Introduction
Cervical cancer, which accounts for 8% of all cancer
deaths, is the third most common cancer in women after breast and
colorectal cancer (1,2). The human papilloma virus (HPV) is
known to be an essential cause of cervical cancer, among which 60%
or more are with HPV type 16 (HPV16) (3). Carcinogenesis by HPV16 is
primarily attributed to the continuous expression of viral protein
E6/E7. After infection of HPV, the integration of the viral
E6/E7 genes occur to the genome of the cervical epithelium,
and the continued expression of E6/E7 not only enhances the
neoplastic progression to the cervical epithelium, but also drives
the cervical cancer cells to malignant phenotype (4–6). There
has been convincing evidence provided by previous studies that the
overexpression of HPV16E7 is associated with the development
of cervical cancer (3,7). Therefore, the HPV16E7 is
considered to be potential therapeutic target for cervical cancer
treatment.
Treatment strategies for cervical cancer today are
focused on surgical operation or chemo-radiation. Although a number
of chemotherapeutic drugs for treating cervical cancer can be used
to control the growth of cancer and have shown certain therapeutic
efficacy, the strong side-effects limit their application. Besides,
only one-third of women with metastatic cervical cancer respond to
chemotherapy and this response is short-lived (1,6).
Therefore, to develop novel natural substances with curative
selectivity for cervical cancer without showing significant toxic
effect to normal cells is becoming much more necessary.
There has been a recent focus on the use of Chinese
medicinal herbs to treat a number of diseases. Oxymatrine, which is
a quinolizidine alkaloid extracted from the root of traditional
Chinese herbal medicine Sophora japonica (Sophora flavescens
Ait), has been found to possess several biological effects such as
anti-inflammation, inhibiting immune reaction, anti-virus and
antitumor (8–13). Different from the traditional
chemotherapy medicine, oxymatrine has been reported to inhibit the
proliferation of a number of cancer cells in vitro and also
inhibited viral-induced tumor formation in mice, with little
influence to some normal cells (14,15).
As far as we know, there are few studies on the
application of oxymatrine in the treatment of cervical cancer
(10,16). Besides, we found no report
concerning the putative relationship between oxymatrine and
HPV16E7 in anticancer study. In the present study, we
investigated the effect of oxymatrine on cervical cancer cell line
CaSki by evaluating cell proliferation, cell apoptosis, cell cycle,
the mRNA and protein expression levels of HPV16E7 gene in
vitro. The aim of this study was to explore the mechanisms
underlying the antitumor effect of oxymatrine on cervical cancer
cells and support experimental data for the application of
oxymatrine in the prevention and treatment of cervical cancer.
Materials and methods
Oxymatrine
We purchased oxymatrine from Chia Tai Tianqing
Pharmaceutical Group Co., Ltd. (Nanjing, China). Before the
application, we tested this oxymatrine and found that its purity is
>99% indicated by SDS-PAGE analysis.
Cell line and culture
CaSki cells of human cervical carcinoma (American
Type Culture Collection, Manassas, VA, USA) were maintained in
RPMI-1640 medium (Gibco, Carlsbad, CA, USA) supplemented with 10%
fetal bovine serum (FBS; Gibco) penicillin (100 μg/ml) and
streptomycin (100 U/ml) in 5% CO2 atmosphere at 37°C.
The cells were passaged every 2–4 days to keep a proper density and
cells in the logarithmic growth phase were used in the
experiment.
Proliferation assay
CaSki cells in logarithmic growth phase were seeded
in 96-well plates with 1×105 cells each well, and
cultured cells with RPMI-1640 complete medium. Twenty-four hours
later, the medium was replaced with RPMI-1640 complete medium with
various concentrations of oxymatrine (2, 4 and 6 mg/ml) and
cultured continuously. In addition, control cells were incubated
with medium only. After exposure to oxymatrine, the proliferation
of CaSki cells was assessed by using Cell Counting kit-8 assay.
After 24, 48 and 72 h, each well of cells were treated with 10
μl of Cell Counting kit-8 solution (Dojindo Laboratories,
Kumamoto, Japan) and incubated for 1 h at 37°C. Spectrometric
absorbance was measured at 450 nm by an enzyme immunoassay analyzer
(Bio-Rad Laboratories, Hercules, CA, USA).
Colony formation assay
Colony formation assay was performed to observe the
effect of oxymatrine on colony formation ability of CaSki cells.
Cells (1×103) were plated in each well of 6-well plate
and cultured with RPMI-1640 complete mediumin a humidified
atmosphere with 5% CO2 at 37°C. After 2 weeks, the
colonies were fixed by 4% paraformaldehyde (PFA) for 15 min and
stained with Giemsa for 20 min. Cell aggregate consisting of 50 or
more cells were defined as one colony. Clonies were manually
counted by microscope (Olympus, Tokyo, Japan) with x40 field.
Apoptosis assay
Apoptosis assay kit (Multi Sciences, Harbin, China)
was used to treat the cell samples and the procedure followed the
instructions of the kit. CaSki cells were treated with various
concentrations of oxymatrine (0, 2, 4 and 6 mg/ml) for 72 h, and
the cells were collected, washed, successively incubated with 5
μl of Annexin V-FITC and 5 μl of propidium iodide
(PI) for 15 min at room temperature. Before being subjected to flow
cytometric analysis, apoptosic ratio of fixed cell sample was
measured by flow cytometry (FACSCalibur™; BD Biosciences, San Jose,
CA, USA).
Cell cycle analysis
CaSki cells were treated with different
concentrations of oxymatrine (0, 2, 4 and 6 mg/ml) for 72 h. The
cells were harvested by trypsinization, then were fixed with 70%
ethanol and resuspended in 20 mg/Ml PI for 30 min. Flow cytometer
(FACSCalibur™; BD Biosciences) was applied to detect DNA content.
Based on the flow cytometry data, we determined the relative
proportions of cells in the individual cell-cycle phase
fraction.
Quantitative real-time PCR
Total RNA was extracted from CaSki cells which had
been treated with 4 mg/ml of oxymatrine for 72 h or the control
cells using TRIzol RNA reagent (Invitrogen, Carlsbad, CA, USA), and
then was treated with DNAse I (Roche, Basel, Switzerland) to remove
contaminating genomic and adenoviral DNA. MMLV reverse
transcriptase kit (Promega, Madison, WI, USA) was used to convert
the prepared total RNA to cDNA, which was then subjected to
quantitative real-time PCR (qRT-PCR) using a SYBR-Green Master Mix
kit on a Bio-Rad connect real-time PCR platform. Taq DNA polymerase
was used. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used
to normalize the mRNA levels. The sequences of primers were as
follows: HPV16E7 (157 bp), sense: 5′-ATGCATGGAGATACACCT-3′ and
anti-sense: 5′-TTATGGTTTCTGAGAACA-3′; GAPDH (101 bp), sense:
5′-ACAACTTTGGTATCGTGGAAGG-3′ and reverse,
5′-GCCATCACGCCACAGTTTC-3′. Data were analyzed using the
2−ΔΔCt method.
Western blot analysis
The protein was extracted from CaSki cells treated
with 4 mg/ml of oxymatrine for 72 h and the control cells. The
cells were lysed by lysis buffer (150 mM NaCl, 0.1% SDS, 0.5%
sodium deoxycholate, 1% Nonidet P-40 and 50 mM Tris, pH 8.0), with
the addition of 2 mM phenylmethylsulfonyl fluoride, and then
centrifuged at 12,000 × g for 30 min at 4°C. Protein concentrations
were determined by protein assay kit (Sigma, St. Louis, MO, USA).
Equal amounts of proteins (20 μg) were boiled for 10 min,
and loaded onto a 15% SDS-PAGE gel and then transferred to the
polyvinylidenedifluoride (PVDF) membranes. The membranes were
incubated for 1 h with blocking buffer (PBS with 5% skim milk and
0.1% Tween-20) and then with anti-HPV16E7 primary antibody (Abcam,
Cambridge, MA, USA) overnight at 4°C. Secondary antibody (Abcam)
was used to incubate the membranes for 1 h at room temperature.
Before each step, membranes were washed 3 times by PBST. The
protein bands were visualized by enhanced chemiluminescence. We
used β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) as the
internal control.
RNA interference
The siRNA for HPV16E7 was designed and
synthesized by Sangon Biotech Co., Ltd., (Shanghai, China), and the
sequences were as follows (5′-3′): GCACACACGTAGACATTCGdTdT;
(3′-5′): dTdTCGUGUGUGCAUCUGUAAGC. A scrambled siRNA which had no
homology with human genome was also produced for use as a negative
control. The siRNA duplex (50 nM) was transfected using
Lipofectamine 2000 reagent (Invitrogen) following the instructions
of the manufacturer. The CaSki cells were effected by
HPV16E7 siRNA for 72 h before the analysis of cell
proliferation and HPV16E7 expression.
Tumorigenicity assay
The ethics approval for this protocol was obtained
from The Institutional Animal Care and use Committee at Hubei
university of Medicine. CaSki cells treated with 4 mg/ml of
oxymatrine for 72 h and control CaSki cells were injected
subcutaneously into 4-week old nude mice (3×106
cells/mouse), respectively. Four weeks after the injection, mice
were sacrificed by injecting excessive chloral hydrate and tumors
were separated and weighed.
Statistical analysis
All experiments were performed for at least three
times, and data are shown as the mean ± SD. One-way ANOVA was used
to determine statistically significant differences between groups,
and P<0.05 was considered statistically significant. Analyses
were carried out using GraphPad Prism version 5.0 (Graphpad
Software, Inc., La Jolla, CA, USA).
Results
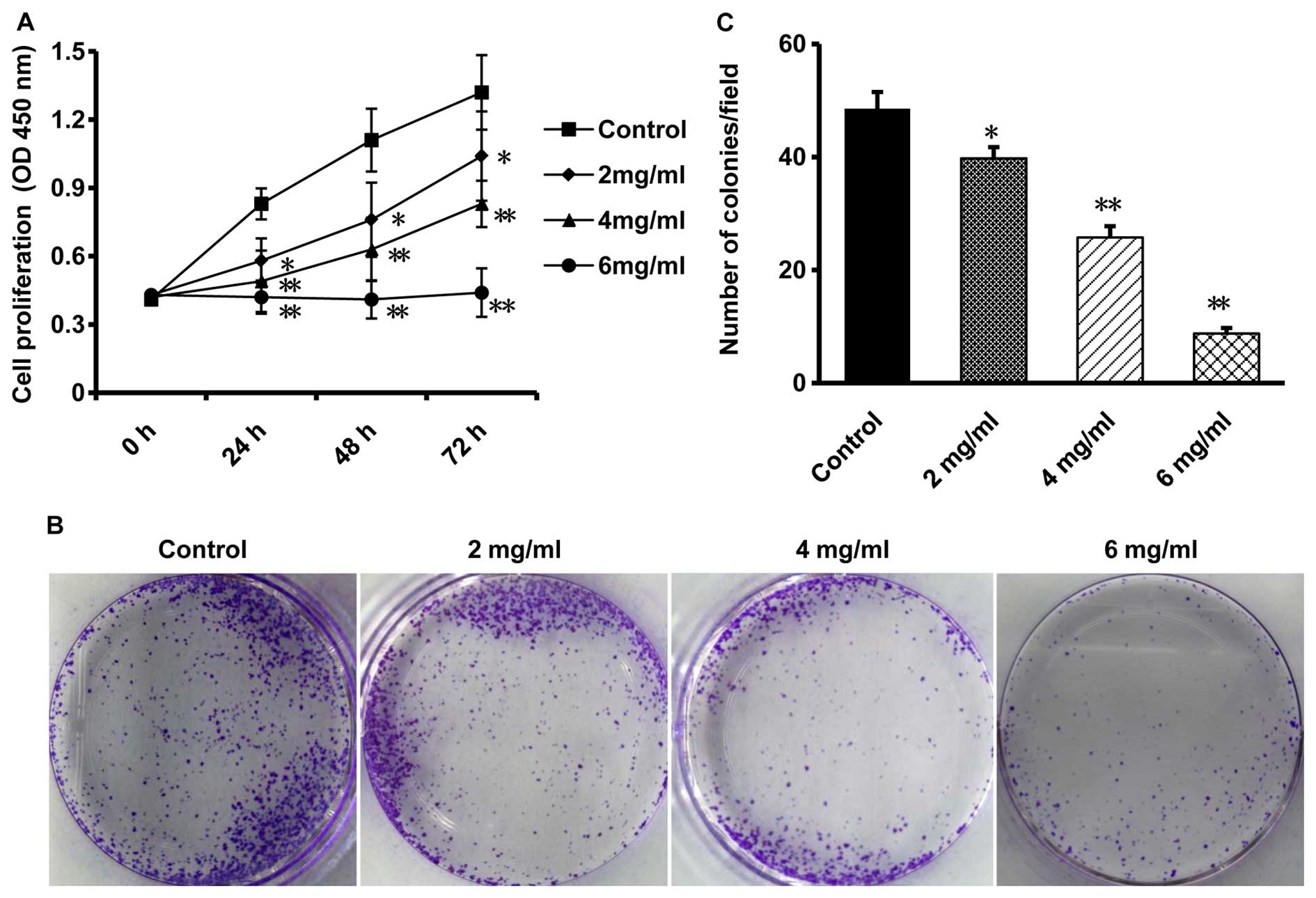
Oxymatrine inhibits proliferation and
clonogenicity of CaSki cells
CaSki cells were treated with various concentrations
of oxymatrine (0, 2, 4 and 6 mg/ml) for 24, 48 and 72 h. CCK-8
assay was performed to determine the effect of oxymatrine on cell
proliferation and found a time-dependent and
concentration-dependent inhibition of oxymatrine to the growth of
CaSki cells, compared with that in the control cells (P<0.05,
n=6; Fig. 1A). Especially when the
CaSki cells were treated with oxymatrine for 72 h, the inhibitory
effect was much more significant than that treated for 24 h
(P<0.05, n=6; Fig. 1A).
Therefore, we chose the time-point of 72 h for further
investigation.
The inhibitory effect of oxymatrine on CaSki cells
was then confirmed by colony formation assay. The results showed
that after treated with concentrations of oxymatrine (0, 2, 4 and 6
mg/ml), the colony formation ability of CaSki cells was
significantly decreased in a dose-dependent manner, compared with
the control group (P<0.05, n=6; Fig.
1B and C), indicating that oxymatrine is able to inhibit the
growth of cervical cancer cells.
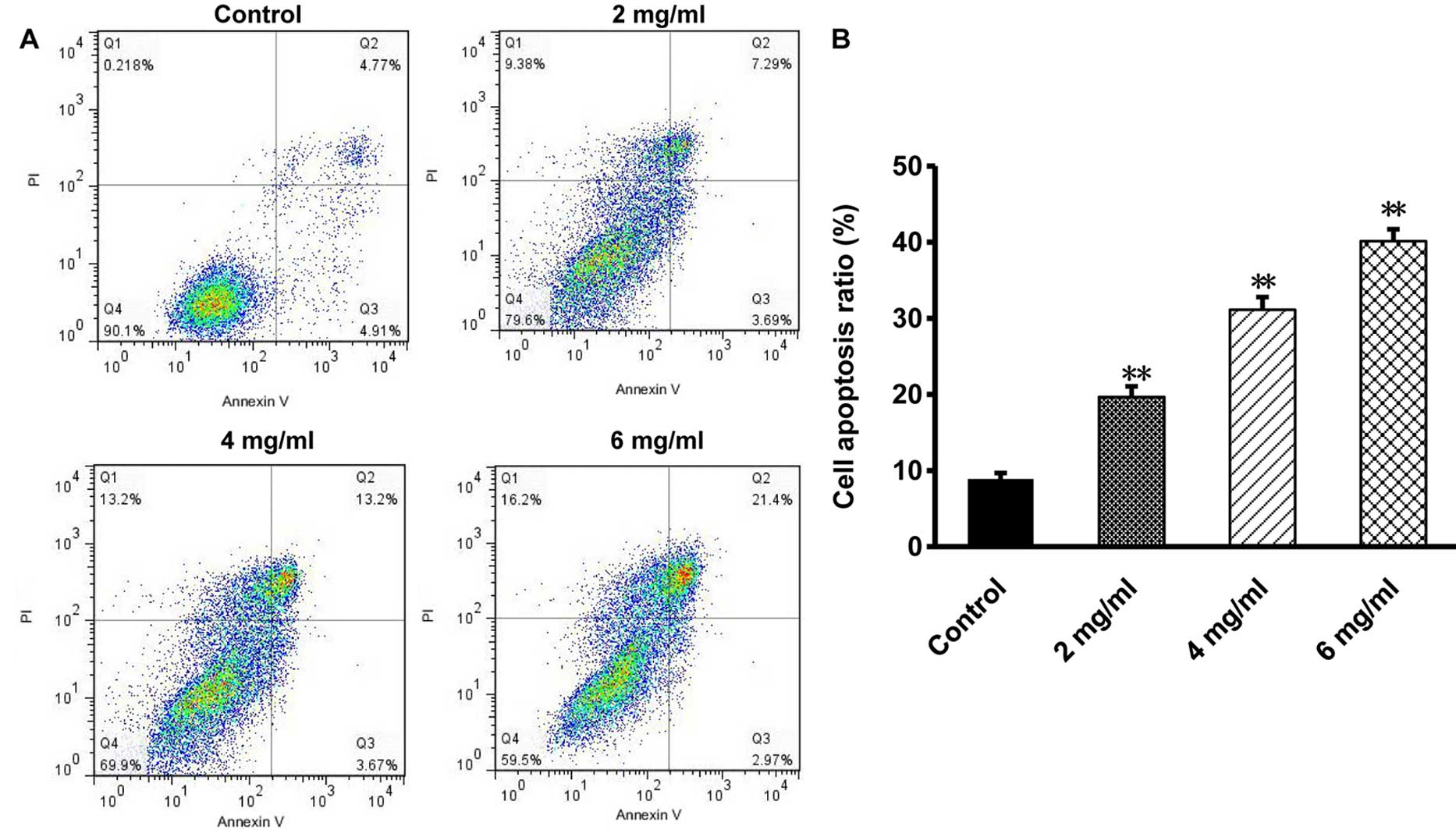
Oxymatrine induces apoptosis to CaSki
cells
The effect of oxymatrine on cell death of CaSki
cells was investigated by evaluating the rate of apoptosis with
flow cytometry. Results showed that after treatment with 2, 4 and 6
mg/ml of oxymatrine for 72 h, the rate of CaSki apoptosis was
19.62±1.71, 31.84±2.46 and 40.94±2.39%, respectively. The apoptosis
rate of the control group was 9.64±0.98%. In contrast with the
control group, the apoptosis rate of oxymatrine treated groups
increased significantly in a dose-dependent manner (P<0.01, n=6;
Fig. 2). As the results show,
oxymatrine may suppress CaSki cell proliferation by causing cell
death.
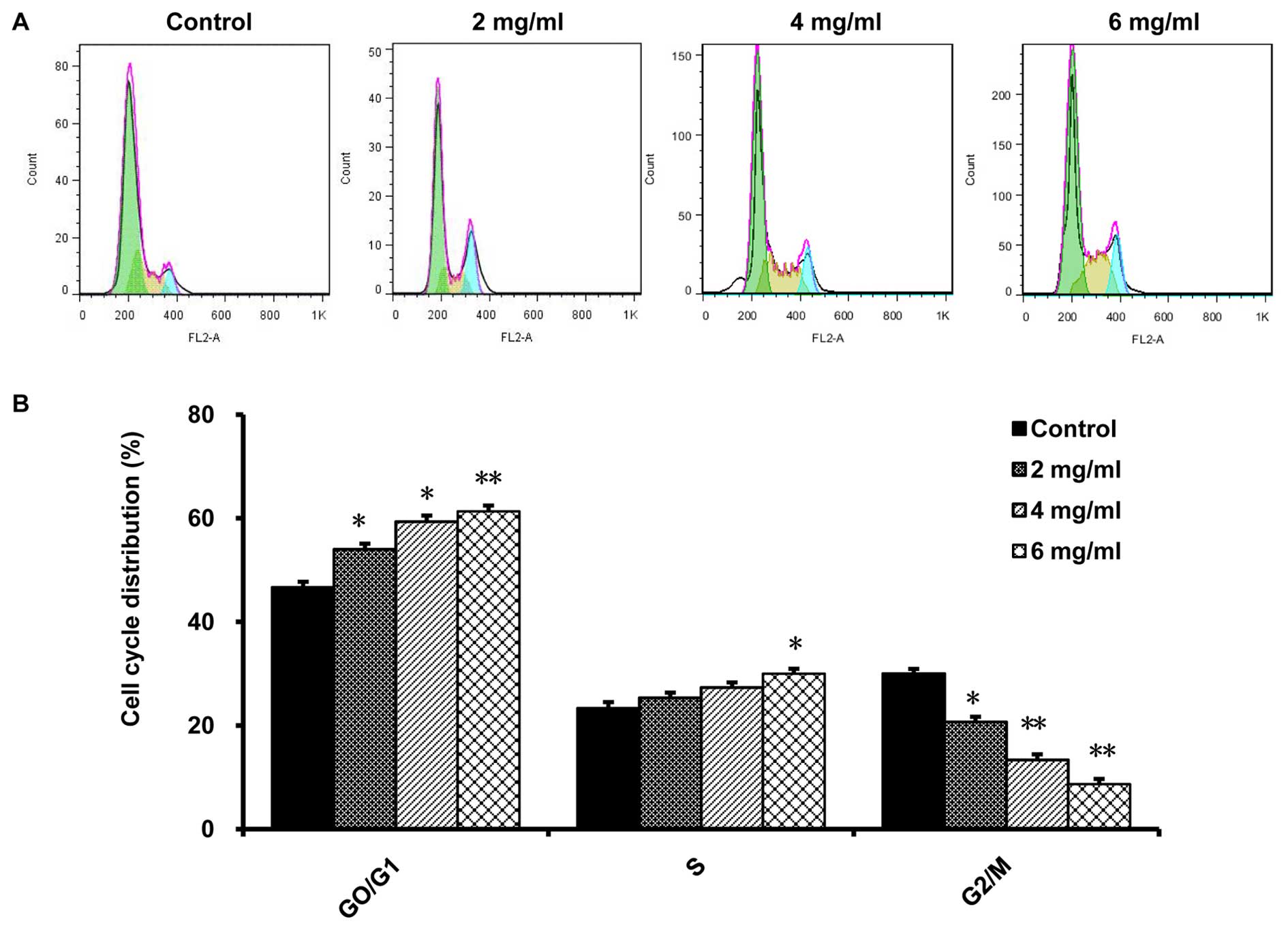
Oxymatrine changes the cell cycle
distribution of CaSki cells
We performed FCM analysis to evaluate the effect of
oxymatrine on the cell cycle of CaSki cells. After treatment with
2, 4 and 6 mg/ml of oxymatrine for 72 h, the proportion of G0/G1
phase was 54.91±2.08, 59.35±2.17 and 65.07±3.22%, respectively,
while the percentage of G0/G1 phase control group was 44.35±1.78%.
Compared with that in the control group, CaSki cells of the treated
group in G0/G1 phase were markedly increased in a dose-dependent
manner, while the cells in G2/M phase were decreased in the same
way (P<0.01, n=6; Fig. 3).
Besides, we found that 6 mg/ml oxymatrine could significantly
increase the number of CaSki cells in S phase, compared to the
control cells (P<0.01, n=6; Fig.
3). The results demonstrate that oxymatrine arrested CaSki
cells in G0/G1 phase and S phase.
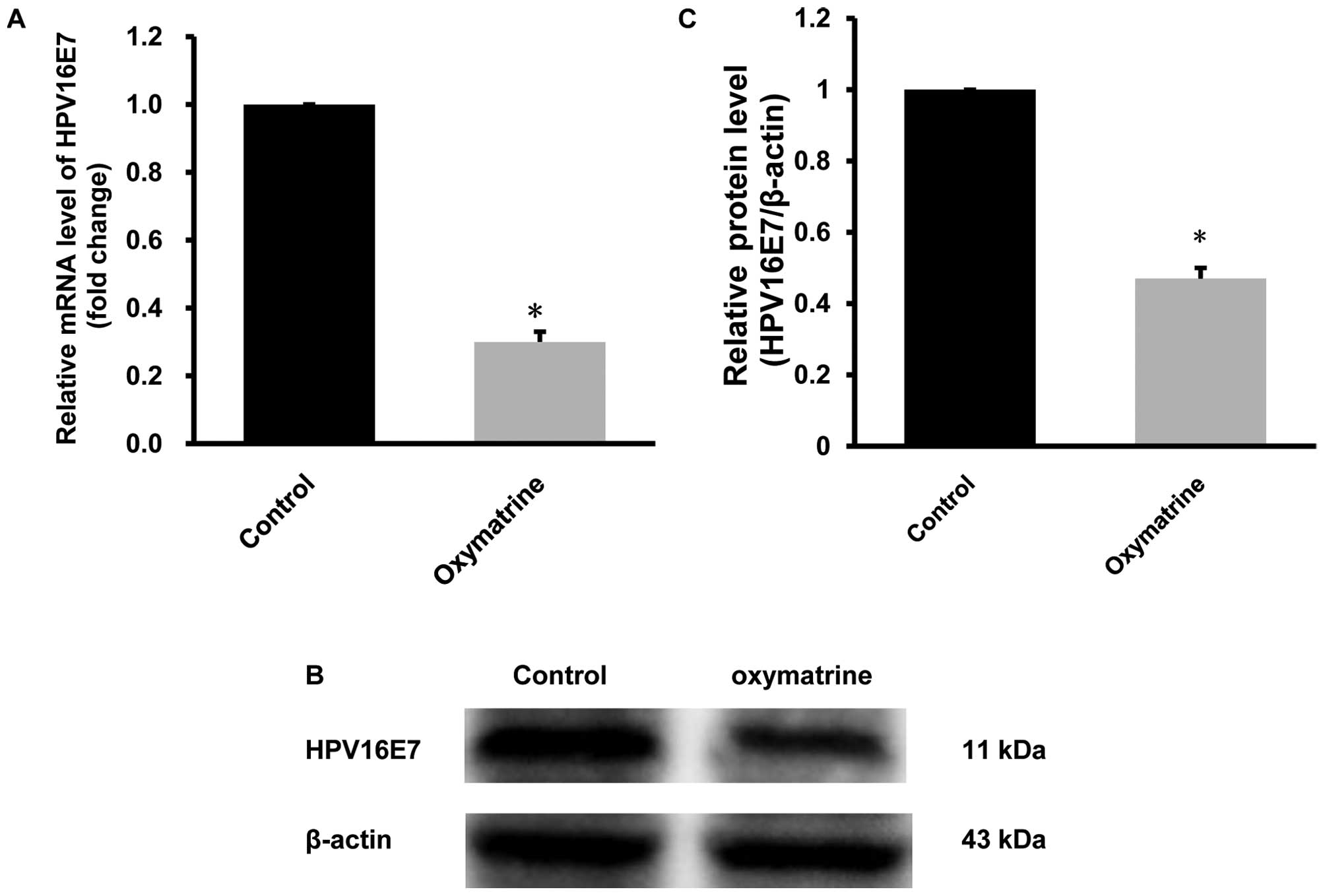
Oxymatrine downregulates the mRNA and
protein levels of HPV16E7 in CaSki cells
We performed qRT-PCR and western blot analysis to
examine the effect of oxymatrine on the expression of
HPV16E7 gene. The results showed that after treated with 4
mg/ml of oxymatrine, mRNA levels of HPV16E7 gene in the
CaSki cells was significantly decreased by 64.5±2.8% (P<0.01,
n=6; Fig. 4A) and protein level was
prominently decreased by 52.3±3.2% (P<0.01, n=6; Fig. 4B and C), compared with the control
group. These data demonstrate that oxymatrine could markedly
downregulate the expression of HPV16E7 in CaSki cells.
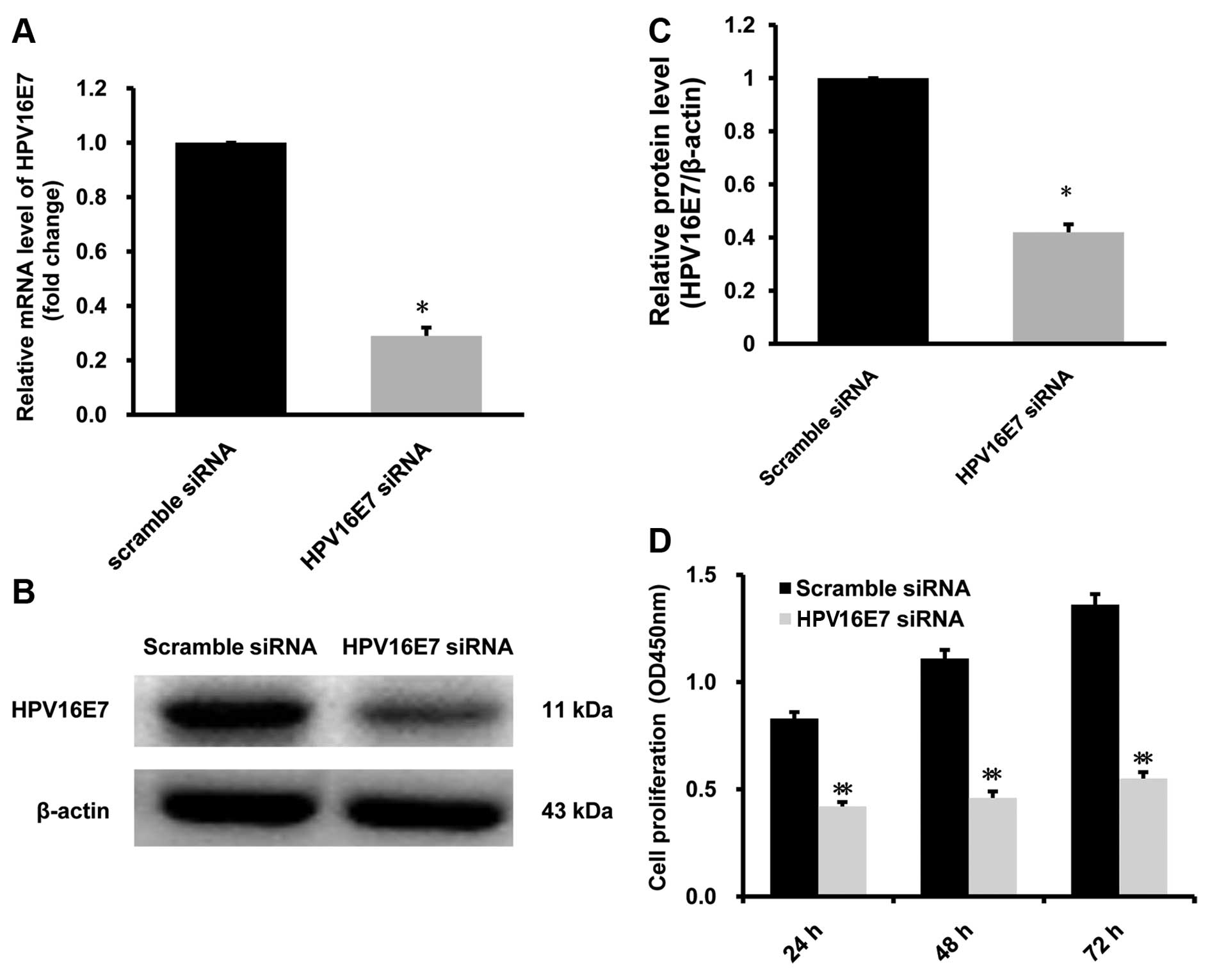
HPV16E7 siRNA suppresses the
proliferation of CaSki cells
We used HPV16E7 siRNA to knock down
HPV16E7 mRNA level to investigate the effect of
downregulating HPV16E7 on the proliferation of CaSki cells.
Our results show that after treated with HPV16E7 siRNA, the
mRNA and protein level of HPV16E7 in CaSki were reduced by
66.4±2.7 and 57.2±6.1% respectively, in contrast with the control
group treated with scramble siRNA (Fig.
5A–C). After treated with HPV16E7 siRNA, the
proliferation of CaSki cells was suppressed in a time-dependent
manner, compared with control siRNA (P<0.05, n=6; Fig. 5D). Our data demonstrate that
knocking down HPV16E7 expression could suppress the
proliferation of CaSki cells.
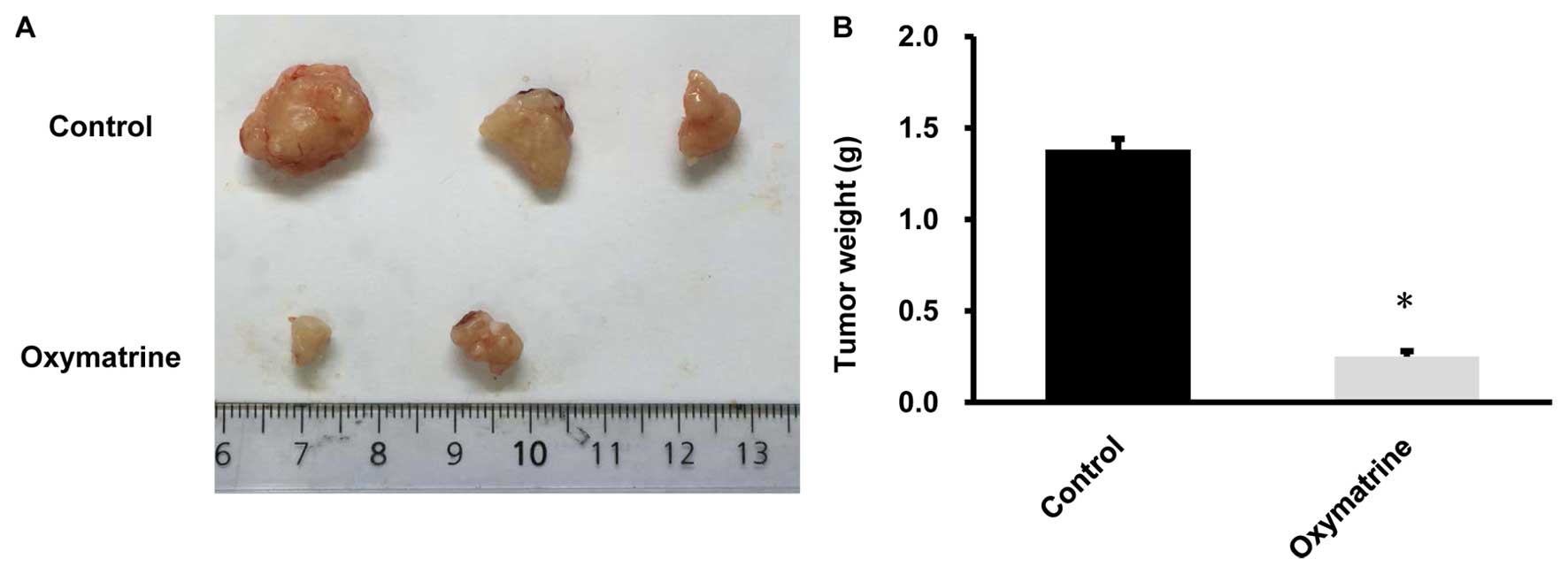
Oxymatrine suppressed the growth of CaSki
cells in vivo
CaSki cells (3×106) treated with
oxymatrine or control cells were injected subcutaneously into each
athymic nude mouse. Four weeks after the injection, mice were
sacrificed and tumors were separated. As showed in the tumor
images, all 3 mice injected with control CaSki cells formed
xenografts. In contrast, 2 out of 3 mice injected with
oxymatrine-treated CaSki cells formed smaller xenografts. The
weight of tumors from oxymatrine treated CaSki cells was
significantly lower than those from the control CaSki cells
(P<0.05, n=3; Fig. 6B). The
results suggest that oxymatrine could suppress tumorigenicity of
cervical cancer cells in vivo.
Discussion
Oxymatrine is a major alkaloid component found in
the roots of Sophora species (17).
It is commonly used for the treatment of liver disorders and other
diseases such as arrhythmia, eczema and skin disorders, leukopenia
and bronchitis (13,18–21).
Some studies have also reported oxymatrine showed anticancer
activity in human gastric cancer cells, pancreatic, ovarian cancer,
and human breast cancer cells (14,16,22).
However, to the best of our knowledge, the mechanisms of the
antitumor effect of oxymatrine on cervical cancer have not yet been
elucidated. In the present study, various concentrations of
oxymatrine were used to treat cervical cancer cell line CaSki
cells, and we found that oxymatrine treatment could induce
inhibition of proliferation and apoptosis in cervical cancer CaSki
cells in vitro and in vivo.
One of the main reasons for tumorigenesis is loss of
control of cell proliferation, which is involved both in tumor
initiation and progression (23).
Therefore, we applied CCK-8 assay and found that oxymatrine could
obviously inhibit cell proliferation in cervical cancer CaSki cells
in a time-dependent and dose-dependent manner in vitro.
Besides, our colony formation assay results also showed similar
inhibition effect of oxymatrine on the colony forming ability of
CaSki cells in a dose-dependent manner. In vivo, we
performed analysis of tumorigenesis and found that the weight of
tumors resulted from CaSki cells treated with oxymatrine were
significantly reduced, compared with the tumors derived from
control CaSki cells. These data indicate that oxymatrine can
suppress the growth of cervical cancer cells in vitro and
in vivo.
Apoptosis is the process of cell death, with a
series of cellular and molecular changes, such as
phosphatidylserine externalization, chromatin condensation and cell
shrinkage. Apoptosis plays important functions in organ
development, homeostasis and immune defense. Uncontrolled cell
death is another main reason for tumorigenesis (24,25).
Therefore, inducing cell apoptosis of tumor cells is always of
great importance in developing anticancer treating methods. In the
present study, flow cytometry was applied to the effect of
oxymatrine on the apotosis of CaSki cells and the results
demonstrated that CaSki cells could be induced to apotosis by
oxymatrine in a dose-dependent manner.
Since the change of cell proliferation and apotosis
is intimately correlated with the change of the cell cycle
(23,24). In this study, in order to
investigate the cell cycle related mechanism of the effect of
oxymatrine on the proliferation and apotosis on CaSki cells, we
also performed flow cytometry and found that the oxymatrine treated
CaSki cells were significantly blocked in G0/G1 phase. After
treated with increasing concentration of oxymatrine, CaSki cells in
G0/G1 phase were prominently increased while cells in the G2/M
phase were obviously decreased, in contrast with control CaSki
cells. Moreover, we found that oxymatrine could increase the number
of CaSki cells in S phase to a certain extent, and 6 mg/ml made a
significant difference compared to the control cells. When arrested
in G0/G1 and S phase, the CaSki cells tend to decrease the
proliferation rate with more apoptosis, which is also in accordance
with our proliferation and apoptosis assay data. These data suggest
that the growth inhibition and apoptosis induction of CaSki cells
may result from the effect of oxymatrine on arresting CaSki cells
in G0/G1 phase.
According to previous studies, over 60% of all
cervical cancers are closely correlated with HPV16, which
encodes the essential genes for virus replication, E6 and
E7 oncogenes (7). The viral
protein E7 can cause destabilization and the disruption of
Rb/E2F complexes, which is essential for driving the process
of cell cycle into S phase, and upregulating the anti-apoptotic
protein Bcl-2 to promote cell survival and proliferation
(3,26). Several researches have reported that
inhibition of the expression of E7 oncogene was efficient in
HPV-associated cancer therapy (15,27,28).
It is reported by previous studies that oxymatrine showed
anti-hepatitis virus effects (29–31).
Although no report on the anti-HPV effect was found, our results
demonstrated that oxymatrine could decrease both the mRNA and
protein levels of HPV16E7. Since the expression of
HPV16E7 was closely correlated with the proliferation and
apoptosis in CaSki cells, we speculated that oxymatrine could
inhibit proliferation and enhance apoptosis in CaSki cells via
downregulating HPV16E7. We also used HPV16E7 siRNA to
knock-down HPV16E7 expression, and the results indicated
that downregulating HPV16E7 expression could suppress the
growth of CaSki cells, which was in accordance with our
speculation. We also hypothesized that oxymatrine is able to treat
cervical cancer through the anti-HPV effect, which is going
to be the subject of our further investigation.
In conclusion, our data showed that oxymatrine
significantly induced cell apoptosis, and suppressed cell growth of
cervical cancer CaSki cells both in vitro and in
vivo. Oxymatrine could decrease the CaSki cells in G2/M phase
and arrest the CaSki cells in G0/G1 and S phase, which partly
mediated proliferation inhibition and apoptosis induction. Besides,
we a found that oxymatrine could downregulate the expression of
HPV16E7 at the mRNA and protein level, which offered the
molecular explanation to the cell growth inhibitory effect of
oxymatrine on CaSki cells. Therefore, we believe that oxymatrine
can be developed to a potential preventive and therapeutic
candidate for cervical cancer treatment. Our data may provide
theoretical support for the clinical anticancer application with
oxymatrine. However, the toxicity of oxymatrine and its effect on
the proliferation of normal cells were not assessed in the present
study. In future, we will continue our study on the toxicity of
oxymatrine and other effects of oxymatrine in carcinoma cells in
vivo and in vitro.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81401447).
Abbreviations:
|
HPV
|
human papilloma virus
|
|
FBS
|
fetal bovine serum
|
|
PFA
|
paraformaldehyde
|
|
PI
|
propidium iodide
|
|
GAPDH
|
glyceraldehyde-3-phosphate
dehydrogenase
|
|
PVDF
|
polyvinylidenedifluoride
|
References
|
1
|
Aggarwal P: Cervical cancer: Can it be
prevented? World J Clin Oncol. 5:775–780. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang KL: Human papillomavirus and
vaccination in cervical cancer. Taiwan J Obstet Gynecol.
46:352–362. 2007. View Article : Google Scholar
|
|
3
|
Haedicke J and Iftner T: Human
papillomaviruses and cancer. Radiother Oncol. 108:397–402. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Almajhdi FN, Senger T, Amer HM, Gissmann L
and Öhlschläger P: Design of a highly effective therapeutic HPV16
E6/E7-specific DNA vaccine: Optimization by different ways of
sequence rearrangements (shuffling). PLoS One. 9:e1134612014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen S, Liao C, Lai Y, Fan Y, Lu G, Wang
H, Zhang X, Lin MC, Leng S and Kung HF: De-oncogenic HPV e6/e7
vaccine gets enhanced antigenicity and promotes tumoricidal synergy
with cisplatin. Acta Biochim Biophys Sin (Shanghai). 46:6–14. 2014.
View Article : Google Scholar
|
|
6
|
Duenas-Gonzalez A, Serrano-Olvera A,
Cetina L and Coronel J: New molecular targets against cervical
cancer. Int J Womens Health. 6:1023–1031. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boulet GA, Horvath CA, Berghmans S and
Bogers J: Human papillomavirus in cervical cancer screening:
important role as biomarker. Cancer Epidemiol Biomarkers Prevy.
17:810–817. 2008. View Article : Google Scholar
|
|
8
|
Fei ZW, Qiu MK, Qi BQ, Dai YX, Wang SQ,
Quan ZW, Liu YB and Ou JM: Oxymatrine suppresses proliferation and
induces apoptosis of hemangioma cells through inhibition of HIF-1α
signaling. Int J Immunopathol Pharmacol. 28:201–208. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guo B, Zhang T, Su J, Wang K and Li X:
Oxymatrine targets EGFR(p-Tyr845) and inhibits EGFR-related
signaling pathways to suppress the proliferation and invasion of
gastric cancer cells. Cancer Chemother Pharmacol. 75:353–363. 2015.
View Article : Google Scholar
|
|
10
|
Li M, Su BS, Chang LH, Gao Q, Chen KL, An
P, Huang C, Yang J and Li ZF: Oxymatrine induces apoptosis in human
cervical cancer cells through guanine nucleotide depletion.
Anticancer Drugs. 25:161–173. 2014. View Article : Google Scholar
|
|
11
|
Wang B, Han Q and Zhu Y: Oxymatrine
inhibited cell proliferation by inducing apoptosis in human lung
cancer A549 cells. Biomed Mater Eng. 26(Suppl 1): S165–S172.
2015.PubMed/NCBI
|
|
12
|
Zhang Y, Sun S, Chen J, Ren P, Hu Y, Cao
Z, Sun H and Ding Y: Oxymatrine induces mitochondria dependent
apoptosis in human osteosarcoma MNNG/HOS cells through inhibition
of PI3K/Akt pathway. Tumour Biol. 35:1619–1625. 2014. View Article : Google Scholar
|
|
13
|
Guzman JR, Koo JS, Goldsmith JR, Mühlbauer
M, Narula A and Jobin C: Oxymatrine prevents NF-κB nuclear
translocation and ameliorates acute intestinal inflammation. Sci
Rep. 3:16292013. View Article : Google Scholar
|
|
14
|
Wang W, You RL, Qin WJ, Hai LN, Fang MJ,
Huang GH, Kang RX, Li MH, Qiao YF, Li JW, et al: Anti-tumor
activities of active ingredients in Compound Kushen Injection. Acta
Pharmacol Sin. 36:676–679. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ying XJ, Jin B, Chen XW, Xie J, XU HM and
Dong P: Oxymatrine downregulates HPV16E7 expression and inhibits
cell proliferation in laryngeal squamous cell carcinoma Hep-2 cells
in vitro. BioMed Res Int. 2015:1503902015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun M, Cao H, Sun L, Dong S, Bian Y, Han
J, Zhang L, Ren S, Hu Y, Liu C, et al: Antitumor activities of
kushen: Literature review. Evid Based Complement Alternat Med.
2012:3732192012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamazaki M: The pharmacological studies on
matrine and oxymatrine. Yakugaku Zasshi. 120:1025–1033. 2000.In
Japanese. PubMed/NCBI
|
|
18
|
Jiang G, Liu X, Wang M, Chen H, Chen Z and
Qiu T: Oxymatrine ameliorates renal ischemia-reperfusion injury
from oxidative stress through Nrf2/HO-1 pathway. Acta Cir Bras.
30:422–429. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu L, Lu W, Ma Z and Li Z: Oxymatrine
attenuates bleomycin-induced pulmonary fibrosis in mice via the
inhibition of inducible nitric oxide synthase expression and the
TGF-β/Smad signaling pathway. Int J Mol Med. 29:815–822.
2012.PubMed/NCBI
|
|
20
|
Zhang TZ, Fu Q, Chen T and Ma SP:
Anti-asthmatic effects of oxymatrine in a mouse model of allergic
asthma through regulating CD40 signaling. Chin J Nat Med.
13:368–374. 2015.PubMed/NCBI
|
|
21
|
Zhao P, Zhou R, Li HN, Yao WX, Qiao HQ,
Wang SJ, Niu Y, Sun T, Li YX and YU JQ: Oxymatrine attenuated
hypoxic-ischemic brain damage in neonatal rats via improving
antioxidant enzyme activities and inhibiting cell death. Neurochem
Int. 89:17–27. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Y, Bi T, Dai W, Wang G, Qian L, Gao Q
and Shen G: Effects of oxymatrine on the proliferation and
apoptosis of human hepatoma carcinoma cells. Technol Cancer Res
Treat. May 24–2015.Epub ahead of print.
|
|
23
|
Cohen SM: Cell proliferation and
carcinogenesis. Drug Metab Rev. 30:339–357. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Suda T: Physiological and pathological
roles of apoptosis. Nihon Rinsho. 63(Suppl 4): 395–400. 2005.
|
|
25
|
Zörnig M, Hueber A, Baum W and Evan G:
Apoptosis regulators and their role in tumorigenesis. Biochim
Biophys Acta. 1551:F1–F37. 2001.PubMed/NCBI
|
|
26
|
Cheng YM, Chou CY, Hsu YC, Chen MJ and
Wing LY: The role of human papillomavirus type 16 E6/E7
oncoproteins in cervical epithelial-mesenchymal transition and
carcinogenesis. Oncol Lett. 3:667–671. 2012.PubMed/NCBI
|
|
27
|
Hamada K, Shirakawa T, Gotoh A, Roth JA
and Follen M: Adenovirus-mediated transfer of human papillomavirus
16 E6/E7 antisense RNA and induction of apoptosis in cervical
cancer. Gynecol Oncol. 103:820–830. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou J, Peng C, Li B, Wang F, Zhou C, Hong
D, Ye F, Cheng X, Lü W and Xie X: Transcriptional gene silencing of
HPV16 E6/E7 induces growth inhibition via apoptosis in vitro and in
vivo. Gynecol Oncol. 124:296–302. 2012. View Article : Google Scholar
|
|
29
|
Wu XN and Wang GJ: Experimental studies of
oxymatrine and its mechanisms of action in hepatitis B and C viral
infections. Chin j Dig Dis. 5:12–16. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yao N and Wang X: In vitro
immunomodulatory activity of oxymatrine on Toll-like receptor 9
signal pathway in chronic hepatitis B. Am J Chin Med. 42:1399–1410.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin M, Yang LY, Li WY, Peng YP and Zheng
JK: Inhibition of the replication of hepatitis B virus in vitro by
oxymatrine. J Int Med Res. 37:1411–1419. 2009. View Article : Google Scholar : PubMed/NCBI
|