Introduction
Paclitaxel (PTX) is the first of a class of
microtubule-stabilizing agents and has significant antitumor
activity in clinical treatments against a broad range of solid
tumors, especially against breast, lung and ovarian cancer
(1,2). The first injectable dosage form of PTX
is Taxol®, which is composed of a mixture of
Cremophor® EL and dehydrated alcohol (1:1, v/v) due to
its low solubility. This vehicle may result in a variety of side
effects such as hypersensitivity, nephrotoxicity and neurotoxicity,
attributed mainly to Cremophor EL (3,4). To
overcome the problems caused by Cremophor EL and improve the drug
efficacy, a variety of formulations have been investigated to
administer PTX in a more safe and convenient manner, including
liposomes, emulsions, nanoparticles and polymeric micelles
(5–8). However, two common drawbacks of most
nanomedicines which limit their clinical use are that the materials
used are potentially toxic/allergic and that they are unable to be
up-scaled by industrialized production (9,10).
Lipid emulsion offers potential advantages for the
delivery of poorly water-soluble drugs owing to many favorable
properties, such as nanometer size range with larger surface area,
manufacturing with biocompatible materials, and easy production on
a large scale (11,12). Furthermore, lipid emulsion is a
commonly acceptable formulation used in the clinic for years. Over
the last decade, several studies reported on the benefits of a
cholesterol-rich emulsion termed LDE to deliver therapeutic agents
to cancers (13–16). The LDE resembles the low density
lipoprotein (LDL) lipid portion structure and binds to LDL
receptors on the cancer cell surface. Increased LDL requirements
and LDL receptor numbers have been observed in many types of
tumors, including breast, human glioblastoma and lung tumors
(17–19). Although the LDE has been confirmed
to possess tumor-targeting effects mediated by LDL receptors and
shows good drug loading capacity of PTX, it is stable only 8 days
at 4°C (13). The instability of
LDE-PTX, which may be attributed to the poor lipophilicity of PTX
(14), makes it less promising for
clinical evaluation.
Previous efforts on improving its stability have
been focused on developing the paclitaxel-cholesterol prodrug with
increased lipophilicity (20,21).
What we found is that the lipid emulsion loaded with the
paclitaxel-cholesterol prodrug exhibited long-term stability and
high intratumoral accumulation (20), but the in vivo antitumor
effect was greatly reduced in comparision to Taxol (unpublished
data), which may attribute to the low concentration of PTX released
from the paclitaxel-cholesterol prodrug by in vivo enzymatic
cleavage. Alternatively, a paclitaxel-cholesterol complex connected
by intermolecular hydrogen bonds was prepared and a novel lipid
emulsion (PTX-CH Emul), which was composed of a
paclitaxel-cholesterol complex surrounded by a phospholipid
monolayer, was engineered in our previous study (22). Compared with the tumor-targeting
LDE-PTX loaded with PTX and cholesteryl ester reported previously
(13), substitution of the
paclitaxel-cholesterol complex for PTX and cholesteryl ester of
LDE-PTX would be doubly advantageous: the paclitaxel-cholesterol
complex not only greatly improves PTX solubility in the oil phase,
but can also function as the component of LDL-cholesterol. The
resulting lipid emulsion survives autoclaving and is more stable
than LDE-PTX (12 months at 6°C vs. 8 days at 4°C). The long-term
stability provides conditions for further clinical treatment.
Additionally, combined with the particularly abundant expression
profile of the LDL receptors in breast tumor cells (19), we speculate that the PTX-CH Emul
that mimics the receptor-mediated binding and uptake properties of
LDL could act as an ideal breast tumor-targeting PTX delivery
system.
In this study, we prepared a PTX-CH Emul and
investigated its antitumor activity by comparing it to a
conventional PTX-loaded lipid emulsion (PTX Emul) and Taxol in
vitro and in vivo using breast tumor models, including
2D monolayer tumor cells, 3D multicellular tumor spheroids and
xenograft tumor models. Because PTX is used to treat patients with
triple-negative breast cancer (TNBC) and LDL receptor mRNA is more
abundant in TNBC cells than in non-TNBC cells (19,23),
MDA-MB-231, a human TNBC cell line, was chosen for treatment with
the 3 PTX formulations. Apart from comparing the antitumor
activity, the tumor-targeting effect of PTX-CH Emul was also
investigated by monitoring the intratumoral accumulation in
xenograft tumor models. Interestingly, the results showed that our
present study may provide a successful paradigm for the treatment
of breast tumors and for further clinical applications.
Materials and methods
Materials
Paclitaxel and Taxol® were purchased from
Beijing Union Pharmaceutical Factory (Beijing, China). RPMI-1640
medium, fetal bovine serum (FBS), 0.25% (w/v) trypsin solution and
antibiotic agents were purchased from Invitrogen Life Technologies
(Carlsbad, CA, USA). Phosphate-buffered saline (PBS, pH, 7.4) was
purchased from Hyclone (Logan, UT, USA). Cell Counting Kit-8
(CCK-8) was obtained from Dojindo Laboratories (Tokyo, Japan). Nile
red, fluorescein and Hoechst 33258 were procured from Sigma-Aldrich
(St. Louis, MO, USA). DIR was obtained from AAT Bioquest, Inc.
(Sunnyvale, CA, USA). All solvents such as acetone and acetonitrile
were of analytical or high performance liquid chromatography (HPLC)
grade and were used according to the manufacturer's
instructions.
Preparation of the PTX-CH Emul
PTX-CH Emul was prepared using a solvent evaporation
method and high-pressure homogenization, respectively, as
previously reported (22). The PTX
Emul was prepared using the same method except that the process of
dissolving PTX in oil was different. Briefly, PTX was dissolved in
acetone. Then soybean oil-medium chain triglyceride (1:1, w/w) and
vitamin E acetate were added to this organic phase. Acetone was
removed by evaporation and the oily paste was used as the oil phase
of emulsion. Fluorescein-labeled emulsion (FL emulsion) was
prepared using the same procedure, except that fluorescein or the
fluorescein-cholesterol complex was dissolved in oil in
advance.
For the in vivo tumor-targeting imaging
studies, DiR-encapsulated PTX-CH Emul and DiR-encapsulated PTX Emul
were prepared using the same method as that of PTX-CH Emul and PTX
Emul, respectively.
Characterization of PTX-CH Emul
The emulsion particle size was measured by a PSS
NICOMP Particle Sizing System (PSS, Port Richey, FL, USA) after
appropriate dilution with double-distilled water. The paclitaxel
content of the emulsion was measured using an HPLC system with a UV
detector (Agilent Technologies Inc., Cotati, CA, USA). The emulsion
encapsulation efficiency was determined by measuring free
paclitaxel in the aqueous phase.
Confocal laser scanning microscopy (CLSM) (FV1000;
Olympus Corp., Tokyo, Japan) was employed to visualize the
microenvironment of the major components in the emulsion, lipid
based depot and lipid nanoparticles (6,24). The
fluorescent emulsions were labeled with two fluorescent probes,
Nile red and fluorescein-cholesterol complex. Nile red- and
fluorescein-labeled emulsions were prepared at a load ratio of
0.005% (w/v), diluted with appropriate quantities of distilled
water, and a droplet of the emulsion dispersion was then sealed
between two coverslips for CLSM measurement.
Cell line and cell culture
MDA-MB-231 cells were purchased from the Department
of Cell Resources, Institute of Basic Medical Sciences, Chinese
Academy of Medical Sciences & Peking Union Medical College
(Beijing, China). The cell line was authenticated by the suppliers
and cells were passaged in the laboratory. Cells were maintained in
a 37°C/5% CO2 humidified chamber in RPMI-1640 media
supplemented with 10% FBS, 100 u/ml penicillin and 100 µg/ml
streptomycin.
Animals and human tumor xenografts
Female BALB/c nude mice with a body weight of 21 to
25 g and ages ranging from 6 to 8 weeks were obtained from Beijing
HFK Bioscience Co., Ltd. (Beijing, China). All animal protocols
were approved by the Institutional Animal Care and use Committee.
Female BALB/c nude mice were inoculated subcutaneously in the right
armpits with 0.2 ml (2×106) MDA-MB-231 cell suspension.
Cells were propagated in vivo as solid tumors. The tumors
were implanted subcutaneously as fragments and allowed to grow to a
median size of 100–200 mm3 before treatment was
initiated.
In vitro cytotoxicity assay
The cytotoxicity of Blank Emul, PTX-CH Emul, PTX
Emul and Taxol in the MDA-MB-231 cells was measured using CCK-8
(25). Cells were seeded in 96-well
plates at a density of 3,000 cells/well. After 24 h, the cells were
incubated with PTX-CH Emul, PTX Emul or Taxol at different PTX
concentrations. The sequence of Blank Emul concentrations was
exactly the same as PTX-CH Emul. After 72 h of incubation, the
number of viable cells was determined using CCK-8 according to the
manufacturer's instructions. Untreated control cells were
considered to be 100% viable. The concentration required for 50% or
70% growth inhibition (IC50 or IC70 values,
respectively) was determined with SPSS19.0 software (SPSS Inc.,
Chicago, IL, USA) as the mean ± standard deviation (SD).
In vitro cellular uptake
MDA-MB-231 cells were seeded into 6-well chamber
slides and allowed to attach overnight. The medium was replaced
with culture medium containing fluorescein-DMSO solution (FL-DMSO),
FL emulsion or emulsion loaded with fluorescein-cholesterol complex
(FL-CH emulsion). After incubation for 4 h at 37°C, cells were
washed three times with cold PBS, fixed with 4% paraformaldehyde
(PFA), and the nuclei were counterstained using Hoechst 33258. The
slides were observed using an Olympus FV1000 CLSM (26).
Growth inhibition of 3D breast tumor
spheroids
Ex vitro 3D breast tumor spheroids of
MDA-MB-231 cells were developed using a liquid overlay system
(27). An agarose solution (1.5%,
w/v) was prepared in serum-free RPMI-1640 media by heating at 80°C
for 30 min, followed by sterilization in an autoclave. Each well in
the 96-well plates was coated with a thin layer (50 µl) of
the sterilized agarose solution. For optimal spheroid formation,
2,000 cells were added to each well and medium containing 2.5%
Matrigel (BD Biosciences, San Jose, CA, USA) was added. The plate
was centrifuged for 10 min at 1000 × g and cultured in a standard
cell incubator at 37°C with 5% CO2. Cell culture medium
was changed every 2 days. After 7 days, separate groups of
spheroids were incubated with culture medium, PTX-CH Emul, PTX Emul
or Taxol, each at the PTX concentration of 0.5 µg/ml. growth
inhibition was monitored by measuring the size of the spheroids
using an inverted phase microscope at 0, 1, 3, 5 and 7 days of
treatment. The major (dmax) and minor (dmin)
diameter of each spheroid was determined, and spheroid volume was
calculated using the following formula: V = (π × dmax ×
dmin)/6. The spheroid volume ratio was calculated using
the following formula: R (%) = (Vdayi/Vday0)
× 100, where Vdayi is the spheroid volume at the nth day
(day 1, 3, 5 or 7) of treatment, and Vday0 is the
spheroid volume prior to treatment.
In vivo tumor-targeting imaging
DiR, a near-infrared fluorescent probe, was
encapsulated into PTX Emul and PTX-CH Emul. To investigate the
tumor-targeting efficiency of PTX-CH Emul in the MDA-MB-231
tumor-bearing nude mice, PBS, DiR-encapsulated PTX Emul or
DiR-encapsulated PTX-CH Emul was injected into the mice via the
tail vein at a dose of 200 µg of DiR/kg body weight. The
in vivo imaging was performed at different time points (2,
4, 8, 12 and 24 h) post-injection using the In vivo IVIS
spectrum imaging system (Perkin-Elmer, Wellesley, MA, USA). At 24 h
post-injection, the mice were sacrificed and the tumors and other
major organs (heart, liver, spleen, lung and kidney) were
harvested. The ex vivo imaging of the organs was also
carried out.
Maximum tolerated dose (MTD)
determination
Healthy female BALB/c nude mice were used to
determine the MTD of PTX-CH Emul and Taxol after intravenous
administration. Eight groups of 5–8 female BALB/c nude mice
received intravenous injections of Taxol (20, 30 or 45 mg/kg),
PTX-CH Emul (30, 45, 67.5 or 101.25 mg/kg) or saline (as a control)
via the tail vein on days 0, 4 and 8. The effects of the treatments
were investigated by close observation of survival rates. The MTD
was defined as the dose that caused neither death resulting from
toxic effects nor significant changes in general clinical signs
within 1 week after intravenous administration of the
aforementioned formulations.
In vivo antitumor effect
The antitumor efficacy and toxicity profiles of the
different PTX formulations were evaluated in a subcutaneous
MDA-MB-231 xenograft model in female BALB/c nude mice. The
treatments commenced when the tumors in the nude mice reached a
volume of 100–200 mm3, and this day was designated as
day 0. On day 0, the mice were randomly divided into 3 groups of
8–9 mice each. The mice were intravenously administered PBS, Taxol
(20 mg/kg) or PTX-CH Emul (45 mg/kg), respectively. The treatments
were conducted every 4 days for a total of 3 doses. Tumor size was
measured with a digital caliper every 3 days. Tumor volume was
calculated by the formula of (LxW2)/2, where L is the
longest tumor dimension (mm) and W is the shortest tumor dimension
(mm). The time required for a tumor to double in volume was
calculated based on the initial tumor volume at the beginning of
the treatment. Tumor recurrence was defined as the first
observation of increased tumor size following tumor regression. The
toxicity after treatment was monitored by observing behavior and
measuring body weight every 3 days.
Statistical analysis
All data subjected to statistical analysis were
obtained from at least 3 parallel experiments, and the results are
expressed as mean ± standard deviation (SD). Tumor doubling time
and time to tumor recurrence were analyzed by Kaplan-Meier
analysis, according to GraphPad Prism version 5.01 for Windows
(GraphPad®, Inc., Software, San Diego, CA, USA).
Statistical analysis was performed by Student's t-test for two
groups, and one way ANOVA for multiple groups. A value of P<0.05
was considered statistically significant.
Results
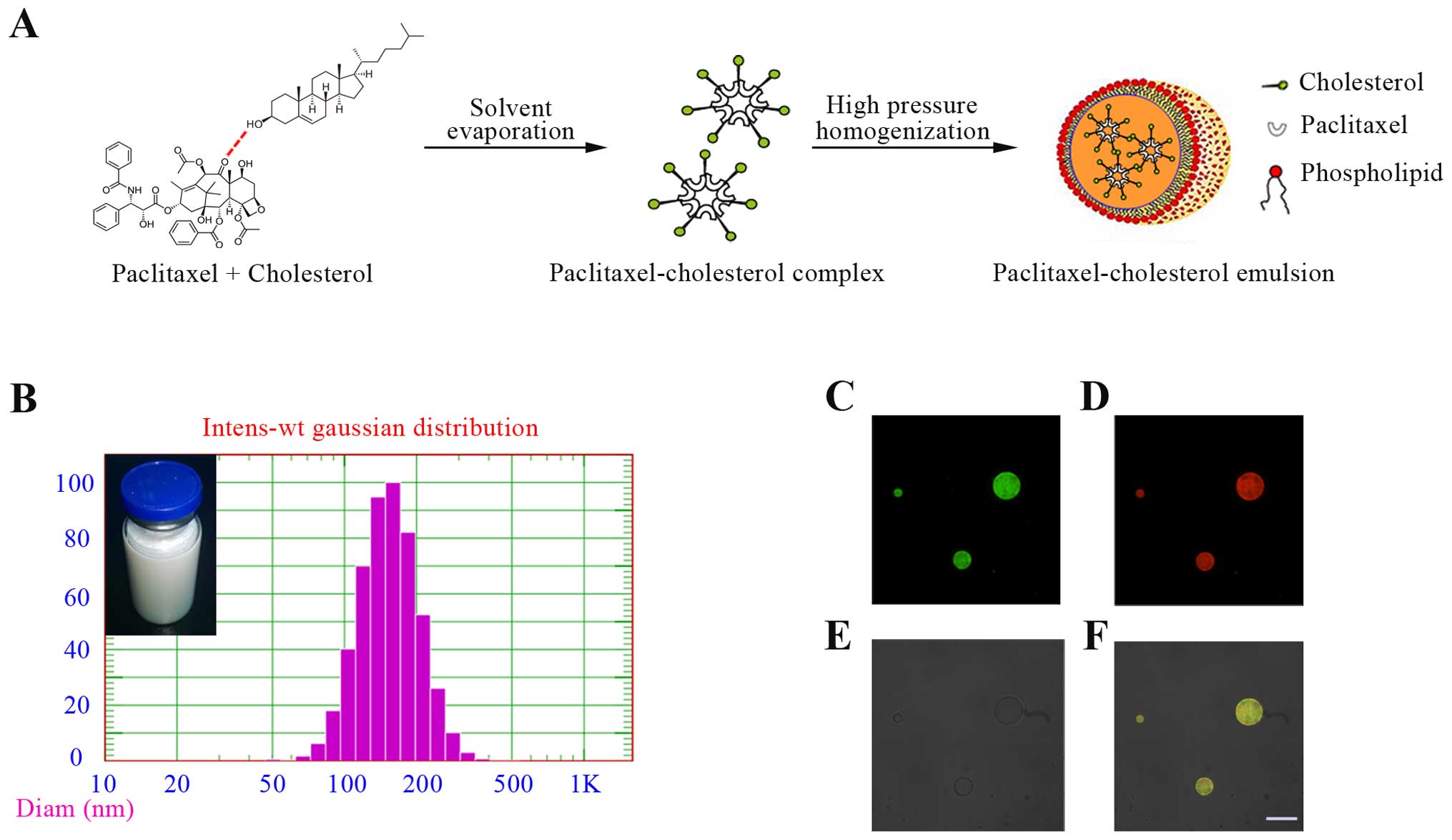
Characterization of PTX-CH Emul
The characteristics of PTX-CH Emul were previously
investigated (22). PTX-CH Emul,
which is composed of a paclitaxel-cholesterol complex surrounded by
a phospholipid monolayer, resembles the lipid structure of native
LDL (Fig. 1A). The resulting
emulsion had an encapsulation efficiency of 97.3% and a mean
particle size of 150 nm (Fig. 1B).
Moreover, the formulation survived autoclaving at 115°C for 30 min
and remained stable for at least 12 months at 6°C. PTX-CH Emul was
also substantially better tolerated than the corresponding dosages
of Taxol in guinea pigs, as no evident hypersensitivity reaction
was observed. These results demonstrated that PTX-CH Emul has
excellent potential for industrial-scale production and clinical
application.
CLSM is a useful tool in the investigation of the
microstructure of emulsions (26,27).
In our study, the distribution of the fluorescein-cholesterol
complex and oil in the emulsion was determined by CLSM. Oil was
labeled with a lipid probe, Nile red, a hydrophobic fluorescent
marker with special fluorescent properties. Nile red emitted red
light and was excited to visualize the distribution of the oil core
in the emulsion. Fluorescein emitted green light was excited to
visualize the distribution of the fluorescein-cholesterol complex
in the emulsion. Yellow light was observed when Nile red and
fluorescein were both excited and localized in the same region. The
micrographs revealed a uniform distribution of Nile red and
fluorescein in the emulsion. The green and red regions completely
overlapped, indicating that the fluorescein-cholesterol complex and
oil phase were evenly distributed throughout the emulsion, without
phase separation (Fig. 1C–F).
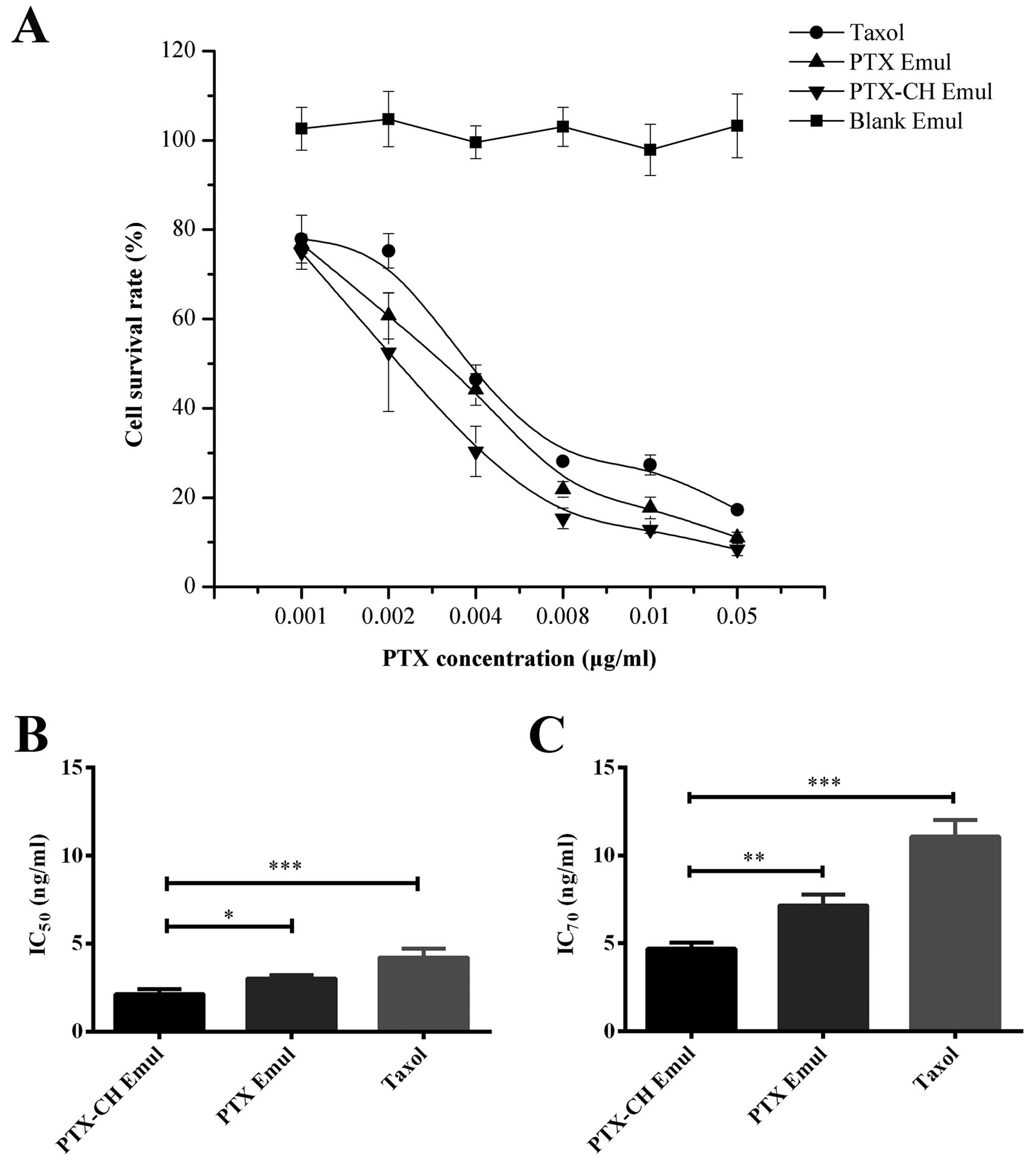
In vitro cytotoxicity assay
The cytotoxicity of Blank Emul, PTX-CH Emul, PTX
Emul and Taxol was evaluated using MDA-MB-231 cells. PTX decreased
cell viability in a concentration-dependent manner in the
concentration range of 0.001–0.05 µg/ml (Fig. 2A). The cell-survival rate in the
group treated with PTX-CH Emul was significantly lower than that in
the groups treated with PTX Emul and Taxol after 72 h,
demonstrating that PTX-CH Emul had better anticancer efficacy than
PTX Emul and Taxol.
An important parameter for quantitatively evaluating
the in vitro cytotoxicity of an anticancer drug is the
IC50 or IC70, which represents the drug
concentration needed to kill 50% or 70% of the cancer cells at a
designated time (28,29). Fig. 2B
and C shows the IC50 and IC70 values of
PTX-CH Emul, PTX Emul and Taxol in the MDA-MB-231 cells after a
72-h incubation, respectively. Among the different PTX
formulations, PTX-CH Emul had the lowest IC50 value
(2.13±0.28 ng/ml), followed by PTX Emul (3.02±0.19 ng/ml,
P<0.05) and Taxol (4.22±0.52 ng/ml, P<0.001). If
IC70 value was used, the differences were more
pronounced since the IC70 value of PTX-CH Emul
(4.69±0.36 ng/ml) was significantly lower than that of all the
other formulations, only ~1.5- and 2.4-fold lower than that of PTX
Emul (7.14±0.63 ng/ml, P<0.01) and Taxol (11.07±0.93 ng/ml,
P<0.001) respectively. These results demonstrated that PTX-CH
Emul exhibited superior cytotoxicity as compared to PTX Emul and
Taxol. Additionally, there was no cellular cytotoxicity caused by
the Blank Emul at the investigated concentrations, eliminating the
possibility that the lipid emulsions themselves were responsible
for the cytotoxicity.
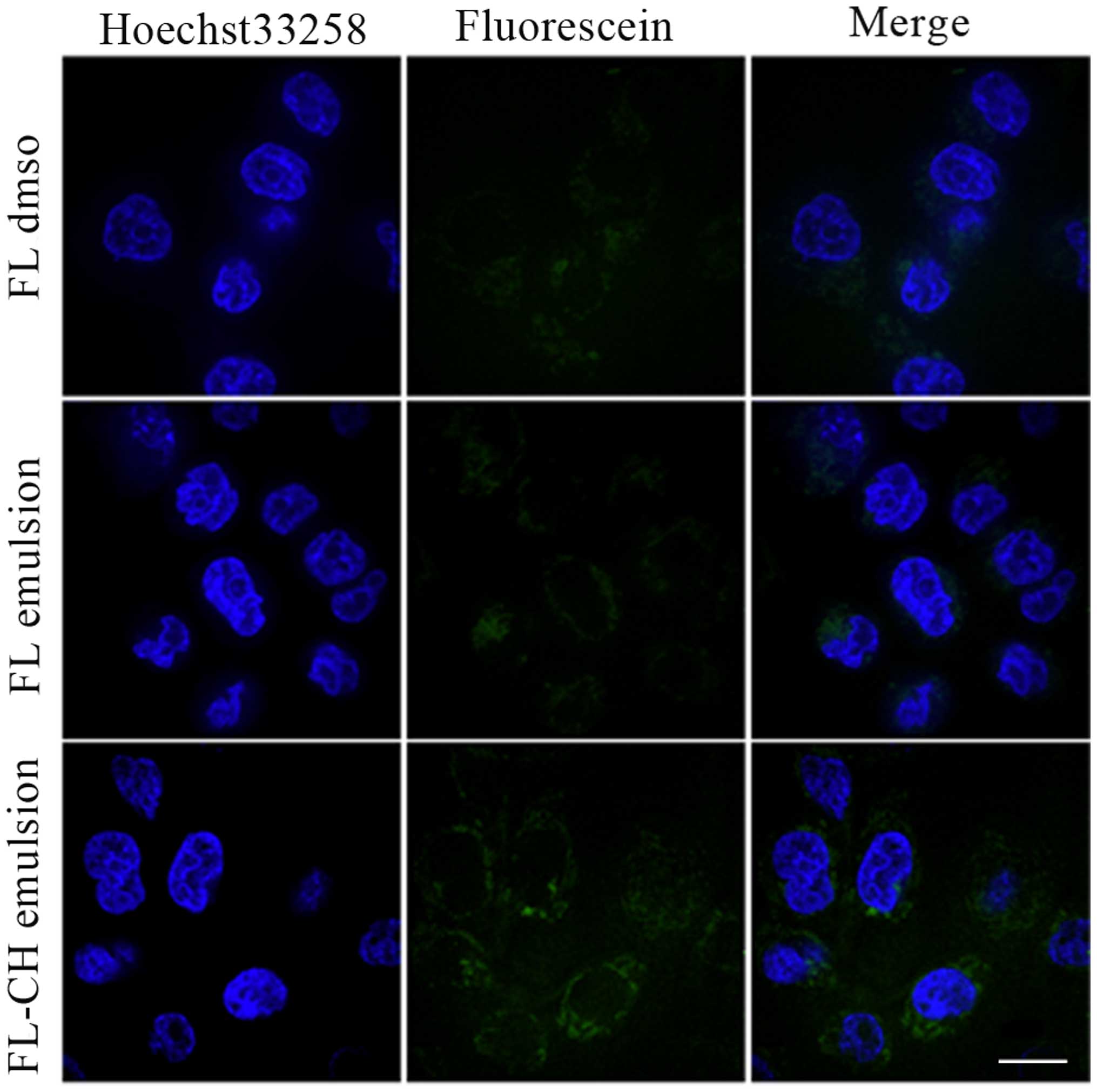
In vitro cellular uptake
The cellular uptake profiles of FL-DMSO, FL emulsion
and FL-CH emulsion in the MDA-MB-231 cells were assessed
qualitatively using CLSM. As shown in Fig. 3, FL-CH emulsion exhibited
significantly greater uptake in comparison with FL-DMSO and FL
emulsion. This result demonstrated that emulsion loaded with a
fluorescein-cholesterol complex significantly facilitated the
uptake of fluorescein by the MDA-MB-231 cells.
Growth inhibition of 3D breast tumor
spheroids
In vitro 3D tumor spheroids provide an
important link between monolayer cell cultures and animal models
for evaluating drug delivery efficiency (30,31).
3D tumor spheroids generated by the liquid overlay technique
contain aggregates of cells in close contact and an organized
extracellular matrix consisting of fibronectin, laminin and
collagen, which is similar to the extracellular matrix of tumors
in vivo (32). Therefore, 3D
breast tumor spheroids were used to evaluate the inhibitory effect
of PTX-CH Emul on cell growth. 3D tumor spheroid growth was
evaluated following the treatment of cells with culture medium
containing PTX-CH Emul, PTX Emul or Taxol. In regular cell culture
medium, 3D tumor spheroids of MDA-MB-231 cells grew rapidly and
became more compact with the increase in culture time. However, in
the presence of PTX Emul or Taxol, the tumor spheroids stopped
growing or even became smaller. Distinctly, treatment of cells with
PTX-CH Emul resulted in shrinkage of the spheroids, with the
concomitant loss of 3D structures due to the dissociation of some
cells from the spheroids, indicating that the PTX-CH Emul
effectively inhibited tumor cell proliferation (Fig. 4A). After 7 days of treatment, the
tumor spheroid volume ratios were 190.2±21.2% for the control
group, 69.5±11.8% for the Taxol group, 54.8±6.2% for the PTX Emul
group and 19.9±4.0% for the PTX-CH Emul group (Fig. 4B). All things considered, PTX-CH
Emul exhibited much stronger inhibitory effects on tumor spheroids
in comparison with Taxol and PTX Emul.
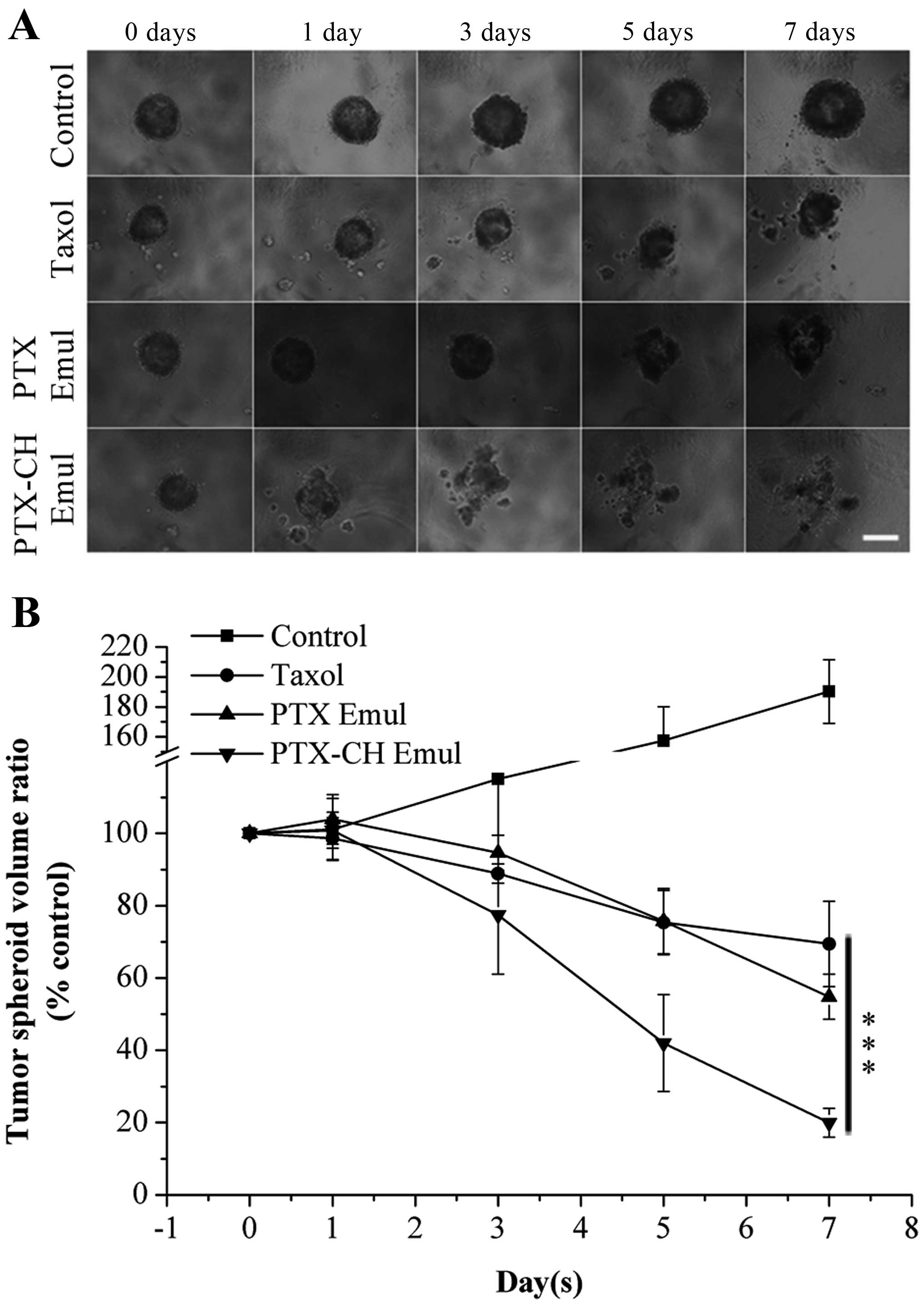 | Figure 4(A) Inhibition of MDA-MB-231 tumor
spheroid growth was evaluated following the treatment with PTX-CH
Emul, PTX Emul and Taxol, respectively, at a paclitaxel
concentration of 0.5 µg/ml with those treated with culture
medium as the blank control. The images were obtained at 0, 1, 3, 5
and 7 days under inverted microscope. Scale bar, 300 µm. (B)
Changes in MDA-MB-231 tumor spheroid volumes (%) after treatment
with various paclitaxel formulations (PTX-CH Emul, PTX Emul and
Taxol) and culture medium. Each value represents the mean ± SD
(n=6). ***P<0.001. PTX Emul, paclitaxel emulsion;
PTX-CH Emul, paclitaxel-cholesterol complex emulsion. |
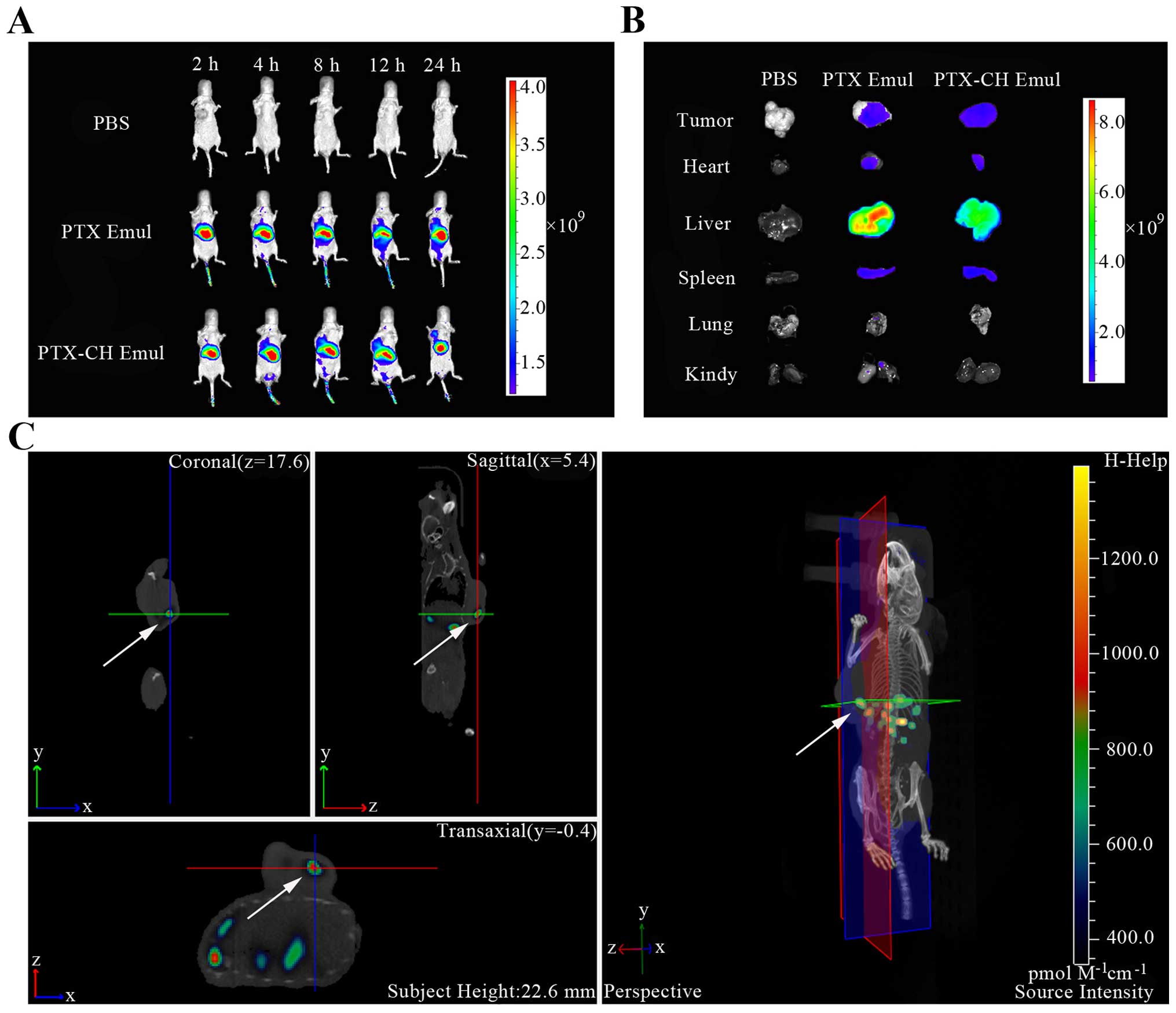
In vivo tumor-targeting imaging
The tumor-targeting efficiency of PTX-CH Emul was
evaluated in nude mice bearing MDA-MB-231 tumors. After the
DiR-labeled emulsions were injected through the tail vein, the
time-dependent biodistribution of different emulsions was monitored
using non-invasive NIR fluorescence imaging in live animals. As
shown in Fig. 5A, at all time
points post-injection, the tumor fluorescence in DiR-PTX-CH
Emul-treated mice was higher than that in the DiR-PTX Emul-treated
mice at equivalent doses of DiR. Twenty-four hours after injection,
3D reconstruction of DiR-PTX-CH Emul-treated mice was performed. As
shown in Fig. 5C, the fluorescence
was primarily found at tumor sites, indicating that the DiR-PTX-CH
Emul may serve as an effective tumor-targeting vehicle by which to
deliver chemotherapeutic drugs to tumor tissues. Additionally, the
ex vivo fluorescent image from the excised tumors also
clearly showed higher fluorescence from the DiR-PTX-CH Emul-treated
group over that from the DiR-PTX Emul-treated one (Fig. 5B), which was further evidence of the
higher tumor-targeting efficiency of PTX-CH Emul. All these
findings indicated that PTX-CH Emul enabled specific
tumor-targeting efficiency, which could contribute to the selective
increase in the therapeutic efficiency.
Maximum tolerated dose
The MTD of Taxol in healthy BALB/c nude mice is 20
mg/kg, consistent with the previously reported results (33). PTX-CH Emul was significantly less
toxic than Taxol, and the MTD of PTX-CH Emul was 2.25-fold higher
than that of Taxol (45 mg/kg vs. 20 mg/kg, respectively). A
previous study reported that the MTD of Abraxane® is
2.24-fold higher than that of Taxol (30 mg/kg vs. 13.4 mg/kg,
respectively) (34), which is
essentially the same as that reported here. Based on these results,
MTD values of 45 mg/kg and 20 mg/kg were chosen for PTX-CH Emul and
Taxol, respectively, for the in vivo antitumor efficiency
study.
In vivo antitumor efficiency
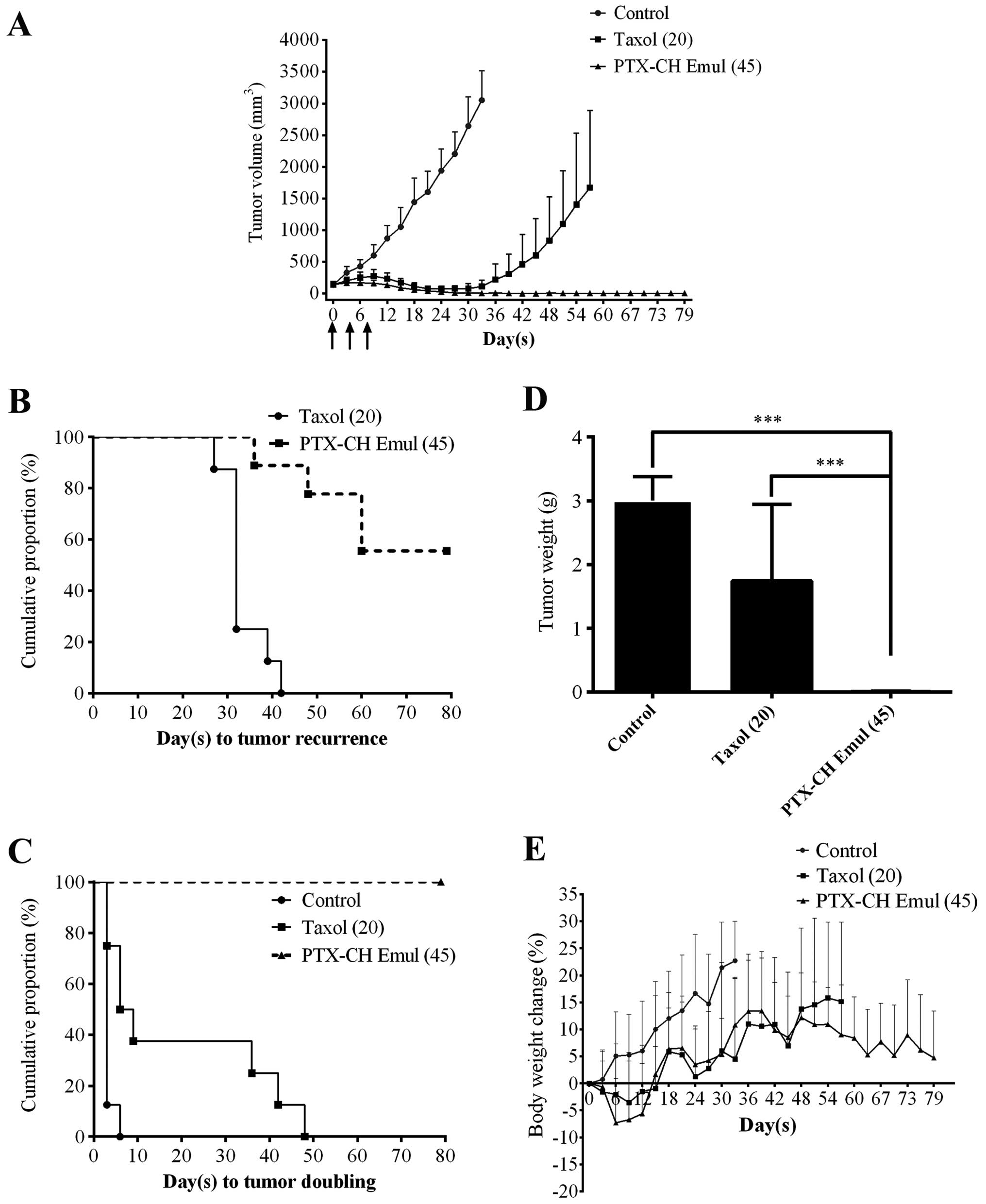
Both Taxol and PTX-CH Emul significantly inhibited
tumor growth in comparison with the control group (Fig. 6A). At the MTDs of PTX-CH Emul and
Taxol (45 and 20 mg/kg, respectively), the proportion of tumor-free
survivors in the PTX-CH Emul group (7 of 9) was higher than that in
the Taxol group (0 of 8) (Table I).
Equally as important, PTX-CH Emul showed superior antitumor
activity to Taxol as measured by median time to tumor recurrence
[>79 days vs. 32 days (P=0.0001)] (Fig. 6B and Table I), tumor doubling time [>79 days
vs. 7.5 days (P<0.0001)] (Fig.
6C and Table I), tumor volume
(P<0.01) (Fig. 6A) and tumor
weight (P<0.001) (Fig. 6D).
These results demonstrated that PTX-CH Emul possessed higher
antitumor efficacy as compared with Taxol at the MTDs.
 | Table IAntitumor activity of PTX-CH Emul and
Taxol at the MTDs |
Table I
Antitumor activity of PTX-CH Emul and
Taxol at the MTDs
| Xenograft
treatment | Tumor-free
survivors | Recurrence
(days)a | P-valuec | Tumor doubling
(days)b | P-valuec |
|---|
| Control | 0/8 | – | – | 3.0 | <0.0001 |
| Taxol®
(20 mg/kg) | 0/8 | 32 | 0.0001 | 7.5 | <0.0001 |
| PTX-CH Emul (45
mg/kg) | 7/9 | >79 | – | >79.0 | – |
Toxicity was assessed by direct observation of
animal behavior and body weight. Mice treated with 20 mg/kg Taxol
showed reduced overall activity, which was likely a sign of a
hypersensitivity reaction to the diluent. In contrast, the mice
treated with PTX-CH Emul tolerated the regimens well. As shown in
Fig. 6E, there was no significant
difference in body weight loss between mice treated with PTX-CH
Emul and Taxol at the MTDs. Mice had reduced body weight initially
after treatment with the aforementioned formulations but gradually
began to grow normally after they adapted to the challenges.
Discussion
It has been previously reported that an artificial
emulsion resembling the lipid structure of LDL, designated as LDE,
has the ability to bind to LDL receptors. Upon intravenous
administration, LDE picks up circulating apolipoproteins (apo),
such as apo E, from the native lipoproteins. The LDL receptor
recognizes and binds apo E with high affinity, thereby coupling the
LDE particles to the receptor and enabling internalization of LDE
into the cytoplasm (13,35). Accumulated evidence has demonstrated
that many tumor cells have high LDL requirements and LDL receptors
are overexpressed in many tumor cells due to the rapid growth of
these malignant cells and their requirement for structural lipids
(17–19). In a previous study, Rodrigues et
al reported that PTX can be associated at high rates with LDE
(LDE-PTX), which is developed for the targeted treatment of lung
carcinoma. However, LDE-PTX is stable only for 8 days at 4°C and
tends to dissociate in the bloodstream after injection into mice
(13,14). Long-term stability is one of the
essential elements for druggability (6). The instability of LDE-PTX makes it
less promising for clinical evaluation. In our previous study, we
successfully developed a PTX-CH Emul resembling the LDL lipid
structure, which is composed of a paclitaxel-cholesterol complex
surrounded by a phospholipid monolayer (22). Compared with the mixture of PTX and
cholesteryl ester in LDE-PTX, the paclitaxel-cholesterol complex
connected by intermolecular hydrogen bonds between PTX and
cholesterol is used as the drug carrier of the PTX-CH Emul. The
paclitaxel-cholesterol complex not only dramatically improves the
lipophilicity of PTX, but can also function as the component of
LDL-cholesterol. The resulting emulsion exhibits an ideal particle
size, high drug loading capability, high drug encapsulation
efficiency and excellent stability (12 months at 6°C). The
long-term stability of PTX-CH Emul is of critical importance not
only for therapeutic purposes but also for the development of the
formulation since stability is of major concern to the
pharmaceutical industry.
Since LDL receptors have been identified to be
overexpressed in breast cancer cells and the LDL receptor mRNA
level in TNBC cells is more abundant than the level in non-TNBC
cells (19), MDA-MB-231, a human
TNBC cell line was used in this study. TNBC is an aggressive,
heterogeneous subclass of breast cancer typically of basal origin,
accounting for 15–20% of all invasive breast cancers.
Unfortunately, TNBC presents a difficult clinical challenge as it
is ultimately refractory to chemotherapy, and displays a shorter
median time to relapse and death than other subtypes of breast
cancer (36,37). Therefore, treatment of the
MDA-MB-231 xenograft tumor model with PTX-CH Emul provides a
stringent assessment of the potential clinical utility of this
formulation.
In order to evaluate and compare the in vitro
antitumor efficacy of PTX-CH Emul and Taxol®, 2D
monolayer tumor cells and 3D multicellular tumor spheroids were
used as the models. Our in vitro cytotoxicity study of 2D
monolayer cells demonstrated that no cytotoxicity of Blank Emul was
observed in the MDA-MB-231 cells. When lipid emulsions were loaded
with the paclitaxel-cholesterol complex, they exhibited superior
cytotoxicity compared to Taxol as reflected by the corresponding
IC50 and IC70 values. Previous studies
reported that the LDE, which is basically composed of a cholesteryl
ester core surrounded by a phospholipid monolayer, could bind to
LDL receptors (13,35). Therefore, the PTX-CH Emul reported
here, which is composed of a paclitaxel-cholesterol complex core
surrounded by a phospholipid monolayer, may serve as a
tumor-targeting vehicle for PTX directed against MDA-MB-231 cells
with abundant LDL receptors thus greatly improving the in
vitro cytotoxicity of PTX. In order to verify this hypothesis,
a comparative in vitro experiment was performed to test the
antitumor effects of PTX-CH Emul and PTX Emul. In contrast to
PTX-CH Emul, PTX Emul, which is basically composed of a paclitaxel
core, does not resemble the lipid structure of LDL. As reflected by
the corresponding IC50 and IC70 values,
PTX-CH Emul showed better cytotoxicity compared to PTX Emul.
Additionally, the result of in vitro cellular uptake
demonstrated that FL-CH emulsion exhibited significantly greater
uptake in MDA-MB-231 cells as compared to FL solution and FL
emulsion. Therefore, we surmise that the superior cytotoxicity of
PTX-CH Emul over other tested PTX formulations may be attributed to
the tumor-targeting effect of PTX-CH Emul. However, whether the
tumor-targeting effect of PTX-CH Emul is mediated by LDL receptors
still remains to be confirmed in our future studies.
Compared with the conventional 2D monolayer model,
the 3D tumor spheroid model has a closer resemblance to cells
growing in the in vivo tissue microenvironment by mimicking
many of the physiological characteristics of the native tumor
environment, including complex multicellular architecture, barriers
to mass transport, and extracellular matrix deposition. More
importantly, 3D tumor spheroids are more chemo-resistant compared
to monolayer cells and thus could serve as excellent models for
evaluating the efficiency of drug delivery systems (38,39).
Similar to the results of in vitro cytotoxicity in the 2D
monolayer tumor cells, PTX-CH Emul exhibited stronger and faster
inhibitory effects on 3D tumor spheroids as compared with PTX Emul
and Taxol. All in all, the enhanced 3D multicellular tumor spheroid
growth inhibition of PTX-CH Emul may be attributed to the superior
cytotoxicity in the 2D monolayer MDA-MB-231 cells and the
capability of LDL receptors to facilitate tumor penetration and
tumor accumulation of PTX-CH Emul in 3D tumor spheroids.
In order to evaluate tumor-targeting efficiency and
monitor the real-time biodistribution of PTX-CH Emul in
vivo, non-invasive near infrared fluorescence optical imaging
technology was employed. Compared to the conventional PTX Emul,
PTX-CH Emul primarily accumulated in tumors in the mice-bearing
MDA-MB-231 xenografts after intravenous administration, possibly as
a result of both enhanced permeability and retention (EPR) effects
and LDL receptor-mediated tumor-targeting effects.
To evaluate the in vivo antitumor efficiency
of PTX-CH Emul on MDA-MB-231 tumor-bearing mice, PBS, PTX-CH Emul
(45 mg/kg) or Taxol (20 mg/kg) was intravenously injected into
mice. As chemotherapy is generally administered at the highest
tolerated dose, the comparison of MTDs, rather than equal doses, is
considered to be more clinically relevant (34). The MTD of PTX-CH Emul was 2.25-fold
of that of Taxol (45 vs. 20 mg/kg, respectively), which was
basically the same as the fold ratio of the MTD of Abraxane to that
of Taxol (30 vs. 13.4 mg/kg, 2.24-fold) as previously reported
(34). The result of MTD indicated
that PTX-CH Emul exhibited better safety profiles in vivo
than Taxol. At the MTDs, PTX-CH Emul exhibited higher antitumor
activity in the TNBC models as compared to Taxol. There are several
possible explanations for the enhanced antitumor efficacy of PTX-CH
Emul after intravenous injection. Firstly, PTX-CH Emul has a high
tumor-targeting ability, thus resulting in a higher tumor
accumulation as compared with PTX Emul. Secondly, the much stronger
and faster inhibitory effects of PTX-CH Emul on 2D monolayer cells
and 3D multicellular tumor spheroids should contribute to the
enhanced antitumor efficacy of PTX-CH Emul.
In conclusion, the results of the present study
suggest that the lipid emulsion based on the paclitaxel-cholesterol
complex reported here has great clinical potential for the
treatment of breast tumors.
Acknowledgments
This study was financially supported by the national
Mega-project for Innovative Drugs (no. 2012ZX09301002-001) and
Beijing Municipal Science & Technology Commission Preclinical
Research Projects (no. 500101009).
References
|
1
|
Rowinsky EK and Donehower RC: Paclitaxel
(Taxol). N Engl J Med. 332:1004–1014. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rowinsky EK, Cazenave LA and Donehower RC:
Taxol: a novel investigational antimicrotubule agent. J Natl Cancer
Inst. 82:1247–1259. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weiss RB, Donehower RC, Wiernik PH, Ohnuma
T, Gralla RJ, Trump DL, Baker JR Jr, Van Echo DA, Von Hoff DD and
Leyland-jones B: Hypersensitivity reactions from Taxol. J Clin
Oncol. 8:1263–1268. 1990.PubMed/NCBI
|
|
4
|
Singla AK, Garg A and Aggarwal D:
Paclitaxel and its formulations. Int J Pharm. 235:179–192. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koudelka S and Turanek J: Liposomal
paclitaxel formulations. Journal Control Release. 163:322–334.
2012. View Article : Google Scholar
|
|
6
|
Jing X, Deng L, Gao B, Xiao L, Zhang Y, Ke
X, Lian J, Zhao Q, Ma L, Yao J, et al: A novel polyethylene glycol
mediated lipid nanoemulsion as drug delivery carrier for
paclitaxel. Nanomedicine. 10:371–380. 2014.
|
|
7
|
Zhang Z and Feng SS: The drug
encapsulation efficiency, in vitro drug release, cellular uptake
and cytotoxicity of paclitaxel-loaded poly(lactide)-tocopheryl
polyethylene glycol succinate nanoparticles. Biomaterials.
27:4025–4033. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huh KM, Min HS, Lee SC, Lee HJ, Kim S and
Park K: A new hydrotropic block copolymer micelle system for
aqueous solubilization of paclitaxel. J Control Release.
126:122–129. 2008. View Article : Google Scholar
|
|
9
|
Joshi MD and Muller RH: Lipid
nanoparticles for parenteral delivery of actives. Eur J Pharm
Biopharm. 71:161–172. 2009. View Article : Google Scholar
|
|
10
|
Lammers T, Kiessling F, Hennink WE and
Storm G: Drug targeting to tumors: principles, pitfalls and (pre-)
clinical progress. J Control Release. 161:175–187. 2012. View Article : Google Scholar
|
|
11
|
Constantinides PP, Tustian A and Kessler
DR: Tocol emulsions for drug solubilization and parenteral
delivery. Adv Drug Deliv Rev. 56:1243–1255. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Collins-gold L, Lyons R and Bartholow L:
Parenteral emulsions for drug delivery. Adv Drug Deliv Rev.
5:189–208. 1990. View Article : Google Scholar
|
|
13
|
Rodrigues DG, Covolan CC, Coradi ST,
Barboza R and Maranhão RC: Use of a cholesterol-rich emulsion that
binds to low-density lipoprotein receptors as a vehicle for
paclitaxel. J Pharm Pharmacol. 54:765–772. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rodrigues DG, Maria DA, Fernandes DC,
Valduga CJ, Couto RD, Ibañez OC and Maranhão RC: Improvement of
paclitaxel therapeutic index by derivatization and association to a
cholesterol-rich microemulsion: in vitro and in vivo studies.
Cancer Chemother Pharmacol. 55:565–576. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pires LA, Hegg R, Valduga CJ, Graziani SR,
Rodrigues DG and Maranhão RC: Use of cholesterol-rich nanoparticles
that bind to lipoprotein receptors as a vehicle to paclitaxel in
the treatment of breast cancer: pharmacokinetics, tumor uptake and
a pilot clinical study. Cancer Chemother Pharmacol. 63:281–287.
2009. View Article : Google Scholar
|
|
16
|
Teixeira RS, Valduga CJ, Benvenutti LA,
Schreier S and Maranhão RC: Delivery of daunorubicin to cancer
cells with decreased toxicity by association with a lipidic
nanoemulsion that binds to LDL receptors. J Pharm Pharmacol.
60:1287–1295. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vitols S, Peterson C, Larsson O, Holm P
and Aberg B: Elevated uptake of low density lipoproteins by human
lung cancer tissue in vivo. Cancer Res. 52:6244–6247.
1992.PubMed/NCBI
|
|
18
|
Maletínská L, Blakely EA, Bjornstad KA,
Deen DF, Knoff LJ and Forte TM: Human glioblastoma cell lines:
levels of low-density lipoprotein receptor and low-density
lipoprotein receptor-related protein. Cancer Res. 60:2300–2303.
2000.PubMed/NCBI
|
|
19
|
Stranzl A, Schmidt H, Winkler R and
Kostner GM: Low-density lipoprotein receptor mRNA in human breast
cancer cells: influence by PKC modulators. Breast Cancer Res Treat.
42:195–205. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xia X, Song X, Xu J, He J, Peng J, Zhang
X, Jin D, Abliz Z and Liu Y: Development of a validated
LC-APCI-MS/MS method to study the plasma and tumor distribution of
CHO-PTX intravenous lipid emulsion. J Pharm Biomed Anal.
117:532–543. 2016. View Article : Google Scholar
|
|
21
|
Stevens PJ, Sekido M and Lee RJ: A folate
receptor-targeted lipid nanoparticle formulation for a lipophilic
paclitaxel prodrug. Pharm Res. 21:2153–2157. 2004. View Article : Google Scholar
|
|
22
|
Xia XJ, Guo RF, Liu YL, Zhang PX, Zhou CP,
Jin DJ and Wang RY: Formulation, characterization and
hypersensitivity evaluation of an intravenous emulsion loaded with
a paclitaxel-cholesterol complex. Chem Pharm Bull (Tokyo).
59:321–326. 2011. View Article : Google Scholar
|
|
23
|
Torrisi R, Balduzzi A, Ghisini R, Rocca A,
Bottiglieri L, Giovanardi F, Veronesi P, Luini A, Orlando L, Viale
G, et al: Tailored preoperative treatment of locally advanced
triple negative (hormone receptor negative and HER2 negative)
breast cancer with epirubicin, cisplatin, and infusional
fluorouracil followed by weekly paclitaxel. Cancer Chemother
Pharmacol. 62:667–672. 2008. View Article : Google Scholar
|
|
24
|
Wang Q, Gong T, Sun X and Zhang Z:
Structural characterization of novel phospholipid lipid
nanoparticles for controlled drug delivery. Colloids Surf B
Biointerfaces. 84:406–412. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang R, Luo K, Yang J, Sima M, Sun Y,
Janát-Amsbury MM and Kopeček J: Synthesis and evaluation of a
backbone biodegradable multiblock HPMA copolymer nanocarrier for
the systemic delivery of paclitaxel. J Control Release. 166:66–74.
2013. View Article : Google Scholar :
|
|
26
|
Xiao K, Li Y, Lee JS, Gonik AM, Dong T,
Fung G, Sanchez E, Xing L, Cheng HR, Luo J, et al: 'OA02' peptide
facilitates the precise targeting of paclitaxel-loaded micellar
nanoparticles to ovarian cancer in vivo. Cancer Res. 72:2100–2110.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nagelkerke A, Bussink J, Sweep FC and Span
PN: Generation of multicellular tumor spheroids of breast cancer
cells: how to go three-dimensional. Anal Biochem. 437:17–19. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bonneau C, Rouzier R, Geyl C, Cortez A,
Castela M, Lis R, Daraï E and Touboul C: Predictive markers of
chemoresistance in advanced stages epithelial ovarian carcinoma.
Gynecol Oncol. 136:112–120. 2015. View Article : Google Scholar
|
|
29
|
Bernabeu E, Helguera G, Legaspi MJ,
Gonzalez L, Hocht C, Taira C and Chiappetta DA: Paclitaxel-loaded
PCL-TPGS nanoparticles: in vitro and in vivo performance compared
with Abraxane®. Colloids Surf B Biointerfaces.
113:43–50. 2014. View Article : Google Scholar
|
|
30
|
Mehta G, Hsiao AY, Ingram M, Luker GD and
Takayama S: Opportunities and challenges for use of tumor spheroids
as models to test drug delivery and efficacy. J Control Release.
164:192–204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pampaloni F, Reynaud EG and Stelzer EH:
The third dimension bridges the gap between cell culture and live
tissue. Nat Rev Mol Cell Biol. 8:839–845. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Davies CD, Müller H, Hagen I, Gårseth M
and Hjelstuen MH: Comparison of extracellular matrix in human
osteosarcomas and melanomas growing as xenografts, multicellular
spheroids, and monolayer cultures. Anticancer Res. 17:4317–4326.
1997.
|
|
33
|
Kim SC, Kim DW, Shim YH, Bang JS, Oh HS,
Wan Kim S and Seo MH: In vivo evaluation of polymeric micellar
paclitaxel formulation: toxicity and efficacy. J Control Release.
72:191–202. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Desai N, Trieu V, Yao Z, Louie L, Ci S,
Yang A, Tao C, De T, Beals B, Dykes D, et al: Increased antitumor
activity, intratumor paclitaxel concentrations, and endothelial
cell transport of cremophor-free, albumin-bound paclitaxel,
ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res.
12:1317–1324. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Maranhão RC, Cesar TB, Pedroso-Mariani SR,
Hirata MH and Mesquita CH: Metabolic behavior in rats of a
nonprotein microemulsion resembling low-density lipoprotein.
Lipids. 28:691–696. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bauer KR, Brown M, Cress RD, Parise CA and
Caggiano V: Descriptive analysis of estrogen receptor
(ER)-negative, progesterone receptor (PR)-negative, and
HER2-negative invasive breast cancer, the so-called triple-negative
phenotype: a population-based study from the California Cancer
Registry. Cancer. 109:1721–1728. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hudis CA and Gianni L: Triple-negative
breast cancer: an unmet medical need. Oncologist. 16(Suppl 1):
1–11. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lei H, Hofferberth SC, Liu R, Colby A,
Tevis KM, Catalano P, Grinstaff MW and Colson YL: Paclitaxel-loaded
expansile nanoparticles enhance chemotherapeutic drug delivery in
mesothelioma 3-dimensional multicellular spheroids. J Thorac
Cardiovasc Surg. 149:1417–1425. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xin H, Sha X, Jiang X, Zhang W, Chen L and
Fang X: Anti-glioblastoma efficacy and safety of paclitaxel-loading
Angiopep-conjugated dual targeting PEG-PCL nanoparticles.
Biomaterials. 33:8167–8176. 2012. View Article : Google Scholar : PubMed/NCBI
|