Introduction
Mesenchymal stem cells (MSCs), known as pluripotent
stem cells, were initially isolated from the bone marrow and named
bone marrow-derived mesenchymal stem cells (BMSCs) (1–3).
In-depth analysis has been performed for BMSCs (4,5), which
exist in a wide range of tissues (6–8). Many
researchers (6,8,9)
believe that MSCs originate from adult stem cells.
MSCs possess the ability of self-renewal, multiple
differentiation potential, specific homing to tumors, and low
immunogenicity (3,10,11).
MSCs are being used increasingly for cancer treatment (12–14).
Various reports state that MSCs can promote the progression of
breast cancer and colon cancer (15,16).
Other reports have demonstrated that MSCs can inhibit the growth of
pancreatic cancer, Kaposi's sarcoma and breast cancer (13,17,18).
In breast cancer, the effect of MSCs is controversial. Studies
reported that the regulated self-renewal of stem cells mediated by
the Wnt/β-catenin signaling pathway might be subverted in cancer
cells to allow malignant proliferation (19,20).
Likewise, the stem cell microenvironment plays an essential role in
preventing carcinogenesis by inhibiting proliferation (21). MSCs were found to inhibit tumor
proliferation through secretion of dickkopf-1 (DKK-1), which acts
as an inhibitor of the Wnt/β-catenin signaling pathway (22).
The rat rib perichondrium contains osteoprogenitor
cells, a type of adult stem cells. In the present study, we first
determined whether MSCs could be isolated from the perichondrium
and whether perichondrium MSCs (PMSCs) present biological
characteristics similar to those of BMSCs. We next explored the
effect of PMSCs on rat SHZ-88 breast cancer cells in vitro
and in vivo. We also investigated whether PMSCs affect
breast cancer cells through the DKK-1/Wnt/β-catenin signaling
pathway. Our findings demonstrated that MSCs could be derived from
the perichondrium and that they inhibited the growth of SHZ-88
breast cancer cells through the DKK-1/Wnt/β-catenin signaling
pathway.
Materials and methods
Cell culture
Specific pathogen-free 4-week-old female
Sprague-Dawley (SD) rats were obtained from the Experimental animal
Center of Xi'an Jiaotong University School of Medicine. All animals
were cared for in accordance with the institutional guidelines for
the use of experimental animals. The perichondrium was separated
under a dissecting microscope and treated with 5 ml of 0.2%
collagenase II (Sigma-Aldrich St. Louis, MO, USA) for 3 h in a 37°C
incubator shaker. We then collected the supernatant via 200 mesh
sieves and added 1 ml fetal bovine serum (FBS; HzSjq Co., Ltd.,
Hangzhou, China) to stop the digestion. After centrifugation at
1,200 × g for 10 min, the perichondrium cells were cultured in
Dulbecco's modified Eagle's medium (DMEM)/F12 (HyClone Co., Logan,
UT, USA) with 10% FBS. Bone marrow cells were isolated from femurs
and tibias as previously described (8), and cultured in DMEM/F12 with 10% FBS.
SHZ-88 cells were obtained from Shanghai Cell Research Institute
(Shanghai, China) and cultured in Roswell Park Memorial Institute
(RPMI)-1640 medium (HyClone) with 10% newborn calf serum (NBCS;
HzSjq Co. Ltd.) and 100 µg/ml penicillin/streptomycin (Gibco
Life Technologies, Rockville, MD, USA). All cell lines were
maintained in a humidified atmosphere at 37°C with 5%
CO2 and routinely passaged using trypsin (Invitrogen,
Camarillo, CA, USA) when nearly confluent.
Immunofluorescence staining
The primary to 3rd-generation perichondrium cells
and BMSCs were seeded at 4×105/ml in 24-well plates
covered with glass slides. When 70% confluency was reached, the
slides were removed and the cells were fixed with 4%
paraformaldehyde phosphate-buffered saline (PBS) for 30 min and
stained with the following primary antibodies overnight at 4°C:
anti-type II collagen rabbit polyclonal antibody (bs-8859R),
anti-CD34 rabbit polyclonal antibody (bs-0646R), anti-CD90 rabbit
polyclonal antibody (bs-0778R) (1:200 dilution), and anti-CD105
antibody rabbit polyclonal (bs-0579R; 1:100 dilution) (both from
Bioss Biological Technology Co., Beijing, China). They were then
incubated with the conjugated secondary CY3 antibody (Ba1032; 1:500
dilution; Boster Biological Technology Co., Wuhan, China).
4′,6-Diamidino-2-phenylindole (DAPI; C-0033; 1:10,000 dilution;
Bioss) was used to stain the cell nuclei.
Adipogenic and osteogenic
differentiation
The 3rd-generation PMSCs and BMSCs were seeded at
4×104/ml in 24-well plates covered with glass slides.
After 3 days, we changed the culture medium to the corresponding
conditioned medium for adipogenic or osteogenic differentiation
(23–26). After 14 days of adipogenic and 21
days of osteogenic induction, the slides were removed and the cells
were fixed for 30 min with 4% paraformaldehyde. The lipid droplets
were stained by Oil Red O (Sigma-Aldrich). The mineralization nodes
were stained by 5% silver nitrate (Sigma-Aldrich) and exposed to an
ultraviolet lamp within 60 min. Hematoxylin was used to stain the
cell nuclei.
Treatment of SHZ-88 cells with
conditioned medium derived from PMSCs
The 3rd-generation PMSCs were cultured as indicated
above. When the cells grew to full confluency, the medium was
replaced by serum-free medium for 48 h. The supernatants derived
from the PMSC cultures were harvested by centrifugation at 800 × g
for 5 min. The SHZ-88 cells were treated with a mixture of
RPMI-1640 and PMSC-conditioned medium (5:5) containing 10% NBCS for
72 h, during which time, the culture medium was replaced every 24
h. SHZ-88 cells were cultured in normal medium as a control group.
To neutralize DKK-1, PMSC-conditioned medium was incubated with
rabbit-anti-DKK-1 polyclonal antibody (Bioworld Technology, Inc.,
St. Louis Park, MN, USA) at a final concentration of 100 ng/ml at
4°C for 24 h (27,28), and subsequently used to treat SHZ-88
cells for 72 h. Normal rabbit IgG was used as a negative
control.
Cell proliferation assessment
Colorimetric
3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay was used to assess the proliferation of cells. SHZ-88 cells
(100 µl) were seeded at a density of 4×104
cells/ml in 96-well plates and treated with PMSC-conditioned medium
(control without PMSC-conditioned medium) for 24, 48 and 72 h. Ten
microliters of a 5 mg/ml solution of MTT (Sigma-Aldrich) in PBS was
added to each well and the plates were incubated for 4 h at 37°C.
One hundred fifty microliters of dimethylsulfoxide (Sigma, St.
Louis, MO, USA) was added to each well and the plates were shaken
for 15 min before reading the optical density (OD) of each well at
570 nm on a microplate reader.
Cell migration and invasion assays
After treatment of SHZ-88 cells with 50%
PMSC-conditioned medium for 48 h, migration and invasion assays
were conducted using Transwell plates with 8-µm pore size
membranes (Millipore Inc., Billerica, MA, USA) as previously
described (29,30). After incubation for 16 h (for
migration assays) or 24 h (for invasion assays), cells remaining on
the upper side of the filter were removed with cotton swabs. The
cells that attached to the lower surface were fixed, stained using
crystal violet and washed with PBS. Cells were counted in five
high-power fields/membrane. The results are presented as the mean
number of cells that migrated per field per membrane. All
experiments were conducted in triplicate.
SHZ-88 cell transplantation in SD
rats
SHZ-88 cells in the log-phase were diluted in normal
saline (NS) and were subcutaneously injected into the right armpit
of 10-day-old SD female rats. Every rat was injected with
5×105 cells/0.2 ml. When subcutaneous tumors reached 5–8
mm in size, SD rats were randomly divided into PMSC and NS groups
(n=16 rats/group). Animals in the PMSC group were injected with
3×106/0.2 ml PMSCs (diluted in NS) via the tail vein
every 3 days. Animals in the NS group were injected with 0.2 ml NS
via the tail vein every 3 day as a control group. We measured the
size of subcutaneous tumors every 6 days. The tumor volume was
calculated using the following formula: Tumor volume = ½(long tumor
diameter × short tumor diameter2). The tumor growth
curve was drawn by plotting tumor volumes according to the time in
each group. Finally, 6 rats in each group were euthanized after 30
days and tumor growth inhibitory rate was calculated using the
following formula: Tumor inhibition rate = [(average volume of
control group − average volume of experimental group)/average
volume of control group] × 100%. A piece of the tumor tissue from
each animal was fixed in 4% paraformaldehyde while the remaining
tumor tissue was stored at 80°C. Surviving animals (the remaining
10 animals in each group) were observed daily for 60 days.
Western blot analyses
The proteins were extracted from the SHZ-88 cells
treated with MSC-conditioned medium for 72 h and frozen tumors from
the tumor-bearing rats according to standard procedures. Proteins
were examined with specific primary antibodies: anti-DKK-1 rabbit
polyclonal antibody (BS7731), anti-Bcl-2 rabbit polyclonal antibody
(BS1031), anti-PCNA rabbit polyclonal antibody (BS6438),
anti-survivin rabbit polyclonal antibody (BS8456) (1:1,000
dilution; Bioworld Technology), anti-β-catenin rabbit polyclonal
antibody (51067-2-AP) (1:500 dilution; Proteintech, Huhan, China),
anti-c-Myc rabbit polyclonal antibody (10057-1-AP) (1:100 dilution;
Proteintech), and anti-β-actin rabbit polyclonal antibody
(bs-0061R; 1:1,000 dilution; Bioss), followed by a conjugated
secondary antibody: goat-anti-rabbit IgG antibody (1:3,000
dilution; Bioss). The reactions were visualized using the enhanced
chemiluminescence (ECL) reagent (Millipore). The band intensity of
western blotting was measured by densitometry using the Quantity
One software (Bio-Rad Laboratories, Hercules, CA, USA). The protein
levels were normalized to the protein level of β-actin which was
used as a loading control.
Immunohistochemical (IHC) staining
Tumor tissues fixed in 4% paraformaldehyde were
processed for paraffin embedding and sectioned (5-µm
thickness). Formalin-fixed paraffin-embedded tumor sections from NS
and PMSC-treated animals were analyzed by IHC staining using an
anti-β-catenin rabbit polyclonal antibody (1:200; Proteintech) and
a biotin-conjugated secondary antibody. The staining was performed
following the SP kit procedure (Golden Bridge International,
Beijing, China). As a control, the primary antibody was replaced by
PBS. IHC staining results were assessed independently by two
pathologists in a semi-quantitative manner, scored by a
semi-quantitative immunoreactivity scoring (IRS) system, and
divided into high and low expression specimens.
Statistical analysis
All data are expressed as means with standard error
(SE). One-way ANOVA and Student's t-test were used to test
differences between the groups. The Kaplan-Meier method was used to
test the survival time of tumor-bearing rats in different groups.
P<0.05 was considered to indicate a significant result. All
analyses were performed using SPSS software version 18.0 (IBM,
Corp., New York, NY, USA).
Results
Biological characteristics of the PMSCs
and BMSCs Growth and morphology
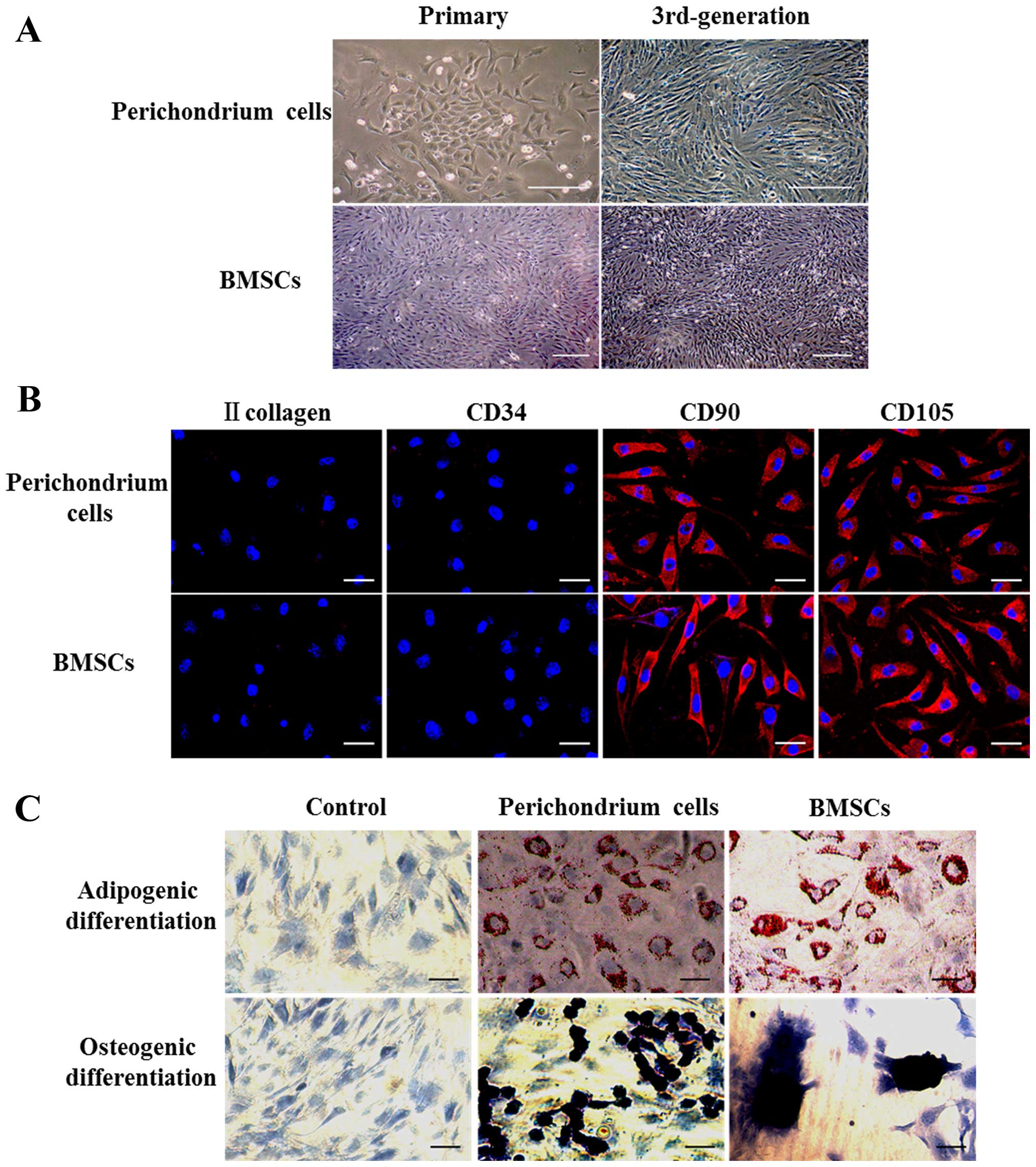
The primary perichondrium cells began to adhere to
the plate after 12 h of culture. They were passaged after 5–6 days
of culture when they presented a round or polygon shape (Fig. 1A). The primary bone marrow cells
began to adhere to the plate after 4–5 h of culture and were
passaged after 8–10 days of culture when they presented a spindle
or polygon shape (Fig. 1A). As the
generations increased, from 3rd-generation, two types of cells
showed spindle-shape with whirlpool arrangement (Fig. 1A) and the generation times of
perichondrium cells and BMSCs were 1–2 and 4–5 days, respectively.
The perichondrium cells were passaged for 30 generations and
retained their shape (data not shown).
Cell surface marker expression
Immunofluorescence staining indicated that type II
collagen, CD34, CD90 and CD105 proteins were not expressed in
primary and first generation perichondrium cells. From the
2nd-generation, cells began to express CD90 and CD105 proteins. The
primary bone marrow cells were weakly positive for CD34, CD90 and
CD105 proteins, but not for type II collagen. Subsequently, the
cells only expressed CD90 and CD105 proteins. From the
3rd-generation, the two cell types did not expressed type II
collagen and CD34 proteins, but strongly expressed CD90 and CD105
proteins (Fig. 1B). The 30th
generation of perichondrium cells still strongly expressed CD90 and
CD105, but did not expressed CD34 and type II collagen (data not
shown).
Multipotent differentiation ability
No change in term of the induction of
differentiation was observed in the control group (Fig. 1C). After 14 days of adipogenic
induction of the 3rd-generation cells, perichondrium cells and
BMSCs became round. Lipid droplets were observed in the cytoplasm
of these cells after Oil Red O staining (Fig. 1C). After 21 days of osteogenic
induction, the perichondrium cells grew as a stratified layer, some
black mineralized nodules stained by silver nitrate were observed
on the cell surface (Fig. 1C).
BMSCs grew as a simple layer, but mineralized nodules gathered as
bone nodules (Fig. 1C). The 30th
generation perichondrium cells still presented lipid droplets and
black mineralized nodules after induction (data not shown).
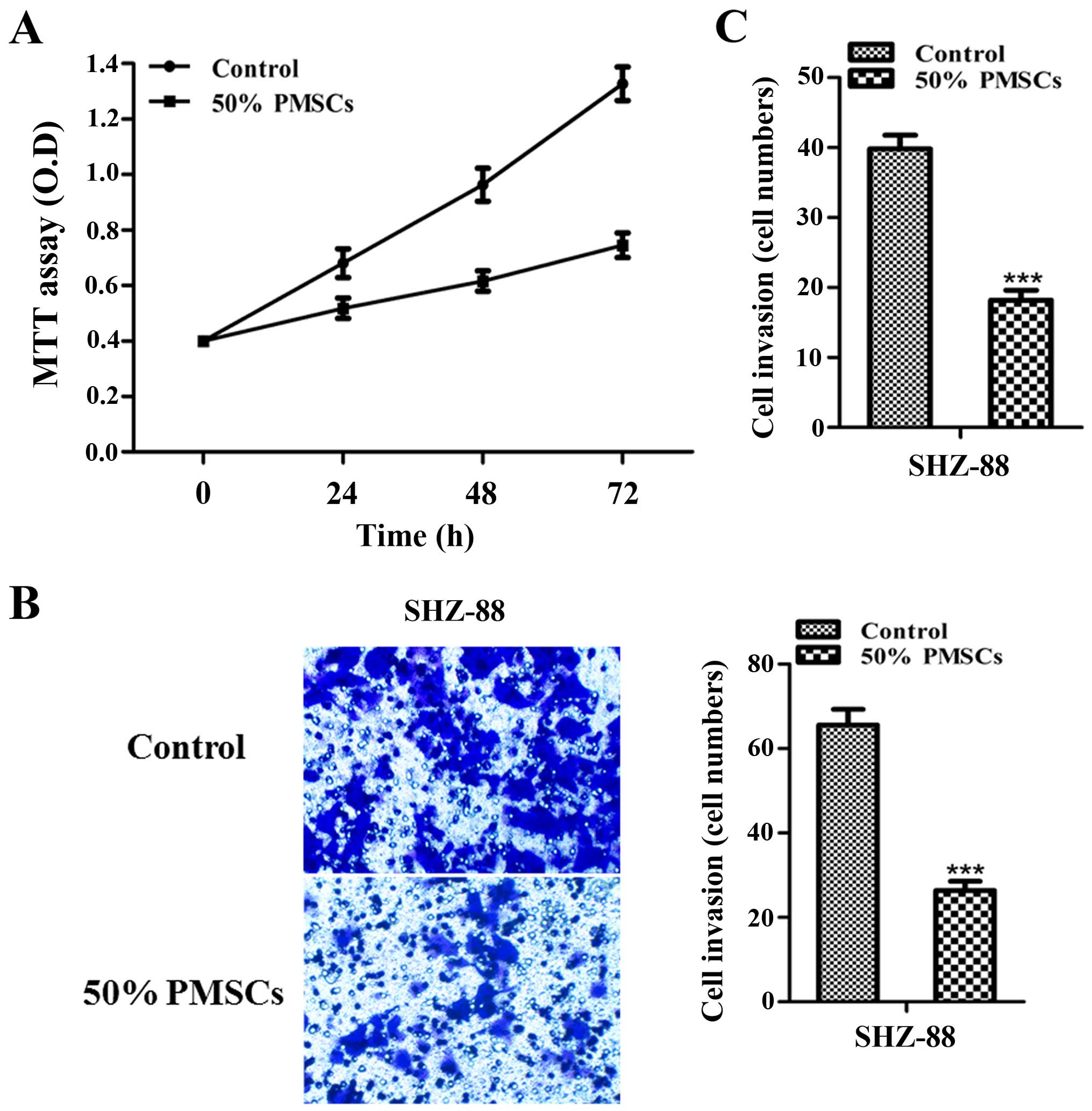
PMSC-conditioned media inhibits SHZ-88
cell growth, migration and invasion
Human MSCs can inhibit tumor growth (22,28).
However, no study has reported such information regarding PMSCs. In
the present study, we explored the effect of PMSCs on breast
cancer. The stem cell microenvironment plays an essential role in
preventing carcinogenesis by inhibiting proliferation (21). Thus, we investigated the inhibitory
effect of 50% PMSC-conditioned medium on SHZ-88 cells in
vitro. MTT assay showed that PMSC-conditioned medium inhibited
the proliferation of SHZ-88 cells in a time-dependent manner
(P<0.001 at 72 h; Fig. 2A). To
further analyze the effect of 50% PMSC-conditioned medium on SHZ-88
cells, cell migration and invasion assays were conducted after
treating SHZ-88 cells with 50% PMSC-conditioned medium for 48 h
in vitro. SHZ-88 cell migration and invasion were
significantly reduced by the PMSC-conditioned medium (P<0.001;
Fig. 2B and C). These data
suggested that some factors in the conditioned medium from PMSCs
were responsible for the inhibition of tumor cell proliferation,
migration and invasion.
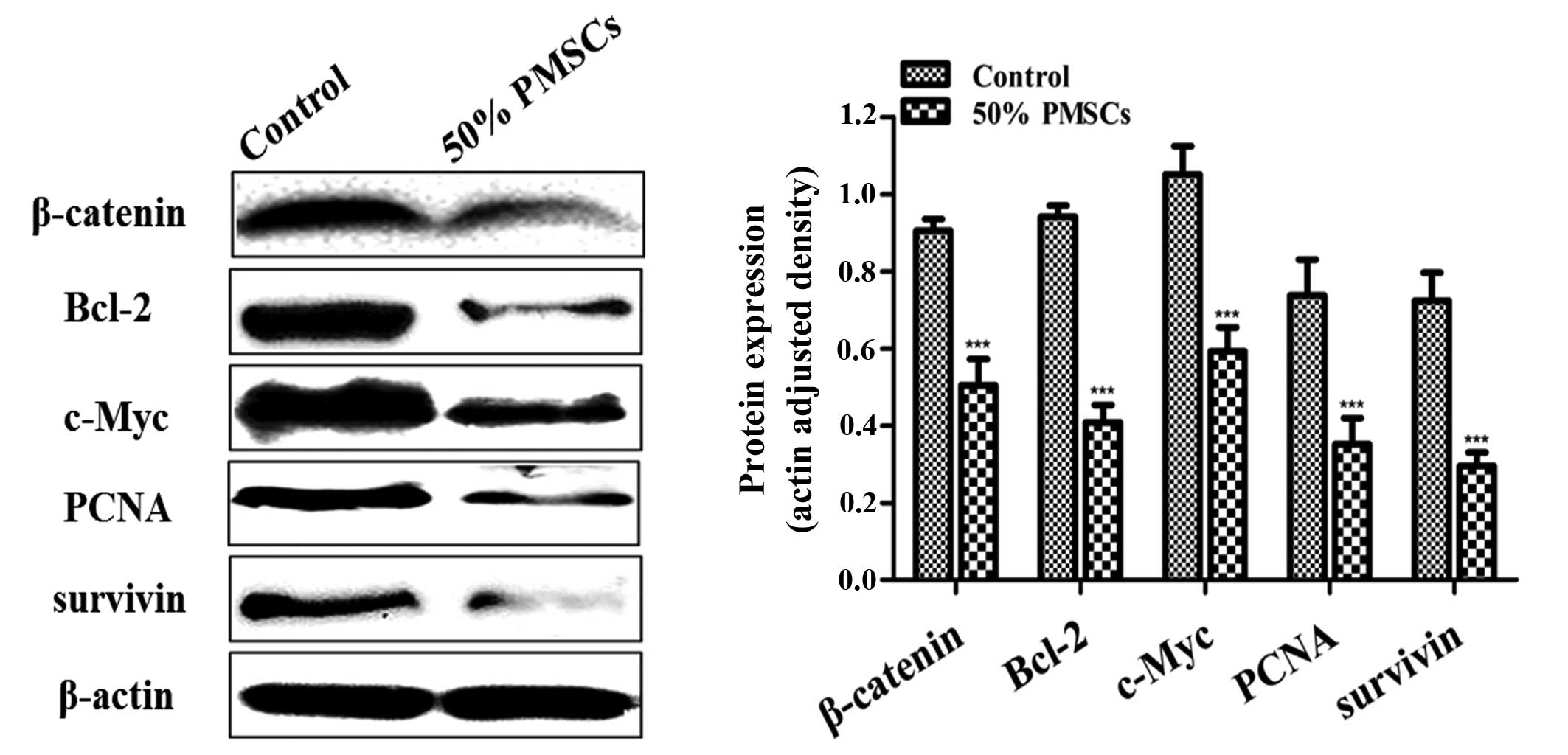
The Wnt/β-catenin signaling pathway is
suppressed in the SHZ-88 cells treated with PMSC-conditioned
medium
Studies have indicated that the self-renewal of stem
cells by the Wnt/β-catenin signaling pathway was subverted in
cancer cells, resulting in tumor progression (19,20).
The Bcl-2, c-Myc, PCNA and survivin genes are all targets of the
Wnt/β-catenin signaling pathway. Western blot analysis showed that
β-catenin, Bcl-2, c-Myc, PCNA and survivin proteins were
downregulated in SHZ-88 cells treated with 50% PMSC-conditioned
medium (P<0.001; Fig. 3). These
results indicated that some soluble exocrine factors in the
MSC-conditioned medium are involved in the inhibition of the
Wnt/β-catenin signaling pathway in SHZ-88 cells.
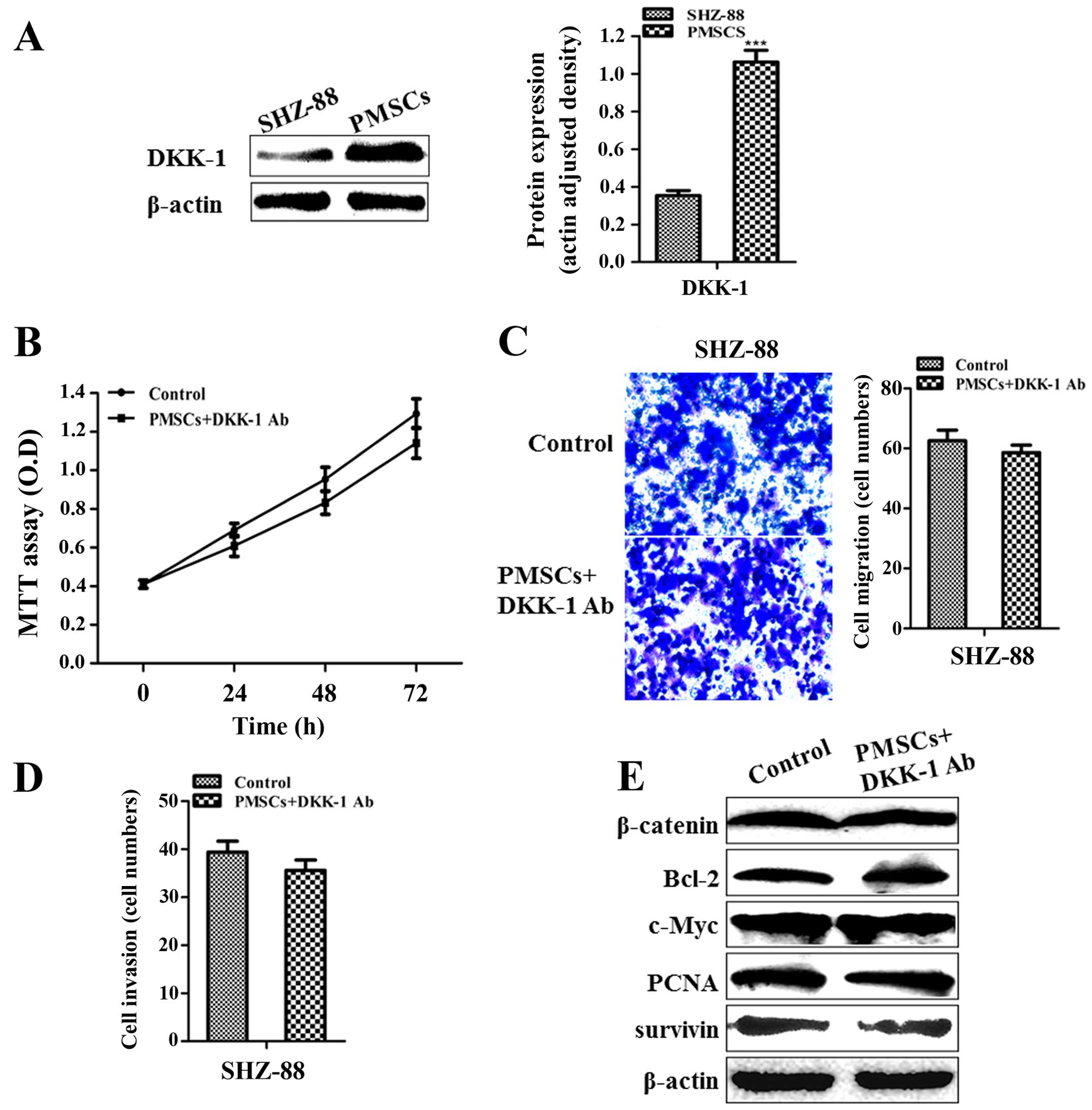
DKK-1 derived from PMSCs contributes to
the inhibition of SHZ-88 cells
MSCs have been shown to inhibit tumor proliferation
by secreting DKK-1, which acts as an inhibitor of the Wnt/β-catenin
signaling pathway (28,31). Thus,
we determined the expression levels of DKK-1 in the SHZ-88 cells
and PMSCs. Western blot analysis indicated that the DKK-1
expression level was higher in the PMSCs than that in the SHZ-88
cells (P<0.001; Fig. 4A). DDK-1
neutralization by the rabbit antibody against the rat DKK-1 in the
PMSC-conditioned medium abolished the inhibitory effect of the
PMSC-conditioned medium on SHZ-88 cell proliferation as
demonstrated by MTT assay (P>0.05; Fig. 4B). In addition, the inhibitory
effect of the PMSC-conditioned medium on migration and invasion of
SHZ-88 cells was also lost (P>0.05; Fig. 4C and D). Moreover, western blot
analysis indicated that neutralization of DKK-1 in the
PMSC-conditioned medium abrogated the downregulating effect of the
conditioned medium on the expression of β-catenin, Bcl-2, c-Myc,
PCNA and survivin in SHZ-88 cells (P>0.05; Fig. 4E). These data indicated that the
inhibition of SHZ-88 cell growth, migration and invasion mediated
by PMSC-conditioned medium involved DKK-1 secreted by PMSCs in the
medium.
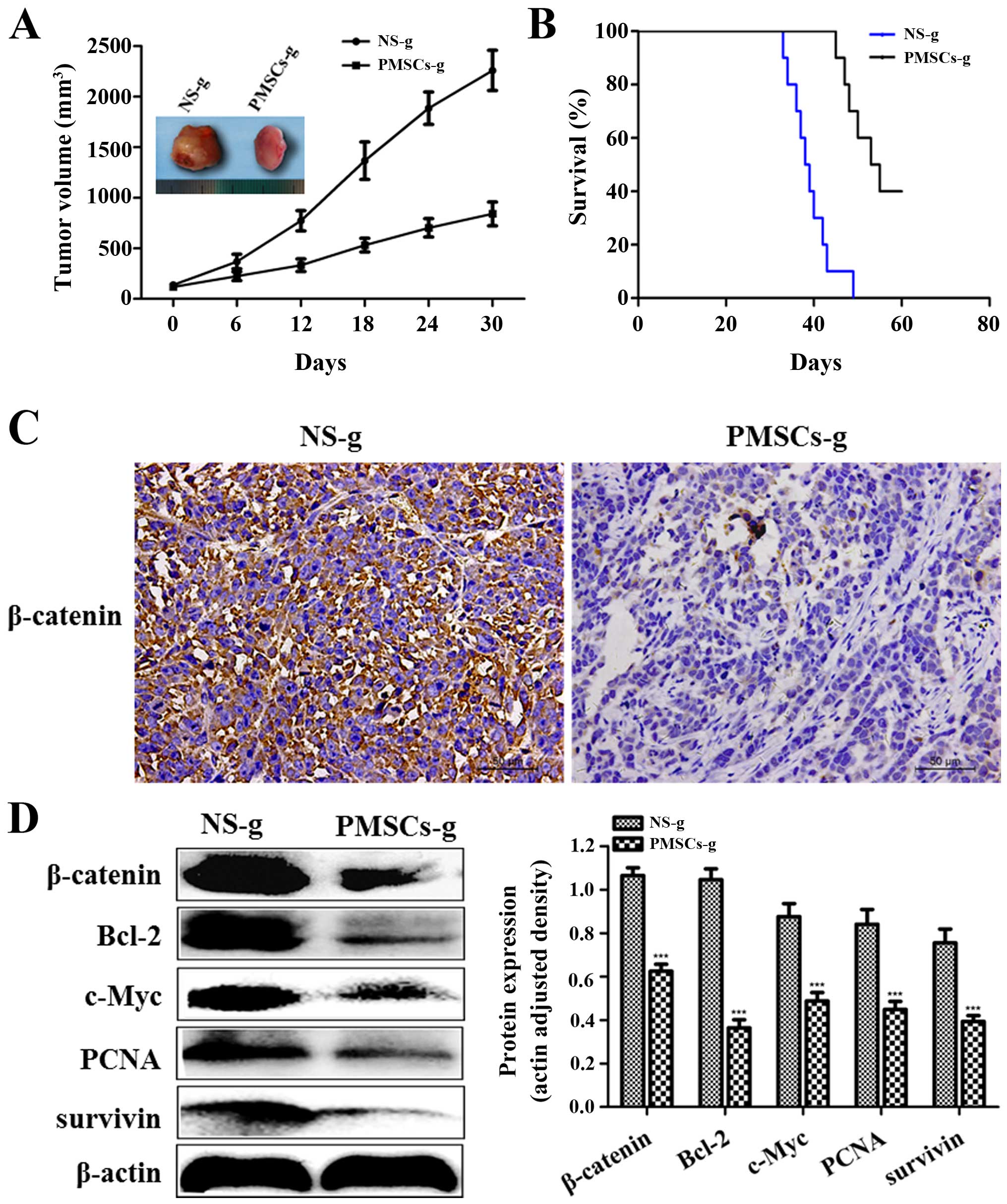
PMSCs inhibit the growth of breast cancer
in vivo PMSCs inhibit the growth of transplanted tumors
To determine the effect of PMSCs on transplanted
tumors in vivo, we established a subcutaneous tumor model of
breast cancer in SD rats. When the tumor nodules reached 5–8 mm in
length, the rats were injected with either PMSCs or NS (control
group). Tumor volume gradually increased and the tumor growth rate
was faster in the NS group than that determined in the PMSC group.
On day 30, no necrosis was observed in the tumors from rats treated
with PMSCs, while partial necrosis and a hemorrhagic tendency on
the surface of tumors were observed in the tumors from rats treated
with NS. Additionally, the size of the tumors in the NS group was
larger than that of the tumors from the PMSC group (Fig. 5A). We measured the size of the
subcutaneous tumors every 6 days and constructed the tumor growth
curve based on tumor volumes (Fig.
5A). The tumor inhibition rate in the PMSC group reached 62.8%.
These data indicated that treatment of the rats with PMSCs resulted
in a significant growth inhibition in vivo when compared
with the control group (P<0.001). The mean survival time of the
rats in the NS group was 39.7±1.2 days, while that of the rats in
the PMSC group was 53.4±2.0 days. The survival time of the rats in
the NS group was significantly shorter than that of the rats
treated with PMSCs (P<0.001; Fig.
5B).
PMSCs inhibit the growth of transplanted
tumors via the Wnt/β-catenin signaling pathway
We further investigated the molecular mechanisms
underlying the inhibitory effect of PMSCs on breast cancer.
Immunohistochemistry staining indicated that the β-catenin protein
in the tumors from rats treated with PMSCs was expressed at low
levels, while it was highly expressed in the tumors of rats treated
with NS when using the IRS system (Fig.
5C). Western blot analyses showed that β-catenin, Bcl-2, c-Myc,
PCNA and survivin proteins were downregulated in the PMSC group
(P<0.001; Fig. 5D). These
results indicated that PMSCs inhibited the growth of transplanted
tumors via the Wnt/β-catenin signaling pathway in vivo.
Discussion
Many researchers (8,10,14)
believe that MSCs originate from adult stem cells. The rib
perichondrium contains osteoprogenitor cells, a type of adult stem
cells. In the present study, we investigated whether MSCs could be
isolated from the perichondrium. Our results showed that, from the
3rd-generation, the perichondrium cells presented a typical
spindle-shape with whirlpool arrangement and strongly expressed
CD90 and CD105 proteins (32).
Additionally, adipogenic and osteogenic differentiation could be
induced in these cells. These data demonstrated that perichondrium
cells presented biological characteristics similar to those of
BMSCs. These findings strongly suggest that we successfully derived
stem cells from the rib perichondrium, which were named PMSCs.
MSCs possess the ability to specifically home to
tumors and to self-renew, and present low immunogenicity (3,10,11,16).
Thus, they have been used for cancer treatment (13,33,34).
However, the effects of MSCs on tumors are controversial. Various
reports state that MSCs promote the progression of breast cancer
and colon cancer cells (15,16).
Other reports indicate that MSCs can inhibit the growth of
pancreatic cancer, Kaposi's sarcoma, and breast cancer cells
(13,17,18).
In the present study, using MTT, cell migration and invasion
assays, we found that PMSC-conditioned medium inhibited the growth,
migration and invasion of SHZ-88 cells (P<0.001). Our findings
are consistent with several reports (18,35).
The Wnt/β-catenin signaling pathway plays an important role in the
self-renewal of stem cells (19),
but abnormal activation of the Wnt/β-catenin signaling pathway can
lead to human cancer (20). The
Wnt/β-catenin pathway is related to the efficacy of MSCs in
suppressing hepatoma and breast cancer (18,28,35).
The Bcl-2, c-Myc, PCNA and survivin
genes are all targets of the Wnt/β-catenin signaling pathway
(36,37). In the present study, western blot
analyses confirmed that the Wnt/β-catenin signaling pathway in
SHZ-88 cells was blocked by PMSC-conditioned medium. These results
are consistent with the above report. These data suggest that
various soluble factors in PMSC-conditioned medium are responsible
for the inhibition of the growth, migration, invasion and
Wnt/β-catenin signaling pathway in SHZ-88 cells.
Next, we attempted to identify the inhibitors of the
Wnt/β-catenin signaling pathway in the PMSC-conditioned medium. It
was previously reported that MSCs inhibit tumor proliferation via
secretion of DKK-1, which acts as a negative regulator of the
Wnt/β-catenin signaling pathway (22,28).
Accordingly, we speculated that DKK-1 secreted by PMSCs was
involved in the inhibition of the Wnt/β-catenin signaling pathway
in SHZ-88 cells. Western blot analyses showed that DKK-1 expression
level in PMSCs was higher than that in SHZ-88 cells, which provided
evidence that DKK-1 from PMSCs may play a vital role in controlling
the Wnt/β-catenin signaling in SHZ-88 cells. These findings are
consistent with various reports (18,22,28).
In order to further confirm the role of DKK-1 secreted by PMSCs in
the inhibition of the Wnt/β-catenin signaling pathway in SHZ-88
cells, we neutralized DKK-1 in the PMSC-conditioned medium using an
antibody against DKK-1 and demonstrated that the inhibition of
SHZ-88 cell growth, migration and invasion mediated by
PMSC-conditioned medium was reduced by DKK-1 neutralization.
Additionally, the treated conditioned medium lost the ability to
downregulate the expression of β-catenin, Bcl-2, c-Myc, PCNA and
survivin in SHZ-88 cells. This provided evidence that DKK-1
secreted by PMSCs contributed to the inhibition of the
Wnt/β-catenin signaling pathway in SHZ-88 cells. Moreover, we
showed that PMSCs inhibited the growth of transplanted tumors in
vivo. When compared with the NS group, injection of PMSCs
significantly inhibited tumor growth and prolonged the survival
time of tumor-bearing rats. Wnt/β-catenin and its target genes were
downregulated in the tumors from rats treated with PMSCs. These
results indicate that PMSCs inhibit the growth of transplanted
breast tumors through the Wnt/β-catenin signaling pathway as
observed in vivo.
In summary, the present study is the first to report
the isolation of mesenchymal stem cells from the perichondrium
(PMSCs) of rat. Furthermore, PMSCs were found to inhibit breast
cancer cell growth through the DKK-1/Wnt/β-catenin signaling
pathway. The present study provided novel information, which can be
useful for the development of new therapeutics for breast cancer
treatment.
Acknowledgments
The authors would like to thank Chen Huang of the
Department of Genetics and Molecular Biology, Medical School, Xi'an
Jiaotong University for his technical support. The present study
was supported by a grant from the National Natural Science
Foundation of China (no. 81471710).
References
|
1
|
Friedenstein AJ, Piatetzky-Shapiro II and
Petrakova KV: Osteogenesis in transplants of bone marrow cells. J
Embryol Exp Morphol. 16:381–390. 1966.PubMed/NCBI
|
|
2
|
Friedenstein AJ, Petrakova KV, Kurolesova
AI and Frolova GP: Heterotopic of bone marrow. Analysis of
precursor cells for osteogenic and hematopoietic tissues.
Transplantation. 6:230–247. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Caplan AI: Mesenchymal stem cells. J
Orthop Res. 9:641–650. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xia Z, Zhang C, Zeng Y, Wang T and Ai G:
Transplantation of BMSCs expressing hVEGF165/hBD3
promotes wound healing in rats with combined radiation-wound
injury. Int Wound J. 11:293–303. 2014. View Article : Google Scholar
|
|
5
|
Zhang L, He A, Yin Z, Yu Z, Luo X, Liu W,
Zhang W, Cao Y, Liu Y and Zhou G: Regeneration of human-ear-shaped
cartilage by co-culturing human microtia chondrocytes with BMSCs.
Biomaterials. 35:4878–4887. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rossi B, Merlo B, Colleoni S, Iacono E,
Tazzari PL, Ricci F, Lazzari G and Galli C: Isolation and in vitro
characterization of bovine amniotic fluid derived stem cells at
different trimesters of pregnancy. Stem Cell Rev. 10:712–724. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zarrabi M, Mousavi SH, Abroun S and
Sadeghi B: Potential uses for cord blood mesenchymal stem cells.
Cell J. 15:274–281. 2014.PubMed/NCBI
|
|
8
|
Yoshimura H, Muneta T, Nimura A, Yokoyama
A, Koga H and Sekiya I: Comparison of rat mesenchymal stem cells
derived from bone marrow, synovium, periosteum, adipose tissue, and
muscle. Cell Tissue Res. 327:449–462. 2007. View Article : Google Scholar
|
|
9
|
Lu T, Hu P, Su X, Li C, Ma Y and Guan W:
Isolation and characterization of mesenchymal stem cells derived
from fetal bovine liver. Cell Tissue Bank. 15:439–450. 2014.
View Article : Google Scholar
|
|
10
|
Reger RL, Tucker AH and Wolfe MR:
Differentiation and characterization of human MSCs. Methods in
Molecular Biology. Prockop DJ, Phinney DG and Bunnell BA: pp.
93–107. 2008, View Article : Google Scholar
|
|
11
|
Si YL, Zhao YL, Hao HJ, Fu XB and Han WD:
MSCs: Biological characteristics, clinical applications and their
outstanding concerns. Ageing Res Rev. 10:93–103. 2011. View Article : Google Scholar
|
|
12
|
Horwitz EM: Oncolytic virotherapy for ALL:
MSCs to the rescue. Blood. 123:1286–1287. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moniri MR, Dai LJ and Warnock GL: The
challenge of pancreatic cancer therapy and novel treatment strategy
using engineered mesenchymal stem cells. Cancer Gene Ther.
21:12–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hong IS, Lee HY and Kang KS: Mesenchymal
stem cells and cancer: Friends or enemies? Mutat Res. 768:98–106.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kikuchi H, Yagi H, Hasegawa H, Ishii Y,
Okabayashi K, Tsuruta M, Hoshino G, Takayanagi A and Kitagawa Y:
Therapeutic potential of transgenic mesenchymal stem cells
engineered to mediate anti-high mobility group box 1 activity:
Targeting of colon cancer. J Surg Res. 190:134–143. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Goldstein RH, Reagan MR, Anderson K,
Kaplan DL and Rosenblatt M: Human bone marrow-derived MSCs can home
to orthotopic breast cancer tumors and promote bone metastasis.
Cancer Res. 70:10044–10050. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Khakoo AY, Pati S, Anderson SA, Reid W,
Elshal MF, Rovira II, Nguyen AT, Malide D, Combs CA, Hall G, et al:
Human mesenchymal stem cells exert potent antitumorigenic effects
in a model of Kaposi's sarcoma. J Exp Med. 203:1235–1247. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qiao L, Xu Z, Zhao T, Zhao Z, Shi M, Zhao
RC, Ye L and Zhang X: Suppression of tumorigenesis by human
mesenchymal stem cells in a hepatoma model. Cell Res. 18:500–507.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reya T and Clevers H: Wnt signalling in
stem cells and cancer. Nature. 434:843–850. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Moon RT, Kohn AD, De Ferrari GV and Kaykas
A: WNT and beta-catenin signalling: Diseases and therapies. Nat Rev
Genet. 5:691–701. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livraghi T, Meloni F, Frosi A, Lazzaroni
S, Bizzarri TM, Frati L and Biava PM: Treatment with stem cell
differentiation stage factors of intermediate-advanced
hepatocellular carcinoma: An open randomized clinical trial. Oncol
Res. 15:399–408. 2005.
|
|
22
|
Zhu Y, Sun Z, Han Q, Liao L, Wang J, Bian
C, Li J, Yan X, Liu Y, Shao C, et al: Human mesenchymal stem cells
inhibit cancer cell proliferation by secreting DKK-1. Leukemia.
23:925–933. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sekiya I, Larson BL, Vuoristo JT, Cui JG
and Prockop DJ: Adipogenic differentiation of human adult stem
cells from bone marrow stroma (MSCs). J Bone Miner Res. 19:256–264.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jaiswal N, Haynesworth SE, Caplan AI and
Bruder SP: Osteogenic differentiation of purified, culture-expanded
human mesenchymal stem cells in vitro. J Cell Biochem. 64:295–312.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sen A, Lea-Currie YR, Sujkowska D,
Franklin DM, Wilkison WO, Halvorsen YD and Gimble JM: Adipogenic
potential of human adipose derived stromal cells from multiple
donors is heterogeneous. J Cell Biochem. 81:312–319. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kang Y, Kim S, Bishop J, Khademhosseini A
and Yang Y: The osteogenic differentiation of human bone marrow
MSCs on HUVEC-derived ECM and β-TCP scaffold. Biomaterials.
33:6998–7007. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yamaguchi Y, Itami S, Watabe H, Yasumoto
K, Abdel-Malek ZA, Kubo T, Rouzaud F, Tanemura A, Yoshikawa K and
Hearing VJ: Mesenchymal-epithelial interactions in the skin:
Increased expression of dickkopf1 by palmoplantar fibroblasts
inhibits melanocyte growth and differentiation. J Cell Biol.
165:275–285. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qiao L, Xu ZL, Zhao TJ, Ye LH and Zhang
XD: Dkk-1 secreted by mesenchymal stem cells inhibits growth of
breast cancer cells via depression of Wnt signalling. Cancer Lett.
269:67–77. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Clarke MR, Imhoff FM and Baird SK:
Mesenchymal stem cells inhibit breast cancer cell migration and
invasion through secretion of tissue inhibitor of
metalloproteinase-1 and -2. Mol Carcinog. 54:1214–1219. 2015.
View Article : Google Scholar
|
|
30
|
Huo C, Kao YH and Chuu CP: Androgen
receptor inhibits epithelial-mesenchymal transition, migration, and
invasion of PC-3 prostate cancer cells. Cancer Lett. 369:103–111.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fedi P, Bafico A, Nieto Soria A, Burgess
WH, Miki T, Bottaro DP, Kraus MH and Aaronson SA: Isolation and
biochemical characterization of the human Dkk-1 homologue, a novel
inhibitor of mammalian Wnt signaling. J Biol Chem. 274:19465–19472.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Screven R, Kenyon E, Myers MJ, Yancy HF,
Skasko M, Boxer L, Bigley EC III, Borjesson DL and Zhu M:
Immunophenotype and gene expression profile of mesenchymal stem
cells derived from canine adipose tissue and bone marrow. Vet
Immunol Immunopathol. 161:21–31. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Du J, Zhang Y, Xu C and Xu X:
Apoptin-modified human mesenchymal stem cells inhibit growth of
lung carcinoma in nude mice. Mol Med Rep. 12:1023–1029.
2015.PubMed/NCBI
|
|
35
|
Kolluri KK, Laurent GJ and Janes SM:
Mesenchymal stem cells as vectors for lung cancer therapy.
Respiration. 85:443–451. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Abdel aziz MT, El Asmar MF, Atta HM,
Mahfouz S, Fouad HH, Roshdy NK, Rashed LA, Sabry D, Hassouna AA and
Taha FM: Efficacy of mesenchymal stem cells in suppression of
hepatocarcinorigenesis in rats: Possible role of Wnt signaling. J
Exp Clin Cancer Res. 30:492011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Joubert A, Bianchi P, Maritz C and Joubert
F: Influence of prostaglandin a2 on Bax, Bcl-2 and PCNA expression
in MCF-7 cells. Biomed Res. 27:157–162. 2006. View Article : Google Scholar : PubMed/NCBI
|