Introduction
Lung cancer accounts for the majority of
cancer-related death worldwide, with more than 226,000 new cases in
the US in 2012. The predominant type of lung cancer is non-small
cell lung cancer (NSCLC) which includes adenocarcinoma and squamous
cell carcinoma, which makes up ~85% of all new diagnoses (1–3).
Despite recent advances in surgical treatment, radiotherapy and
chemotherapy, the prognosis of lung cancer is still unfavorable,
with a 5-year overall survival rate of ~11–15% after diagnosis
(4,5). Thus, a greater understanding of the
molecular mechanisms underlying NSCLC development and progression
is essential for improving diagnosis, prevention and treatment of
this disease.
Recently, studies using high-throughput
transcriptome analysis have revealed that over 90% of the total
mammalian genome can be transcribed, whereas only 2% of the
transcribed genome codes for protein (5), with the remaining short or long
non-coding RNAs (lncRNAs) with limited or no protein-coding
capacity (6,7). lncRNAs are non-coding RNAs that are
longer than 200 nucleotides in length, with a large range of
functions in diverse biological processes including regulation of
cellular development and differentiation, modulation of
proliferation, apoptosis and invasiveness of tumors, and
reprogramming of induced pluripotent stem cells (8–12).
However, few studies have characterized the mechanisms involved in
the functions of lncRNAs, which involve the regulation of gene
expression by chromatin remodeling, regulation of mRNA splicing,
histone protein modification and acting as sponges for microRNAs
(9–12).
Accumulating evidence suggests that dysregulation of
lncRNAs occur in various types of cancers, such as hepatocellular
carcinoma (HCC), breast, bladder, melanoma and prostate cancer
(13–18). Recent studies have also found that
lncRNAs play important roles in cancer development, metastasis and
chemotherapy resistance. Moreover, previous studies have also
demonstrated that lncRNAs act as proto-oncogenes or
tumor-suppressor genes (19,20).
For example, lncRNA HOX antisense intergenic RNA (HOTAIR) has been
reported as a negative prognostic indicator in breast, liver and
pancreatic cancer, and is associated with breast cancer metastasis
(21,22). Another study found that lncRNA GAS5
was downregulated in HCC tissues and may be an independent
prognostic factor and potential valuable biomarker for HCC patients
(23). Similar results were found
for lncRNA HOTAIR, which was significantly upregulated and acted as
an independent prognostic factor of recurrence in stage Ta/T1
urothelial carcinoma (24). There
is also growing evidence indicating that lncRNAs may be involved in
the pathogenesis of NSCLC, providing new insights into the biology
of this disease (25,26).
Metastasis-associated lung adencarcinoma transcript
1 (MALAT1), mapped to human chromosome 11q13, is an evolutionarily
highly conserved lncRNA which cannot be translated into protein
in vivo. MALAT1 was originally demonstrated as a prognostic
marker for metastasis and patient survival in NSCLC, although it is
a well-described lncRNA widely expressed in normal tissues
(27–29). However, it is particularly
overexpressed in various carcinomas including lung, cervical, liver
and bladder (26,30,31),
and also confers proliferative and metastatic phenotypes to tumor
cells (26,32). In addition, MALAT1 may be a
candidate biomarker for NSCLC, particularly in early-stage
metastasizing NSCLC (33). The
mechanisms of MALAT1-induced tumor growth and metastasis are still
unknown, including binding to the active regions of chromosomes
(14), recruiting SR family
proteins (13) and regulating
alternative splicing of oncogenic mRNAs (15), depending on tissue contexts. There
is also much evidence suggesting that MALAT1 may be involved in
cell cycle regulation, which contributes to uncontrolled tumor
growth.
The role of MALAT1 in lung cancer has been widely
researched, yet its role in bone metastasis has not yet been
investigated. The present study aimed to investigate the role of
lncRNA MALAT1 in the bone metastasis of NSCLC, including the
expression pattern in tumor tissues and its effect on the
apoptosis, proliferation, migration and invasion of NSCLC
cells.
Materials and methods
The procedures followed have been approved by the
Ethics Committee of the Affiliated Hospital of Weifang Medical
College, Weifang, China. Informed written consents were provided by
all patients who participated in the present study. Tissue samples
of NSCLC were obtained from 40 patients who underwent primary
surgical resection or needle biopsy of NSCLC between 2012 and 2014
collected at the Affiliated Hospital of Weifang Medical College.
Among the patients, 20 patients were diagnosed with NSCLC of stage
I and the remaining 20 patients were diagnosed with NSCLC with bone
metastasis. None of the patients had received radiotherapy or
chemotherapy prior to surgery. NSCLC tissues were immediately
snap-frozen in liquid nitrogen and stored at −80°C until total RNA
was extracted.
Cell lines
A normal human lung adenocarcinoma cell line
(SPC-A1), a normal human bronchial epithelial cell line (16HBE),
and a human lung adenocarcinoma cell line (ACC-LC-319/bone2) with
high bone metastatic ability were purchased from Shanghai
Institutes for Biological Sciences, Chinese Academy of Sciences
(Shanghai, China). The cells were cultured in Dulbecco's modified
Eagle's medium (DMEM), supplemented with 10% fetal bovine serum
(FBS), antibiotic-antimycotic mixture and incubated at 37°C in 5%
CO2.
RNA isolation and quantitative real-time
PCR
Tissues were homogenized and total RNA was isolated
using the TR RNA isolation kit (Invitrogen, Carlsbad, CA, USA).
Then, RNA was reversely transcribed into cDNAs with a reverse
transcription kit (Promega, Madison, WI, USA) according to the
manufacturer's protocol. The expression of lncRNA MALAT1 was
determined by quantitative real-time PCR using the following primer
sequences: MALAT1 forward, 5′-GAATTGCGTCATTTAAAGCCTAGTT-3′ and
reverse, 5′-GTTTCATCCTACCACTCCCAATTAAT-3′. GAPDH was also included
as an internal control, and the relative expression level of MALAT1
was normalized to GAPDH. qRT-PCR was performed using the FastStart
Universal SYBR-Green Master Mix kit (Roche, San Francisco, CA, USA)
according to the manufacturer's instructions. Each experiment was
carried out in triplicate. Differences in gene expression,
expressed as fold-changes, were calculated using the
2−ΔΔCt method.
Small interfering RNA and cell
transfection
The small interfering RNAs (siRNAs) against MALAT1
(si-MALAT1) and the negative control (si-NC) were employed and
synthesized by GenePharma (Shanghai, China). The siRNA sequences
were: 5′-GAGGUGUAAAGGGAUUUAUTT-3′. Exponentially growing cells
(1.5×105) were seeded into 12-well plates overnight, and
then transfected with siRNA or the negative control at a final
concentration of 30 nM using X-tremeGENE transfection reagent
(Ambion, Austin, TX, USA). The transfection efficiency was
determined by qRT-PCR 48 h after transfection.
Cell proliferation assay
The proliferation of the ACC-LC-319/bone2 cells was
assessed by MTT assay (Sigma) using the Cell Counting Kit-8 (CCK-8)
(Dojindo, Japan) according to the manufacturer's instructions 24 h
after transfection. The cells were trypsinized, counted and seeded
into 96-well plates with the cell density adjusted to
4×103/well. At 12, 24 and 48 h, 100 µg of MTT
reagent was added to each well and incubation was carried out for 1
h at 37°C. The solution absorbance was measured at 450 nm using the
MRX II absorbance reader (Dynex Technologies, Chantilly, VA, USA).
Three independent experiments were performed and data are presented
as mean ± standard deviation (SD).
Cell apoptosis assay
ACC-LC-319/bone2 cells transfected with si-MALAT1
were harvested 48 h after transfection. The cells were resuspended,
fixed, resuspended in staining solution and were finally cultured
in 6-well plates at a density of 1×105cells/well. The
cells were double stained with Annexin V-FITC and propidium iodide
(PI) according to the manufacturer's recommendations, and the cells
were analyzed with flow cytometry (KeyGen Biotech, Co., Ltd.)
equipped with CellQuest software (BD Biosciences). Cells were
characterized as viable, dead, early apoptotic and apoptotic cells,
and then the relative ratio of early apoptotic cells was compared
with the control transfectant from each experiment.
Cell migration and invasion assays
To determine cell migration, similar sized wounds
were introduced to monolayer cells using a sterile white pipette
tip. Wounded monolayer cells were washed 3 times by
phosphate-buffered saline (PBS) to remove cell debris and were then
cultured. The speed of wound closure was monitored and photographed
at 48 h. To determine cell invasion ability, the ACC-LC-319/bone2
cells transfected with either si-MALAT1 or si-Con were seeded into
24-well plates with a Matrigel-coated membrane with 8-mm pore size
(Costar) chamber inserts. Cells were suspended in 0.2 ml of DMEM
without FBS when they were seeded into the upper chamber. In the
lower chamber, 0.6 ml of DMEM supplemented with 10% FBS was added.
After incubation for 48 h at 37°C in 5% CO2, the
non-invaded cells on the upper membrane surface were removed with a
cotton tip, and the cells that passed through the filter were fixed
and stained using 0.1% crystal violet for 10 min and placed on a
glass slide. The numbers of invaded cells were counted in 3
randomly selected high-power fields under a microscope
(Olympus).
Tumor formation assay in a nude mouse
model
Female athymic BALB/c nude mice (4-weeks old) were
maintained under pathogen-free conditions and maintained according
to the protocols approved by the Shanghai Medical Experimental
Animal Care Commission. ACC-LC-319/bone2 cells transfected with
either si-MALAT1 or si-Con were injected into a single side of the
posterior flank of each mouse. Mice were euthanized and the
subcutaneous growth of each tumor was examined 18 days after
injection. Tumor volume (V) was calculated using the equation: V =
0.5 × D × d2 (D, longitudinal diameter; d, latitudinal
diameter).
Statistical analysis
Statistical Package for Social Sciences software
(SPSS, Inc., Chicago, IL, USA), version 16.0 for Windows was used
for statistical analysis. The data are presented as the mean ± SD,
and comparison between groups was assessed by the Student's t-test.
Categorical data were analyzed using the two-sided Chi-square test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
MALAT1 expression in NSCLC tissues and
cell lines
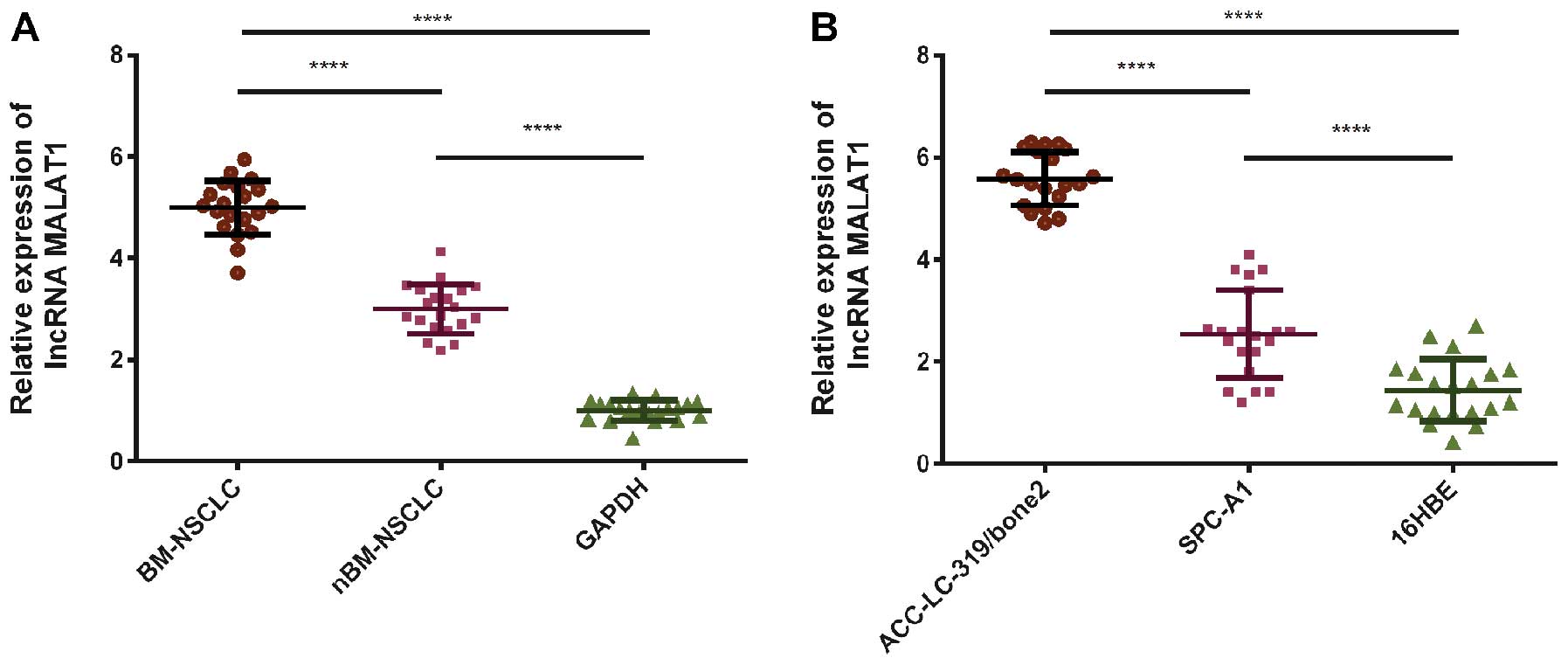
qRT-PCR was used to detect MALAT1 expression levels
in cell lines and clinical samples, which were normalized to GAPDH.
To investigate the potential role of MALAT1 in the bone metastasis
of NSCLC, the expression of lncRNA-MALAT1 was assessed in 40 tissue
samples from NSCLC patients with (BM-NSCLC, n=20) or without
(nBM-NSCLC, n=20) bone metastasis. The relative expression level of
lncRNA-MALAT1 was significantly higher in the lung tumor tissues
with bone metastasis compared with the level in the tumor tissues
without bone metastasis (P<0.0001, Fig. 1A). At the same time, the two human
lung adenocarcinoma cell lines ACC-LC-319/bone2 (P<0.0001,
Fig. 1B) and SPC-A1 (P<0.0001,
Fig. 1B) were also found to exhibit
significantly higher expression of MALAT1 than the level in the
normal cell line 16HBE. The expression of MALAT1 in the human lung
adenocarcinoma cell line ACC-LC-319/bone2 with high bone metastatic
ability was also significantly higher than the expression in the
normal human lung adenocarcinoma cell line SPC-A1 (P<0.0001,
Fig. 1B).
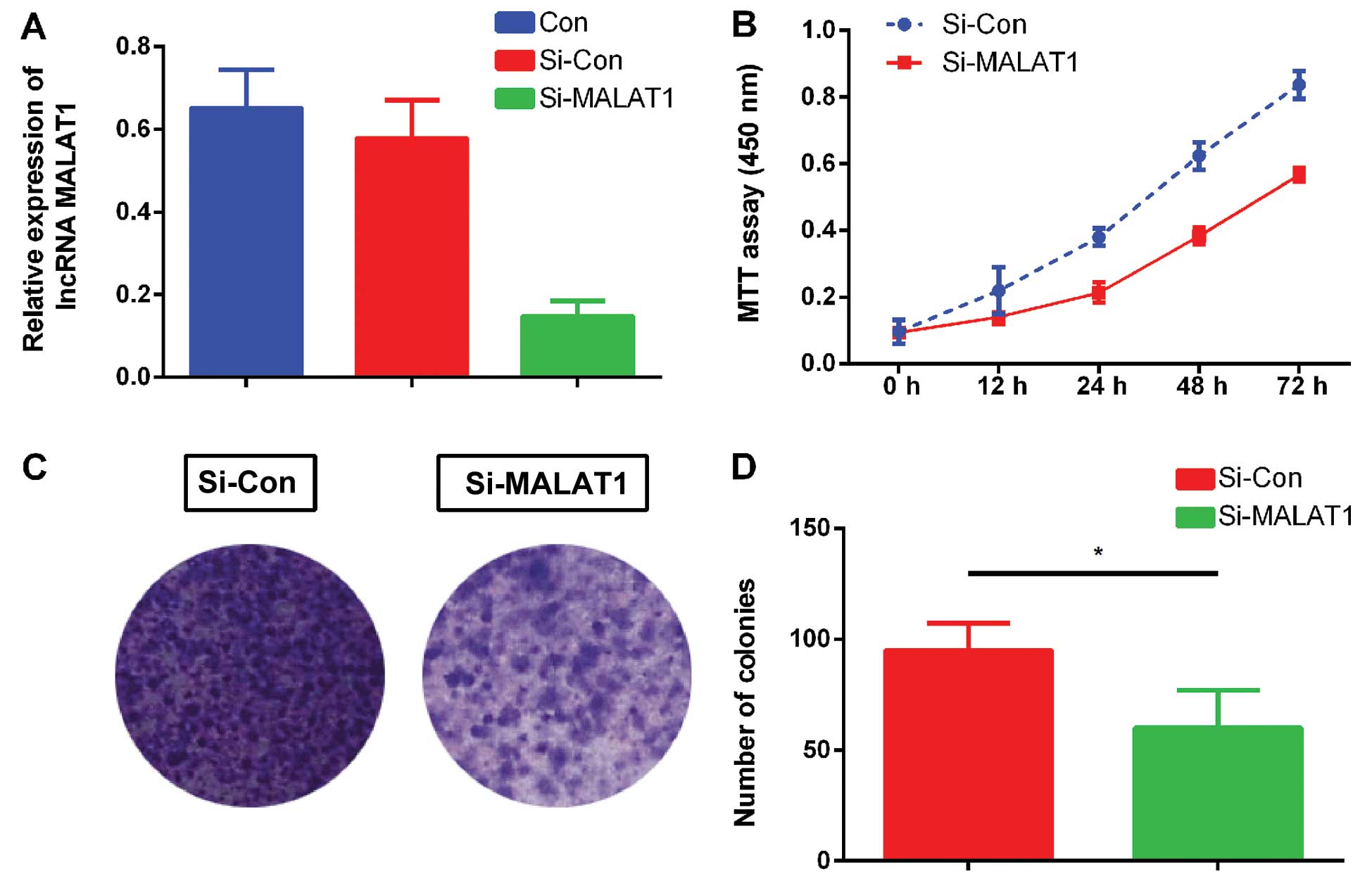
MALAT1 expression and the proliferation
ability of NSCLC
Human lung adenocarcinoma cell line ACC-LC-319/bone2
with high bone metastatic ability was chosen for the proliferation
ability assessment. lncRNA-MALAT1 was downregulated with siRNA as
previously described (Fig. 2A).
Fig. 2B shows that the
proliferation abilities of the ACC-LC-319/bone2 cells decreased
significantly after incubation with si-MALAT1. Additionally, the
colony formation assay also showed that silencing of MALAT1
significantly decreased the number of colonies formed by the
ACC-LC-319/bone2 cells (Fig. 2C and
D) compared with the si-Con group. These data suggest that
MALAT1 knockdown had the ability to inhibit ACC-LC-319/bone2 cell
proliferation.
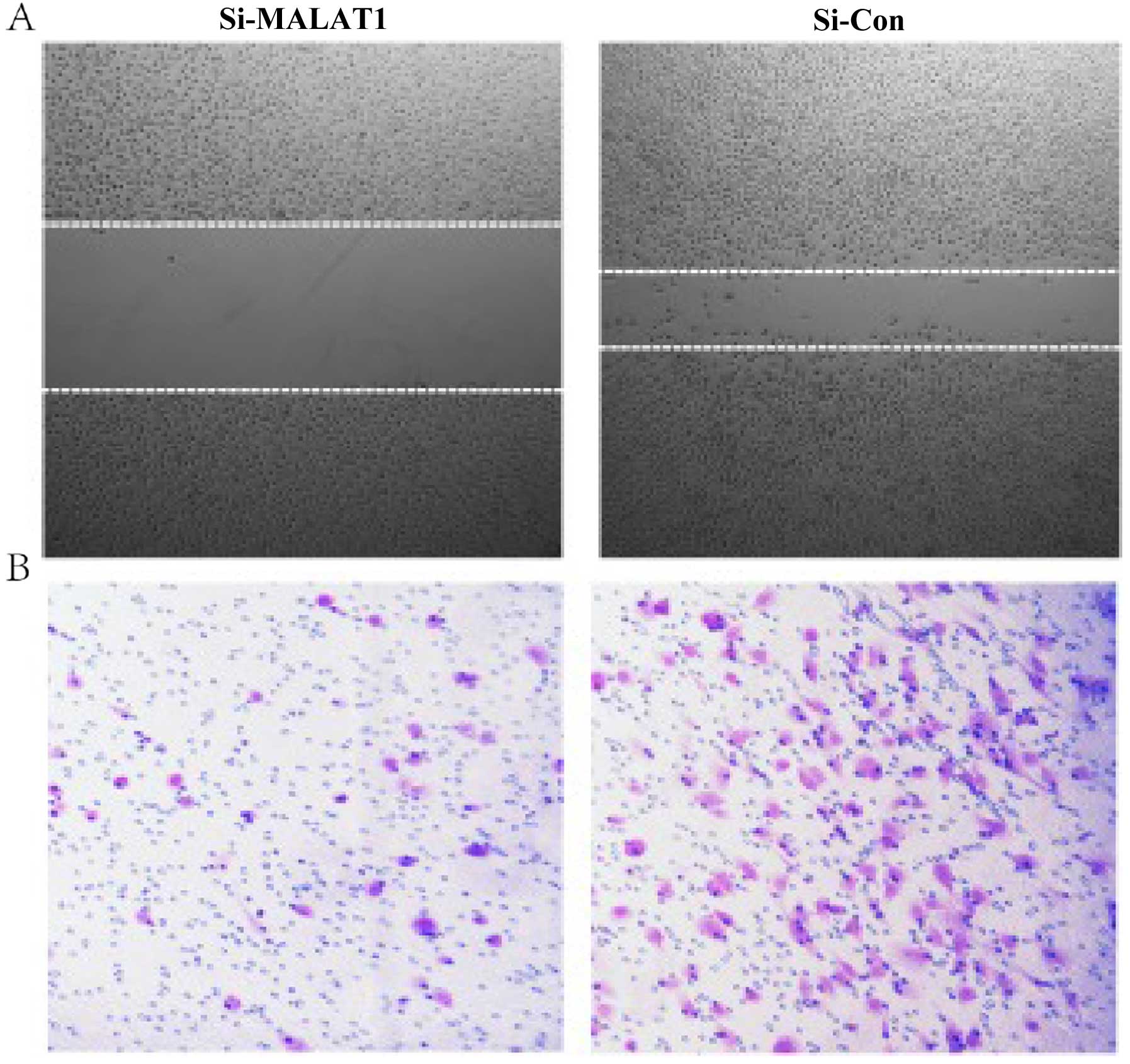
MALAT1 expression and the migratory and
invasive abilities of NSCLC cells
Cell invasion is a significant aspect of cancer
progression, and involves the migration of tumor cells into
contiguous and distant tissues. The present study performed
Transwell and wound-healing assays to determine the effect of
MALAT1 knockdown on NSCLC cell invasion and metastasis. As shown in
Fig. 3, the silencing of MALAT1
expression in ACC-LC-319/bone2 cells decreased the migratory
ability significantly compared with that in the normal
ACC-LC-319/bone2 cells (Fig. 3A).
Furthermore, a Transwell assay was performed to determine the
ability of cells to invade a matrix barrier and the representative
micrographs are presented in Fig.
3B. The invasive cell count demonstrated that invasive
potential was significantly reduced in the Si-MALAT1 group relative
to the si-Con group.
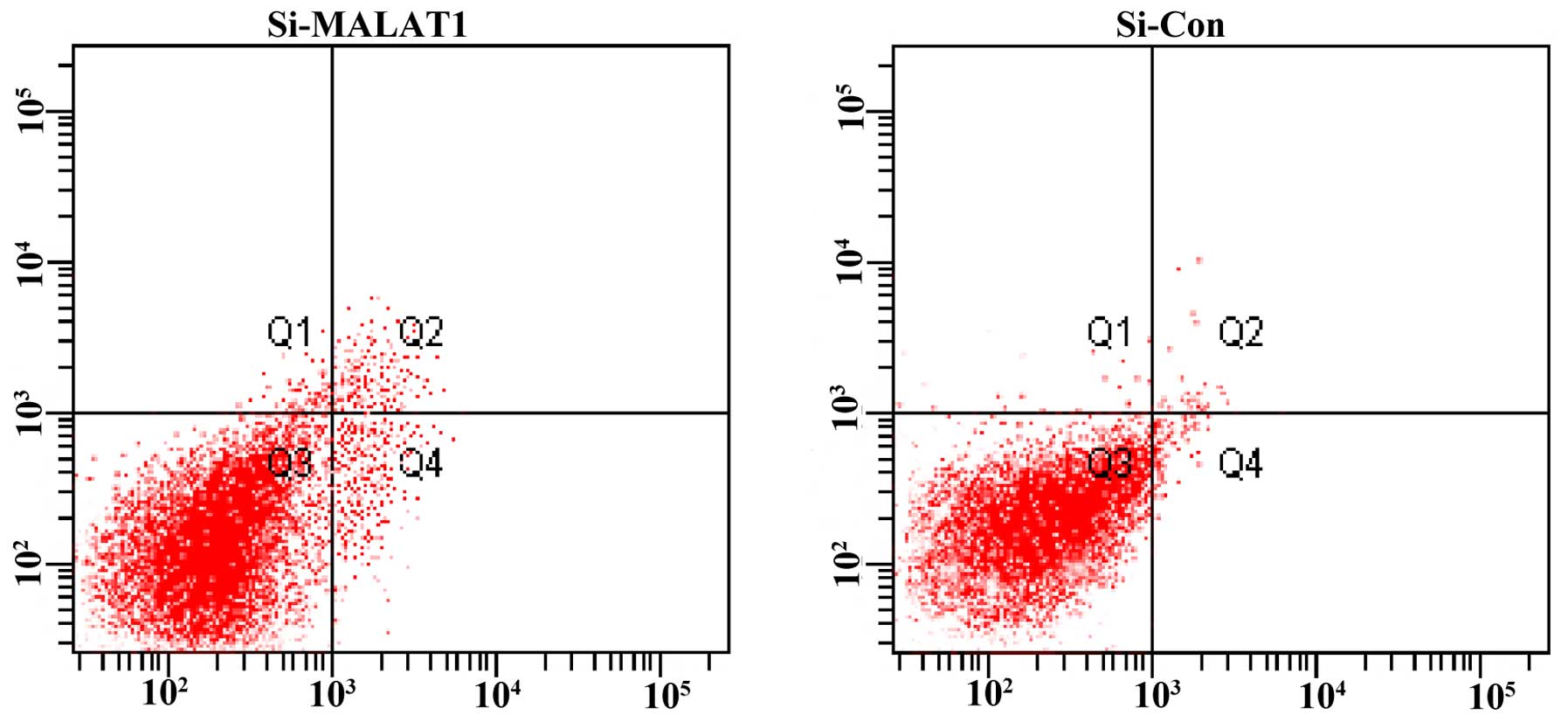
MALAT1 expression and apoptosis of
NSCLC
As shown in Fig. 4,
after treatment with the siRNA for 48 h, the percentage of
apoptotic cells was significantly increased in the ACC-LC-319/bone2
cells in comparison with the negative controls.
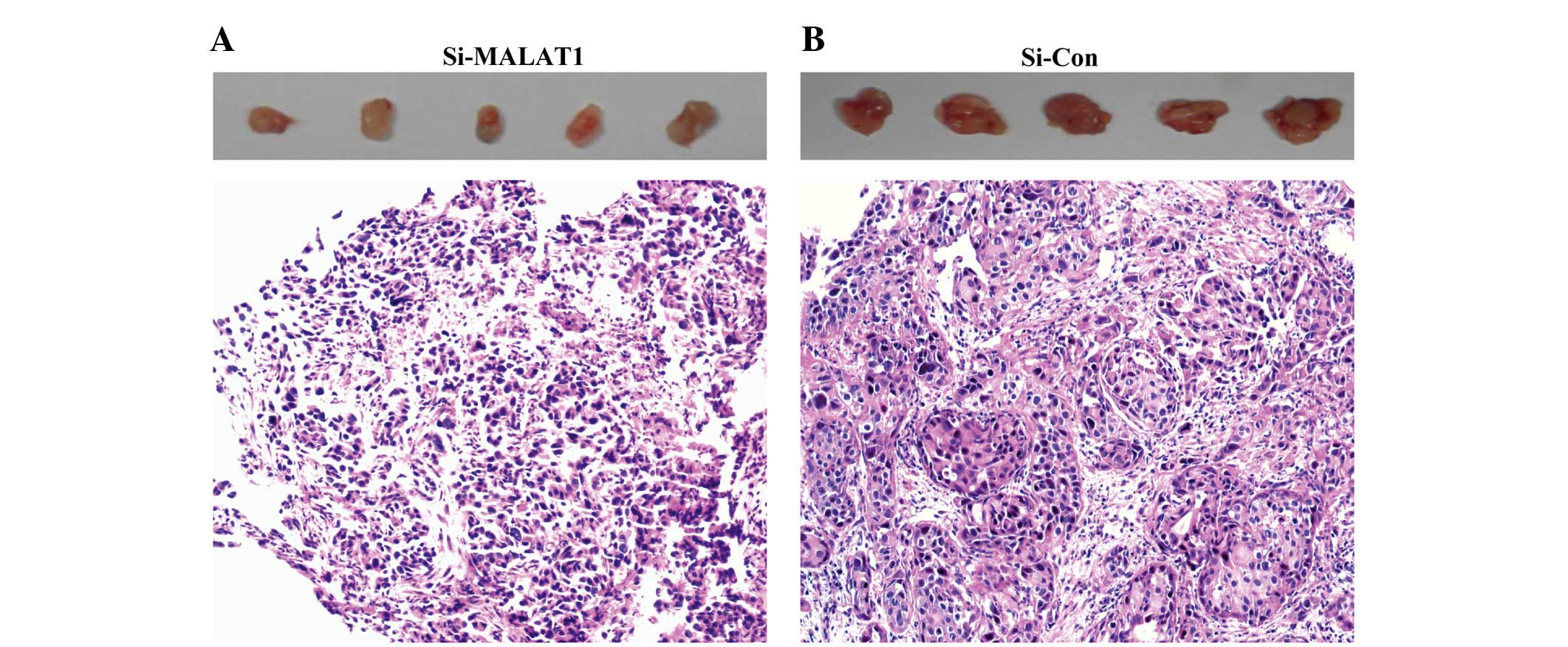
Downregulation of MALAT1 inhibits NSCLC
cell tumorigenesis in vivo
To explore whether the level of MALAT1 expression
affects tumorigenesis, ACC-LC-319/bone2 cells stably transfected
with si-MALAT1 or the empty vector were inoculated into nude mice.
Eighteen days after the injection, the tumors formed in the
si-MALAT1 group were substantially smaller than those that formed
in the control group (Fig. 5).
Discussion
In the present study, we investigated the clinical
significance of MALAT1 in NSCLC patients with bone metastasis for
the first time. Using qRT-PCR, our results indicated that lncRNA
MALAT1 was upregulated in NSCLC patients with bone metastasis and
lung cancer cells with high bone metastatic ability when compared
with the normal NSCLC tumor tissues, normal human lung
adenocarcinoma cell line SPC-A1 and normal human bronchial
epithelial cell line 16HBE. The present study also found that
expression of lncRNA-MALAT1 was involved in increasing the cellular
proliferation ability and inhibiting apoptosis of the NSCLC cells.
Moreover, lncRNA-MALAT1 promoted the migration, invasion and
tumorigenesis in vivo of NSCLC cells which suggest its
important role in the bone metastasis of NSCLC.
Long non-coding RNAs, which are >200 nt in
length, are unable to be translated into proteins. Recently, more
and more studies have shown that dysregulation of lncRNAs is
associated with the progression of cancer, such as HOX antisense
intergenic RNA (HOTAIR), cancer-upregulated drug resistant (CUDR),
prostate-specific transcript 1 (PCGEM1), and can be used as
biomarkers and prognosis factors (11). There is also increasing evidence
suggesting that these lncRNAs are involved in the biological
behavior of cancer cells, including proliferative capability and
replicative immortality, activation of invasion and metastasis, and
induction of angiogenesis and resistance of cell death (34,35).
MALAT1, also known as nuclear-enriched transcript 2,
was originally identified in 2003 via subtractive hybridization as
a prognostic marker for lung cancer metastasis. Currently, more and
more evidence has linked MALAT1 to several other human tumor
entities. Gutschner et al showed that MALAT1-deficient lung
cancer cells demonstrated impaired migratory ability and formed
fewer tumors (36). Ji et al
reported the increased proliferation and migration effect of MALAT1
in LoVo and HCT116 cells (27). A
recent loss-of-function study also unraveled the regulatory effect
of MALAT1 in gene expression governing hallmarks of lung cancer
metastasis (36). Ren et al
reported the increased expression of MALAT-1 in prostate cancer
which was correlated with Gleason score, prostate-specific antigen,
tumor stage and castration-resistant prostate cancer. Moreover,
downregulation of MALAT-1 significantly inhibited the cell growth,
invasion and migration of prostate cancer cells (37).
Alhough the effect of lncRNA MALAT1 in tumor growth
and invasion is well known, the effective mechanism still needs
further investigation. MALAT1 is specifically retained in nuclear
speckles, and MALAT1 functions as storage for small RNAs which is
broadly expressed in human tissues (38–41).
Another study reported that MALAT1 regulates the alternative
splicing of pre-mRNAs by modulating the levels of active
serine/arginine splicing factors. Recent studies have also reported
that the effects of lncRNA MALAT1 are associated with
tumor-suppressor gene SFPQ and proto-oncogene PTBP2. MALAT1 was
found to inhibit the combination of SFPQ protein with
proto-oncogene GAGE6 transcriptional regulatory regions, thus
releasing PTBP2 from the SFPQ/PTBP2 complex and promoting cell
proliferation and migration (42–44).
Ying et al demonstrated that MALAT1 promoted bladder cancer
cell migration by activating the Wnt pathway (45), and Wu et al suggested that
MALAT1 promotes the proliferation and metastasis of gallbladder
cancer cells by activating the ERK/MAPK pathway (46). Another study indicated that MALAT1
suppresses tumor growth and metastasis via the PI3K/AKT signaling
pathway (47). In addition,
mutations enriched in the 3′-end of MALAT1 in the MALAT1 gene were
recently discovered in breast cancer and CRC (40,48),
and these mutations were able to promote invasive behavior
(40). Guo et al showed that
the effect of MALAT1 was achieved through the regulation of gene
expression, such as caspase-3 and -8, Bcl-2, Bax and Bcl-xL
(31). In contrast, Tripathi et
al reported a critical involvement of MALAT1 in genome
stability and cell cycle checkpoint [MALAT1 depletion results in an
increased level of γH2AX (a DNA damage indicator) and accumulation
of the G2/M population]. Previous studies have also found that the
expression level of MALAT1 is controlled by methylation of histone
H3 (49), transcriptional factors
(50) and microRNAs (51,52);
however, evidence to explain its overexpression in diverse tumor
tissues is lacking.
In conclusion, the present study demonstrated that
lncRNA MALAT1 was upregulated in NSCLC tissues with bone metastasis
and in lung cancer cell lines with high bone metastatic ability.
The present study also found that expression of lncRNA MALAT1 was
involved in increasing the cellular proliferation ability and
inhibiting apoptosis of the NSCLC cells. In adddition, lncRNA
MALAT1 promoted the migration, invasion and tumorigenesis in
vivo of NSCLC cells which suggest the important role of MALAT1
in the bone metastasis of NSCLC.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gridelli C, Rossi A, Maione P, Ferrara ML,
Castaldo V and Sacco PC: Vaccines for the treatment of non-small
cell lung cancer: A renewed anticancer strategy. Oncologist.
14:909–920. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Verdecchia A, Francisci S, Brenner H,
Gatta G, Micheli A, Mangone L and Kunkler I; EUROCARE-4 Working
Group: Recent cancer survival in Europe: A 2000-02 period analysis
of EUROCARE-4 data. Lancet Oncol. 8:784–796. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pastorino U: Lung cancer screening. Br J
Cancer. 102:1681–1686. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wilusz JE, Sunwoo H and Spector DL: Long
noncoding RNAs: Functional surprises from the RNA world. Genes Dev.
23:1494–1504. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mercer TR, Dinger ME and Mattick JS: Long
non-coding RNAs: Insights into functions. Nat Rev Genet.
10:155–159. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gupta RA, Shah N, Wang KC, Kim J, Horlings
HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al: Long
non-coding RNA HOTAIR reprograms chromatin state to promote cancer
metastasis. Nature. 464:1071–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Loewer S, Cabili MN, Guttman M, Loh YH,
Thomas K, Park IH, Garber M, Curran M, Onder T, Agarwal S, et al:
Large intergenic non-coding RNA-RoR modulates reprogramming of
human induced pluripotent stem cells. Nat Genet. 42:1113–1117.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Esteller M: Non-coding RNAs in human
disease. Nat Rev Genet. 12:861–874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gibb EA, Brown CJ and Lam WL: The
functional role of long non-coding RNA in human carcinomas. Mol
Cancer. 10:382011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang J, Liu X, Wu H, Ni P, Gu Z, Qiao Y,
Chen N, Sun F and Fan Q: CREB up-regulates long non-coding RNA,
HULC expression through interaction with microRNA-372 in liver
cancer. Nucleic Acids Res. 38:5366–5383. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Khaitan D, Dinger ME, Mazar J, Crawford J,
Smith MA, Mattick JS and Perera RJ: The melanoma-upregulated long
noncoding RNA SPRY4-IT1 modulates apoptosis and invasion. Cancer
Res. 71:3852–3862. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang F, Zhang L, Huo XS, Yuan JH, Xu D,
Yuan SX, Zhu N, Zhou WP, Yang GS, Wang YZ, et al: Long noncoding
RNA high expression in hepatocellular carcinoma facilitates tumor
growth through enhancer of zeste homolog 2 in humans. Hepatology.
54:1679–1689. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cui Z, Ren S, Lu J, Wang F, Xu W, Sun Y,
Wei M, Chen J, Gao X, Xu C, et al: The prostate cancer-up-regulated
long noncoding RNA PlncRNA-1 modulates apoptosis and proliferation
through reciprocal regulation of androgen receptor. Urol Oncol.
31:1117–1123. 2013. View Article : Google Scholar
|
|
17
|
Wang Y, Chen W, Yang C, Wu W, Wu S, Qin X
and Li X: Long non-coding RNA UCA1a(CUDR) promotes proliferation
and tumorigenesis of bladder cancer. Int J Oncol. 41:276–284.
2012.PubMed/NCBI
|
|
18
|
Jin G, Sun J, Isaacs SD, Wiley KE, Kim ST,
Chu LW, Zhang Z, Zhao H, Zheng SL, Isaacs WB, et al: Human
polymorphisms at long non-coding RNAs (lncRNAs) and association
with prostate cancer risk. Carcinogenesis. 32:1655–1659. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kotake Y, Nakagawa T, Kitagawa K, Suzuki
S, Liu N, Kitagawa M and Xiong Y: Long non-coding RNA ANRIL is
required for the PRC2 recruitment to and silencing of
p15INK4B tumor suppressor gene. Oncogene. 30:1956–1962.
2011. View Article : Google Scholar
|
|
20
|
Zhou Y, Zhang X and Klibanski A: MEG3
noncoding RNA: A tumor suppressor. J Mol Endocrinol. 48:R45–R53.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Geng YJ, Xie SL, Li Q, Ma J and Wang GY:
Large intervening non-coding RNA HOTAIR is associated with
hepatocellular carcinoma progression. J Int Med Res. 39:2119–2128.
2011. View Article : Google Scholar
|
|
22
|
Kim K, Jutooru I, Chadalapaka G, Johnson
G, Frank J, Burghardt R, Kim S and Safe S: HOTAIR is a negative
prognostic factor and exhibits pro-oncogenic activity in pancreatic
cancer. Oncogene. 32:1616–1625. 2013. View Article : Google Scholar
|
|
23
|
Tu ZQ, Li RJ, Mei JZ and Li XH:
Down-regulation of long non-coding RNA GAS5 is associated with the
prognosis of hepatocellular carcinoma. Int J Clin Exp Pathol.
7:4303–4309. 2014.PubMed/NCBI
|
|
24
|
Yan TH, Lu SW, Huang YQ, Que GB, Chen JH,
Chen YP, Zhang HB, Liang XL and Jiang JH: Upregulation of the long
noncoding RNA HOTAIR predicts recurrence in stage Ta/T1 bladder
cancer. Tumour Biol. 35:10249–10257. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu XH, Lu KH, Wang KM, Sun M, Zhang EB,
Yang JS, Yin DD, Liu ZL, Zhou J, Liu ZJ, et al: MicroRNA-196a
promotes non-small cell lung cancer cell proliferation and invasion
through targeting HOXA5. BMC Cancer. 12:3482012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gutschner T, Hämmerle M, Eissmann M, Hsu
J, Kim Y, Hung G, Revenko A, Arun G, Stentrup M, Gross M, et al:
The noncoding RNA MALAT1 is a critical regulator of the metastasis
phenotype of lung cancer cells. Cancer Res. 73:1180–1189. 2013.
View Article : Google Scholar
|
|
27
|
Ji P, Diederichs S, Wang W, Böing S,
Metzger R, Schneider PM, Tidow N, Brandt B, Buerger H, Bulk E, et
al: MALAT-1, a novel noncoding RNA, and thymosin beta4 predict
metastasis and survival in early-stage non-small cell lung cancer.
Oncogene. 22:8031–8041. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hutchinson JN, Ensminger AW, Clemson CM,
Lynch CR, Lawrence JB and Chess A: A screen for nuclear transcripts
identifies two linked noncoding RNAs associated with SC35 splicing
domains. BMC Genomics. 8:392007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tseng JJ, Hsieh YT, Hsu SL and Chou MM:
Metastasis associated lung adenocarcinoma transcript 1 is
up-regulated in placenta previa increta/percreta and strongly
associated with trophoblast-like cell invasion in vitro. Mol Hum
Reprod. 15:725–731. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin R, Maeda S, Liu C, Karin M and
Edgington TS: A large noncoding RNA is a marker for murine
hepatocellular carcinomas and a spectrum of human carcinomas.
Oncogene. 26:851–858. 2007. View Article : Google Scholar
|
|
31
|
Guo F, Li Y, Liu Y, Wang J, Li Y and Li G:
Inhibition of metastasis-associated lung adenocarcinoma transcript
1 in CaSki human cervical cancer cells suppresses cell
proliferation and invasion. Acta Biochim Biophys Sin. 42:224–229.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zheng HT, Shi DB, Wang YW, Li XX, Xu Y,
Tripathi P, Gu WL, Cai GX and Cai SJ: High expression of lncRNA
MALAT1 suggests a biomarker of poor prognosis in colorectal cancer.
Int J Clin Exp Pathol. 7:3174–3181. 2014.PubMed/NCBI
|
|
33
|
Schmidt LH, Spieker T, Koschmieder S,
Schäffers S, Humberg J, Jungen D, Bulk E, Hascher A, Wittmer D and
Marra A: The long noncoding MALAT-1 RNA indicates a poor prognosis
in non-small cell lung cancer and induces migration and tumor
growth. J Thorac Oncol. 6:1984–1992. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yuan SX, Yang F, Yang Y, Tao QF, Zhang J,
Huang G, Yang Y, Wang RY, Yang S, Huo XS, et al: Long noncoding RNA
associated with microvascular invasion in hepatocellular carcinoma
promotes angiogenesis and serves as a predictor for hepatocellular
carcinoma patients' poor recurrence-free survival after
hepatectomy. Hepatology. 56:2231–2241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gutschner T and Diederichs S: The
hallmarks of cancer: A long non-coding RNA point of view. RNA Biol.
9:703–719. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gutschner T, Hämmerle M and Diederichs S:
MALAT1 - a paradigm for long noncoding RNA function in cancer. J
Mol Med Berl. 91:791–801. 2013. View Article : Google Scholar
|
|
37
|
Ren S, Liu Y, Xu W, Sun Y, Lu J, Wang F,
Wei M, Shen J, Hou J, Gao X, et al: Long noncoding RNA MALAT-1 is a
new potential therapeutic target for castration resistant prostate
cancer. J Urol. 190:2278–2287. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wilusz JE, Freier SM and Spector DL: 3′
end processing of a long nuclear-retained noncoding RNA yields a
tRNA-like cytoplasmic RNA. Cell. 135:919–932. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tripathi V, Ellis JD, Shen Z, Song DY, Pan
Q, Watt AT, Freier SM, Bennett CF, Sharma A, Bubulya PA, et al: The
nuclear-retained noncoding RNA MALAT1 regulates alternative
splicing by modulating SR splicing factor phosphorylation. Mol
Cell. 39:925–938. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xu C, Yang M, Tian J, Wang X and Li Z:
MALAT-1: A long non-coding RNA and its important 3′ end functional
motif in colorectal cancer metastasis. Int J Oncol. 39:169–175.
2011.PubMed/NCBI
|
|
41
|
Birney E, Stamatoyannopoulos JA, Dutta A,
Guigó R, Gingeras TR, Margulies EH, Weng Z, Snyder M, Dermitzakis
ET, Thurman RE, et al Children's Hospital Oakland Research
Institute: Identification and analysis of functional elements in 1%
of the human genome by the ENCODE pilot project. Nature.
447:799–816. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li L, Feng T, Lian Y, Zhang G, Garen A and
Song X: Role of human noncoding RNAs in the control of
tumorigenesis. Proc Natl Acad Sci USA. 106:12956–12961. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ji Q, Zhang L, Liu X, Zhou L, Wang W, Han
Z, Sui H, Tang Y, Wang Y, Liu N, et al: Long non-coding RNA MALAT1
promotes tumour growth and metastasis in colorectal cancer through
binding to SFPQ and releasing oncogene PTBP2 from SFPQ/PTBP2
complex. Br J Cancer. 111:736–748. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Meissner M, Dechat T, Gerner C, Grimm R,
Foisner R and Sauermann G: Differential nuclear localization and
nuclear matrix association of the splicing factors PSF and PTB. J
Cell Biochem. 76:559–566. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ying L, Chen Q, Wang Y, Zhou Z, Huang Y
and Qiu F: Upregulated MALAT-1 contributes to bladder cancer cell
migration by inducing epithelial-to-mesenchymal transition. Mol
Biosyst. 8:2289–2294. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wu XS, Wang XA, Wu WG, Hu YP, Li ML, Ding
Q, Weng H, Shu YJ, Liu TY, Jiang L, et al: MALAT1 promotes the
proliferation and metastasis of gallbladder cancer cells by
activating the ERK/MAPK pathway. Cancer Biol Ther. 15:806–814.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dong Y, Liang G, Yuan B, Yang C, Gao R and
Zhou X: MALAT1 promotes the proliferation and metastasis of
osteosarcoma cells by activating the PI3K/Akt pathway. Tumour Biol.
36:1477–1486. 2015. View Article : Google Scholar
|
|
48
|
Ellis MJ, Ding L, Shen D, Luo J, Suman VJ,
Wallis JW, Van Tine BA, Hoog J, Goiffon RJ, Goldstein TC, et al:
Whole-genome analysis informs breast cancer response to aromatase
inhibition. Nature. 486:353–360. 2012.PubMed/NCBI
|
|
49
|
Tee AE, Ling D, Nelson C, Atmadibrata B,
Dinger ME, Xu N, Mizukami T, Liu PY, Liu B, Cheung B, et al: The
histone demethylase JMJD1A induces cell migration and invasion by
up-regulating the expression of the long noncoding RNA MALAT1.
Oncotarget. 5:1793–1804. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Fan Y, Shen B, Tan M, Mu X, Qin Y, Zhang F
and Liu Y: TGF-β-induced upregulation of malat1 promotes bladder
cancer metastasis by associating with suz12. Clin Cancer Res.
20:1531–1541. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Han Y, Liu Y, Zhang H, Wang T, Diao R,
Jiang Z, Gui Y and Cai Z: Hsa-miR-125b suppresses bladder cancer
development by down-regulating oncogene SIRT7 and oncogenic long
non-coding RNA MALAT1. FEBS Lett. 587:3875–3882. 2013. View Article : Google Scholar
|
|
52
|
Leucci E, Patella F, Waage J, Holmstrøm K,
Lindow M, Porse B, Kauppinen S and Lund AH: microRNA-9 targets the
long non-coding RNA MALAT1 for degradation in the nucleus. Sci Rep.
3:25352013. View Article : Google Scholar : PubMed/NCBI
|