nogo-A | 29.2 |
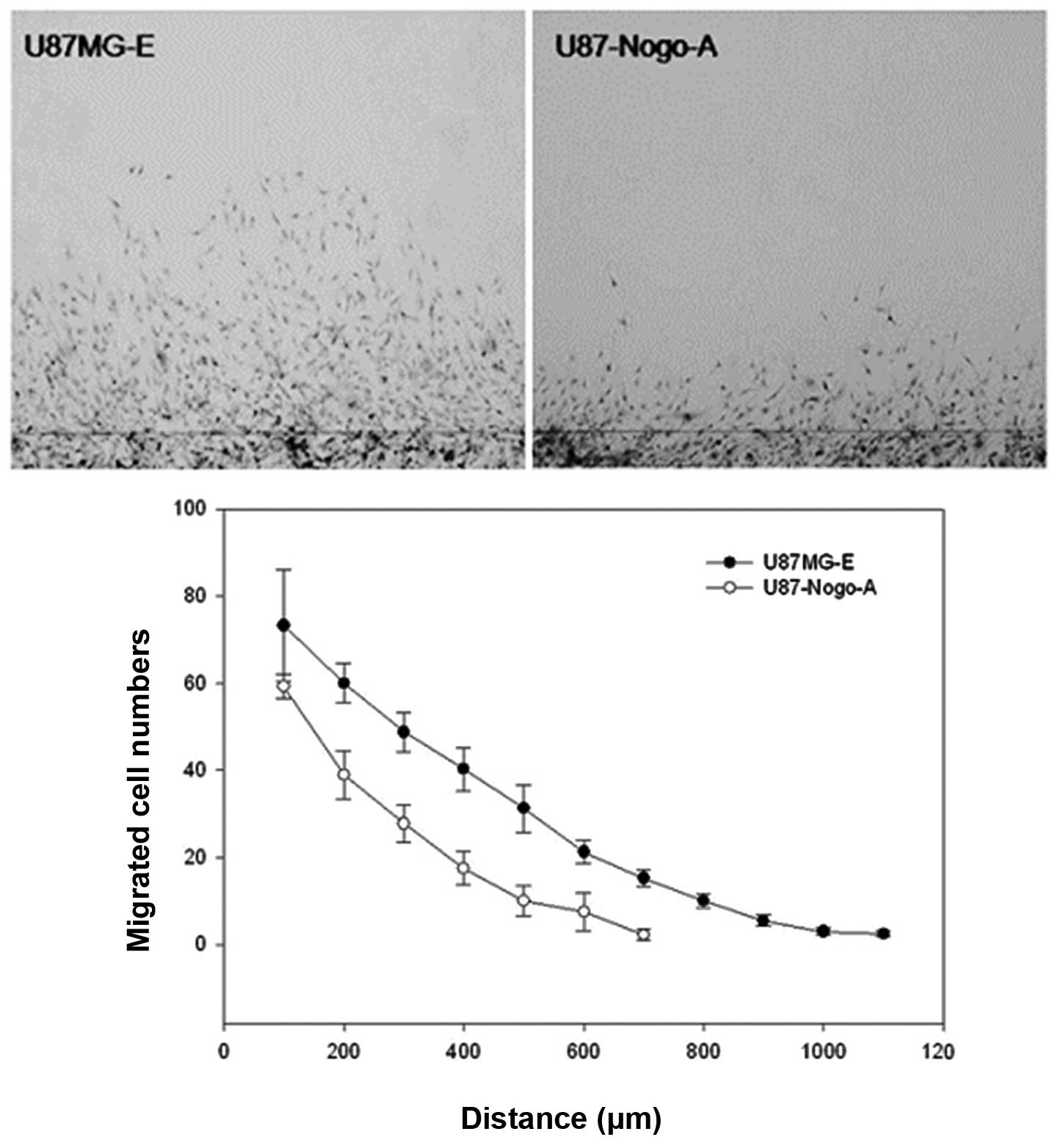
Effect of Nogo-A on migration ability; a
simple scratch test
The motility of the cell lines was detected using a
simple scratch technique. The difference in proliferation between
the two cell lines may affect the results of the in vitro
motility assay. To exclude this factor, we pretreated the cells
with hydroxyurea, which causes cell cycle arrest. As shown in
Fig. 5 and Table III, the total number of cells that
migrated from the wound in the U87MG-E and U87-Nogo-A cell lines
was 311±18.4 and 163.3±31, respectively. In addition, the maximum
distance of migration from the wound in the U87MG-E and U87-Nogo-A
cells was 1,100 and 700 µm, respectively. These results
suggest that the expression of Nogo-A in the U87MG cells is
inversely associated with the migration ability (P=0.005) of the
tumor cells.
 | Table IIIThe results of the scratch test. |
Table III
The results of the scratch test.
| Cell line | Cell number | Maximum distance
(µm) |
|---|
| U87MG-E | 311±18.4 | 1,100 |
| U87-Nogo-A | 163.3±31 | 700 |
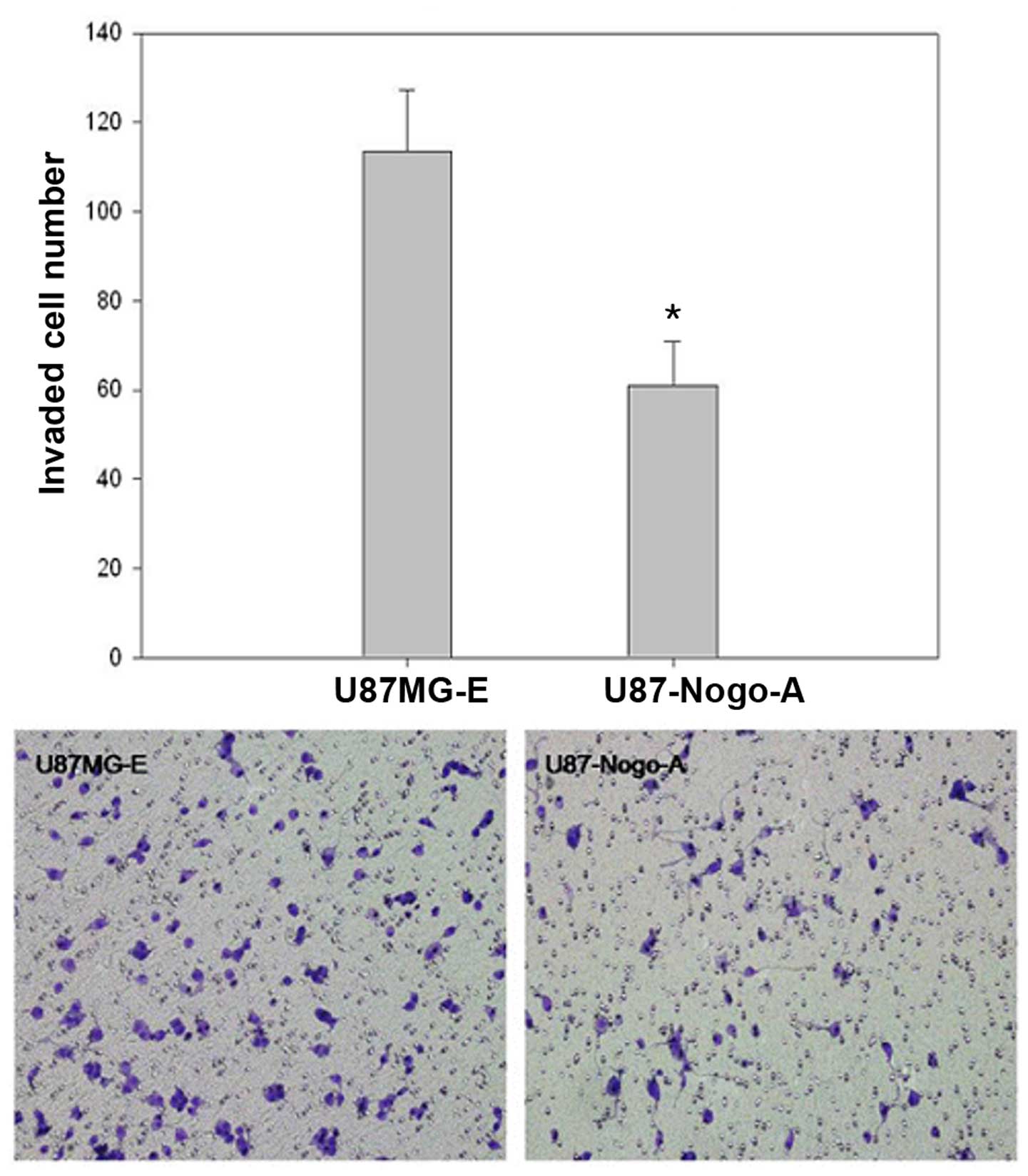
Effect of Nogo-A on invasion; the Boyden
chamber assay
The effect of Nogo-A on the invasion of tumor cells
was examined using a Boyden chamber assay. This method is for
quantitative analysis of the invasion ability of tumor cells. It is
estimated by counting the number of cells invading through the
Matrigel-coated membrane. The total number of U87MG-E and
U87-Nogo-A cells was 113.5±13.8 and 60.9±9.9, respectively.
Invasiveness of U87MG cells was significantly decreased by
overexpression of Nogo-A (P<0.001; Fig. 6 and Table IV).
 | Table IVResults of the Matrigel assay. |
Table IV
Results of the Matrigel assay.
| Cell line | Cell number |
|---|
| U87MG-E | 113.5±13.8 |
| U87-Nogo-A | 60.9±9.9 |
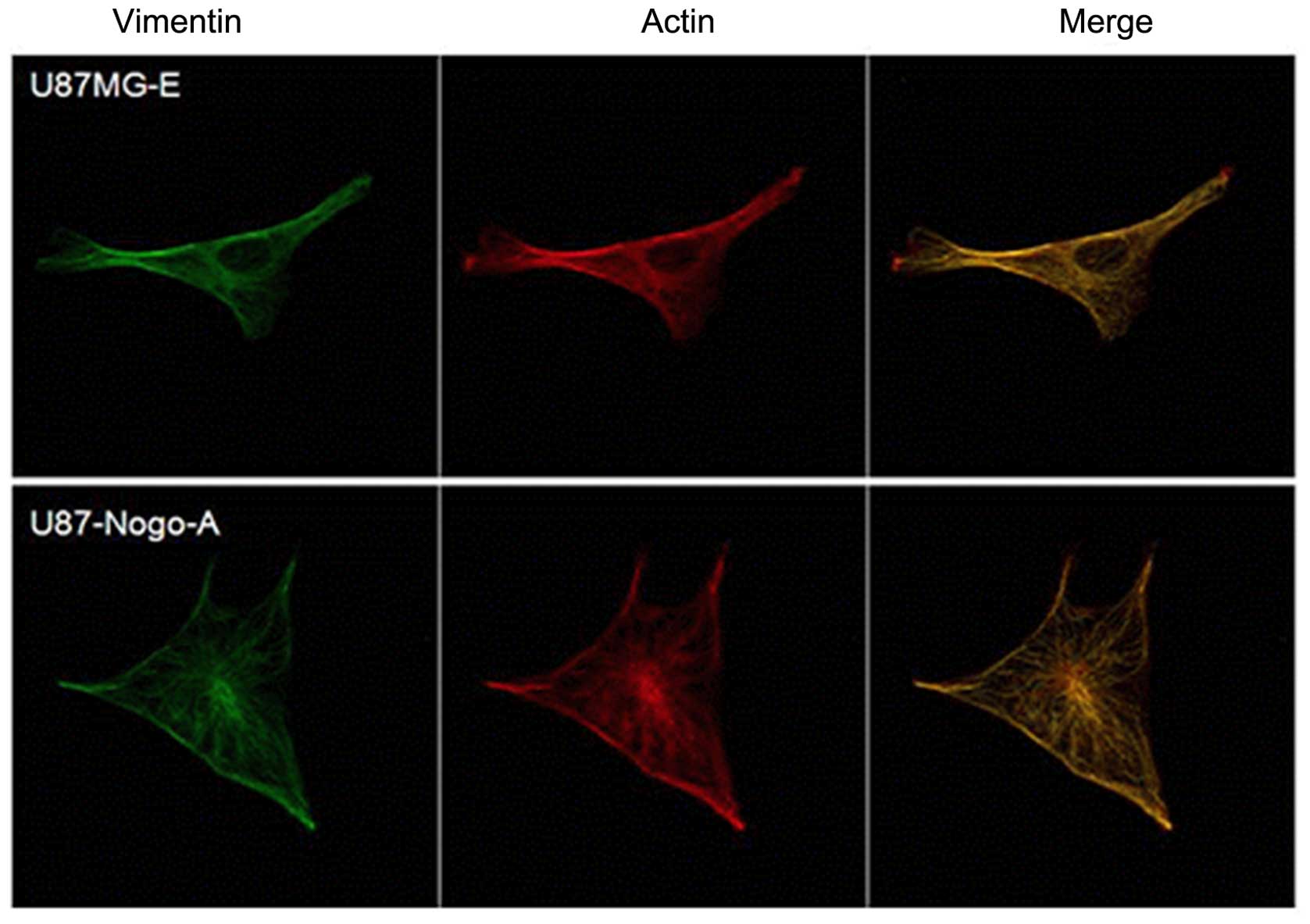
Cytoskeletal actin/vimentin changes
Immunofluorescence staining of actin and vimentin
was performed to evaluate whether the differences in cytoskeletal
alterations were associated with the cell motility and invasion of
malignant glioma cells. U87-Nogo-A cells became round and flat,
while U87MG-E cells showed a bipolar shape. U87-Nogo-A cells with
less motility showed fewer stress fibers, shorter lamellipodia and
the cytoskeletal proteins were mainly concentrated around the
nucleus and became entangled, compared with the U87MG-E cells
(Fig. 7).
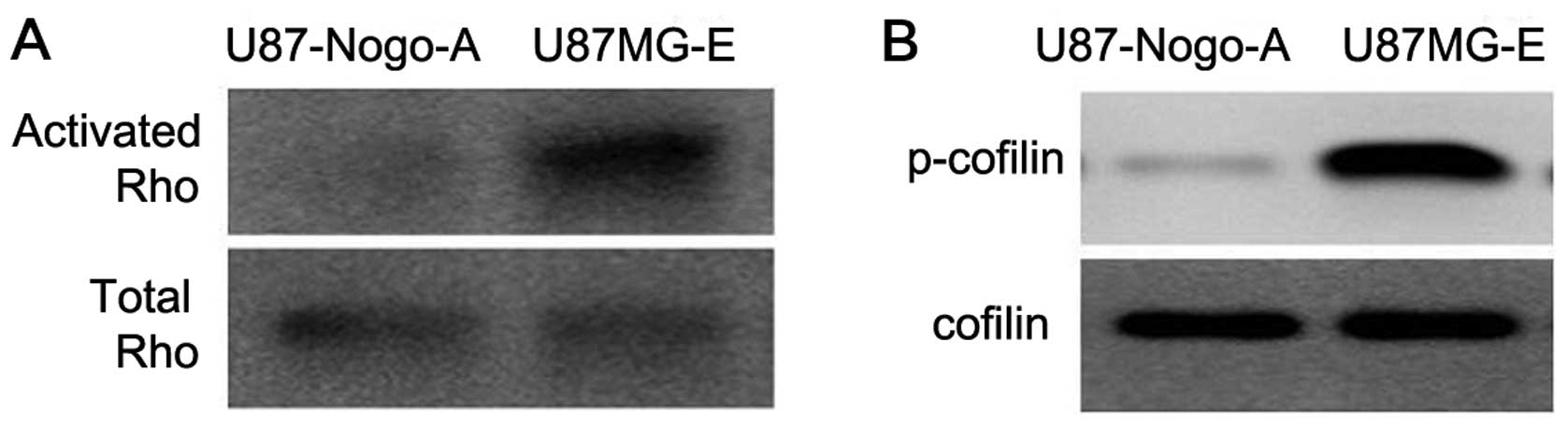
Nogo-A decreases Rho GTPase activity and
cofilin phosphorylation
Rho proteins are well established factors which
regulate cell shape and motility. We investigated Rho activity and
expression of total Rho GTPase (RhoA, B and C) in the U87MG-E and
U87-Nogo-A cells. Rho GTPases cycle between an active GTP-bound and
an inactive GDP-bound conformation. In the GTP-bound state, Rho
GTPases interact with downstream targets to elicit cellular
responses. The U87-Nogo-A cells showed decreased GTP-bound Rho
compared with the U87MG-E cells (Fig.
8A). Cofilin, an actin severing protein inactivated through
phosphorylation, is a downstream factor of the Rho GTPases. In our
results, phosphorylated cofilin was also decreased in the
U87-Nogo-A cells, compared with the control (Fig. 8B).
Discussion
The complete removal of gliomas is usually
microscopically impossible due to the insidious infiltration of
tumor cells into the surrounding normal brain tissue. Glioma cells
use myelinated nerve fiber tracts, vessel basement membranes and
the subependymal layers as major routes for brain invasion
(17). This is the primary cause of
therapeutic failure.
Proteins of the reticulon family are membrane-bound
proteins present in all eukaryotic organisms examined, and range in
size from 200 to 1,200 amino acids. Vertebrate reticulons are
attracting more attention due to their roles in various disorders
including neurodegenerative diseases. The nomenclature of
reticulons originally referred to a neuroendocrine-specific
protein, anchored mainly on the membrane of smooth endoplasmic
reticulum (18).
In the adult CNS, Nogo-A is expressed in
oligodendrocytes, in the inner and outer loops of myelin sheaths,
and in some neuronal populations such as dorsal root ganglion
cells, retinal ganglion cells and ventral horn neurons in the
spinal cord (5,7,19).
During development, Nogo-A is expressed in neurons, where it has
been suggested to play a role in neuronal migration and cortical
development (20). In the present
study, we identified that expression of Nogo-A was found in a
higher percentage of oligodendrogliomas than in glioblastomas,
using western blotting and IHC, in accordance with previous results
(11). These results may indicate a
correlation between Nogo-A expression and tumor grade. Nogo-A was
found to have a negative relationship with the malignancy of
oligodendroglial tumors (12) and
furthermore, Nogo was found to enhance the adhesion of
olfactory-ensheathing cells and to inhibit their migration
(21). These results suggest that
Nogo-A may be involved in the migration and invasion of
glioblastoma cells.
In the present study, we investigated whether Nogo-A
is associated with glioma cell migration and invasion. Firstly, we
selected the U87MG cell line with high motility and no expression
of Nogo-A. The 'U87-Nogo-A' cell line, continuously overexpressing
Nogo-A and the 'U87MG-E' cell line as a control were established.
The effect of Nogo-A on the migratory and invasive abilities was
evaluated by an in vitro assay that involved the simple
scratch technique and the Boyden chamber system. The results showed
that the motility and invasion ability of the U87-Nogo-A cells were
significantly decreased compared with the control cells.
Gliomas, even low grade gliomas, invade surrounding
normal tissues at a very early stage. Cell migration involves
multiple processes that are regulated by various signaling
molecules (22), and incudes
changes in the cytoskeleton, cell-substrate adhesion, and the
extracellular matrix. The actin cytoskeleton and its regulatory
proteins are crucial for cell migration in most cells. During cell
migration, the actin cytoskeleton is dynamically remodeled, and
this reorganization produces the force necessary for cell migration
(23).
Thus, alterations in cytoskeletal proteins, actin
and vimentin were examined by immunofluorescence staining in the
U87-Nogo-A cells overexpressing Nogo-A. Expression patterns of
actin and vimentin in the U87-Nogo-A cells were observed to
concentrate around the nucleus, and the cells had fewer stress
fibers and shorter lamellipodia.
Rho proteins play a role in organelle development,
cytoskeletal dynamics, cell movement, and other common cellular
functions. Rho family small GTPases play pivotal roles in the
reorganization of the actin cytoskeleton during cell migration
(23,24). Higher vertebrates have 3 Rho GTPases
(RhoA, RhoB and RhoC), which share 85% amino acid sequence
identity. Although RhoB and RhoC were characterized at the same
time as RhoA, RhoB and RhoC have received less attention due to
their extensive homology with RhoA (25). Nogo-A inhibits neurite outgrowth
through the NgR receptor, subsequently activating RhoA. RhoA is
involved in diverse cellular functions including cytokinesis
(26), cellular migration (27) and adhesion (28), and biological processes central to
tumorigenesis. Increased RhoA expression has been demonstrated in a
variety of malignancies (29–33)
and the expression is pronounced in high-grade astrocytomas
(34).
Cofilin, an actin depolymerization factor, is
inactivated through phosphorylation downstream of RhoA signaling.
It can directly affect the rate of actin polymerization, actin
filopodia formation, growth cone motility (35,36),
and as recently shown, neurite outgrowth (37). Since Rho regulates the bundling of
actin filaments into stress fibers and the formation of focal
adhesion complexes (38), a
decrease in Rho activity should influence cell stress fiber
formation. A decrease in phosphorylated cofilin clearly leads to an
increase in severing actin, followed by actin reorganization, and
consequently cytoskeleton changes and alterations in cell migration
and invasion.
Based on these studies, we aimed to ascertain
whether Nogo-A-overexpressing cells have altered Rho activity and
phosphorylated cofilin using western blotting. As expected, Rho
activity and p-cofilin expression were decreased in the U87-Nogo-A
cells, compared with the U87MG-E cells. These results suggest that
migration and invasion decreased by over-expression of Nogo-A may
be the result of changes in Rho activity in the U87MG malignant
glioma cells.
In conclusion, Nogo-A negatively modulated the
motility and invasion through regulation of RhoA and cofilin in the
U87MG malignant glioma cell line. Since there are numerous
downstream molecules which can influence cytoskeleton
re-organization through the RhoA signaling pathway, further
experiments must be performed to build upon our hypothesis.
Acknowledgments
The present study was supported by the Leading
Foreign Research Institute Recruitment Program through the National
Research Foundation of Korea (NRF) funded by the Ministry of
Education, Science and Technology (MEST) (2011-0030034).
References
|
1
|
Maher EA, Furnari FB, Bachoo RM, Rowitch
DH, Louis DN, Cavenee WK and DePinho RA: Malignant glioma: Genetics
and biology of a grave matter. Genes Dev. 15:1311–1333. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guo L, Qiu Y, Ge J and Zhou D:
Glioblastoma multiforme with subcutaneous metastases, case report
and literature review. J Korean Neurosurg Soc. 52:484–487. 2012.
View Article : Google Scholar
|
|
3
|
Oertle T, Klinger M, Stuermer CA and
Schwab ME: A reticular rhapsody: Phylogenic evolution and
nomenclature of the RTN/Nogo gene family. FASEB J. 17:1238–1247.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
GrandPré T, Li S and Strittmatter SM:
Nogo-66 receptor antagonist peptide promotes axonal regeneration.
Nature. 417:547–551. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huber AB, Weinmann O, Brösamle C, Oertle T
and Schwab ME: Patterns of Nogo mRNA and protein expression in the
developing and adult rat and after CNS lesions. J Neurosci.
22:3553–3567. 2002.PubMed/NCBI
|
|
6
|
Hunt D, Coffin RS, Prinjha RK, Campbell G
and Anderson PN: Nogo-A expression in the intact and injured
nervous system. Mol Cell Neurosci. 24:1083–1102. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang X, Chun SJ, Treloar H, Vartanian T,
Greer CA and Strittmatter SM: Localization of Nogo-A and Nogo-66
receptor proteins at sites of axon-myelin and synaptic contact. J
Neurosci. 22:5505–5515. 2002.PubMed/NCBI
|
|
8
|
Josephson A, Widenfalk J, Widmer HW, Olson
L and Spenger C: NOGO mRNA expression in adult and fetal human and
rat nervous tissue and in weight drop injury. Exp Neurol.
169:319–328. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Niederöst B, Oertle T, Fritsche J,
McKinney RA and Bandtlow CE: Nogo-A and myelin-associated
glycoprotein mediate neurite growth inhibition by antagonistic
regulation of RhoA and Rac1. J Neurosci. 22:10368–10376.
2002.PubMed/NCBI
|
|
10
|
Boureux A, Vignal E, Faure S and Fort P:
Evolution of the Rho family of ras-like GTPases in eukaryotes. Mol
Biol Evol. 24:203–216. 2007. View Article : Google Scholar
|
|
11
|
Kuhlmann T, Gutenberg A, Schulten HJ,
Paulus W, Rohde V and Bruck W: Nogo-a expression in glial CNS
tumors: A tool to differentiate between oligodendrogliomas and
other gliomas? Am J Surg Pathol. 32:1444–1453. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xiong NX, Zhao HY, Zhang FC and He ZQ:
Negative correlation of Nogo-A with the malignancy of
oligodendroglial tumor. Neurosci Bull. 23:41–45. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jung TY, Jung S, Lee KH, Cao VT, Jin SG,
Moon KS, Kim IY, Kang SS, Kim HS and Lee MC: Nogo-A expression in
oligodendroglial tumors. Neuropathology. 31:11–19. 2011. View Article : Google Scholar
|
|
14
|
Schwab ME: Myelin-associated inhibitors of
neurite growth and regeneration in the CNS. Trends Neurosci.
13:452–456. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Teng FY and Tang BL: No go for brain
tumors? J Mol Neurosci. 25:1–6. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Walker JM: The bicinchoninic acid (BCA)
assay for protein quantitation. Methods Mol Biol. 32:5–8.
1994.PubMed/NCBI
|
|
17
|
Giese A and Westphal M: Glioma invasion in
the central nervous system. Neurosurgery. 39:235–252. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
van de Velde HJ, Roebroek AJ, Senden NH,
Ramaekers FC and Van de Ven WJ: NSP-encoded reticulons,
neuroendocrine proteins of a novel gene family associated with
membranes of the endoplasmic reticulum. J Cell Sci. 107:2403–2416.
1994.PubMed/NCBI
|
|
19
|
Buss A, Sellhaus B, Wolmsley A, Noth J,
Schwab ME and Brook GA: Expression pattern of NOGO-A protein in the
human nervous system. Acta Neuropathol. 110:113–119. 2005.
View Article : Google Scholar
|
|
20
|
Mingorance-Le Meur A, Zheng B, Soriano E
and del Río JA: Involvement of the myelin-associated inhibitor
Nogo-A in early cortical development and neuronal maturation. Cereb
Cortex. 17:2375–2386. 2007. View Article : Google Scholar
|
|
21
|
Su Z, Cao L, Zhu Y, Liu X, Huang Z, Huang
A and He C: Nogo enhances the adhesion of olfactory ensheathing
cells and inhibits their migration. J Cell Sci. 120:1877–1887.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ridley AJ, Schwartz MA, Burridge K, Firtel
RA, Ginsberg MH, Borisy G, Parsons JT and Horwitz AR: Cell
migration: Integrating signals from front to back. Science.
302:1704–1709. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pollard TD and Borisy GG: Cellular
motility driven by assembly and disassembly of actin filaments.
Cell. 112:453–465. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Raftopoulou M and Hall A: Cell migration:
Rho GTPases lead the way. Dev Biol. 265:23–32. 2004. View Article : Google Scholar
|
|
25
|
Yamazaki D, Kurisu S and Takenawa T:
Regulation of cancer cell motility through actin reorganization.
Cancer Sci. 96:379–386. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Glotzer M: The molecular requirements for
cytokinesis. Science. 307:1735–1739. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ridley AJ: Rho GTPases and cell migration.
J Cell Sci. 114:2713–2722. 2001.PubMed/NCBI
|
|
28
|
Chrzanowska-Wodnicka M and Burridge K:
Rho-stimulated contractility drives the formation of stress fibers
and focal adhesions. J Cell Biol. 133:1403–1415. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fritz G, Just I and Kaina B: Rho GTPases
are over-expressed in human tumors. Int J Cancer. 81:682–687. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Horiuchi A, Imai T, Wang C, Ohira S, Feng
Y, Nikaido T and Konishi I: Up-regulation of small GTPases, RhoA
and RhoC, is associated with tumor progression in ovarian
carcinoma. Lab Invest. 83:861–870. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kamai T, Arai K, Tsujii T, Honda M and
Yoshida K: Overexpression of RhoA mRNA is associated with advanced
stage in testicular germ cell tumour. BJU Int. 87:227–231. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kamai T, Tsujii T, Arai K, Takagi K, Asami
H, Ito Y and Oshima H: Significant association of Rho/ROCK pathway
with invasion and metastasis of bladder cancer. Clin Cancer Res.
9:2632–2641. 2003.PubMed/NCBI
|
|
33
|
Liu N, Bi F, Pan Y, Sun L, Xue Y, Shi Y,
Yao X, Zheng Y and Fan D: Reversal of the malignant phenotype of
gastric cancer cells by inhibition of RhoA expression and activity.
Clin Cancer Res. 10:6239–6247. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yan B, Chour HH, Peh BK, Lim C and
Salto-Tellez M: RhoA protein expression correlates positively with
degree of malignancy in astrocytomas. Neurosci Lett. 407:124–126.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bernard O: Lim kinases, regulators of
actin dynamics. Int J Biochem Cell Biol. 39:1071–1076. 2007.
View Article : Google Scholar
|
|
36
|
Endo M, Ohashi K, Sasaki Y, Goshima Y,
Niwa R, Uemura T and Mizuno K: Control of growth cone motility and
morphology by LIM kinase and Slingshot via phosphorylation and
dephosphorylation of cofilin. J Neurosci. 23:2527–2537.
2003.PubMed/NCBI
|
|
37
|
Endo M, Ohashi K and Mizuno K: LIM kinase
and slingshot are critical for neurite extension. J Biol Chem.
282:13692–13702. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ridley AJ: Rho GTPases and actin dynamics
in membrane protrusions and vesicle trafficking. Trends Cell Biol.
16:522–529. 2006. View Article : Google Scholar : PubMed/NCBI
|