Introduction
Hepatocellular carcinoma (HCC) is the most common
type of liver cancer and 86% of cases occur in developing
countries. It ranks fifth for incidence and second for mortality in
developing countries and for developed countries it ranks 11th for
incidence and seventh for mortality, with an estimated 792,000
incident cases and 818,000 related deaths occurring globally in
2013 (1). Surgery is currently the
most effective treatment but many patients are diagnosed in an
advanced stage that was not appropriate for surgical treatment. The
effects of alternative treatments such as traditional
chemotherapeutic agents is still unsatisfactory due to their
side-effects and the development of drug resistance (2,3).
Therefore, it is necessary and urgent to identify new agents or
therapeutic strategies with lower toxicity that are active against
HCC for the prevention and treatment of HCC.
Many agents extracted from natural plants have shown
their effects in the prevention and treatment of cancer (4). Among these, many of the medicines
derive from Traditional Chinese Medicine (TCM) and have been
confirmed to be effective in the treatment of a number of tumors
via targeting and regulating multiple molecular pathways (5,6).
Emodin is an active ingredient derived from root and rhizome of
Rheum palmatum L, which is a plant widely used in Chinese
medicine as a laxative for thousands of years (7,8). The
molecular formula of emodin is
C15H10O5 and its molecular weight
is 270.24. In recent decades, increased attention was focused on
anticancer activities of emodin since studies have reported that it
exhibits antiproliferative and apoptosis-inducing effects in a
number of human cancers such as colon, cervical and gastric cancer
(9–11). It may also suppress migration and
metastasis of cancer such as breast cancer and HCC (12,13).
However, there is little information demonstrating the possible
effects of emodin on the proliferation and apoptosis of HCC at
present. Therefore, further interpretations are still needed to
elucidate the exact mechanisms.
Thus, in the present study, we examined the effects
of emodin on the proliferation and apoptosis of HCC cells in
vitro and in vivo, as well as the molecular mechanisms
involved.
Materials and methods
Materials
Emodin (1,3,8-trihydroxy-6-methyl-anthraquinine),
dimethyl sulfoxide (DMSO) and Cell Counting kit-8 (CCK-8) were
purchased from Sigma (St. louis, MO, USA). Emodin was dissolved in
100% DMSO to prepare stock solutions of 5, 12.5, 25, 50 and 100
mmol/l, respectively, which were diluted in high glucose Dulbecco's
modified Eagle's medium (DMEM) to the indicated concentrations
before each assay. DMSO (0.2%) was used as vehicle control for all
the experiments. The Annexin V-FITC apoptosis detection kit was
purchased from Nanjing KeyGen Biotech. Co., Ltd, Nanjing, China.
Rabbit phosphorylated (p)-Akt and total (t)-Akt polyclonal
antibody, rabbit p-extracellular-signal-regulated kinase (ERK) 1/2
and t-ERK1/2 monoclonal antibody, rabbit p-p38 and t-p38 monoclonal
antibody, rabbit p-c-Jun-N-terminal kinase (p-JNK) and t-JNK
monoclonal antibody, rabbit anti-cleaved caspase-3, -9 and
pro-caspase-3, -9 monoclonal antibody, mouse anti-β-actin and PCNA
monoclonal antibody were all purchased from Cell Signaling
Technology (Boston, MA, USA). Rabbit anti-Ki-67 antibody was
purchased from Abcam (Cambridge, MA, USA). Terminal
deoxynucleotidyl transferase-mediated dUTP nick-end labelling
(TUNEL) assay kit was purchased from Roche Diagnostics (Meylan,
France). Alanine aminotransferase (ALT), aspartate aminotransferase
(AST), alkaline phosphatase (AKP), gamma-glutamyltransferase (GGT),
creatinine (Cr) and blood urea nitrogen (BUN) colorimeter testing
kits were obtained from Nanjing Jiancheng Bioengineering Research
Institute (Nanjing, China).
Cell culture
The HCC cell line SMMC-7721 was obtained from the
Cell Bank of the Chinese Academy of Sciences Committee Type Culture
Collection (Shanghai, China) and cultured in DMEM (Thermo Fisher
Scientific, Shanghai, China) with 10% fetal bovine serum (FBS;
Gibco-Life Technologies, Carlsbad, CA, USA). The cells were
cultured in an incubator at 37°C in a humidified atmosphere of 5%
CO2 and sub-cultured when the cell density reached
80–90%.
Cell proliferation assay
Cell proliferation was measured with CCK-8 according
to the manufacturer's instructions. Briefly, SMMC-7721 cells were
seeded at a density of 3×103 cells/well in a 96-well
plate and cultured for 24 h. Emodin was then added to the wells
with final concentrations of 0, 10, 25, 50, 100 and 200
μmol/l and incubated for 12, 24 and 48 h. Before detecting
the absorbance, 10 μl CCK-8 was added to each well and
incubated for an additional 1.5 h at 37°C. Finally, the absorbance
at 450 nm was measured using a Multiskan Spectrum microplate reader
(Thermo Fisher Scientific, Waltham, MA, USA).
Flow cytometric analysis of apoptosis
assay
Cell apoptosis was detected using the Annexin V-FITC
apoptosis detection kit following the manufacturer's instructions.
In brief, SMMC-7721 cells were seeded into the 6-well plates with
3×105 cells in each well. After incubation at 37°C for
24 h, different concentrations (25, 50 and 100 μmol/l) of
emodin were added to the wells. Then, cells of each sample were
collected in a centrifuge tube after an additional 24 h. After
washed with phosphate-buffered saline (PBS), the cells were
suspended in 500 μl of Annexin V binding buffer (1X), 5
μl of Annexin V-FITC and propidium iodide (PI) were added
and incubated with for 15 min at room temperature away from the
light. The stained cells were analyzed by flow cytometry on
FACSCalibur (BD Biosciences, San Jose, CA, USA).
Western blot assay
After treatment with 100 μmol/l emodin for
indicated time, the cells were washed three times with ice-cold PBS
to stop the stimulation. Then, the cells were lysed in RIPA protein
lysis buffer containing 1 mM PMSF on ice. The cell lysates were
centrifuged at 12,000 × g for 15 min at 4°C and the supernatant was
collected as the total proteins and transferred to a new tube. The
protein concentration was determined using a BCA assay kit
(Beyotime Institute of Biotechnology, Shanghai, China) and equal
amounts of proteins (30 μg) were separated by 10%
SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to a
PVDF membrane. After blocked by Tris-buffered saline and Tween-20
(TBST) buffer containing 5% BSA for 1 h at room temperature, the
PVDF membrane was incubated with appropriate concentrations of
primary antibodies (dilution, 1:1,000) at 4°C overnight. After
washing the membrane with TBST three times for 15 min, the membrane
was incubated with corresponding secondary antibody labeled with
horseradish peroxidase-conjugated (goat anti-mouse, 1:5,000, goat
anti-rabbit, 1:2,000) for 2 h at room temperature. Following three
washes with TBST for 15 min, the immunoreactive bands were detected
using an enhanced chemiluminescence detection kit (Sigma). β-actin
was used as the internal control and the relative values of target
protein were corrected in accordance with the absorbency of the
internal control.
Antitumor activity in vivo
All work performed with animals was in accordance
with the Guide for the Care and Use of Laboratory Animals of the
National Institutes of Health and were approved by the Committee on
the Ethics of Animal Experiments of the Second Military Medical
University. Four-week-old male BALB/c-nu nude mice were obtained
from the Shanghai SIPPR-BK Laboratory Animal Co., Ltd., (Shanghai,
China) and maintained under standard conditions in the Laboratory
Animal Center of the Second Military Medical University. SMMC-7721
cells were harvested and resuspended in PBS. The mice were
subcutaneously inoculated with 5×106 cells into the
right flank. Tumor volume was calculated using the following
formula: Larger diameter × (smaller diameter) 2/2. The
mice were randomly divided into three groups (n=5) when the tumor
volume reached 75–100 mm3 and then treated every day for
two weeks with intraperitoneal injection of either vehicle (DMSO),
25 or 50 mg/kg emodin. Body weights were measured every week. The
mice were euthanized at the end of the experiment and the tumor
masses were removed and weighed.
The xenograft tumors were isolated and fixed in a
10% formalin solution, dehydrated and embedded in paraffin. The
samples were then sectioned at a 5-μm thickness and either
stained with hematoxylin and eosin (H&E) to reveal tumor tissue
necrosis or immunohistochemistry (IHC) stained using antibodies
against Ki-67 (1:400) or PCNA (1:500). The apoptosis of
paraffin-embedded sections of the tumors was detected by a TUNEL
assay kit according to the manufacturer's protocol. All slides were
detected under a phase-contrast microscope (magnification,
×200).
Function tests of the liver and
kidney
In other to determine the safety of emodin in
treating the nude mice bearing liver cancer, we collected 1 ml of
blood through eye-bleeding at the time of necropsy. The blood was
centrifuged at 3,000 rpm for 10 min to obtain sera and the sera
were analyzed for the levels of ALT, AST, AKP, GGT, Cr and BUN
using the respective colorimeter testing kits following the
manufacturer's protocol.
Statistical analysis
All experiments were performed at least three
independent times and the results are presented as the mean ±
standard deviation (SD). Statistical analysis was performed with
SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA). The statistical
analysis was performed using a one-way analysis of variance (ANOVA)
and Dunnett's test. P<0.05 was considered statistically
significant.
Results
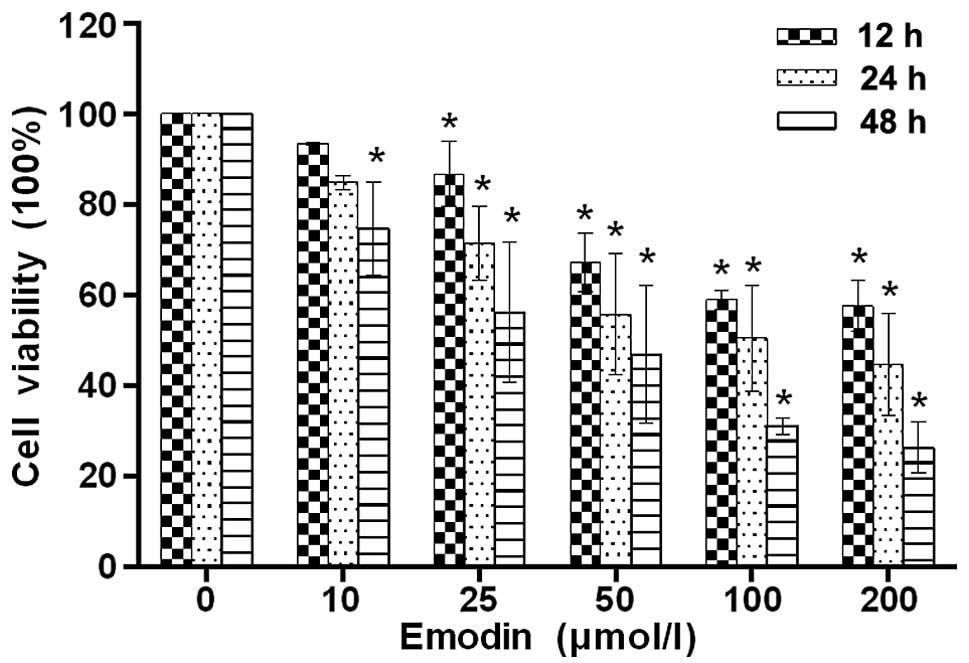
Emodin inhibits the proliferation of
SMMC-7721 cells
In order to investigate the anticancer activity of
emodin on liver cancer, we first investigated the effect of emodin
on the proliferation of SMMC-7721 cells using the CCK-8 assay. As
shown in Fig. 1, the viability of
the cells decreased evidently as the concentrations of emodin
increased from 10 to 200 μmol/l. In addition, emodin also
showed the antiproliferative effect on SMMC-7721 cells in a
time-dependent manner compared with the control.
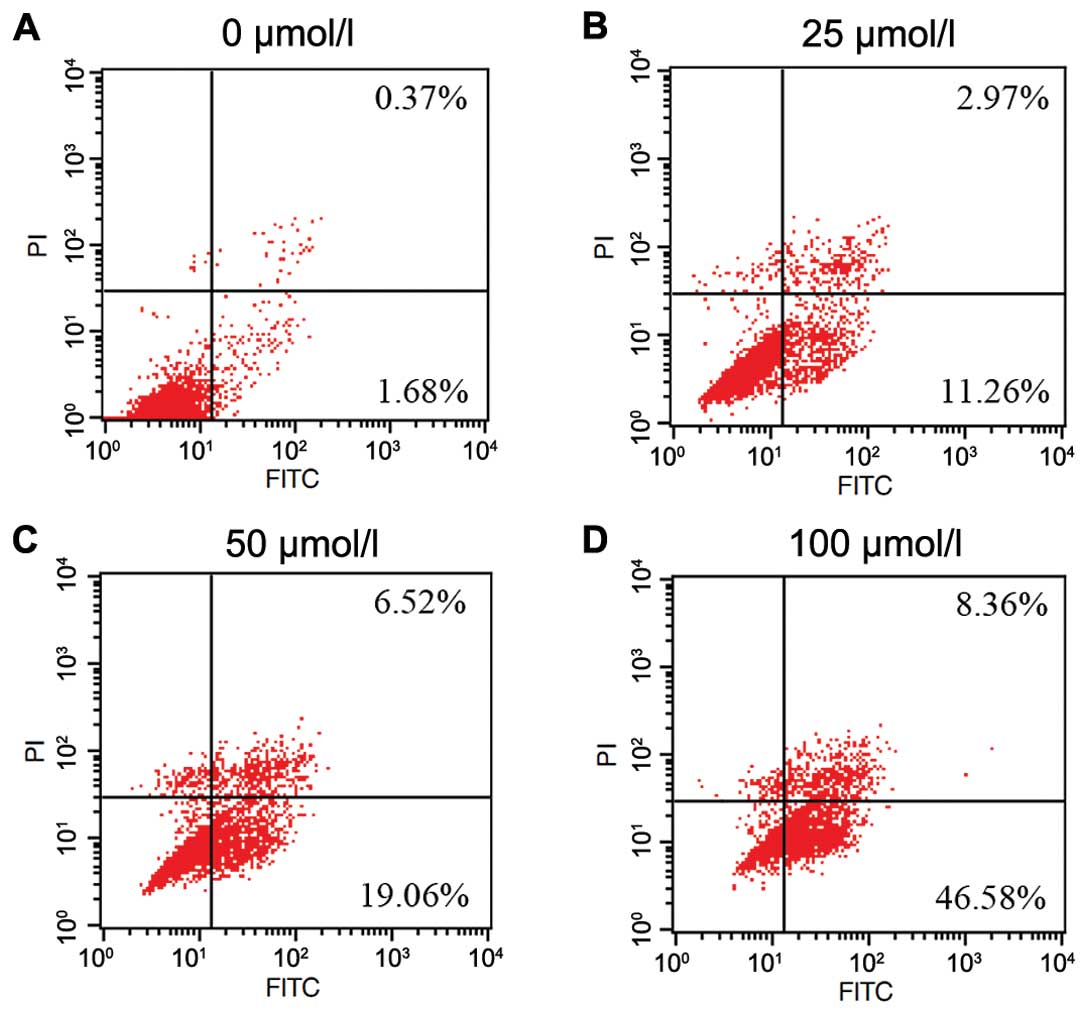
Emodin induced apoptosis of SMMC-7721
cells
To determine the effect of emodin on apoptosis
induction in SMMC-7721 cells, flow cytometry was used to assess the
effect after treatment with emodin at different concentrations (25,
50 and 100 μmol/l) for 24 h. As shown in Fig. 2, the percentage of apoptotic cells
(including early and late apoptotic cells) was shown to
significantly increase as the emodin concentration increased
(2.75±0.88, 17.40±2.81, 28.30±2.40 and 60.19±4.62, respectively).
These data clearly showed that emodin treatment may significantly
induce apoptosis of SMMC-7721 cells in a dose-dependent manner.
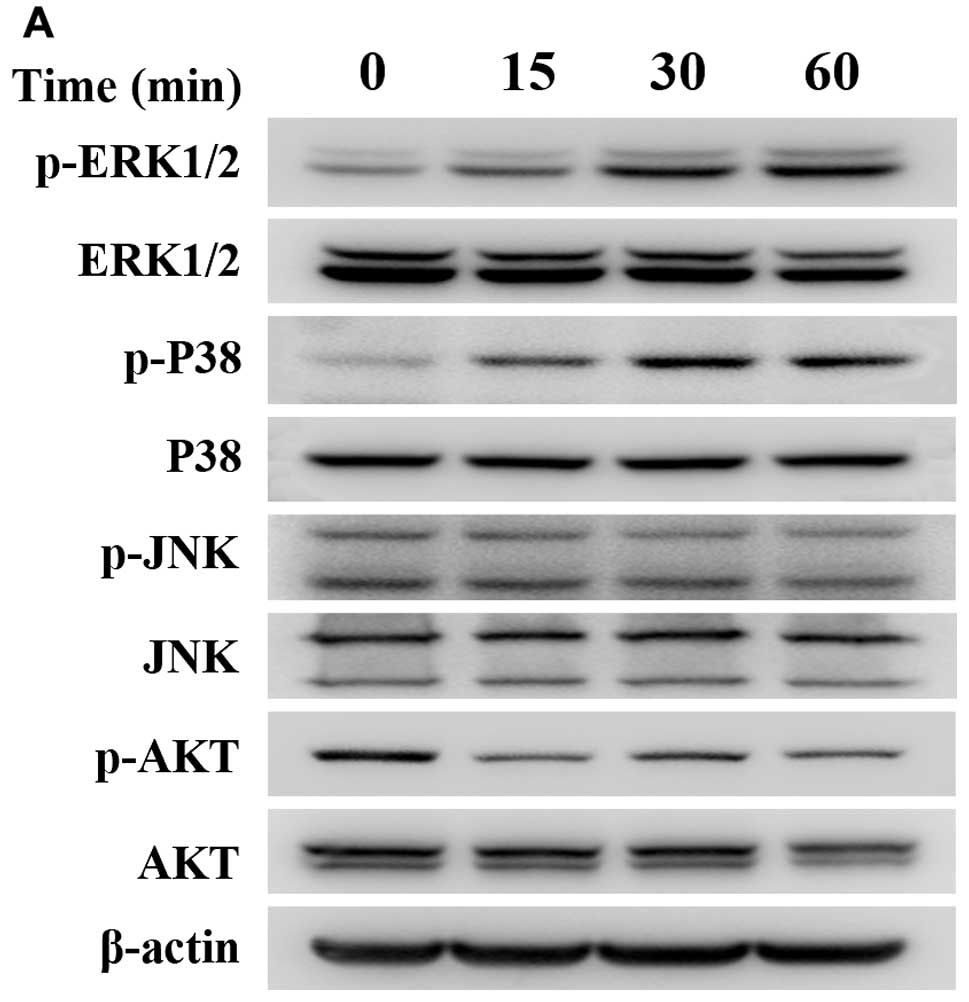
Effect of emodin on the expression of
proteins associated with tumor apoptosis
To further determine the probable mechanism(s)
underlying the decrease in cell viability caused by emodin, we used
western blotting to detect the protein expression of MAPK and
PI3K/AKT pathways since they act as key regulators of cellular
survival and apoptosis in various human cancers. The results
indicate that emodin may significantly promote the expression of
p-ERK and p-p38 after treatment of 100 μmol/l emodin for 0,
15, 30 and 60 min, respectively. However, the protein lever of
p-JNK was suppressed mildly by emodin after treatment for more than
30 min. Whereas, the total ERK, p38 and JNK remained unchanged. On
the other hand, emodin also inhibited the expression of p-AKT but
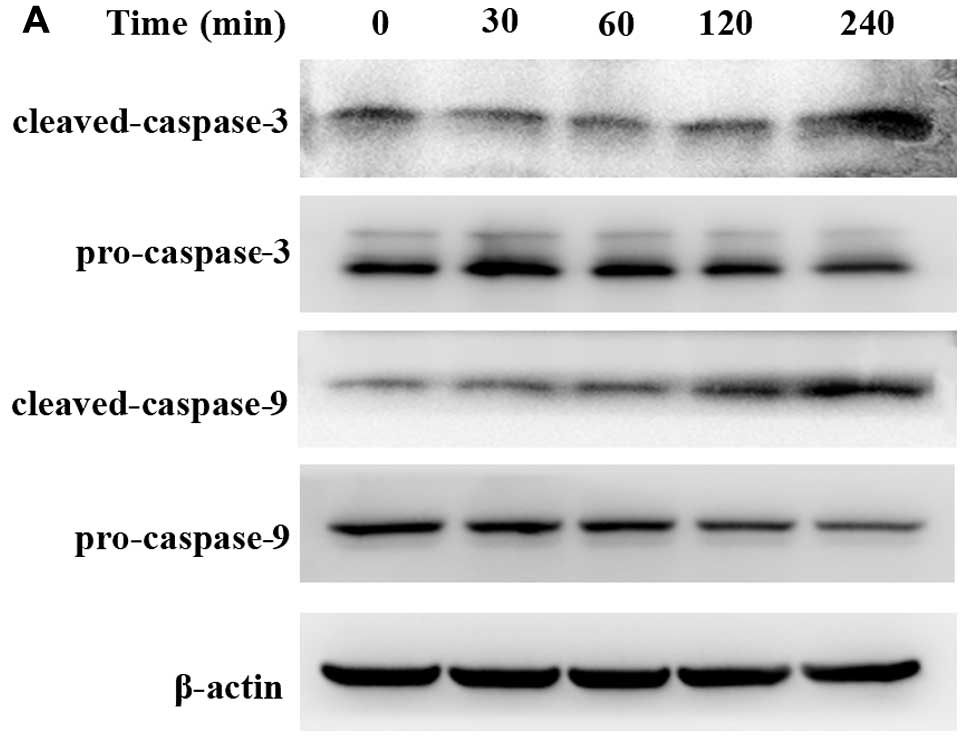
did not affect the total AKT under the same condition (Fig. 3). In addition, because caspase
activation is considered to be a hallmark of apoptosis, we further
examined and found that cleaved caspase-3 and cleaved caspase-9
were clearly increased in emodin-treated cells in a time-dependent
manner (30, 60, 120 and 240 min). However, pro-caspase-3 and -9
were mildly decreased with treatment of 100 μmol/l emodin
after 120 min (Fig. 4).
Emodin inhibits the growth of SMMC 7721
cell xenografts in nude mice
Based on the above in vitro results, we next
investigated whether emodin has the potential anticancer effect
in vivo by using a xenograft tumor model of liver cancer. As
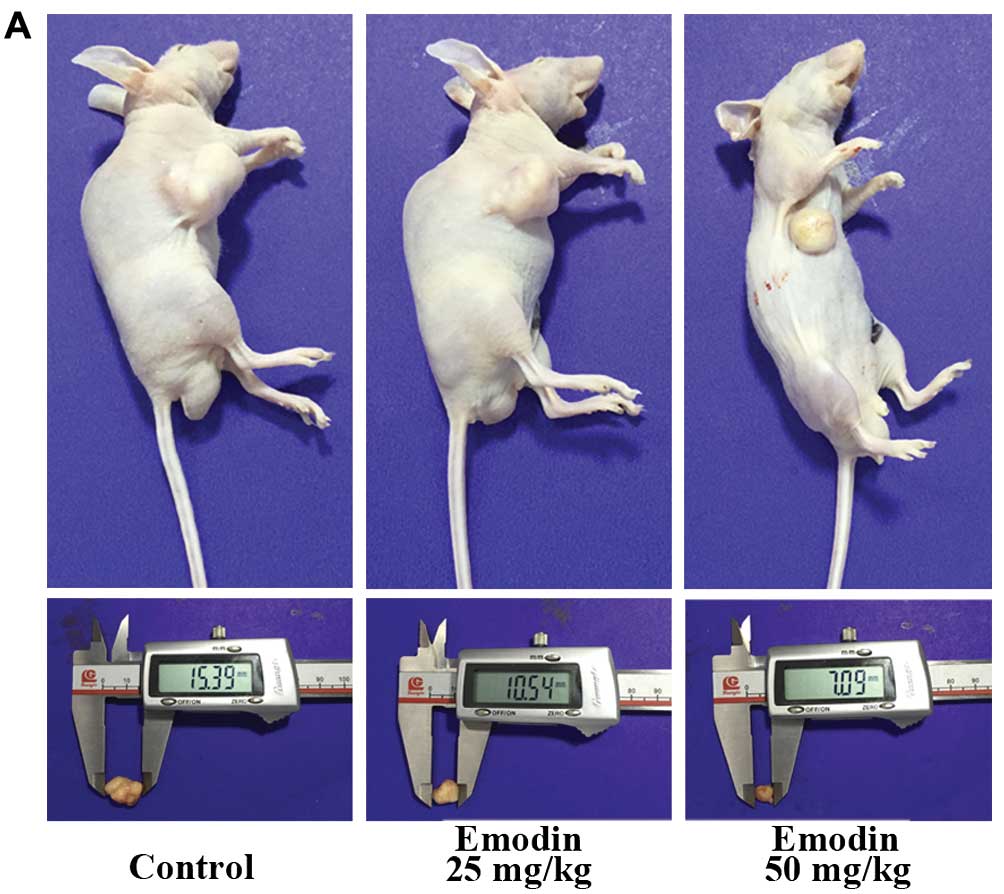
expected, emodin suppressed the tumor growth in a dose-dependent
manner, while little influence on the body weight of mice was
observed (Fig. 5). To detect cell
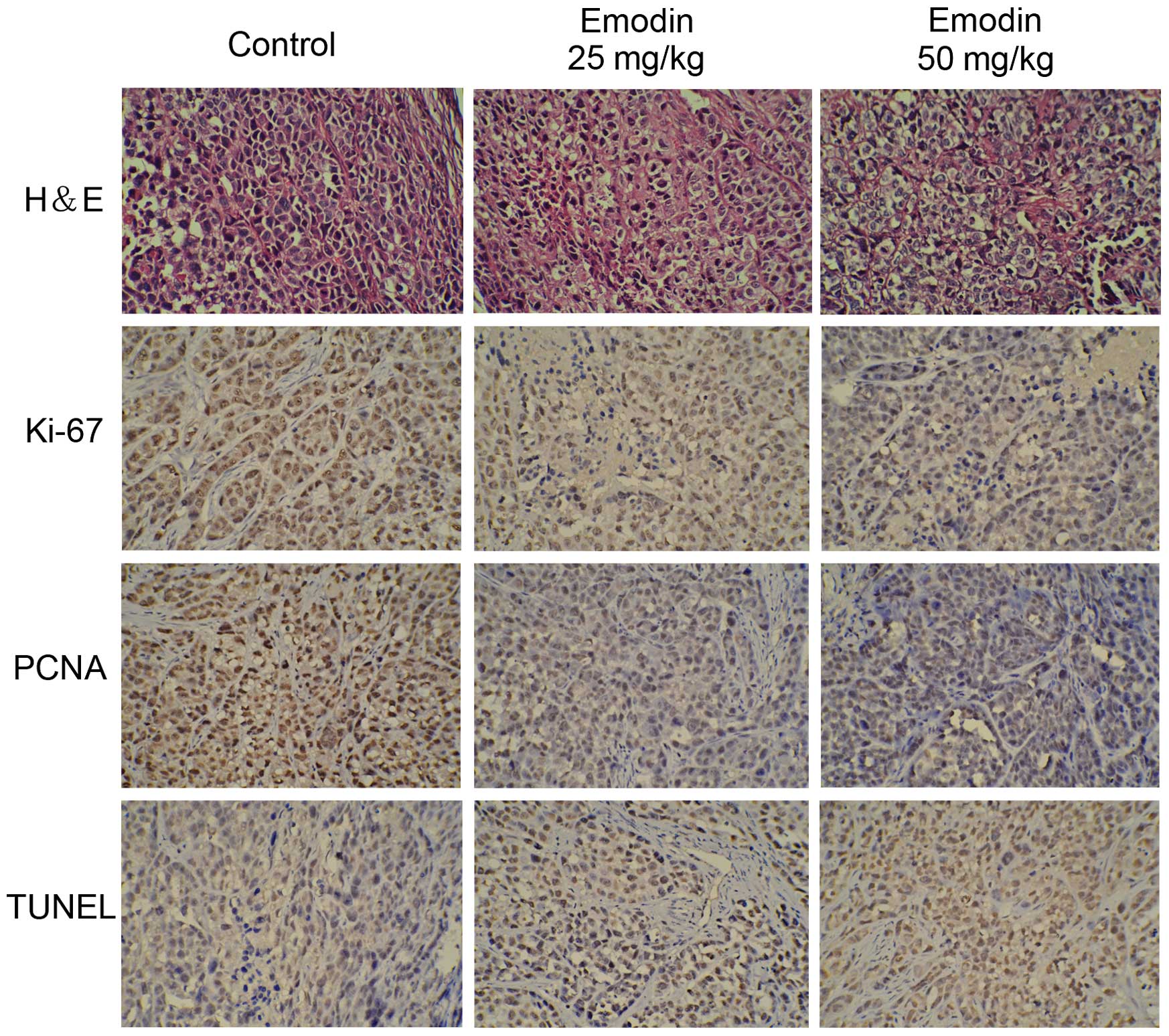
necrosis and the expression of Ki-67 and PCNA in tumor tissues,
H&E staining and IHC analysis were performed. H&E staining
showed that emodin may induced cell death and caused symptoms of
necrosis in the tumor masses. In IHC analysis, Ki-67 and PCNA,
which are markers of cell proliferation, showed significant
reduction with treatment of emodin in a dose-dependent manner.
Moreover, TUNEL staining was used to detect the apoptosis of the
tumor sections and the result indicated that the apoptotic index
was significantly increased as determined by the percentage of
TUNEL stained nuclei (Fig. 6).
Emodin treatment improves the liver and
kidney function in mice
Previous studies have shown that emodin may inhibit
lung metastasis in mice with no obvious changes in liver and kidney
functions (14). In the present
study, we also demonstrated that emodin does not cause obvious
toxicity in nude mice since there were no obvious changes in their
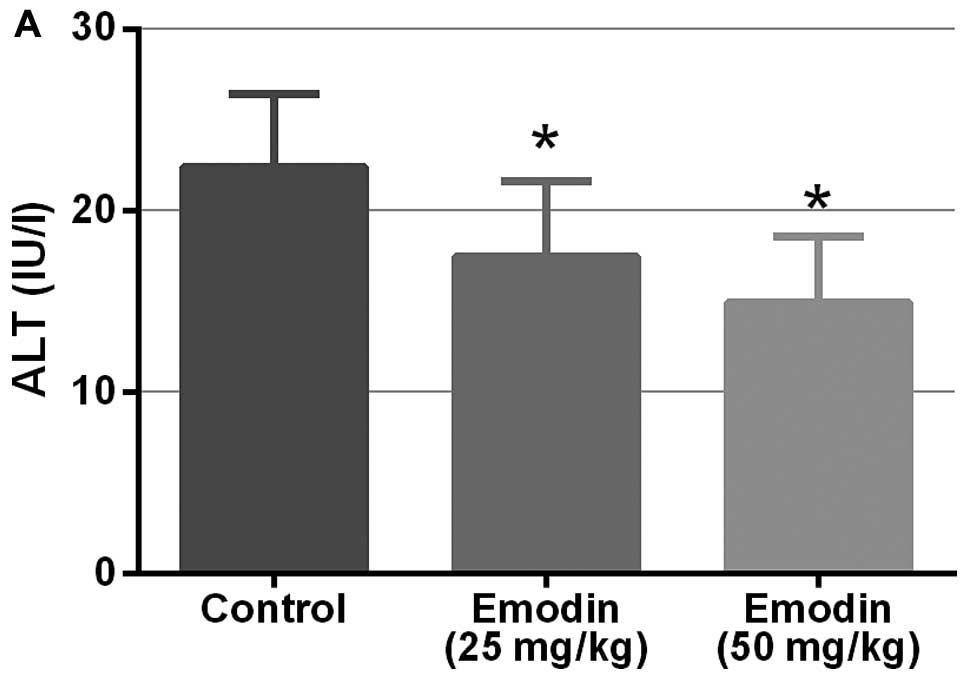
body weight. Furthermore, as showed in Fig. 7, emodin may decrease the levels of
serum ALT, AST, AKP, GGT, Cr and BUN, which indicated the
improvement of the liver and kidney function in mice.
Discussion
Despite increasing progress in treatment methods in
recent years, the prognosis of HCC has not substantially improved
since many patients were detected in an advanced stage when there
are limited therapeutic options. Sorafenib is the only drug for
treating advanced HCC that has been approved by the USA Food and
Drug Administration in the past decade (15). However, many patients may not
benefit from this drug due to its side-effects and rapidly
development of drug resistance. Therefore, it is still imperative
to identify novel drugs for HCC treatment.
Increased evidence shows that many Chinese herbs
have antitumor properties and induction of apoptosis is one of the
mechanisms (5). Emodin, which was
extracted from traditional Chinese medicine Rheum palmatum
L, was found effective in suppressing cancer proliferation,
invasion and metastasis in different types of cancer (16). Although Subramaniam et al
(17) have demonstrated that emodin
may inhibit growth and induce apoptosis of HCC in vitro and
in vivo through the inhibition of the STAT3 signaling
cascade, further studies of the underlying molecular target and
mechanism are still necessary.
In the present study, we assessed and validated the
efficacy of emodin on HCC in vitro and in vivo.
Emodin inhibited the proliferation of SMMC-7721 cells in a dose-and
time-dependent manner and induced apoptosis of cells in a
concentration-dependent manner after treatment for 24 h. In
vivo, we found that emodin may suppress the tumor growth in
experimental mice without an obvious change in body weight, which
may work through the antiproliferation and apoptosis inducing
effects. Moreover, emodin may also improve the liver and kidney
function in mice, revealing that emodin may improve the quality of
life of the mice with implanted tumors. Thus, these findings
indicate that emodin may be a potential effective and safe drug to
induce apoptosis of HCC.
Mitogen-activated protein kinase (MAPK) is an
important signaling pathway, which is mainly composed of three
subfamilies such as ERK, p38 and JNK signaling pathways (18,19).
Significant attention has been focused on the important role of the
MAPK pathway since it is critically involved in tumor cell
proliferation, apoptosis, invasion and tumor metastasis (14,20).
Thus, we investigated whether emodin could mediate its anticancer
effects in part through the MAPK signaling pathway by detecting the
protein expression of ERK, p38 and JNK and their phosphorylation
events. We found that emodin induced the activation of ERK and p38
via promoting their phosphorylation, which is contrary to some
cases that activation of the ERK or p38 pathway has been associated
with proliferation and the apoptotic signaling pathways of HCC
(21,22). Nevertheless, the involvement of ERK
or p38 MAPK pathway in the proliferation and apoptosis remains
somewhat controversial. In an experiment performed by Zhao et
al (23), they found that
Methyl CpG-binding protein 2 (MeCP2) promotes cell proliferation by
activating ERK1/2 and inhibiting p38 activity in HCC. Lu et
al (24) also discovered that
the suppression of AAA domain containing 2 (ATAD2) increased
interactions of MKK3/6 with p38 that led to p38 activation and
subsequently apoptosis. Emodin may also induce apoptosis of
colorectal cancer cells through activating p53/p38/Puma pathway by
triggering ROS production (25).
However, according to the study by Liu et al (26), aspafilioside B may induce G2 phase
arrest and apoptosis by upregulate ERK and p38 phosphorylation,
which is consistent with our result that the activation of ERK and
p38 may induce apoptosis of HCC. We think the roles of the ERK and
p38 are partly associated with cell type. On the contrary, JNK
signaling pathway was considered to induce HCC cell cycle arrest
and induced HCC apoptosis (27). In
the present study we also found that emodin may suppress the
activation of JNK mildly, this indicates the JNK pathway may not
play a major role in emodin's effect.
The phosphoinositide 3-kinase (PI3K) signaling
cascade is another critical pathway in cancer as it promotes cell
survival and growth (28,29). According to the clinical research,
p-AKT was higher in tumor (53%) than in cirrhotic tissues (12%)
while it was absent in normal liver. Inhibitors of this pathway are
under active development as anticancer therapeutics (30). As expected, the phosphorylation of
AKT was suppressed by emodin in a time-dependent manner, which may
reveal partly the possible mechanism of apoptosis inducing effect
of emodin on HCC.
In summary, the present study demonstrated that
emodin inhibited proliferation and induced apoptosis of HCC in
vitro and in vivo. The mechanisms may be transduced
through MAPK and PI3K/AKT signaling pathways. Our results shed some
light on the mechanisms behind the effect of emodin on HCC and
suggested that emodin could be a potential safe candidate for the
treatment of HCC due to its high efficacy and less systemic
side-effects.
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
MAPK
|
mitogen-activated protein kinases
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
|
ERK
|
extracellular signal regulated
kinase
|
|
JNK
|
c-Jun-N-terminal kinase
|
|
ALT
|
alanine aminotransferase
|
|
AST
|
aspartate aminotransferase
|
|
AKP
|
alkaline phosphatase
|
|
GGT
|
gamma-glutamyltransferase
|
|
Cr
|
creatinine
|
|
BUN
|
blood urea nitrogen
|
Acknowledgments
The present study was supported by the National
Nature Science Foundation of China (grant no. 30901986), the
Foundation for the Author of National Excellent Doctoral
Dissertation of China (grant no. 201366), the Shanghai Rising-Star
Program (grant no. 15QA1404800) and the Shanghai Municipal Natural
Science Foundation (grant no. 13ZR1448900).
References
|
1
|
Fitzmaurice C, Dicker D, Pain A, Hamavid
H, Moradi-Lakeh M, MacIntyre MF, Allen C, Hansen G, Woodbrook R,
Wolfe C, et al Global Burden of Disease Cancer Collaboration: The
Global Burden of Cancer 2013. JAMA Oncol. 1:505–527. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maluccio M and Covey A: Recent progress in
understanding, diagnosing, and treating hepatocellular carcinoma.
CA Cancer J Clin. 62:394–399. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ling CQ, Yue XQ and Ling C: Three
advantages of using traditional Chinese medicine to prevent and
treat tumor. J Integr Med. 12:331–335. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Block KI, Gyllenhaal C, Lowe L, Amedei A,
Amin AR, Amin A, Aquilano K, Arbiser J, Arreola A, Arzumanyan A, et
al: Designing a broad-spectrum integrative approach for cancer
prevention and treatment. Semin Cancer Biol. 35(Suppl): S276–S304.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu H, Zhao X, Liu X, Xu P, Zhang K and Lin
X: Antitumor effects of traditional Chinese medicine targeting the
cellular apoptotic pathway. Drug Des Devel Ther. 9:2735–2744.
2015.PubMed/NCBI
|
|
6
|
Wang X, Wang N, Cheung F, Lao L, Li C and
Feng Y: Chinese medicines for prevention and treatment of human
hepatocellular carcinoma: Current progress on pharmacological
actions and mechanisms. J Integr Med. 13:142–164. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qu W, Wang Y, Wu Q, Liu J and Hao D:
Emodin inhibits HMGB1-induced tumor angiogenesis in human
osteosarcoma by regulating SIRT1. Int J Clin Exp Med.
8:15054–15064. 2015.PubMed/NCBI
|
|
8
|
Ma L and Li W: Emodin inhibits LOVO
colorectal cancer cell proliferation via the regulation of the
Bcl-2/Bax ratio and cytochrome c. Exp Ther Med. 8:1225–1228.
2014.PubMed/NCBI
|
|
9
|
Xie MJ, Ma YH, Miao L, Wang Y, Wang HZ,
Xing YY, Xi T and Lu YY: Emodin-provoked oxidative stress induces
apoptosis in human colon cancer HCT116 cells through a
p53-mitochondrial apoptotic pathway. Asian Pac J Cancer Prev.
15:5201–5205. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yaoxian W, Hui Y, Yunyan Z, Yanqin L, Xin
G and Xiaoke W: Emodin induces apoptosis of human cervical cancer
HeLa cells via intrinsic mitochondrial and extrinsic death receptor
pathway. Cancer Cell Int. 13:712013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun ZH and Bu P: Downregulation of
phosphatase of regenerating liver-3 is involved in the inhibition
of proliferation and apoptosis induced by emodin in the SGC-7901
human gastric carcinoma cell line. Exp Ther Med. 3:1077–1081.
2012.PubMed/NCBI
|
|
12
|
Jia X, Yu F, Wang J, Iwanowycz S, Saaoud
F, Wang Y, Hu J, Wang Q and Fan D: Emodin suppresses pulmonary
metastasis of breast cancer accompanied with decreased macrophage
recruitment and M2 polarization in the lungs. Breast Cancer Res
Treat. 148:291–302. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Manu KA, Shanmugam MK, Ong TH, Subramaniam
A, Siveen KS, Perumal E, Samy RP, Bist P, Lim LH, Kumar AP, et al:
Emodin suppresses migration and invasion through the modulation of
CXCR4 expression in an orthotopic model of human hepatocellular
carcinoma. PLoS One. 8:e570152013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun Y, Wang X, Zhou Q, Lu Y, Zhang H, Chen
Q, Zhao M and Su S: Inhibitory effect of emodin on migration,
invasion and metastasis of human breast cancer MDA-MB-231 cells in
vitro and in vivo. Oncol Rep. 33:338–346. 2015.
|
|
15
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al SHARP Investigators Study Group: Sorafenib in advanced
hepatocellular carcinoma. N Engl J Med. 359:378–390. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wei WT, Lin SZ, Liu DL and Wang ZH: The
distinct mechanisms of the antitumor activity of emodin in
different types of cancer (Review). Oncol Rep. 30:2555–2562.
2013.PubMed/NCBI
|
|
17
|
Subramaniam A, Shanmugam MK, Ong TH, Li F,
Perumal E, Chen L, Vali S, Abbasi T, Kapoor S, Ahn KS, et al:
Emodin inhibits growth and induces apoptosis in an orthotopic
hepatocellular carcinoma model by blocking activation of STAT3. Br
J Pharmacol. 170:807–821. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aroui S, Aouey B, Chtourou Y, Meunier AC,
Fetoui H and Kenani A: Naringin suppresses cell metastasis and the
expression of matrix metalloproteinases (MMP-2 and MMP-9) via the
inhibition of ERK-P38-JNK signaling pathway in human glioblastoma.
Chem Biol Interact. 244:195–203. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang M and Huang CZ: Mitogen-activated
protein kinase signaling pathway and invasion and metastasis of
gastric cancer. World J Gastroenterol. 21:11673–11679. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang SH, Sharrocks AD and Whitmarsh AJ:
MAP kinase signalling cascades and transcriptional regulation.
Gene. 513:1–13. 2013. View Article : Google Scholar
|
|
21
|
Liu Y, Bi T, Dai W, Wang G, Qian L, Gao Q
and Shen G: Oxymatrine synergistically enhances the inhibitory
effect of 5-fluorouracil on hepatocellular carcinoma in vitro and
in vivo. Tumour Biol. Dec 18–2015.Epub ahead of print.
|
|
22
|
Chan LK, Chiu YT, Sze KM and Ng IO:
Tensin4 is up-regulated by EGF-induced ERK1/2 activity and promotes
cell proliferation and migration in hepatocellular carcinoma.
Oncotarget. 6:20964–20976. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao LY, Zhang J, Guo B, Yang J, Han J,
Zhao XG, Wang XF, Liu LY, Li ZF, Song TS, et al: MECP2 promotes
cell proliferation by activating ERK1/2 and inhibiting p38 activity
in human hepatocellular carcinoma HEPG2 cells. Cell Mol Biol
(Noisy-legrand). (Suppl 59): OL1876–OL1881. 2013.
|
|
24
|
Lu WJ, Chua MS and So SK: Suppression of
ATAD2 inhibits hepatocellular carcinoma progression through
activation of p53- and p38-mediated apoptotic signaling.
Oncotarget. 6:41722–41735. 2015.PubMed/NCBI
|
|
25
|
Liu B, Yuan B, Zhang L, Mu W and Wang C:
ROS/p38/p53/Puma signaling pathway is involved in emodin-induced
apoptosis of human colorectal cancer cells. Int J Clin Exp Med.
8:15413–15422. 2015.PubMed/NCBI
|
|
26
|
Liu W, Ning R, Chen RN, Huang XF, Dai QS,
Hu JH, Wang YW, Wu LL, Xiong J, Hu G, et al: Aspafilioside B
induces G2/M cell cycle arrest and apoptosis by up-regulating H-Ras
and N-Ras via ERK and p38 MAPK signaling pathways in human hepatoma
HepG2 cells. Mol Carcinog. 55:440–457. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang C, Zhang J, Li X, Sun N, Yu R, Zhao
B, Yu D, Cheng Y and Liu Y: Huaier aqueous extract induces
hepatocellular carcinoma cells arrest in S phase via JNK signaling
pathway. Evid Based Complement Alternat Med. 2015:1713562015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wong KK, Engelman JA and Cantley LC:
Targeting the PI3K signaling pathway in cancer. Curr Opin Genet
Dev. 20:87–90. 2010. View Article : Google Scholar :
|
|
29
|
Engelman JA: Targeting PI3K signalling in
cancer: Opportunities, challenges and limitations. Nat Rev Cancer.
9:550–562. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kunter I, Erdal E, Nart D, Yilmaz F,
Karademir S, Sagol O and Atabey N: Active form of AKT controls cell
proliferation and response to apoptosis in hepatocellular
carcinoma. Oncol Rep. 31:573–580. 2014.
|