Introduction
Breast cancer is one of the most common malignancies
among women, with 458,000 annual deaths worldwide (1,2).
Treatment strategies for breast cancer include surgery,
radiotherapy, hormone therapy, chemotherapy or a combination of
these methods (3). A range of
chemotherapeutic drugs are employed in the treatment of breast
cancer, in which platinum agents represent a class of common
chemotherapeutic drugs, such as cisplatin or carboplatin (4). Cisplatin is currently the most
effective chemotherapeutic drug used to treat breast cancer.
Cisplatin is a genotoxic agent and the mechanism of action includes
induction of DNA damages; therefore it is considered to be
dose-limiting (6). The efficacy of
this chemotherapeutic agent is often low due to adverse side
effects and drug resistance (7–10).
High resistance to cisplatin is a major challenge in the successful
treatment of breast cancer, and there is currently no effective
cure for patients with advanced stage of the disease. Consequently,
strategies designed to sensitize breast cancer cells to cisplatin
are still under investigation.
Berberine (BBR) is an isoquinoline alkaloid
extracted from the rhizomes of a variety of valuable medicinal
plants, including Coptis chinensis and Coptis
japonica (11). BBR has been
reported to possess a wide variety of pharmacological activities as
an anti-microbial and anti-inflammatory agent (12–15).
Currently, the anticancer activities of BBR have been reported in a
range of cancers including hepatoma, prostate cancer, glioblastoma,
ovarian cancer, leukemia and breast cancer (16–24).
BBR achieves its antitumor effect through inhibition of cell
proliferation and induction of tumor cell apoptosis although the
underlying molecular mechanisms of BBR involved in the inhibition
of cancer cell growth have not been fully elucidated (25–29).
BBR has been demonstrated to directly bind with DNA and interfere
with DNA replication as a DNA topoisomerase I inhibitor, through
which BBR eventually induces cellular apoptosis. Studies have also
shown that BBR binds to DNA, and radiosensitized lung cancer and
esophageal cancer cells by regulating the expression of DNA
repair-associated proteins (30–33),
and BBR was found to modulate the anticancer effects of doxorubicin
and rapamycin in human cancer cells (34,35).
Although the mechanisms through which BBR sensitizes cancer cells
to radiation or chemotherapy agents remain unclear, it is likely
that BBR increases DNA damage induced by various therapeutic
drugs.
As resistance to cisplatin of breast cancer is still
a major challenge for the successful treatment of this disease, in
the present study, we focused on the effects of BBR on the
sensitivity of breast cancer cells to cisplatin and the mechanisms
through which BBR functions in breast cancer cells. In combination
with cisplatin, a low dose of BBR suppressed the proliferation of
MCF-7 cells, increased apoptotic-associated protein expression, and
more importantly, BBR increased the DNA breaks induced by
cisplatin. In conclusion, our findings demonstrated that BBR
increased the genotoxic ability of cisplatin and sensitized breast
cancer cells to cisplatin, which could be a potential strategy for
the treatment of breast cancer patients with cisplatin
resistance.
Materials and methods
Cell culture
The human breast cancer MCF-7 cell line was obtained
from the American Type Culture Collection (ATCC, Manassas, VA,
USA). The cells were cultured in RPMI-1640 culture medium
supplemented with 10% heat-inactivated fetal bovine serum (FBS)
(both from Hyclone, Waltham, MA, USA), 100 u/ml penicillin and 100
mg/ml streptomycin (Thermo Fisher Scientific, Waltham, MA,
USA).
Antibodies and reagents
Berberine (BBR), cisplatin and DMSO were purchased
from Sigma (St. Louis, MO, USA). Antibodies to GAPDH were purchased
from ProteinTech group, Inc. (Chicago, IL, USA) and the antibody to
γH2AX was obtained from CST (Boston, MA, USA).
Cell viability assay
Cell viability was determined by the MTT assay.
Briefly, breast cancer cells were seeded at 4×103
cells/well in 96-well plates overnight, cultured in fresh medium
containing various concentrations of BBR and cisplatin was
dissolved in DMSO. After incubation for 44 h, MTT (0.5 mg/ml;
Sigma-Aldrich) was added and 4 h later the growth of the cells was
measured at 492 nm using a microplate photometer (Thermo Fisher
Scientific). The effect of the drugs on cell viability was assessed
as the percentages of cell viability compared with the control
cells which were arbitrarily assigned as having 100% viability.
Wound-healing assay
The cells were grown to full confluency in 6-well
plates and incubated overnight. Cell monolayers were wounded with a
sterile 10-μl pipette tip, washed with PBS, and treated with
the indicated dose of BBR (13 μM) or cisplatin (3.3
μM) or the combination in complete medium. After a 48-h
incubation, the medium was replaced with PBS, and the wound gap was
observed and photographed using an Olympus microscope (Olympus,
Tokyo, Japan).
Anchorage-independent colony formation
assay
MCF-7 cells were treated with BBR (13 μM) and
cisplatin (3.3 μM) for 48 h. The cells were washed with PBS
and trypsinized with trypsin (0.25% trypsin, EDTA) and 400 cells
were seeded into a well of the 6-well plates. The cultures were
maintained in an incubator at 37°C with 5% CO2 for 10
days. The cells were washed with PBS twice, fixed with methanol for
15 min, stained with Giemsa for 15 min, washed with water and
air-dried. The colonies with more than 50 cells were counted under
an ordinary optical microscope.
Western blot analysis
After incubation with 13 μM BBR and 3.3
μM cisplatin for 48 h, the cells were lysed in RIPA lysis
buffer. Whole cell proteins were quantified using the BCA protein
assay (KangChen Bio-tech, Shanghai, China), separated by
electrophoresis using 10% SDS-PAGE and transferred to a PVDF
membrane. Western blot analyses were probed with the specific
antibodies at dilution conditions as follows: mouse anti-GAPDH
(1:4,000), β-actin (1:4,000), caspase-9 (1:500), rabbit
anti-caspase-3 (1:500), Bcl2 (1:500), anti-mouse and rabbit IgG
(H+L) secondary antibodies (1:5,000); all the antibody were
purchased from ProteinTech group, Inc.
Immunofluorescence analysis
Cells grown on chamber slides were treated with BBR
(13 μM) in combination with cisplatin (3.3 μM). After
48 h, the cells were washed with PBS and then fixed with 4%
paraformaldehyde at room temperature for 30 min, and then washed
with PBS for three times. After permeabilization in 0.2% Triton
X-100 for 30 min, the cells were washed twice in PBS and blocked
for 1 h in PBS containing 1% BSA (all from Solarbio, Beijing,
China). The cell pellet was suspended in 100 μl of 1% BSA
containing either 1:100 diluted anti-γH2AX polyclonal Ab (CST). The
cells were then incubated overnight at 4°C. On the following day,
the cells were washed twice with PBS and incubated in 100 μl
of 1:100 diluted Alexa Fluor 488-conjugated anti-rabbit IgG (Thermo
Fischer Scientific) for 2 h at room temperature in the dark. After
washing with PBS three times, the cells were dyed with Hoechst
33342 (Sigma, St. Louis, MO, USA) for 3 min, and washed with PBS
for three times, and then photographed under a microscope
(Olympus).
Statistical analysis
Data analysis was carried out using SPSS 6.0
software. one-Way ANOVA was used to determine the significance of
the differences in multiple comparisons; p<0.05, p<0.01,
p<0.001, p<0.0001 were considered statistically significant.
All experiments were performed in triplicate. Data are expressed as
the mean ± SD. We used Image J and IPP6.0 software to process and
analysis the immunofluorescence image.
Results
Berberine in combination with cisplatin
suppresses MCF-7 cell proliferation
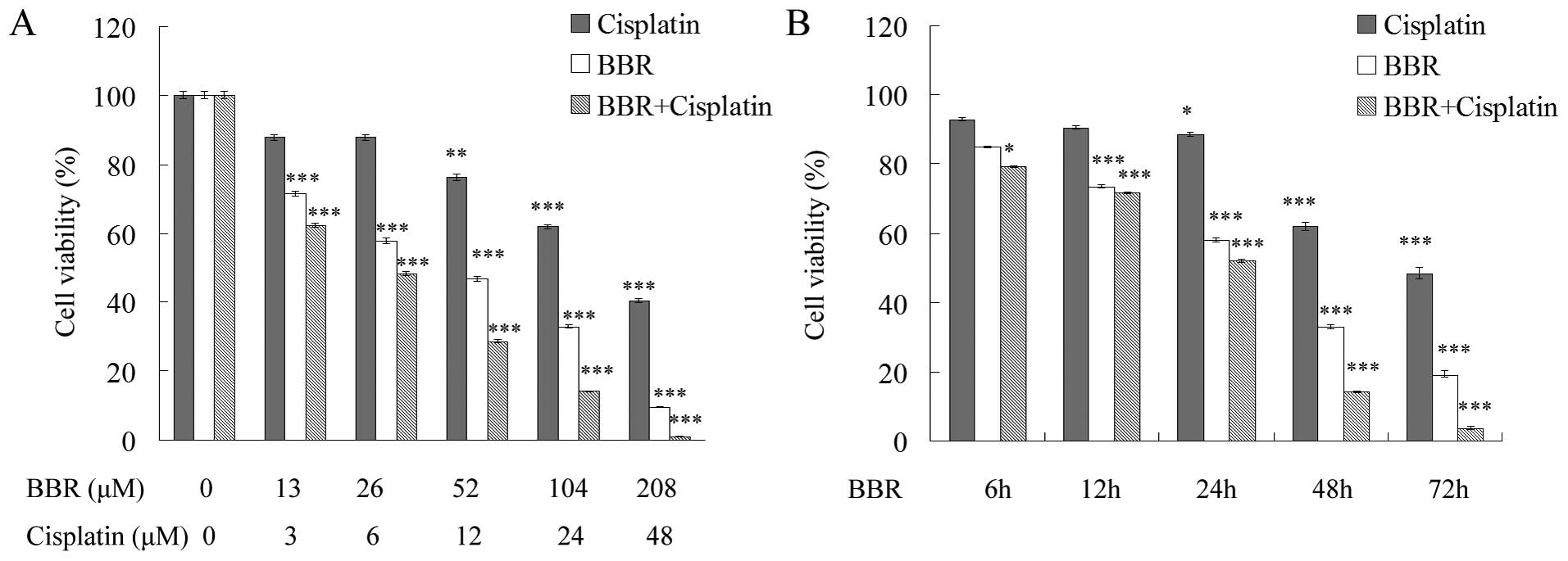
We analyzed the effect of BBR in combination with
cisplatin on human breast cancer MCF-7 cell proliferation by MTT
assay. After a 48-h BBR treatment, the IC50 value of BBR
in the MCF-7 cells was 52.178±1.593 μM and the
IC50 value of cisplatin was 49.541±1.618 μM. In
contrast, following combination with 26 μM BBR, the
IC50 value of cisplatin was 5.759±0.76 μM
(Fig. 1A). BBR increased the
sensitivity of MCF-7 cells to cisplatin in a dose and
time-dependent manner (Fig. 1A and
B).
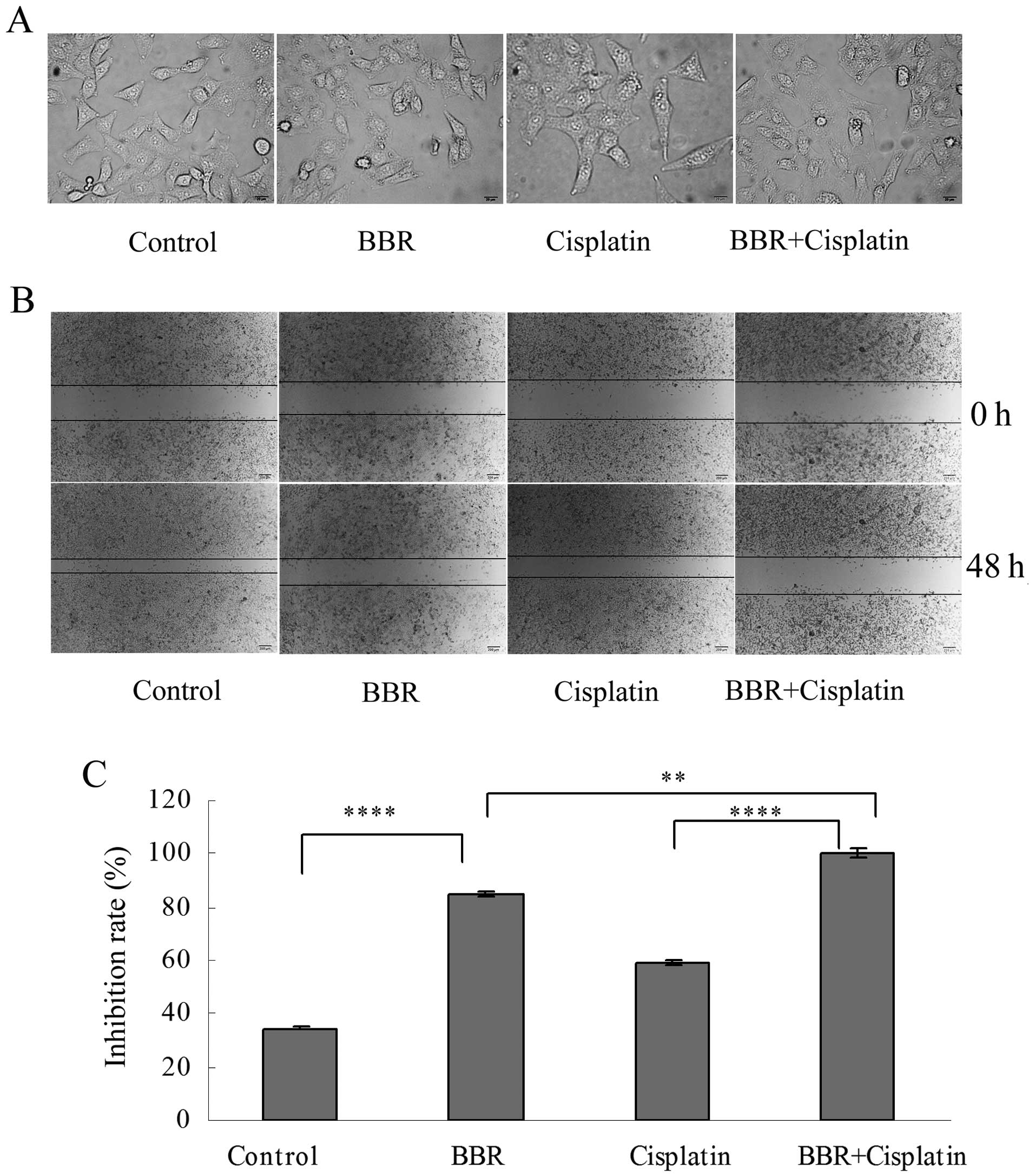
Berberine modifies cell morphology and
inhibits cell migration and colony formation
Following treatment of the MCF-7 cells with BBR at
the dose of 13 μM and with cisplatin at 3.3 μM,
reduced cell-cell contact and the formation of filopodia were
observed (Fig. 2A). The wound
healing assay showed that BBR and cisplatin inhibited the migration
of MCF-7 cells. BBR in combination with cisplatin further inhibited
the migration of MCF-7 cells (Fig. 2B
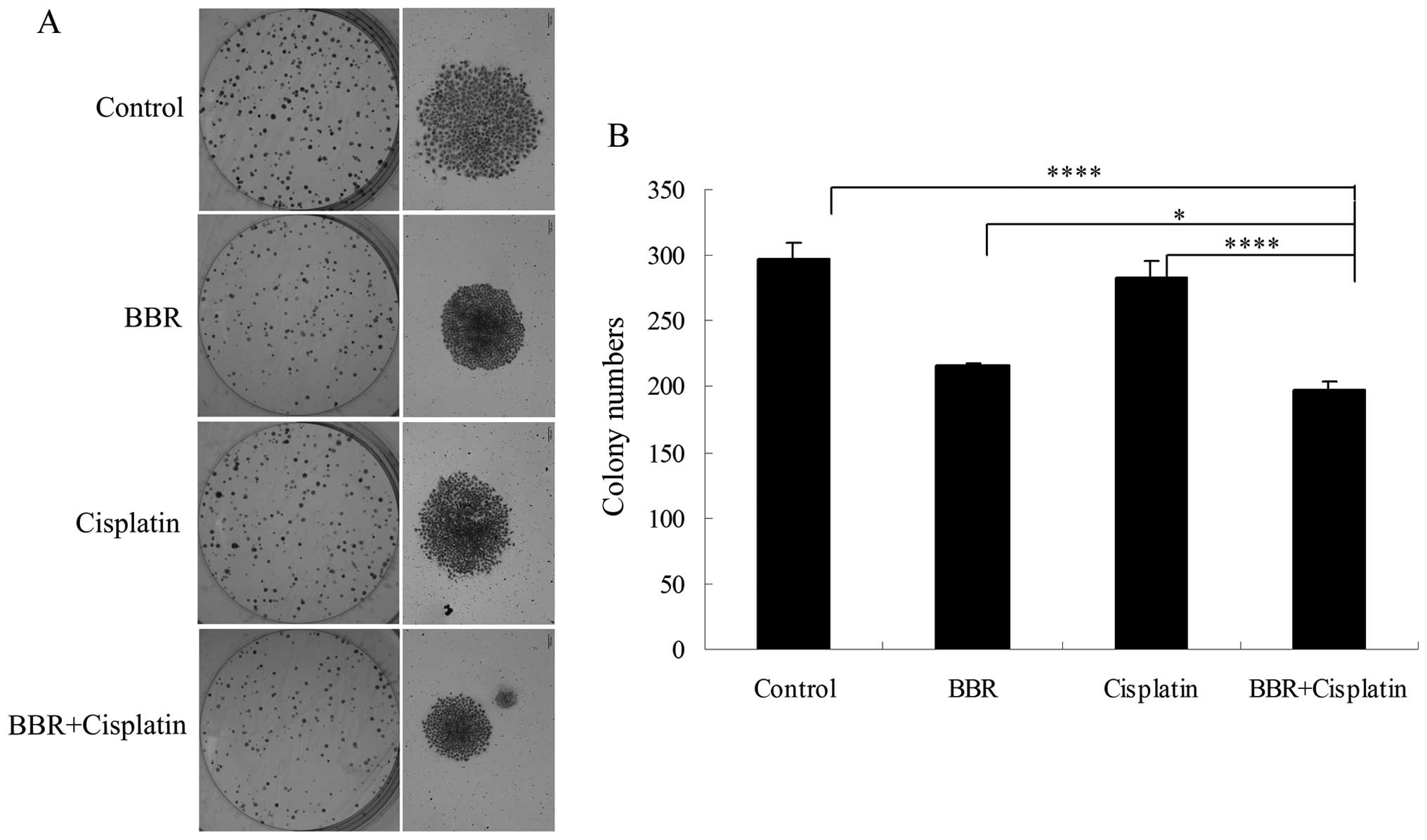
and C). Each drug administered alone suppressed cell colony
formation. BBR in combination with cisplatin further suppressed
MCF-7 cell colony formation (Fig. 3A
and B).
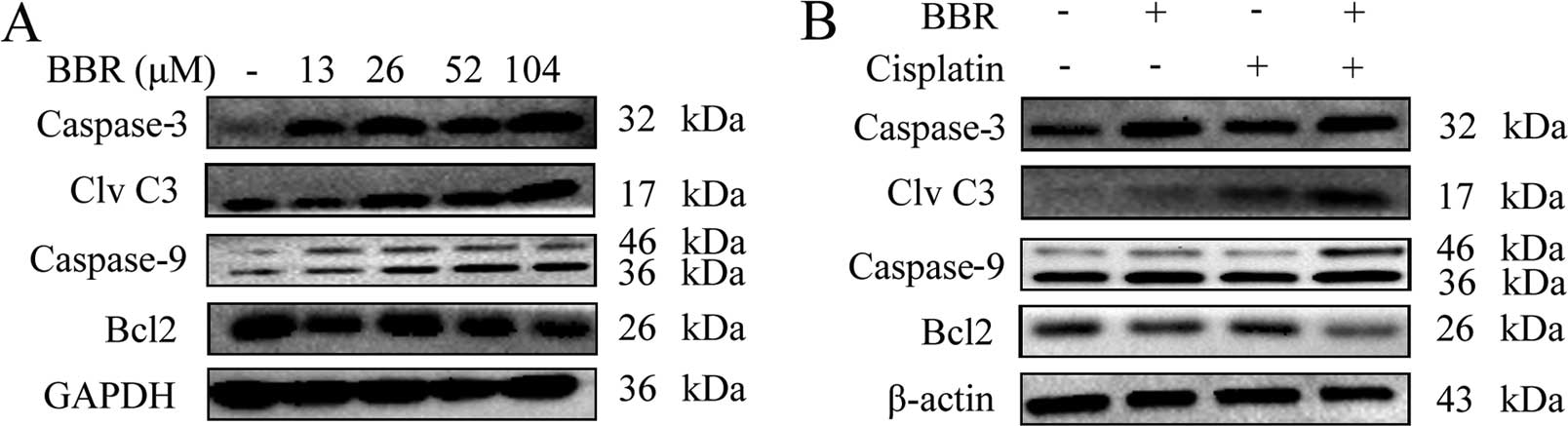
Berberine sensitizes MCF-7 cells to
cisplatin through the caspase-3-dependent apoptotic pathway
We next tested whether BBR and cisplatin induce
apoptotic-associated proteins. The expression levels of
pro-apoptotic proteins, caspase-3 and caspase-9 and anti-apoptotic
protein Bcl-2 in MCF-7 cells were analyzed by western blot
analysis. BBR increased the expression levels of caspase-3 and
caspase-9 compared with these levels in the control group (Fig. 4A). A low dose of BBR (13 μM)
in combination with cisplatin increased the expression of cleaved
caspase-3 and caspase-9, but decreased expression of Bcl-2 compared
with these levels in the cells treated with cisplatin alone (3.3
μM) (Fig. 4B). The results
indicate that BBR sensitized MCF-7 breast cancer cells to cisplatin
through a caspase-3-dependent apoptotic pathway.
Berberine increases DNA breaks and
restrains the expression of PCNA
We used immunofluorescence analysis to test γH2AX
foci in the cells. The cells were cultured with BBR and cisplatin
for 48 h, and γH2AX foci are shown in Fig. 5A. The result showed that cisplatin
induced DNA breaks, and a low dose of BBR increased the DNA breaks
induced by cisplatin. We also detected the effect of BBR on
expression of PCNA, an important factor in DNA replication and DNA
repair. BBR extensively reduced the expression of PCNA (Fig. 5B), suggesting that BBR may regulate
the cellular DNA repair pathway to increase DNA breaks and
sensitize cells to cisplatin.
Discussion
Currently, breast cancer treatment includes surgery,
chemotherapy, hormone therapy, radiotherapy, and combinations of
these methods. Conventional cisplatin is still the most effective
chemotherapeutic agent in breast cancer treatment. However, the
resistance of tumor cells to cisplatin is a considerable obstacle
to effective breast cancer therapy. Due to the genotoxicity of
cisplatin, the drug is often considered to be dose-limiting.
Therefore, it would be beneficial for chemotherapeutic treatment if
alternative reliable agents can sensitize cancer cells to
cisplatin. Berberine is a traditional Chinese medicine and has been
demonstrated to function in anticancer therapy with minor side
effects. Thus, we evaluated the sensitization of MCF-7 cells to BBR
in combination with cisplatin and the mechanisms of BBR action
involved in the inhibition of breast cancer cells.
BBR inhibited breast cancer MCF-7 cell growth, and
suppressed breast cancer cell colony formation and migration. We
investigated the effect of a low level of BBR in combination with
cisplatin on apoptosis and DNA breaks. A low level of BBR increased
apoptotic caspase-3 and caspase-9 expression, reduced Bcl2
expression in combination with cisplatin. The results demonstrated
that a low level of BBR greatly increased cisplatin-induced
caspase-3 activation although this dose of BBR had a limited effect
on the cell proliferation of the MCF-7 cells. To study the
mechanism of BBR-induced apoptosis, we investigated the DNA breaks
induced by BBR and cisplatin. A low level of BBR had a limited
effect on cell growth, however, BBR greatly increased the
sensitivity of the cells to genotoxic cisplatin. BBR in combination
with cisplatin induced more γH2AX foci, suggesting that BBR
increased the DNA damage induced by cisplatin. The increased
cellular DNA damage may result in subsequent apoptosis and
suppression of MCF-7 cell proliferation. BBR was reported to bind
to DNA directly and to interfere with DNA replication (33), which would be a possible explanation
for the ability of BBR to sensitize breast cancer cells to
chemotherapeutic cisplatin. To address the role of BBR in
regulating cellular DNA repair, we detected the effect of BBR on
expression of proliferating cell nuclear antigen (PCNA), a DNA
sliding clamp required for DNA polδ to replicate DNA and is crucial
in DNA repair (36). BBR
extensively restrained the expression level of PCNA, suggesting
that BBR may decrease the cellular DNA repair ability to sensitize
cells to genotoxic cisplatin.
In conclusion, our data demonstrated that BBR
suppressed breast cancer MCF-7 cell proliferation, colony formation
and migration. A low level of BBR sensitized breast cancer cells to
cisplatin, regulated cleaved caspase-3, caspase-9, Bcl-2 protein
expression, and more importantly, BBR increased the DNA damages
induced by cisplatin and reduced the cellular PCNA level. These
results suggest that a low level of BBR can regulate cellular DNA
repair and promote the DNA breaks induced by cisplatin, further
potentiating the breast cancer cells to cisplatin-induced
apoptosis, which could be one of the mechanisms of BBR action in
antitumor activity. Given the wide application of cisplatin and
other platinum-based drugs in cancer treatment and the relatively
limited side effects of a low dose of BBR, our studies suggest an
alternative approach to circumvent the cancer resistance to
cisplatin and to improve the efficacy of platinum-based
chemotherapeutic treatment. Further studies are needed to determine
the clinical relevance of BBR in combination with cisplatin.
Acknowledgments
We thank the Department of Biotechnology and Cancer
and Stem Cell Research Center, Dalian Medical University for
technical support. This study is supported by Chinese NSF grant
nos. 31371254 and 81201563.
References
|
1
|
Harris JR, Lippman ME, Veronesi U and
Willett W: Breast cancer. N Engl J Med. 327:473–480. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Jemal A, Grey N, Ferlay J and
Forman D: Global cancer transitions according to the Human
Development Index (2008–2030): a population-based study. Lancet
Oncol. 13:790–801. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Buzdar AU: Role of biologic therapy and
chemotherapy in hormone receptor- and HER2-positive breast cancer.
Ann Oncol. 20:993–999. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cohen SM, Mukerji R, Cai S, Damjanov I,
Forrest ML and Cohen MS: Subcutaneous delivery of nanoconjugated
doxorubicin and cisplatin for locally advanced breast cancer
demonstrates improved efficacy and decreased toxicity at lower
doses than standard systemic combination therapy in vivo. Am J
Surg. 202:646–652; discussion 652–653. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fuertes MA, Castilla J, Alonso C and Pérez
JM: Cisplatin biochemical mechanism of action: from cytotoxicity to
induction of cell death through interconnections between apoptotic
and necrotic pathways. Curr Med Chem. 10:257–266. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Leonard BJ, Eccleston E, Jones D, Todd P
and Walpole A: Antileukaemic and nephrotoxic properties of platinum
compounds. Nature. 234:43–45. 1971. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Köberle B, Tomicic MT, Usanova S and Kaina
B: Cisplatin resistance: preclinical findings and clinical
implications. Biochim Biophys Acta. 1806:172–182. 2010.PubMed/NCBI
|
|
8
|
Galluzzi L, Senovilla L, Vitale I, Michels
J, Martins I, Kepp O, Castedo M and Kroemer G: Molecular mechanisms
of cisplatin resistance. Oncogene. 31:1869–1883. 2012. View Article : Google Scholar
|
|
9
|
Shen DW, Pouliot LM, Hall MD and Gottesman
MM: Cisplatin resistance: a cellular self-defense mechanism
resulting from multiple epigenetic and genetic changes. Pharmacol
Rev. 64:706–721. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu FS: Mechanisms of chemotherapeutic
drug resistance in cancer therapy-a quick review. Taiwan J Obstet
Gynecol. 48:239–244. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Imanshahidi M and Hosseinzadeh H:
Pharmacological and therapeutic effects of Berberis vulgaris and
its active constituent, berberine. Phytother Res. 22:999–1012.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kheir MM, Wang Y, Hua L, Hu J, Li L, Lei F
and Du L: Acute toxicity of berberine and its correlation with the
blood concentration in mice. Food Chem Toxicol. 48:1105–1110. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Satou T, Akao N, Matsuhashi R, Koike K,
Fujita K and Nikaido T: Inhibitory effect of isoquinoline alkaloids
on movement of second-stage larvae of Toxocara canis. Biol Pharm
Bull. 25:1651–1654. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kuo CL, Chi CW and Liu TY: The
anti-inflammatory potential of berberine in vitro and in vivo.
Cancer Lett. 203:127–137. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chi L, Peng L, Hu X, Pan N and Zhang Y:
Berberine combined with atorvastatin downregulates LOX-1 expression
through the ET-1 receptor in monocyte/macrophages. Int J Mol Med.
34:283–290. 2014.PubMed/NCBI
|
|
16
|
Zhu Y, Ma N, Li HX, Tian L, Ba YF and Hao
B: Berberine induces apoptosis and DNA damage in MG 63 human
osteosarcoma cells. Mol Med Rep. 10:1734–1738. 2014.PubMed/NCBI
|
|
17
|
Huang ZH, Zheng HF, Wang WL, Wang Y, Zhong
LF, Wu JL and Li QX: Berberine targets epidermal growth factor
receptor signaling to suppress prostate cancer proliferation in
vitro. Mol Med Rep. 11:2125–2128. 2015.
|
|
18
|
Wu K, Yang Q, Mu Y, Zhou L, Liu Y, Zhou Q
and He B: Berberine inhibits the proliferation of colon cancer
cells by inactivating Wnt/β-catenin signaling. Int J oncol.
41:292–298. 2012.PubMed/NCBI
|
|
19
|
Ortiz LM, Lombardi P, Tillhon M and
Scovassi AI: Berberine, an epiphany against cancer. Molecules.
19:12349–12367. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hwang JM, Kuo HC, Tseng TH, Liu JY and Chu
CY: Berberine induces apoptosis through a mitochondria/caspases
pathway in human hepatoma cells. Arch Toxicol. 80:62–73. 2006.
View Article : Google Scholar
|
|
21
|
Lin CC, Yang JS, Chen JT, Fan S, Yu FS,
Yang JL, Lu CC, Kao MC, Huang AC, Lu HF, et al: Berberine induces
apoptosis in human HSC-3 oral cancer cells via simultaneous
activation of the death receptor-mediated and mitochondrial
pathway. Anticancer Res. 27:3371–3378. 2007.PubMed/NCBI
|
|
22
|
Choi MS, Oh JH, Kim SM, Jung HY, Yoo HS,
Lee YM, Moon DC, Han SB and Hong JT: Berberine inhibits
p53-dependent cell growth through induction of apoptosis of
prostate cancer cells. Int J Oncol. 34:1221–1230. 2009.PubMed/NCBI
|
|
23
|
Kim S, Han J, Kim NY, Lee SK, Cho DH, Choi
MY, Kim JS, Kim JH, Choe JH, Nam SJ, et al: Effect of berberine on
p53 expression by TPA in breast cancer cells. Oncol Rep.
27:210–215. 2012.
|
|
24
|
Kim JB, Yu JH, Ko E, Lee KW, Song AK, Park
SY, Shin I, Han W and Noh DY: The alkaloid Berberine inhibits the
growth of Anoikis-resistant MCF-7 and MDA-MB-231 breast cancer cell
lines by inducing cell cycle arrest. Phytomedicine. 17:436–440.
2010. View Article : Google Scholar
|
|
25
|
Katiyar SK, Meeran SM, Katiyar N and
Akhtar S: p53 cooperates berberine-induced growth inhibition and
apoptosis of non-small cell human lung cancer cells in vitro and
tumor xenograft growth in vivo. Mol Carcinog. 48:24–37. 2009.
View Article : Google Scholar
|
|
26
|
Tan W, Li Y, Chen M and Wang Y: Berberine
hydrochloride: anticancer activity and nanoparticulate delivery
system. Int J Nanomedicine. 6:1773–1777. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kang JX, Liu J, Wang J, He C and Li FP:
The extract of Huanglian, a medicinal herb, induces cell growth
arrest and apoptosis by upregulation of interferon-beta and
TNF-alpha in human breast cancer cells. Carcinogenesis.
26:1934–1939. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu J, He C, Zhou K, Wang J and Kang JX:
Coptis extracts enhance the anticancer effect of estrogen receptor
antagonists on human breast cancer cells. Biochem Biophys Res
Commun. 378:174–178. 2009. View Article : Google Scholar
|
|
29
|
Tsang CM, Lau EP, Di K, Cheung PY, Hau PM,
Ching YP, Wong YC, Cheung AL, Wan TS, Tong Y, et al: Berberine
inhibits Rho GTPases and cell migration at low doses but induces G2
arrest and apoptosis at high doses in human cancer cells. Int J Mol
Med. 24:131–138. 2009.PubMed/NCBI
|
|
30
|
Singh T, Vaid M, Katiyar N, Sharma S and
Katiyar SK: Berberine, an isoquinoline alkaloid, inhibits melanoma
cancer cell migration by reducing the expressions of
cyclooxygenase-2, prostaglandin E2 and prostaglandin
E2 receptors. Carcinogenesis. 32:86–92. 2011. View Article : Google Scholar
|
|
31
|
Krey AK and Hahn FE: Berberine: Complex
with DNA. Science. 166:755–757. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Peng PL, Kuo WH, Tseng HC and Chou FP:
Synergistic tumor-killing effect of radiation and berberine
combined treatment in lung cancer: the contribution of autophagic
cell death. Int J Radiat Oncol Biol Phys. 70:529–542. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu Q, Jiang H, Liu Z, Wang Y, Zhao M, Hao
C, Feng S, Guo H, Xu B, Yang Q, et al: Berberine radiosensitizes
human esophageal cancer cells by downregulating homologous
recombination repair protein RAD51. PLoS One. 6:e234272011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tong N, Zhang J, Chen Y, Li Z, Luo Y, Zuo
H and Zhao X: Berberine sensitizes mutliple human cancer cells to
the anticancer effects of doxorubicin in vitro. Oncol Lett.
3:1263–1267. 2012.PubMed/NCBI
|
|
35
|
Guo N, Yan A, Gao X, Chen Y, He X, Hu Z,
Mi M, Tang X and Gou X: Berberine sensitizes rapamycin mediated
human hepatoma cell death in vitro. Mol Med Rep. 10:3132–3138.
2014.PubMed/NCBI
|
|
36
|
Zhu Q, Chang Y, Yang J and Wei Q:
Post-translational modifications of proliferating cell nuclear
antigen: a key signal integrator for DNA damage response (Review).
Oncol Lett. 7:1363–1369. 2014.PubMed/NCBI
|