Introduction
Nasopharyngeal carcinoma (NPC) is one of the most
common head and neck malignancies in Southeast Asia and Southern
China (1). Radiotherapy is a
routine treatment for NPC patients (2). With more accurate intensity-modulated
radiation therapy and adjuvant chemotherapy, the 5-year survival of
NPC patients has reached more than 60% (3). Clinically, posttreatment recurrence
and residual mass are still obstacles to successful treatment in a
small population of NPC cases. NPC patients with residue are always
considered to be refractory to salvage irradiation and chemotherapy
and present with higher local or distant metastasis, resulting in a
worse prognosis (4). However, the
underlying mechanisms and malignant behaviors of residual NPC
remains unclear.
Several situations restrict the study of these
patients. In routine clinical practice, recurrence and residual NPC
tissues are difficult to obtain for the reason that surgery is not
the first line therapeutic choice for these NPC patients.
Meanwhile, the residues are usually located in the deeper
para-pharyngeal space covered with fibrotic scar and necrotic
tissue after full course radiotherapy, which also restrict
successful biopsy. Thus, we established post-irradiation residual
NPC cells by exposure to irradiation in our previous study, which,
to some extent, imitate the patients with a residual mass after a
full course of radiotherapy (5,6). Using
these cells, we detected their malignant alteration in regards to
radioresistance, chemoresistance, motility and invasion
capabilities.
Epithelial-mesenchymal transition (EMT) is a process
by which cells change their original epithelial morphology and are
dispersed with the disappearance of inter-cellular connections, and
change into long fibroblast-like cells (7). It is well known that EMT gifts tumor
cells with more powerful metastatic potency (7). Recently, compelling evidence indicates
that EMT transition in tumor cells also contributes to other
malignant behaviors, such as chemoresistance and radioresistance
(8,9). On the other hand, cancer stem cells
(CSCs) are also considered as another crucial mechanism for the
tumor to survive in extreme environments, such as irradiation
(10) or chemotherapeutic agents
(11). These intra-tumoral cells
are generated and are maintained in a small proportion, but have
the ability to self-renew and to differentiate in multi-directions
which include EMT (10). Thus, we
simultaneously focused on cellular morphological changes, detected
EMT and CSC biomarker levels, and aimed to identify the underlying
mechanisms to reverse the occurrence and residues in NPC.
Materials and methods
Cell culture
The poorly differentiated NPC cell lines CNE-2 and
6-10B were provided by the Cell Center of Central South University
(Changsha, China). The radioresistant NPC cells derived from the
CNE-2 and 6-10B cells were established as previously described
(5) and were termed CNE-2-Rs and
6-10B-Rs, respectively. Cells were cultured in RPMI-1640 medium
(Hyclone, Waltham, MA, USA) supplemented with 10% fetal bovine
serum (FBS) and 1% penicillin and streptomycin (all from Gibco, MA,
USA). Cell cultures were maintained in a humidified atmosphere of
5% CO2 at 37°C. Cell morphology was monitored with an
inverted phase-contrast microscope (Leica, Wetzlar, Germany).
Patients and tissue preparation
Three coupled NPC tissues before/post-radiotherapy
were obtained from October 2011 to October 2014 at the Department
of Otolaryngology Head and Neck Surgery, Xiangya Hospital of
Central South University (Changsha, China). None of the patients
had a previous malignancy and did not undergo chemo/radiotherapy.
All specimens were snap-frozen immediately and stored in liquid
nitrogen for further protein extraction. The present study was
approved by the Ethics Committee of Xiangya Hospital of Central
South University. Prior patient consent was obtained from all the
patients.
Cell viability assay
Cell viability was assessed by the CCK-8 kit
(Beyotime, Shanghai, China) as described previously (12). Briefly, the cells were seeded in
96-well plates at 5,000/well and allowed to adhere to the plate
overnight. The cells were then exposed to different concentrations
of paclitaxel or cisplatin (both from Sigma, USA) for another 48 h.
Absorbance values were expressed as percentages relative to the
controls. Resistant index was calculated by IC50
(resistant cells)/IC50 (parental cells) where
IC50 is the half maximal inhibitory concentration. Each
experiment was performed in triplicate.
Wound-healing assay
Wound-healing assay was performed as previously
described (13). The cells were
seeded in 6-well plates at 2×105/well and allowed to
grow to almost confluency. The cells were washed twice with PBS and
incubated in serum-free medium for another 24 h. The cell monolayer
was wounded with a sterile 10-µl pipette tip. Then the cells
were cultured in medium supplemented with 2% FBS for another 48 h.
Cell migration was calculated by measuring the distance covered by
migrating cells and further divided by that of the original wound.
The experiment was carried out in triplicate.
Invasion assay
The invasion assay was also described previously
(13). In brief, chambers coated
with Matrigel (BD Biosciences, Bedford, MA, USA) were incubated at
37°C for 30 min. The cells were seeded in the upper well and
incubated for 24 or 48 h. The cells in the upper well were removed
and the cells that had invaded to the lower side of the filter were
fixed with methanol and stained with crystal violet. The number of
invading cells was quantified by counting in 5 random fields (×200
field). This experiment was performed in triplicate.
Western blotting
Western blot assay was performed as previously
described (12). In brief, the
total protein was extracted and separated by SDS-PAGE. Then the
separated proteins were transferred to PVDF membranes. The
membranes were incubated with the corresponding primary antibodies
followed by the relevant secondary antibody. Primary antibodies
used in the present study were monoclonal rabbit anti-E-cadherin
(1:1,000), anti-vimentin (1:500) (both from Cell Signalling
Technology, Danvers, MA, USA) and monoclonal mouse anti-β-actin
(1:1,000; Beyotime).
Quantitative real-time reverse
transcription-PCR (qRT-PCR)
Briefly, cDNA was synthesized from total RNA using a
PrimeScript RT reagent kit with a DNA Eraser (Takara, Shiga,
Japan). Primers for genes related to cancer stem cells were
designed and synthesized. Then, qPCR assays were performed using a
Bio-Rad IQ5™ Multicolor Real-Time qRT-PCR detection system
(Bio-Rad, Hercules, CA, USA). The mRNA expression levels were
detected as previously described (5) using specific primer sequences
(Table I). The expression levels
were measured in terms of the cycle threshold (Ct) and were then
normalized to GAPDH expression using the 2−ΔΔCt method
(5,14).
 | Table IGene primers. |
Table I
Gene primers.
| Gene | Primer
sequence |
|---|
| Lgr5 | F:
5′-CTCTTCCTCAAACCGTCTGC-3′ |
| R:
5′-GATCGGAGGCTAAGCAACTG-3′ |
| c-myc | F:
5′-GTCAGTATCACGCCCGTTTT-3′ |
| R:
5′-GCTTCCTTTACGCACTTGGT-3′ |
| CD117 | F:
5′-GCACAGCCTTGAGCCTACTC-3′ |
| R:
5′-TACGAATGCATGGGCAGTAA-3′ |
| Bmi1 | F:
5′-CCAGGGCTTTTCAAAAATGA-3′ |
| R:
5′-CCGATCCAATCTGTTCTGGT-3′ |
| ALDH1 | F:
5′-AAGCCAAGTGCTCTATCA-3′ |
| R:
5′-TCAACATCCTCCTTATCTC-3′ |
| CD133 | F:
5′-TTGTGGCAAATCACCAGGTA-3′ |
| R:
5′-TCAGATCTGTGAACGCCTTG-3′ |
| PD-L1 | F:
5′-TATGGTGGTGCCGACTACAA-3′ |
| R:
5′-TGCTTGTCCAGATGACTTCG-3′ |
| CD24 | F:
5′-GCCAGTCTCTTCGTGGTCTC-3′ |
| R:
5′-CCTGTTTTTCCTTGCCACAT-3′ |
| GAPDH | F:
5′-TCCAAAATCAAGTGGGGCGA-3′ |
| R:
5′-AGTAGAGGCAGGGATGATGT-3′ |
Immunofluorescent staining and flow
cytometry assay
The cells were washed with phosphate-buffered saline
(PBS, Hyclone, Waltham, MA, USA) and blocked with 0.05% bovine
serum albumin (Beyotime, Shanghai, China) in PBS. The cells were
diluted to 10^6–10^7/ml and stained with rabbit anti-LGR5-FITC
(Bioss, Beijing, China; cat. no: bs-1117R-FITC, 1:100 dilution) at
4°C for 1 h. After being washed with PBS, the cells were used for a
FACScalibur through a flow cytometer (Becton Dickinson, San Jose,
CA, USA) and analyzed using WinMDI software.
Statistical analysis
The statistical analyses were performed using SPSS
17.0 software. The quantitative data are presented as the mean ±
standard deviation (SD). Statistical comparisons between two groups
were performed using the Student's t-test. In all cases, p<0.05
was considered statistically significant.
Results
Irradiation induces chemoresistance in
NPC cells
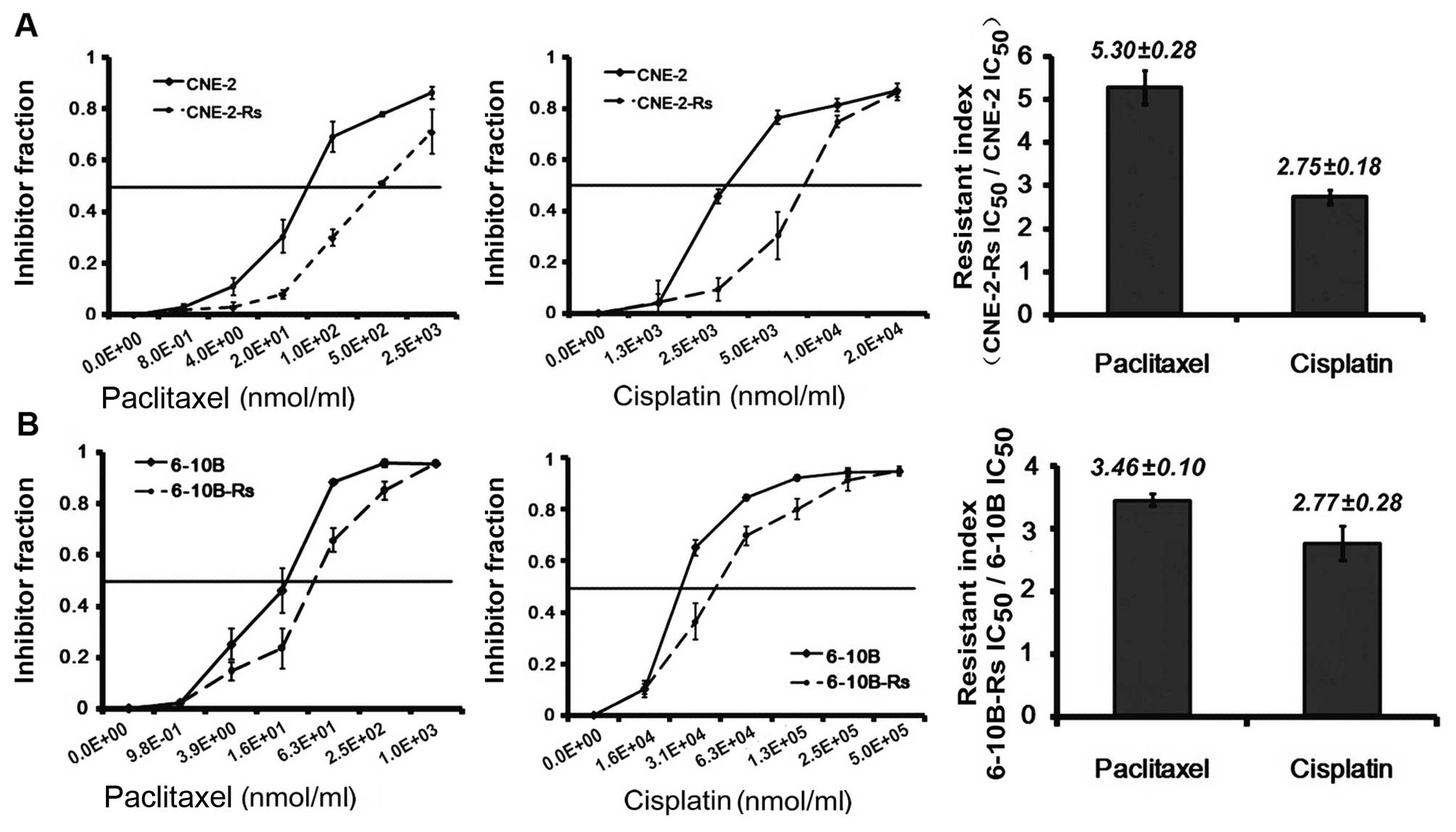
To investigate the potential impact of irradiation
on chemosensitivity, NPC radioresistant cells CNE-2-Rs and
6-10B-Rs, established via exposure to gradually increasing doses of
irradiation in our previous study (5), were used in the following experiments.
We initially applied the CCK-8 assay to evaluate the sensitivity of
radioresistant NPC cells to routine chemoagents including
paclitaxel and cisplatin. Our results revealed that both the
IC50 values of paclitaxel and cisplatin in the
radioresistant NPC CNE-2-Rs were increased 5.28–(38.2±6.09 vs.
7.24±1.29 nmol/ml) and 2.75-fold (8.32±0.83 vs. 3.02±0.34 nmol/ml),
compared with the parental CNE-2 cells (Fig. 1A). Meanwhile, similar data were
obtained in the radioresistant 6-10B-Rs cells, which showed
3.46–(60.12±3.71 vs. 17.38±0.59 nmol/ml) and 2.77-fold (4.68±0.37
vs. 1.67±0.24 nmol/ml) more resistance to paclitaxel and cisplatin
(Fig. 1B). Collectively, these
results indicate that the radioresistant NPC cells acquired
resistance to paclitaxel and cisplatin after surviving from
exposure to irradiation.
Irradiation promotes the migration of the
NPC cells
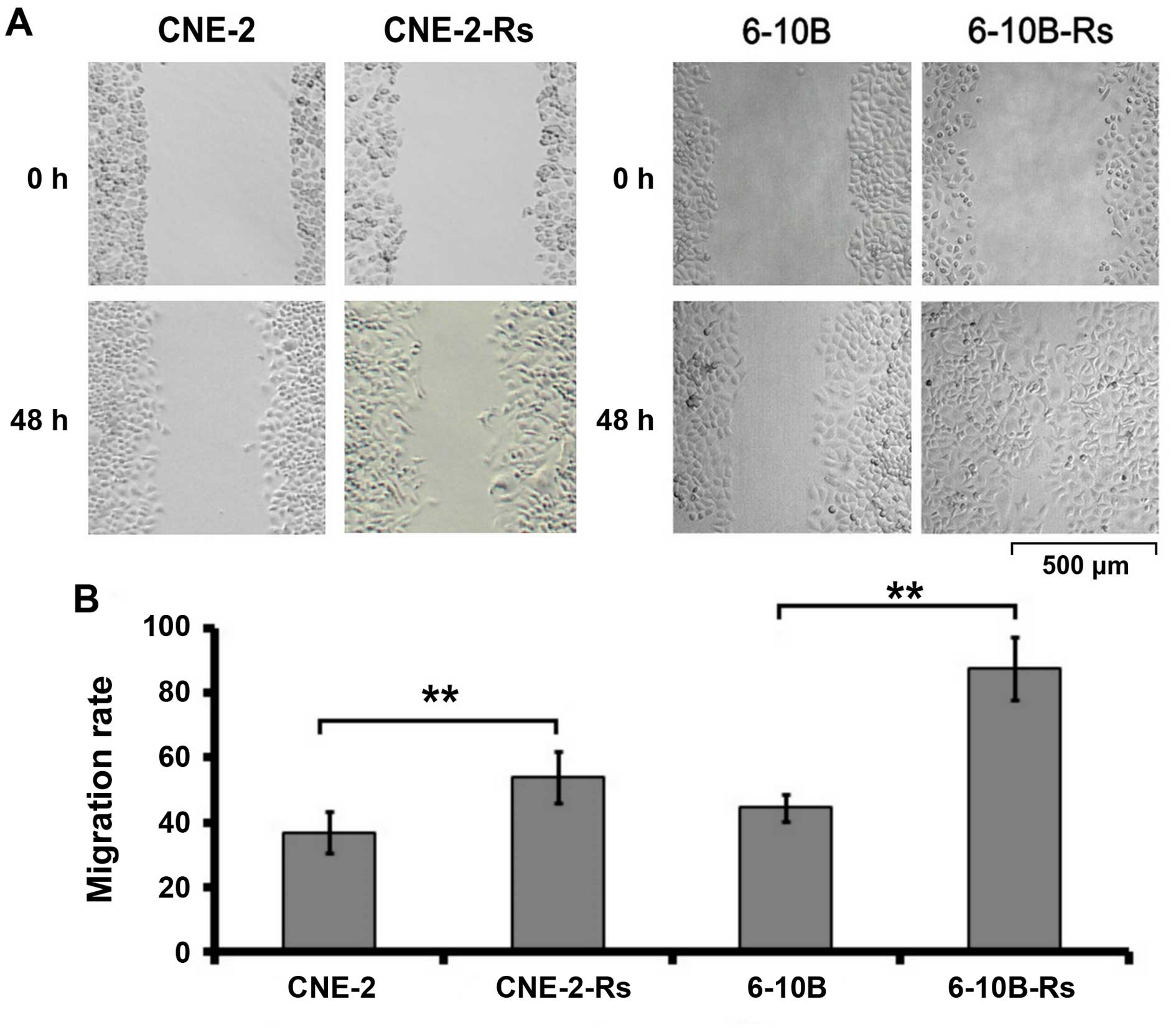
The migratory capacity of the radioresistant NPC
cells was then investigated by wound healing assay. As indicated in
Fig. 2, the migration of NPC cells
was observed and photographed at 0 and 48 h, respectively. Our data
demonstrated that the migration rate was obviously increased in the
radioresistant NPC cells compared to the parental NPC cells
(CNE-2-Rs cells: 58.04±7.22% vs. CNE-2 cells: 38.23±6.15%,
p<0.05; 6-10B-Rs cells: 87.09±10.72% vs. 6-10B cells
44.71±3.98%, p<0.05). These results indicate that irradiation
can promote the migration of NPC cells in vitro.
Irradiation enhances the invasion of the
NPC cells
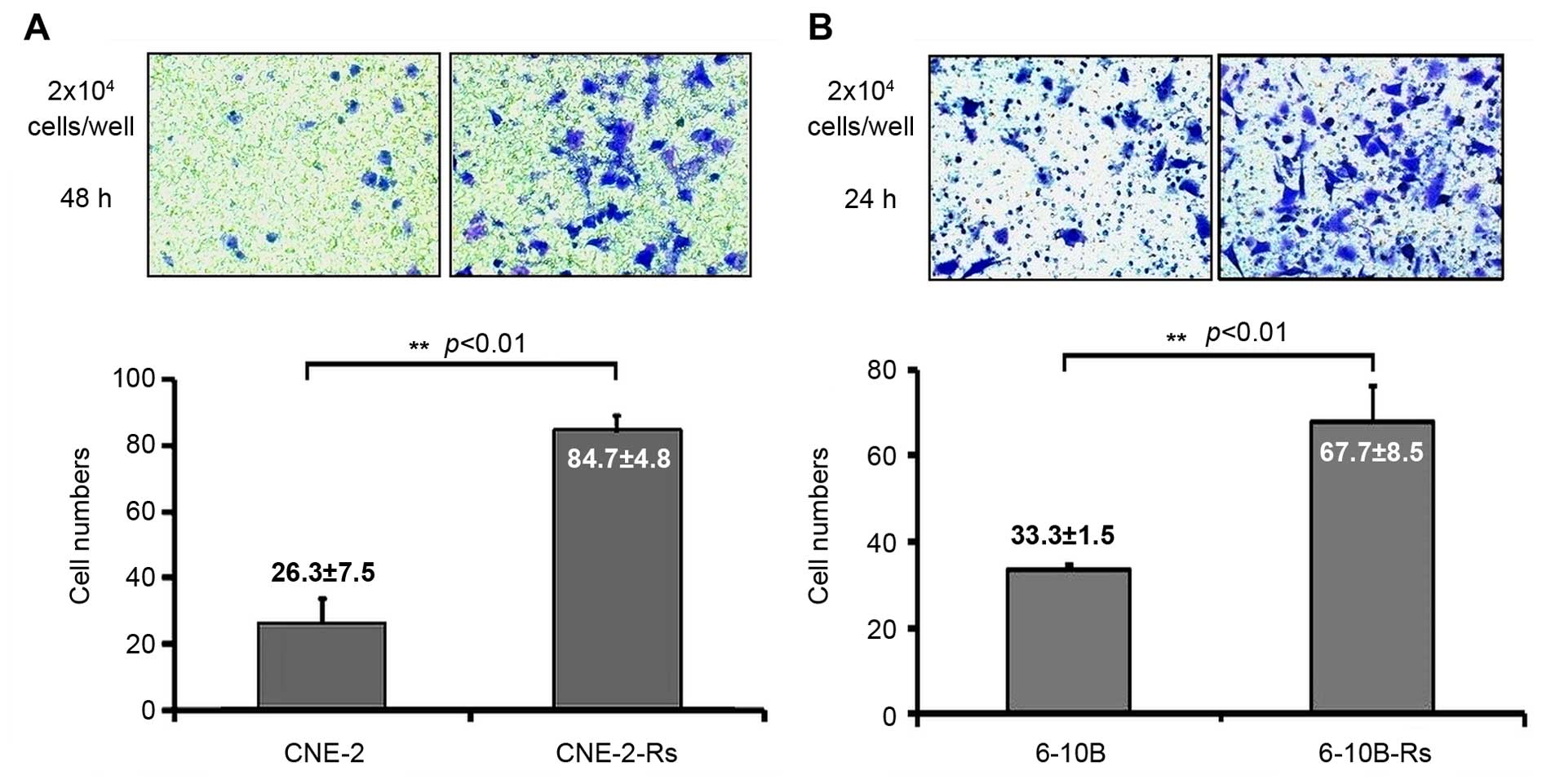
The invasive capacity of the radioresistant NPC
cells was also examined by Transwell assay. As indicated in
Fig. 3A, the numbers of invading
CNE-2 and CNE-2-Rs cells on the bottom sides of the Matrigel-coated
membrane after 48 h were 26.3±7.5 and 84.7±4.8 (p<0.01),
respectively. Similarly, the numbers of invading 6-10B and 6-10B-Rs
cells after 24 h were 33.3±1.5 and 67.7±8.5 (p<0.01),
respectively (Fig. 3B). Taken
together, the above results suggest that irradiation can enhance
the metastatic phenotype including migration and invasion in the
NPC cells.
Irradiation leads to EMT change in the
NPC cells
Our above results revealed that irradiation induces
chemoresistance to paclitaxel and cisplatin, and promotes migration
and invasion of NPC cells in vitro. However, the underlying
mechanisms that promote radioresistance, chemoresistance and
metastasis in NPC remain to be clarified. In the phase of
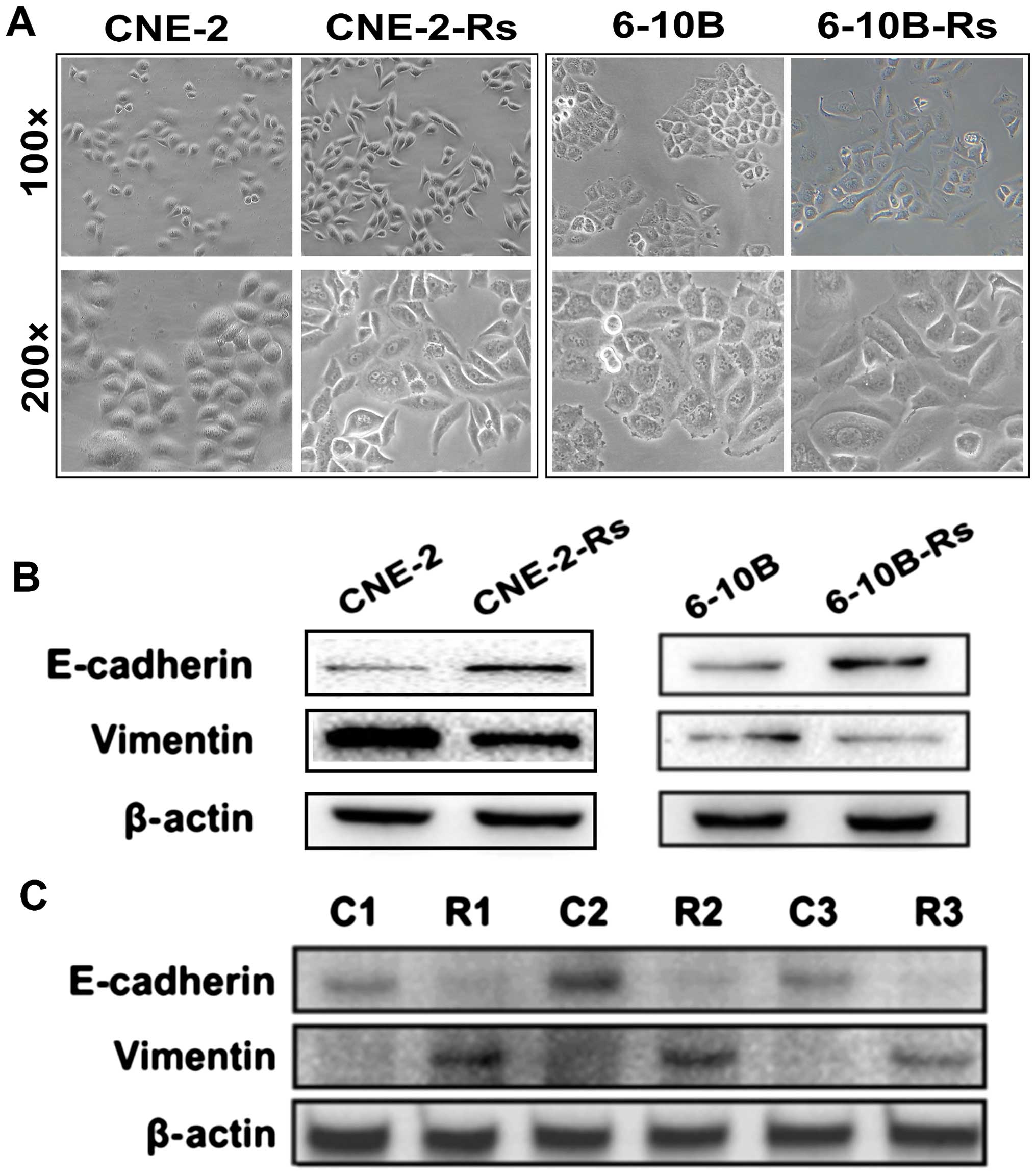
establishing radioresistant NPC cells, we observed significant
morphological changes in the radioresistant NPC cells. Under
phase-contrast microscopy, radioresistant CNE-2-Rs and 6-10B-Rs
cells were found to become elongated with pseudopodia formation, a
decrease in cell-to-cell contact, similar to the shape of
fibroblasts (Fig. 4A). These
morphological changes were consistent with canonical EMT formation,
which has been reported to tightly correlate with radioresistance,
chemoresistance and metastasis (15). Therefore, the molecules associated
with EMT were further examined. Our data clearly indicated that
CNE-2-Rs and 6-10B-Rs cells displayed higher expression of
mesenchymal marker vimentin and lower expression of epithelial
protein E-cadherin (Fig. 4B). These
findings were further validated in clinical NPC samples that
experienced irradiation. Three residual NPC specimens
post-radiotherapy were obtained. Western blot analysis detected
decreased expression of E-cadherin and elevated expression of
vimentin in the NPC residues, compared with the NPC samples before
radiotherapy (Fig. 4C). These
results suggest that irradiation promotes the emergence of EMT,
which may be involved in the observed chemoresistance and
metastasis in NPC.
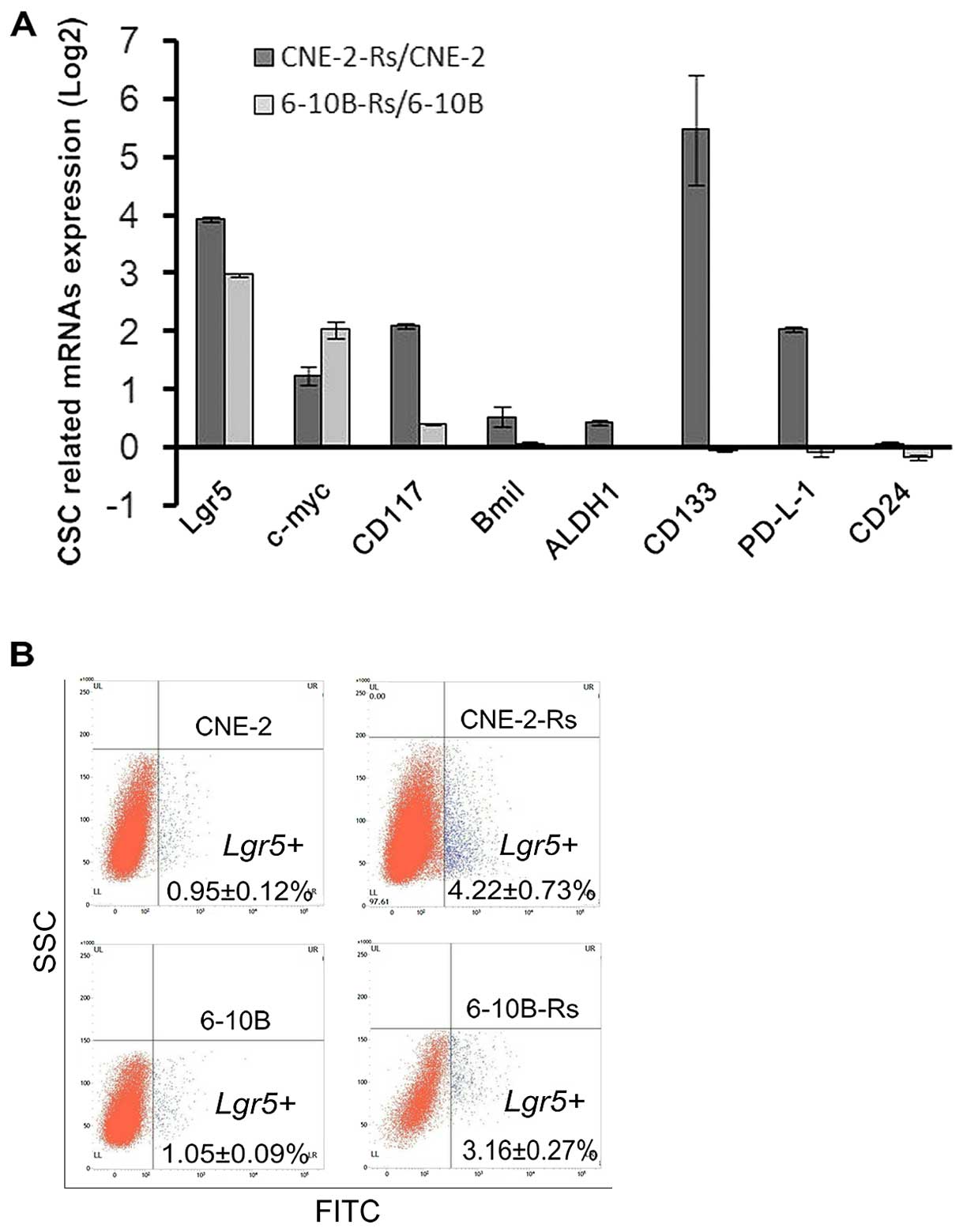
Irradiation increases molecules
associated with cancer stem cells (CSCs)
CSCs persist in tumors as a distinct population and
cause cancer progression and relapse by giving rise to new tumors
(16). CSCs are involved in diverse
cancer malignant behaviors including metastasis, radioresistance
and chemoresistance (16).
Therefore, we hypothesized that irradiation may also increase the
number of CSCs and promote chemoresistance and metastasis. To test
this hypothesis, qRT-PCR assays were used to evaluate molecular
markers associated with CSCs. Our results showed that Lgr5 and
c-myc had consistent changes (fold-change >2) in the CNE-2-Rs
and 6-10B-Rs cells, when compared with the parental cells (Fig. 5A). Then, Lgr5+ proportions of the
cells were detected by immunofluorescent staining and flow
cytometry. The Lgr5+ proportion was 4.22±0.73% and 0.95±0.12%
(p<0.05) in the CNE-2-Rs and CNE-2 cells, respectively, and the
Lgr5+ proportion was 3.16±0.27% and 1.05±0.09% (p<0.05) in the
6-10B-Rs and 6-10B cells, respectively (Fig. 5B). The data suggest that irradiation
may induce chemoresistance and metastasis via increasing CSCs,
which is indicated by increased expression of specific CSC
markers.
Discussion
Clinically, the difficulty in obtaining specimens is
still an obstacle for the study of NPC post-irradiation residue and
recurrence. In order to better understand the underlying mechanism,
the establishment of post-irradiation residual NPC cells is the
main choice for many laboratories and research teams. Three methods
have been widely accepted to obtain post-irradiation NPC cell
lines, including low-dose repeated irradiation (17), sublethal dose irradiation (18) and gradient increasing irradiation
(6). Compared with the first two
patterns, accumulating doses in a gradient irradiation pattern of
up to 60–70 Gy most closely resembles the clinical doses for NPC
patients. Therefore, our group chose a gradient increasing
irradiation pattern and successfully established two
post-irradiation residual NPC cell lines (5,6).
In some cases, post-irradiation residual cells were
found to be also accompanied with advanced radioresistance, and
were considered to be radioresistant cells (6,17).
Similarly, two residual NPC cell lines screened in our previous
research were named radioresistant CNE-2-Rs and 6-10B-Rs cells,
respectively (6). Previous research
has only focused on the mechanism of radioresistance rather than
other malignant behaviors. Our results showed chemoresistance as
well as aggressive motility and invasion capability in residual NPC
cells. These findings, to some extent, may be the answer for
treatment failure and the poorer prognosis of NPC patients with
post-irradiation residue and recurrence.
Notably, following the total irradiation dose of the
gradually accumulation, NPC cells transformed from epithelial cells
into a mesenchymal phenotype. Concurrently, a loss of epithelial
marker E-cadherin and an increase in the mesenchymal molecule
vimentin were also observed in the post-irradiation cells. Thus, we
considered that the NPC cells went through an EMT process, which
partially explains the higher invasion and metastasis observed in
the post-irradiation cells. In addition, in three pairs of patient
tissues from pre- and post-irradiation, EMT markers exhibited the
same alterations as in the residual cells, further supporting that
irradiation may induce EMT in NPC. Several reports suggested the
potential connections between the EMT phenotype and
post-irradiation residues, in malignancies of the stomach (19), prostate (20) and cervical cancer (21). The mechanisms involved included
activation of the WNT/β-catenin (22), PI3K/Akt/mTOR (23), and NF-κB pathways (24). Inspiringly, our group firstly found
this connection in NPC cells, and showed that cells with an EMT
phenotype not only had a greater radioresistance, but was also
accompanied by more aggressive invasion and migration abilities.
Our previous studies discovered that the WNT signaling pathway was
increasingly activated in radioresistance NPC cells (6,12).
Reversely, following WNT2B knockout, the NPC radioresistance and
their invasion and migration capacities weakened (data not shown),
along with corresponding changes in EMT markers (12). All the above findings suggest that
WNT-mediated EMT may be a potential mechanism in NPC malignancy and
warrants further investigation.
Chemotherapy is an indispensable method for the
treatment of NPC, and our research firstly investigated the
chemosensitivity of post-irradiation residual NPC cells. As
first-line chemotherapeutics used in NPC treatment, paclitaxel and
cisplatin were used in our study and multidrug resistance to both
chemotherapeutic agents was observed in the radioresistant cells.
Irradiation-induced multidrug resistance or an increase in related
genes were also reported in other tumors, such as breast (25), oral (26) and colon (27). Even a low irradiation dose also led
to chemoresistance (28,29), suggesting that insufficient
irradiation dose and residues may cause present or subsequent
chemotherapy failure. In recent years, the role of EMT in drug
resistance has attracted attention. In NPC, EMT is necessary for
acquired resistance to cisplatin (30). The altered expression of FOXC2 and
P53 could also affect NPC chemoresistance via regulation of EMT
(31,32).
The cancer stem cell (CSC) is considered as another
crucial reason for advanced tumor malignancy. Via self-renewal and
multi-direction differentiation, CSCs can survive in extreme
situations such as exposure to irradiation or chemo-agents. The
related mechanisms attributed to the CSC-mediated survival include
high DNA damage repair capacity, cell cycle regulation and enhanced
reactive oxygen species (ROS) defenses (8,11). In
our study, CSC-related genes Lgr5 and c-Myc were significantly
upregulated in both radioresistance cell lines. In addition, higher
proportions of Lgr5+ cells were observed in radioresistant cells.
Lgr5 is a classic CSC biomarker and is associated with WNT pathway
activation in many malignancies (33). c-Myc is reported as a key
transcription factor for CSC phenotype maintenance (34,35).
Furthermore, during the therapy process, CSC trait transition and
self-renewal could also elicit tumor adaptive responses by
irradiation and the drug agents themselves (36).
Our present results indicate that co-existence of
EMT and CSCs may be the common mechanisms for NPC radioresistance,
chemotherapy tolerance, invasion and metastasis. In fact, the EMT
phenotype may be the result of CSC multidirectional differentiation
and may be a bridge to connect CSCs and treatment resistance
(37). Thus, reversing the
progression of EMT and CSCs may become a method to restrict
multiple malignant bio-behaviors of NPC cells, and become an
effective strategy to improve the prognosis and life quality of NPC
patients with a residual and recurrent mass. These findings warrant
further study.
Acknowledgments
Grants were provided by the National Natural Science
Foundation of China (nos. 81372426, 81202128 and 81172558), the
National Natural Science Foundation of Hunan Province (nos.
2015JJ3137 and 14JJ2018), and the Research Fund for the Doctoral
Program of Higher Education of China (no. 20120162120049).
References
|
1
|
Yu MC and Yuan JM: Epidemiology of
nasopharyngeal carcinoma. Semin. Cancer Biol. 12:421–429. 2002.
View Article : Google Scholar
|
|
2
|
Caponigro F, Longo F, Ionna F and Perri F:
Treatment approaches to nasopharyngeal carcinoma: A review.
Anticancer Drugs. 21:471–477. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang WY, Lin CL, Lin CY, Jen YM, Lo CH,
Sung FC and Kao CH: Survival outcome of patients with
nasopharyngeal carcinoma: A nationwide analysis of 13 407 patients
in Taiwan. Clin Otolaryngol. 40:327–334. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li JX, Huang SM, Jiang XH, Ouyang B, Han
F, Liu S, Wen BX and Lu TX: Local failure patterns for patients
with nasopharyngeal carcinoma after intensity-modulated
radiotherapy. Radiat Oncol. 9:872014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li G, Qiu Y, Su Z, Ren S, Liu C, Tian Y
and Liu Y: Genome-wide analyses of radioresistance-associated miRNA
expression profile in nasopharyngeal carcinoma using next
generation deep sequencing. PLoS One. 8:e844862013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li G, Liu Y, Su Z, Ren S, Zhu G, Tian Y
and Qiu Y: MicroRNA-324-3p regulates nasopharyngeal carcinoma
radioresistance by directly targeting WNT2B. Eur J Cancer.
49:2596–2607. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: At the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marie-Egyptienne DT, Lohse I and Hill RP:
Cancer stem cells, the epithelial to mesenchymal transition (EMT)
and radioresistance: Potential role of hypoxia. Cancer Lett.
341:63–72. 2013. View Article : Google Scholar
|
|
9
|
Kajiyama H, Shibata K, Terauchi M,
Yamashita M, Ino K, Nawa A and Kikkawa F: Chemoresistance to
paclitaxel induces epithelial-mesenchymal transition and enhances
metastatic potential for epithelial ovarian carcinoma cells. Int J
Oncol. 31:277–283. 2007.PubMed/NCBI
|
|
10
|
Rycaj K and Tang DG: Cancer stem cells and
radioresistance. Int J Radiat Biol. 90:615–621. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Di C and Zhao Y: Multiple drug resistance
due to resistance to stem cells and stem cell treatment progress in
cancer (review). Exp Ther Med. 9:289–293. 2015.PubMed/NCBI
|
|
12
|
Li G, Wang Y, Liu Y, Su Z, Liu C, Ren S,
Deng T, Huang D, Tian Y and Qiu Y: miR-185-3p regulates
nasopharyngeal carcinoma radioresistance by targeting WNT2B in
vitro. Cancer Sci. 105:1560–1568. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu C, Liu Y, Tan H, Li G, Su Z, Ren S, Zhu
G, Tian Y, Qiu Y and Zhang X: Metadherin regulates metastasis of
squamous cell carcinoma of the head and neck via AKT signalling
pathway-mediated epithelial-mesenchymal transition. Cancer Lett.
343:258–267. 2014. View Article : Google Scholar
|
|
14
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kaufhold S and Bonavida B: Central role of
Snail1 in the regulation of EMT and resistance in cancer: A target
for therapeutic intervention. J Exp Clin Cancer Res. 33:622014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Spillane JB and Henderson MA: Cancer stem
cells: A review. ANZ J Surg. 77:464–468. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li WF, Zhang L, Li HY, Zheng SS and Zhao
L: WISP-1 contributes to fractionated irradiation-induced
radioresistance in esophageal carcinoma cell lines and mice. PLoS
One. 9:e947512014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Feng XP, Yi H, Li MY, Li XH, Yi B, Zhang
PF, Li C, Peng F, Tang CE, Li JL, et al: Identification of
biomarkers for predicting nasopharyngeal carcinoma response to
radiotherapy by proteomics. Cancer Res. 70:3450–3462. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang X, Zheng L, Sun Y, Wang T and Wang
B: Tangeretin enhances radiosensitivity and inhibits the
radiation-induced epithelial-mesenchymal transition of gastric
cancer cells. Oncol Rep. 34:302–310. 2015.PubMed/NCBI
|
|
20
|
Ni J, Cozzi PJ, Hao JL, Beretov J, Chang
L, Duan W, Shigdar S, Delprado WJ, Graham PH, Bucci J, et al: CD44
variant 6 is associated with prostate cancer metastasis and
chemo-/radioresistance. Prostate. 74:602–617. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
de Jong MC, Ten Hoeve JJ, Grénman R,
Wessels LF, Kerkhoven R, Te Riele H, van den Brekel MW, Verheij M
and Begg AC: Pretreatment microRNA expression impacting on
epithelial-to-mesenchymal transition predicts intrinsic
radio-sensitivity in head and neck cancer cell lines and patients.
Clin Cancer Res. 21:5630–5638. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cojoc M, Peitzsch C, Kurth I, Trautmann F,
Kunz-Schughart LA, Telegeev GD, Stakhovsky EA, Walker JR, Simin K,
Lyle S, et al: Aldehyde dehydrogenase is regulated by β-catenin/TCF
and promotes radioresistance in prostate cancer progenitor cells.
Cancer Res. 75:1482–1494. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chang L, Graham PH, Hao J, Ni J, Bucci J,
Cozzi PJ, Kearsley JH and Li Y: Acquisition of
epithelial-mesenchymal transition and cancer stem cell phenotypes
is associated with activation of the PI3K/Akt/mTOR pathway in
prostate cancer radioresistance. Cell Death Dis. 4:e8752013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yan S, Wang Y, Yang Q, Li X, Kong X, Zhang
N, Yuan C, Yang N and Kong B: Low-dose radiation-induced
epithelial-mesenchymal transition through NF-κB in cervical cancer
cells. Int J Oncol. 42:1801–1806. 2013.PubMed/NCBI
|
|
25
|
Bottke D, Koychev D, Busse A, Heufelder K,
Wiegel T, Thiel E, Hinkelbein W and Keilholz U: Fractionated
irradiation can induce functionally relevant multidrug resistance
gene and protein expression in human tumor cell lines. Radiat Res.
170:41–48. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ng IO, Lam KY, Ng M, Kwong DL and Sham JS:
Expression of P-glycoprotein, a multidrug-resistance gene product,
is induced by radiotherapy in patients with oral squamous cell
carcinoma. Cancer. 83:851–857. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bartkowiak D, Stempfhuber M, Wiegel T and
Bottke D: Radiation- and chemoinduced multidrug resistance in colon
carcinoma cells. Strahlenther Onkol. 185:815–820. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Eichholtz-Wirth H and Hietel B: Cisplatin
resistance in mouse fibrosarcoma cells after low-dose irradiation
in vitro and in vivo. Br J Cancer. 70:579–584. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hill BT, Moran E, Etiévant C, Perrin D,
Masterson A, Larkin A and Whelan RD: Low-dose twice-daily
fractionated X-irradiation of ovarian tumor cells in vitro
generates drug-resistant cells overexpressing two multidrug
resistance-associated proteins, P-glycoprotein and MRP1. Anticancer
Drugs. 11:193–200. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang P, Liu H, Xia F, Zhang QW, Zhang YY,
Zhao Q, Chao ZH, Jiang ZW and Jiang CC: Epithelial-mesenchymal
transition is necessary for acquired resistance to cisplatin and
increases the metastatic potential of nasopharyngeal carcinoma
cells. Int J Mol Med. 33:151–159. 2014.
|
|
31
|
Zhou Z, Zhang L, Xie B, Wang X, Yang X,
Ding N, Zhang J, Liu Q, Tan G, Feng D, et al: FOXC2 promotes
chemoresistance in nasopharyngeal carcinomas via induction of
epithelial mesenchymal transition. Cancer Lett. 363:137–145. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shen YA, Lin CH, Chi WH, Wang CY, Hsieh
YT, Wei YH and Chen YJ: Resveratrol impedes the stemness,
epithelial-mesenchymal transition, and metabolic reprogramming of
cancer stem cells in nasopharyngeal carcinoma through p53
activation. Evid Based Complement Alternat Med. 2013:5903932013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang L, Tang H, Kong Y, Xie X, Chen J,
Song C, Liu X, Ye F, Li N, Wang N, et al: LGR5 promotes breast
cancer progression and maintains stem-like cells through activation
of Wnt/β-catenin signaling. Stem Cells. 33:2913–2924. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Civenni G, Malek A, Albino D,
Garcia-Escudero R, Napoli S, Di Marco S, Pinton S, Sarti M, Carbone
GM and Catapano CV: RNAi-mediated silencing of Myc transcription
inhibits stem-like cell maintenance and tumorigenicity in prostate
cancer. Cancer Res. 73:6816–6827. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Moumen M, Chiche A, Decraene C, Petit V,
Gandarillas A, Deugnier MA, Glukhova MA and Faraldo MM: Myc is
required for β-catenin-mediated mammary stem cell amplification and
tumorigenesis. Mol Cancer. 12:1322013. View Article : Google Scholar
|
|
36
|
Jachetti E, Mazzoleni S, Grioni M,
Ricupito A, Brambillasca C, Generoso L, Calcinotto A, Freschi M,
Mondino A, Galli R, et al: Prostate cancer stem cells are targets
of both innate and adaptive immunity and elicit tumor-specific
immune responses. OncoImmunology. 2:e245202013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dave B, Mittal V, Tan NM and Chang JC:
Epithelial-mesenchymal transition, cancer stem cells and treatment
resistance. Breast Cancer Res. 14:2022012. View Article : Google Scholar : PubMed/NCBI
|