Introduction
Osteosarcoma alone comprises more than 50% of the
primary bone tumors and is detected mostly in children. In Japan
every year more than 100 cases of osteosarcoma are detected. In
osteosarcoma various changes are observed in the chromosomes and
the gene mutations are also extensive resulting in the development
of complex tumors (1,2). Numerous studies have been performed to
understand the mechanism underlying the development of osteosarcoma
(1–6). Despite advancement in the techniques
including, wide tumor excision and aggressive chemotherapy the
prognosis in osteosarcoma patients is very poor (7,8). Thus,
the screening of molecules for effective treatment of osteosarcoma
is desired (9).
Apoptosis is the controlled and programmed death of
cells and is vital for the treatment of carcinoma and removal of
unwanted cells from the body (10,11).
Regulation of various factors involved in the process of apoptosis
is mediated through phosphorylation (12). One more factor involved in the
regulation of apoptosis in various types of carcinoma cells is the
nuclear factor-kappa B (NF-κB) (13–17).
Activation of IκBα followed by its degradation induces
translocation of NF-κB into the cell nucleus from cytoplasm where
it influences several genes.
Tumstatin, is a well-known anti-angiogenic agent
possessing promising antitumor potential (18,19).
Various reports have demonstrated that tumstatin treatment prevents
tumor proliferation in glioma and melanoma tumor models (20–22).
In addition, tumstatin exhibits a strong tendency to suppress
proliferation and angiogenesis of tumor in the head and neck
carcinoma models. Furthermore, in oral squamous cancer model
treatment with tumstatin inhibited metastasis of carcinoma cells to
the lymph nodes (23). The present
study was aimed to investigate the effect of tumstatin on tendency
of proliferation and apoptosis in Saos-2 osteosarcoma cells. The
results demonstrated that tumstatin treatment in Saos-2 cells
inhibited cell proliferation and induced apoptosis through
activation of p65NF-κB.
Materials and methods
Chemicals and reagents
Tumstatin and TNF-α were purchased from
Sigma-Aldrich (St. Louis, MO, USA). The stock solution of tumstatin
was prepared in dimethyl sulfoxide and stores at −15°C before use
in the experiment. Fetal bovine serum (FBS) and Dulbecco's modified
Eagle's minimum essential medium (D-MEM) were obtained from
Gibco-BRL (Gaithersburg, MD, USA).
Cell culture
Saos-2 osteosarcoma cell line and human embryonic
kidney HEK293 cells were obtained from the American Type Culture
Collection (ATCC; Rockville, MD, USA). The cells were cultured in
D-MEM containing 10% FBS, 2 mM glutamine, 100 U/ml penicillin and
100 µg/ml streptomycin. Both cell lines were cultured in an
incubator of humidified atmosphere with 5% CO2 at
37°C.
Analysis of cell proliferation
The effect of tumstatin on proliferation of Saos-2
cells was analyzed by
3-(4,5-dimeth-ylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Sigma-Aldrich) assay. The cells were distributed at density of
2×106 cells/well in 2 ml DMEM medium supplemented with
10% FBS in 6-well plates (Nunc A/S Plastfabrikation, Roskilde,
Denmark). Following 12-h incubation, the medium was replaced with
new medium containing 5, 10, 15, 20, 25 or 30 µM
concentrations of tumstatin and incubated for 48 h. Then, 50
µl of MTT (5 µg/ml) solution was added to each well
and incubation was continued for 2 h. To each well of the plate,
150 µl of DMSO was added and incubated for 5 min. The well
optical density (OD) was recorded at 570 nm using an EL800
Universal Microplate Reader (BioTek Instruments, Inc., Winooski,
VT, USA).
DNA fragmentation assay
Saos-2 cells were seeded in T-75 flasks at a density
of 2×105 cells/flask and cultured for 12 h. Then, medium
was replaced with new medium containing 25 µM concentration
of tumstatin for 48 h. QIAamp DNA Mini kit (Qiagen) was used for
the preparation of genomic DNA in accordance with the guidelines on
the user manual. Electrophoresis of the DNA samples was performed
on 1.8% agarose gel at 50 V for 2 h. For gel staining ethidium
bromide (Sigma-Aldrich) was used, whereas visualization was
achieved by ultraviolet (UV) transilluminator (Wealtech Corp.,
Reno, NV, USA).
DNA isolation and agarose gel
electrophoresis
Saos-2 cells after incubation with tumstatin for 48
h were rinsed three times in PBS and lysed in lysis buffer [10 mM
Tris-HCl buffer (pH 7.5), 10 mM EDTA and 0.5% Triton X-100). The
cell lysates were centrifuges at 12,000 × g for 45 min to remove
the insoluble material. The lysate was then treated with DNase and
subjected to incubation at 37°C for 45 min. The lysates were
treated with proteinase K for 50 min followed by addition of
2-propanol and NaCl to precipitate the DNA. DNA was suspended in
TE-buffer and then electrophoresed using agarose gel. The UV
transilluminator (Vilber Lourmat, Marne la Vallee, France) was used
for the analysis of apoptotic changes.
Real-time reverse transcription
polymerase chain reaction (RT-PCR)
The expression of mRNA in Saos-2 osteosarcoma cells
was determines by using real-time RT-PCR. For this purpose, a total
of 2×106 cells were cultured in 100-mm dishes containing
DMEM medium and tumstatin and incubated for 48 h. The cells were
then collected to extract the RNA using an RNeasy Plus Mini kit
(Qiagen, Waco, TX, USA). RT-PCR analysis was carried out using
SuperScript III First-Strand Synthesis SuperMix for qRT-PCR
(Invitrogen, Carlsbad, CA, USA). NanoDrop 1000 (Thermo Fisher
Scientific, Wilmington, DE, USA) was used for the determination of
concentration of each cDNA after adjustment to 40 ng/ml using
diethylpyrocarbonate (DPEC) water. FAM-labeled TaqMan probes and
TaqMan Universal Master Mix (Applied Biosystems) using Chromo4
(Bio-Rad Laboratories, Cambridge, MA, USA) were employed to carry
out the real-time PCR with the primers listed in Table I. The PCR sequence involved 2-min
incubation at 50°C, 10-min denaturation at 50°C followed by 15 sec
50 cycles at 95°C and then 1 min at 60°C. GAPDH was used as an
internal control. Analysis of the PCR products was performed on 2%
agarose gel using ethidium bromide and a UV illuminator was used
for the visualized.
 | Table IThe primers used in RT-PCR. |
Table I
The primers used in RT-PCR.
| Primer | Forward | Reverse |
|---|
| PTEN |
5′-ACCGCCAAATTTAATTGCAG-3′ |
5′-GGGTCCTGAATTGGAFFAAT-3′ |
| FasL |
5′-TCTCAGACGTTTTTCGGCTT-3′ |
5′-AAGACAGTCCCCCTTGAGGT-3′ |
| FasR |
5′-CAAGGGATTGGAATTGAGGA-3′ |
5′-GACAAAGCCACCCCAAGTTA-3′ |
| GAPDH |
5′-ACCACAGTCCATGCCATCAC-3′ |
5′-TCCACCACCCTGTTGCTGTA-3′ |
Western blot analysis
Tumstatin, TNF-α-treated or untreated control Saos-2
cells were suspended in DMEM medium containing 1.5 µM
aprotinin, 5 µM AEBSF, 0.01 µM leupeptin, 10
µM E-64 and phosphatase inhibitors [1 mM sodium
orthovanadate (Na2VO4); 1 mM sodium molybdate
(Na2MoO4) 4 mM sodium tartrate dihydrate; 2
mM imidazole were obtained from Sigma-Aldrich]. The cell plates
were kept for a period of 30 min on ice prior to centrifugation at
12,000 × g for 30 min to collect the supernatant. The bicinchoninic
protein assay kit (Pierce, Rockford, IL, USA) was used for the
analysis of the concentration of proteins. The 30 µg protein
samples were isolated using 10% SDS-PAGE gel followed by transfer
to polyvinylidene difluoride membrane (Bio-Rad Laboratories,
Hercules, CA, USA) using electroblotting. Following incubation for
1 h in a blocking solution for phosphorylated proteins (Blocking
One-P; Nacalai Tesque, Kyoto, Japan), the membranes were washed
with PBS-Tween followed by overnight incubation with primary
antibodies. Then the membranes were rinsed twice for 10 min each
time in PBS and 0.05% Tween-20 before incubation with horseradish
peroxidase-conjugated polyclonal horse anti-rabbit (1:2,000; Cell
Signaling Technology, Inc., Danvers, MA, USA) for 1 h. The blot was
developed using an enhanced chemiluminescence kit (Intron
Biotechnology, Inc., Seongnam, Korea).
DNA construction and transfection
HEK293 cells seeded in plastic dishes were cultured
in DMEM medium supplemented with FBS until attaining 80%
confluence. The cells were washed with PBS and then treated with a
mixture of DNA and Opti-MEM. QuickChange II Site-Directed
Mutagenesis kit (Stratagene, La Jolla, CA, USA) was used for the
transfection of p65NF-κB S536A carrying alanine instead of serine
at 536 position. The cells were then incubated with tumstatin for
180 min at 37°C in D-MEM containing 10% FBS.
Statistical analysis
For the analysis of the data the unpaired Student's
t-test (Graph Pad Prism V.4) was used and P-values of <0.05 were
considered statistically significant.
Results
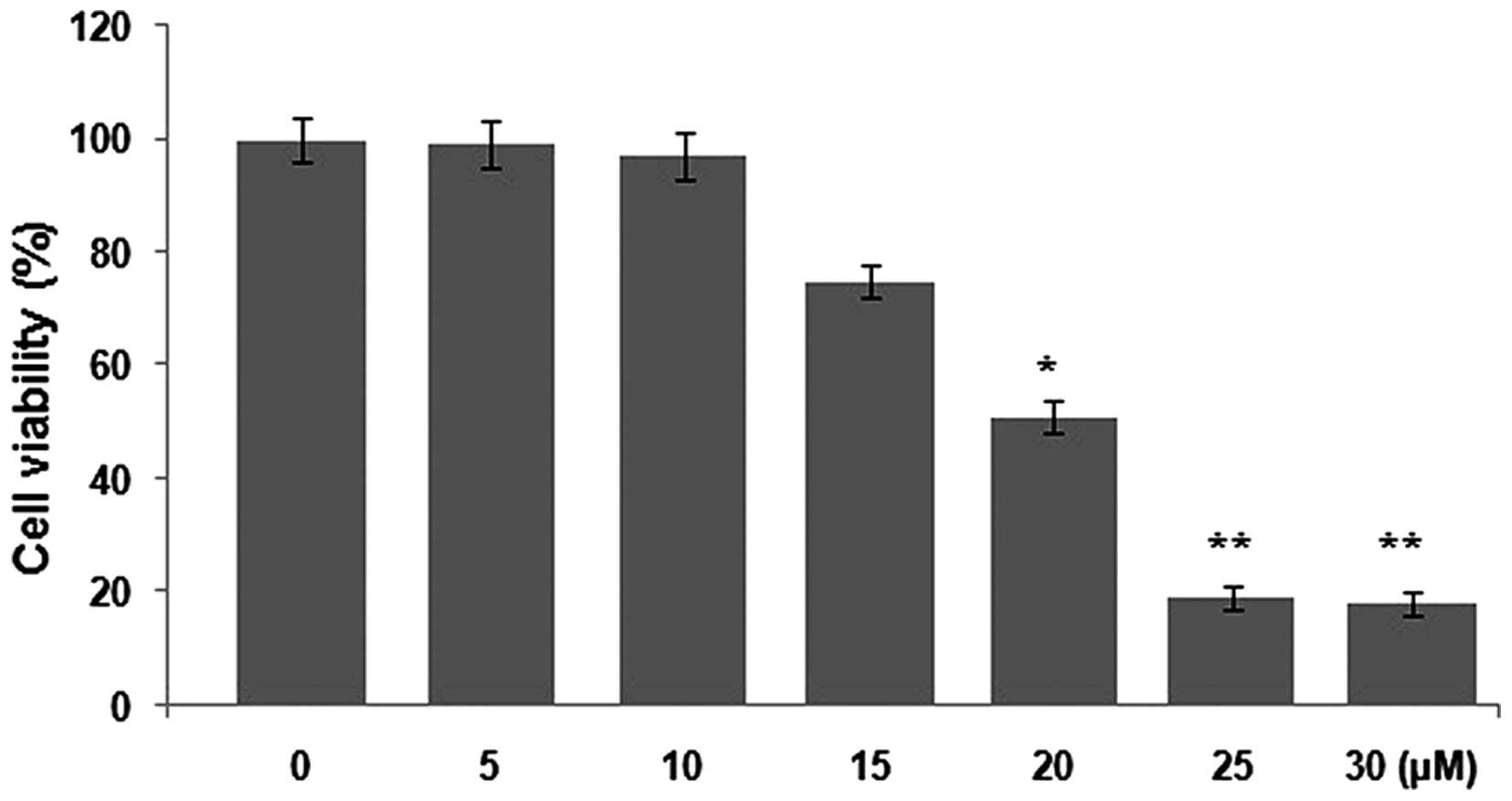
Inhibition of Saos-2 cell viability by
tumstatin
Saos-2 cells were treated with various
concentrations (5, 10, 15, 20, 25 and 30 µM) of tumstatin
for 48 h and then analyzed by MTT assay. Tumstatin treatment
reduced the viability of Saos-2 cells in a concentration-dependent
manner after 48 h. No significant effect was observed on the
viability of Saos-2 cells treated with 5 and 10 µM
concentrations of tumstatin for 48 h. However, cell viability was
reduced significantly by the concentration of 15 µM with
maximum inhibition at 25 µM (Fig. 1). At 25 µM concentration of
tumstatin after 48 h, viability of Saos-2 cells was reduced to 19%
compared to 100% in the control cultures (Fig. 1).
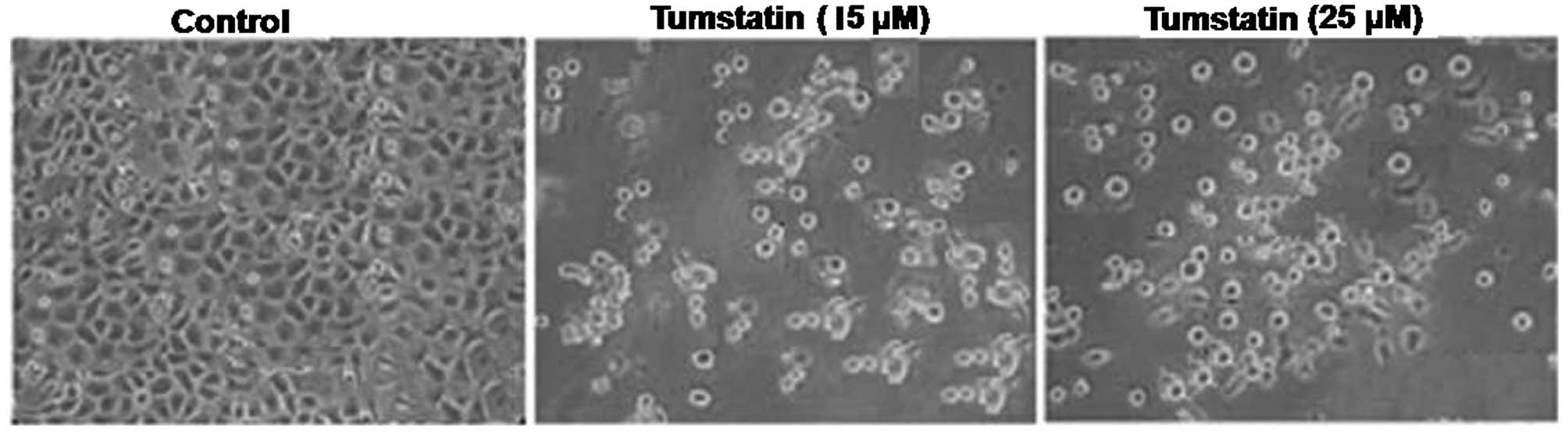
Tumstatin induces apoptosis in Saos-2
cells
Analysis of the apoptosis induction in Saos-2 cells
using Hoechst 33342 staining revealed presence of characteristic
apoptotic nuclei on treatment with 25 µM concentration of
tumstatin after 48 h. The cells were seen to be rounded in shape
and shrunken in size. However, no apoptotic nuclei were observed in
the control Saos-2 cell cultures after 48 h (Fig. 2). Increase in the concentration of
tumstatin from 15 to 25 µM enhanced the proportion of
apoptotic cells significantly compared to the control cells. The
DNA fragmentation pattern showed formation of ladder like
structures on tumstatin treatment for 48 h (Fig. 2).
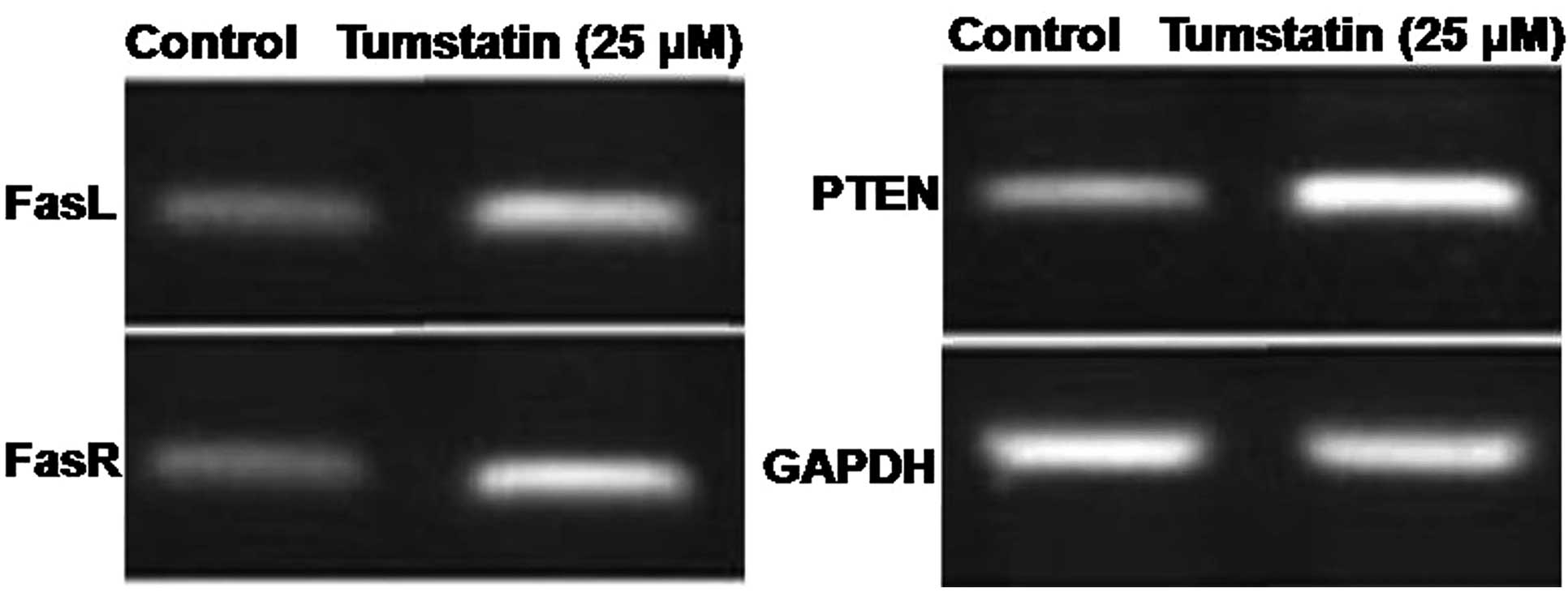
Effect of tumstatin on the expression
level of PTEN, FasL and FasR mRNA in Saos-2 cells
The effect of tumstatin on the expression of PTEN,
FasL and FasR mRNA following amplification of cDNA for 40 cycles
revealed a significant increase after 30 min (Fig. 3). However, tumstatin exhibited no
effect on the expression of mRNA corresponding to GAPDH which was
constitutively expressed in Saos-2 cells (Fig. 3).
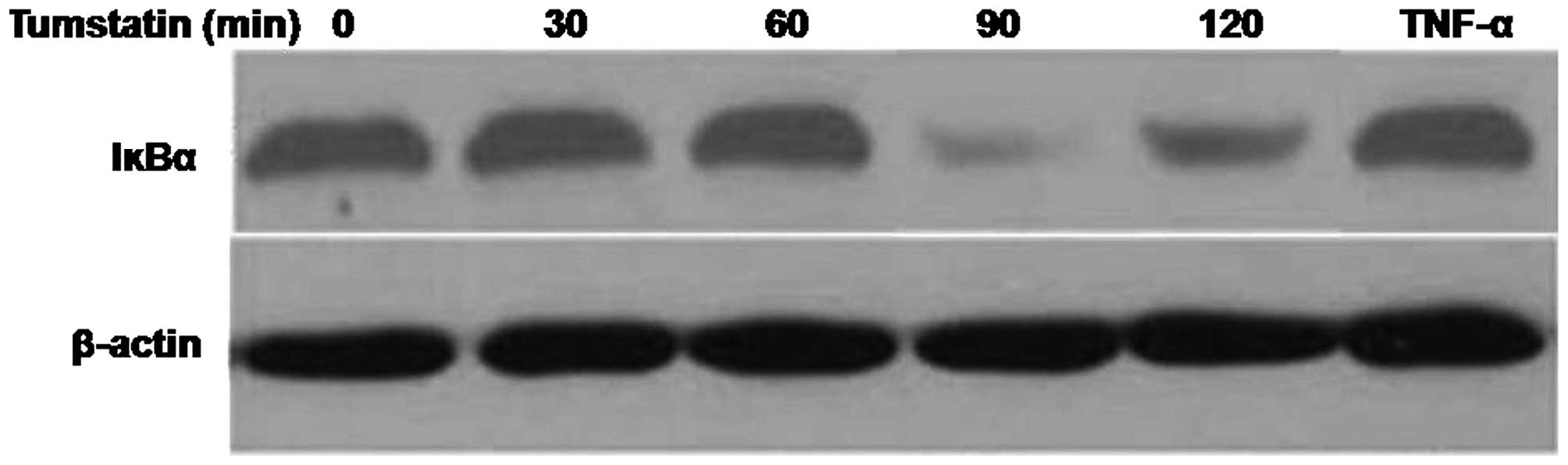
Effect of tumstatin on Iκ-Bα regulation
in Saos-2 cells
The effect of tumstatin and TNF-α on the expression
of IκBα in Saos-2 cells was analyzed using western blot assay. The
results revealed that expression of IκBα was reduced up to 90 min
by tumstatin treatment and was then increased by 180 min. However,
treatment of Saos-2 cells with 10 ng/ml TNF-α as the control
inhibited the expression of IκBα after 30 min (Fig. 4). The expression level of IκBα
increased by 60 min and became similar to those of untreated
control cells.
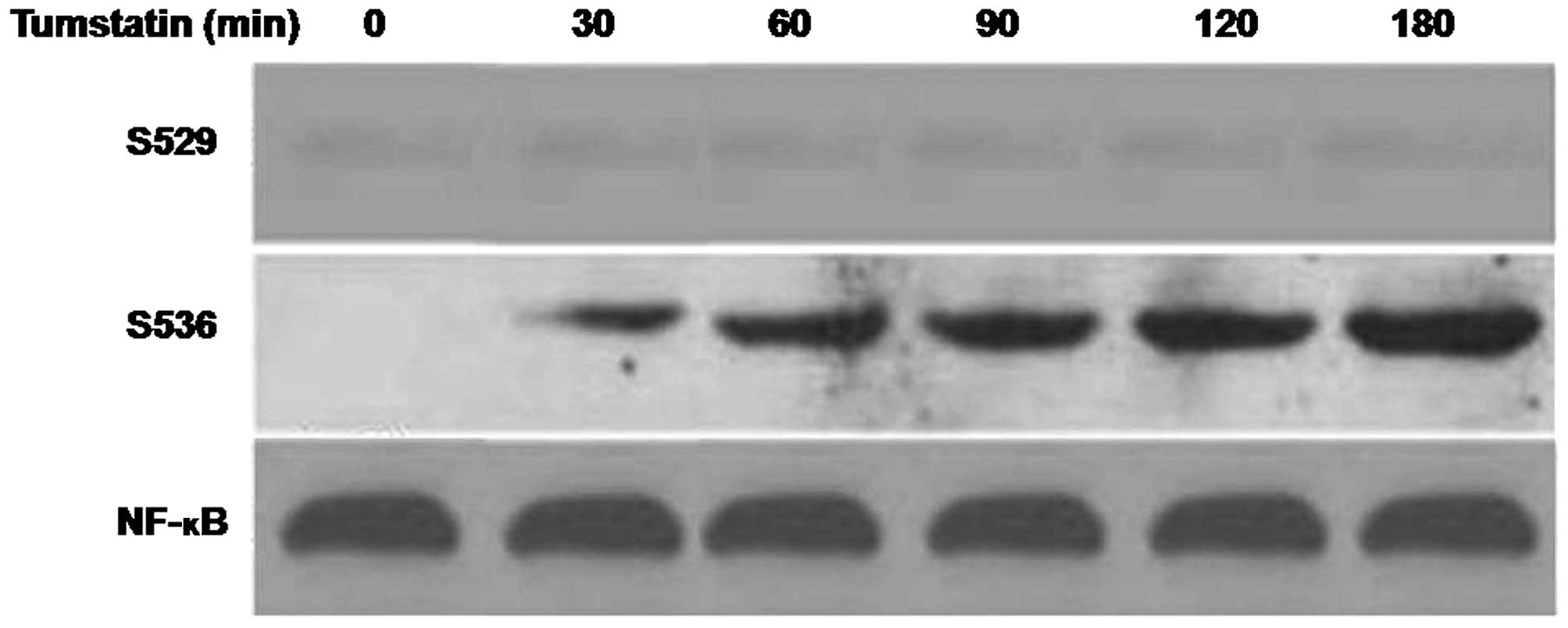
Effect of tumstatin on phosphorylation of
NF-κB
The interaction of anti-phospho-Ser536 p65NF-κB
antibody. Incubation with anti-p65NF-κB antibody also led to a
strong interaction with 65-kDa band. The interaction of
anti-phospho-Ser529 p65NF-κB and anti-p65NF-κB antibodies with
65-kDa band remained independent on treatment with tumstatin
(Fig. 5). The interaction of
anti-phospho-Ser536 p65NF-κB antibody with proteins of tumstatin
treated cells showed a concentration dependent increase (Fig. 5). However, the interaction was found
to be very weak in case of untreated control cells. Following
removal of anti-phospho-Ser536 p65NF-κB antibody and then
incubation with anti-p65NF-κB antibody, interaction was observed.
Treatment of the cells with 10 ng/ml concentration of TNF-α
resulted interaction of antiphospho-Ser529 p65NF-κB and
anti-phospho-Ser536 antibodies with 65-kDa protein. The interaction
increased after 5 min and was maximum after 15 and 60 min,
respectively for antiphospho-Ser529 p65NF-κB and
anti-phospho-Ser536 antibodies (Fig.
5).
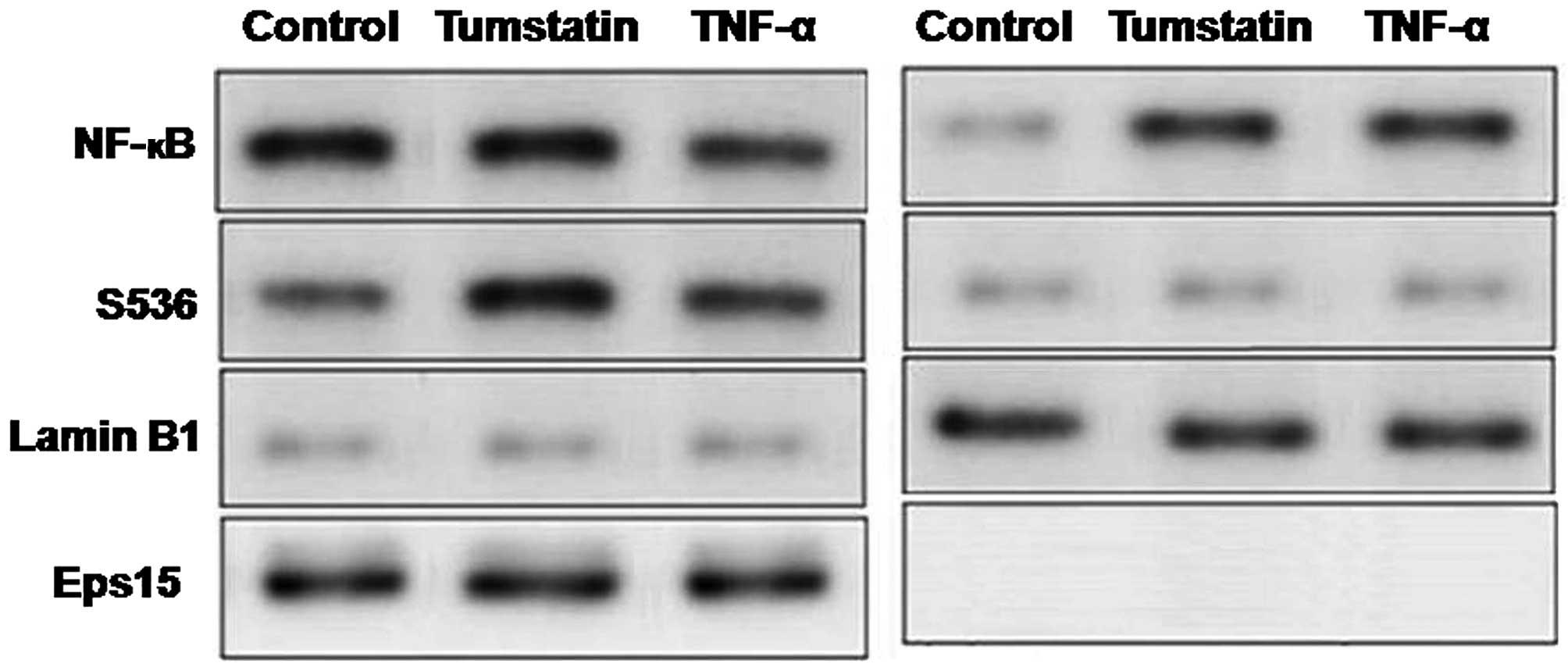
Effect of tumstatin on translocation of
NF-κB in Saos-2 cells
Saos-2 cells were treated with 25 µM
concentration of tumstatin and 10 ng/ml concentration of TNF-α.
Interaction of anti-p65NF-κB antibody and 65-kDa protein band was
observed in the cytosolic fractions of both untreated and
tumstatin-treated cells (Fig. 6).
However, this interaction was observed at higher level in the
nuclear fraction of only tumstatin-treated but not in untreated
control cells. In untreated control cells none of the proteins
interacted with anti-phospho-Ser536 p65NF-κB antibody (Fig. 6). In tumstatin treated cells
interaction of anti-phospho-Ser536 p65NF-κB antibody with proteins
was significant both in the cytosolic and nuclear fractions. The
interaction of anti-phospho-Ser536 p65NF-κB antibody with the
proteins of cytosolic and nuclear fractions was also observed in
the TNF-α treated cells. In both tumstatin and TNF-α-treated cells
interaction of anti-Eps15 antibody was found in cytosolic but not
in nuclear fraction (Fig. 6).
Analysis of the interaction of anti-Lamin B1 antibody with 68-kDa
protein band was found in the nuclear, but not in cytosolic
fraction.
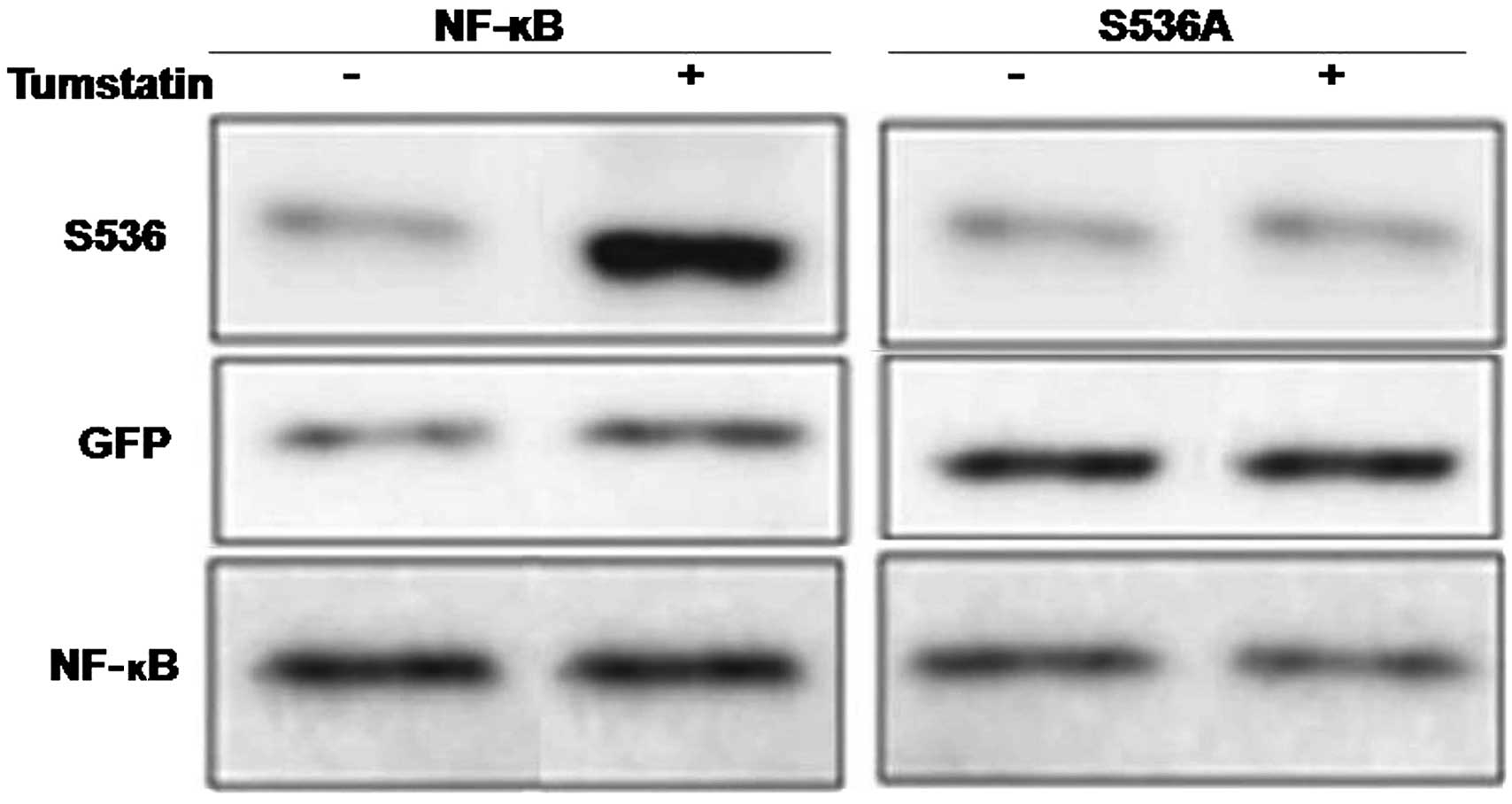
In vitro dephosphorylation and
phosphorylation of mutant gene products
Activation of the Ser536 of p65NF-κB by tumstatin
treatment was confirmed by transfection of HEK293 cells with mutant
p65NF-κB gene possessing alanine (GFP-S536A) instead of serine at
536. In both control and tumstatin treated cells transfected with
GFP-S536A mutant gene, no interaction of anti-phospho-Ser536
p65NF-κB antibody was observed with any of the proteins (Fig. 7). On the contrary, interaction of
anti-phospho-Ser536 p65NF-κB antibody with proteins of tumstatin
treated cells increased significantly.
Discussion
The present study demonstrates the viability of
inhibitory and apoptosis inducing effect of tumstatin on Saos-2
osteoblastic carcinoma cells. MTT assay was used for analysis of
reduction in viability and phase-contrast microscopy for analysis
of alterations in morphology of Saos-2 cells following tumstatin
treatment. The results from viability assay revealed significant
reduction in concentration-dependent manner on treatment with
tumstatin for 48 h. The cells became round and shrunken, DNA showed
fragmentation and ladder-like pattern following tumstatin treatment
at 25 µM. Therefore, tumstatin treatment significantly
induced apoptosis in Saos-2 cells in a dose-dependent manner.
Studies have demonstrated that PTEN, FasR and FasL play a vital
role in the induction of apoptosis through NF-κB pathway (24). Various cell apoptosis is induced via
the NF-κB pathway by increase in the expression of PTEN, FasR and
FasL (25,26). Investigation of the mRNA expression
corresponding to PTEN, FasL and FasR in Saos-2 cells revealed
significant increase in tumstatin-treated cells. These findings
indicated that tumstatin exhibited apoptosis inducing effect
through increase in the expression level of PTEN, FasL and
FasR.
Results from the present study revealed that
tumstatin treatment activated p65NF-κB by phosphorylation of serine
on position 536. TNF-α also led to the activation of p65NF-κB by
phosphorylation of serine at position 536 in Saos-2 cells.
Phosphorylation of p65NF-κB at position 536 serine is well known
and this study also demonstrated the same (27–29).
Therefore, tumstatin induced phosphorylation of p65NF-κB at
position 536 serine observed in the present study is also
confirmed. In most of the studies on p65NF-κB, it has been observed
that serine is phosphorylated on position 536 (27,30,31).
Overexpression studies with the activated Akt revealed that IKK is
necessary for enhanced p65NF-κB transactivation, whereas mutation
of Ser536 abolishes this effect (31). Our results revealed that tumstatin
treatment also induced activation of p65NF-κB in Saos-2 cells by
phosphorylation of serine present on position 536. Thus, NF-κB is
activated by phosphorylation of serine on position 536 in the
Saos-2 cells treated with tumstatin. The present study also
demonstrated that activation of p65NF-κB induced its translocation
into the nucleus in Saos-2 cells. Phosphorylation of Ser536 on
p65NF-κB can be detected both in the cytoplasm and the nucleus.
Activation of NF-κB stimulates the expression of
IκBα which exhibits inhibitory effect on the phosphorylation of
NF-κB (32). The present study
revealed that treatment of Saos-2 cells with TNF-α inhibited IκBα
expression and therefore enhanced the nuclear translocation of
NF-κB. Tumstatin treatment also inhibited the expression of IκBα in
Saos-2 cells, but the effect was weak compared to TNF-α. Therefore,
tumstatin treatment leads to the activation of NF-κB in a manner
independent of IκBα.
In conclusion, the present study demonstrates that
tumstatin inhibits viability and induces apoptosis in Saos-2
osteosarcoma cells through NF-κB activation pathway. Therefore,
tumstatin shows promise for the treatment of osteosarcoma.
References
|
1
|
Tarkkanen M, Karhu R, Kallioniemi A,
Elomaa I, Kivioja AH, Nevalainen J, Böhling T, Karaharju E,
Hyytinen E, Knuutila S, et al: Gains and losses of DNA sequences in
osteosarcomas by comparative genomic hybridization. Cancer Res.
55:1334–1338. 1995.PubMed/NCBI
|
|
2
|
Al-Romaih K, Bayani J, Vorobyova J,
Karaskova J, Park PC, Zielenska M and Squire JA: Chromosomal
instability in osteosarcoma and its association with centrosome
abnormalities. Cancer Genet Cytogenet. 144:91–99. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ragland BD, Bell WC, Lopez RR and Siegal
GP: Cytogenetics and molecular biology of osteosarcoma. Lab Invest.
82:365–373. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
He JP, Hao Y, Wang XL, Yang XJ, Shao JF,
Guo FJ and Feng JX: Review of the molecular pathogenesis of
osteosarcoma. Asian Pac J Cancer Prev. 15:5967–5976. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Broadhead ML, Clark JC, Myers DE, Dass CR
and Choong PF: The molecular pathogenesis of osteosarcoma: A
review. Sarcoma. 2011:9592482011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kansara M and Thomas DM: Molecular
pathogenesis of osteosarcoma. DNA Cell Biol. 26:1–18. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tabone MD, Kalifa C, Rodary C, Raquin M,
Valteau-Couanet D and Lemerle J: Osteosarcoma recurrences in
pediatric patients previously treated with intensive chemotherapy.
J Clin Oncol. 12:2614–2620. 1994.PubMed/NCBI
|
|
8
|
Kempf-Bielack B, Bielack SS, Jurgens H,
Branscheid D, Berdel WE, Exner GU, Göbel U, Helmke K, Jundt G,
Kabisch SF, et al: Osteosarcoma relapse after combined modality
therapy: An analysis of unselected patients in the Cooperative
Osteosarcoma Study Group (COSS). J Clin Oncol. 23:559–568. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Davis AM, Bell RS and Goodwin PJ:
Prognostic factors in osteosarcoma: A critical review. J Clin
Oncol. 12:423–431. 1994.PubMed/NCBI
|
|
10
|
Arends MJ and Wyllie AH: Apoptosis:
mechanisms and roles in pathology. Int Rev Exp Pathol. 32:223–254.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jacobson MD, Weil M and Raff MC:
Programmed cell death in animal development. Cell. 88:347–354.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cross TG, Scheel-Toellner D, Henriquez NV,
Deacon E, Salmon M and Lord JM: Serine/threonine protein kinases
and apoptosis. Exp Cell Res. 256:34–41. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Aggarwal BB and Takada Y: Pro-apoptotic
and anti-apoptotic effects of tumor necrosis factor in tumor cells.
Role of nuclear transcription factor NF-κB. Cancer Treat Res.
126:103–127. 2005. View Article : Google Scholar
|
|
14
|
Graham B and Gibson SB: The two faces of
NF-κB in cell survival responses. Cell Cycle. 4:1342–1345. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lamkanfi M, Declercq W, Van den Berghe T
and Van den Abeele P: Caspases leave the beaten track:
caspase-mediated activation of NF-κB. J Cell Biol. 173:165–171.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Piva R, Belardo G and Santoro MG: NF-κB: a
stress-regulated switch for cell survival. Antioxid Redox Signal.
8:478–486. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Radhakrishnan SK and Kamalakaran S:
Pro-apoptotic role of NF-κB: implications for cancer therapy.
Biochim Biophys Acta. 1766:53–62. 2006.PubMed/NCBI
|
|
18
|
Maeshima Y, Colorado PC, Torre A, Holthaus
KA, Grunkemeyer JA, Ericksen MB, Hopfer H, Xiao Y, Stillman IE and
Kalluri R: Distinct antitumor properties of a type IV collagen
domain derived from basement membrane. J Biol Chem.
275:21340–21348. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hamano Y and Kalluri R: Tumstatin, the NC1
domain of α3 chain of type IV collagen, is an endogenous inhibitor
of pathological angiogenesis and suppresses tumor growth. Biochem
Biophys Res Commun. 333:292–298. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pasco S, Brassart B, Ramont L, Maquart FX
and Monboisse JC: Control of melanoma cell invasion by type IV
collagen. Cancer Detect Prev. 29:260–266. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pasco S, Ramont L, Venteo L, Pluot M,
Maquart FX and Monboisse JC: In vivo overexpression of tumstatin
domains by tumor cells inhibits their invasive properties in a
mouse melanoma model. Exp Cell Res. 301:251–265. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kawaguchi T, Yamashita Y, Kanamori M,
Endersby R, Bankiewicz KS, Baker SJ, Bergers G and Pieper RO: The
PTEN/Akt pathway dictates the direct alphaVbeta3-dependent
growth-inhibitory action of an active fragment of tumstatin in
glioma cells in vitro and in vivo. Cancer Res. 66:11331–11340.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chung IS, Son YI, Ko YJ, Baek CH, Cho JK
and Jeong HS: Peritumor injections of purified tumstatin delay
tumor growth and lymphatic metastasis in an orthotopic oral
squamous cell carcinoma model. Oral Oncol. 44:1118–1126. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tanaka Y, Singh S and Aggarwal BB:
Identification of a p65 peptide that selectively inhibits NF-κB
activation induced by various inflammatory stimuli and its role in
down-regulation of NF-κB-mediated gene expression and up-regulation
of apoptosis. J Biol Chem. 279:15096–15104. 2004. View Article : Google Scholar
|
|
25
|
Fujita M, Goto K, Yoshida K, Okamura H,
Morimoto H, Kito S, Fukuda J and Haneji T: Okadaic acid stimulates
expression of Fas receptor and Fas ligand by activation of nuclear
factor kappa-B in human oral squamous carcinoma cells. Oral Oncol.
40:199–206. 2004. View Article : Google Scholar
|
|
26
|
Bertram J, Peacock JW, Tan C, Mui AL,
Chung SW, Gleave ME, Dedhar S, Cox ME and Ong CJ: Inhibition of the
phosphatidylinositol 3′-kinase pathway promotes autocrine
Fas-induced death of phosphatase and tensin homologue-deficient
prostate cancer cells. Cancer Res. 66:4781–4788. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang F, Tang E, Guan K and Wang CY: IKKβ
plays an essential role in the phosphorylation of RelA/p65 on
serine 536 induced by lipopolysaccharide. J Immunol. 170:5630–5635.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Doyle SL, Jefferies CA and O'Neill LA:
Bruton's tyrosine kinase is involved in p65-mediated
transactivation and phosphorylation of p65 on serine 536 during
NFκB activation by lipopolysaccharide. J Biol Chem.
280:23496–23501. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shiraki K, Yamanaka T, Inoue H, Kawakita
T, Enokimura N, Okano H, Sugimoto K, Murata K and Nakano T:
Expression of TNF-related apoptosis-inducing ligand in human
hepatocellular carcinoma. Int J Oncol. 26:1273–1281.
2005.PubMed/NCBI
|
|
30
|
Sakurai H, Chiba H, Miyoshi H, Sugita T
and Toriumi W: IκB kinases phosphorylate NF-κB p65 subunit on
serine 536 in the transactivation domain. J Biol Chem.
274:30353–30356. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Madrid LV, Mayo MW, Reuther JY and Baldwin
AS Jr: Akt stimulates the transactivation potential of the RelA/p65
subunit of NF-κB through utilization of the IκB kinase and
activation of the mitogen-activated protein kinase p38. J Biol
Chem. 276:18934–18940. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sasaki CY, Barberi TJ, Ghosh P and Longo
DL: Phosphorylation of RelA/p65 on serine 536 defines an
IκBα-independent NF-κB pathway. J Biol Chem. 280:34538–34547. 2005.
View Article : Google Scholar : PubMed/NCBI
|