Introduction
Gliomas are the most common primary central nervous
system tumors and account for more than 46% of all brain tumors
(1). Despite comprehensive
treatment, the prognosis of patients with glioma remains poor
(2). The prognosis of glioma
patients is associated with various clinical and biological
factors, such as disease stage, patient age and genetic and
epigenetic molecular features of the tumor (3). In general, changes in the expression
of oncogenes and tumor-suppressor genes are associated with glioma
initiation and progression. However, the involved genes are
ambiguous.
Epigenetic regulation plays a key role in the
dysregulation of gene expression in tumors. Epigenetic regulation
is defined as a stably heritable phenotype resulting from changes
in a chromosome without alterations in the DNA sequence (4), including DNA methylation and histone
modification. Aberrant promoter methylation of CpG
island-associated genes is the most common epigenetic alteration
associated with the inactivation of tumor suppressor and other
genes in human cancers (5–7).
Maspin, identified in 1994, is a member of the
serpin superfamily of serine protease inhibitors (8). Multiple studies have shown that maspin
plays a tumor-suppressor role in various cancers, including breast
cancer (9), prostate cancer
(10) and other cancers (11). Although the maspin gene is
frequently silenced in breast cancer cells (9), maspin deletions and mutations have not
been reported (8,12). Maspin can be silenced by an
epigenetic mechanism that involves aberrant methylation in breast
cancer cells (13). Findings have
suggested that DNA methylation is probably involved in regulating
the expression of maspin in cancers (14,15).
Although maspin is expressed in normal brain tissue (16), the expression level of maspin in
glioma has been rarely reported, and the role of the maspin gene in
glioma is unknown.
In the present study, the expression level and
promoter methylation status of maspin in glioma were investigated.
We also explored the effect of maspin on the proliferation and
migration of glioma cells.
Materials and methods
Human tissue samples
All human normal brain and glioma tissues were
collected from patients treated at the Department of Neurosurgery,
The Second Affiliated Hospital of Soochow University. Normal brain
tissues were obtained from patients with cerebral trauma. Glioma
tissues were obtained and verified following diagnosis of the
clinical and pathological grade. Prior consent was obtained from
all patients, and the study was approved by the institutional
research boards of the affiliated institutions.
Cell culture
The human glioma cell lines U87, U251, and U343, and
the human prostate cancer cell line PC3 were purchased from the
Cell Bank of the Chinese Academy of Science. PC3 cells were used as
the opposite control for maspin expression and methylation status
studies. All cells were maintained in Dulbecco's modified Eagle's
medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and
antibiotics (100 U/ml penicillin and 100 U/ml streptomycin; Gibco,
Grand Island, NY, USA). The cells were grown in a 37°C incubator
with 5% CO2.
RNA extraction and reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA from tissues and cell lines was extracted
using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), and the
isolated RNA was reverse transcribed into complementary DNA using
the RevertAid™ First Strand cDNA Synthesis kit (Fermentas, Vilnius,
Lithuania) following the manufacturer's instructions. The primers
for the PCR were as follows: maspin forward,
5′-GCTTTTGCCGTTGATCTGTTC-3′ and reverse, 5′-GATCTGACCTTT
CGTTTTCTTC-3′; and GAPDH forward, 5′-GGAAGGTGAAGGTCGGAGTC-3′
and reverse, 5′-GAGGCATTGCTGATGATCTTGA-3′. The PCR conditions were
as follows: an initial denaturation at 94°C for 5 min followed by
33 cycles of 94°C for 30 sec, 52°C for 30 sec, and 72°C for 30 sec,
and a final extension at 72°C for 7 min. The RT-PCR products were
analyzed using Quantity One software (Bio-Rad, Hercules, CA, USA),
and the images were collected and saved. The housekeeping gene
GAPDH was used as the internal control.
Western blot analysis
Tissues and cells were lysed in lysis buffer, and
whole proteins were extracted by incubation with the western blot
assay buffer (Beyotime, Nantong, China). Protein concentration was
measured using a BCA protein assay kit (Beyotime, Nantong, China).
A total of 50 µg of each protein sample was subjected to 10%
SDS-PAGE, and the proteins were transferred to nitrocellulose
membranes. The membranes were blocked with 5% skim milk in TBST for
1 h at room temperature, and then incubated with a 1:1,200 dilution
of anti-maspin monoclonal antibody (BD Pharmingen, San Diego, CA,
USA) overnight at 4°C. The HRP-conjugated secondary antibody was
incubated for 1 h at 37°C. Specific bands were visualized using the
enhanced chemiluminescence detection kit for HRP (Biological
Industries, Kibbutz Beit Haemek, Israel) and Kodak X-OMAT LS film
(Eastman Kodak, Rochester, NY, USA). Quantitative data were
obtained using Quantity One software.
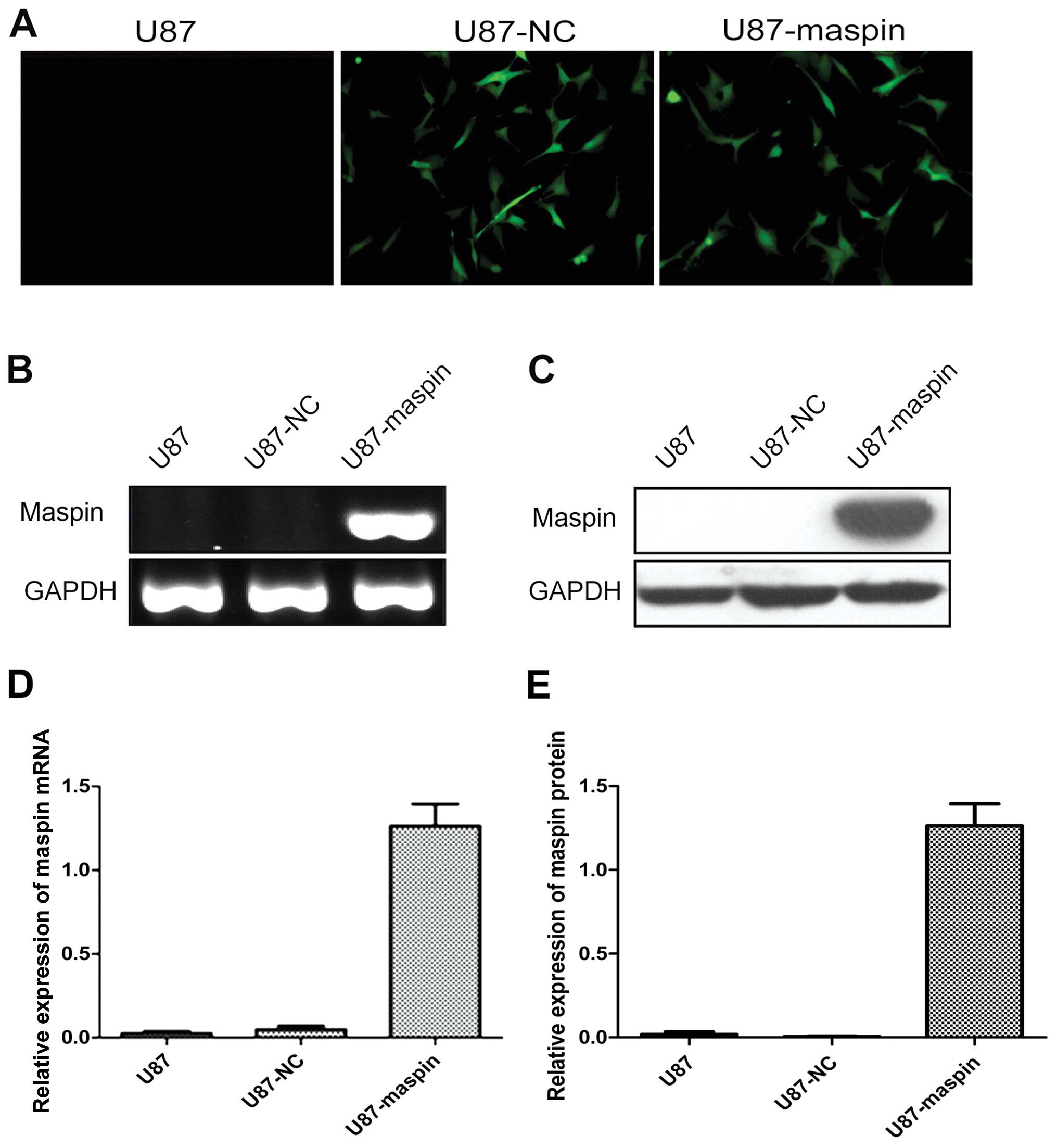
Lentivirus infection and confirmation of
maspin expression
Lentiviral vectors expressing maspin (LV-maspin) and
a non-silenced control lentivirus (LV-NC) were designed and
constructed by GenePharma (Shanghai, China). Cell infection was
conducted according to the recommendations of GenePharma.
U87-LV-maspin (U87-maspin) cells represent U87 cells infected with
LV-maspin, and the negative control U87-LV-NC (U87-NC) cells
represent U87 cells infected with LV-NC. GFP expression was
observed under a fluorescence microscope after infection. For
further validation, the expression of maspin was confirmed by
RT-PCR and western blotting.
Migration and invasion assay
For the migration assay, U87, U87-NC and U87-maspin
cells were suspended in 120 µl of serum-free medium. A total
of 2×104 cells/well were seeded into the upper chambers
of the 24-well Transwell inserts with 8-µm pores (Corning,
Corning, NY, USA). Next, 600 µl of DMEM containing 10% FBS
was added to the lower chambers. After 48 h, the cells on the upper
surface were removed, and the cells on the lower surface were fixed
using a crystal violet cell colony staining kit (Genemed, Shanghai,
China). The stained cells were counted in five randomly selected
fields per filter under a microscope (×400 magnification). For the
invasion assay, the Transwell inserts were coated with Matrigel (40
µl/well; BD Biosciences, San Jose, CA, USA). The following
procedures were the same as those for the migration assay.
Proliferation assay
The number of viable cells was assayed using the
Cell Counting Kit-8 (CCK-8; Dojindo Laboratories, Kumamoto, Japan)
as per the manufacturer's protocol. U87, U87-NC and U87-maspin
cells were seeded onto the 96-well plates at a density of
2×103 cells/well. After 24 h, 10 µl of CCK-8
solution was added to each well. After 2 h, the optical density
(OD) at 450 nm was measured using a microplate reader
(Bio-Rad).
BrdU incorporation assay
Cell proliferation was also determined using
5-ethynyl-2-deoxyuridine (EdU) using the Cell-Light™ EdU
Apollo® 567 In Vitro Imaging kit (RiboBio, Guangzhou,
China) according to the manufacturer's protocol. Briefly, cells
seeded in 96-well culture plates were incubated with 50 µM
EdU for 3 h before fixation, permeabilization, and Apollo staining.
Cell nuclei were stained with Hoechst 33342 at a concentration of 5
µg/ml for 30 min. The cells were observed using a
fluorescence microscope (Olympus, Tokyo, Japan).
5-Aza-2′-deoxycytidine treatment
U87 and U251 cells were treated with various
concentrations of 5-Aza-2′-deoxycytidine (5-Aza-dC; Sigma-Aldrich,
St. Louis, MO, USA). U87 cells were treated with 12 µM
5-Aza-dC for 96 h. U251 cells were treated with 15 µM
5-Aza-dC for 96 h. After treatment with 5-Aza-dC, the maspin mRNA
expression level was investigated in the U87 and U251 cell
lines.
DNA extraction and bisulphite
modification
Genomic DNA from cells and tissues was isolated
using the Genomic DNA purification kit (Thermo, Waltham, MA, USA).
Bisulphite modification was performed using the EZ DNA
Methylation-Gold™ kit (D5005; Zymo Research, Orange, CA, USA)
following the manufacturer's instructions.
Methylation-specific PCR (MSP)
The primers for maspin promoter methylation analysis
by PCR were designed by MethPrimer (http://www.urogene.org/methprimer/). The primers were
as follows: methylated maspin (Mmaspin) forward,
5′-TTTTATCGAATATTTTATTTTTCGG-3′ and reverse,
5′-GATAACTCACCTAAACAACACCG-3′; and unmethylated Maspin (Umaspin)
forward, 5′-TTTTATTTTATTGAATATTTTATTTTTTGG-3′ and reverse,
5′-CAATAACTCACCTAAACAACACCAC-3′. The DNA for this PCR was treated
as previously described. The PCR reaction conditions were as
follows: 94°C for 5 min; 40 cycles of 94°C for 30 sec, 57°C for 30
sec, 72°C for 30 sec; and a 72°C - extension for 7 min. MSP
products were analyzed by Quantity One software.
Bisulphite genomic sequencing
DNA CpG islands of the maspin promoter were analyzed
by EMBOSS Cpgplot (http://www.ebi.ac.uk/). The parameters for CpG island
searching were set at Mayor >100 bp, CpG/expected CpG >0.6,
GC% >50% for the EMBOSS Cpgplot. The primers for this PCR were
designed from the predicted sequence of the maspin promoter CpG
island using MethPrimer as previously described. The primers were
5′-GAGAAATTTGTAGTGTTATTATTATTATAT-3′ (forward) and
5′-ATAACTCACCTAAACAACACC-3′ (reverse), amplifying a 321-bp product
including 16 expected CpG sites. The PCR conditions were as
follows: 94°C for 5 min; 40 cycles of 94°C for 30 sec, 57°C for 30
sec, 72°C for 30 sec; and a 72°C extension for 7 min.
Bisulphite-sequencing PCR products were analyzed using Quantity One
software. The PCR products were purified directly using the TIANgel
Midi Purification kit (Tiangen Biotech, Beijing, China). The
purified products were sequenced by Suzhou Genewiz, Inc. (Suzhou,
China).
Statistical analysis
Data are presented as the mean ± standard error of
the mean (mean ± SEM). Statistical analyses were conducted using
Statistical Package for the Social Sciences (SPSS) software version
19.0 (Chicago, IL, USA). The differences between groups were
analyzed using the Student's t-test or one-way analysis of variance
(ANOVA). Differences were considered statistically significant at a
P-value <0.05.
Results
Expression of maspin is silenced in
glioma
We first investigated the expression of maspin in
glioma cells and tissues. The mRNA and protein levels of maspin in
the glioma cell lines and tissues were explored by RT-PCR and
western blotting, respectively. PC3 cells were used as the positive
control for maspin expression. As shown in Fig. 1A and C, maspin transcripts were
present in the PC3 cells and normal brain tissues, but not in the
glioma cell lines and tissues. Consistent with this result, maspin
protein was not detected in the above glioma cell lines and tissues
(Fig. 1B and D). These results
indicated that the expression of maspin was silenced in glioma.
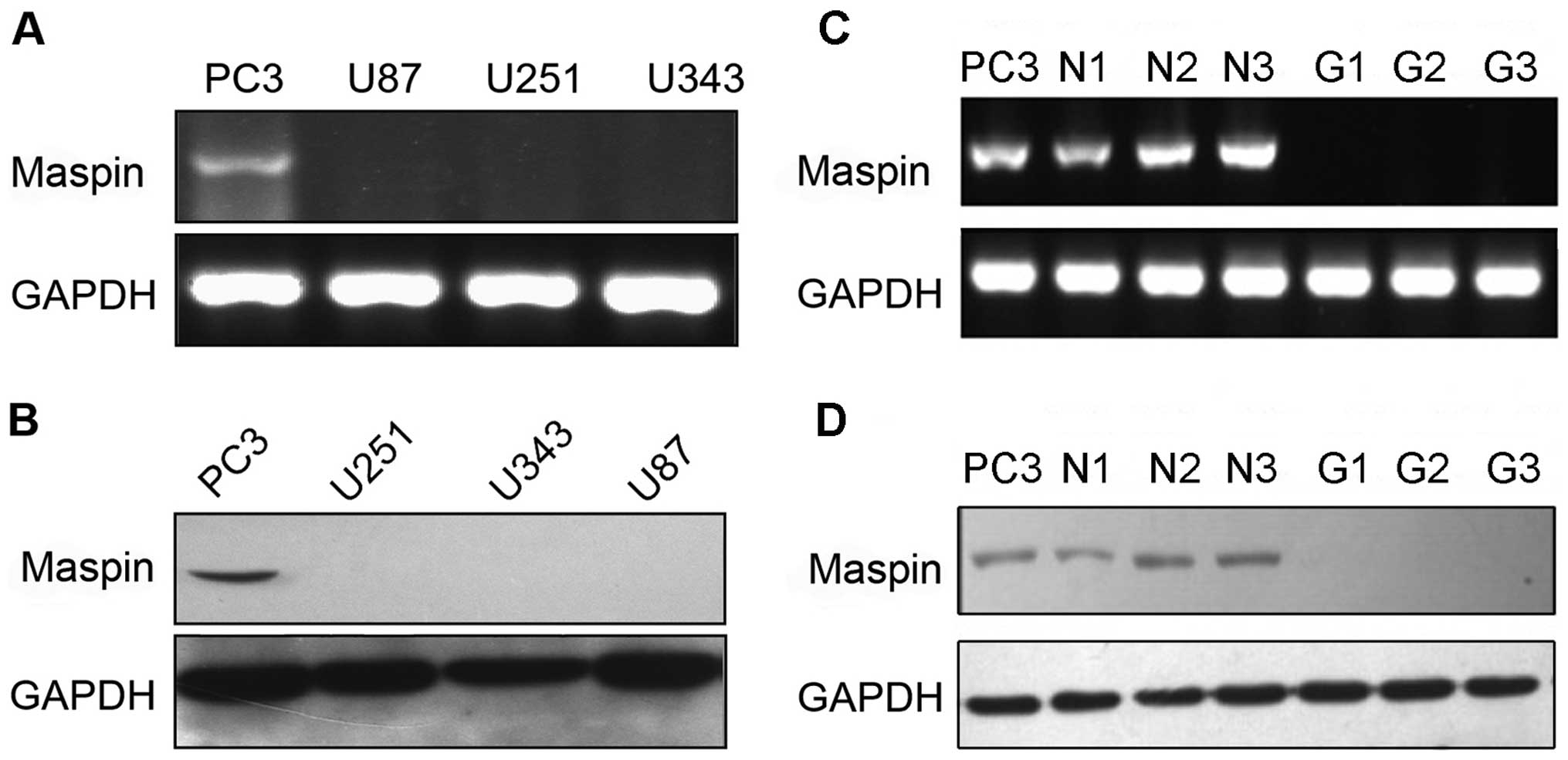 | Figure 1Differential maspin expression in
glioma cell lines and tissues. (A) Maspin expression in PC3
(control), U87, U251 and U343 cells as assessed by RT-PCR. (B)
Maspin expression in the PC3, U251, U343 and U87 cell lines as
determined by western blot analysis. (C) Maspin expression in PC3
cells (control), 3 normal brain tissues (N1, N2 and N3) and 3
glioma tissues (G1, G2 and G3) as assessed by RT-PCR. (D) Maspin
expression in PC3 cells, 3 normal brain tissues (N1, N2 and N3) and
3 glioma tissues (G1, G2 and G3) as determined by western blot
analysis. |
Establishment of stable
maspin-overexpressing cells
To explore the function of maspin, the coding region
of maspin was cloned and then subcloned into an overexpression
vector (with GFP tag). To perform the cell function experiments,
the stable glioma cell lines with overexpression of maspin and the
control cells were screened (Fig.
2A). Expression of maspin in the parental U87, U87-NC and
U87-maspin cells was verified by RT-PCR and Western blotting. As
shown in Fig. 2B–E, the mRNA and
protein levels of maspin were both significantly upregulated in the
U87-maspin cells compared with the control cells (P<0.01). These
results indicated that the stable cell line with overexpression of
maspin and the control cell line were successfully established.
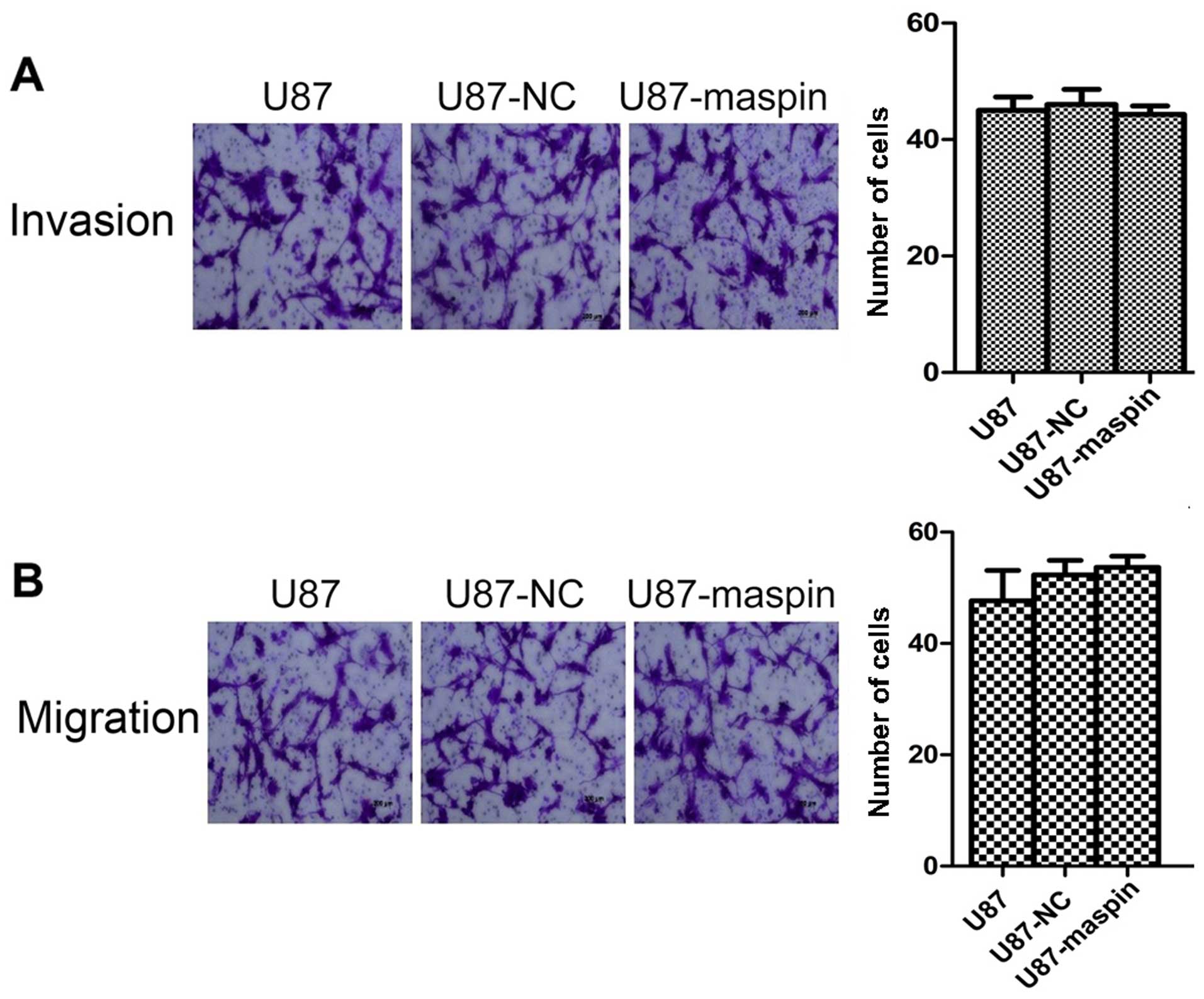
Overexpression of maspin does not affect
the migration and invasion of the U87 cells
We next investigated the effect of maspin
overexpression on cell migration and invasion. Parental U87 cells,
U87 cells infected with the control lentivirus (U87-NC) and U87
cells with maspin overexpression (U87-maspin) were used for the
Transwell migration and invasion assays. The numbers of invasive
U87, U87-NC and U87-maspin cells were 45.0±4.0, 46.0±4.6 and
44.3±2.5, respectively. As shown in Fig. 3A, no significant difference was
noted between the U87-maspin cells and control cells regarding the
invasion capability (P>0.05). The numbers of migrated U87,
U87-NC and U87-maspin cells were 47.7±9.5, 52.3±4.5 and 53.7±3.5,
respectively. The number of U87-maspin cells that transferred
through the chambers was almost the same as that of the control
group (P>0.05; Fig. 3B). These
data indicate that maspin had no effect on the invasion and
migration of the U87 cells.
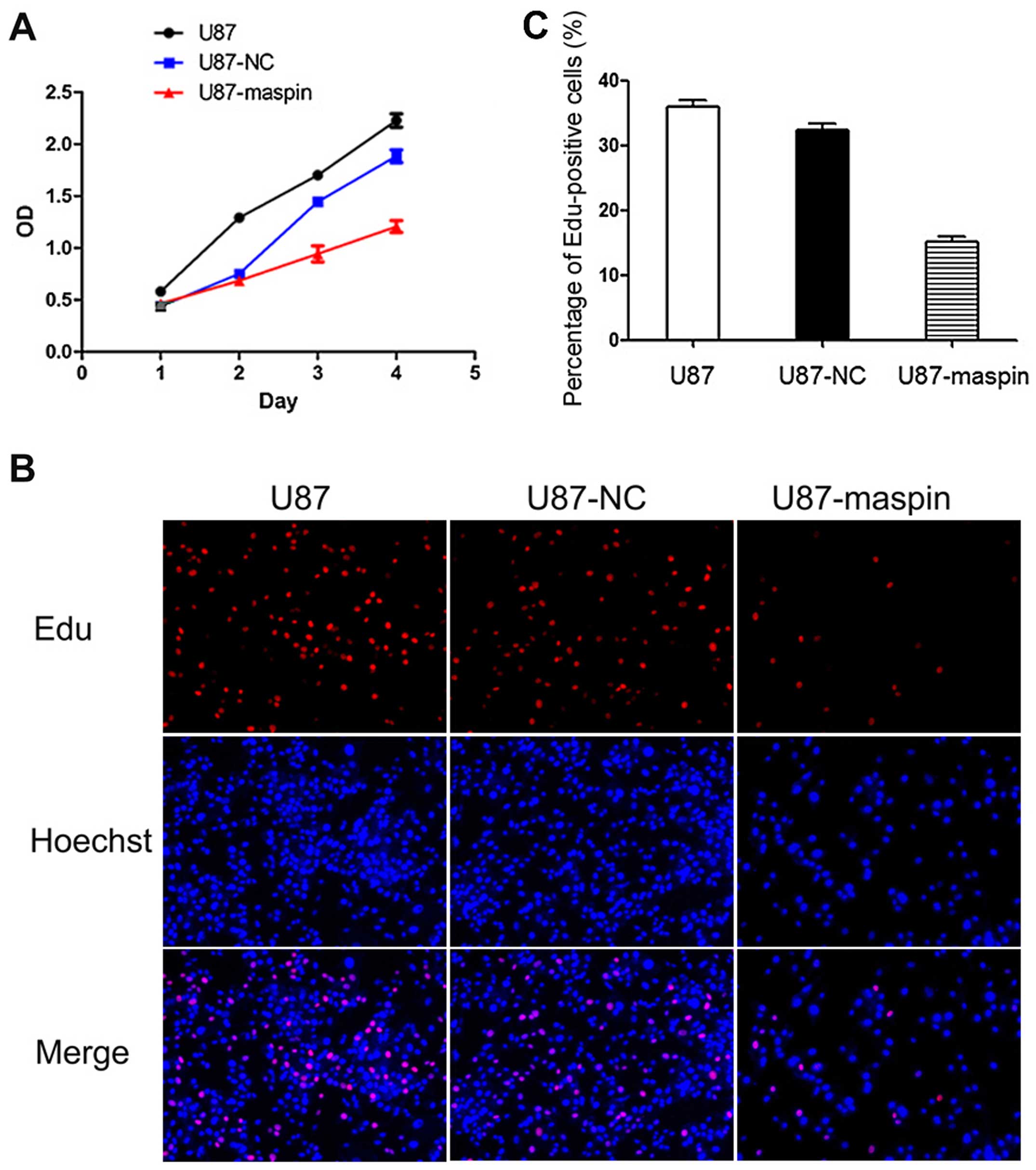
Effects of maspin overexpression on the
cell proliferation of U87 cells
We next investigated the effect of maspin on the
proliferation of glioma cells. To this end, the CCK-8-based assay
was used. As shown in Fig. 4A,
U87-maspin cells grew markedly slower than the control group
starting from the second day. Thus, maspin inhibited cell
proliferation in the U87 cells in vitro. To further
characterize the effect of maspin on the proliferation of glioma
cells, the EdU proliferation assay was also performed (Fig. 4B). The percentage of EdU-positive
cells was quantified. The percentage of EdU-positive cells was
significantly downregulated in the U87-maspin cells when compared
with the control cell lines (P<0.01; Fig. 4C). These results indicated that
maspin contributed to the reduced proliferation of the U87
cells.
5-Aza-dC induces the expression of maspin
in glioma cell lines
Gene sequences of maspin were analyzed using EMBOSS
Cpgplot (http://www.ebi.ac.uk/). After
bioinformatic analysis, a CpG island in the 5′ promoter region and
5 CpG islands in the gene body were predicted (Fig. 5A). The presence of a CpG-rich island
in the 5′ promoter region suggested the possibility that the maspin
gene might be regulated through DNA methylation. To demonstrate a
functional association between maspin promoter methylation and its
gene inactivation, a DNA-demethylating agent, 5-Aza-dC, was used to
treat the U87 and U251 cells. After treatment with 5-Aza-dC, a
significant increase in maspin mRNA expression was noted in both
the U87 and U251 cells (Fig.
5B).
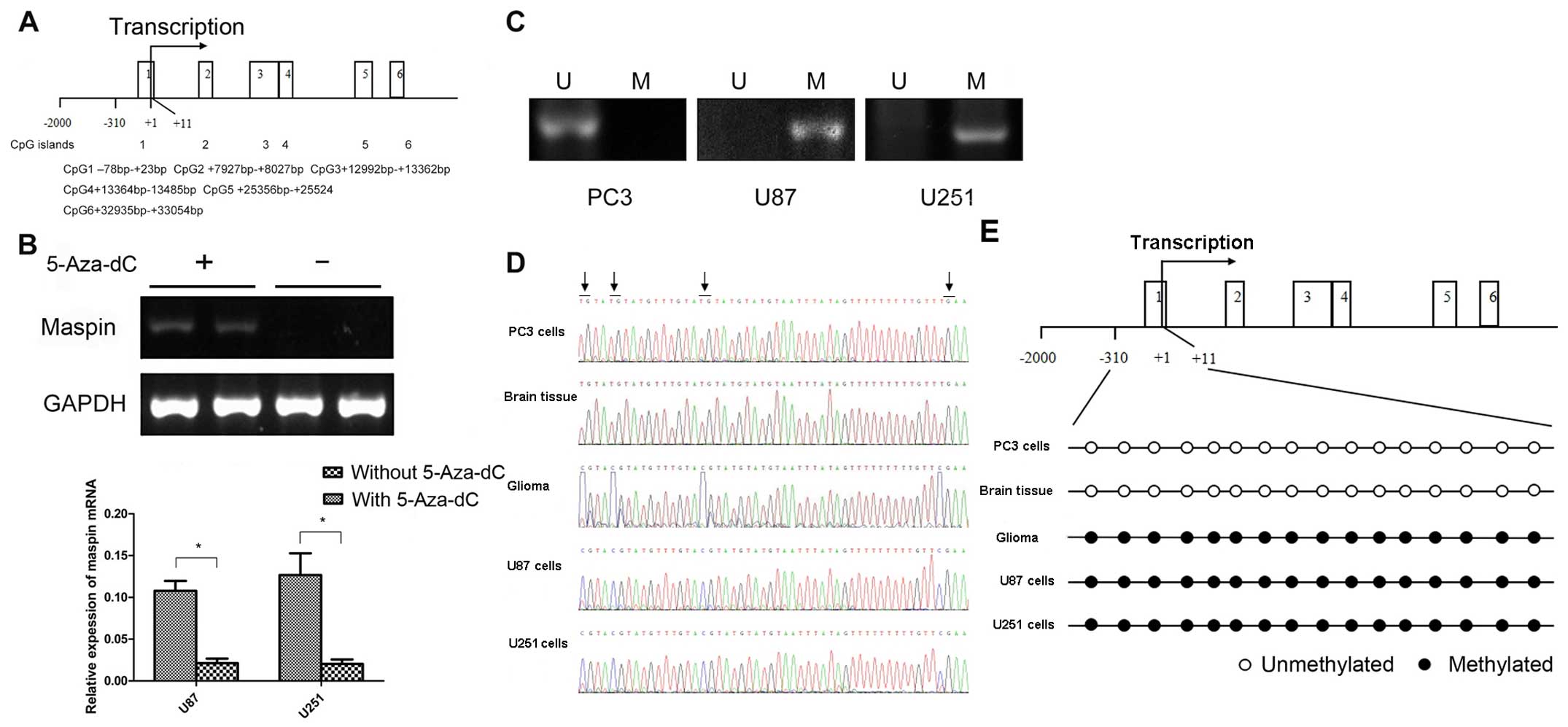 | Figure 5Regulation of maspin expression by
DNA methylation. (A) The gene sequences of maspin were analyzed
using EMBOSS Cpgplot; 6 CpG islands were found, and one of them was
in the 5′ promoter region. (B) The effect of 5-Aza-dC on maspin
expression. Restoration of maspin expression in U87 and U251 cells
after treatment with 5-Aza-dC. (+) represents cells treated with
5-Aza-dC. (−) represents cells untreated with 5-Aza-dC. Maspin mRNA
expression was analyzed by RT-PCR, and a statistically significant
difference was observed (P<0.01). (C) MSP was used to determine
the methylation status of maspin. U, unmethylated; M, methylated.
The glioma cell lines U87 and U251 were methylated in the DNA
promoter region of maspin, and PC3 was shown to be unmethylated.
(D) Representative bisulphite sequencing results of maspin promoter
methylation in the PC3 cells, normal brain tissues, glioma tissues,
U87 and U251 cells. (E) Bisulphite sequencing detected methylation
in the PC3 cells, normal brain tissues, glioma tissues, U87 and
U251 cells, showing a partially methylated sequence (arrows
indicate CpG sites). |
DNA hypermethylation in the maspin
promoter
Since maspin could be induced by 5-Aza-dC in the U87
and U251 cells, it could be suggested that the aberrant promoter
methylation might contribute to maspin inactivation in glioma. To
test this hypothesis, MSP was used to examine the methylation
status of the maspin promoter. The results from MSP showed that the
maspin promoter was methylated in the U87 and U251 cells but not in
the PC3 cells (Fig. 5C).
Furthermore, to determine a more detailed map of the methylation in
the maspin promoter, we performed bisulphite sequencing around the
promoter region of the maspin gene in the glioma cell lines and
tissues. The maspin gene was amplified with bisulphite-treated DNA
dinucleotides in the promoter, including 16 putative CpG sites. In
the U87 and U251 cells as well as three glioma tissues, 16 CpG
dinucleotides of the promoter were found to be methylated, whereas
in the PC3 cells and three normal brain tissues, the CpG
dinucleotides in the maspin promoter were unmethylated.
Representative sequencing results are shown in Fig. 5D and E. Thus, DNA methylation is
involved in the suppression of maspin promoter activity in
glioma.
Discussion
Exploration of the molecular mechanisms involved in
glioma initiation and development affords the opportunity to
identify molecules that may provide new targets for the therapy of
malignant tumors. Many known oncogenes and tumor-suppressor genes,
including c-Myc, p53 and RB, have been identified and characterized
in glioma and contribute to our understanding of this malignancy
(17–19). In the present study, we
investigated, for the first time, the role of maspin in glioma.
Maspin was first discovered as a tumor suppressor in
breast cancer (8). Studies have
further revealed that treatment with exogenous recombinant maspin
in breast and prostate cancer cells leads to less invasive and
aggressive phenotypes (20,21). Loss of maspin expression in these
cancers is correlated with tumor invasiveness and poor prognoses
(22,23). By contrast, maspin expression
correlates with better prognosis and better overall patient
survival (23,24). Consistent with these clinical data,
functional studies have demonstrated the tumor-suppressive
functions of maspin in biological processes in cancer cells,
including cell differentiation, cell migration, invasion, apoptosis
and angiogenesis (25–27). Several candidate maspin targets have
been identified under various experimental conditions, including
histone deacetylase 1 (HDAC1) (28)
and pro-urokinase type plasminogen activator (pro-uPA) (29). In addition, interferon regulatory
factor 6 (IRF6) (30), β1-integrin
(31), collagen I (32) and glutathione S-transferase (GST)
(33) have been identified as
maspin-associated proteins. For example, studies have suggested
that, in prostate carcinoma cells, the loss of endogenous
inhibition of HDAC1 by maspin may lead to the silencing of other
tumor suppressors such as GSTp, thus representing a significant
gain of function in tumor progression (33).
Although ample studies have reported that maspin may
play important roles in the formation and progression of human
tumors, the role of maspin in glioma remains unknown. Our results,
for the first time, showed that maspin was silenced in
glioma-derived U87, U251 and U343 cells and glioma tissues. To
explore the functions of maspin in glioma cell lines, we infected
lentiviral vectors overexpressing maspin into U87 cells. The
results revealed that maspin had no effect on the migration and
invasion in vitro of U87 human glioblastoma cells.
Nevertheless, overexpression of maspin significantly inhibited
tumor cell proliferation. In breast cancer, maspin is the only
pro-apoptotic serpin implicated in apoptosis regulation.
Intracellular maspin can translocate to the mitochondria to induce
cytochrome c release and caspase activation or modulate the
expression of Bcl-2 family members (34,35).
In human prostate cancer, the progressive decrease in invasive
cancer is associated with the capability of maspin to reduce tumor
growth, osteolysis and angiogenesis. Furthermore, there is evidence
that maspin inhibits prostate cancer-induced bone matrix
remodelling and induces prostate cancer glandular redifferentiation
(25,26). Although our result was reconcilable
with those research data, the different roles of maspin in
different tumors suggest that maspin may demonstrate different
activities in different cell types.
We next explored the mechanisms underlying maspin
silencing in glioma. Alterations in DNA methylation, usually in the
CpG island region of the gene promoter, is one of the common
reasons that leads to gene silencing, and could contribute to
oncogenesis (36,37). A putative CpG island in the 5′
promoter and 5 CpG islands in the maspin gene body were found by
EMBOSS Cpgplot. In this study, U87 and U251 cells were treated with
5-Aza-2-dC, and the mRNA expression of maspin was restored in both
cell lines. Based on these findings, it could be reasonably
speculated that DNA methylation in the CpG island region of the
maspin promoter is involved in the regulation of the expression of
maspin, influencing the proliferative abilities of the glioma
cells. The molecular mechanism by which DNA methylation silences
gene expression is not fully understood. DNA methylation may
directly interfere with the binding of transcription factors,
resulting in the transcriptional repression of the associated gene
(37). In addition, methyl-binding
domain-containing proteins (MBDs) may bind to areas of dense DNA
methylation and recruit histone deacetylases and transcriptional
repressor complexes that are refractory to transcription (38).
To clarify the mechanism of maspin inactivation in
these two cell lines, MSP was used to examine the methylation
status of the maspin promoter. Both U87 and U251 cell lines showed
methylation of the maspin promoter. To confirm the results of MSP,
we performed bisulphite sequencing analysis of a 321-bp fragment,
including 16 expected CpG sites. The 16 CpG sites in the promoter
were completely methylated in the U87 and U251 cells and glioma
tissues, while in normal brain tissues, the CpG dinucleotides were
unmethylated. Taken together, these findings suggested that
promoter methylation is an important mechanism involved in the
inactivation of maspin in glioma. Combined with the effect of CpG
island methylation on maspin silencing in breast cancer cells
(13), our research result appears
to be convincing. However, other epigenetic processes, such as
histone deacetylation, also played a role in the gene silencing in
the breast cancer cells (39), and
maspin expression may be regulated by hormone receptors in prostate
cells (40). Moreover, some
researchers have reported that the differential expression of
maspin in tumor progression may be mediated by factors such as p53
and IKK-α (41–43). It is possible that other mechanisms,
such as histone deacetylation, may also modulate maspin expression
in glioma cell lines. Clearly, more studies are needed to clarify
the issue.
In conclusion, we found that methylation-induced
silencing of maspin contributes to the proliferation of human
glioma cells, and maspin may be exploited as a potential
therapeutic target for glioma.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (81072058), the Natural Science
Foundation of Jiangsu Province (BK2010230), the Foundation Program
of Suzhou Science and Technology Project (SYSD2014092), Research
and Innovation Project for College Graduates of Jiangsu Province
(2014–1263), and the Foundation of Young Member of the Second
Affiliated Hospital of Soochow University (SDFEYQN1409).
References
|
1
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huse JT, Holland E and DeAngelis LM:
Glioblastoma: Molecular analysis and clinical implications. Annu
Rev Med. 64:59–70. 2013. View Article : Google Scholar
|
|
3
|
Cloughesy TF, Cavenee WK and Mischel PS:
Glioblastoma: From molecular pathology to targeted treatment. Annu
Rev Pathol. 9:1–25. 2014. View Article : Google Scholar
|
|
4
|
Burgess DJ: Epigenetics. Dissecting
driving DNA methylations. Nat Rev Cancer. 12:448–449. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cahill N and Rosenquist R: Uncovering the
DNA methylome in chronic lymphocytic leukemia. Epigenetics.
8:138–148. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sulaiman L, Juhlin CC, Nilsson IL, Fotouhi
O, Larsson C and Hashemi J: Global and gene-specific promoter
methylation analysis in primary hyperparathyroidism. Epigenetics.
8:646–655. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mueller S, Phillips J, Onar-Thomas A,
Romero E, Zheng S, Wiencke JK, McBride SM, Cowdrey C, Prados MD,
Weiss WA, et al: PTEN promoter methylation and activation of the
PI3K/Akt/mTOR pathway in pediatric gliomas and influence on
clinical outcome. Neuro-oncol. 14:1146–1152. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zou Z, Anisowicz A, Hendrix MJ, Thor A,
Neveu M, Sheng S, Rafidi K, Seftor E and Sager R: Maspin, a serpin
with tumor-suppressing activity in human mammary epithelial cells.
Science. 263:526–529. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sharma G, Mirza S, Parshad R, Srivastava
A, Gupta SD, Pandya P and Ralhan R: Clinical significance of Maspin
promoter methylation and loss of its protein expression in invasive
ductal breast carcinoma: Correlation with VEGF-A and MTA1
expression. Tumour Biol. 32:23–32. 2011. View Article : Google Scholar
|
|
10
|
McKenzie S, Sakamoto S and Kyprianou N:
Maspin modulates prostate cancer cell apoptotic and angiogenic
response to hypoxia via targeting AKT. Oncogene. 27:7171–7179.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yoshizawa K, Nozaki S, Kitahara H, Kato K,
Noguchi N, Kawashiri S and Yamamoto E: Expression of urokinase-type
plasminogen activator/urokinase-type plasminogen activator receptor
and maspin in oral squamous cell carcinoma: Association with mode
of invasion and clinicopathological factors. Oncol Rep.
26:1555–1560. 2011.PubMed/NCBI
|
|
12
|
Bodenstine TM, Seftor RE, Khalkhali-Ellis
Z, Seftor EA, Pemberton PA and Hendrix MJ: Maspin: Molecular
mechanisms and therapeutic implications. Cancer Metastasis Rev.
31:529–551. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu Y, Alvarez M, Slamon DJ, Koeffler P and
Vadgama JV: Caspase 8 and maspin are downregulated in breast cancer
cells due to CpG site promoter methylation. BMC Cancer. 10(32)2010.
View Article : Google Scholar
|
|
14
|
Alvarez Secord A, Darcy KM, Hutson A,
Huang Z, Lee PS, Jewell EL, Havrilesky LJ, Markman M, Muggia F and
Murphy SK: The regulation of MASPIN expression in epithelial
ovarian cancer: association with p53 status, and MASPIN promoter
methylation: a gynecologic oncology group study. Gynecol Oncol.
123:314–319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rose SL, Fitzgerald MP, White NO, Hitchler
MJ, Futscher BW, De Geest K and Domann FE: Epigenetic regulation of
maspin expression in human ovarian carcinoma cells. Gynecol Oncol.
102:319–324. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang W and Zhang M: Tissue microarray
analysis of maspin expression and its reverse correlation with
mutant p53 in various tumors. Int J Oncol. 20:1145–1150.
2002.PubMed/NCBI
|
|
17
|
Xie R, Yang H, Xiao Q, Mao F, Zhang S, Ye
F, Wan F, Wang B, Lei T and Guo D: Downregulation of LRIG1
expression by RNA interference promotes the aggressive properties
of glioma cells via EGFR/Akt/c-Myc activation. Oncol Rep.
29:177–184. 2013.
|
|
18
|
Squatrito M, Brennan CW, Helmy K, Huse JT,
Petrini JH and Holland EC: Loss of ATM/Chk2/p53 pathway components
accelerates tumor development and contributes to radiation
resistance in gliomas. Cancer Cell. 18:619–629. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chow LM, Endersby R, Zhu X, Rankin S, Qu
C, Zhang J, Broniscer A, Ellison DW and Baker SJ: Cooperativity
within and among Pten, p53, and Rb pathways induces high-grade
astrocytoma in adult brain. Cancer Cell. 19:305–316. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stark AM, Schem C, Maass N, Hugo HH, Jonat
W, Mehdorn HM and Held-Feindt J: Expression of metastasis
suppressor gene maspin is reduced in breast cancer brain metastases
and correlates with the estrogen receptor status. Neurol Res.
32:303–308. 2010. View Article : Google Scholar
|
|
21
|
Dzinic SH, Chen K, Thakur A, Kaplun A,
Bonfil RD, Li X, Liu J, Bernardo MM, Saliganan A, Back JB, et al:
Maspin expression in prostate tumor elicits host anti-tumor
immunity. Oncotarget. 5:11225–11236. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Prasad CP, Rath G, Mathur S, Bhatnagar D
and Ralhan R: Expression analysis of maspin in invasive ductal
carcinoma of breast and modulation of its expression by curcumin in
breast cancer cell lines. Chem Biol Interact. 183:455–461. 2010.
View Article : Google Scholar
|
|
23
|
Machtens S, Serth J, Bokemeyer C, Bathke
W, Minssen A, Kollmannsberger C, Hartmann J, Knüchel R, Kondo M,
Jonas U, et al: Expression of the p53 and Maspin protein in primary
prostate cancer: Correlation with clinical features. Int J Cancer.
95:337–342. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Machowska M, Wachowicz K, Sopel M and
Rzepecki R: Nuclear location of tumor suppressor protein maspin
inhibits proliferation of breast cancer cells without affecting
proliferation of normal epithelial cells. BMC Cancer. 14:1422014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cher ML, Biliran HR Jr, Bhagat S, Meng Y,
Che M, Lockett J, Abrams J, Fridman R, Zachareas M and Sheng S:
Maspin expression inhibits osteolysis, tumor growth, and
angiogenesis in a model of prostate cancer bone metastasis. Proc
Natl Acad Sci USA. 100:7847–7852. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bernardo MM, Meng Y, Lockett J, Dyson G,
Dombkowski A, Kaplun A, Li X, Yin S, Dzinic S, Olive M, et al:
Maspin reprograms the gene expression profile of prostate carcinoma
cells for differentiation. Genes Cancer. 2:1009–1022. 2011.
View Article : Google Scholar
|
|
27
|
Kaplun A, Dzinic S, Bernardo M and Sheng
S: Tumor suppressor maspin as a rheostat in HDAC regulation to
achieve the fine-tuning of epithelial homeostasis. Crit Rev
Eukaryot Gene Expr. 22:249–258. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li X, Yin S, Meng Y, Sakr W and Sheng S:
Endogenous inhibition of histone deacetylase 1 by tumor-suppressive
maspin. Cancer Res. 66:9323–9329. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yin S, Lockett J, Meng Y, Biliran H Jr,
Blouse GE, Li X, Reddy N, Zhao Z, Lin X, Anagli J, et al: Maspin
retards cell detachment via a novel interaction with the
urokinase-type plasminogen activator/urokinase-type plasminogen
activator receptor system. Cancer Res. 66:4173–4181. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bailey CM, Khalkhali-Ellis Z, Kondo S,
Margaryan NV, Seftor RE, Wheaton WW, Amir S, Pins MR, Schutte BC
and Hendrix MJ: Mammary serine protease inhibitor (Maspin) binds
directly to interferon regulatory factor 6: Identification of a
novel serpin partnership. J Biol Chem. 280:34210–34217. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Endsley MP, Hu Y, Deng Y, He X, Warejcka
DJ, Twining SS, Gonias SL and Zhang M: Maspin, the molecular bridge
between the plasminogen activator system and beta1 integrin that
facilitates cell adhesion. J Biol Chem. 286:24599–24607. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Blacque OE and Worrall DM: Evidence for a
direct interaction between the tumor suppressor serpin, maspin, and
types I and III collagen. J Biol Chem. 277:10783–10788. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li X, Kaplun A, Lonardo F, Heath E, Sarkar
FH, Irish J, Sakr W and Sheng S: HDAC1 inhibition by maspin
abrogates epigenetic silencing of glutathione S-transferase pi in
prostate carcinoma cells. Mol Cancer Res. 9:733–745. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Latha K, Zhang W, Cella N, Shi HY and
Zhang M: Maspin mediates increased tumor cell apoptosis upon
induction of the mitochondrial permeability transition. Mol Cell
Biol. 25:1737–1748. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Toillon RA, Lagadec C, Page A, Chopin V,
Sautière PE, Ricort JM, Lemoine J, Zhang M, Hondermarck H and Le
Bourhis X: Proteomics demonstration that normal breast epithelial
cells can induce apoptosis of breast cancer cells through
insulin-like growth factor-binding protein-3 and maspin. Mol Cell
Proteomics. 6:1239–1247. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang S, Hao J, Xie F, Hu X, Liu C, Tong
J, Zhou J, Wu J and Shao C: Downregulation of miR-132 by promoter
methylation contributes to pancreatic cancer development.
Carcinogenesis. 32:1183–1189. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sarkar S, Goldgar S, Byler S, Rosenthal S
and Heerboth S: Demethylation and re-expression of epigenetically
silenced tumor suppressor genes: Sensitization of cancer cells by
combination therapy. Epigenomics. 5:87–94. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jaenisch R and Bird A: Epigenetic
regulation of gene expression: How the genome integrates intrinsic
and environmental signals. Nat Genet. 33(Suppl): 245–254. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Maass N, Biallek M, Rösel F, Schem C,
Ohike N, Zhang M, Jonat W and Nagasaki K: Hypermethylation and
histone deacetylation lead to silencing of the maspin gene in human
breast cancer. Biochem Biophys Res Commun. 297:125–128. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Reddy GP, Barrack ER, Dou QP, Menon M,
Pelley R, Sarkar FH and Sheng S: Regulatory processes affecting
androgen receptor expression, stability, and function: Potential
targets to treat hormone-refractory prostate cancer. J Cell
Biochem. 98:1408–1423. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Eitel JA, Bijangi-Vishehsaraei K,
Saadatzadeh MR, Bhavsar JR, Murphy MP, Pollok KE and Mayo LD: PTEN
and p53 are required for hypoxia induced expression of maspin in
glioblastoma cells. Cell Cycle. 8:896–901. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jiang R, Xia Y, Li J, Deng L, Zhao L, Shi
J, Wang X and Sun B: High expression levels of IKKalpha and IKKbeta
are necessary for the malignant properties of liver cancer. Int J
Cancer. 126:1263–1274. 2010. View Article : Google Scholar
|
|
43
|
Zhang M: PTEN in action: Coordinating with
p53 to regulate maspin gene expression. Cell Cycle. 8:1112–1113.
2009. View Article : Google Scholar : PubMed/NCBI
|