Introduction
Breast cancer is the most frequent tumor in women
and is the second cause of death from malignant diseases among
women worldwide (1). Early
diagnosis and development of targeted therapies have contributed to
a reduction in breast cancer mortality. However, after an initial
response to treatment, a high proportion of breast cancer patients
become non-responsive to therapy. This drug resistance phenomenon
has been observed with both traditional and targeted therapies and
is the main cause of breast cancer mortality (2,3).
Therefore, an understanding of the mechanisms of drug resistance is
crucial for patient stratification and to develop novel targeted
therapies. Cancer stem cells (CSCs), a small subpopulation of
cancer cells with self-renewal, differentiation, and tumorigenic
capabilities (4), have been
suggested to explain many of the features of drug resistant tumors
(5). CSCs have an increased
resistance to a variety of chemotherapeutics in comparison to
non-CSCs and are thought to drive tumor growth after an initial
response to therapy (5). Although
targeting CSCs is currently difficult, CSCs represent a promising
target for novel anticancer drug development (6).
The phosphatidylinositol-3-kinase (PI3K) pathway
plays a key role in the regulation of cell survival, growth,
migration and proliferation of normal cells. Importantly, it is the
most frequently misregulated signalling pathway in cancer (7). It has been implicated in the
development, progression, and therapy resistance of breast cancer
(8), mainly due to the activation
of its major downstream effector Akt (9). PI3Kα is a heterodimer with adaptor
function, made up of a regulatory subunit of 85 kDa (p85α) and one
of three possible catalytic subunits of 110 kDa (p110) encoded by
the PIK3CA (p110α), PIK3CB (p110β) and PIK3CD
(p110γ) genes (10). Activation of
PI3K stimulates phosphorylation of
phosphatidylinositol-4,5-diphosphate (PIP2), a phospholipid
component of the cell membrane, and generation of
phosphatidylinositol-3,4,5-triphosphate (PIP3), which bind
pleckstrin homology domains of various signalling proteins. An
inhibitory effect is exerted by the tumor suppressor PTEN
(phosphatase and tensin homologue) which hydrolyzes and thus
inactivates PIP3 (10).
Importantly, activating mutations in the catalytic subunit of PI3K
(p110α) and inactivating mutations in PTEN are frequently found in
cancer (7).
NVP-BKM120 (BKM120) is a 2,6-dimorpholino pyrimidine
derivative that is a potent pan-class I PI3K inhibitor, highly
selective against other kinases including mammalian target of
rapamycin (mTOR) (11). It shows
anti-proliferative activity and induces apoptosis in cancer cell
lines through inhibiting the PI3K/Akt signalling pathway (12,13).
Phase I clinical trials indicate that BKM120 is safe at the
maximum-tolerated dose with a favourable pharmacokinetic profile in
several solid tumors (14) and has
been reported to overcome trastuzumab resistance in several breast
cancer cell lines (15).
Importantly, BKM120 has shown enhanced antitumor effect in mouse
models when combined with inhibitors of other signalling pathways
(16,17).
An important downstream effector of PI3K/Akt is
mTOR, a key activator of protein synthesis, a process which is
frequently enhanced in cancer cells (18). Thus, the rapamycin analogue, and
mTOR inhibitor, RAD001 (Everolimus) has gained attention as an
anticancer agent and has been used in advanced renal cancer after
failure of therapy to target vascular endothelial growth factor
(19). Importantly, in addition to
mTOR signalling inhibition, rapamycin analogues cause Akt
activation and attenuation of their therapeutic efficacy (20,21).
Thus, it has been suggested that the combination of BKM120 and
RAD001 may overcome these effects and has shown positive results in
lung cancer mouse models (16).
In this study we demonstrate the efficacy of BKM120
combined with either trastuzumab or RAD001 targeting breast cancer
stem cells. BKM120 displays antitumor activity by inhibiting the
PI3K/Akt signalling pathway. The combination of BKM120 with either
trastuzumab or RAD001 leads also to a decrease in the generation of
drug resistant derivatives in vitro and excellent tumor
response in xenograft mouse models.
Materials and methods
Cell lines and chemicals
Luminal A group MCF-7, claudin-low group
triple-negative MDA-MB-231, triple-negative CAL51 (22), and HER2 group trastuzumab-responsive
SK-BR-3 breast cancer cell lines were used (23). Cells were maintained in Dulbecco's
modified Eagle's medium (DMEM) supplemented with 1 g/l glucose, 10%
foetal calf serum and 4 mM L-glutamine (Life Technologies).
NVB-BKM120 and RAD001 were a kind gift of Novartis. The
HER2-inhibitor trastuzumab was obtained from Roche.
Flow cytometry
For stem cell markers, FITC-conjugated anti-CD44 and
phycoerythrin-conjugated anti-CD24 antibodies, or their respective
isotype controls, all from BD Biosciences were used essentially as
described (24). An Aldefluor assay
kit (StemCell Technologies) was used for the determination of
aldehyde dehydrogenase (ALDH) activity by flow cytometry
essentially as described (24).
Briefly, cells were resuspended in assay buffer (106
cells/ml) and activated aldefluor substrate (5 µl) was added
to samples and incubated at 37°C for 45 min to allow substrate
conversion. A sample with the ALDH inhibitor
diethylaminobenzaldehyde was used as a negative control.
Mammosphere formation
Mammospheres were grown as described (24). In brief, cells (1×103)
were plated in each well of an ultralow attachment 6-well plate
(Corning) with 3 ml serum-free mammary epithelial growth medium
(MEGM; BioWhittaker), supplemented with 2% B27 (Invitrogen), 20
ng/ml EGF and 20 ng/ml bFGF (BD Biosciences). Mammospheres were
grown for 10 days and phase contrast images were obtained using
Nikon TS100 microscope (Nikon, Shanghai, China). Where indicated,
mammospheres were collected by centrifugation at 115 × g for 10 min
at room temperature, trypsinized, counted and used in further
experiments (25).
Cell viability analysis
MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]
assays were performed to evaluate the cell growth inhibitory effect
in response to drug treatments and were used to determine the
concentration of drug that inhibited cell growth by 50%
(IC50) after 3 days of treatment (26). For drug combination experiments, a
combination index (CI) was calculated using the CalcuSyn software
(Biosoft) based on the Chou and Talalay method (27). CI values between 0.1 and 0.9 define
different grades of synergism, with values between 0.9 and 1.1
being additive, whereas values >1.1 are antagonistic.
Drug resistance clonogenic assay
Cells (2×105/well of a 6-well plate) were
treated with a single drug or a combination of drugs as indicated
for 1 week. Drug resistant proliferating clones were fixed with 4%
paraformaldehyde and stained with 0.2% crystal violet. Crystal
violet retained within the cells was quantified by solubilization
with 0.5% acetic acid and measurement of optical density at 592 nm
(28).
Protein extraction and western
blotting
A modified RIPA buffer (50 mM Tris-HCl, 150 mM NaCl,
0.25% SDS, 1% Triton X-100, 0.25% sodium deoxycholate, 1 mM EDTA, 1
mM EGTA, 1 mM dithiothreitol) with protease inhibitor cocktail
(Sigma) was used for protein isolation from cells. Protein
concentrations were determined using the BCA Protein Assay kit
(Pierce). Cell lysates containing 50 µg of protein were
resolved on 12% (w/v) polyacrylamide gels, transferred to
nitrocellulose membranes (Millipore) and blocked with 5% blotting
grade milk (Bio-Rad) in PBST (0.1% Tween-20 in PBS). Membranes were
then incubated with primary antibodies to phospho-Akt (D9E), Akt1
(C73H10), phospho-S6 (D57.2.2E), S6 (54D2) and β-actin (13E5) (Cell
Signaling Technology) at 1:1,000 dilution at 4°C overnight,
followed by HRP-conjugated secondary antibodies (Cell Signaling
Technology) at 1:2,000 dilution for 2 h at room temperature.
Signals were visualized using SuperSignal West Pico
Chemiluminescent substrate (Pierce) according to the manufacturer's
instructions.
In vivo assays
Cells (5×106) were trypsinized and
resuspended in a total volume of 100 µl PBS containing 50%
Matrigel (BD Biosciences) and were injected into the mammary fat
pad of nude mice (5–6 weeks of age). Tumor sizes were measured
every three days in two dimensions using callipers, and the tumor
volume calculated [tumor volume (mm3) = 0.5 ×
ab2; a and b being the longest and shortest diameters of
the tumor, respectively]. Fifteen days after cell injection, the
tumor-bearing mice were randomly divided into four groups (five
animals per group) and received: group 1, saline (control group);
group 2, 50 mg/kg BKM120 (BKM120 group); group 3, either 5 mg/kg
trastuzumab (trastuzumab group) or 2 mg/kg RAD001 (RAD001 group);
and group 4, a combination of BKM120 and either trastuzumab or
RAD001 (at the above doses; combination group). Drugs were injected
intraperitoneally every there days and tumor volume and mouse
weight monitored until mice were sacrificed in a humane manner. All
mice were maintained as required under the National Institutes of
Health guidelines for the Care and Use of Laboratory Animals. The
use of animals in this study was approved by the Animal Care and
Use Committee of Tianjin Cancer Hospital.
Statistical analysis
Statistical evaluations were performed by Student's
t-test for paired data and by ANOVA for sets of data with multiple
comparison points. Statistical significance was considered at
p<0.05.
Results
BKM120 effectively inhibits the growth of
breast cancer stem-like cells
Given the importance of the PI3K pathway in cancer,
we asked whether BKM120 had a differential effect in the stem-like
sub-populations from several breast cancer cell lines. Breast
stem-like cells (SCs) exhibit the ability to survive and grow as
mammospheres in low attachment plates (29,30),
are characterized by a
CD44+/CD24−/ALDH1+ phenotype and
show strong tumorigenicity in NOD/SCID mouse models (31,32).
We isolated SCs from SK-BR-3, MDA-MB-231, MCF-7 and CAL51 cells
after proliferation in low attachment plates. In all cases, the
proportion of CD44+/CD24− and
ALDH1+ cells was higher in SCs than in the original cell
population (Figs. 1A and 5A). Next we tested the effect of BKM120 on
cell survival using MTT assays. As expected, the pan-PI3K inhibitor
had a dose-dependent effect on cell proliferation, both in the SC
subpopulation and, to a greater extent, in the total cells
(Figs. 1B and 5C). SCs resistance ratios varied between
4.26 in MCF-7 and 6.79 in CAL51 cells (Table I). Comparison with the
IC50 obtained previously by us on similar cell
sub-populations (33) indicates
that BKM120 is more effective targeting SCs than docetaxel, a
taxane type drug affecting cell proliferation by disruption of
microtubules. As drug resistant cells have been proposed to arise
from the selection of a small population of cells with stem-like
properties (5), we asked whether
BKM120 could inhibit the formation of drug resistant clones. For
this, SCs were left to grow as monolayers up to one week with or
without BMK120 and the cell mass determined by crystal violet
staining. The pan-PI3K inhibitor decreased the proliferation of
resistant cells in a dose-dependent manner in MDA-MB-231, MCF-7 and
SK-BR-3 cells (Fig. 1C). As BKM120
inhibited growth on monolayer cultures, we also performed
mammosphere forming assays to detect whether BKM120 could eliminate
SC growth. Indeed, the mammosphere-forming efficiency (MFE)
decreased in a dose-dependent manner in MDA-MB-231, SK-BR-3, MCF-7
(Fig. 1D) and CAL51 (Fig. 5E) cells. Thus, BKM120 inhibits the
growth of breast cancer SCs.
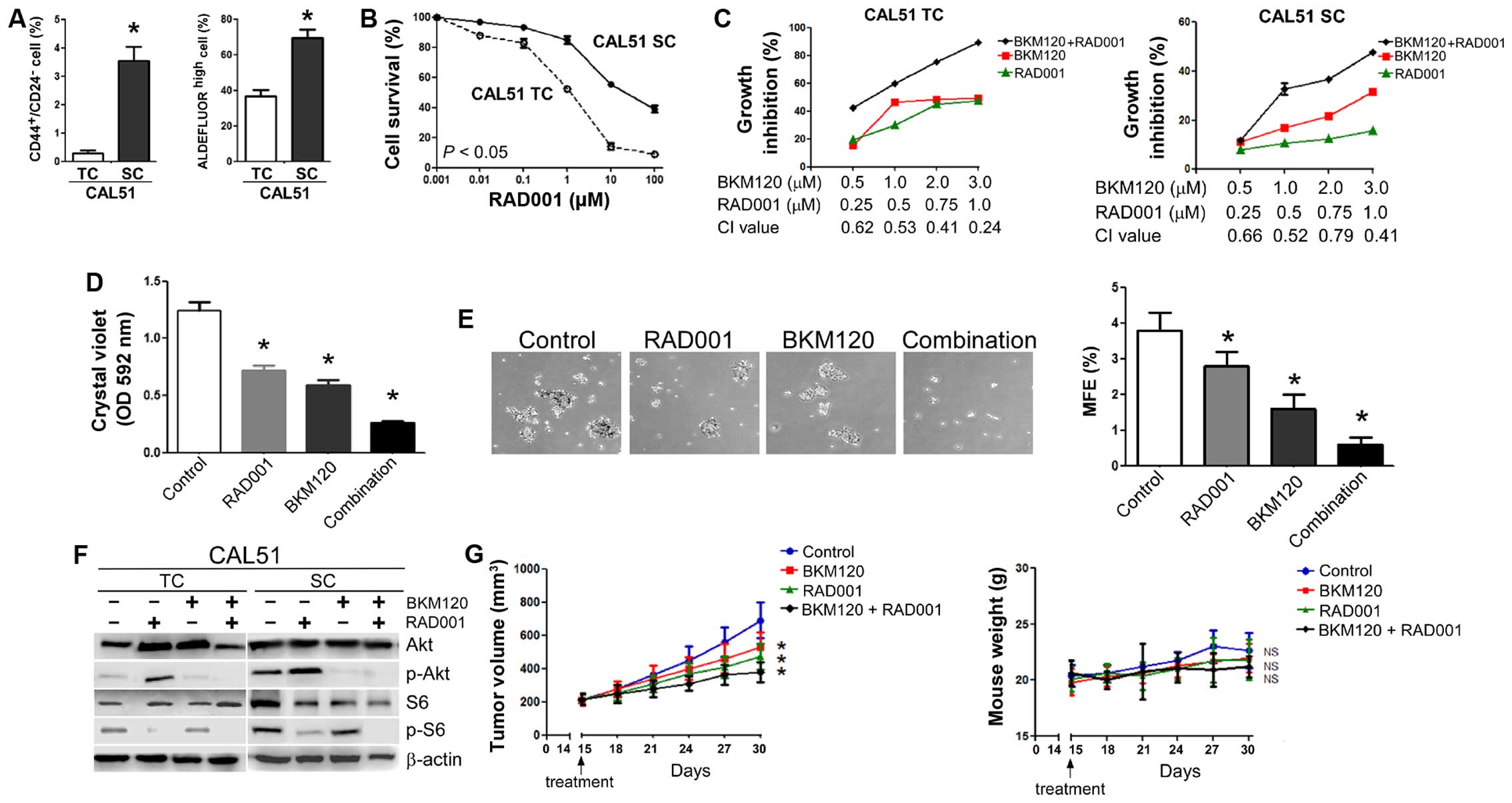 | Figure 5The combination of BKM120 and RAD001
synergistically inhibits the growth of triple-negative CAL51 cells
both in vitro and in vivo. (A) Quantitative flow
cytometry data indicating the average percentages of
CD44+/CD24− (left panel) and ALDH+
cells (right panel) in CAL51 total cells (TC) and stem-like
cells (SC) after their isolation by mammosphere cultures.
(B) Dose-response curves of RAD001. (C) The combination of BKM120
and RAD001 synergistically inhibits the growth of CAL51 TCs (left
panel) and CAL51 SCs (right panel). Cells were treated with
different combinations of BKM120 and RAD001 for three days and the
effect on cellular proliferation determined by MTT assays.
Combination index (CI) for each set of drug concentrations
is indicated on the abscissa. (D) Long-term drug resistance. Cells
were treated with vehicle, 1 µM BKM120, 0.5 µM RAD001
or a combination of the two drugs for seven days and stained with
crystal violet. After dye solubilization, the optical density at
592 nm was determined. (E) Effect of a combination of RAD001 and
BKM120 on the growth of mammospheres from CAL51 cells. Cells were
treated with vehicle, 1 µM BKM120, 0.5 µM RAD001 or a
combination of the two drugs for ten days. Mammosphere formation
was visualized at ×20 magnification (left panel) and MFE calculated
(right panel). (F) Effect of BKM120 and RAD001 on Akt and S6
phosphorylation in CAL51 cells. Both TCs (left panel) and SCs
(right panel) were treated with 1 µM RAD001 in the absence
or presence of 1 µM BKM120 for 24 h and the levels of Akt,
S6 and their phosphorylated forms analyzed by western blotting.
β-actin was used as a loading control. (G) Antitumor activity of
BKM120 combined with RAD001 in CAL51 xenograft tumors. Growth of
CAL51 xenograft tumors (expressed as tumor volume) after treatment
with PBS (control), RAD001, BKM120, or BKM120 plus RAD001 (left
panel, see Materials and methods for experimental details). Data
represent mean tumor size ± SD of five tumors per group. Right
panel illustrates body weight of nude mice bearing CAL51
xenografts. Data indicate mean body weight ± SD of five mice per
group. Other numerical data represent the mean ± SD of three
independent experiments (*P<0.05; NS, not
significant). Pictorial data were repeated at least in triplicate
and a representative picture is shown. |
 | Table ISensitivity of breast cancer cell
lines to BKM120. |
Table I
Sensitivity of breast cancer cell
lines to BKM120.
| Cell line | IC50
(µM)
| Stem-like cells
resistance ratio |
|---|
| Total cells | Stem-like
cells |
|---|
| MCF-7 | 1.71±0.05 | 7.29±0.51 | 4.26 |
| MDA-MB-231 | 3.07±0.14 | 20.01±3.46 | 6.52 |
| SK-BR-3 | 1.64±0.16 | 9.83±1.05 | 5.99 |
| CAL51 | 1.21±0.12 | 8.22±0.43 | 6.79 |
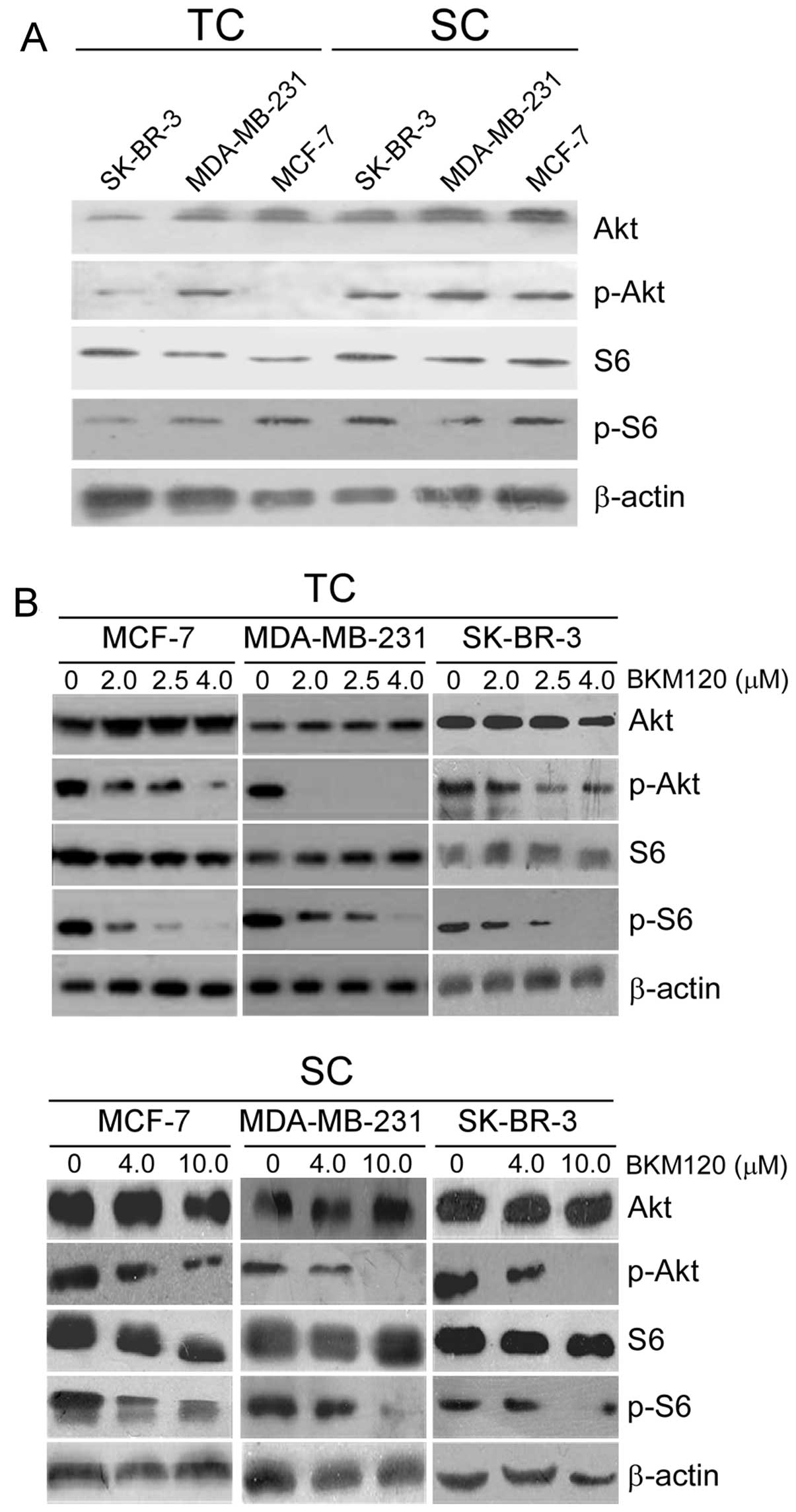
BKM120 effectively inhibits the
PI3K/Akt/mTOR signalling pathway
As BKM120 is a pan-PI3K inhibitor, we next sought to
determine its effect on the PI3K/Akt/mTOR axis in breast cancer
cells. For this, we analyzed by western blotting the total and
phosphorylated levels of Akt and ribosomal protein S6, both in SCs
and the total cellular population. Both phosphorylated Akt and S6
levels were higher in SCs than in the whole cell population,
although there were variations among cells. MCF-7 and SK-BR-3 SCs
showed higher activation of Akt than MDA-MB-231 SCs. Ribosomal
protein S6 was clearly activated in SK-BR-3 SCs, although the
activation was less robust in the other two SC subpopulations
(Fig. 2A).
Having confirmed an activation of the PI3K/Akt/mTOR
pathway in SCs, we treated both SCs and the whole cell population
with a range of BKM120 concentrations for 24 h and determined the
extent of the above proteins by western blotting. As expected,
higher doses of BKM120 were necessary to decrease the levels of
phospho-Akt and phospho-S6 in SCs than in the whole cell
population. For instance, 2 µM BKM120 completely inhibited
the Akt pathway in MDA-MB-231 cells whereas in the SC subpopulation
a partial inhibition was obtained after treatment with 4 µM
and a total inhibition was achieved only with 10 µM BKM120.
Similarly, phospho-S6 was absent in cells treated with up to 4
µM BKM120, whereas 10 µM BKM120 partly inhibited S6
activation in SCs (Fig. 2B).
Therefore, BKM120 exerts potent suppressive effects
on PI3K/Akt/mTOR signalling in both total and SCs subpopulations of
breast cancer cells.
The combination of BKM120 and trastuzumab
synergistically inhibits the growth of SK-BR-3 cells and eliminates
the SC subpopulation
Alterations in the PI3K/Akt signalling pathway have
been associated with therapy-induced resistance in breast cancer
patients, including endocrine-based therapy and combined
chemotherapy and HER2-targeted-therapy (34,35).
Recent studies demonstrate that targeting the PI3K/Akt pathway in
combination with trastuzumab, a monoclonal antibody that interferes
with the HER2/neu receptor, is beneficial in trastuzumab-resistant
breast cancer (15). As BKM120 has
been shown to have robust anticancer properties in breast cancer
SCs, we asked whether BKM120 could synergize with trastuzumab in
SK-BR-3, a HER2+ breast cancer cell line, especially in
its SC subpopulation. For this, SK-BR-3 total cells and SCs were
treated with increasing concentrations of trastuzumab, either alone
or in combination with BKM120. As expected, trastuzumab decreased
cell survival, although the effect was more noticeable in the whole
SK-BR-3 population (IC50 ~10 µg/ml) than in SCs
(IC50 >100 µg/ml) (Fig. 3A). When used in combination, the CI
values ranged from 0.3 to 0.6 (Fig.
3B), indicating that trastuzumab and BKM120 act synergistically
both in total SK-BR-3 cells and SCs (27). Importantly, trastuzumab in
combination with BKM120 had a greater effect suppressing the
generation of resistant cells (Fig.
3C) and mammospheres (SCs; Fig.
3D) than the individual drugs acting alone. Western blot
analyses also indicated a stronger effect on the PI3K/Akt/mTOR
pathway when the drugs were combined. Both phospho-Akt and
phospho-S6 levels decreased to a high extent in SCs, whilst, as
expected, the effect was stronger in the whole cell population
(Fig. 3E).
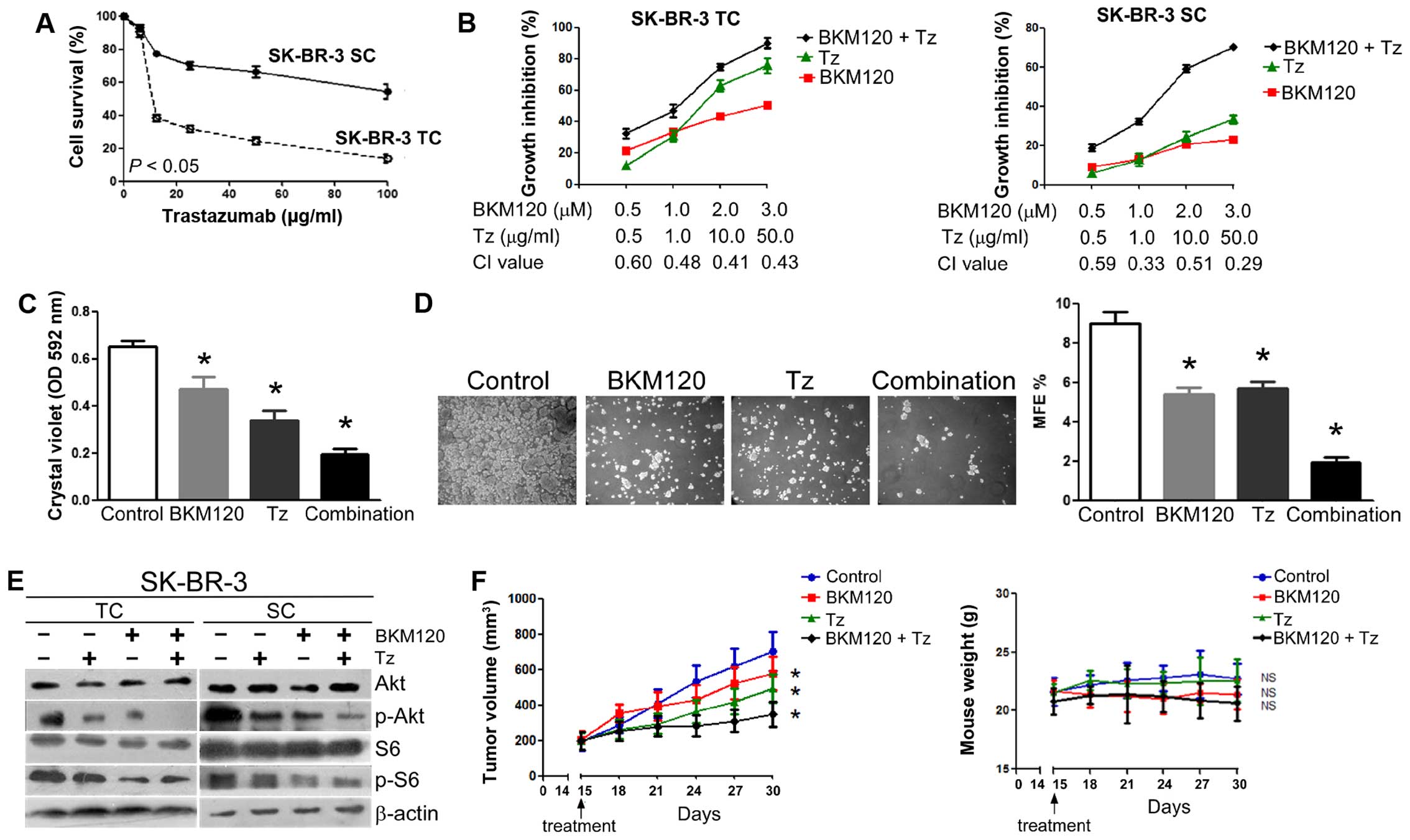 | Figure 3The combination of BKM120 and
trastuzumab synergistically inhibits the growth of HER2+
SCs in vitro and the formation of tumors in vivo. (A)
Dose-response curves of trastuzumab for SK-BR-3 cells. TC, total
cell population; SC, stem-like population. (B) The combination of
BKM120 and trastuzumab synergistically inhibits the growth of
SK-BR-3 TCs (left panel) and SK-BR-3 SCs (right panel). Cells were
treated with different combinations of BKM120 and trastuzumab for
three days and the effect on cellular proliferation determined by
MTT assays. Combination index (CI) for each set of drug
concentrations is indicated on the abscissa. (C) Long-term drug
resistance. Cells were treated with vehicle, 1 µM BKM120, 1
µg/ml trastuzumab or a combination of the two drugs for
seven days and stained with crystal violet. After dye
solubilization, the optical density at 592 nm was determined. (D)
Effect of a combination of trastuzumab and BKM120 on the growth of
mammospheres from SK-BR-3 cells. Cells were treated with vehicle, 1
µM BKM120, 1 µg/ml trastuzumab or a combination of
the two drugs for ten days. Mammosphere formation was visualized at
×20 magnification (left panel) and MFE calculated (right panel).
(E) Effect of BKM120 and trastuzumab on Akt and S6 phosphorylation
in SK-BR-3 cells. Both TCs and SCs were treated with 100
µg/ml trastuzumab in the absence or presence of 4 µM
BKM120 for 24 h and the levels of Akt, S6 and their phosphorylated
forms analyzed by western blotting. β-actin was used as a loading
control. (F) Antitumor activity of BKM120 combined with trastuzumab
in SK-BR-3 xenograft tumors (right panel). Growth of SK-BR-3
xenograft tumors (expressed as tumor volume) after treatment with
PBS (control), trastuzumab, BKM120, or BKM120 plus trastuzumab
(left panel, see Materials and methods for experimental details).
Data represent mean tumor size ± SD of five tumors per group. Right
panel illustrates body weight of nude mice bearing SK-BR-3
xenografts. Data indicate mean body weight ± SD of five mice per
group. Other numerical data represent the mean ± SD of three
independent experiments (*P<0.05; NS, not
significant). Pictorial data were repeated at least in triplicate
and a representative picture is shown. |
Next, we used a xenograft model to assess the
efficacy of this drug combination against the growth of
SK-BR-3-derived tumors. For this, SK-BR-3 SCs were injected into
the mammary fat pad of female nude mice. Mice were then randomly
divided into four groups 14 days after injection and treated with
vehicle, BKM120, trastuzumab, and a combination of the two. As
expected, tumor growth followed a steady progress during the
following 15 days in the control group, whereas the tumor volume
increased at lower rates in the BKM120 and trastuzumab groups.
Importantly, the group receiving both BKM120 and trastuzumab showed
a slight tumor growth with no significant mouse body weight loss,
indicating that the drug combination is well tolerated (Fig. 3F).
In summary, BKM120 in combination with trastuzumab
acts synergistically inhibiting the PI3K/Akt/mTOR pathway, the
growth of HER2+ cells, the generation of drug-resistant
SCs in vitro, and the formation of tumors in
vivo.
The combination of BKM120 and RAD001
synergistically inhibits the growth of triple-negative breast
cancer cells both in vitro and in vivo
We have previously demonstrated that the combination
of RAD001 with docetaxel, latrozole or trastuzumab has enhanced
growth-inhibitory effects against breast cancer SCs (33,36,37).
As RAD001 has been reported to act synergistically with BKM120 in
lung cancer models (16), we asked
whether a similar effect would be observed in triple-negative
breast cancer (TNBC). TNBC remains a challenging clinical problem
due to a lack of targeted therapies and, consequently, a high
mortality rate. For this purpose, total cells and SCs of MDA-MB-213
and CAL51 cell lines were treated with increasing concentrations of
RAD001, either alone or in combination with a range of doses of
BKM120. As expected, RAD001 decreased cell survival, although the
effect was more noticeable in the total cell population than in SCs
(Figs. 4A and 5B). When used in combination, RAD001 and
BKM120 CI values ranged from 0.3 to 0.7 in MDA-MB-231 (Fig. 4B) and from 0.2 to 0.8 and CAL51
cells (Fig. 5C). This indicates
that RAD001 and BKM120 act synergistically when used in
combination, both in the total population and in the SC
subpopulations of these two cell lines (27). Importantly, RAD001 in combination
with BKM120 had a greater effect on the generation of resistant
cells (Figs. 4C and 5D) and mammosphere formation (Figs. 4D and 5E) than the drugs individually.
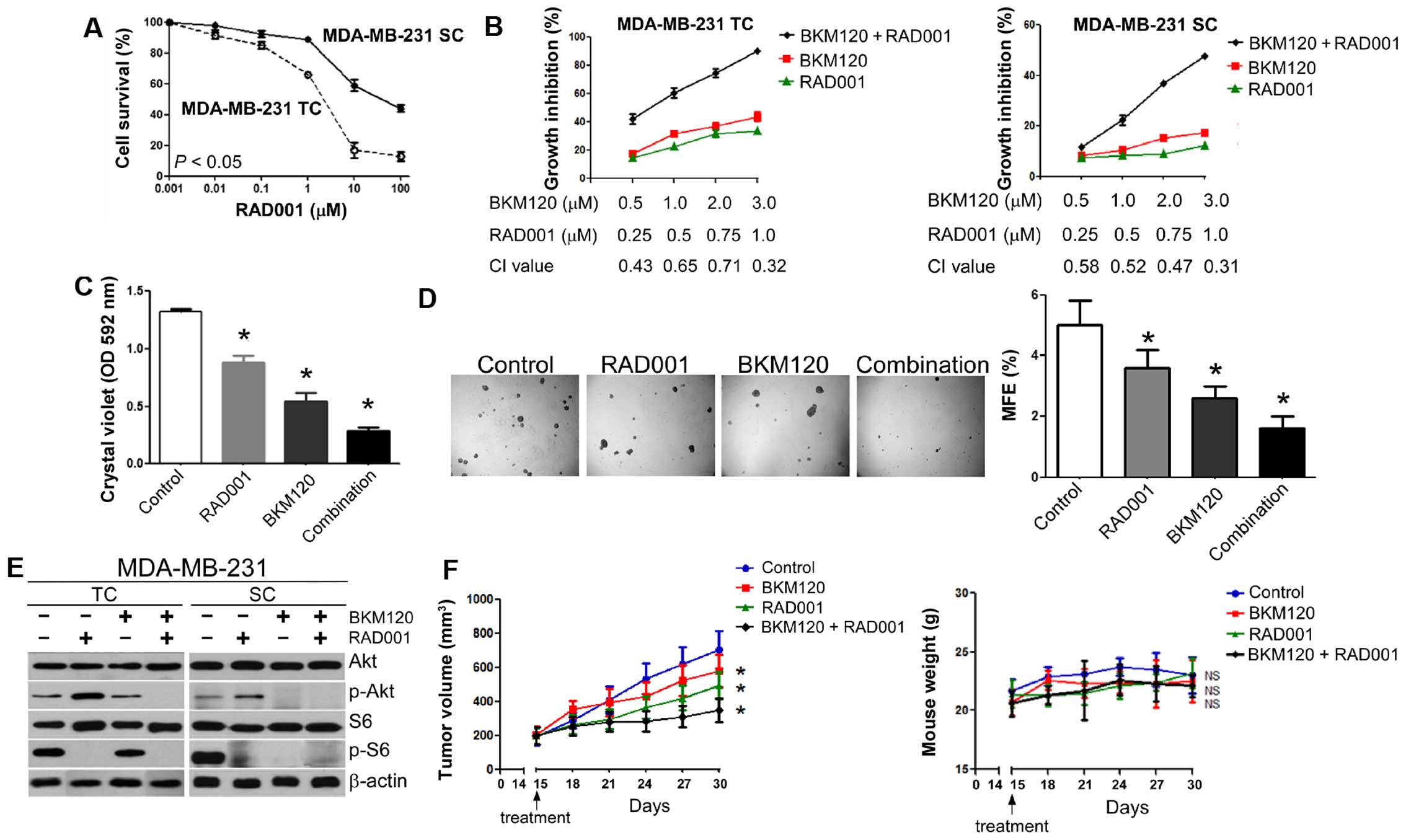 | Figure 4The combination of BKM120 and RAD001
synergistically inhibits the growth of triple negative MDA-MB-231
cells both in vitro and in vivo. (A) Dose-response
curves of RAD001 for MDA-MB-231 cells. TC, total cell
population; SC, stem-like population. (B) The combination of
BKM120 and RAD001 synergistically inhibits the growth of MDA-MB-231
TCs (left panel) and MDA-MB-231 SCs (right panel). Cells were
treated with different combinations of BKM120 and RAD001 for three
days and the effect on cellular proliferation determined by MTT
assays. Combination index (CI) for each set of drug
concentrations is indicated on the abscissa. (C) Long-term drug
resistance. Cells were treated with vehicle, 1 µM BKM120,
0.5 µM RAD001 or a combination of the two drugs for seven
days and stained with crystal violet. After dye solubilization, the
optical density at 592 nm was determined. (D) Effect of a
combination of RAD001 and BKM120 on the growth of mammospheres from
MDA-MB-231 cells. Cells were treated with vehicle, 1 µM
BKM120, 0.5 µM RAD001 or a combination of the two drugs for
ten days. Mammosphere formation was visualized at ×20 magnification
(left panel) and MFE calculated (right panel). (E) Effect of BKM120
and RAD001 on Akt and S6 phosphorylation in MDA-MB-231 cells. Both
TCs (left panel) and SCs (right panel) were treated with 1
µM RAD001 in the absence or presence of 3 µM BKM120
for 24 h and the levels of Akt, S6 and their phosphorylated forms
analyzed by western blotting. β-actin was used as a loading
control. (F) Antitumor activity of BKM120 combined with RAD001 in
MDA-MB-231 xenograft tumors (right panel). Growth of MDA-MB-231
xenograft tumors (expressed as tumor volume) after treatment with
PBS (control), RAD001, BKM120, or BKM120 plus RAD001 (left panel,
see Materials and methods for experimental details). Data represent
mean tumor size ± SD of five tumors per group. Right panel
illustrates body weight of nude mice bearing MDA-MB-231 xenografts.
Data indicate mean body weight ± SD of five mice per group. Other
numerical data represent the mean ± SD of three independent
experiments (*P<0.05; NS, not significant). Pictorial
data were repeated at least in triplicate and a representative
picture is shown. |
Inhibition of mTOR leads to feedback reactivation of
PI3K activity in a variety of systems (21,38).
We confirmed that RAD001 treatment, as a single agent, increased
Akt phoshphorylation in MDA-MB-231 (Fig. 4E) and CAL51 cells (Fig. 5F). However, RAD001 failed to
activate PI3K activity in the presence of BKM120, both in the total
cell population and SCs. The combination of BKM120 and RAD001 also
showed more activity in reducing phospho-S6 levels than either
single agent did when acting alone (Figs. 4E and 5F). Thus, the combination of BKM120 and
RAD001 blocks RAD001-induced phosphorylation of Akt and exerts
enhanced effects on suppression of phospho-S6.
Because of the growth-inhibitory effects of the
BKM120 and RAD001 combination in TNBC SCs in vitro (Figs. 4B and 5C), we sought to determine whether the
same effect could be found in vivo. For this, MDA-MB-231 SCs
or CAL51 SCs were injected into the mammary fat pad of female nude
mice. Mice were then randomly divided into four groups 14 days
after injection and treated with vehicle, BKM120, RAD001, and a
combination of the two drugs. As expected, tumor growth followed a
steady progress during the following 15 days in the control group,
whereas the tumor volume increased at lower rates in the BKM120 and
RAD001 groups. Importantly, the group receiving both BKM120 and
RAD001 showed the slowest tumor growth and absence of mouse body
weight loss, indicating that the drug combination is well tolerated
(Figs. 4F and 5G).
In summary, BKM120 in combination with RAD001 acts
synergistically inhibiting the PI3K/Akt/mTOR pathway, the growth of
TNBC cells, the generation of drug-resistant derivatives in
vitro, and the formation of tumors in vivo.
Discussion
The development of cancer targeted therapies has
been of paramount importance for the increase in patient survival
achieved during the last few decades (39,40).
However, most targeted therapies, as well as traditional
non-targeted cytotoxic and radiation therapies, are encumbered by
the acquisition of resistance. Accumulating evidence indicates that
CSCs play a crucial role in therapy resistance and recurrence of
breast cancers (5,32). Therapy resistance is a complex
phenomenon involving multiple mechanisms, including activation of
signalling pathways such as the PI3K/Akt/mTOR axis (41), and the activation of this pathway is
crucial for maintaining the stemness and chemoresistance of breast
CSCs (42). Hence, breast CSCs are
critical therapeutic targets and their elimination may improve the
prognosis and outcome of cancer therapy (43). PI3K inhibition has recently been
shown to sensitize CSCs to chemotherapy and targeted therapy in
several cancers including leukemia (44), hepatocellular carcinoma (45) and breast cancer (33). In line with these studies, we
present data indicating that the PI3K inhibitor BKM120 is also
effective in eliminating breast CSCs. After BKM120 treatment, the
in vitro tumorigenicity of breast cancer cells is highly
impaired. Moreover, BKM120 exerts tumor inhibiting effect on breast
SCs-derived xenograft models in vivo, further confirming the
potency of BKM120 in CSCs.
Aberrant activation of several signalling pathways
downstream of HER2, including the MAPK (46), Notch (47) and PI3K/Akt pathways (48), leads to HER2-targeted therapy
resistance. Since HER2 mediates signal transduction through the
PI3K/Akt pathway, inhibition of components of this pathway is a
reasonable approach to overcome resistance to HER2-targeted therapy
(15,49). Indeed, the combination of PI3K
inhibitor BAY 80-6946 with HER2-targeted therapy inhibits
HER2-positive breast cancer cell growth more effectively than
either therapy used alone (50),
and the phase Ib study of BKM120 plus trastuzumab in HER2-positive
breast cancer patients has shown promising results (51). Here, we report that BKM120 has a
synergistic effect with trastuzumab on HER2-positive breast SCs
in vitro and, importantly, that the drug combination is well
tolerated in mouse models. This adds weight to the design of future
trials with a combination of BKM120 and transtuzumab in
HER2-positive breast cancer patients.
TNBC is a heterogeneous disease comprised of several
biologically distinct subtypes (52). In addition to our poor understanding
of the molecular characteristics of each TNBC subtype, we lack
effective targeting strategies, leading to a poor prognosis for
TNBC patients. However, anti-angiogenic, EGFR-targeted, PARP
inhibitors, PI3K/Akt/mTOR inhibitors and Src inhibiting therapies
have demonstrated promising results (53). It has been suggested that TNBC
patients might benefit from the combined effect of BKM120 with PARP
inhibitors, as BKM120 sensitizes BRCA-proficient TNBC to PARP
inhibition (54). Experimental data
indicate that RAD001 has favourable activity against basal-like
TNBCs (55) and phase 2 clinical
trials show that RAD001 combined with carboplatin is efficacious in
metastatic TNBC (56). Here, we
demonstrated that the combination of BKM120 and RAD001 enhances the
suppressive growth effect of TNBC cells, including SCs both in
vitro and in vivo. It is also important to emphasize
that BKM120 inhibits RAD001-induced Akt phosphorylation in TNBC, in
line with studies using other systems (21,57).
Accordingly, we suggest that the combination of BKM120 and RAD001
may be an effective regimen for TNBC treatment.
In summary, we demonstrate that the combination of a
pan-PI3K inhibitor, BKM120, with either trastuzumab or RAD001, is
effective in targeting breast cancer SCs in vivo and offers
the rationale to develop further clinical trials for HER2-positive
and TNBC, respectively.
Acknowledgments
This study was supported by the Chinese National
Natural Sciences Foundation (81402480 to Y.H.), Tianjin municipal
Major Scientific and Technological Special Project for Significant
Anticancer Development (12ZCDZSY15700 to J. Zhang), Tianjin
municipal Natural Sciences Foundation (15JCYBJC28300 to S.Z.) and
Tianjin Medical University Cancer Institute and Hospital Foundation
(1416 to J. Zhao).
Abbreviations:
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide
|
|
ALDH
|
aldehyde dehydrogenase
|
|
bFGF
|
basic fibroblast growth factor
|
|
CI
|
combination index
|
|
IC50
|
drug concentration necessary to kill
50% of cells
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
EGF
|
epidermal growth factor
|
|
mTOR
|
mammalian target of rapamycin
|
|
BKM120
|
NVP-BKM120
|
|
PBS
|
phosphate-buffered saline
|
|
RIPA
|
radioimmunoprecipitation assay
|
|
SC
|
stem-like cell
|
|
TNBC
|
triple-negative breast cancer
|
References
|
1
|
Mendes D, Alves C, Afonso N, Cardoso F,
Passos-Coelho JL, Costa L, Andrade S and Batel-Marques F: The
benefit of HER2-targeted therapies on overall survival of patients
with metastatic HER2-positive breast cancer - a systematic review.
Breast Cancer Res. 17:1402015. View Article : Google Scholar
|
|
2
|
Fan W, Chang J and Fu P: Endocrine therapy
resistance in breast cancer: Current status, possible mechanisms
and overcoming strategies. Future Med Chem. 7:1511–1519. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen Z, Shi T, Zhang L, Zhu P, Deng M,
Huang C, Hu T, Jiang L and Li J: Mammalian drug efflux transporters
of the ATP binding cassette (ABC) family in multidrug resistance: A
review of the past decade. Cancer Lett. 370:153–164. 2016.
View Article : Google Scholar
|
|
4
|
Ailles LE and Weissman IL: Cancer stem
cells in solid tumors. Curr Opin Biotechnol. 18:460–466. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dean M, Fojo T and Bates S: Tumour stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takebe N, Harris PJ, Warren RQ and Ivy SP:
Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog
pathways. Nat Rev Clin Oncol. 8:97–106. 2011. View Article : Google Scholar
|
|
7
|
Yuan TL and Cantley LC: PI3K pathway
alterations in cancer: Variations on a theme. Oncogene.
27:5497–5510. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baselga J: Targeting the
phosphoinositide-3 (PI3) kinase pathway in breast cancer.
Oncologist. 16(Suppl 1): 12–19. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Burris HA III: Overcoming acquired
resistance to anticancer therapy: Focus on the PI3K/AKT/mTOR
pathway. Cancer Chemother Pharmacol. 71:829–842. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Engelman JA, Luo J and Cantley LC: The
evolution of phosphatidylinositol 3-kinases as regulators of growth
and metabolism. Nat Rev Genet. 7:606–619. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Maira SM, Pecchi S, Huang A, Burger M,
Knapp M, Sterker D, Schnell C, Guthy D, Nagel T, Wiesmann M, et al:
Identification and characterization of NVP-BKM120, an orally
available pan-class I PI3-kinase inhibitor. Mol Cancer Ther.
11:317–328. 2012. View Article : Google Scholar
|
|
12
|
Zheng Y, Yang J, Qian J, Zhang L, Lu Y, Li
H, Lin H, Lan Y, Liu Z, He J, et al: Novel phosphatidylinositol
3-kinase inhibitor NVP-BKM120 induces apoptosis in myeloma cells
and shows synergistic anti-myeloma activity with dexamethasone. J
Mol Med Berl. 90:695–706. 2012. View Article : Google Scholar
|
|
13
|
Amrein L, Shawi M, Grenier J, Aloyz R and
Panasci L: The phosphatidylinositol-3 kinase I inhibitor BKM120
induces cell death in B-chronic lymphocytic leukemia cells in
vitro. Int J Cancer. 133:247–252. 2013. View Article : Google Scholar
|
|
14
|
Bendell JC, Rodon J, Burris HA, de Jonge
M, Verweij J, Birle D, Demanse D, De Buck SS, Ru QC, Peters M, et
al: Phase I, dose-escalation study of BKM120, an oral pan-Class I
PI3K inhibitor, in patients with advanced solid tumors. J Clin
Oncol. 30:282–290. 2012. View Article : Google Scholar
|
|
15
|
O'Brien NA, McDonald K, Tong L, von Euw E,
Kalous O, Conklin D, Hurvitz SA, di Tomaso E, Schnell C, Linnartz
R, et al: Targeting PI3K/mTOR overcomes resistance to HER2-targeted
therapy independent of feedback activation of AKT. Clin Cancer Res.
20:3507–3520. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ren H, Chen M, Yue P, Tao H, Owonikoko TK,
Ramalingam SS, Khuri FR and Sun SY: The combination of RAD001 and
NVP-BKM120 synergistically inhibits the growth of lung cancer in
vitro and in vivo. Cancer Lett. 325:139–146. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jane EP, Premkumar DR, Morales A, Foster
KA and Pollack IF: Inhibition of phosphatidylinositol 3-kinase/AKT
signaling by NVP-BKM120 promotes ABT-737-induced toxicity in a
caspase-dependent manner through mitochondrial dysfunction and DNA
damage response in established and primary cultured glioblastoma
cells. J Pharmacol Exp Ther. 350:22–35. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Silvera D, Formenti SC and Schneider RJ:
Translational control in cancer. Nat Rev Cancer. 10:254–266. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Albiges L, Kube U, Eymard JC, Schmidinger
M, Bamias A, Kelkouli N, Mraz B, Florini S, Guderian G, Cattaneo A,
et al: Everolimus for patients with metastatic renal cell carcinoma
refractory to anti-VEGF therapy: Results of a pooled analysis of
non-interventional studies. Eur J Cancer. 51:2368–2374. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
O'Reilly KE, Rojo F, She QB, Solit D,
Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL, et al:
mTOR inhibition induces upstream receptor tyrosine kinase signaling
and activates Akt. Cancer Res. 66:1500–1508. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang X, Yue P, Kim YA, Fu H, Khuri FR and
Sun SY: Enhancing mammalian target of rapamycin (mTOR)-targeted
cancer therapy by preventing mTOR/raptor inhibition-initiated,
mTOR/rictor-independent Akt activation. Cancer Res. 68:7409–7418.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gioanni J, Le François D, Zanghellini E,
Mazeau C, Ettore F, Lambert JC, Schneider M and Dutrillaux B:
Establishment and characterisation of a new tumorigenic cell line
with a normal karyotype derived from a human breast adenocarcinoma.
Br J Cancer. 62:8–13. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Holliday DL and Speirs V: Choosing the
right cell line for breast cancer research. Breast Cancer Res.
13:2152011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lombardo Y, Filipović A, Molyneux G,
Periyasamy M, Giamas G, Hu Y, Trivedi PS, Wang J, Yagüe E, Michel
L, et al: Nicastrin regulates breast cancer stem cell properties
and tumor growth in vitro and in vivo. Proc Natl Acad Sci USA.
109:16558–16563. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lombardo Y, de Giorgio A, Coombes CR,
Stebbing J and Castellano L: Mammosphere formation assay from human
breast cancer tissues and cell lines. J Vis Exp. 97:e526712015.
|
|
26
|
Hu Y, Cheng X, Li S, Zhou Y, Wang J, Cheng
T, Yang M and Xiong D: Inhibition of sorcin reverses multidrug
resistance of K562/A02 cells and MCF-7/A02 cells via regulating
apoptosis-related proteins. Cancer Chemother Pharmacol. 72:789–798.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chou TC: Drug combination studies and
their synergy quantification using the Chou-Talalay method. Cancer
Res. 70:440–446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou Y, Hu Y, Yang M, Jat P, Li K,
Lombardo Y, Xiong D, Coombes RC, Raguz S and Yagüe E: The
miR-106b~25 cluster promotes bypass of doxorubicin-induced
senescence and increase in motility and invasion by targeting the
E-cadherin transcriptional activator EP300. Cell Death Differ.
21:462–474. 2014. View Article : Google Scholar
|
|
29
|
Ponti D, Costa A, Zaffaroni N, Pratesi G,
Petrangolini G, Coradini D, Pilotti S, Pierotti MA and Daidone MG:
Isolation and in vitro propagation of tumorigenic breast cancer
cells with stem/progenitor cell properties. Cancer Res.
65:5506–5511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Grimshaw MJ, Cooper L, Papazisis K,
Coleman JA, Bohnenkamp HR, Chiapero-Stanke L, Taylor-Papadimitriou
J and Burchell JM: Mammosphere culture of metastatic breast cancer
cells enriches for tumorigenic breast cancer cells. Breast Cancer
Res. 10:R522008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ginestier C, Hur MH, Charafe-Jauffret E,
Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG,
Liu S, et al: ALDH1 is a marker of normal and malignant human
mammary stem cells and a predictor of poor clinical outcome. Cell
Stem Cell. 1:555–567. 2007. View Article : Google Scholar
|
|
32
|
Calcagno AM, Salcido CD, Gillet JP, Wu CP,
Fostel JM, Mumau MD, Gottesman MM, Varticovski L and Ambudkar SV:
Prolonged drug selection of breast cancer cells and enrichment of
cancer stem cell characteristics. J Natl Cancer Inst.
102:1637–1652. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang X, Zhang S, Liu Y, Liu J, Ma Y, Zhu
Y and Zhang J: Effects of the combination of RAD001 and docetaxel
on breast cancer stem cells. Eur J Cancer. 48:1581–1592. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Miller TW, Balko JM and Arteaga CL:
Phosphatidylinositol 3-kinase and antiestrogen resistance in breast
cancer. J Clin Oncol. 29:4452–4461. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ghebeh H, Al-Khaldi S, Olabi S, Al-Dhfyan
A, Al-Mohanna F, Barnawi R, Tulbah A, Al-Tweigeri T, Ajarim D and
Al-Alwan M: Fascin is involved in the chemotherapeutic resistance
of breast cancer cells predominantly via the PI3K/Akt pathway. Br J
Cancer. 111:1552–1561. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu Y, Zhang X, Liu J, Hou G, Zhang S and
Zhang J: Everolimus in combination with letrozole inhibit human
breast cancer MCF-7/Aro stem cells via PI3K/mTOR pathway: An
experimental study. Tumour Biol. 35:1275–1286. 2014. View Article : Google Scholar
|
|
37
|
Zhu Y, Zhang X, Liu Y, Zhang S, Liu J, Ma
Y and Zhang J: Antitumor effect of the mTOR inhibitor everolimus in
combination with trastuzumab on human breast cancer stem cells in
vitro and in vivo. Tumour Biol. 33:1349–1362. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Passacantilli I, Capurso G, Archibugi L,
Calabretta S, Caldarola S, Loreni F, Delle Fave G and Sette C:
Combined therapy with RAD001 e BEZ235 overcomes resistance of PET
immortalized cell lines to mTOR inhibition. Oncotarget.
5:5381–5391. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bareschino MA, Schettino C, Rossi A,
Maione P, Sacco PC, Zeppa R and Gridelli C: Treatment of advanced
non small cell lung cancer. J Thorac Dis. 3:122–133. 2011.
|
|
40
|
Harris CA, Ward RL, Dobbins TA, Drew AK
and Pearson S: The efficacy of HER2-targeted agents in metastatic
breast cancer: A meta-analysis. Ann Oncol. 22:1308–1317. 2011.
View Article : Google Scholar
|
|
41
|
Holohan C, Van Schaeybroeck S, Longley DB
and Johnston PG: Cancer drug resistance: An evolving paradigm. Nat
Rev Cancer. 13:714–726. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhou J, Wulfkuhle J, Zhang H, Gu P, Yang
Y, Deng J, Margolick JB, Liotta LA, Petricoin E III and Zhang Y:
Activation of the PTEN/mTOR/STAT3 pathway in breast cancer
stem-like cells is required for viability and maintenance. Proc
Natl Acad Sci USA. 104:16158–16163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gupta PB, Onder TT, Jiang G, Tao K,
Kuperwasser C, Weinberg RA and Lander ES: Identification of
selective inhibitors of cancer stem cells by high-throughput
screening. Cell. 138:645–659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Airiau K, Mahon FX, Josselin M, Jeanneteau
M and Belloc F: PI3K/mTOR pathway inhibitors sensitize chronic
myeloid leukemia stem cells to nilotinib and restore the response
of progenitors to nilotinib in the presence of stem cell factor.
Cell Death Dis. 4:e8272013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang XQ, Ongkeko WM, Chen L, Yang ZF, Lu
P, Chen KK, Lopez JP, Poon RT and Fan ST: Octamer 4 (Oct4) mediates
chemotherapeutic drug resistance in liver cancer cells through a
potential Oct4-AKT-ATP-binding cassette G2 pathway. Hepatology.
52:528–539. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Peiró G, Ortiz-Martínez F, Gallardo A,
Pérez-Balaguer A, Sánchez-Payá J, Ponce JJ, Tibau A, López-Vilaro
L, Escuin D, Adrover E, et al: Src, a potential target for
overcoming trastuzumab resistance in HER2-positive breast
carcinoma. Br J Cancer. 111:689–695. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pandya K, Wyatt D, Gallagher B, Shah D,
Baker A, Bloodworth J, Zlobin A, Pannuti A, Green A, Ellis IO, et
al: PKCα attenuates Jagged-1-mediated Notch signaling in
ErbB-2-positive breast cancer to reverse trastuzumab resistance.
Clin Cancer Res. 22:175–186. 2016. View Article : Google Scholar
|
|
48
|
Berns K, Horlings HM, Hennessy BT,
Madiredjo M, Hijmans EM, Beelen K, Linn SC, Gonzalez-Angulo AM,
Stemke-Hale K, Hauptmann M, et al: A functional genetic approach
identifies the PI3K pathway as a major determinant of trastuzumab
resistance in breast cancer. Cancer Cell. 12:395–402. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhu Y, Tian T, Zou J, Wang Q, Li Z, Li Y,
Liu X, Dong B, Li N, Gao J, et al: Dual PI3K/mTOR inhibitor BEZ235
exerts extensive antitumor activity in HER2-positive gastric
cancer. BMC Cancer. 15:8942015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Elster N, Cremona M, Morgan C, Toomey S,
Carr A, O'Grady A, Hennessy BT and Eustace AJ: A preclinical
evaluation of the PI3K alpha/delta dominant inhibitor BAY 80–6946
in HER2-positive breast cancer models with acquired resistance to
the HER2-targeted therapies trastuzumab and lapatinib. Breast
Cancer Res Treat. 149:373–383. 2015. View Article : Google Scholar
|
|
51
|
Saura C, Bendell J, Jerusalem G, Su S, Ru
Q, De Buck S, Mills D, Ruquet S, Bosch A, Urruticoechea A, et al:
Phase Ib study of Buparlisib plus Trastuzumab in patients with
HER2-positive advanced or metastatic breast cancer that has
progressed on Trastuzumab-based therapy. Clin Cancer Res.
20:1935–1945. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lehmann BD, Bauer JA, Chen X, Sanders ME,
Chakravarthy AB, Shyr Y and Pietenpol JA: Identification of human
triple-negative breast cancer subtypes and preclinical models for
selection of targeted therapies. J Clin Invest. 121:2750–2767.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Rodler E, Korde L and Gralow J: Current
treatment options in triple negative breast cancer. Breast Dis.
32:99–122. 2010.
|
|
54
|
Ibrahim YH, García-García C, Serra V, He
L, Torres-Lockhart K, Prat A, Anton P, Cozar P, Guzmán M, Grueso J,
et al: PI3K inhibition impairs BRCA1/2 expression and sensitizes
BRCA-proficient triple-negative breast cancer to PARP inhibition.
Cancer Discov. 2:1036–1047. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yunokawa M, Koizumi F, Kitamura Y,
Katanasaka Y, Okamoto N, Kodaira M, Yonemori K, Shimizu C, Ando M,
Masutomi K, et al: Efficacy of everolimus, a novel mTOR inhibitor,
against basal-like triple-negative breast cancer cells. Cancer Sci.
103:1665–1671. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Singh J, Novik Y, Stein S, Volm M, Meyers
M, Smith J, Omene C, Speyer J, Schneider R, Jhaveri K, et al: Phase
2 trial of everolimus and carboplatin combination in patients with
triple negative metastatic breast cancer. Breast Cancer Res.
16:R322014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue
P, Fu H and Khuri FR: Activation of Akt and eIF4E survival pathways
by rapamycin-mediated mammalian target of rapamycin inhibition.
Cancer Res. 65:7052–7058. 2005. View Article : Google Scholar : PubMed/NCBI
|