Introduction
Anaplastic thyroid cancer (ATC) is a very aggressive
malignancy with a poor prognosis, with a median survival of
approximately 3–5 months following diagnosis. Although ATC accounts
for less than 1–3% of all thyroid cancers, it is responsible for
14–39% of thyroid cancer-related deaths (1–9). Thus,
it is important to clarify the molecular mechanism underlying the
highly aggressive nature of ATC. Recently, several clinical trials
have tested the efficacy of small molecule tyrosine kinase
inhibitors, antiangiogenesis agents, and vascular-disrupting
agents; these show promise as additional drugs to combat ATC, in
addition to the multimodal therapy, including surgical resection,
radiotherapy and chemotherapy, that has long been used (10). However, a standard therapy for ATC
remains to be established.
The human (h)-prune protein is a member of the
desert hedgehog homolog (DHH) protein superfamily, and it is known
to have a cAMP phosphodiesterase (PDE) activity (11). Its overexpression in breast,
colorectal and gastric cancers is correlated with depth of invasion
and a high degree of lymph-node metastasis (12–14).
We previously identified h-prune as a binding protein of a
serine/threonine kinase, glycogen synthase kinase-3 (GSK-3)
(15). The binding of h-prune to
GSK-3 was involved in the regulation of the disassembly of focal
adhesions to promote cell migration. H-prune protein expression was
correlated with depth of invasion and degree of lymph-node
metastasis in colorectal and pancreatic cancers.
Thus, although expression of h-prune is correlated
with progression and aggressiveness in various cancers, the role of
h-prune in thyroid cancer has not been investigated. In the present
study, we investigated whether h-prune affects the ability of
invasion and metastasis of anaplastic thyroid cancer cells.
Materials and methods
Tissue samples and
immunohistochemistry
Tissue samples were collected from 15 ATC patients
who underwent surgery between 2003 and 2010 at Tsuchiya General
Hospital (Hiroshima, Japan). We investigated the expression of
h-prune by immunohistochemical analysis of ATC tissues,
corresponding non-tumor thyroid follicle epithelium and metastatic
lymph node tissues. Histological classification was based on the
General Rules for the Description of Thyroid Cancer (16). For immunohistochemical analysis,
archival formalin-fixed and paraffin-embedded tissues were used.
h-prune was detected using a polyclonal antibody raised in our
laboratory (15). The specificity
of the anti-h-prune antibody has previously been characterized in
detail (15). A Histofine
SAD-PO® kit (Nichirei Biosciences, Inc., Tokyo, Japan)
was used for immunohistochemical analyses. Sections were incubated
with the rabbit polyclonal anti-h-prune antibody (diluted 1:100)
overnight at 4°C, then incubated with biotinylated anti-rabbit IgG
and peroxidase-labeled streptavidin for 30 min each. In all tumors,
expression of h-prune was classified as positive or negative. When
>50% of tumor cells were strongly or diffusely stained, the
immunostaining was considered positive for h-prune. Written
informed consent was obtained from all patients. The procedure to
protect the identity of the patients was subject to approval by the
institutional review committee and met the guidelines of the
responsible governmental authority.
Cell lines and culture conditions
Cells of the human ATC cell line 8505C were
purchased from the Riken Cell Bank (RIKEN BioResource Center,
Tsukuba, Japan). KTC-3 and ACT-1 cells were kindly provided by
Junichi Kurebayashi (Departments of Breast and Thyroid Surgery,
Kawasaki Medical School) and Takanori Miyoshi (Department of
Thoracic, Endocrine Surgery and Oncology, The University of
Tokushima Graduate School), respectively (17). 8505C and KTC-3 cells were maintained
in RPMI-1640 supplemented with 10% fetal bovine serum (FBS), and
ACT-1 cells were maintained in Dulbecco's modified Eagle's medium
(DMEM) with 10% FBS.
Inhibitors and cell transfection
Dipyridamole, a selective h-prune cAMP-PDE inhibitor
and 3-isobuty-1-methylxanthine (IBMX), a non-selective cAMP-PDE
inhibitor, were purchased from Sigma-Aldrich, Tokyo, Japan
(18). For small interfering RNA
(siRNA) analyses, the sense strand for h-prune targeting had the
following sequence: 5′-GGCGUCAAGGUGGCCAUUATT-3′. Small duplex RNAs
containing the same but scrambled nucleotides (siRNA SCR) were used
as a negative control. siRNA transfection was performed using
Lipofectamine™ RNAiMAX (Invitrogen, Carlsbad, CA, USA).
Transfection with the pGL4.51 [luc/CMV/Hyglo] vector (Promega K.K.,
Tokyo, Japan) was performed using a jetPRIME kit (Funakoshi, Co.,
Ltd., Tokyo, Japan).
Western blot analysis
Protein samples obtained from the cell lysis buffer
(Cell Signaling Technology Japan, Tokyo, Japan) were resolved using
10% sodium dodecyl sulfate polyacrylamide gel electrophoresis
(SDS-PAGE) buffer and electrophoretically transferred onto
nitrocellulose membranes. The membranes were blocked with 5% skim
milk, probed with the polyclonal anti h-prune antibody (diluted
1:2,000, raised in our laboratory), with anti-rabbit IgG antibody
(ELC) at 1:2,000 as the secondary antibody. Blots were also probed
with an anti-β actin antibody (diluted 1:5,000; Abcam, Cambridge,
UK). Amersham ECL Plus™ Western Blotting Detection system reagents
(GE Healthcare Japan, Tokyo, Japan) were used for detection of
antigen-antibody reactions.
Real-time reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA was collected from samples using
RNeasy® Mini kits (Qiagen, Limburg, The Netherlands).
cDNA samples were obtained from 2 ng of total RNA with buffer, Mg,
RNase inhibitor, a random primer (6-mer), 10 mM dNTPs, and
reverse-transcriptase (SuperScript II), then amplified using an
intercalation procedure with Power SYBR® Green PCR
Master Mix (Life Technologies, Carlsbad, CA, USA). Results were
analyzed using a relative quantitative method (ΔΔCt).
Cell migration assay
To measure cell migration activity, Transwell and
wound healing assays were performed. The Transwell migration assay
was performed using a Boyden chamber (6.5 mm in diameter, with
8-µm pores; Costar Life Sciences, Corning, MA, USA) the
bottom face of which was coated with 10 µg/ml fibronectin.
Cells (1×104) suspended in serum-free medium containing
0.1% bovine serum albumin (BSA), with or without inhibitors, were
applied to the upper chamber. The concentration of 0.1% dimethyl
sulfoxide (DMSO) was adjusted to the same concentration as the
inhibitors. After 4 h, the number of the cells that had migrated to
the lower side of the upper chamber were counted automatically
using a fluorescence microscope (model BZ-9000; Keyence Corp.,
Osaka, Japan), and relative cell migration was expressed as the
percentage of migrating cells with treatment compared to without
treatment. To perform the wound-healing assay, fully confluent
monolayer of cells on 24-well fibronectin-coated dishes were
scratched manually, and the healing rate was measured after 12 or
24 h.
Cell invasion assay
Invasion assays were performed using BD BioCoat™
Matrigel Invasion chambers (8-µm pore, PET membrane; BD
Biosciences, Franklin Lakes, NJ, USA). Cells (2×104)
suspended in serum-free medium containing 0.1% BSA, with or without
inhibitors, were applied to the upper chambers, which were inserted
in 24-well plates containing 10% FBS/RPMI, with or without
inhibitors. The concentrations of 0.1% DMSO were adjusted to the
same concentration as the inhibitors. After 12 or 24 h, the number
of cells that had invaded the lower side of the upper chamber
through the matrigel were counted automatically using a
fluorescence microscope (model BZ-9000; Keyence), and relative cell
invasion was expressed as the percentage of invaded cells with
treatment compared to that without treatment.
Animal experiments
Six-week-old NOG/Jic (NOD/Shi-scid, IL-2RγKO) female
mice (CLEA Japan, Inc., Tokyo, Japan) were used as models of
orthotopic tumor implantation. Approximately 5×105 cells
resuspended in 20 µl of serum-free medium 199 were injected
into the right thyroid gland of each mouse using a Hamilton syringe
attached to a 27-gauge standard needle. In the dipyridamole-treated
group, 0.3 mg/kg dipyridamole was administered intraperitoneally
each day after cell injection. The same volume of
phosphate-buffered saline (PBS) was administered to the control
group in the same manner. For live imaging, 8505C-luc cells
expressing the luciferase gene allowed measurement of
bioluminescent activity as a surrogate for tumor growth.
D-luciferin (150 mg/kg) was intraperitoneally administered once a
week, and luciferase activity was estimated with a cooled CCD
camera (NightOWL II LB 983; BMS, Tokyo, Japan). Emitted photons
were measured for 180 sec. Images were analyzed using the Indigo 2
software (Berthold Technologies, Baden-Wurttemberg, Germany). All
mice were necropsied on day 25. The extent of thyroid tumor
invasion was estimated using hematoxylin-eosin (H&E) stained
resected specimens. The rate of pulmonary metastasis for the lung
field was obtained from average dimensions, counted automatically
using a microscope (model BZ-9000; Keyence) for 5 sections cut from
paraffin block of each mouse randomly. All animal studies were
performed according to the guidelines set by the US National
Institutes of Health (1985). This experimental protocol was
approved by the ethics review committee for animal experimentation
of the Graduate School of Biomedical Sciences at Hiroshima
University.
Statistical analysis
Statistical analyses were conducted using JMP 11.0.0
(SAS Institute, Inc.) with the Student's t-test, the Fisher's exact
probability test and the Mann-Whitney U test. P-values <0.05
were considered statistically significant. The data are presented
as the mean ± standard error.
Results
Expression of h-prune in anaplastic
thyroid cancer tissues
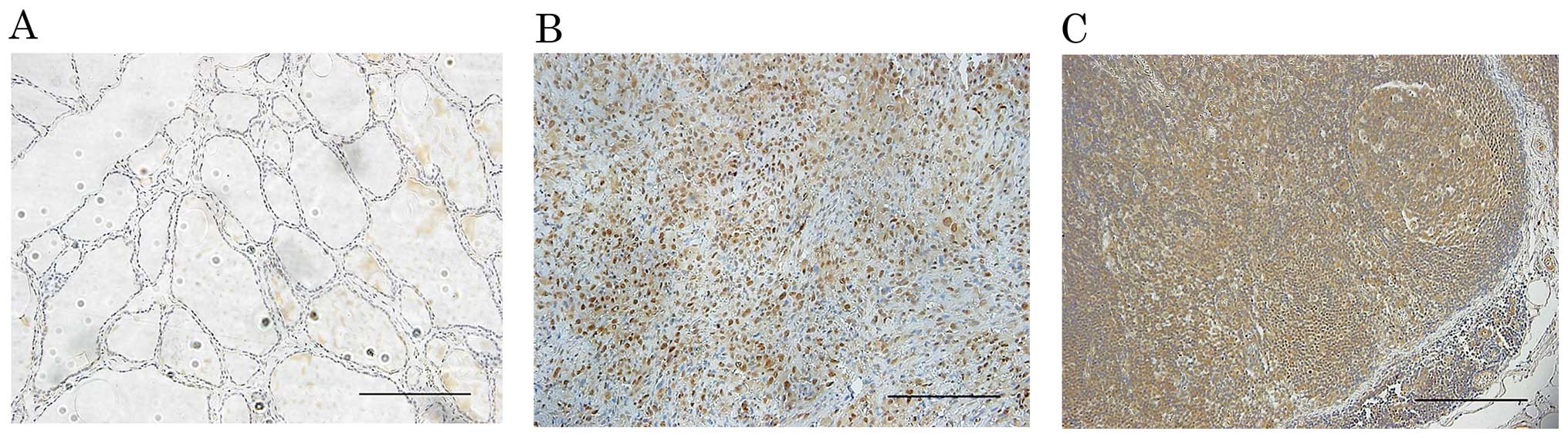
In corresponding non-tumor thyroid follicle
epithelium, weak focal or no staining of h-prune was observed
(Fig. 1A). In contrast, all 15 ATC
tissues showed diffuse staining (Fig.
1B). Furthermore, h-prune-positive cells were observed in all 8
corresponding ATC lymph node metastasis tissue samples (Fig. 1C).
Involvement of h-prune PDE activity in
ATC cell motility
To investigate whether the cAMP-PDE activity of
h-prune is necessary for ATC cell motility, 8505C and KTC-3 cells
treated with DMSO, dipyridamole, and IBMX were subjected to
Transwell migration and wound healing assays. In the wound healing
assay, dipyridamole suppressed 8505C cell migration in a
concentration-dependent manner (Fig. 2A
and B). Dipyridamole also suppressed 8505C and KTC-3 cell
migration in a concentration-dependent manner in the Transwell
migration assay (Fig. 2C–E).
Furthermore, 8505C cells treated with dipyridamole showed
concentration-dependent reduced cell invasion (Fig. 2F and G).
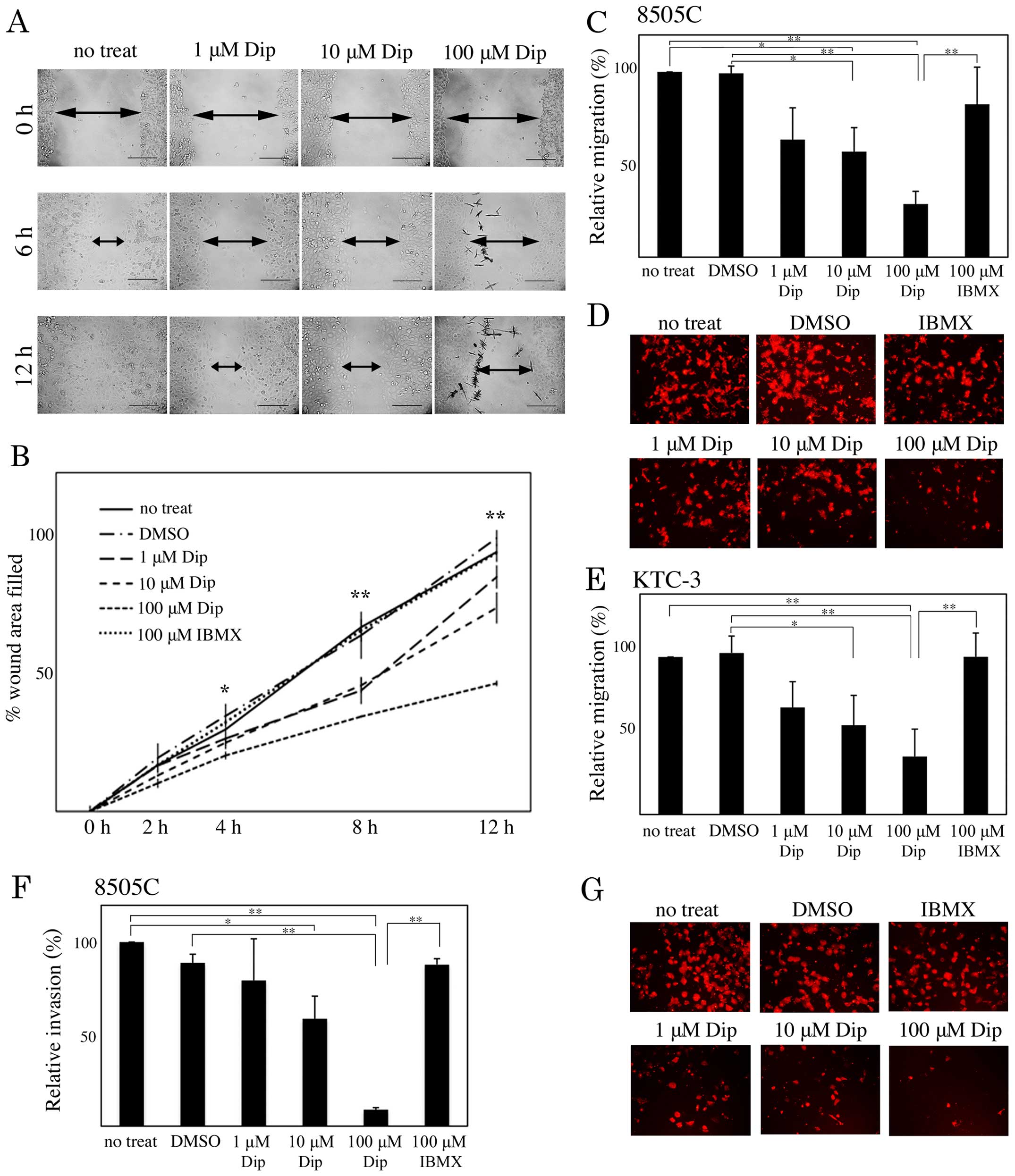 | Figure 2Involvement of cAMP-PDE activity in
human ATC cell motility. (A and B) Monolayers of 8505C cells were
scratched manually, and wounded cells were treated with dimethyl
sulfoxide (DMSO), 1, 10 and 100 µM dipyridamole (Dip), or
100 µM 3-isobuty-1-methylxanthine (IBMX), and allowed to
heal for 12 h. Wound-healing rates were measured at 2, 4, 8 and 12
h. The width of wounds are indicated with arrows. (C-E) 8505C (C
and D) and KTC-3 (E) cells treated with DMSO, 1, 10 and 100
µM Dip, and 100 µM IBMX were subjected to a Transwell
cell migration assay. (F and G) 8505C cells were subjected to a
Transwell cell invasion assay. The results are shown as a ratio
compared with no treatment (no treat), as means ± SE. Error bars
indicate the SE. *P<0.05, **P<0.01. |
Involvement of h-prune expression in ATC
cell motility
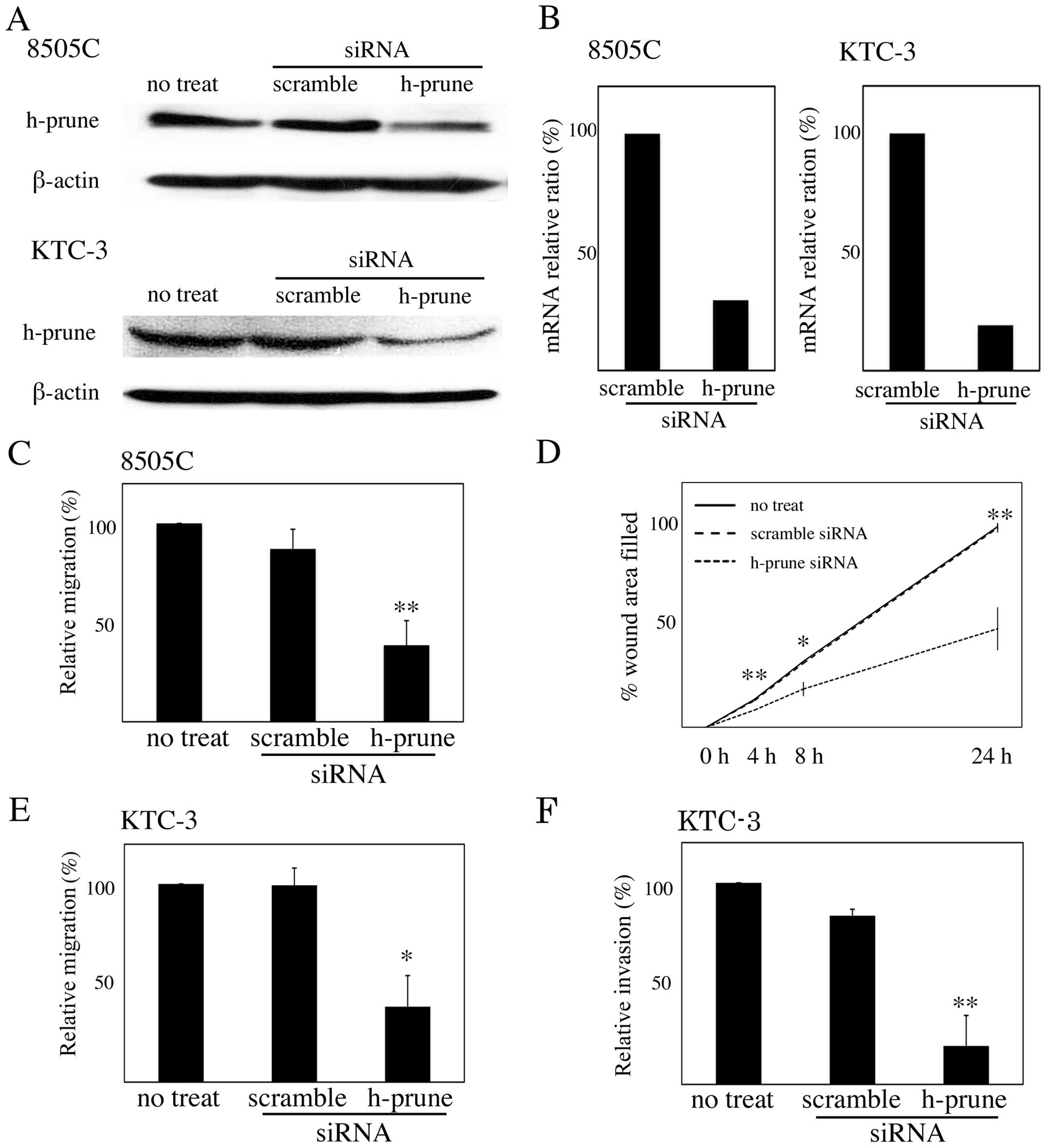
To investigate whether h-prune protein expression is
necessary for ATC cell motility, we depleted endogenous h-prune in
8505C and KTC-3 cells by RNA interference. Treatment with siRNA for
h-prune reduced the protein levels but not levels of β-actin
(Fig. 3A). Quantitative RT-PCR
showed that expression of h-prune mRNA was also reduced by
treatment with siRNA for h-prune (Fig.
3B). The Transwell migration assay revealed that the reduction
of h-prune by RNAi in 8505C and KTC-3 cells resulted in reduced
cell migration (Fig. 3C and D), and
the wound healing assay revealed that the reduction of h-prune by
RNAi in 8505C cells resulted in reduced cell migration (Fig. 3E). We also found that reduction of
h-prune by RNAi in KTC-3 cells resulted in reduced cell invasion
(Fig. 3F).
The effect of inhibition of h-prune PDE
activity on thyroid tumor growth, invasion, and pulmonary
metastasis in an orthotopic mouse model
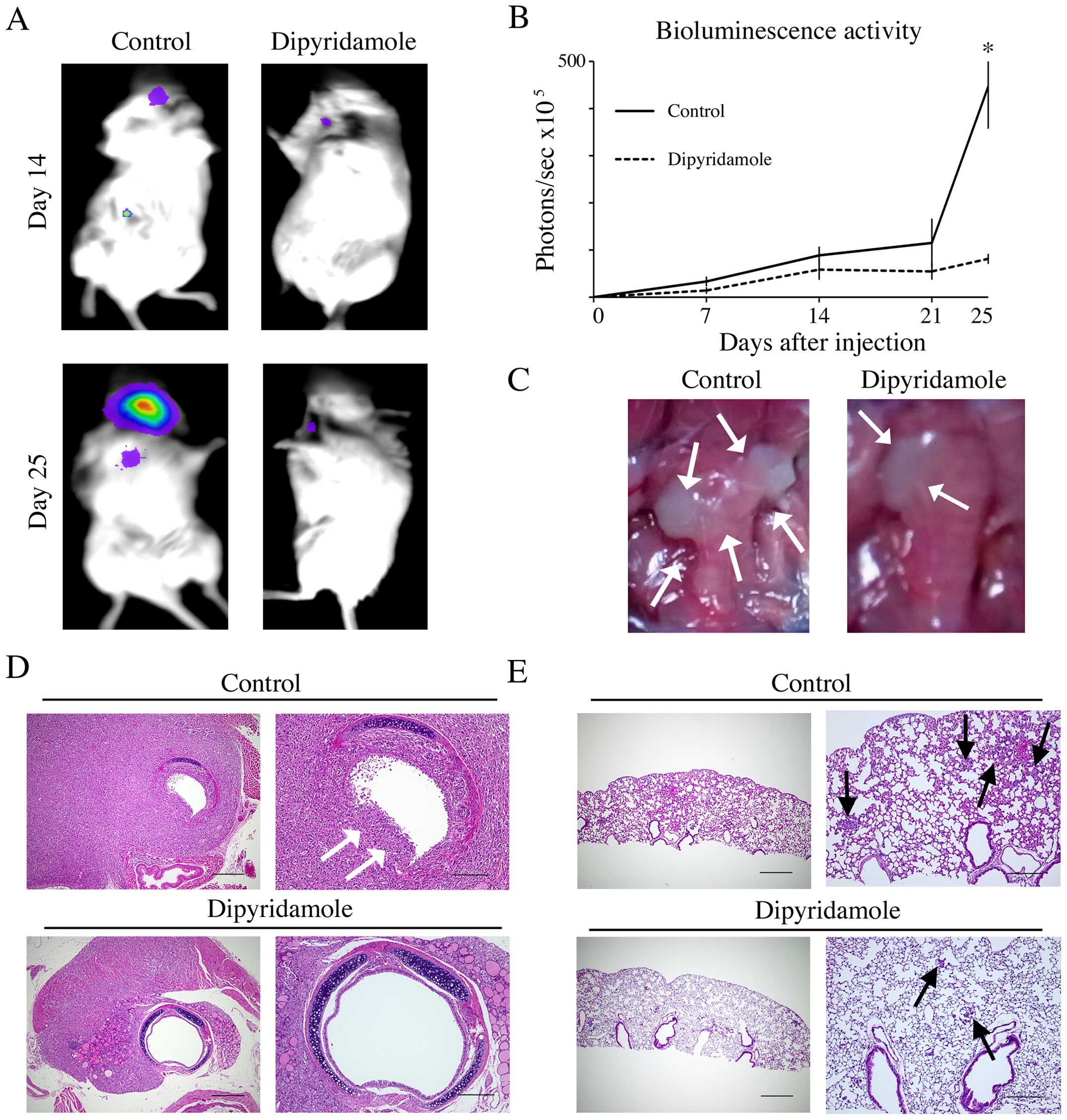
To clarify the ability of dipyridamole to inhibit
tumor invasion and pulmonary metastasis in vivo, we
generated 8505C-luc orthotopic xenografts in mice. Luciferase
activity and representative images of orthotopic tumors at the time
of necropsy showed enlargement of tumor around the trachea and
surrounding tissue in mice administered PBS as control while
dipyridamole-treated mice showed a significantly suppressed tumor
growth as measured by bioluminescence activity at day 25
(P<0.05; Fig. 4A and B) and
suppressed invasion into the trachea and the surrounding tissue
(Fig. 4C). In pathological
analysis, while trachea invasion with breaking tracheal mucosa and
cartilages and multiple pulmonary metastasis were found in control
mice, dipyridamole-treated mice showed significantly suppressed
trachea invasion and pulmonary metastasis (Fig. 4D and E; Table I).
 | Table IEffect of inhibition of cAMP-PDE
activity and h-prune expression on thyroid cancer invasion and
pulmonary metastasis in an orthotopic mouse model. |
Table I
Effect of inhibition of cAMP-PDE
activity and h-prune expression on thyroid cancer invasion and
pulmonary metastasis in an orthotopic mouse model.
| Characteristics | 8505C-luc
control
(n=3) | 8505C-luc
dipyridamole
(n=3) | P-value |
|---|
| Trachea invasion | | | 0.051 |
| Modest/mild | 0 (0%) | 2 (66.7%) | |
| Moderate/severe | 3 (100%) | 1 (33.3%) | |
| Esophagus
invasion | | | 0.410 |
| No | 1 (33.3%) | 2 (66.7%) | |
| Yes | 2 (66.7%) | 1 (33.3%) | |
| Pulmonary metastasis
area for total lung field (%) | 2.771±0.113 | 0.645±0.066 | <0.001 |
|
| Characteristics | 8505C
(n=8) | ACT-1
(n=8) | P-value |
|
| Trachea invasion | | | 0.0070 |
| Modest/mild | 2 (25%) | 8 (100%) | |
| Moderate/severe | 6 (75%) | 0 (0%) | |
| Esophagus
invasion | | | 0.0769 |
| No | 4 (50%) | 8 (100%) | |
| Yes | 4 (50%) | 0 (0%) | |
| Pulmonary metastasis
area for total lung field (%) | 2.814±0.978 | 0 | <0.001 |
The effect of h-prune expression on
thyroid tumor growth, invasion and pulmonary metastasis in an
orthotopic mouse model
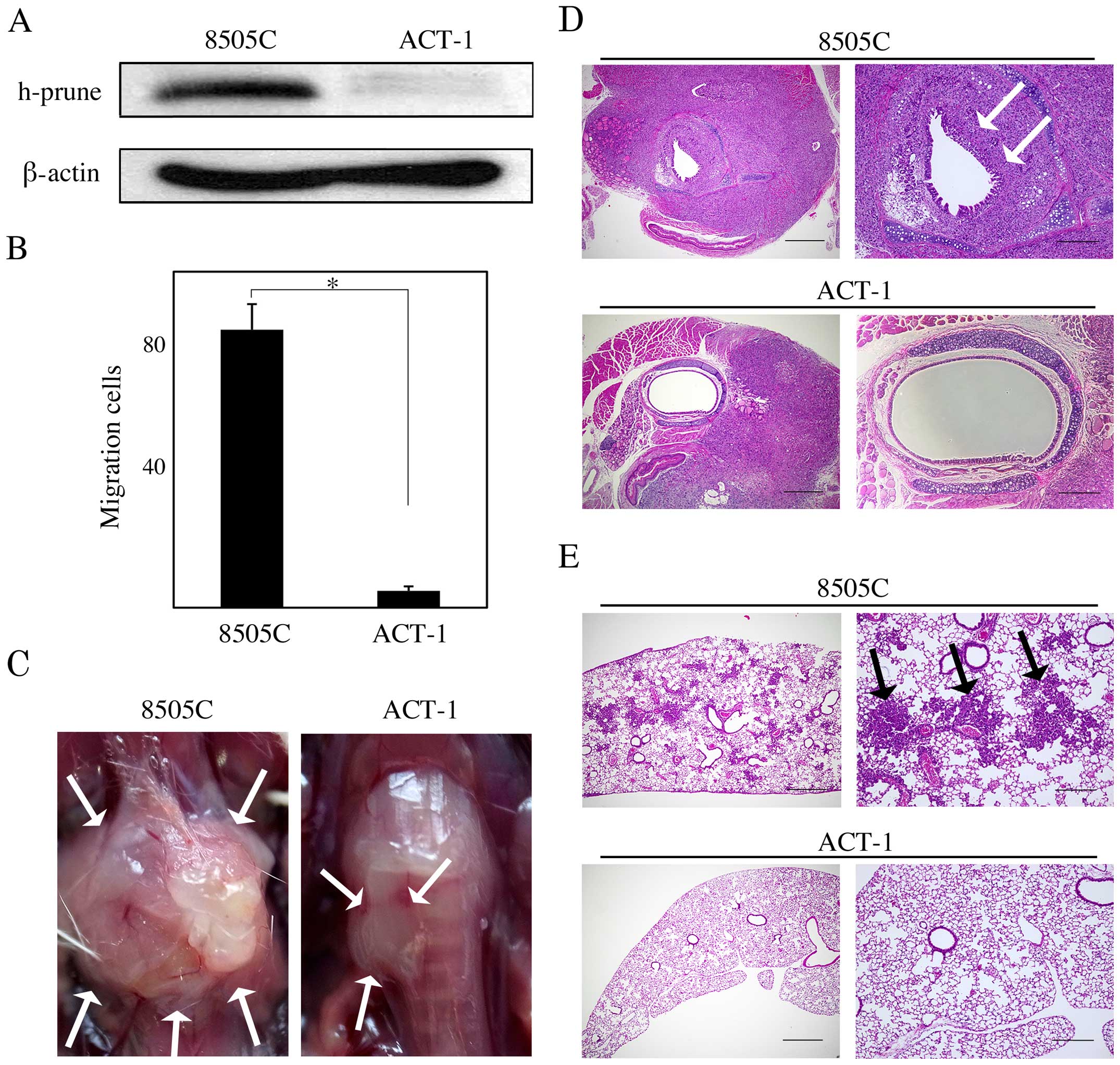
ACT-1 cells exhibited lower h-prune expression than
8505C cells (Fig. 5A) and the cell
migration assay revealed that ACT-1 cells showed impaired cell
migration ability compared to 8505C cells (P<0.05; Fig. 5B). Thus, we tested whether h-prune
protein expression levels affect tumor growth, invasion, and
pulmonary metastasis in vivo, by using 8505C an ACT-1 murine
orthotopic xenograft model. The 8505C mice had larger tumors that
involved the trachea and surrounding tissue than the ACT-1 mice
(Fig. 5C). In the pathological
analysis, more severe tracheal and esophageal invasion and more
frequent pulmonary metastasis were observed in the 8505C mice than
in the ACT-1 mice (Fig. 5D and E;
Table I).
Discussion
Previous reports have shown that in several types of
cancer, h-prune overexpression is correlated with advanced tumor
stage and poor prognosis. Forus et al (18) reported amplification of the PRUNE
gene in aggressive sarcoma subtypes. Zollo et al (12) reported that overexpression of
h-prune in breast cancer patients was correlated with advanced
lymph node status and the presence of distant metastases. We
previously showed that h-prune protein expression was correlated
with depth of invasion and degree of lymph node metastasis in
colorectal and pancreatic cancers (15). Patients with h-prune-positive
gastric cancer had a significantly worse survival rate than
patients with h-prune-negative gastric cancer (13). In addition, in esophageal squamous
cell carcinoma patients, h-prune-positive staining was
significantly correlated with tumor stage and independent
predictors of survival (14). These
results suggest that h-prune can be used as a marker for the
identification of subsets of cancer patients with highly aggressive
tumors. In the present study, although h-prune is not appropriate
as a marker for the identification of highly aggressive subsets of
thyroid cancer patients, h-prune was frequently expressed in ATC
tissues and corresponding lymph node metastasis.
Present study showed that inhibition of both
expression and PDE activity of h-prune downregulated thyroid cancer
cell migration and invasion in vitro, consistent with
previous reports (15,19). Although the molecular mechanisms by
which h-prune regulates cell motility remain to be sufficiently
defined, previous reports have suggested that h-prune might
regulate cell motility by two different means of action: through
its PDE activity and its interactions with protein partners.
One mechanism by which h-prune stimulates cell
motility and metastasis is through its PDE activity, which can be
suppressed by dipyridamole, a pyrimido[5,4-d]pyrimidine analogue
(19). H-prune has been reported to
possess cAMP-PDE activity, with a preferential affinity for cAMP
over cGMP as a substrate, with Km values of 0.9±0.03 and 2.3±0.11
M, respectively (19). Dypiridamole
has been used as an anti-platelet-aggregation agent, and its
activity as a selective PDE inhibitor has been tested in various
studies. Inhibition of PDE activity with dipyridamole suppressed
cell motility, indicating that h-prune PDE activity might be
critical for cellular motility (19). In the present study, dipyridamole
suppressed ATC cell motility and invasiveness in vitro, and
also suppressed cancer invasiveness and lung metastasis in an
orthotopic mouse model. These results suggested that the PDE
activity of h-prune might be necessary for cancer invasion and
metastasis.
In the present study, h-prune protein expression
levels were involved in migration and invasion of ATC cell lines,
and metastasis in murine orthotopic xenograft model. H-prune has
been reported to interact with several proteins, including nm23-H1,
GSK-3 and ASAP1 (15,20–22).
These proteins have been reported to act as a regulator of cancer
metastasis (20,23). Thus, h-prune may promote anaplastic
cancer cell motility through an interaction with several protein
partners.
A limitation of the present study is that
immunohistochemistry showed that h-prune was frequently expressed
in all ATC tissues. According to this result, h-prune may not be
correlated with aggressiveness. Thus, the inhibition of h-prune
function may critically affect the maintenance of the cancer cell
itself. However, the MTT assay showed that dipyridamole had no
significant effect on cell proliferation (data not shown).
In conclusion, h-prune is frequently expressed in
ATC cells and lymph node metastasis, and promotes migration and
invasion of ATC cells and metastasis in an anaplastic thyroid
cancer model. Thus, h-prune shows promise as a targeting candidate
against ATC.
Acknowledgments
We are grateful to Junichi Kurebayashi and Takanori
Miyoshi, for donating cells. We thank the Analysis Center of Life
Science, Hiroshima University for the use of their facilities.
References
|
1
|
Ain KB: Anaplastic thyroid carcinoma: A
therapeutic challenge. Semin Surg Oncol. 16:64–69. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Demeter JG, De Jong SA, Lawrence AM and
Paloyan E: Anaplastic thyroid carcinoma: risk factors and outcome.
Surgery. 110:956–961. 1991.PubMed/NCBI
|
|
3
|
Hundahl SA, Fleming ID, Fremgen AM and
Menck HR: A National Cancer Data Base report on 53,856 cases of
thyroid carcinoma treated in the U.S., 1985–1995 [see commetns].
Cancer. 83:2638–2648. 1998. View Article : Google Scholar
|
|
4
|
Kebebew E, Greenspan FS, Clark OH, Woeber
KA and McMillan A: Anaplastic thyroid carcinoma. Treatment outcome
and prognostic factors. Cancer. 103:1330–1335. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nel CJ, van Heerden JA, Goellner JR,
Gharib H, McConahey WM, Taylor WF and Grant CS: Anaplastic
carcinoma of the thyroid: A clinicopathologic study of 82 cases.
Mayo Clin Proc. 60:51–58. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tan RK, Finley RK III, Driscoll D,
Bakamjian V, Hicks WL Jr and Shedd DP: Anaplastic carcinoma of the
thyroid: A 24-year experience. Head Neck. 17:41–47; discussion
47–48. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Venkatesh YS, Ordonez NG, Schultz PN,
Hickey RC, Goepfert H and Samaan NA: Anaplastic carcinoma of the
thyroid. A clinicopathologic study of 121 cases. Cancer.
66:321–330. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Voutilainen PE, Multanen M, Haapiainen RK,
Leppäniemi AK and Sivula AH: Anaplastic thyroid carcinoma survival.
World J Surg. 23:975–978; discussion 978–979. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shaha AR: Implications of prognostic
factors and risk groups in the management of differentiated thyroid
cancer. Laryngoscope. 114:393–402. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nagaiah G, Hossain A, Mooney CJ,
Parmentier J and Remick SC: Anaplastic thyroid cancer: A review of
epidemiology, pathogenesis, and treatment. J Oncol.
2011:5423582011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marino N and Zollo M: Understanding
h-prune biology in the fight against cancer. Clin Exp Metastasis.
24:637–645. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zollo M, Andrè A, Cossu A, Sini MC,
D'Angelo A, Marino N, Budroni M, Tanda F, Arrigoni G and Palmieri
G: Overexpression of h-prune in breast cancer is correlated with
advanced disease status. Clin Cancer Res. 11:199–205.
2005.PubMed/NCBI
|
|
13
|
Oue N, Yoshida K, Noguchi T, Sentani K,
Kikuchi A and Yasui W: Increased expression of h-prune is
associated with tumor progression and poor survival in gastric
cancer. Cancer Sci. 98:1198–1205. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Noguchi T, Oue N, Wada S, Sentani K,
Sakamoto N, Kikuchi A and Yasui W: h-Prune is an independent
prognostic marker for survival in esophageal squamous cell
carcinoma. Ann Surg Oncol. 16:1390–1396. 2009. View Article : Google Scholar
|
|
15
|
Kobayashi T, Hino S, Oue N, Asahara T,
Zollo M, Yasui W and Kikuchi A: Glycogen synthase kinase 3 and
h-prune regulate cell migration by modulating focal adhesions. Mol
Cell Biol. 26:898–911. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
The Japanese Society of Thyroid Surgery:
General Rules for the Description of Thyroid Cancer. 6th edition.
Kanehara Press; Tokyo, Japan: 2005
|
|
17
|
Kurebayashi J, Okubo S, Yamamoto Y, Ikeda
M, Tanaka K, Otsuki T and Sonoo H: Additive antitumor effects of
gefitinib and imatinib on anaplastic thyroid cancer cells. Cancer
Chemother Pharmacol. 58:460–470. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Forus A, D'Angelo A, Henriksen J, Merla G,
Maelandsmo GM, Flørenes VA, Olivieri S, Bjerkehagen B, Meza-Zepeda
LA, del Vecchio Blanco F, et al: Amplification and overexpression
of PRUNE in human sarcomas and breast carcinomas - a possible
mechanism for altering the nm23-H1 activity. Oncogene.
20:6881–6890. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
D'Angelo A, Garzia L, André A, Carotenuto
P, Aglio V, Guardiola O, Arrigoni G, Cossu A, Palmieri G, Aravind
L, et al: Prune cAMP phosphodiesterase binds nm23-H1 and promotes
cancer metastasis. Cancer Cell. 5:137–149. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Steeg PS: Metastasis suppressors alter the
signal transduction of cancer cells. Nat Rev Cancer. 3:55–63. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Carotenuto M, De Antonellis P, Liguori L,
Benvenuto G, Magliulo D, Alonzi A, Turino C, Attanasio C, Damiani
V, Bello AM, et al: H-Prune through GSK-3β interaction sustains
canonical WNT/β-catenin signaling enhancing cancer progression in
NSCLC. Oncotarget. 5:5736–5749. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Müller T, Stein U, Poletti A, Garzia L,
Rothley M, Plaumann D, Thiele W, Bauer M, Galasso A, Schlag P, et
al: ASAP1 promotes tumor cell motility and invasiveness, stimulates
metastasis formation in vivo, and correlates with poor survival in
colorectal cancer patients. Oncogene. 29:2393–2403. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin D, Watahiki A, Bayani J, Zhang F, Liu
L, Ling V, Sadar MD, English J, Fazli L, So A, et al: ASAP1, a gene
at 8q24, is associated with prostate cancer metastasis. Cancer Res.
68:4352–4359. 2008. View Article : Google Scholar : PubMed/NCBI
|