Introduction
p54nrb/NONO is known to be involved in a
variety of biological events including pre-mRNA splicing,
transcriptional regulation (1),
nuclear retention of defective RNA (2,3), DNA
unwinding and pairing (4), DNA
damage repair (5,6). p54nrb/NONO was recently
shown to be a component of a novel nuclear domain termed
paraspeckles (7,8).
Paraspeckles are ribonucleoprotein bodies found in
the interchromatin space of mammalian cell nuclei. The core
paraspeckle proteins contain polypyrimidine tract-binding
protein-associated splicing factor (PSF)/splicing factor
proline/glutamine-rich (SFPQ), p54nrb/NONO and
paraspeckle protein 1 (PSPC1). These proteins, together with the
long non-protein-coding RNA NEAT1 (MEN-ε/β), associate to form
paraspeckles (9,10). The core paraspeckle proteins have
been shown to bind to both double and single-stranded DNA and RNA,
and have been involved in numerous nucleus events including
transcription and splicing. The function relevant to paraspeckles
is the involvement of PSF/SFPQ and p54nrb/NONO in the
nuclear retention of RNA, specifically preventing A to I
hyperedited RNA from leaving the nucleus (11,12).
Recent research hints at a more generic retention-release mechanism
that exists for transcripts containing hyperedited inverted repeats
in their 3′ UTR (12,13). Adenosine-to-inosine conversion
(A-to-I editing) contributes to extensive transcriptome diversity
(14). Disturbance in RNA editing
has been implicated in various pathologic disorders, including
cancer. Abnormal A-to-I editing was involved in cancer development
(15).
Recent studies suggest that as a core paraspeckle
protein and multifunctional protein, p54nrb/NONO may be
implicated in tumor progress and matastasis. Phosphorylated β-cell
differentiation transcription factor HLXB9 promoted insulinoma cell
proliferation through interaction with NONO protein (16). Expression of p54nrb was
increased in breast cancer with estrogen receptor (17). The protooncogene Spi-1/PU.1 is
involved in the erythroleukemic process via impeding the binding of
p54nrb to RNA and alters the splicing process (18). Acute myeloid leukemia (AML) is a
type of heterogeneous disease derived from haematopoietic stem
cells. Whether p54nrb/NONO plays a role in the progress
of human acute monocytic leukemia remains unknown. In the present
study, we examined the effects of p54nrb silencing on
the proliferation, migration, invasion and TNF-α release of human
acute monocytic leukemia THP1 cells.
Materials and methods
Materials
Lipopolysaccharide (LPS) from E. coli,
puromycin, Brefeldin A, propidium iodide (PI),
p54nrb/NONO and β-actin antibodies, horseradish
peroxidase (HRP)-conjugated secondary antibodies were purchased
from Sigma-Aldrich (St. Louis, MO, USA). Proliferating cell nuclear
antigen (PCNA) and TNF-α antibodies were purchased from Cell
Signaling Technology Inc. (Beverly, MA, USA). RPMI-1640 and fetal
bovine serum (FBS) were purchased from Gibco-Life Technologies
(Carlsbad, CA, USA). Matrigel and 8 µm pore-sized Transwell
chambers were purchased from BD Biosciences (San Jose, CA, USA).
RNeasy Mini kit was purchased from Invitrogen (San Diego, CA, USA).
ReverTra Ace-α-™ Kit was purchased from Toyobo Co., Ltd. (Osaka,
Japan). Human TNF-α Immunoassay Valukine™ ELISA kit was purchased
from R&D Systems, Inc. (Minneapolis, MN, USA). Cell Counting
kit-8 (CCK-8) was purchased from Dojindo Laboratories (Kumamoto,
Japan). RIPA lyses buffer was purchased from Beijing Leagene
Biotech Co., Ltd. (Beijing, China). Other reagents were produced in
China and purchased from local suppliers.
Cell cultures and establishment of
p54nrb silencing THP1 cell line
THP1 cells were obtained from the Department of
Biochemistry (Guangdong Medical University, Guangdong, China). THP1
cells were maintained in RPMI-1640 media supplemented with 15% FBS
and penicillin (100 U/ml) and streptomycin (10 µg/ml).
p54nrb-silencing THP1 cell line and negative control
THP1 cell line were established in our laboratory. The cell lines
were established as follow: packaged lentiviruses containing
p54nrb-specific short hairpin RNA (shRNA)-expressing GFP
lentiviral vector LV3 (H1/GFP&Puro-NONO) (sense sequence
5′-GGCGAAGUCUUCAUUCAUATT-3′) or with negative control shRNA
lentiviral vector LV3 (H1/GFP&Puro) (sense sequence
5′-UUCUCCGAACGUGUCACGUUUC-3′) were provided by Shanghai GenePharma
Co., Ltd., (Shanghai, China). Exponentially growing THP1 cells were
infected with p54nrb-specific shRNA-containing
lentiviruses or negative control shRNA-containing lentiviruses for
24 h, then the cells were cultured in completed RPMI-1640 media
containing 1 µg/ml puromycin for 14 days. The efficiency of
p54nrb silencing was determined by reverse transcription
polymerase chain reaction (RT-PCR) and western blotting. Stable
p54nrb-silencing THP1 cell line
(THP1-p54nrb-si) and negative control THP1 cell line
(THP1-NC) were maintained in similar media to THP1 supplemented
with 0.5 µg/ml puromycin. Cells were grown at 37°C in a
humidified atmosphere of 5% CO2, 95% air.
RT-PCR
Total RNA was isolated using RNeasy Mini kit
(Qiagen). The first strand cDNAs were synthesized using the
ReverTra Ace-α-™ kit (Toyobo). The levels of p54nrb
transcripts in THP1, THP1-p54nrb-si and THP1-NC cells
were assessed by semi-quantitative PCR, respectively. The specific
primers for PCR (p54nrb-sense,
5′-ATGCAGAGTAATAAAACTTTTAACTTGG-3′ and p54nrb-antisense,
5′-GTATCGGCGACGTTTGTTTGG-3′. β-actin sense,
5′-GAGACCTTCAACACCCCAGC-3′ and β-actin-antisense,
5′-ATGTCACGCACGATTTCCCT-3′) were synthetized by BGI Tech Solutions
Co. (Shenzhen, China). Semi-quantitative PCR was performed with
MasterCycler® Gradient Thermal cycler (Eppendorf,
Hamburg, Germany). PCR amplification was performed as follows: 94°C
for 2 min, then 30 cycles of 94°C for 30 sec, 56°C for 30 sec, 68°C
for 1 min, last 68°C for 5 min. For PCR analysis β-actin served as
loading control. GoldView I-stained bands were visualized by UV
using InGenius LHR gel documentation and analysis system (InGene,
Fredericktown, MO, USA), and the relative grey scale intensity of
p54nrb/β-actin was quantified using ImageJ2x
software.
Western blot analysis
THP1, THP1-p54nrb-si and THP1-NC cells
(5×106 cells/well), respectively were seeded in 6-well
culture plates. To test the protein expression of PCNA and
p54nrb in THP1, THP1-p54nrb-si and THP1-NC
cells, the cells were collected for western blot analysis. To test
the effect of p54nrb silencing on expression of TNF-α
induced by LPS, the experiments were divided into three groups: i)
blank group untreated; ii) LPS treatment group treated with 5
µg/ml LPS for 6 h; iii) BFA and LPS treatment group treated
with 5 µg/ml LPS for 6 h and simultaneously protein
secretion inhibitor Brefeldin A (10 µg/ml) for last 5 h.
Western blot analyses were performed as follow: the cells were
collected and lysed with RIPA lyses buffer. Total proteins were
subjected to sodium dodecyl sulfate polyacrylamide gel
electrophoresis (SDS-PAGE), and then transferred onto
polyvinylidene fluoride (PVDF) membranes. The membranes were
incubated with 5% skimmed milk in TBST for 2 h at room temperature.
After washing with TBST, the membranes were respectively incubated
overnight at 4°C with the first antibodies against human
p54nrb (1:800), PCNA (1:1,000), tumor necrosis factor α
(TNF-α) (1:1,000) or β-actin (1:1,000), followed by incubation with
an HRP-conjugated secondary antibodies at room temperature for 1 h.
Enhanced chemiluminescence (ECL) was used to detect the results.
The expression of β-actin was used as loading control. The relative
grey scale intensity was quantified using ImageJ2x software. Data
are representative of three independent experiments.
Cell cycle analysis
THP1, THP1-p54nrb-si and THP1-NC cells
were seeded in 6-well plates and cultured for 48 h. After washing
with PBS, the cells were fixed with 70% ice-cold ethanol for 12 h.
After being washed twice with PBS, the cells were stained with PI
for 30 min. The cell cycle distributions were then analyzed by flow
cytometry and the percentage of cells in G1/G0, S or G2/M phase was
calculated using ModFit LT software. Data represent the mean value
derived from triplicate experiments.
Cell proliferation assay
Cell proliferation was assessed by the Cell Counting
kit-8 (CCK-8) assay, in accordance with the manufacturer's
instructions. Briefly, THP1, THP1-p54nrb-si and THP1-NC
cells (2×104 cells/well) were seeded in 96-well culture
plates and cultured for either 48 or 72 h, then 10 µl CCK-8
reagent was added per well and incubated for 3 h at 37°C.
Absorbance was subsequently measured at 450 nm using Synergy 2
Multi-Mode microplate reader (BioTek Instruments, Inc., Winooski,
VT, USA). The assay was conducted in quadruplicate for each sample
and three parallel experiments were performed.
Cell invasion and motility assay
In vitro invasion assay was performed by
using Matrigel-coated Transwell chambers. Pore-sized polycarbonate
membranes (8 µm) were coated with Matrigel (100
µg/cm2) and incubated overnight. THP1,
THP1-p54nrb-si or THP1-NC cells (1×106 cells)
suspended in PRMI-1640 medium supplemented with 1% BSA were
respectively seeded in upper chamber which was placed over the
lower chamber. The PRMI-1640 medium supplemented with 15% FBS in
lower chamber was used as a chemoattractant. Invasion was allowed
to proceed for 28 h, then the invaded cells in lower chamber were
collected and counted. Transwell motility assay was performed
similar to the above invasion assay, with the exception that
Transwell insert was not coated with Matrigel. Migration was
allowed to proceed for 19 h, then the migrated cells in the lower
chamber were collected and counted. All the experiments were
repeated three times.
TNF-α release and content assay
THP1, THP1-p54nrb-si and THP1-NC cells
(5×106 cells/well) were seeded in 6-well culture plates,
then 5 µg/ml LPS was added per well and incubated for 0, 3,
6 and 9 h. The cells were subsequently centrifuged at 2,000 rpm for
15 min, then TNF-α-containing supernatants were collected and
measured with Human TNF-α Immunoassay Valukine™ ELISA kit, in
accordance with the manufacturer's instruction. The assay was
conducted in triplicate for each sample and three parallel
experiments were performed.
Statistical analysis
The SPSS version 16.0 software package and GraphPad
Prism were used for the statistical analysis and data plotting. The
data were expressed as mean ± SD. ANOVA was carried out followed by
the Student-Newman-Keuls and LSD tests. P<0.05 was considered to
indicate a statistically significant result.
Results
Expression of p54nrb mRNA and
protein in THP1, THP1-p54nrb-si and THP1-NC cell
lines
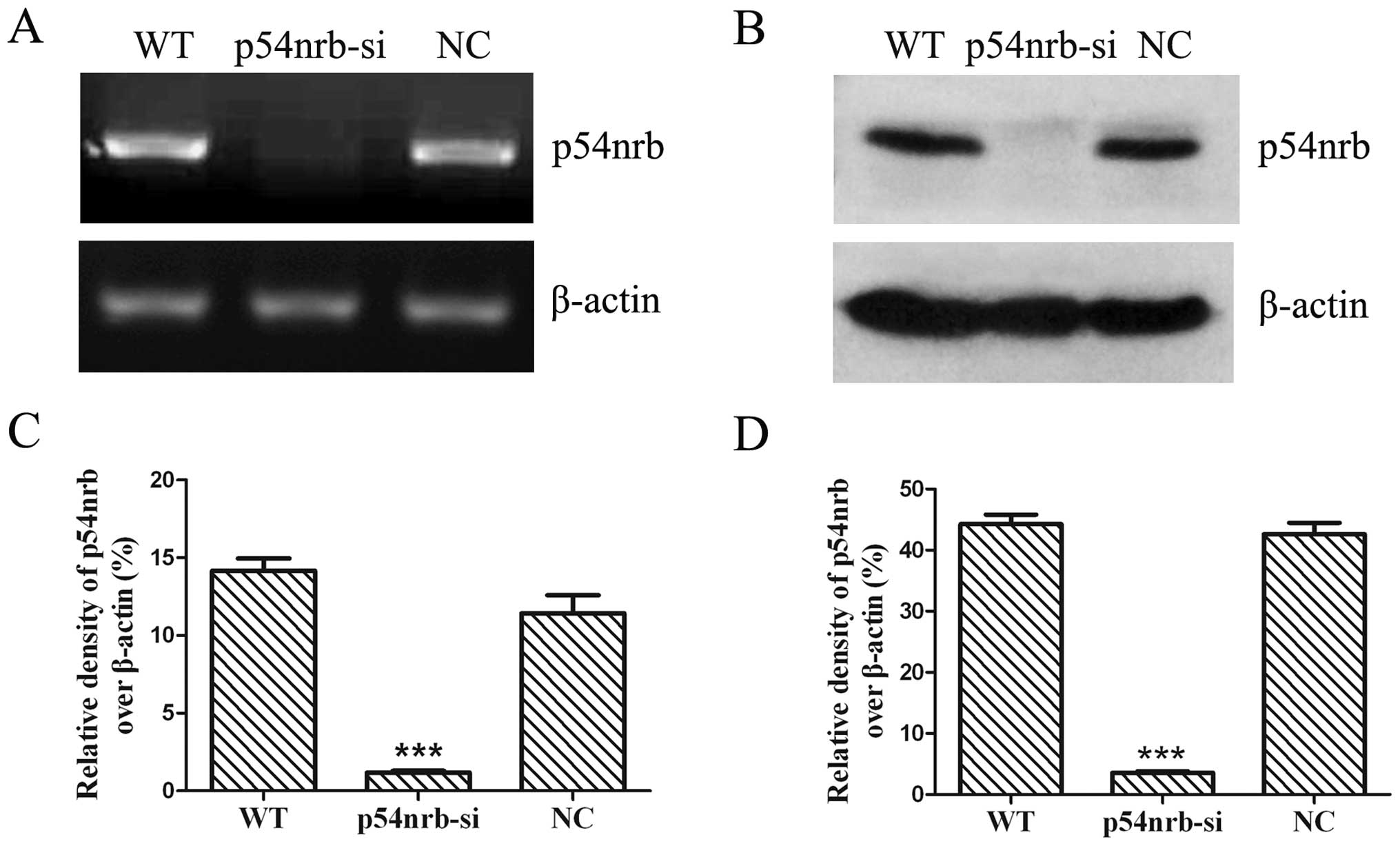
To test the interference efficiency of
p54nrb-specific shRNA-containing lentiviruses, THP1
cells, THP1-p54nrb-si and THP1-NC cells were harvested.
The expression levels of p54nrb mRNA were assessed by
semi-quantitative RT-PCR. The result showed that the expression of
p54nrb mRNA in THP1-p54nrb-si cells was
significantly decreased compared with wild-type or negative control
THP1 cells (Fig. 1A). The mRNA
level of p54nrb in THP1-p54nrb-si cells was
89.76% decreased compared with negative control THP1 cells
(Fig. 1C). To confirm the
efficiency of p54nrb knockdown, the p54nrb
proteins were assessed by western blot analysis. The result showed
that the expression of p54nrb protein was significantly
decreased in THP1-p54nrb-si cells compared with
wild-type and negative control THP1 cells (Fig. 1B). The expression of
p54nrb protein showed 91.59% reduction in
THP1-p54nrb-si cells compared with negative control THP1
cells (Fig. 1D).
Knockdown of p54nrb promotes
THP1 cell proliferation
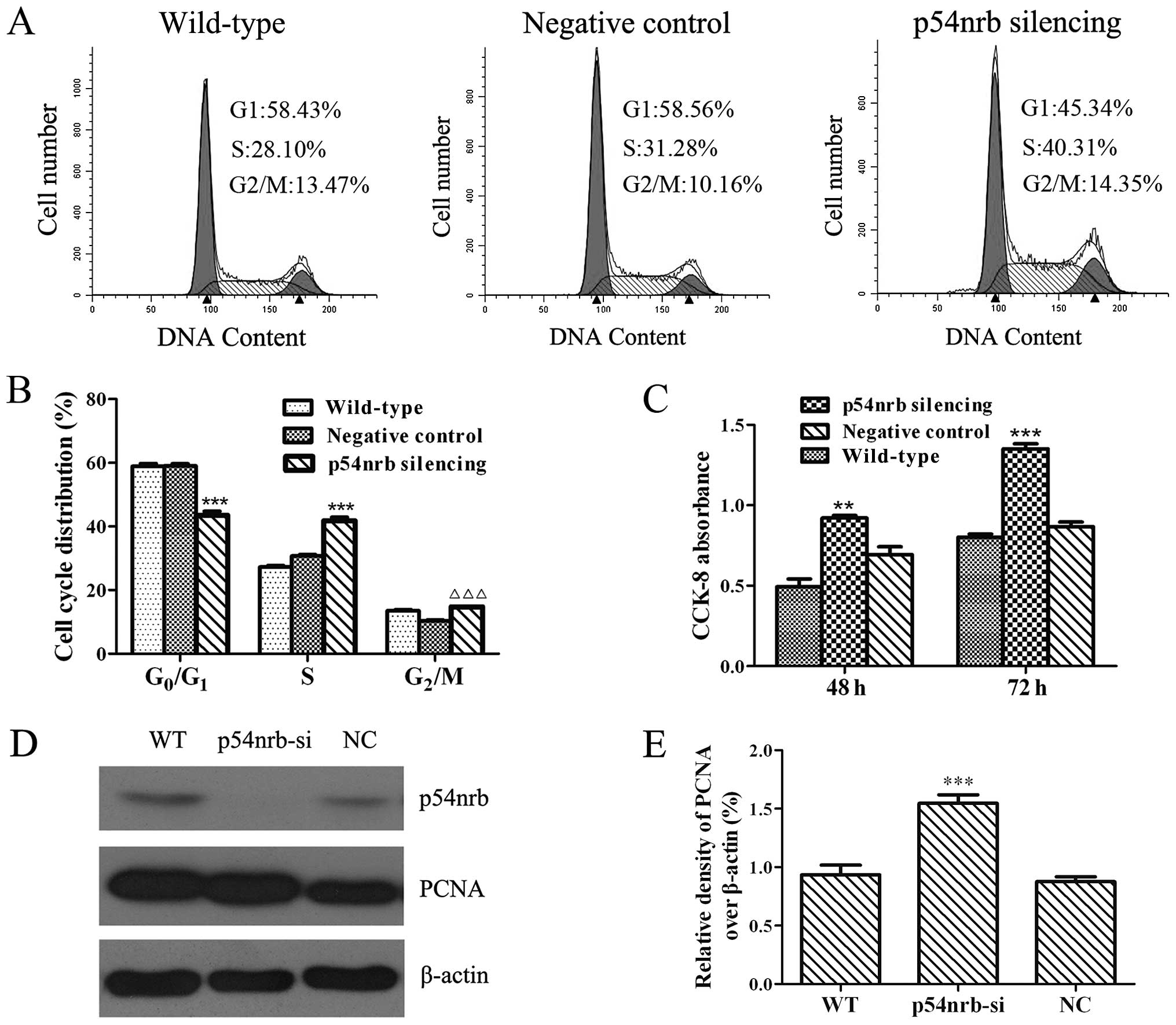
The effect of p54nrb silencing on THP1
cell cycle distribution was analyzed by flow cytometric assay. As
shown in Fig. 2A and B,
THP1-p54nrb-si cells showed a decrease of cells in G0/G1
phase, and an increase of cells in the S and G2/M phases compared
with wild-type or negative control THP1 cells, which indicated that
p54nrb silencing can accelerate cell cycle progression
to promote the proliferation of THP1 cells. This alteration was
further confirmed by using CCK-8 assay (Fig. 2C). Moreover, expression of PCNA, a
marker of cell proliferation, was increased in
p54nrb-silencing THP1 cells compared with wild-type or
negative control THP1 cells (Fig. 2D
and E). These data demonstrated that p54nrb
silencing slightly promoted the proliferation of THP1 cells.
Knockdown of p54nrb inhibits
the invasion and motility of THP1 cells
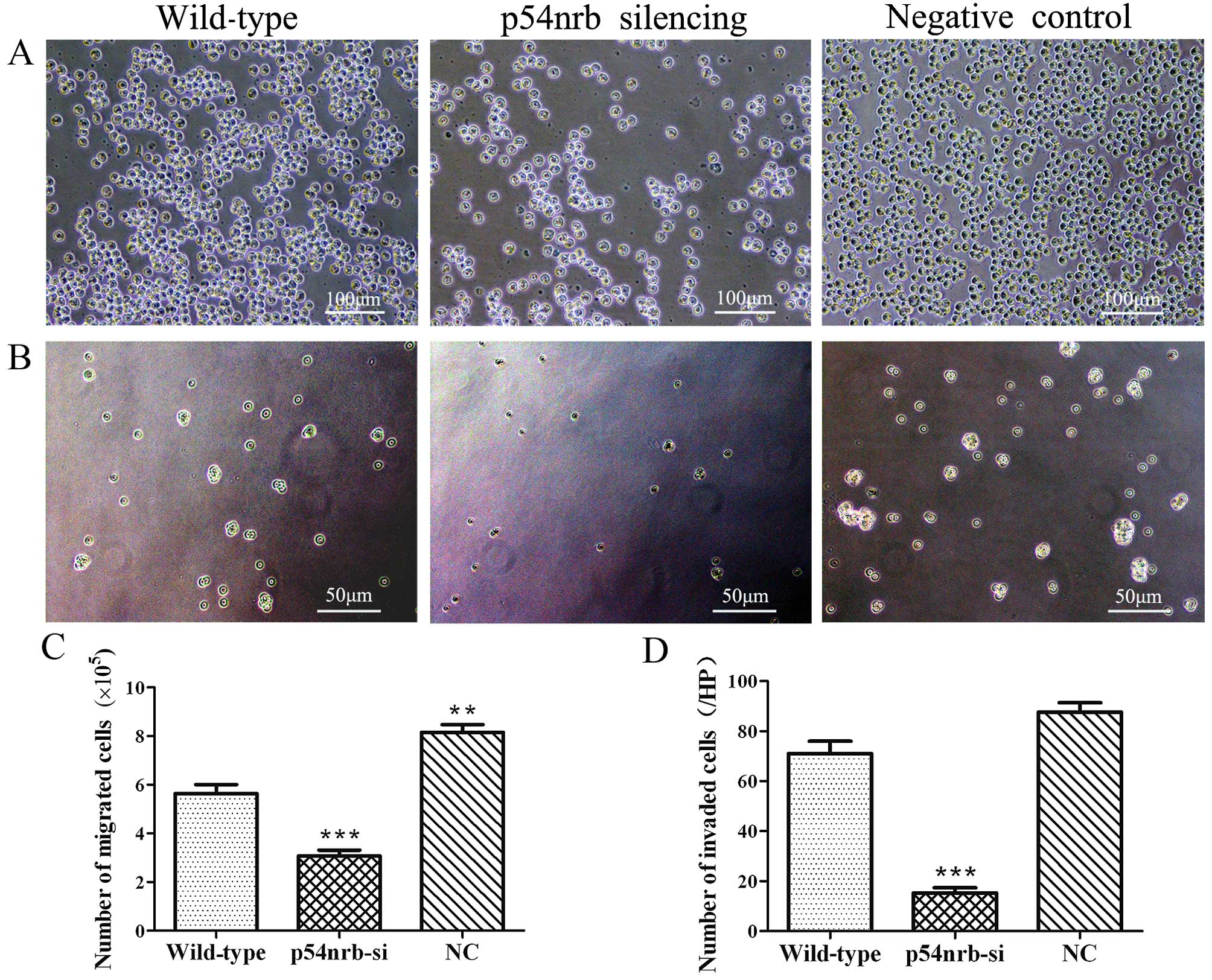
To detect the effect of p54nrb silencing
on THP1 cell motility, Transwell assay was performed. As shown in
Fig. 3A and C, the migration of
THP1-p54nrb-si cells was significantly reduced compared
with wild-type and negative control THP1 cells. Similar effect was
observed for the invasion assay. Matrigel-coated Transwell chambers
were used to measure the effect of p54nrb silencing on
THP1 cell invasion. The result showed that the invasion of
THP1-p54nrb-si cells was significantly reduced compared
with wild-type and negative control THP1 cells (Fig. 3B and D). Above results showed that
knockdown of p54nrb significantly inhibited the invasion
and migration of THP1 cells. The result also displayed that THP1-NC
cells infected negative control shRNA lentiviruses displayed
stronger migration than wild-type THP1 cells (Fig. 3A and C).
Knockdown of p54nrb inhibits
the release of TNF-α induced by LPS from THP1 cells
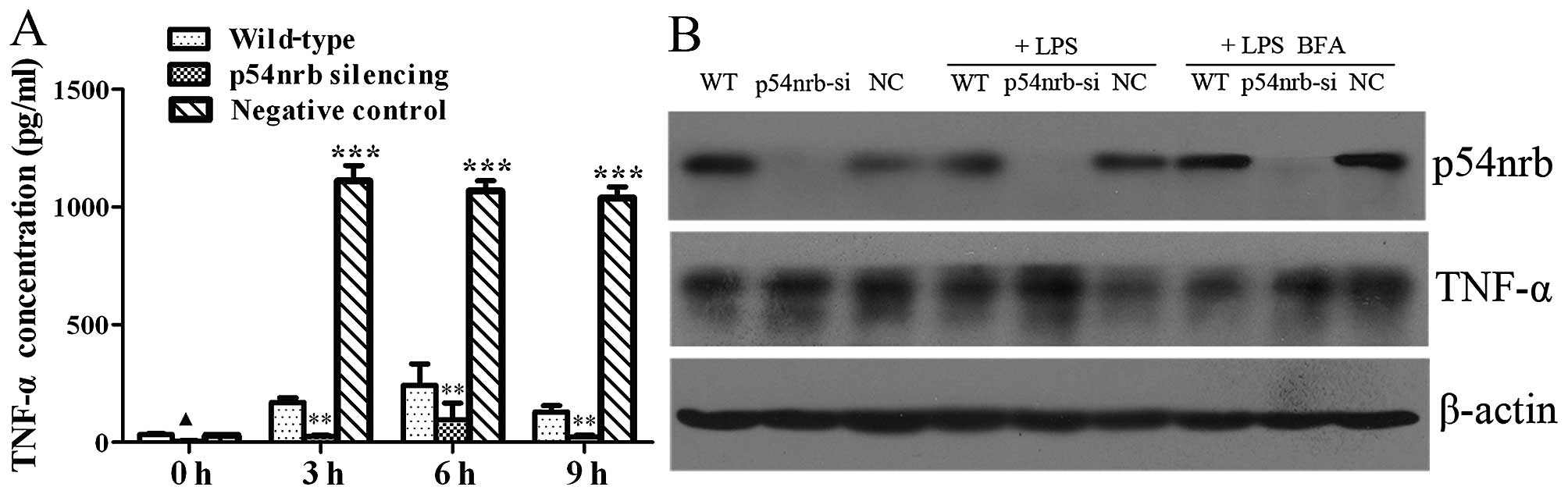
The effect of p54nrb knockdown on the
release of TNF-α induced by LPS from THP1 cells was measured with
Human TNF-α Immunoassay Valukine™ ELISA kit. As shown in Fig. 4A, the content of TNF-α in
supernatant of THP1-p54nrb-si cells with or without LPS
treatment significantly decreased compared with THP1 or THP1-NC
cells, which indicated that knockdown of p54nrb
significantly inhibited the release of TNF-α from THP1 cells,
especially for the release of TNF-α induced by LPS. Moreover, we
found that content of TNF-α in supernatant of THP1-NC cells treated
with LPS was remarkably higher than that of wild-type THP1 cells,
which indicated that infection of negative control lentiviruses
promoted the release of TNF-α induced by LPS from THP1 cells.
In order to explore the mechanism involving in
reduction of TNF-α release in p54nrb silencing THP1
cells, we tested the expression of TNF-α protein in THP1,
THP1-p54nrb-si and THP1-NC cells. Western blot analysis
showed that the protein level of TNF-α was similar in THP1,
THP1-p54nrb-si and THP1-NC cells without treatment.
After LPS treatment, the protein level of TNF-α in
THP1-p54nrb-si cells was significantly higher than that
of THP1 and THP1-NC cells. TNF-α can be released from THP1 cells
when THP1 cells are stimulated by LPS. Therefore, the protein level
of TNF-α was monitored after different THP1 cells were
simultaneously treated with LPS and protein secretion inhibitor
Brefeldin A. As shown in Fig. 4B,
the protein level of TNF-α was similar in THP1-p54nrb-si
and THP1-NC cells upon simultaneously treated with LPS and
Brefeldin A. The results demonstrated that knockdown of
p54nrb decreased the content of TNF-α in culture
supernatant by inhibiting certain process of TNF-α secretion from
THP1-p54nrb-si cells, instead of effecting the TNF-α
protein expression.
Discussion
AML is a heterogeneous disease derived from
haematopoietic stem cells. To identify new genes involved in tumor
progress is important for the diagnosis and treatment of AML
(19). In the present study, we
analyzed the effects of p54nrb silencing on the
biological characteristics of human acute monocytic leukemia THP1
cells.
Cell cycle and cell proliferation assay showed that
knockdown of p54nrb slightly promoted proliferation, and
western blotting displayed that knockdown of p54nrb
increased the expression of PCNA protein that is a marker of cell
proliferation (20). The above
results indicated that knockdown of p54nrb slightly
promoted the proliferation of THP1 cells. As a core paraspeckle
protein and multifunctional protein, p54nrb/NONO was
reported to be implicated in tumor progress and metastasis.
Previous research demonstrated that p54nrb played
diverse roles in cell proliferation. Knockdown of p54nrb
in melanoma cell lines led to reduced proliferation rates (21). β-cell differentiation transcription
factor HLXB9 promoted insulinoma cell proliferation through
interaction with NONO protein (16). Spi-1/PU.1 blocks the differentiation
of proerythroblast and promotes their malignant transformation in
the Friend erythroleukemia, and Spi-1/PU.1 might be involved in
leukemogenesis via impeding the binding of p54nrb to RNA
and alters the splicing process (18). NONO bound to the p16-Ink4A cell
cycle checkpoint gene and potentiated its circadian activation.
Loss of NONO abolished this activation and circadian expression of
p16-Ink4A and eliminated circadian cell cycle gating. Fibroblasts
from NONO gene-trapped mice showed increased proliferation and
decreased senescence (22). Our
results displayed that knockdown of p54nrb slightly
promoted proliferation of THP1 cells, human umbilical vein
endothelial cells (HUVEC). Various research demonstrated that
p54nrb promotes or inhibits cell proliferation in
different tumor cells. These alterations may be responsible for
functional diversity of p54nrb protein including mRNA
splicing and transcription. As a core paraspeckle protein,
p54nrb/NONO involved in the nuclear retention of RNA,
specifically for A to I hyper-edited RNA (11,12).
The common dysregulation of A-to-I editing in human cancers may
contribute to the altered transcriptional program necessary to
sustain carcinogenesis (23–25).
In hematologic malignancies, abnormal A to-I editing of
hematopoietic cell phosphatase (PTPN6) gene was associated to AML
(26). Whether
p54nrb/NONO affects the proliferation of THP1 cells by
the nuclear retention of A to I hyper-edited RNA remains
unknown.
Cell motility and invasion assay showed that
knockdown of p54nrb significantly inhibited the motility
and invasion of THP1 cells. p54nrb/NONO shows diverse
roles in cell proliferation in different tumor cells and research,
but its role in migration was very consistent and obvious.
Knockdown of p54nrb significantly inhibited migration of
melanoma cell lines (21).
Overexpression of p54nrb increased migration of HUVEC
and HeLa cells (27). Our results
indicated that knockdown of p54nrb strongly decreased
the motility and invasion of THP1, HUVEC, SKBR-3 and CNE2 cells
(partial data not shown). All these studies showed that
p54nrb is a powerful molecule involving in the
regulation of cell motility. To date, only a few reports on the
mechanism of p54nrb participating in cell motility have
been reported. Fibroblast growth factor 1 (FGF1) functions as a
modifier of endothelial cell migration and proliferation.
Heterogeneous nuclear ribonucleoprotein M (hnRNPM) and
p54nrb present in protein complexes bound to the FGF1
promoter and to the mRNA internal ribosome entry site. Knockdown of
either p54nrb or hnRNPM blocks endogenous FGF1 induction
and myotube formation (28).
Angiopoietin-1 (Ang1) regulates angiogenesis as a ligand of Tie 2
receptor tyrosine kinase. p54nrb was the tyrosine
phosphorylated protein in Ang-1 induced signaling pathway in HUVEC
cells. p54nrb was validated as a molecule involved in
cell migration of HUVEC cells, which can be supported by the
results obtained from microarray analysis: overexpression of
p54nrb significantly upregulated the genes involved in
cell motility and structural functions in HUVEC cells (data not
shown) (27). In vivo, NONO
gene-trapped mice showed defective wound repair. Considering the
above results, we speculate that p54nrb might be a key
molecule involving in cell motility via effecting the expression or
function of certain cytoskeleton proteins.
THP1 cells are often used as an inflammatory cell
model in the study of inflammation and some leukemia is relative to
infection. Therefore, we also analyzed the effect of
p54nrb silencing on the release and expression of
inflammatory mediator TNF-α in THP1 cells. The results indicated
that knockdown of p54nrb had no effect on the expression
of TNF-α protein, but significantly inhibited the release of TNF-α
from THP1 cells, especially for the release of TNF-α induced by
LPS, the mechanism of which is still obscure. The secretion of
TNF-α from cells is an exocytosis process which depends on
microtubules (29–31). Furthermore, knockdown of
p54nrb blocks endogenous FGF1 induction and myotube
formation (28). Overexpression of
p54nrb significantly upregulated the genes involved in
cell motility and structural functions in HUVEC cells (27). Thus, we speculate that knockdown of
p54nrb might inhibit the release of TNF-α from THP1
cells via effecting the exocytosis of TNF-α which depends on
cytoskeleton protein microtubules. Cell movement and organelle
transport all depends on the activities of cytoskeleton proteins.
There may be some connection between p54nrb silencing
inhibiting the motility of THP1 cells and the release of TNF-α from
THP1 cells. Moreover, the infection of negative control
shRNA-containing lentiviruses promoted the migration and the
release of TNF-α induced by LPS in THP1 cells. We speculate that
increase of the migration and the release of TNF-α in THP-1 cells
infected by control vector lentiviruses is a defensive reaction of
THP1 cells to viruses.
Based on the present study, it is suggest that
p54nrb slightly inhibited THP1 cell proliferation, but
significantly promoted migration, invasion and release of TNF-α in
THP1 cells. Our and other recent studies indicate that
p54nrb may be a powerful molecule involving in the
regulation of cell motility, and it may promote metastasis,
invasion of tumor cells and angiogenesis. p54nrb is more
likely to promote the release of inflammatory mediators and the
motility of inflammatory cells. The occurrence of many tumors is
closely related to infection and inflammation (32), and we also speculate involvement in
tumor processes and inflammation.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (no. 81441118), the Science and
Technology Planning Project of Guangdong Province, China (no.
2014A020212294), the Sci-Tech Project Foundation of Zhanjiang City,
China (nos. 2011C3105011 and 2008C01010), and the Key Cultivation
Project of Guangdong Medical University, China (2013).
References
|
1
|
Emili A, Shales M, McCracken S, Xie W,
Tucker PW, Kobayashi R, Blencowe BJ and Ingles CJ: Splicing and
transcription-associated proteins PSF and p54nrb/nonO bind to the
RNA polymerase II CTD. RNA. 8:1102–1111. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Peng R, Dye BT, Pérez I, Barnard DC,
Thompson AB and Patton JG: PSF and p54nrb bind a conserved stem in
U5 snRNA. RNA. 8:1334–1347. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Izumi H, McCloskey A, Shinmyozu K and Ohno
M: p54nrb/NonO and PSF promote U snRNA nuclear export by
accelerating its export complex assembly. Nucleic Acids Res.
42:3998–4007. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Straub T, Knudsen BR and Boege F:
PSF/p54nrb stimulates 'jumping' of DNA topoisomerase I
between separate DNA helices. Biochemistry. 39:7552–7558. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Salton M, Lerenthal Y, Wang SY, Chen DJ
and Shiloh Y: Involvement of Matrin 3 and SFPQ/NONO in the DNA
damage response. Cell Cycle. 9:1568–1576. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li S, Kuhne WW, Kulharya A, Hudson FZ, Ha
K, Cao Z and Dynan WS: Involvement of p54nrb, a PSF
partner protein, in DNA double-strand break repair and
radioresistance. Nucleic Acids Res. 37:6746–6753. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu JY and Sewer MB: p54nrb/NONO regulates
cyclic AMP-dependent glucocorticoid production by modulating
phosphodiesterase mRNA splicing and degradation. Mol Cell Biol.
35:1223–1237. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Naganuma T and Hirose T: Paraspeckle
formation during the biogenesis of long non-coding RNAs. RNA Biol.
10:456–461. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Souquere S, Beauclair G, Harper F, Fox A
and Pierron G: Highly ordered spatial organization of the
structural long noncoding NEAT1 RNAs within paraspeckle nuclear
bodies. Mol Biol Cell. 21:4020–4027. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bond CS and Fox AH: Paraspeckles: Nuclear
bodies built on long noncoding RNA. J Cell Biol. 186:637–644. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Z and Carmichael GG: The fate of
dsRNA in the nucleus: A p54nrb-containing complex
mediates the nuclear retention of promiscuously A-to-I edited RNAs.
Cell. 106:465–475. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qi L, Chan TH, Tenen DG and Chen L: RNA
editome imbalance in hepatocellular carcinoma. Cancer Res.
74:1301–1306. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen LL and Carmichael GG: Altered nuclear
retention of mRNAs containing inverted repeats in human embryonic
stem cells: Functional role of a nuclear noncoding RNA. Mol Cell.
35:467–478. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Weissbach R and Scadden AD: Tudor-SN and
ADAR1 are components of cytoplasmic stress granules. RNA.
18:462–471. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Osenberg S, Dominissini D, Rechavi G and
Eisenberg E: Widespread cleavage of A-to-I hyperediting substrates.
RNA. 15:1632–1639. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Desai SS, Kharade SS, Parekh VI, Iyer S
and Agarwal SK: Pro-oncogenic roles of HLXB9 protein in insulinoma
cells through interaction with Nono protein and down-regulation of
the c-Met inhibitor Cblb (casitas B-lineage lymphoma b). J Biol
Chem. 290:25595–25608. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hof D, Cheung K, Roossien HE, Pruijn GJ
and Raats JM: A novel subtractive antibody phage display method to
discover disease markers. Mol Cell Proteomics. 5:245–255. 2006.
View Article : Google Scholar
|
|
18
|
Hallier M, Tavitian A and Moreau-Gachelin
F: The transcription factor Spi-1/PU.1 binds RNA and interferes
with the RNA-binding protein p54nrb. J Biol Chem. 271:11177–11181.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu J, Hong X, Li Z and Lu Q: Acute
monocytic leukaemia with t(11;12) (p15;q13) chromosomal changes: A
case report and literature review. Oncol Lett. 10:2307–2310.
2015.PubMed/NCBI
|
|
20
|
Hall PA, Levison DA, Woods AL, Yu CC,
Kellock DB, Watkins JA, Barnes DM, Gillett CE, Camplejohn R, Dover
R, et al: Proliferating cell nuclear antigen (PCNA)
immunolocalization in paraffin sections: An index of cell
proliferation with evidence of deregulated expression in some
neoplasms. J Pathol. 162:285–294. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schiffner S, Zimara N, Schmid R and
Bosserhoff AK: p54nrb is a new regulator of progression
of malignant melanoma. Carcinogenesis. 32:1176–1182. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kowalska E, Ripperger JA, Hoegger DC,
Bruegger P, Buch T, Birchler T, Mueller A, Albrecht U, Contaldo C
and Brown SA: NONO couples the circadian clock to the cell cycle.
Proc Natl Acad Sci USA. 110:1592–1599. 2013. View Article : Google Scholar :
|
|
23
|
Dominissini D, Moshitch-Moshkovitz S,
Amariglio N and Rechavi G: Adenosine-to-inosine RNA editing meets
cancer. Carcinogenesis. 32:1569–1577. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chan TH, Lin CH, Qi L, Fei J, Li Y, Yong
KJ, Liu M, Song Y, Chow RK, Ng VH, et al: A disrupted RNA editing
balance mediated by ADARs (Adenosine DeAminases that act on RNA) in
human hepatocellular carcinoma. Gut. 63:832–843. 2014. View Article : Google Scholar :
|
|
25
|
Chen L, Li Y, Lin CH, Chan TH, Chow RK,
Song Y, Liu M, Yuan YF, Fu L, Kong KL, et al: Recoding RNA editing
of AZIN1 predisposes to hepatocellular carcinoma. Nat Med.
19:209–216. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Beghini A, Ripamonti CB, Peterlongo P,
Roversi G, Cairoli R, Morra E and Larizza L: RNA hyperediting and
alternative splicing of hematopoietic cell phosphatase (PTPN6) gene
in acute myeloid leukemia. Hum Mol Genet. 9:2297–2304. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim YM, Seo J, Kim YH, Jeong J, Joo HJ,
Lee DH, Koh GY and Lee KJ: Systemic analysis of tyrosine
phosphorylated proteins in angiopoietin-1 induced signaling pathway
of endothelial cells. J Proteome Res. 6:3278–3290. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ainaoui N, Hantelys F, Renaud-Gabardos E,
Bunel M, Lopez F, Pujol F, Planes R, Bahraoui E, Pichereaux C,
Burlet-Schiltz O, et al: Promoter-dependent translation controlled
by p54nrb and hnRNPM during myoblast differentiation.
PLoS One. 10:e01364662015. View Article : Google Scholar
|
|
29
|
Yuan T, Liu L, Zhang Y, Wei L, Zhao S,
Zheng X, Huang X, Boulanger J, Gueudry C, Lu J, et al:
Diacylglycerol guides the hopping of clathrin-coated pits along
microtubules for exoendocytosis coupling. Dev Cell. 35:120–130.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li-Villarreal N, Forbes MM, Loza AJ, Chen
J, Ma T, Helde K, Moens CB, Shin J, Sawada A, Hindes AE, et al:
Dachsous1b cadherin regulates actin and microtubule cytoskeleton
during early zebrafish embryogenesis. Development. 142:2704–2718.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Graffe M, Zenisek D and Taraska JW: A
marginal band of micro-tubules transports and organizes
mitochondria in retinal bipolar synaptic terminals. J Gen Physiol.
146:109–117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|