Introduction
Small cell lung cancer (SCLC), which accounts for
~15% of all lung cancer cases, is the most aggressive metastatic
form of lung cancer and does not respond well to surgery or
radiotherapy (1). Chemotherapeutic
resistance is closely associated with multidrug resistance (MDR).
Although a relatively good response can be achieved in the initial
stages of lung cancer chemotherapy, chemotherapeutic resistance can
develop quickly after initial chemotherapy (2–4).
Hence, chemotherapeutic resistance, particularly MDR, is a major
obstacle for successful SCLC chemotherapy.
Tumor tissues are composed of tumor cells,
fibroblasts and immune cells, which secret pro-inflammatory
cytokines such as IL-2, IL-1 and IL-6, and thereby affect the
abilities of cell proliferation in the microenvironment (5,6).
Hence, hypoxia always exists in the process of tumor tissue
development. It was reported that hypoxia-induced acidification may
cause this resistance by decreasing cellular uptake along with a
lowered cytotoxicity due to pH-dependent topoisomerase type II
activity (7). Meanwhile, MDR is
also characterized by a reversal of the pH gradient across cell
membranes leading to an acidification of the outer milieu and an
alkalinization of the cytosol that is maintained by the proton pump
vacuolar-type ATPase (V-ATPase) (8,9).
ATP-binding cassette transporter proteins such as ATP-binding
cassette (ABC) transporters P-glycoprotein (P-gp; MDR1), multidrug
resistance-associated protein (MRP) and breast cancer resistance
protein (BCRP/ABCG2) (10,11), transporting a wide variety of
chemical compounds in an ATP-dependent manner, have been found to
contribute to MDR formation in a variety of tumors arising from
gastric, renal, endometrium, melanoma and soft tissue (12,13).
Our previous studies showed that the upregulation of ABCG2
facilitates MDR formation in lung cancer (14); however, the role of ABCG2 in
acidification associated MDR formation is still uncertain.
The PI3K/AKT/mTOR pathway is an intracellular
signaling pathway important in regulating the cell cycle.
Therefore, it is directly related to cellular quiescence,
proliferation, cancer and longevity (15). Apart from the key roles of Akt in
regulating co-stimulator molecule expression in dendritic cells
(16–18), the activation of Akt has been
documented to regulate V-ATPase expression and induce MDR in
different types of tumor (19-21).
Hence, the activation of Akt may be a key regulator in tumor MDR
formation. However, to date, the role of PI3K-Akt activation in
acidification-induced chemoresistance is still unclear.
In the present study, we modified the pH value of
the medium to mimic the tumor acidic microenvironment and
investigated the effects of acidification on cell viability, the
expression of ATP-binding cassette transporter proteins, and
activation of PI3K-Akt. We demonstrated that acidification
obviously increased the expression of ABCG2, myeloid cell
leukemia-1 (Mcl-1) via PI3K-Akt-mTOR-S6 pathway activation and
contributed to MDR. The inhibition of PI3K-Akt activity efficiently
abolished the effect of acidification on cell viability, indicating
that the PI3K-Akt pathway may include potential therapeutic target
molecules in acidized microenvironment-associated lung cancer
chemotherapeutic resistance.
Materials and methods
Reagents and antibodies
2-(N-morpholino)ethanesulfonic acid (MES
monohydrate) and primers were purchased from Sangon Biotech
(Shanghai, China). Cisplatin was purchased from Calbiochem (San
Diego, CA, USA). Antibody to ABCG2, antibody to Mcl-1, antibodies
to phospho and total kinases were acquired from Cell Signaling
Technology (Beverly, MA, USA). Annexin V/propidium iodide (PI)
apoptosis detection kit was obtained from KeyGen Biotech (Nanjing,
China). RPMI-1640 medium, Dulbecco's modified Eagle's medium (DMEM)
and fetal bovine serum (FBS) were acquired from HyClone (Logan, UT,
USA). SYBR Premix Ex Taq, TRIzol and PrimeScript reverse
transcriptase were obtained from Takara Biotechnology (Dalian,
China).
Cell lines
LTEP-a-2 and A549 cells were maintained in our
laboratory as previously described (14). Cells were synchronized by serum
starvation for at least 6 h before acidification treatment.
Flow cytometric measurements
Cell apoptosis was assayed as previously described
(14). Briefly, A549 and LTEP-a-2
cells were pretreated with pH 6.6 for 2 h. Then, the acidized
medium was replaced with normal medium for 48 h. After that, the
cells were cultured for 24 h in the presence of cisplatin (DDP) (4
µg/ml for A549 cells and 8 µg/ml for LTEP-a-2 cells).
The cells were stained with Annexin V-FITC and PI for 20 min at
room temperature. Flow cytometry was performed using a FACSCalibur
flow cytometer, and the data were analyzed using CellQuest software
(BD Biosciences, San Jose, CA, USA).
Quantitative PCR
The effects of acidification on MDR-related protein
expression were investigated via real-time PCR analyses, as
previously described (14).
Briefly, whole cellular RNA was extracted, and reverse
transcription was performed using PrimeScript reverse
transcriptase. To quantify gene amplification, real-time PCR
analysis was performed using an ABI 7500 Sequence Detection system
in the presence of SYBR-Green (Takara Biotechnology). The cycling
parameters were 95°C for 5 min, followed by 32 cycles of 95°C for 5
sec, 55°C for 30 sec and 72°C for 60 sec, with a final extension at
72°C for 10 min; a melting curve analysis was subsequently
conducted. The relative expression levels (defined as fold-changes)
of the target genes were normalized to the folds of the
corresponding control cells. The primer sequences outlined in
Table I were used in these
assays.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Genes | F/R | Sequence |
|---|
| β-actin | F |
5′-TCAAGATCATTGCTCCTCCTG-3′ |
| R |
5′-CTGCTTGCTGATCCACATCTG-3′ |
| ABCG2 | F |
5′-ACTGGCTTAGACTCAAGCACA-3′ |
| R |
5′-ATAGGCCTCACAGTGATAACCA-3′ |
| Mcl-1 | F |
5′-TGCAGGTGTTGCTGGAGTAG-3′ |
| R |
5′-CCTCTTGCCACTTGCTTTTC-3′ |
Western blot analysis
The cells were treated with pH 6.6 for 2 h and the
expression of related proteins was determined via western blot
analysis as previously described (16,17).
Briefly, proteins were obtained in lysis buffer and loaded onto
SDS-PAGE gels for electrophoresis and transferred onto
polyvinylidene fluoride (PVDF) membranes. After blocking in 5%
fat-free milk in Tris-buffered saline and Tween-20 (TBST) for 90
min, the membranes were incubated with primary antibodies at 4°C
overnight. Subsequently, the membranes were incubated with
corresponding horseradish peroxidase (HRP)-conjugated secondary
antibodies at room temperature for 90 min. After washing 4 times
with TBST (for 10 min each), the bound antibodies were visualized
using enhanced chemiluminescence (ECL). β-actin was used as a
loading control.
Statistical analysis
All experiments were repeated at least three times
to confirm the results. The data are presented as the mean ± SEM.
Student's t-test and one-way ANOVA with the Newman-Keuls post test
were applied. Differences were considered significant at
p<0.05.
Results
Treatment with acidification increases
the chemotherapeutic resistance in A549 and LTEP-a-2 lung cancer
cells
MDR formation is the important factor in lung cancer
therapeutic failure (22). The
tumor microenvironment, which consists of tumor cells,
extracellular matrix, immune cells and fibroblasts, is closely
involved in MDR formation (23). As
the high ability of cell proliferation to blood supply, there was
exactly acidized environment in tumor (9). To explore the effect of acidized
microenvironment on chemotherapeutic resistance, A549 and LTEP-a-2
cells were firstly treated with MES monohydrate and the value of pH
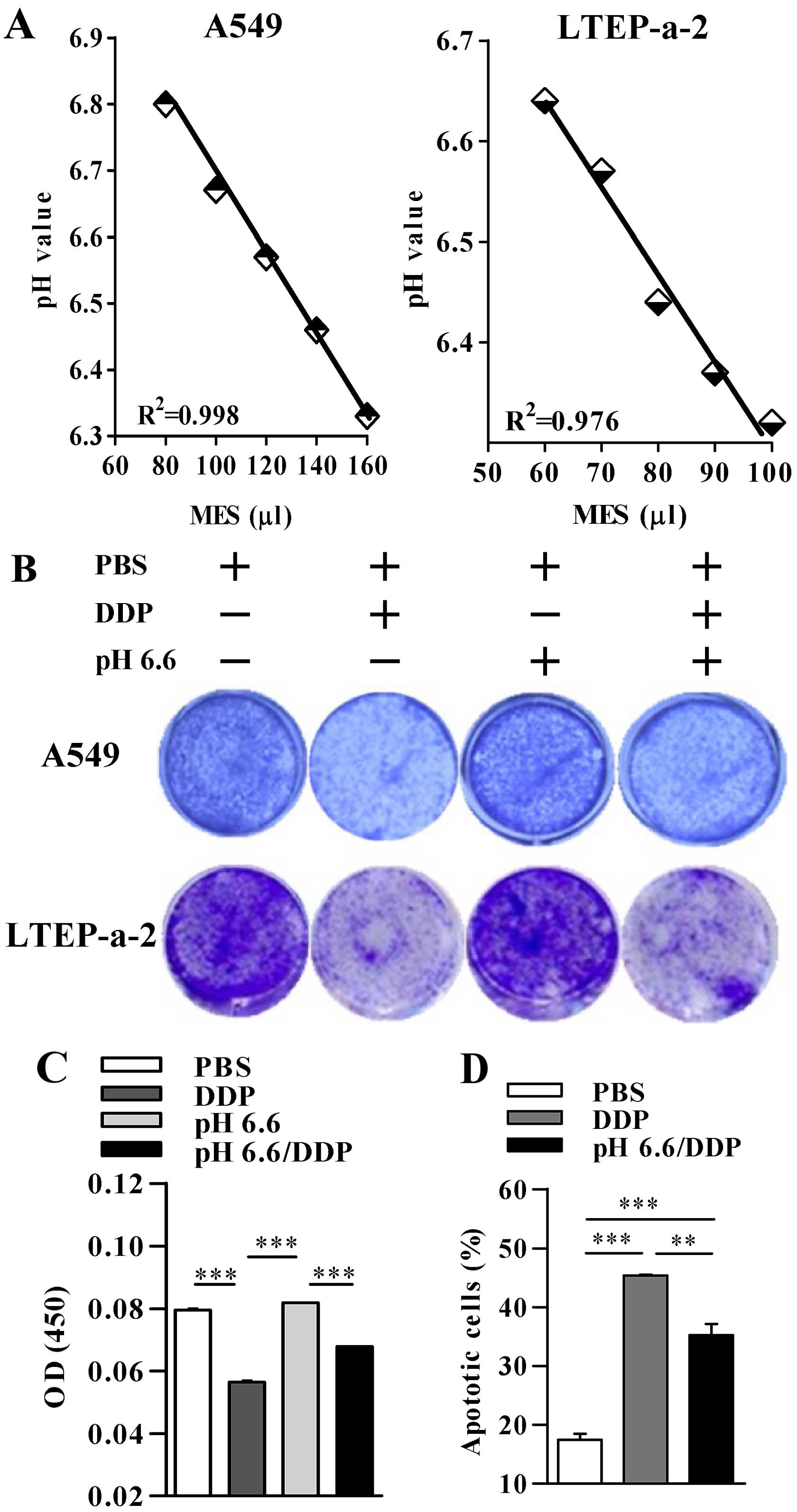
in the medium was determined. As shown in Fig. 1A, the value of pH in the medium of
the A549 and LTEP-a-2 cells was controlled by the addition of
different volumes of MES monohydrate. The co-relationship index of
the standard curve was 0.998 and 0.976 for A549 and LTEP-a-2 cells,
respectively, which indicated that the pH value in the medium of
cultured cells could be exactly modified. Cell viability
observations by crystal violet staining showed that the
modification of the pH value from normal to pH 6.6 obviously
increased the cell viability in the presence of cisplatin in both
the A549 and LTEP-a-2 cells (Fig.
1B). Analyses of the light absorption value also revealed that
a pH 6.6 microenvironment attenuated cisplatin-induced cell death
(Fig. 1C). The flow cytometric
analyses of cell apoptosis by Annexin V/PI staining demonstrated
that whereas the treatment with cisplatin induced ~45% apoptosis in
the A549 cells, pH 6.6 acidification of the medium efficiently
decreased the percentage of Annexin V/PI-positive cells, which
revealed an ~22.2% inhibitory rate (Fig. 1D). All of these results indicate
that an acidized microenvironment contributes to MDR formation in
lung cancer cells.
Acidification of the microenvironment
increases the expression of ABCG2 and Mcl-1 in the A549 and
LTEP-a-2 lung cancer cells
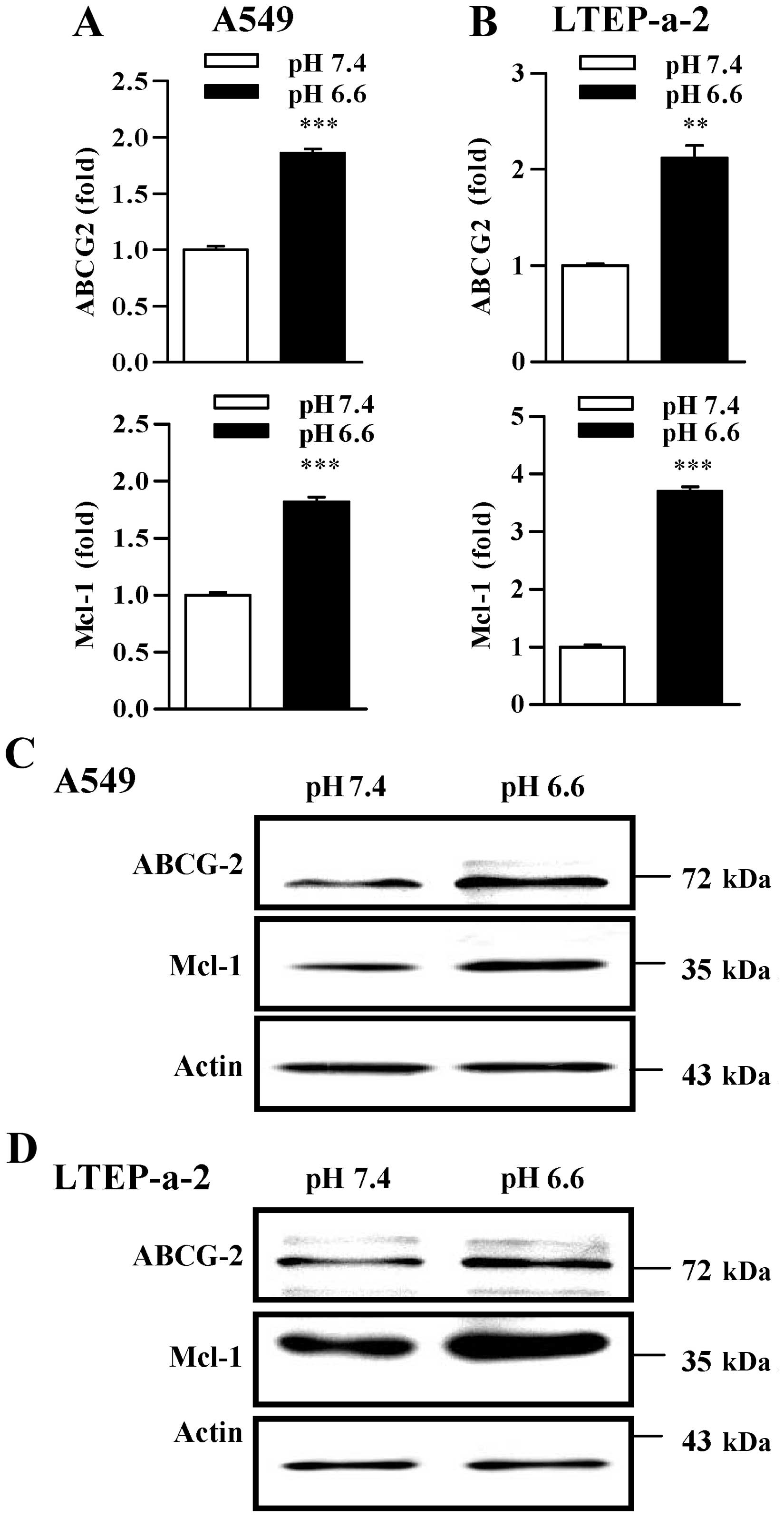
Our previous study showed that ABCG2 and
anti-apoptotic genes such as Mcl-1 and Bcl-2 could be upregulated
and contribute to cisplatin-induced MDR (14). As the treatment with acidic medium
increased lung cancer cell viability and attenuated
cisplatin-induced apoptosis (Fig.
1), we aimed to ascertain whether ABCG2 and anti-apoptotic
proteins are involved in acidification-promoted chemotherapeutic
resistance. Toward this end, we modified the pH value of the
cultured lung cancer cells and analyzed the effect of acidification
on the expression of ABCG2 and Mcl-1. Surprisingly, qPCR analyses
showed that the treatment with pH 6.6 acidification not only
increased ABCG2 expression, but also augmented Mcl-1 upregulation
at the transcription level in both the A549 and LTEP-a-2 lung
cancer cells (Fig. 2A and B).
Importantly, western blot analyses revealed that upregulation of
ABCG2 and Mcl-1 was obviously achieved by the treatment with pH 6.6
acidification (Fig. 2C and D). As
ABCG2 and Mcl-1 are the important proteins mediating MDR, the above
results indicate that the acidic microenvironment could contribute
to lung cancer chemotherapeutic resistance by upregulating the
expression of ABCG2 and Mcl-1.
Acidic microenvironment obviously
increases the phosphorylation of PI3K-Akt-mTOR-S6 in the A549 and
LTEP-a-2 lung cancer cells
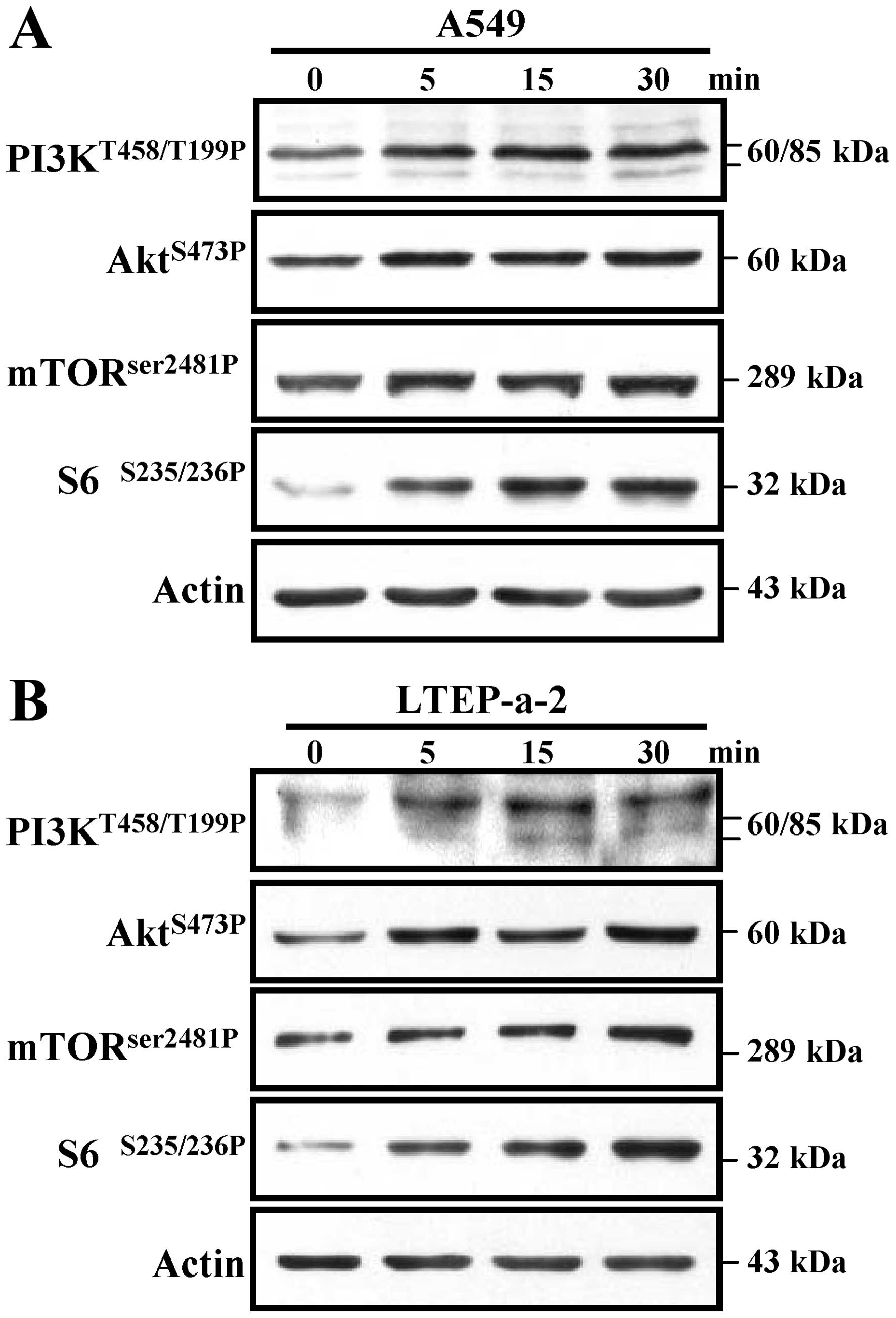
Previous studies showed that the phosphorylation of
the PI3K-Akt pathway is involved in MDR (20). To explore the effect of
acidification on PI3K-Akt kinase phosphorylation, A549 and LTEP-a-2
cells were treated with MES monohydrate to induce pH 6.6
acidification and the phosphorylation of PI3K-Akt was determined by
western blot analyses. The results showed that, not only the
phosphorylation at T458 or T199 of PI3K, but also the
phosphorylation at S473 of Akt could be achieved in both the A549
and LTEP-a-2 cells, which started at 5 min and continue to 30 min
after pH 6.6 acidification (Fig. 3A and
B). Notably, the activation of Akt downstream kinases mTOR and
S6 was also achieved by exposure to pH 6.6 acidification (Fig. 3A and B). All of these results
indicate that the PI3K-Akt-mTOR-S6 pathway could be efficiently
activated in the acidized microenvironment of lung cancer, and may
play a pivotal role in the formation of lung cancer
chemotherapeutic resistance.
Microenvironment of acidification
upregulates the expression of ABCG2 and Mcl-1 via the
PI3K-Akt-mTOR-S6 pathway in A549 and LTEP-a-2 lung cancer
cells
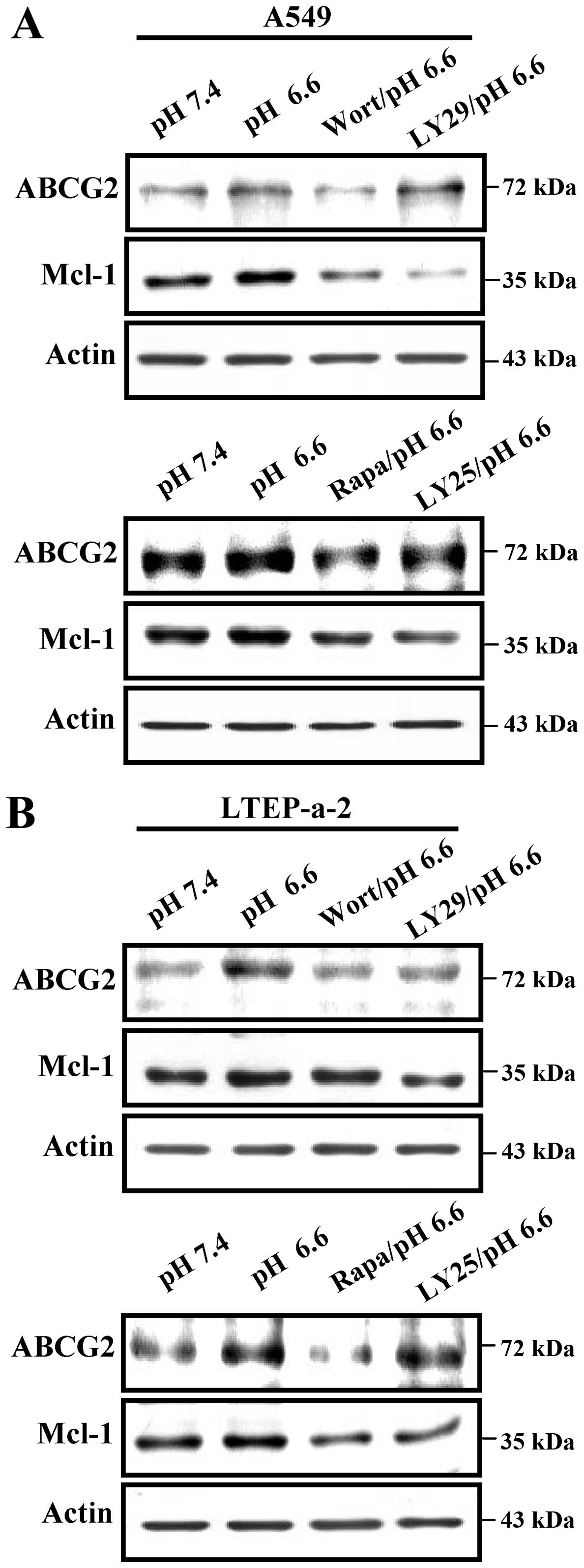
Previous studies have shown that activation of
PI3K-Akt induced by chemotherapeutic agents facilitates the
formation of MDR (20,21). Despite that pH 6.6 acidification
efficiently induces the phosphorylation of the PI3K-Akt-mTOR-S6
pathway, the effects of kinase activation on acidification-median
upregulation of ABCG2 and Mcl-1 are still unclear. To elucidate
this issue, A549 and LTEP-a-2 cells were pretreated with
wortmannin, LY294002, rapamycin and LY2584702 to inhibit kinase
activation. Then, the expression of ABCG2 and Mcl-1 was determined
by western blot analyses. The results showed that not only the
inhibition of PI3K and Akt activities but also the deficiencies of
mTOR and S6 obviously abolished pH 6.6 acidification-mediated
upregulation of the expression of ABCG2 and Mcl-1 in the A549 cells
(Fig. 4A). Western blot analyses of
LTEP-a-2 cells also revealed the similar phenomena (Fig. 4B). All of these results indicate
that the inhibition of the PI3K-Akt-mTOR-S6 pathway may be a
potential strategy for overcoming acidic microenvironment-mediated
lung cancer chemotherapeutic resistance.
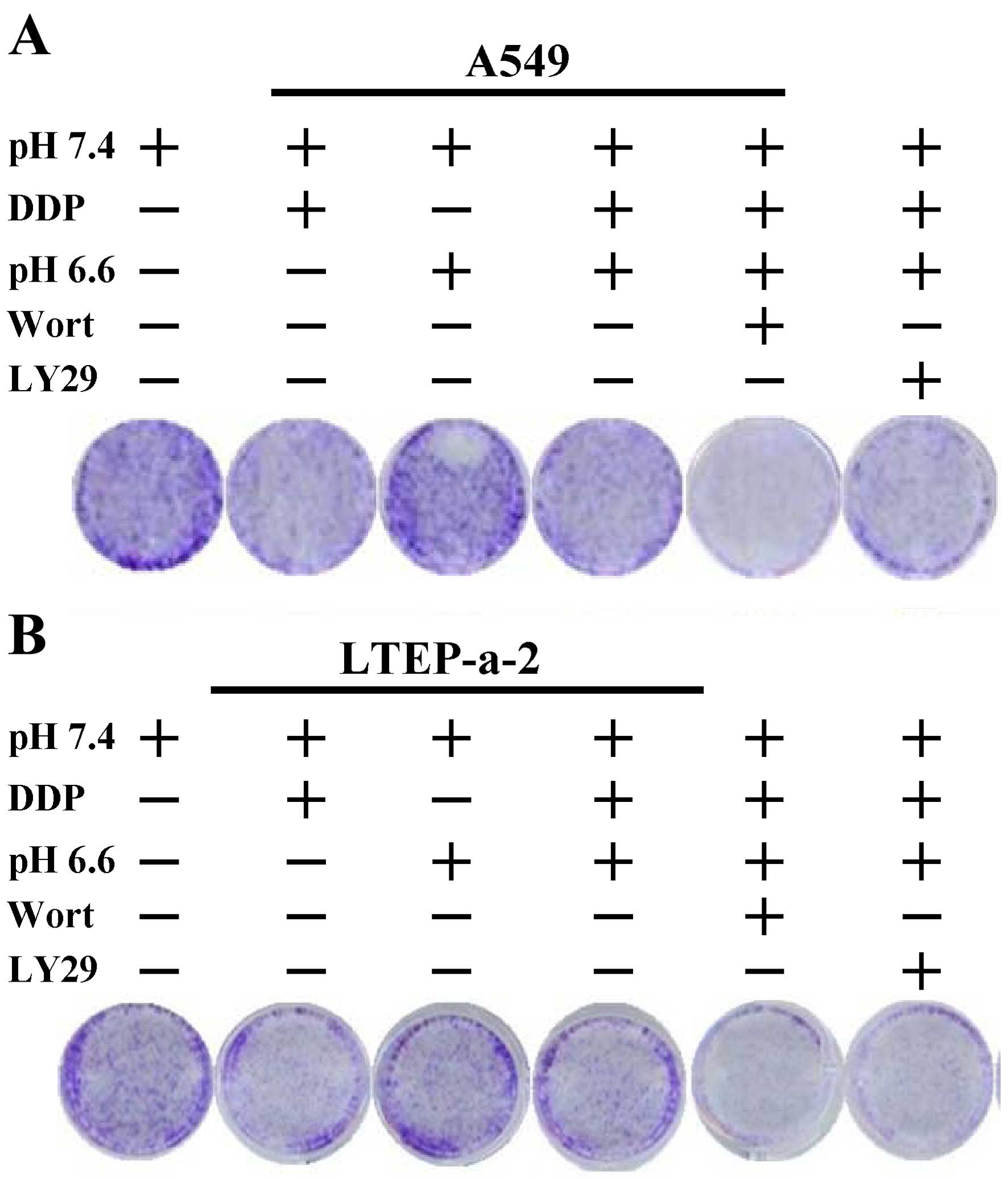
Inhibition of PI3K-Akt-mTOR-S6 activation
decreases acidic microenvironment-associated chemotherapeutic
resistance in lung cancer cells
Due to the phenomena that acidification increases
the activation of PI3K-Akt (Fig.
3), the finding that the inhibition of PI3K-Akt decreased the
acidification effect on the expression of ABCG2 and Mcl-1 motivated
us to ascertain whether the deficiency of PI3K-Akt activity would
be useful to overcome acidification-induced chemotherapeutic
resistance. To address this issue, A549 or LTEP-a-2 cells were
pretreated with wortmannin or LY294002 and the effect of PI3K-Akt
inhibition on acidification-increased cell viability was monitored.
Despite that decreased cell viability was achieved following
treatment with cisplatin, pH 6.6 acidification obviously increased
the survival rate of the cells (Fig.
5A). Importantly, pretreatment with LY294002 and wortmannin
efficiently abrogated the effect of acidification on cell viability
(Fig. 5A). A similar conclusion
could also be derived from the exploration in LTEP-a-2 cells
(Fig. 5B). All of these
observations indicate that PI3K-Akt may include potential molecules
to regulate acidized microenvironment-mediated MDR in lung cancer
chemotherapy.
Discussion
In the present study, we investigated the effects of
an acidized microenvironment on cell viability, the expression of
ATP-binding cassette transporter proteins, and activation of
PI3K-Akt. We demonstrated that acidification at pH 6.6 efficiently
upregulated the expression of ABCG2 and Mcl-1 via phosphorylation
of PI3K-Akt kinases and contributed to multidrug resistance (MDR)
in lung cancer. The decreased cell viability was achieved by the
inhibition of PI3K-Akt activity, indicating that PI3K and Akt
molecules may be potential therapeutic target molecules in acidic
microenvironment-associated chemotherapeutic resistance in lung
cancer.
IL-6, which is secreted by immune cells, has been
demonstrated to be expressed by tumor cells (24–26).
An elevated level of IL-6 has a close relationship with poor
clinical outcome of advanced lung cancer patients (27–29).
Meanwhile, IL-6 reveals anti-apoptotic effects and promotes MDR via
the upregulation of ABCG2 (30–32).
Our previous studies found that lung cancer cells, which have a
high level of IL-6, revealed not only higher MDR, but also a
stronger ability for migration (32,33),
indicating that IL-6 may be an oncogene in the formation and the
development of lung cancer. In the present study, despite that the
acidic microenvironment increased the expression of ABCG2 and
facilitated MDR formation, the effects of acidification on
pro-inflammatory cytokines such as IL-6 and TNF-α are uncertain and
need further investigation.
Ataxia-telangiectasia mutated (ATM), which is
involved in DNA damage response and cell cycle checkpoints
(34), was documented to increase
MDR-associated protein expression, and contribute to
chemotherapeutic resistance (14,35).
Despite that chemotherapeutic agents trigger the phosphorylation of
ATM and initiate the activation of TAK1-IKK-NF-κB (36), our previous studies showed that ATM
could also be activated by the treatment with IL-6 facilitating the
formation of MDR and metastasis in lung cancer (32,33),
indicating that ATM plays an important role in lung cancer
chemotherapeutic resistance. In the present study, despite that the
activation of PI3K-Akt was achieved by the acidic microenvironment,
the exact effects of acidification on PI3K-Akt phosphorylation are
still unknown and need further exploration.
Cancer stem cells are different from common cancer
cells due to their ability to produce tumors and resist
chemoradiation (37). Apart from
ABCB1 (38), ABCG2, CD44 and CD133
were recently recognized as lung cancer stem cell markers (39,40).
Previous studies have revealed that IL-6 treatment increases ABCG2
expression at both the translational and transcriptional levels
(32), and contributes to
chemotherapeutic resistance (32),
indicating that IL-6 treatment facilitates the ability of lung
cancer cells to acquire cancer stem-like phenotypes. Meanwhile,
epigallocatechin gallate, a bioactive polyphenol in green tea, was
documented to inhibit the stem cell characteristics of glioma
stem-like cells by downregulating P-glycoprotein expression and
inhibiting the phosphorylation of Akt (41). In the present study, despite that
the acidic microenvironment increased the expression of ABCG2 via
the PI3K-Akt pathway, the effects of acidification on other
molecules of cancer stem-like phenotypes and the cross-interaction
among the components of tumor tissues are complicated and require
further elucidation.
Taken together, our data provide a new molecular
mechanism for acidification-mediated MDR formation in lung cancer,
which is mediated by the combined action of increased expression of
ABCG2 and Mcl-1 via the PI3K-Akt pathway. This mechanism provides
new insights into the molecular mechanisms of chemotherapeutic
resistance and may thus open new opportunities for therapeutic
intervention in lung cancer therapy.
Acknowledgments
The present study was supported by grants from the
State Key Laboratory of Oncogenes and Related Genes (no. 90-14-05)
and by grants from the National Natural Science Foundation of China
(no. 81273203).
References
|
1
|
Tan XL, Moyer AM, Fridley BL, Schaid DJ,
Niu N, Batzler AJ, Jenkins GD, Abo RP, Li L, Cunningham JM, et al:
Genetic variation predicting cisplatin cytotoxicity associated with
overall survival in lung cancer patients receiving platinum-based
chemotherapy. Clin Cancer Res. 17:5801–5811. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rodriguez E and Lilenbaum RC: Small cell
lung cancer: Past, present, and future. Curr Oncol Rep. 12:327–334.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jackman DM and Johnson BE: Small-cell lung
cancer. Lancet. 366:1385–1396. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lowe JM, Menendez D, Bushel PR, Shatz M,
Kirk EL, Troester MA, Garantziotis S, Fessler MB and Resnick MA:
p53 and NF-κB coregulate proinflammatory gene responses in human
macrophages. Cancer Res. 74:2182–2192. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
O'Reilly S, Ciechomska M, Cant R and van
Laar JM: Interleukin-6 (IL-6) trans signaling drives a
STAT3-dependent pathway that leads to hyperactive transforming
growth factor-β (TGF-β) signaling promoting SMAD3 activation and
fibrosis via Gremlin protein. J Biol Chem. 289:9952–9960. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Greijer AE, de Jong MC, Scheffer GL,
Shvarts A, van Diest PJ and van der Wall E: Hypoxia-induced
acidification causes mitoxantrone resistance not mediated by drug
transporters in human breast cancer cells. Cell Oncol. 27:43–49.
2005.PubMed/NCBI
|
|
8
|
Daniel C, Bell C, Burton C, Harguindey S,
Reshkin SJ and Rauch C: The role of proton dynamics in the
development and maintenance of multidrug resistance in cancer.
Biochim Biophys Acta. 1832:606–617. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tavares-Valente D, Baltazar F, Moreira R
and Queirós O: Cancer cell bioenergetics and pH regulation
influence breast cancer cell resistance to paclitaxel and
doxorubicin. J Bioenerg Biomembr. 45:467–475. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamazaki R, Nishiyama Y, Furuta T, Hatano
H, Igarashi Y, Asakawa N, Kodaira H, Takahashi H, Aiyama R,
Matsuzaki T, et al: Novel acrylonitrile derivatives, YHO-13177 and
YHO-13351, reverse BCRP/ABCG2-mediated drug resistance in vitro and
in vivo. Mol Cancer Ther. 10:1252–1263. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim DH, Sriharsha L, Xu W, Kamel-Reid S,
Liu X, Siminovitch K, Messner HA and Lipton JH: Clinical relevance
of a pharmacogenetic approach using multiple candidate genes to
predict response and resistance to imatinib therapy in chronic
myeloid leukemia. Clin Cancer Res. 15:4750–4758. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Doyle L and Ross DD: Multidrug resistance
mediated by the breast cancer resistance protein BCRP (ABCG2).
Oncogene. 22:7340–7358. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Robey RW, Medina-Pérez WY, Nishiyama K,
Lahusen T, Miyake K, Litman T, Senderowicz AM, Ross DD and Bates
SE: Overexpression of the ATP-binding cassette half-transporter,
ABCG2 (Mxr/BCrp/ABCP1), in flavopiridol-resistant human breast
cancer cells. Clin Cancer Res. 7:145–152. 2001.PubMed/NCBI
|
|
14
|
Ke SZ, Ni XY, Zhang YH, Wang YN, Wu B and
Gao FG: Camptothecin and cisplatin upregulate ABCG2 and MRP2
expression by activating the ATM/NF-κB pathway in lung cancer
cells. Int J Oncol. 42:1289–1296. 2013.PubMed/NCBI
|
|
15
|
King D, Yeomanson D and Bryant HE: PI3King
the lock: Targeting the PI3K/Akt/mTOR pathway as a novel
therapeutic strategy in neuroblastoma. J Pediatr Hematol Oncol.
37:245–251. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jin HJ, Li HT, Sui HX, Xue MQ, Wang YN,
Wang JX and Gao FG: Nicotine stimulated bone marrow-derived
dendritic cells could augment HBV specific CTL priming by
activating PI3K-Akt pathway. Immunol Lett. 146:40–49. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jin HJ, Sui HX, Wang YN and Gao FG:
Nicotine up-regulated 4-1BBL expression by activating Mek-PI3K
pathway augments the efficacy of bone marrow-derived dendritic cell
vaccination. J Clin Immunol. 33:246–254. 2013. View Article : Google Scholar
|
|
18
|
Wang YY, Yang YW, You X, Deng XQ, Hu CF,
Zhu C, Wang JY, Gu JJ, Wang YN, Li Q, et al: Ex vivo nicotine
stimulation augments the efficacy of human peripheral blood
mononuclear cell-derived dendritic cell vaccination via activating
Akt-S6 pathway. Anal Cell Pathol. 2015:7414872015. View Article : Google Scholar
|
|
19
|
Wang SQ, Liu ST, Zhao BX, Yang FH, Wang
YT, Liang QY, Sun YB, Liu Y, Song ZH, Cai Y, et al: Afatinib
reverses multidrug resistance in ovarian cancer via dually
inhibiting ATP binding cassette subfamily B member 1. Oncotarget.
6:26142–26160. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xi G, Hayes E, Lewis R, Ichi S,
Mania-Farnell B, Shim K, Takao T, Allender E, Mayanil CS and Tomita
T: CD133 and DNA-PK regulate MDR1 via the PI3K- or Akt-NF-κB
pathway in multidrug-resistant glioblastoma cells in vitro.
Oncogene. 35:241–250. 2016. View Article : Google Scholar
|
|
21
|
de Souza PS, Cruz AL, Viola JP and Maia
RC: Microparticles induce multifactorial resistance through
oncogenic pathways independently of cancer cell type. Cancer Sci.
106:60–68. 2015. View Article : Google Scholar :
|
|
22
|
Wang R, Zhang J, Chen S, Lu M, Luo X, Yao
S, Liu S, Qin Y and Chen H: Tumor-associated macrophages provide a
suitable microenvironment for non-small lung cancer invasion and
progression. Lung Cancer. 74:188–196. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bhowmick NA, Neilson EG and Moses HL:
Stromal fibroblasts in cancer initiation and progression. Nature.
432:332–337. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang TH, Chan YH, Chen CW, Kung WH, Lee
YS, Wang ST, Chang TC and Wang HS: Paclitaxel (Taxol) upregulates
expression of functional interleukin-6 in human ovarian cancer
cells through multiple signaling pathways. Oncogene. 25:4857–4866.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Poth KJ, Guminski AD, Thomas GP, Leo PJ,
Jabbar IA and Saunders NA: Cisplatin treatment induces a transient
increase in tumorigenic potential associated with high
interleukin-6 expression in head and neck squamous cell carcinoma.
Mol Cancer Ther. 9:2430–2439. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Duan Z, Lamendola DE, Penson RT, Kronish
KM and Seiden MV: Overexpression of IL-6 but not IL-8 increases
paclitaxel resistance of U-2OS human osteosarcoma cells. Cytokine.
17:234–242. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chang CH, Hsiao CF, Yeh YM, Chang GC, Tsai
YH, Chen YM, Huang MS, Chen HL, Li YJ, Yang PC, et al: Circulating
interleukin-6 level is a prognostic marker for survival in advanced
nonsmall cell lung cancer patients treated with chemotherapy. Int J
Cancer. 132:1977–1985. 2013. View Article : Google Scholar
|
|
28
|
Wójcik E, Jakubowicz J, Skotnicki P,
Sas-Korczyńska B and Kulpa JK: IL-6 and VEGF in small cell lung
cancer patients. Anticancer Res. 30:1773–1778. 2010.PubMed/NCBI
|
|
29
|
Nikiteas NI, Tzanakis N, Gazouli M, Rallis
G, Daniilidis K, Theodoropoulos G, Kostakis A and Peros G: Serum
IL-6, TNFalpha and CRP levels in Greek colorectal cancer patients:
Prognostic implications. World J Gastroenterol. 11:1639–1643. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee SO, Lou W, Johnson CS, Trump DL and
Gao AC: Interleukin-6 protects LNCaP cells from apoptosis induced
by androgen deprivation through the Stat3 pathway. Prostate.
60:178–186. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Domingo-Domenech J, Oliva C, Rovira A,
Codony-Servat J, Bosch M, Filella X, Montagut C, Tapia M, Campás C,
Dang L, et al: Interleukin 6, a nuclear factor-κB target, predicts
resistance to docetaxel inhormone-independent prostate cancer and
nuclear docetaxel antitumor activity. Clin Cancer Res.
12:5578–5586. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yan HQ, Huang XB, Ke SZ, Jiang YN, Zhang
YH, Wang YN, Li J and Gao FG: IL-6 augments lung cancer
chemotherapeutics resistance via ATM/NF-kappaB pathway activation.
Cancer Sci. 105:1220–1227. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jiang YN, Yan HQ, Huang XB, Wang YN, Li Q
and Gao FG: Interleukin 6 trigged ataxia-telangiectasia mutated
activation facilitates lung cancer metastasis via MMP-3/MMP-13
up-regulation. Oncotarget. 6:40719–40733. 2015.PubMed/NCBI
|
|
34
|
Shiloh Y and Ziv Y: The ATM protein
kinase: Regulating the cellular response to genotoxic stress, and
more. Nat Rev Mol Cell Biol. 14:197–210. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Svirnovski AI, Serhiyenka TF, Kustanovich
AM, Khlebko PV, Fedosenko VV, Taras IB and Bakun AV: DNA-PK, ATM
and MDR proteins inhibitors in overcoming fludarabine resistance in
CLL cells. Exp Oncol. 32:258–262. 2010.
|
|
36
|
Wu ZH, Wong ET, Shi Y, Niu J, Chen Z,
Miyamoto S and Tergaonkar V: ATM- and NEMO-dependent ELKS
ubiquitination coordinates TAK1-mediated IKK activation in response
to genotoxic stress. Mol Cell. 40:75–86. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen J, Wang J, Chen D, Yang J, Yang C,
Zhang Y, Zhang H and Dou J: Evaluation of characteristics of
CD44+CD117+ ovarian cancer stem cells in
three dimensional basement membrane extract scaffold versus two
dimensional monocultures. BMC Cell Biol. 14:72013. View Article : Google Scholar
|
|
38
|
Grimm M, Krimmel M, Polligkeit J,
Alexander D, Munz A, Kluba S, Keutel C, Hoffmann J, Reinert S and
Hoefert S: ABCB5 expression and cancer stem cell hypothesis in oral
squamous cell carcinoma. Eur J Cancer. 48:3186–3197. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shi Y, Liu C, Liu X, Tang DG and Wang J:
The microRNA miR-34a inhibits non-small cell lung cancer (NSCLC)
growth and the CD44hi stem-like NSCLC cells. PLoS One.
9:e900222014. View Article : Google Scholar
|
|
40
|
Sarvi S, Mackinnon AC, Avlonitis N,
Bradley M, Rintoul RC, Rassl DM, Wang W, Forbes SJ, Gregory CD and
Sethi T: CD133+ cancer stem-like cells in small cell
lung cancer are highly tumorigenic and chemoresistant but sensitive
to a novel neuropeptide antagonist. Cancer Res. 74:1554–1565. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang Y, Wang SX, Ma JW, Li HY, Ye JC, Xie
SM, Du B and Zhong XY: EGCG inhibits properties of glioma stem-like
cells and synergizes with temozolomide through downregulation of
P-glycoprotein inhibition. J Neurooncol. 121:41–52. 2015.
View Article : Google Scholar
|