Introduction
Malignant pleural mesothelioma (MPM) is the most
common and aggressive primary tumor of the pleura (1,2). It is
a rare asbestos-related neoplasm of the serosal membrane (3). It is extremely difficult to treat,
being invariably fatal (4). MPM is
attributed to the widespread use of asbestos as an insulator over
the latter half of the last century (5–7). Other
potential carcinogenic factors are exposure to simian virus 40 and
radiation (8,9). Median survival time for untreated MPM
patients ranges from 4 to 12 months (10,11).
Moreover, its mortality rates are estimated to increase by 5–10%
per year in most industrialized countries until 2020 (1,12).
Specificity protein 1 (Sp1) is the founding member
of a family of zinc finger transcription factors, which play
important physiological roles such as cell cycle regulation, cell
proliferation and cell apoptosis (13–15).
As compared with normal tissues or cells, Sp1 levels are high in
many types of cancer cells, such as breast carcinomas, thyroid
cancer, hepatocellular carcinomas, pancreatic cancer, colorectal
cancer, gastric cancer and lung cancer (13). For these reasons, downregulation of
Sp1 is regarded as a potential strategy for treating MPM.
Manumycin A (Manu A) is a natural product from
Streptomyces parvulus and acts as a potent and selective Ras
farnesyltransferase inhibitor (16,17).
Farnesyl protein transferase is important in activating a variety
of signaling proteins including Ras (17,18).
Recently, the anti-neoplastic activity of Manu A has been
demonstrated in various experimental systems (18). It exerts antitumor activity against
a variety of cancer cells such as human pancreatic cancer cells
(19), anaplastic thyroid cancer
cells (20,21), human colon tumor cells (22) and human hepatocellular carcinoma
HepG2 cells (23), medulloblastoma
cells (24,25), leukemic cells (26), lymphoid tumor and myeloma cell lines
(27,28). However, little is known concerning
the anticancer mechanisms of Manu A by which it can induce
apoptosis in MPM cells.
Apoptosis is a physiological process that causes
selective cell loss, and is an essential regulatory event to
maintain the homeostasis of tissues (29–31).
It can be triggered by various extracellular and intracellular
stimuli via either an extrinsic or intrinsic pathway depending on
cell type (31). The extrinsic
pathway is initiated by cell surface receptors, while the intrinsic
pathway is activated by releasing cytochrome c into the
cytoplasm by loss of mitochondial membrane potential and activating
cascade (30,31).
The aims of the present study were to investigate
the death mode and the engagement of the mitochondrial-mediated
pathway when MPM cells are exposed to Manu A. Based on the reports
that Manu A has antitumor activity against many cancer cell lines,
we investigated whether Manu A might have anticancer activity in
MPM through downregulation of Sp1 and induction of mitochondrial
cell death pathways.
Materials and methods
Purification of manumycin from
Streptomyces sp
All the solvents used in the experiments were of
extra-pure grade. Hexane, ethyl acetate and acetonitrile were
purchased from J.T. Baker (Phillipsburg, NJ, USA). Silica gel for
thin layer chromatography and precoated silica gel plate (Kieselgel
60F254; Merck, NJ, USA) was used. Silica gel for silica gel column,
Kieselgel 60 (70–230 mesh; Merck), was used to purify manumycin.
CS392 was grown on a rotary shaker at 180 rpm in Emerson media for
2–3 days at 28°C. Culture broth (3L) was centrifuged at 6,000 rpm
for 20 min. The supernatant was extracted two times with ethyl
acetate (1:1, v/v). The extracted ethyl acetate fraction was
evaporated and dried using a rotary evaporator at 50°C under
reduced pressure. Purification of the antibiotic was carried out by
silica gel column chromatography (0.8×15 cm). After washing the
column with hexane, active material was eluted from the column with
hexane-ethyl acetate (4:1). Active fractions were collected and
rechromatographed, using a reverse phase-C18 silica gel column
(1.0×15 cm) with 0.01% formic acid-acetonitrile (4:6) to isolate
manumycin.
Cell culture
The human MPM MSTO-211H and H28 cells were purchased
from the American Tissue Culture Collection (ATCC; Manassas, VA,
USA). The MSTO-211H and H28 cells were grown in RPMI-1640 medium,
supplemented with 10% fetal bovine serum, 2 mM L-glutamine and 100
U/ml each of penicillin and streptomycin (Thermo Scientific, Logan,
UT, USA) at 37°C in a humidified atmosphere of 5% CO2
and 95% air.
MTS assay
Cell viability was determined using the
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulphophenyl)-2H-tetrazolium
(MTS) dye-reduction assay. MSTO-211H (3×103) and H28
(2×103) cells were seeded into 96-well plates for 24 and
48 h treated with various concentrations (2.5, 5 and 10 µM)
of Manu A. The cells were incubated with MTS solution for 2 h.
Absorbance was determined using an EnSpire Multimode plate reader
(Perkin-Elmer, Waltham, MA, USA) at 490 nm. Experiments were
carried out in triplicates on different days. The percentage of
viability was calculated as: Viable cells (%) = (Manu A-treated
cell to measure the absorbance - Manu A-treated blank wells (no
cells) to measure the background absorbance)/absorbance of the
untreated sample × 100.
4′,6-diamidino-2-phenylindole (DAPI)
staining
MSTO-211H and H28 cells treated with Manu A were
harvested by trypsin and fixed in 100% methanol at room temperature
(RT) for 2 h. The cells were spread on slides, stained with DAPI
solution (2 µg/ml) and analyzed under a FluoView confocal
laser microscope (Fluoview FV10i; Olympus Corporation, Tokyo,
Japan).
Annexin V staining
The cells were seeded on 6-well plates for
MSTO-211H, H28 and treated with various concentrations of Manu A
for 48 h (2.5, 5 and 10 µM). The cells were harvested by
trypsinization and were incubated with Annexin V/7-aminoactinomycin
(7-AAD) for 20 min at RT in the dark for detection of apoptosis.
Apoptotic and necrotic cells were analyzed by Muse Cell analyzer
(Merck Millipore, Billerica, MA, USA) using the Muse Annexin V
& Dead Cell kit (MCH100105; Merck Millipore). The experiment
was performed in triplicate independently.
RT-PCR
Total RNA was extracted from the cells using
TRIzol® reagent (Life Technologies, Carlsbad, CA, USA),
and 2.5 µg of RNA was used to synthesize cDNA using the
HelixCript™ first Strand cDNA Synthesis kit (NanoHelix, Korea).
Amplimers were obtained by PCR using β-actin-specific and
Sp1-specific primers as described below under following PCR
conditions (25 cycles: 1 min at 95°C, 1 min at 56°C, and 1 min at
72°C). The β-actin primers used were; forward, 5′-GTG GGG CGC CCC
AGG CAC CA-3′ and reverse, 5′-CTC CTT AAT GTC ACG CAC GAT TTC-3′;
and the Sp1 primers were; forward, 5′-ATG CCT AAT ATT CAG TAT CAA
GTA-3′ and reverse, 5′-CCC TGA GGT GAC AGG CTG TGA-3′. PCR products
were analyzed by 2% agarose gel electrophoresis.
Western blotting
Western blot analyses were performed using cell
lysates. Lysates of the treated cells were prepared using PRO-PREP™
protein extraction solution (iNtRON Biotechnology, Korea), followed
by centrifugation and supernatant collection. Protein samples were
separated by 10 or 15% SDS-polyacrylamide gel electrophoresis and
transferred to polyvinylidene difluoride membranes (Millipore,
Darmstadt, Germany) using standard procedures. The primary
antibodies used were: Sp1, actin, poly (ADP-ribose) polymerase
(PARP), cyclin D1, survivin, BID, Bcl-xL, Bax, CHOP, DR4 and DR5
(Santa Cruz Biotechonology, Santa Cruz, CA, USA) antibodies. The
protein bands were detected using ECL Plus Western Blotting
detection system (Santa Cruz Biotechnology).
Mitochondrial membrane potential (MMP)
assay
To investigate the mitochondrial membrane
permeability, the control and Manu A-treated (2.5, 5 and 10
µM) cells were harvested. After washing with
phosphate-buffered saline (PBS), the cells were dissociated with
trypsin. For detection of the depolarized mitochondria of cells
undergoing apoptosis, MitoPotential working solution was added to
the MPM cell culture and then reaction was carried out for 20 min
in the dark. Muse 7-AAD was added to each well and samples were
incubated in the dark at RT for 5 min. The experiment was analyzed
by Muse cell analyzer.
Multi-caspase activity
The process was carried out as instructed in the
Muse Multi-Caspase kit (Merck Millipore). Each group, including
negative and positive controls was harvested to quantitatively
measure caspase activation and cell permeability. Cell samples in
1X caspase buffer with Muse Multi-Caspase reagent working solution
were incubated at 37°C for 30 min. Then, 7-AAD working solution was
added to each triplicate sample and samples were analyzed by Muse
Cell analyzer.
Statistical analysis
Data are presented as mean ± SD of at least three
independent experiments performed in triplicate. Data were analyzed
for statistical significance using a one-way analysis of variance.
A value of p<0.05 was considered significant.
Results
The growth inhibitory effect of Manu A on
malignant mesothelioma cells
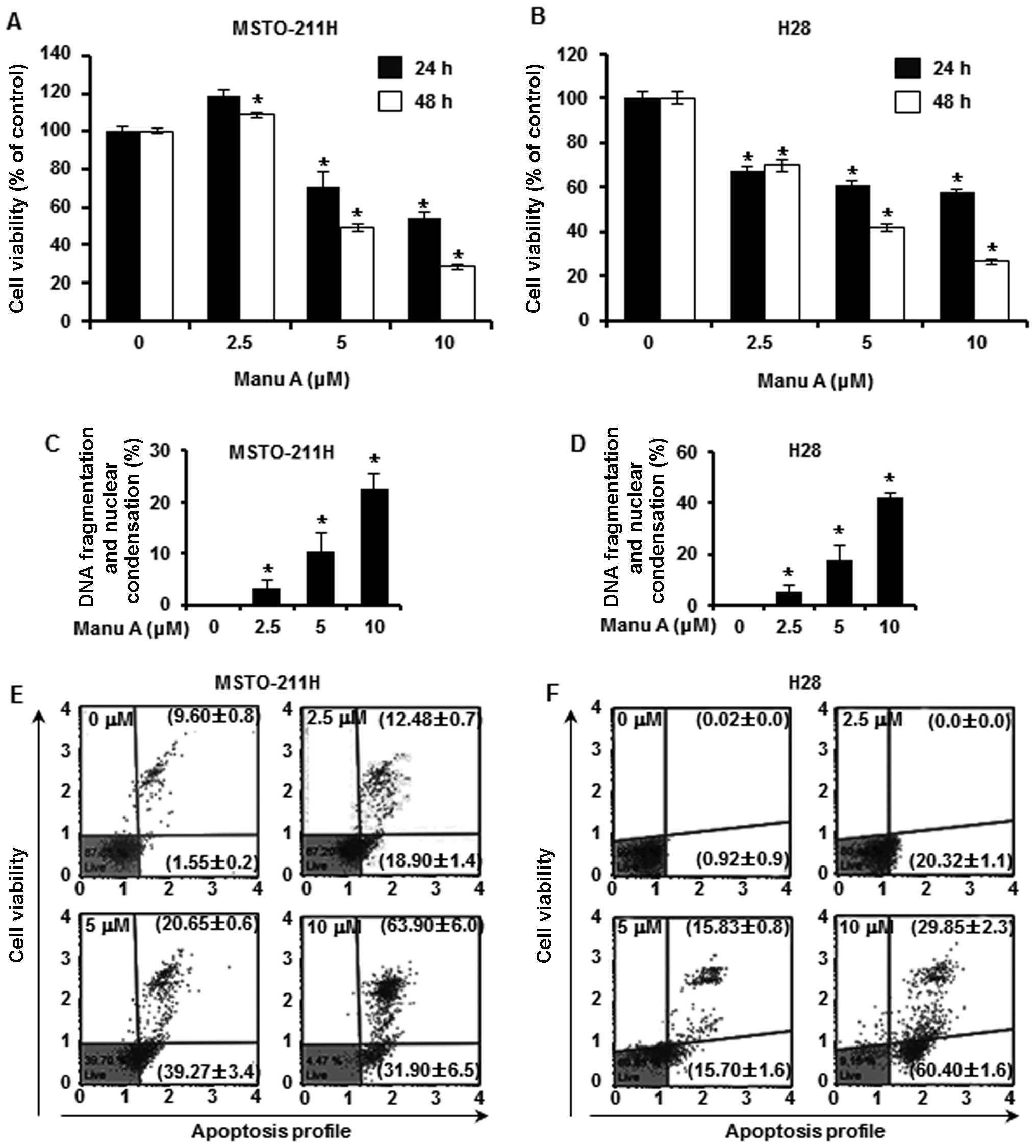
To examine the cytotoxic effect of Manu A on the
MSTO-211H (Fig. 1A) and H28
(Fig. 1B) MPM cell lines, MTS assay
was carried out after treatment with Manu A at various
concentrations (2.5, 5 and 10 µM) for different times (24
and 48 h). The IC50 of Manu A for cytotoxicity in the
MSTO-211H and H28 cells was 8.3 and 4.3 µM, respectively.
Furthermore, to confirm the inhibitory effects of Manu A on cancer
cell proliferation through apoptosis, we investigated morphological
nuclear changes through DAPI staining. Manu A treatment effectively
induced nuclear changes in the MPM cells in a dose-dependent
manner. To quantify the cell death induced by Manu A, MPM cells
were treated with Manu A at various concentrations (2.5, 5 and 10
µM), and then we measured the number of apoptotic and
necrotic cells by flow cytometry after staining with Annexin V and
7-AAD. The numbers of live cell were significantly reduced while
cells undergoing apoptosis were increased in a
concentration-dependent manner. In the MSTO-211H (Fig. 1E) and H28 cells (Fig. 1F), the total apoptotic cell
population was increased from 11.15±0.9 to 95.80±0.6% (MSTO-211H)
and from 0.93±0.9 to 90.25±1.2% (H28), respectively.
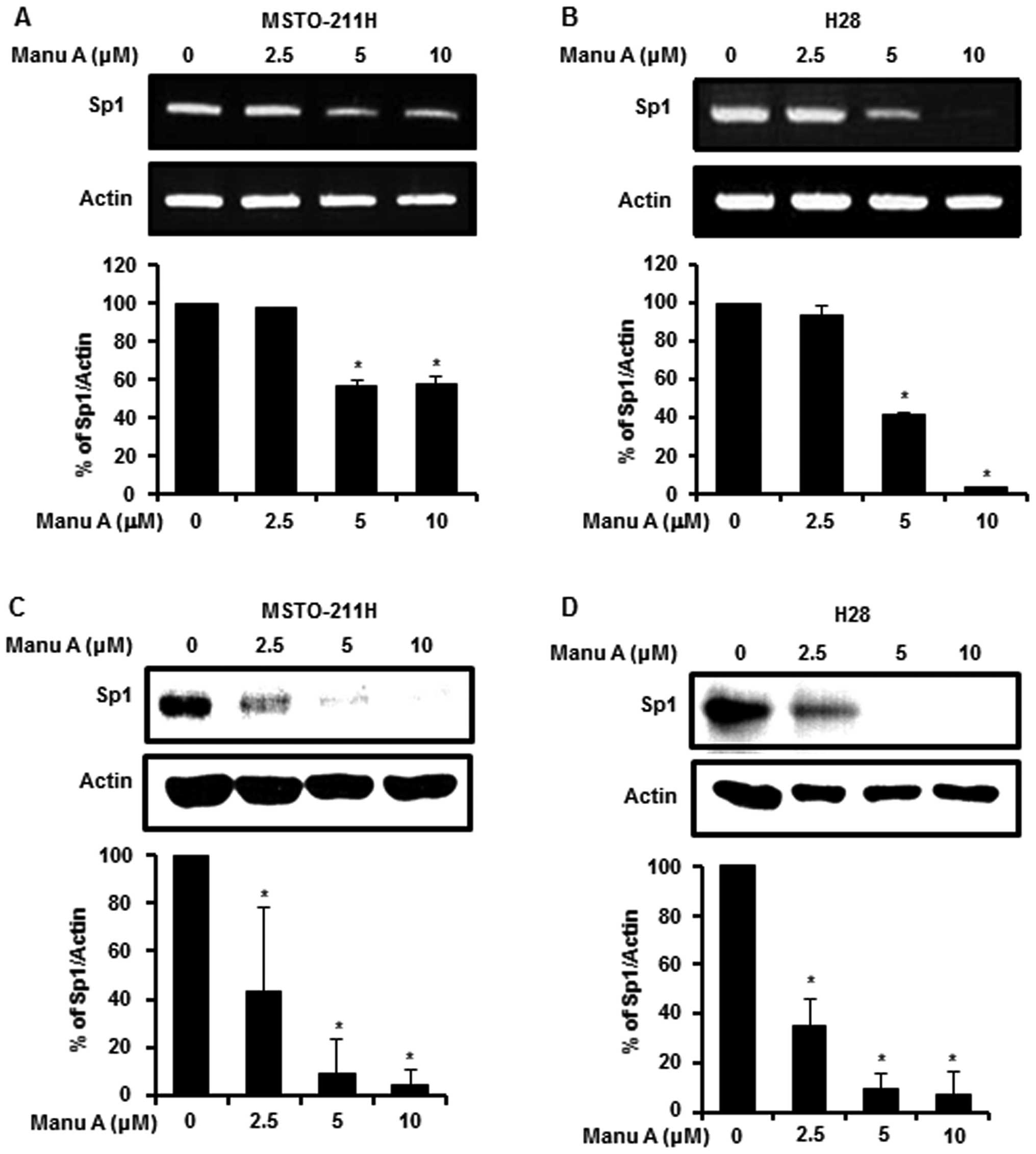
Manu A modulates Sp1 and Sp1-regulated
proteins in the malignant mesothelioma cells
Sp1 plays an important role in oncogenesis,
regulation of cell survival and death (13). To demonstrate the link between Sp1
and apoptosis, we investigated the expression level of Sp1 by
RT-PCR and western blotting after treatment of the MPM cells with
Manu A (2.5, 5 and 10 µM) for 48 h. Sp1 mRNA expression
levels in the MSTO-211H (Fig. 2A)
and H28 (Fig. 2B) cells were
reduced by Manu A in a dose-dependent manner. Consistent with mRNA
levels, the Sp1 protein levels in the MSTO-211H and H28 cells were
decreased in a dose-dependent manner (Fig. 2C and D). Sp1 is a transcription
factor that modulates cell cycle regulation, anti-proliferative,
and apoptosis cell death by regulating the promoter of target genes
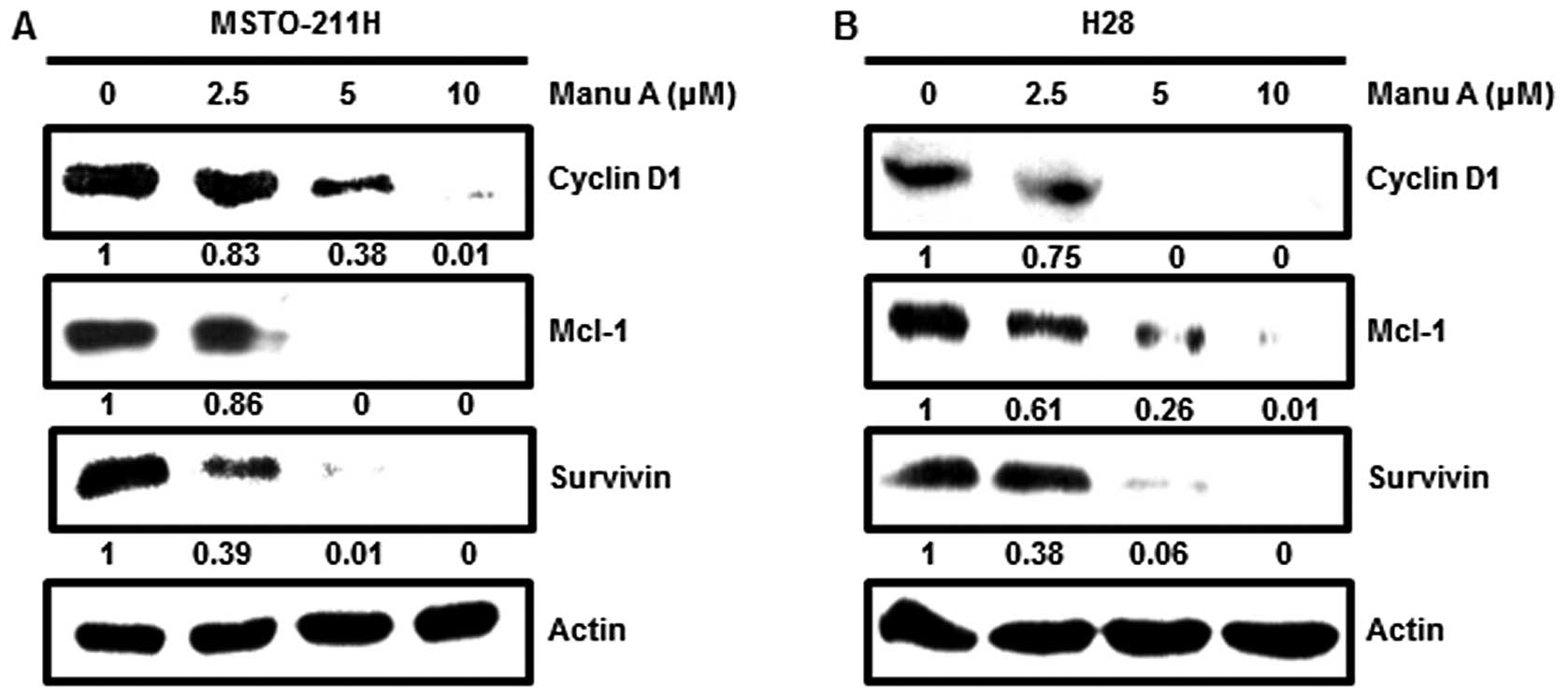
(14,32). Moreover, Sp1 regulates expression of
many proteins such as p21, p27, cyclin D1, Mcl-1 and survivin
(33). Among a variety of genes
involved, in this experiment, cyclin D1, Mcl-1 and survivin were
investigated. After treatment of the cells with Manu A for 48 h,
Sp1 target proteins in the MSTO-211H (Fig. 3A) and H28 cells (Fig. 3B) were downregulated.
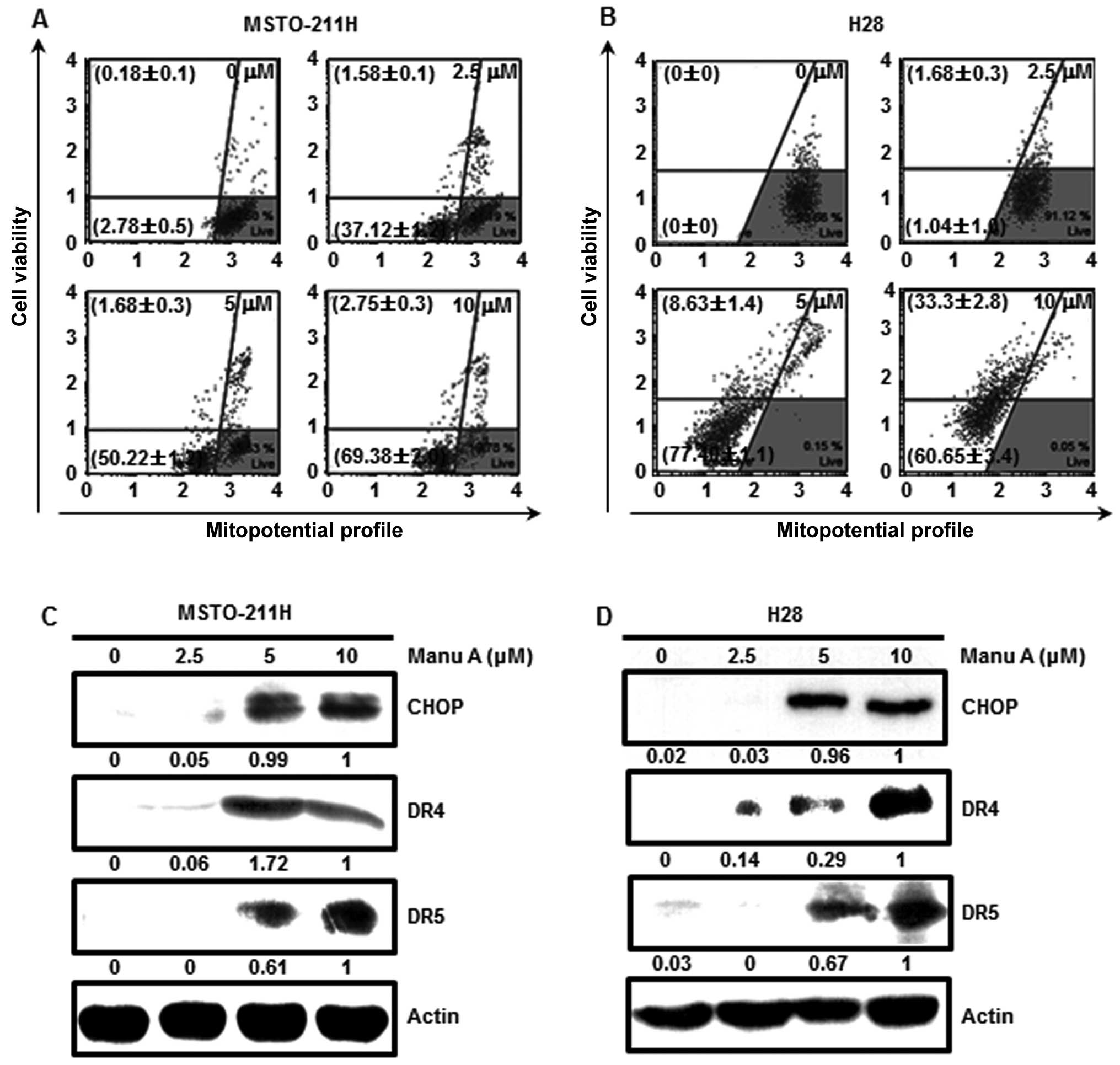
Manu A has an effect on mitochondrial
membrane permeability
There are two pathways which execute cell apoptosis;
that is, the extrinsic pathway through activation of cell death
receptors and the intrinsic pathway through mitochondrial damage
(34,35). To confirm whether the mitochondrial
pathway is activated by Manu A, we measured the degree of
mitochondrial membrane potential. This was measured by staining
with
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine
iodide reagent in the MPM cells. As a result, MMP was significantly
decreased in a concentration-dependent manner. Total depolarized
MSTO-211H cell populations were 38.7±1.3, 51.9±1.2 and 72.1±1.7%
and those of H28 (Fig. 4B) were
2.73±1.2, 86.0±0.5 and 93.9±0.9% at increasing doses, respectively.
To confirm whether Manu A could kill cells by inducing
mitochondrial damage, we treated MPM cells with Manu A (2.5, 5 and
10 µM) for 48 h and then carried out western blot analysis.
CHOP is increased by ER stress and the cell death receptors such as
DRs (DR4 and DR5) are upregulated by cell stress (36,37).
Based on our results, CHOP, DR4 and DR5 were increased in a
concentration-dependent manner (Fig. 4C
and D).
Manu A regulates the expression of
apoptosis-related proteins in the MSTO-211H and H28 cells
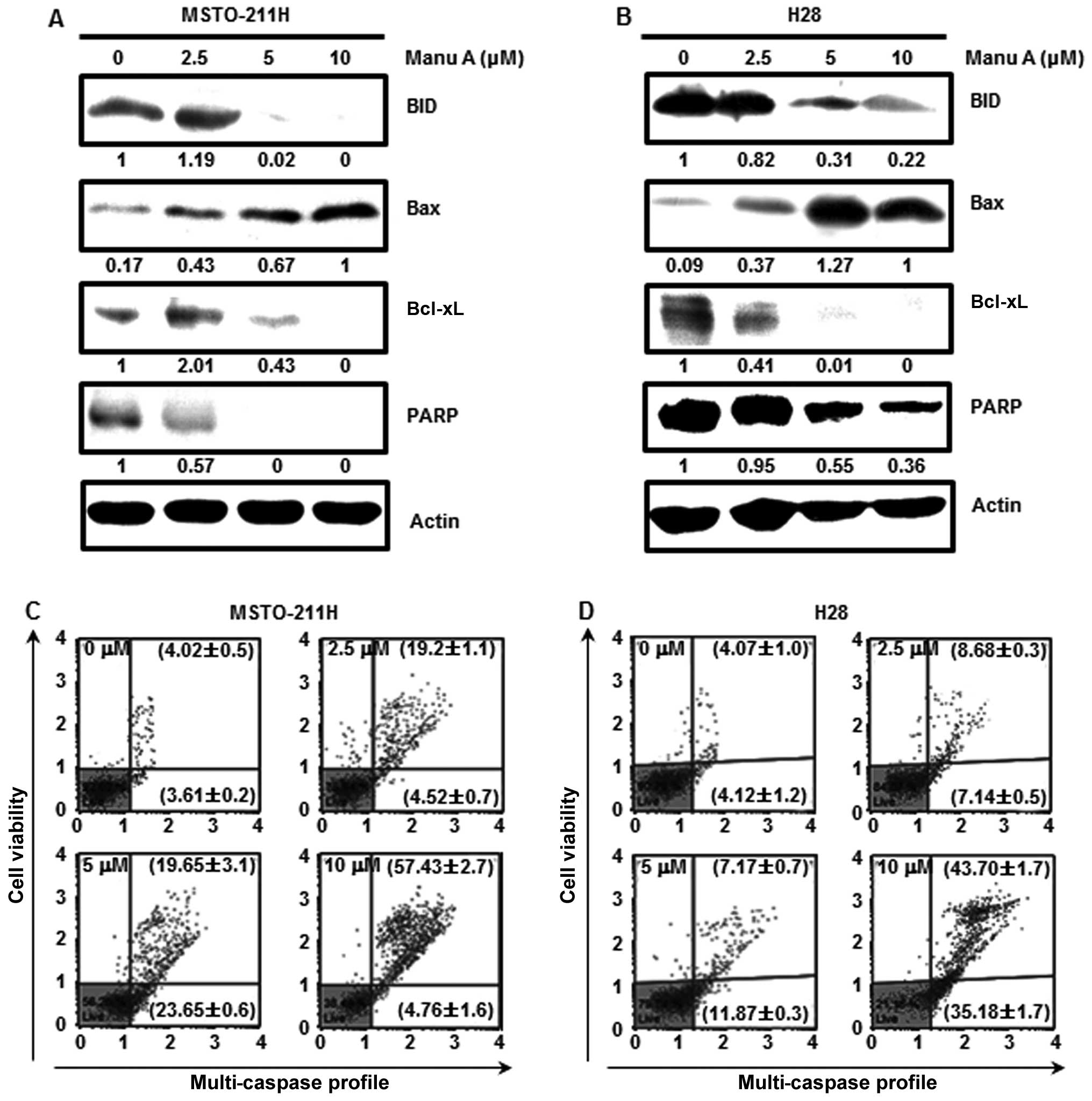
Treatment of the MSTO-211H and H28 cells with Manu A
regulated the expression levels of apoptosis-related proteins. To
clarify the association between Manu A and Sp1-mediated apoptosis,
we performed western blot analysis. When MPM cells were treated
with Manu A, Bax expression was increased in a
concentration-dependent manner, while the expression of Bcl-xL, an
apoptosis inhibitory protein, was reduced in a
concentration-dependent manner (Fig. 5A
and B). PARP that interferes with the apoptosis of cancer cells
is decreased (38). In this
experiment, PARP was decreased in both the MPM cell lines following
treatment with Manu A (Fig. 5A and
B). When cells are exposed to internal and external stimuli,
apoptotic signals are transmitted to induce activation of caspase-8
and -9. Then, procaspase-3 is cleaved into caspase-3, and
inactivates PARP finally inducing apoptosis (35). To examine whether Manu A-mediated
apoptosis could be associated with caspases, the caspase activity
was measured using a multi-caspase kit. Total multi-caspase
activities in the MSTO-211H cells (Fig.
5C) were 23.7±1.3, 43.3±3.4 and 62.2±1.1%, and those in the H28
cells (Fig. 5D) were 15.8±0.7,
19.0±0.5 and 78.9±0.5% at increasing doses. From the above results,
we confirmed that Manu A regulated the expression of
apoptosis-related proteins in the MPM cells.
Discussion
Due to environmental pollution and unhealthy dietary
habits, the incidence of cancer is increasing. For the treatment of
these diseases, various therapeutic anticancer agents have been
developed (39). Among various
cancers, MPM is a rare, but aggressive form of cancer with poor
prognosis (2,7). It is closely associated with exposure
to asbestos, radiation or simian virus 40 (1,2).
Targeting the apoptotic pathway of MPM to effectively halt tumor
progression would be ideal to develop effective treatments for MPM.
Our results showed that Manu A is a potential chemotherapeutic
agent for MPM through the mitochondrial pathway and that Sp1 is a
potential therapeutic target of Manu A.
It has been reported that Sp1 is associated with
tumor growth and is overexpressed in many types of human tumors
(15). A number of studies have
reported that Sp1 is highly expressed in a variety of human tumors
and that the use of natural compounds may be used to inhibit Sp1
expression in cancer (33). The
effectiveness of anticancer agents is closely dependent on
apoptosis induction. Therefore, biochemical mechanisms of apoptosis
for cancer treatment should be well understood (40). The precise apoptotic mechanisms of
Manu A in MPM cells remain undetermined, thus we investigated the
apoptotic effects and pathway in the inhibition of Sp1 protein
expression by Manu A in the MSTO-211H and H28 cells.
First, as shown in the MTS assay, the survival rate
of the MPM cell lines was significantly reduced in a
concentration-dependent manner by Manu A. In order to investigate
whether the cell death would be associated with Manu A, DAPI and
Annexin V/7-AAD staining were performed. Data showed that the
numbers of MPM cells were significantly reduced in a dose-dependent
manner while the number of apoptotic cells increased.
Our results showed that Manu A inhibited Sp1
expression at both the protein and mRNA levels. Since Manu A
modulates the expression of the Sp1 protein, it was also important
to determine the response of key candidates related to apoptosis in
its downstream signaling pathway. Manu A suppressed Sp1 downstream
target genes, including cyclin D1, Mcl-1 and survivin in the
MSTO-211H and H28 cells as detected by western blot analyses.
In the process of cell death induction in cancer
cells, both apoptosis-promoting protein and apoptosis inhibitory
proteins play important roles (31). They are located inside and outside
of the mitochondria and are increased or decreased by cell death
factors activated by apoptosis signaling factors (41,42).
We investigated the mitochondrial membrane permeability during
apoptosis in the MPM cells. Based on the results, Manu A modulated
cell stress and mitochondrial integrity of the MPM cells. Moreover,
we determined the response on core elements related to apoptosis in
the mitochondrial signaling pathway. Therefore, the effect of Manu
A on CHOP, DR4 and DR5 in both cell lines was examined.
To confirm whether Manu A could modulate
apoptosis-related proteins in the MPM cells, western blot analyses
were carried out. As a result, it was confirmed that BID, Bcl-xL
and PARP were reduced. In addition, expression of Bax can be
considered to induce the release of cytochrome c by
abolishing MMP (35,40). A downstream protein of apoptosis
active caspase-3 was increased in a concentration-dependent manner
and PARP protein was decreased in a dose-dependent manner. These
in vitro results suggest that Manu A modulates
apoptosis-related proteins in MPM cells.
Based on the results above, Manu A has therapeutic
and chemopreventive benefits and Sp1 is a therapeutic target in MPM
cells. We conclude that Manu A downregulated Sp1 protein levels,
which in turn induced cell apoptosis of MPM cells through both the
intrinsic and the extrinsic pathways. Therefore, Manu A is
promising as an anticancer agent.
Acknowledgments
This research was supported by the Basic Science
Research Program through the National Research Foundation Korea
(NRF) Funded by the Ministry of Education, Science and Technology
(2014R1A1A2053500) and the Next-Generation BioGreen 21 Program
(PJ01116401) from Rural Development Administration, Republic of
Korea.
Abbreviations:
|
MPM
|
malignant pleural mesothelioma
|
|
Manu A
|
Manumycin A
|
|
Sp1
|
specificity protein 1
|
|
RPMI-1640
|
Roswell Park Memorial Institute-1640
medium
|
|
Mcl-1
|
myeloid cell leukemia-1, PARP,
poly(ADP-ribose) polymerase
|
|
MTS
|
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
|
|
DAPI
|
4′-6-diamidino-2-phenylindole
|
|
RT
|
room temperature
|
|
7-AAD
|
7-aminoactinomycin D
|
|
MMP
|
mitochondrial membrane potential
|
References
|
1
|
Carbone M, Ly BH, Dodson RF, Pagano I,
Morris PT, Dogan UA, Gazdar AF, Pass HI and Yang H: Malignant
mesothelioma: Facts, myths, and hypotheses. J Cell Physiol.
227:44–58. 2012. View Article : Google Scholar
|
|
2
|
Pass HI, Vogelzang N, Hahn S and Carbone
M: Malignant pleural mesothelioma. Curr Probl Cancer. 28:93–174.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Attanoos RL and Gibbs AR: Pathology of
malignant mesothelioma. Histopathology. 30:403–418. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Linton A, Cheng YY, Griggs K, Kirschner
MB, Gattani S, Srikaran S, Chuan-Hao Kao S, McCaughan BC, Klebe S,
van Zandwijk N, et al: An RNAi-based screen reveals PLK1, CDK1 and
NDC80 as potential therapeutic targets in malignant pleural
mesothelioma. Br J Cancer. 110:510–519. 2014. View Article : Google Scholar :
|
|
5
|
Spugnini EP, Bosari S, Citro G, Lorenzon
I, Cognetti F and Baldi A: Human malignant mesothelioma: Molecular
mechanisms of pathogenesis and progression. Int J Biochem Cell
Biol. 38:2000–2004. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tatsuta T, Hosono M, Takahashi K, Omoto T,
Kariya Y, Sugawara S, Hakomori S and Nitta K: Sialic acid-binding
lectin (leczyme) induces apoptosis to malignant mesothelioma and
exerts synergistic antitumor effects with TRAIL. Int J Oncol.
44:377–384. 2014.
|
|
7
|
Sahin AA, Cöplü L, Selçuk ZT, Eryilmaz M,
Emri S, Akhan O and Bariş YI: Malignant pleural mesothelioma caused
by environmental exposure to asbestos or erionite in rural Turkey:
CT findings in 84 patients. AJR Am J Roentgenol. 161:533–537. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Carbone M, Kratzke RA and Testa JR: The
pathogenesis of mesothelioma. Semin Oncol. 29:2–17. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zucali PA, Ceresoli GL, De Vincenzo F,
Simonelli M, Lorenzi E, Gianoncelli L and Santoro A: Advances in
the biology of malignant pleural mesothelioma. Cancer Treat Rev.
37:543–558. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cedrés S, Montero MA, Zamora E, Martínez
A, Martínez P, Fariñas L, Navarro A, Torrejon D, Gabaldon A, Ramon
Y, Cajal S, et al: Expression of Wilms' tumor gene (WT1) is
associated with survival in malignant pleural mesothelioma. Clin
Transl Oncol. 16:776–782. 2014. View Article : Google Scholar
|
|
11
|
Martinis UJ and Radovic VR: Pleural
mesothelioma in patient with pulmonary tuberculosis: Report of a
case. Dis Chest. 47:568–570. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tanaka I, Osada H, Fujii M, Fukatsu A,
Hida T, Horio Y, Kondo Y, Sato A, Hasegawa Y, Tsujimura T, et al:
LIM-domain protein AJUBA suppresses malignant mesothelioma cell
proliferation via Hippo signaling cascade. Oncogene. 34:73–83.
2015. View Article : Google Scholar
|
|
13
|
Safe S and Abdelrahim M: Sp transcription
factor family and its role in cancer. Eur J Cancer. 41:2438–2448.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aslam F, Palumbo L, Augenlicht LH and
Velcich A: The Sp family of transcription factors in the regulation
of the human and mouse MUC2 gene promoters. Cancer Res. 61:570–576.
2001.PubMed/NCBI
|
|
15
|
Black AR, Black JD and Azizkhan-Clifford
J: Sp1 and krüppel-like factor family of transcription factors in
cell growth regulation and cancer. J Cell Physiol. 188:143–160.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tsuda M, Okamoto K, Muguruma N, Sannomiya
K, Nakagawa T, Miyamoto H, Kitamura S, Goji T, Kimura T, Okahisa T,
et al: Suppressive effect of RAS inhibitor manumycin A on aberrant
crypt foci formation in the azoxymethane-induced rat colorectal
carcinogenesis model. J Gastroenterol Hepatol. 28:1616–1623.
2013.PubMed/NCBI
|
|
17
|
Hara M, Akasaka K, Akinaga S, Okabe M,
Nakano H, Gomez R, Wood D, Uh M and Tamanoi F: Identification of
Ras farnesyltransferase inhibitors by microbial screening. Proc
Natl Acad Sci USA. 90:2281–2285. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Singha PK, Pandeswara S, Venkatachalam MA
and Saikumar P: Manumycin A inhibits triple-negative breast cancer
growth through LC3-mediated cytoplasmic vacuolation death. Cell
Death Dis. 4:e4572013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kainuma O, Asano T, Hasegawa M, Kenmochi
T, Nakagohri T, Tokoro Y and Isono K: Inhibition of growth and
invasive activity of human pancreatic cancer cells by a
farnesyltransferase inhibitor, manumycin. Pancreas. 15:379–383.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yeung SC, Xu G, Pan J, Christgen M and
Bamiagis A: Manumycin enhances the cytotoxic effect of paclitaxel
on anaplastic thyroid carcinoma cells. Cancer Res. 60:650–656.
2000.PubMed/NCBI
|
|
21
|
Pan J, Xu G and Yeung SC: Cytochrome c
release is upstream to activation of caspase-9, caspase-8, and
caspase-3 in the enhanced apoptosis of anaplastic thyroid cancer
cells induced by manumycin and paclitaxel. J Clin Endocrinol Metab.
86:4731–4740. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Di Paolo A, Danesi R, Nardini D, Bocci G,
Innocenti F, Fogli S, Barachini S, Marchetti A, Bevilacqua G and
Del Tacca M: Manumycin inhibits ras signal transduction pathway and
induces apoptosis in COLO320-DM human colon tumour cells. Br J
Cancer. 82:905–912. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou JM, Zhu XF, Pan QC, Liao DF, Li ZM
and Liu ZC: Manumycin induces apoptosis in human hepatocellular
carcinoma HepG2 cells. Int J Mol Med. 12:955–959. 2003.PubMed/NCBI
|
|
24
|
Wang W and Macaulay RJ: Apoptosis of
medulloblastoma cells in vitro follows inhibition of farnesylation
using manumycin A. Int J Cancer. 82:430–434. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Selleri C, Maciejewski JP, Montuori N,
Ricci P, Visconte V, Serio B, Luciano L and Rotoli B: Involvement
of nitric oxide in farnesyltransferase inhibitor-mediated apoptosis
in chronic myeloid leukemia cells. Blood. 102:1490–1498. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
She M, Pan I, Sun L and Yeung SC:
Enhancement of manumycin A-induced apoptosis by methoxyamine in
myeloid leukemia cells. Leukemia. 19:595–602. 2005.PubMed/NCBI
|
|
27
|
Frassanito MA, Cusmai A, Piccoli C and
Dammacco F: Manumycin inhibits farnesyltransferase and induces
apoptosis of drug-resistant interleukin 6-producing myeloma cells.
Br J Haematol. 118:157–165. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sears KT, Daino H and Carey GB: Reactive
oxygen species-dependent destruction of MEK and Akt in Manumycin
stimulated death of lymphoid tumor and myeloma cell lines. Int J
Cancer. 122:1496–1505. 2008. View Article : Google Scholar
|
|
29
|
Pathak S, Sharma C, Jayaram HN and Singh
N: Apoptotic signaling induced by benzamide riboside: An in vitro
study. Mol Cell Biochem. 328:67–73. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Reed JC: Apoptosis mechanisms:
Implications for cancer drug discovery. Oncology (Williston Park).
18(Suppl 10): 11–20. 2004.
|
|
31
|
Lowe SW and Lin AW: Apoptosis in cancer.
Carcinogenesis. 21:485–495. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Courey AJ and Tjian R: Analysis of Sp1 in
vivo reveals multiple transcriptional domains, including a novel
glutamine-rich activation motif. Cell. 55:887–898. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cho JJ, Chae JI, Yoon G, Kim KH, Cho JH,
Cho SS, Cho YS and Shim JH: Licochalcone A, a natural chalconoid
isolated from Glycyrrhiza inflata root, induces apoptosis via Sp1
and Sp1 regulatory proteins in oral squamous cell carcinoma. Int J
Oncol. 45:667–674. 2014.PubMed/NCBI
|
|
34
|
Banjerdpongchai R, Wudtiwai B and Pompimon
W: Stigmalactam from Orophea enterocarpa induces human cancer cell
apoptosis via a mitochondrial pathway. Asian Pac J Cancer Prev.
15:10397–10400. 2014. View Article : Google Scholar
|
|
35
|
Lim SW, Loh HS, Ting KN, Bradshaw TD and
Zeenathul NA: Antiproliferation and induction of
caspase-8-dependent mitochondria-mediated apoptosis by
β-tocotrienol in human lung and brain cancer cell lines. Biomed
Pharmacother. 68:1105–1115. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang X, Du T, Wang X, Zhang Y, Hu W, Du X,
Miao L and Han C: IDH1, a CHOP and C/EBPβ-responsive gene under ER
stress, sensitizes human melanoma cells to hypoxia-induced
apoptosis. Cancer Lett. 365:201–210. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ghosh AP, Klocke BJ, Ballestas ME and Roth
KA: CHOP potentially co-operates with FOXO3a in neuronal cells to
regulate PUMA and BIM expression in response to ER stress. PLoS
One. 7:e395862012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kharbanda S, Pandey P, Schofield L,
Israels S, Roncinske R, Yoshida K, Bharti A, Yuan ZM, Saxena S,
Weichselbaum R, et al: Role for Bcl-xL as an inhibitor of cytosolic
cytochrome C accumulation in DNA damage-induced apoptosis. Proc
Natl Acad Sci USA. 94:6939–6942. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wong WW and Puthalakath H: Bcl-2 family
proteins: The sentinels of the mitochondrial apoptosis pathway.
IUBMB Life. 60:390–397. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
41
|
Scatena R: Mitochondria and cancer: A
growing role in apoptosis, cancer cell metabolism and
dedifferentiation. Adv Exp Med Biol. 942:287–308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Martinou JC and Youle RJ: Mitochondria in
apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev
Cell. 21:92–101. 2011. View Article : Google Scholar : PubMed/NCBI
|