Introduction
5-Aminolevulinic acid (5-ALA) is a natural metabolic
precursor in the heme biosynthesis pathway. Oral administration of
a large volume of 5-ALA overloads the heme pathway and induces the
synthesis and selective accumulation of the fluorescent
protoporphyrin IX (PpIX) in tumor cells and epithelial tissue
(1,2). Increased vascular permeability
attributable to disruption of the blood-brain barrier (BBB) in the
tumor and decreased levels of ferrochelatase activity in tumor
cells contribute to this phenomenon in glioblastoma multiforme
(GBM) (3–6). PpIX in GBM tumors, present at higher
levels compared to normal brain tissue, emits red-violet
fluorescence under blue light, visually distinguishing tumor
margins and allowing intraoperative, objective assessment of tumor
infiltration (7,8). Evidence demonstrating the high
sensitivity and specificity of 5-ALA-induced PpIX fluorescence in
GBM provides support for the diagnostic accuracy of 5-ALA. As a
result, 5-ALA fluorescence guidance has come to be widely used to
improve the extent of tumor resection (9).
Since a number of studies have demonstrated that the
extent of resection (EOR) is correlated with improved prognosis of
patients with GBM, neurosurgeons have sought to maximize the EOR,
an objective constrained by the difficulties of resection arising
from the invasive nature of GBM (10–15). A
large, randomized, controlled, multicenter phase III trial has
shown that 5-ALA fluorescence-guided surgery for malignant glioma
leads to a significantly higher resection rate, resulting in
prolonged progression-free survival compared with conventional
microsurgery guided by white light (16). In addition, several techniques,
including intraoperative neuronavigation (17), intraoperative magnetic resonance
(MR) imaging (18), intraoperative
ultrasound (19) and intraoperative
electrical stimulation (20), have
been introduced to facilitate optimal resection, thereby maximizing
safe EOR and improving survival in patients with GBM. Increasing
the EOR of GBM to achieve these survival benefits brings with it a
greater chance of entering the ventricular system during the course
of cytoreduction. In some cases of 5-ALA-guided GBM surgery, upon
ventricular entry we encountered fluorescence in ventricular walls
that lacked enhancement on MR images and were free of macroscopic
invasion of tumor cells. However, the implications of this
5-ALA-induced fluorescence of the ventricle wall are not yet fully
understood.
In this study, we obtained 25 ventricular wall
tissues from 19 patients with newly diagnosed GBM that showed no
enhancement on MR images, and investigated the relationship between
intraoperative 5-ALA fluorescence and histopathology findings of
these non-enhancing ventricular wall tissues.
Materials and methods
Patient information
Nineteen patients who underwent fluorescence-guided
surgery with 5-ALA for newly diagnosed GBM at our hospital from
December 2012 to May 2015 were included in this study. Approval was
given by the Institutional Review Board of Severance Hospital,
Yonsei University College of Medicine. Informed consent was
provided according to the Declaration of Helsinki. Of these 19
patients, 12 were males and 7 were females, and their age ranged
from 45 to 74 years (mean, 58.5 years). All patients were newly
diagnosed with GBM, and had no prior history of treatment with
surgery, chemotherapy, or radiotherapy. All tumors showed typical
enhancement patterns of GBM in MR images after the administration
of contrast medium. In all cases, the ventricle was opened during
resection of the tumor, which was located near the lateral
ventricle. The characteristics of the 19 patients are summarized in
Table I.
 | Table IClinical and molecular
characteristics of the 19 patients with glioblastoma
multiforme. |
Table I
Clinical and molecular
characteristics of the 19 patients with glioblastoma
multiforme.
| Case no. | Age (years),
gender | Tumor location | Extent of
resection | Tumor contact to
lateral ventricle | IDH1 mutation | EGFR | p53 mutation
(%) | Ki-67 LI (%) | MGMT promoter
methylation | 1p LOH/19q LOH |
|---|
| 1 | 61, M | Rt. FT | Subtotal | Yes | No | 2+ | 40 | 60 | Unmethylated | No/Yes |
| 2 | 66, F | Rt. TP | Total | Yes | No | 3+ | 3 | 50 | Unmethylated | Yes/Yes |
| 3 | 61, M | Rt. temporal | Total | Yes | No | 3+ | 5 | 20 | Unmethylated | Yes/No |
| 4 | 70, F | Rt. TPO | Subtotal | Yes | No | 3+ | Negative | 30 | Methylated | No/No |
| 5 | 62, F | Rt. frontal | Supratotal | No | No | 3+ | Negative | 5 | Methylated | No/No |
| 6 | 53, M | Lt. temporal | Total | Yes | No | 3+ | 2 | 60 | Unmethylated | No/No |
| 7 | 45, M | Rt. frontal | Total | Yes | No | 3+ | Negative | 50 | Methylated | Yes/No |
| 8 | 45, M | Rt. TPO | Subtotal | No | No | 3+ | 30 | 40 | Unmethylated | No/No |
| 9 | 58, F | Lt. TP | Total | Yes | No | 2+ | 60 | 30 | Methylated | Yes/Yes |
| 10 | 56, M | Rt. temporal | Supratotal | Yes | No | 1+ | 2 | 5 | Methylated | No/No |
| 11 | 51, M | Lt. FT | Subtotal | Yes | No | 2+ | 40 | 15 | Unmethylated | No/No |
| 12 | 51, M | Lt. frontal | Total | Yes | Yes | 0 | 25 | 7 | Methylated | Yes/Yes |
| 13 | 62, F | Rt. temporal | Subtotal | Yes | No | 1+ | 5 | 20 | Unmethylated | No/No |
| 14 | 58, F | Lt. TP | Total | Yes | No | 3+ | Negative | 3 | Unmethylated | No/No |
| 15 | 67, M | Rt. TPO | Subtotal | Yes | No | 3+ | 90 | 40 | Methylated | Yes/Yes |
| 16 | 74, M | Lt. temporal | Subtotal | Yes | No | 1+ | 70 | 30 | Unmethylated | No/No |
| 17 | 65, F | Rt. temporal | Total | No | No | 0 | 1 | 3 | Unmethylated | No/No |
| 18 | 46, M | Rt. temporal | Total | No | No | 3+ | 5 | 20 | Unmethylated | No/No |
| 19 | 60, M | Rt. PO | Supratotal | Yes | No | 3+ | 20 | 25 | Unmethylated | No/No |
Surgical procedure
Three hours prior to induction of anesthesia, 5-ALA
(Gliolan; Photonamic GmbH & Co. KG, wedel, Germany) was
administered orally at a dose of 20 mg/kg body weight. Patients
were protected from direct exposure to light sources for 24 h after
intake of 5-ALA to avoid skin phototoxicity. Preoperative,
high-resolution, contrast-enhanced, T1-weighted axial MR images
were obtained for each patient on the day of the procedure. All
tumor resections were performed under the guidance of a
neuronavigation system [StealthStation Treon (Medtronic,
Minneapolis, MN, USA) or Stryker (Stryker Instruments, Kalamazoo,
MI, USA)] using the MR images. Additional functional MR images and
diffusion tensor images were used as appropriate, depending on
tumor location. Zeiss OPMI Pentero microscopes (Carl Zeiss Surgical
GmbH, Oberkochen, Germany) equipped with BLUE 400 fluorescence
technology, which enabled switching from conventional standard
white xenon light to filtered violet-blue excitation light for
visualization of fluorescence, were used in all patients. In all
cases, the tumor was resected to the extent possible consistent
with safety, and supratotal resection was performed in some
patients with a tumor in a non-eloquent area. After the opening of
lateral ventricles, the fluorescence of the ventricular wall was
examined with a microscope by switching between white light and
violet-blue excitation light. Regions were annotated as
non-visible, weak, or strong fluorescence for 5-ALA-induced
fluorescence by the operating neurosurgeon, and samples of these
regions were collected for histopathological analysis. Before
sampling, regions of ventricular walls were checked macroscopically
for tumor involvement and confirmed with the aid of the
neuronavigation system. Tumor involvement was defined as the
presence of macroscopic invasion of tumor or enhancement on the
preoperative contrast-enhanced T1-weighted MR images used for
neuronavigation.
Histopathological analysis
Specimens from patients with GBM were freshly
obtained from the operating room. Samples of ventricular walls for
histopathological analysis were categorized according to the
presence or absence of 5-ALA-induced fluorescence by the operating
neurosurgeon and were forwarded to the neuropathology department.
Histopathological analyses were performed on hematoxylin and eosin
(H&E) -stained, formalin-fixed, paraffin-embedded tissues from
the main tumor mass and ventricular wall. Immunohistochemical
staining for glial fibrillary acidic protein (GFAP), the
proliferation marker Ki-67, epidermal growth factor receptor
(EGFR), and p53 was also carried out to establish a definitive
diagnosis. O6-methylguanine DNA methyltransferase (MGMT)
promoter methylation status and isocitrate dehydrogenase 1 (IDH1)
mutations were analyzed by polymerase chain reaction (PCR), and
loss of heterozygosity (LOH) at chromosomes 1p and 19q was
determined by fluorescent in situ hybridization. One
experienced neuropathologist diagnosed the type and grade of each
sample based on the World Health Organization (WHO) 2007 grading
criteria (21). To reduce bias, the
neuropathologist was blinded to the 5-ALA fluorescence status.
Samples in which tumor cells could not be identified and where
increased levels of proliferation were not detectable were
considered tumor-free.
Results
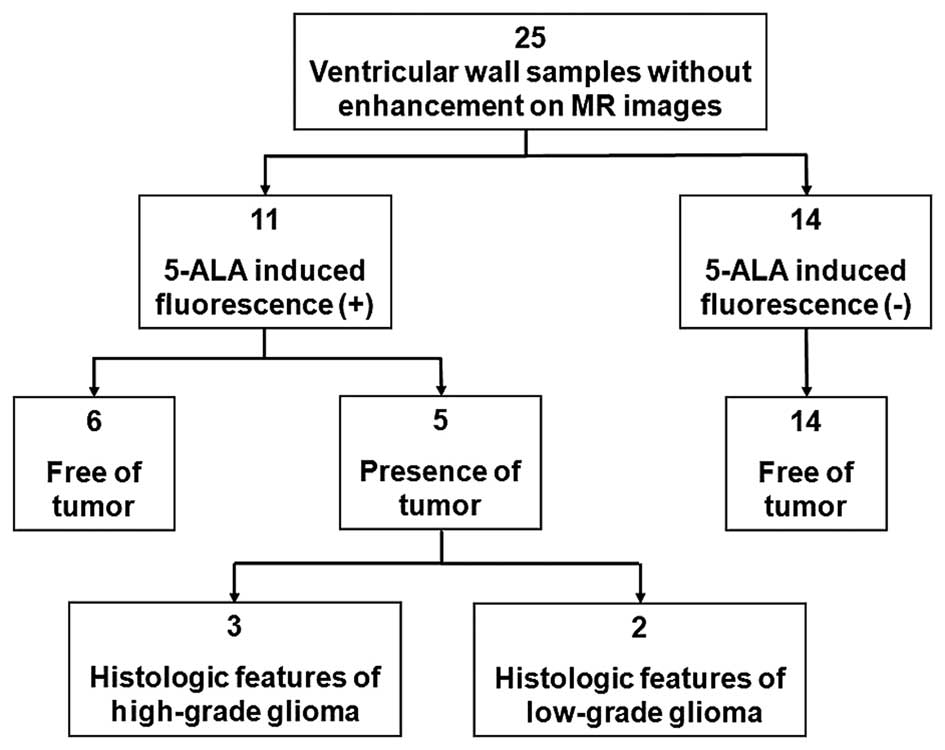
Ventricle wall fluorescence and sample
collection
In each case, the tumor was clearly revealed in the
surgical field and exhibited 5-ALA-induced fluorescence under blue
light. Seventeen cases showed strong fluorescence of the main
tumors and 2 cases showed weak fluorescence of the main tumors.
5-ALA-induced fluorescence in the ventricular wall was identified
in 11 patients (57.9%) after opening the lateral ventricle. The
fluorescent areas of the ventricular wall varied from one case to
another. In these 11 patients, 5 showed strong fluorescence of the
ventricular walls and 6 showed weak fluorescence of the ventricular
walls. However, there was no correlation between the fluorescence
intensity of the ventricular wall and that of the main tumors. A
total of 25 samples of the ventricular wall, which is divided into
5 regions (anterior horn, body, atrium, occipital horn, and
temporal horn) along the rostrocaudal axis (22), were collected intraoperatively from
the 19 GBM patients: five from the anterior horn, one from the
atrium, 2 from the occipital horn, and 12 from the temporal horn.
Of these 25 samples, 11 showed observable intraoperative
5-ALA-induced fluorescence, whereas 14 samples did not (Table II). In all cases, the ventricular
wall areas sampled showed no macroscopic evidence for tumor
involvement and were free of enhancement on MR images.
 | Table II5-ALA fluorescence characteristics
and pathological findings of the 19 patients with glioblastoma
multiforme. |
Table II
5-ALA fluorescence characteristics
and pathological findings of the 19 patients with glioblastoma
multiforme.
| Case no. | Ventricular wall
sampling site | 5-ALA fluorescence
of tumor | Ventricular wall
tissue
| Presence of tumor
cells |
|---|
| No. of samples | 5-ALA
fluorescence |
|---|
| 1 | Temporal horn | Strong | 1 | Strong | Yes
(low-grade) |
| 2 | Temporal horn | Strong | 1 | Strong | No |
| 3 | Temporal horn | Strong | 2 | Weak | Yes
(low-grade) |
| | | | Non-visible | No |
| 4 | Temporal horn | Strong | 2 | Strong | Yes
(high-grade) |
| | | | Non-visible | No |
| 5 | Anterior horn | Strong | 1 | Non-visible | No |
| 6 | Temporal horn | Strong | 2 | Weak | No |
| | | | Non-visible | No |
| 7 | Anterior horn | weak | 1 | Non-visible | No |
| 8 | Occipital horn | Strong | 1 | Weak | No |
| 9 | Temporal horn | weak | 1 | Non-visible | No |
| 10 | Temporal horn | Strong | 2 | Weak | No |
| | | | Non-visible | No |
| 11 | Anterior horn | Strong | 1 | Non-visible | No |
| 12 | Anterior horn | Strong | 1 | Non-visible | No |
| 13 | Temporal horn | Strong | 2 | Weak | Yes
(high-grade) |
| | | | Non-visible | No |
| 14 | Atrium | Strong | 1 | Strong | Yes
(high-grade) |
| 15 | Temporal horn | Strong | 2 | Strong | No |
| | | | Non-visible | No |
| 16 | Temporal horn | Strong | 1 | Non-visible | No |
| 17 | Temporal horn | Strong | 1 | Non-visible | No |
| 18 | Temporal horn | Strong | 1 | Non-visible | No |
| 19 | Occipital horn | Strong | 1 | Weak | No |
Histopathological analysis of ventricular
wall samples
Histopathological assessments of the main tumor mass
confirmed WHO Grade IV GBM in every case (Fig. 1). Eleven ventricular wall samples
with observable intraoperative 5-ALA-induced fluorescence were
analyzed; 5 (45.5%) were positive for the presence of tumor cells,
whereas the remaining 6 (54.5%) were free of tumor cells. Of the 5
ventricular wall samples that showed the presence of tumor cells, 2
(40%) corresponded to low-grade glioma, and 3 (60%) corresponded to
high-grade glioma. Fourteen ventricular wall samples without
observable intraoperative 5-ALA-induced fluorescence were analyzed;
no tumor cells were identified in any of the 14 samples (Table II). 5-ALA exhibited a sensitivity
of 100% and specificity of 70% [95% confidential interval (CI):
45.72–88.11%] in detecting tumor invasion of ventricular wall
samples. Positive predictive values and negative predictive values
were 45.5% (95% CI: 16.75–76.62%) and 100%. The overall accuracy of
this method was 76%. However, we did not find any correlation
between the fluorescence intensity and the pathological finding of
the ventricular wall in this study.
Illustrative cases
Patient 1
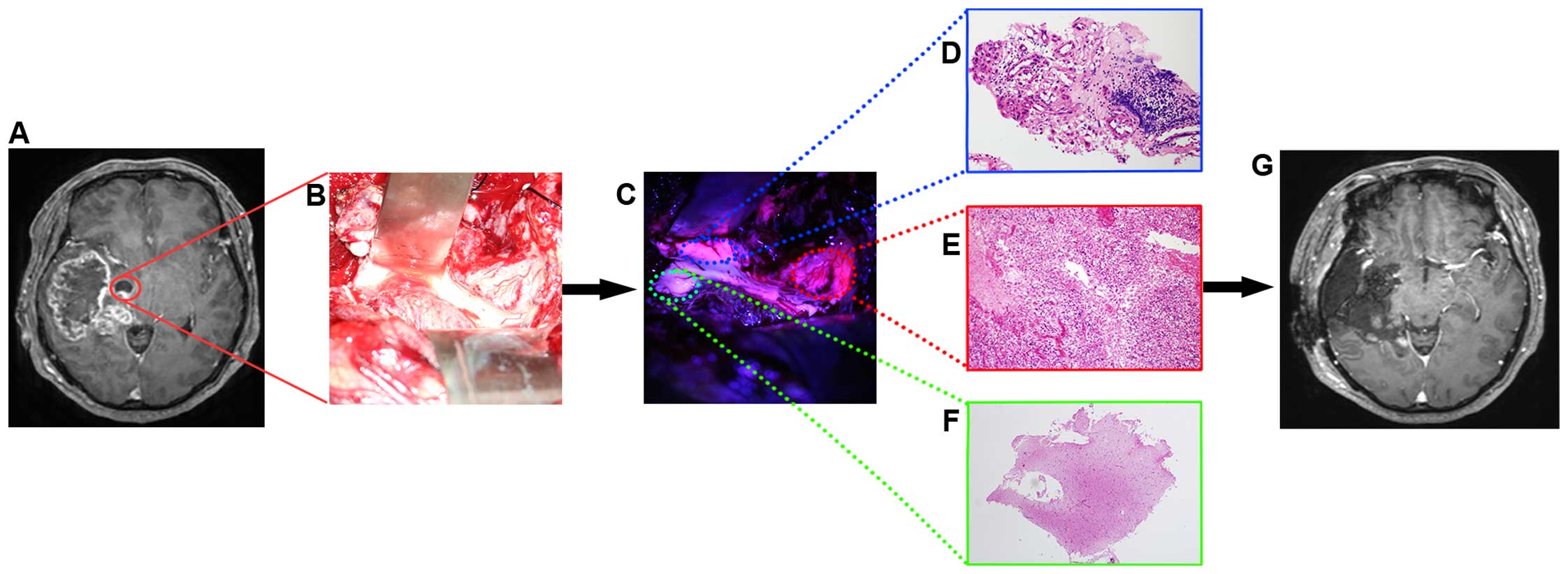
A 62-year-old woman (case 13) presented with a
1-month history of headache and progressive left hemiparesis.
Preoperative MR images revealed a right temporal enhancing mass
extending to the frontal lobe and insula (Fig. 2A). A right fronto-temporal
craniotomy was performed with 5-ALA fluorescence guidance and the
tumor was subtotally removed with a temporal lobectomy. A
histopathological analysis confirmed diagnosis of the main tumor
mass as GBM. During resection of the tumor, we entered the temporal
horn of the left lateral ventricle (Fig. 2B), and detected apparent
fluorescence on some parts of the ventricular wall (Fig. 2C). Ventricular wall samples obtained
as part of the planned temporal lobectomy were analyzed
histopathologically. The presence of tumor consistent with
high-grade glioma was confirmed in a sample of weakly fluorescent
ventricular wall tissue without contrast enhancement on MR images
(Fig. 2D). The pathological
diagnosis of strongly fluorescent ventricular wall tissue with
contrast enhancement on MR images was GBM (Fig. 2E). No tumor cells were identified in
the sample from the non-fluorescent ventricular wall tissue lacking
contrast enhancement on MR images (Fig.
2F). A postoperative MR image showed that the tumor was
resected to the greatest possible extent, and an additional right
temporal lobectomy was performed (Fig.
2G).
Patient 2
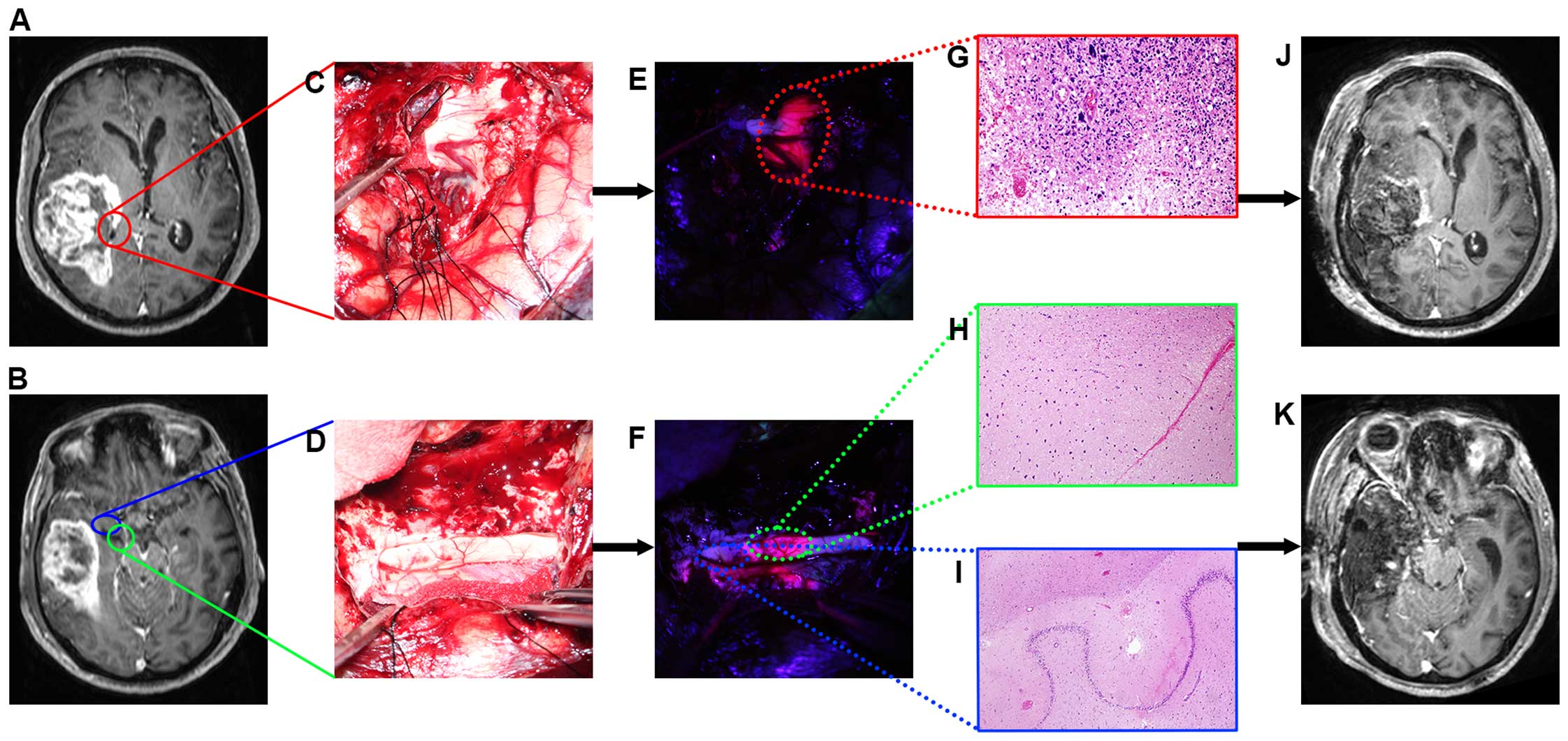
A 67-year-old man (case 15) presented with a 2-week
history of confusion, left homonymous hemianopsia, and left
hemiparesis. Preoperative MR images revealed a right
temporo-parietal enhancing mass that involved the posterior part of
the temporal horn of the right lateral ventricle (Fig. 3A and B). The tumor was resected
under the guidance of 5-ALA fluorescence, and showed strong red
fluorescence under blue light. The histopathological diagnosis of
the main tumor mass was GBM. After the ventricle was opened
(Fig. 3C and D), we encountered
fluorescence in the ventricle walls that were free of enhancement
on MR images (Fig. 3E and F).
Ventricular wall samples obtained as part of the planned
lesionectomy and temporal lobectomy were analyzed
histopathologically. The pathological diagnosis of strongly
fluorescent ventricular wall tissue with contrast enhancement on MR
images was GBM (Fig. 3G). No tumor
cells were identified in the samples from either strongly
fluorescent ventricular wall tissue or non-fluorescent ventricular
wall tissue lacking contrast enhancement on MR images (Fig. 3H and I). A postoperative MR image
showed that the tumor was resected to the extent possible, and an
additional right temporal lobectomy was performed (Fig. 3J and K).
Discussion
Because 5-ALA has been widely used for improving
surgery of malignant gliomas, it is not rare to detect
5-ALA-induced fluorescence of the ventricle wall in cases where the
ventricle is opened during the operation (23,24).
Although the relationships between 5-ALA-induced fluorescence and
pathologic parameters in gliomas and peritumoral tissues have been
extensively investigated, few studies have addressed the
histopathological implications of 5-ALA-induced fluorescence in
ventricular wall tissues that show no tumor involvement. In a
series of 7 patients with periventricular GBMs, Hayashi et
al (23) found that most
ventricle wall tissues with unenhanced MR images that showed
5-ALA-induced fluorescence were positive for the presence of tumor
cells, whereas all tissues showed disruption of ependymal cell
layers of the ventricle wall. On the basis of this study, these
authors suggested that tumor cells in the ventricular wall or
environmental changes around the ventricle could lead to 5-ALA
fluorescence of the ventricular wall. In a separate study,
Tejada-Solís et al (24)
concluded that ventricular wall fluorescence does not always
indicate glioma cell invasion of the ventricular wall, based on the
observation that 3 out of 8 (37.5%) cases that underwent selective
biopsy of fluorescent ventricle wall exhibited an intact ependymal
layer and no tumor cells. However, these studies were limited by
the small number of cases and the lack of negative control
specimens.
In the present study, we compared pathological
findings of fluorescent ventricular walls with those of
non-fluorescent ventricular walls using a relatively large number
of samples in a homogeneous group of patients. To maintain the
homogeneity of the patient population, we only included patients
with newly diagnosed GBM. To eliminate the possibility of
pseudo-positive 5-ALA-induced fluorescence in ventricular wall
samples, we excluded patients diagnosed with recurring GBM that had
undergone prior treatment, including surgery and radiotherapy,
because areas showing infiltration of inflammatory cells associated
with surgical and radiation interventions can also accumulate PpIX
and show 5-ALA-induced fluorescence (25,26).
Compared with the previous studies described above,
we obtained a relatively large number of samples from various
domains of the lateral ventricle, including non-fluorescent
ventricular wall samples as a negative control group. Most of the
ventricular wall samples were excised during surgery as part of the
planned margin of resection surrounding the GBM. Maximizing the
extent of resection likely extends time to progression and
increases survival, and supratotal resection of gliomas in
non-eloquent regions can be beneficial for clinical outcomes
(10,14,15,27–29).
Accordingly, we tried to increase the extent of resection during
neurosurgical procedures. Because we sought to achieve supratotal
resection and additional lobectomy if possible during the removal
of GBMs in non-eloquent areas, most ventricular wall samples were
consequently included in the resected area. Therefore, we could
safely harvest samples from ventricular walls. Additionally, for
safety we collected intraoperative samples from ventricular walls
in accordance with certain a prior criteria. First, we did
not intentionally open the ventricle during the surgical procedure
to check for fluorescence in the ventricular wall or to obtain
ventricular wall samples. Because ventricular opening during
surgical procedures can be a risk factor for postoperative
hydrocephalus and leptomeningeal dissemination, which may
potentially worsen the clinical condition and decrease survival
(30–35), opening of the ventricular system was
performed only in cases where it was needed for radical supratotal
resection of the tumor. Second, ventricular wall samples in the
safe area were collected carefully to prevent postoperative
neurological sequelae. Functionally important areas, including the
fornix, were excluded from ventricular wall sampling, because
neurological sequelae can occur due to direct damage to the area.
We also excluded certain areas with vascular structures, such as
internal cerebral veins and thalamostriate veins, to prevent
ischemic insult. Third, we tried to obtain a sufficient ventricular
wall sample to clearly assess histopathology, while limiting the
depth of tissue resection to <5 mm to prevent damage to
structures under the surface.
In our series, ventricular wall fluorescence was
detected in a majority (57.9%) of patients, but not in all
patients. Considering the variety of locations in the lateral
ventricle that showed fluorescence, the frequency of existence of
ventricular wall fluorescence may be underestimated since we did
not explore the entire lateral ventricle. Five of the 11 (45.5%)
fluorescent ventricular wall samples contained glioma cells,
whereas none of the 14 non-fluorescent samples showed infiltration
of tumor cells (Fig. 1). In
contrast to previous studies of the relationships between 5-ALA
fluorescence and the pathology of tissue in GBM (9,36,37),
the low positive predictive values (PPVs) and high negative
predictive values (NPVs) in our series indicate that
non-fluorescent ventricular walls under blue light should lack
invading tumor cells. On the other hand, fluorescent ventricular
walls could possibly reflect the presence of tumor cells, even
though the fluorescence is not always related to the infiltration
of tumor cells into the ventricular wall. Because the NPV depends
greatly on the non-fluorescent tissue biopsy algorithm, the high
NPV in our series may imply that ventricular wall samples remote
from the tumor and not invaded by tumor cells were properly
collected. The likelihood of not finding tumor cells should be
higher if sampling sites are remote from the tumor than would be
the case if they are close to the tumor. Our results suggest that,
in cases where the ventricular wall is fluorescent, there is a
chance of tumor cell infiltration into the ependymal spaces;
accordingly, we recommend close follow-up with imaging studies and
monitoring of the patient's clinical status after the surgical
resection of GBM if 5-ALA-induced fluorescence of the ventricular
wall is detected during surgery. Since fluorescence can be an
indication of ependymal glioma cell invasion or leptomeningeal
seeding in some cases, a biopsy should be performed to decide
treatment options if feasibility and safety allow. However,
postoperative radiotherapy covering the whole ventricle system
based on the presence of ventricular wall fluorescence should be
avoided because it may not always indicate pathological glioma cell
invasion.
Six of the ventricular wall samples in our series
were falsely fluorescing, showing no evidence of the presence of
tumor cells. This raises questions regarding the meaning of
false-positive fluorescence in the ventricular wall. False-positive
fluorescence previously described by others was related to
infiltration of inflammatory cells and reactive astrocytes,
necrosis, prominent vasculature, or peritumoral edema (26,36,37).
However, we did not observe such findings in our false-positive
ventricular wall samples and found no difference between falsely
fluorescing ventricular wall samples and non-fluorescent control
samples from the standpoint of histopathology. The intraoperative
5-ALA-induced fluorescence in the 2 samples corresponding to
low-grade glioma might be a false-positive finding because the vast
majority of low-grade gliomas do not exhibit visible intraoperative
fluorescence under a surgical microscope (38–40). A
better understanding of 5-ALA-induced fluorescence in the
ventricular wall will require further investigation, including a
molecular characterization, of these falsely fluorescing samples.
In a study with GBM patients by Piccirillo et al (41), the histologic analysis and genomic
characterization revealed that the fluorescent subependymal zone
contained tumor-initiating cells, however most samples from the
fluorescent subependymal zone were truly fluorescing as they were
included in the contrast enhancing lesion on MR images and
pathologically confirmed to be involved by tumor. Studies with
samples from 5-ALA-induced fluorescent ventricular wall tissues
which show no tumor involvement are still lacking.
One limitation of this study is its subjective
assessment of intraoperative 5-ALA-induced ventricular wall
fluorescence. We adopted a trinary approach - non-visible, weak, or
strong fluorescence - to assess fluorescence. However, this
approach is subjective as fluorescence was estimated by a surgeon
using only a surgical microscope with a specific filter. Overcoming
this limitation would require quantitative or semiquantitative
fluorescence measurements capable of objectively discriminating
fluorescence intensity. These modalities, such as intraoperative
spectrometry or confocal microscopy, have proven to be more
sensitive for the determination of 5-ALA-induced fluorescence than
the surgical microscope used here, although these modalities for
quantitative determination of fluorescence still need to be
validated for clinical use (37–39,42,43).
Another limitation of this study is that infiltration of the tumor
was judged based on enhancement on T1-weighted, contrast-enhanced
MR images. GBMs have an infiltrative nature, and their presence is
not always associated with a disrupted BBB; thus, contrast
enhancement may not show invasive areas of GBM because it only
depicts areas with a disruption in the BBB. Accordingly, active
tumor tissue can exist beyond the area of contrast enhancement.
T2-weighted and fluid-attenuated inversion recovery MR images may
depict invasive areas of GBM; however, it is difficult to determine
the extent of the non-enhancing component of the tumor using these
approaches owing to peritumoral edema (44,45).
On this account, there is the possibility of tumor invasion into
ventricular wall samples in some cases, despite the fact that we
obtained ventricular wall samples that were definitely remote from
the enhanced lesion in MR images. Errors in the neuronavigation
system related to image-to-patient registration error and
intraoperative brain shift are also a limitation of this study. In
all cases, the ventricle was opened and a considerable amount of
cerebrospinal fluid was drained out during the surgery, which might
subsequently degrade navigational accuracy over the course of a
surgical procedure. We tried to compensate for such errors using
intracranial anatomical landmarks; however, this method is
subjective and could not eliminate the error completely.
In summary, our data suggest the possibility that
glioma cells are present in ventricle walls exhibiting 5-ALA
fluorescence despite the absence of tumor involvement in MR images.
Ventricular walls lacking 5-ALA fluorescence and enhancement on MR
images may be free of tumor, so a decision to resect
non-fluorescent ventricular walls should be made carefully. Further
investigations of non-tumor cells from tissues exhibiting 5-ALA
fluorescence are needed to understand the nature of ventricular
wall fluorescence.
Acknowledgments
This study was supported by grants from the Basic
Science Research Program through the National Research Foundation
of Korea (NRF) funded by the Ministry of Education, Science and
Technology (NRF-2013R1A1A2055597), and the Korean Health Technology
R&D Project, Ministry of Health & welfare, Republic of
Korea (HI13C1509).
References
|
1
|
Friesen SA, Hjortland GO, Madsen SJ,
Hirschberg H, Engebraten O, Nesland JM and Peng Q: 5-Aminolevulinic
acid-based photodynamic detection and therapy of brain tumors
(Review). Int J Oncol. 21:577–582. 2002.PubMed/NCBI
|
|
2
|
Regula J, MacRobert AJ, Gorchein A,
Buonaccorsi GA, Thorpe SM, Spencer GM, Hatfield AR and Bown SG:
Photosensitisation and photodynamic therapy of oesophageal,
duodenal, and colorectal tumours using 5 aminolaevulinic acid
induced protoporphyrin IX - a pilot study. Gut. 36:67–75. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ohgari Y, Nakayasu Y, Kitajima S, Sawamoto
M, Mori H, Shimokawa O, Matsui H and Taketani S: Mechanisms
involved in delta-aminolevulinic acid (ALA) -induced
photosensitivity of tumor cells: Relation of ferrochelatase and
uptake of ALA to the accumulation of protoporphyrin. Biochem
Pharmacol. 71:42–49. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Teng L, Nakada M, Zhao SG, Endo Y,
Furuyama N, Nambu E, Pyko IV, Hayashi Y and Hamada JI: Silencing of
ferrochelatase enhances 5-aminolevulinic acid-based fluorescence
and photo-dynamic therapy efficacy. Br J Cancer. 104:798–807. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Valdés PA, Moses ZB, Kim A, Belden CJ,
Wilson BC, Paulsen KD, Roberts DW and Harris BT: Gadolinium- and
5-aminolevulinic acid-induced protoporphyrin IX levels in human
gliomas: An ex vivo quantitative study to correlate protoporphyrin
IX levels and blood-brain barrier breakdown. J Neuropathol Exp
Neurol. 71:806–813. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ennis SR, Novotny A, Xiang J, Shakui P,
Masada T, Stummer W, Smith DE and Keep RF: Transport of
5-aminolevulinic acid between blood and brain. Brain Res.
959:226–234. 2003. View Article : Google Scholar
|
|
7
|
Stummer W, Stepp H, Möller G, Ehrhardt A,
Leonhard M and Reulen HJ: Technical principles for
protoporphyrin-IX-fluorescence guided microsurgical resection of
malignant glioma tissue. Acta Neurochir (Wien). 140:995–1000. 1998.
View Article : Google Scholar
|
|
8
|
Stummer W, Stocker S, Wagner S, Stepp H,
Fritsch C, Goetz C, Goetz AE, Kiefmann R and Reulen HJ:
Intraoperative detection of malignant gliomas by 5-aminolevulinic
acid-induced porphyrin fluorescence. Neurosurgery. 42:518–525;
discussion 525–516. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao S, Wu J, Wang C, Liu H, Dong X, Shi
C, Shi C, Liu Y, Teng L, Han D, et al: Intraoperative
fluorescence-guided resection of high-grade malignant gliomas using
5-aminolevulinic acid-induced porphyrins: A systematic review and
meta-analysis of prospective studies. PLoS One. 8:e636822013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chaichana KL, Cabrera-Aldana EE,
Jusue-Torres I, Wijesekera O, Olivi A, Rahman M and
Quinones-Hinojosa A: When gross total resection of a glioblastoma
is possible, how much resection should be achieved? World
Neurosurg. 82:e257–e265. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lacroix M, Abi-Said D, Fourney DR,
Gokaslan ZL, Shi W, DeMonte F, Lang FF, McCutcheon IE, Hassenbusch
SJ, Holland E, et al: A multivariate analysis of 416 patients with
glioblastoma multiforme: Prognosis, extent of resection, and
survival. J Neurosurg. 95:190–198. 2001. View Article : Google Scholar
|
|
12
|
McGirt MJ, Chaichana KL, Gathinji M,
Attenello FJ, Than K, Olivi A, Weingart JD, Brem H and
Quiñones-Hinojosa AR: Independent association of extent of
resection with survival in patients with malignant brain
astrocytoma. J Neurosurg. 110:156–162. 2009. View Article : Google Scholar
|
|
13
|
Pichlmeier U, Bink A, Schackert G and
Stummer W; ALA Glioma Study Group: Resection and survival in
glioblastoma multiforme: An RTOG recursive partitioning analysis of
ALA study patients. Neuro Oncol. 10:1025–1034. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sanai N, Polley MY, McDermott MW, Parsa AT
and Berger MS: An extent of resection threshold for newly diagnosed
glioblastomas. J Neurosurg. 115:3–8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stummer W, Reulen HJ, Meinel T, Pichlmeier
U, Schumacher W, Tonn JC, Rohde V, Oppel F, Turowski B,
Woiciechowsky C, et al ALA-Glioma Study Group: Extent of resection
and survival in glioblastoma multiforme: Identification of and
adjustment for bias. Neurosurgery. 62:564–576; discussion 564–576.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stummer W, Pichlmeier U, Meinel T,
Wiestler OD, Zanella F and Reulen HJ; ALA-Glioma Study Group:
Fluorescence-guided surgery with 5-aminolevulinic acid for
resection of malignant glioma: A randomised controlled multicentre
phase III trial. Lancet Oncol. 7:392–401. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Willems PW, Taphoorn MJ, Burger H,
Berkelbach van der Sprenkel JW and Tulleken CA: Effectiveness of
neuronavigation in resecting solitary intracerebral
contrast-enhancing tumors: A randomized controlled trial. J
Neurosurg. 104:360–368. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Senft C, Bink A, Franz K, Vatter H, Gasser
T and Seifert V: Intraoperative MRI guidance and extent of
resection in glioma surgery: A randomised, controlled trial. Lancet
Oncol. 12:997–1003. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Unsgaard G, Selbekk T, Brostrup Müller T,
Ommedal S, Torp SH, Myhr G, Bang J and Nagelhus Hernes TA: Ability
of navigated 3D ultrasound to delineate gliomas and metastases -
comparison of image interpretations with histopathology. Acta
Neurochir (wien). 147:1259–1269; discussion 1269. 2005. View Article : Google Scholar
|
|
20
|
De Witt Hamer PC, Robles SG, Zwinderman
AH, Duffau H and Berger MS: Impact of intraoperative stimulation
brain mapping on glioma surgery outcome: A meta-analysis. J Clin
Oncol. 30:2559–2565. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rhoton AL Jr: The lateral and third
ventricles. Neurosurgery. 51(Suppl): S207–S271. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hayashi Y, Nakada M, Tanaka S, Uchiyama N,
Hayashi Y, Kita D and Hamada J: Implication of 5-aminolevulinic
acid fluorescence of the ventricular wall for postoperative
communicating hydrocephalus associated with cerebrospinal fluid
dissemination in patients with glioblastoma multiforme: A report of
7 cases. J Neurosurg. 112:1015–1019. 2010. View Article : Google Scholar
|
|
24
|
Tejada-Solís S, Aldave-Orzaiz G,
Pay-Valverde E, Marigil-Sánchez M, Idoate-Gastearena MA and
Diez-Valle R: Prognostic value of ventricular wall fluorescence
during 5-aminolev-ulinic-guided surgery for glioblastoma. Acta
Neurochir (wien). 154:1997–2002; discussion 2002. 2012. View Article : Google Scholar
|
|
25
|
Miyatake S, Kuroiwa T, Kajimoto Y,
Miyashita M, Tanaka H and Tsuji M: Fluorescence of non-neoplastic,
magnetic resonance imaging-enhancing tissue by 5-aminolevulinic
acid: Case report. Neurosurgery. 61:E1101–E1103; discussion
E1103–E1104. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Utsuki S, Oka H, Sato S, Shimizu S, Suzuki
S, Tanizaki Y, Kondo K, Miyajima Y and Fujii K: Histological
examination of false positive tissue resection using
5-aminolevulinic acid-induced fluorescence guidance. Neurol Med
Chir (Tokyo). 47:210–213; discussion 213–214. 2007. View Article : Google Scholar
|
|
27
|
Duffau H: Is supratotal resection of
glioblastoma in noneloquent areas possible? World Neurosurg.
82:e101–e103. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Grabowski MM, Recinos PF, Nowacki AS,
Schroeder JL, Angelov L, Barnett GH and Vogelbaum MA: Residual
tumor volume versus extent of resection: Predictors of survival
after surgery for glioblastoma. J Neurosurg. 121:1115–1123. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wolbers JG: Novel strategies in
glioblastoma surgery aim at safe, supra-maximum resection in
conjunction with local therapies. Chin J Cancer. 33:8–15. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bae JS, Yang SH, Yoon WS, Kang SG, Hong YK
and Jeun SS: The clinical features of spinal leptomeningeal
dissemination from malignant gliomas. J Korean Neurosurg Soc.
49:334–338. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fischer CM, Neidert MC, Péus D, Ulrich NH,
Regli L, Krayenbühl N and Woernle CM: Hydrocephalus after resection
and adjuvant radiochemotherapy in patients with glioblastoma. Clin
Neurol Neurosurg. 120:27–31. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Montano N, D'Alessandris QG, Bianchi F,
Lauretti L, Doglietto F, Fernandez E, Maira G and Pallini R:
Communicating hydrocephalus following surgery and adjuvant
radiochemotherapy for glioblastoma. J Neurosurg. 115:1126–1130.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Saito R, Kumabe T, Jokura H, Shirane R and
Yoshimoto T: Symptomatic spinal dissemination of malignant
astrocytoma. J Neurooncol. 61:227–235. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vertosick FT Jr and Selker RG: Brain stem
and spinal metastases of supratentorial glioblastoma multiforme: A
clinical series. Neurosurgery. 27:516–521; discussion 521–512.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Grabb PA, Albright AL and Pang D:
Dissemination of supratentorial malignant gliomas via the
cerebrospinal fluid in children. Neurosurgery. 30:64–71. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Roberts DW, Valdés PA, Harris BT, Fontaine
KM, Hartov A, Fan X, Ji S, Lollis SS, Pogue BW, Leblond F, et al:
Coregistered fluorescence-enhanced tumor resection of malignant
glioma: Relationships between δ-aminolevulinic acid-induced
protoporphyrin IX fluorescence, magnetic resonance imaging
enhancement, and neuropathological parameters. Clinical article. J
Neurosurg. 114:595–603. 2011. View Article : Google Scholar
|
|
37
|
Stummer W, Tonn JC, Goetz C, Ullrich W,
Stepp H, Bink A, Pietsch T and Pichlmeier U: 5-Aminolevulinic
acid-derived tumor fluorescence: The diagnostic accuracy of visible
fluorescence qualities as corroborated by spectrometry and
histology and postoperative imaging. Neurosurgery. 74:310–319;
discussion 319–320. 2014. View Article : Google Scholar :
|
|
38
|
Sanai N, Snyder LA, Honea NJ, Coons SW,
Eschbacher JM, Smith KA and Spetzler RF: Intraoperative confocal
microscopy in the visualization of 5-aminolevulinic acid
fluorescence in low-grade gliomas. J Neurosurg. 115:740–748. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Utsuki S, Oka H, Sato S, Suzuki S, Shimizu
S, Tanaka S and Fujii K: Possibility of using laser spectroscopy
for the intraoperative detection of nonfluorescing brain tumors and
the boundaries of brain tumor infiltrates. Technical note. J
Neurosurg. 104:618–620. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Widhalm G, Kiesel B, Woehrer A,
Traub-Weidinger T, Preusser M, Marosi C, Prayer D, Hainfellner JA,
Knosp E and Wolfsberger S: 5-Aminolevulinic acid induced
fluorescence is a powerful intraoperative marker for precise
histopathological grading of gliomas with non-significant
contrast-enhancement. PLoS One. 8:e769882013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Piccirillo SG, Spiteri I, Sottoriva A,
Touloumis A, Ber S, Price SJ, Heywood R, Francis NJ, Howarth KD,
Collins VP, et al: Contributions to drug resistance in glioblastoma
derived from malignant cells in the sub-ependymal zone. Cancer Res.
75:194–202. 2015. View Article : Google Scholar :
|
|
42
|
Valdés PA, Kim A, Brantsch M, Niu C, Moses
ZB, Tosteson TD, Wilson BC, Paulsen KD, Roberts DW and Harris BT:
δ-aminolevulinic acid-induced protoporphyrin IX concentration
correlates with histopathologic markers of malignancy in human
gliomas: The need for quantitative fluorescence-guided resection to
identify regions of increasing malignancy. Neurooncol. 13:846–856.
2011.
|
|
43
|
Valdés PA, Leblond F, Kim A, Harris BT,
Wilson BC, Fan X, Tosteson TD, Hartov A, Ji S, Erkmen K, et al:
Quantitative fluorescence in intracranial tumor: Implications for
ALA-induced PpIX as an intraoperative biomarker. J Neurosurg.
115:11–17. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wen PY, Macdonald DR, Reardon DA,
Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert
MR, Lassman AB, et al: Updated response assessment criteria for
high-grade gliomas: Response assessment in neuro-oncology working
group. J Clin Oncol. 28:1963–1972. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cage TA, Pekmezci M, Prados M and Berger
MS: Subependymal spread of recurrent glioblastoma detected with the
intraoperative use of 5-aminolevulinic acid: Case report. J
Neurosurg. 118:1220–1223. 2013. View Article : Google Scholar : PubMed/NCBI
|