Introduction
Oral squamous cell carcinoma (OSCC) is the 6th most
frequent malignant neoplasm and remains a lethal disease for more
than 50% of the patients annually. OSCC patients die of tumor
metastasis and recurrence due to its strong invasion potential
(1). MicroRNAs (miRNAs) are small
non-coding RNAs which are frequently dysregulated in human
malignancies (2). Recently, miRNAs
have emerged as fundamental regulators in gene expression through
silencing gene expression at the post-transcriptional and
translational levels. In this study, microarray analysis of miRNA
expression change in the OSCC patient specimens and OSCC cell lines
was firstly identified. Through the analysis of the multiple
experimental data, miRNAs that displayed >3-fold changes in OSCC
patients and cell lines were recorded. Then, miR-27a-3p was
selected as the most potential gene which might be involved in the
occurrence and development progress of OSCC when combined with the
clinical prognosis and T stage in the statistical analysis.
However, the underlying mechanism of OSCC related to miR-27a-3p
still remains largely unknown.
In this study, we aimed to reveal the biological
function of miR-27a-3p related to OSCC, especially in the tumor
invasion. Through Target prediction algorithms (3) and dual-luciferase reporter assays, we
found that YAP1 (Yes-associated protein-1) was a direct target gene
of miR-27a-3p. Consistently, the expression of active YAP1 was
negatively correlated with that of miR-27a-3p in OSCC cell lines.
YAP1, a transcriptional co-activator, is the oncogenic component of
the Hippo signaling pathway and contributes to cell self-renewal to
modulate the pluripotency of embryonic stem cells in various
organisms (4). We found that YAP1
mRNA and protein were expressed at a higher level in OSCC tumor
specimens and related cell lines. Intriguingly, overexpression of
miR-27a-3p in OSCC cell lines could significantly decrease the
expression level of YAP1 but also, markedly decreased epithelial to
mesenchymal transition (EMT)-related factors simultaneously.
Epithelial to mesenchymal transition (EMT) is a critical step in
the dissemination of malignant diseases (5). In primary tumors, carcinoma cells lose
cell-cell adhesion mediated by E-cadherin repression and break
through the basement membrane with increased invasive properties.
Upregulated expression of EMT-activating transcription factors,
including Snail, Twist families and ZEB, could promote tumor
invasiveness in xenograft tumor models and cancer cell lines
(6). Recently, several studies have
shown that miRNAs could regulate invasiveness and metastasis by
targeting the transcripts of numerous genes involved in EMT
regulation in human cancers (7–9).
However, intrinsic relationship of OSCC progression related to EMT
factors and miRNA regulation still need further research.
In this study, through the gene microarray data
analysis and western blot assays, we found miR-27a-3p in OSCC cell
lines suppressed its target gene YAP1 expression level and
unpredictability decreased the expression level of major
EMT-activating transcription factors. Conversely, miR-27a-3p
inhibitor transfection reversed this process and promoted cancer
invasion by stimulating EMT in OSCC cell lines. Mechanism study
showed that miR-27a-3p inhibit EMT in OSCC cell lines and might be
involved in the regulation of YAP1-OCT4/Sox2 signal axis. We report
that miR-27a-3p acts as an important upstream regulator related to
the EMT via the inhibition of YAP1 in OSCC, which might provide
scientific foundation of clinical diagnostics and biomedical
research.
Materials and methods
Cell lines and tissue specimens
Human OSCC cell lines Tca8113, CAL-27, SCC-4, SCC-9,
SCC-25, HN-6 and human normal oral keratinocytes (hNOK) cell lines
were purchased from the American Type Culture Collection (ATCC).
All cell lines were cultured with the complete medium DMEM-F12
medium (Invitrogen) with 10% heat-inactivated fetal bovine serum
(FBS; Gibco), 100 U/ml of penicillin G sodium, and 100 µg/ml
streptomycin sulfate (Sigma) in a humidified atmosphere containing
5% CO2 and 37°C. Fifty paired samples of OSCC tissue and
adjacent non-cancerous tissue were obtained from patients with oral
squamous cell carcinoma in our department. All the patients
provided written informed consent. The histologic diagnosis of
tumors was made and agreed upon by two senior pathologists based on
World Health Organization (WHO) criteria. This study was approved
by the Institutional Review Board and Human Ethics Committee of the
Hospital. Tissue samples were flash frozen and stored in liquid
nitrogen until used.
Quantitative real-time PCR analysis
Total RNA for quantitative real-time PCR (qRT-PCR)
analysis was extracted using 74104 Rneasy Mini kit (Qiagen) and
reverse-transcribed into cDNA with the Reverse Transcriptase MMLV
(Takara) according to the manufacturer's protocol. Real-time PCR
was performed using SYBR Green reagents on the iQ5 Real-Time PCR
detection system (both from Bio-Rad). For analysis of miR-27a-3p
expression by qRT-PCR, reverse transcription and PCR were carried
out using Bulge-Loop miRNA qPCR Primer Set for hsa-miR-143 and U6
snRNA (both from RiboBio, China) according to the manufacturer's
instructions. The relative expression level was normalized to that
of internal control U6 by using 2−ΔΔCt cycle threshold
method. Primer sequence miR-27a-3p, 5′-ACACTCCAGCTGGGTTCACAGT
GGCTAAG-3′; U6, 5′-CTCGCTTCGGCAGCACA-3′.
miRNA profiling
miRNA expression profiling was performed using total
RNA with the Total miRNA qPCR array (System Biosciences, Mountain
View, CA, USA). All miRNAs were registered with the Sanger miRBase
database. Small nuclear U6 RNA was amplified as normalization
control. qPCR was also performed using SYBR-Green as mentioned
above. The analysis was performed using software provided by System
Biosciences.
Luciferase reporter assay
The 3′-untranslated region (3′UTR) of the human YAP1
gene that was predicted to interact with miR-27a-3p
(pMIR-REPORT-YAP1) was synthesized and inserted into pMIR-REPORT
(Ambion Inc.). Mutations within potential binding sites were
generated by nucleotide replacement of wild-type sequence to
inhibit the miR-27a-3p binding. hsa-miR-27a-3p mimic and the
negative control were purchased from Ribobio®
(miR10000084-1-5). Anti-hsa-miR-27a-3p miScript miRNA inhibitor and
miScript inhibitor negative control purchased from Qiagen
(MIN0000084). Tca8113 cells were plated into 96-well plates and
were co-transfected with 0.4 mg of the reporter construct, 0.2 mg
of control vector, and miR-27a-3p negative controls (NC) after 24 h
incubation. Luciferase values were determined using the
Dual-Luciferase reporter assay system.
Cell invasion assay
Cell invasion assays were performed using millicell
chambers (Millipore). The chamber was cultivated in 5%
CO2 for 24 h after the suspension cells (200 µl,
5×104 cells) added to the upper chamber. FBS medium
(0.5%) with 10% FBS (600 µl) was added to the lower chamber.
Attached cells in the lower section were stained with 0.1% crystal
violet 24 h later. The invasion rate were quantified by counting
the invasion cells in six random fields under a light
microscope.
Western blot analysis
Antibodies: YAP1 (CST8579); OCT4 (CST2750); Sox2
(CST3579); and Snail (CST3879) (all from Cell Signaling Technology,
USA); Twist (AV37997; Sigma); vimentin (CST5741); E-cadherin
(CST5296); and β-actin (CST4970) (all from Cell Signaling
Technology). Cellular lysates were resolved on SDS-PAGE and
electrophoretically transferred to polyvinylidene difluoride
membranes. Membranes were blocked with a buffer containing Tris (10
mmol/l, pH 7.4), NaCl (150 mmol/l), Tween-20 (0.1%) and bovine
serum albumin (5%) and then incubated with the primary antibodies
at 4°C overnight. Subsequently, washed membranes was treated with
appropriate secondary antibodies conjugated to horseradish
peroxidase for 1 h. The immunoreactivities were visualized by
enhanced chemiluminescence reagents (WBKLS0500; Millipore). β-actin
was used as an internal control.
Statistical analysis
All statistical analyses were performed using SPSS
13.0 software. The data are expressed as mean ± SD, and one-way
ANOVA and an unpaired Student's t-test was used to determine the
significant differences of all the results. The level of
significance was set at p<0.05.
Results
miRNA profiling of OSCC tissues and cell
lines
miRNAs are recognized as important regulators of
post-transcriptional gene expression. In light of miRNA functions,
we profiled isolated cells from freshly resected tumors of OSCC
patients (n=5) and OSCC cell lines (n=2; Tca8113 and CAL-27) using
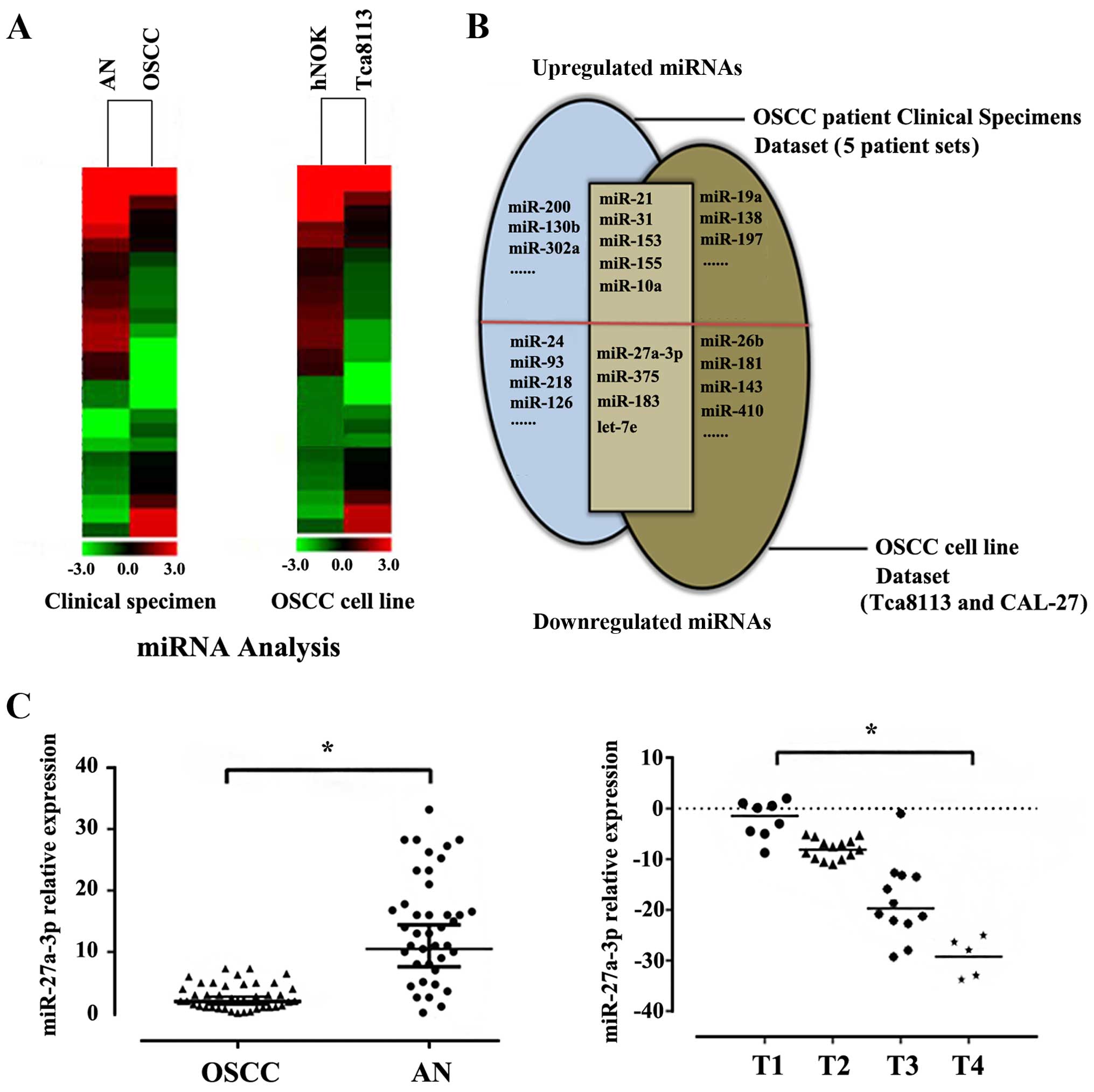
a Total SYBR Green-based qPCR miRNA array (Fig. 1A). Then, we combined the two
different data sets in a Venn diagram and observed
expression-altered miRNAs (Fig.
1B). miRNAs that displayed >3-fold changes in OSCC patients
and common to cell lines were recorded (Table I). Analysis of clinical data and
histopathological findings of 50 OSCC samples identified that lower
expressions of miR-27a-3p was correlated with poorer outcome of
metastatic in OSCC patients. Thus, miR-27a-3p was selected as the
most potential gene involved in the occurrence and development
progress of OSCC for further investigation (Fig. 1C).
 | Table IDysregulated miRNAs in OSCC patients
and OSCC cell lines. |
Table I
Dysregulated miRNAs in OSCC patients
and OSCC cell lines.
| miRNAs | Fold change
|
|---|
| (Clinical) | Tca8113 | CAL-27 |
|---|
| High expression
>3-fold |
| miRNA-21 | 11.13 | 3.53 | 4.03 |
| miRNA-31 | 6.35 | 4.21 | 5.37 |
| miRNA-153 | 6.16 | 3.04 | 3.25 |
| miRNA-155 | 5.53 | 3.48 | 4.13 |
| miRNA-10a | 4.82 | 4.42 | 3.54 |
| Low expression
<0.3-fold |
| miRNA-27a-3p | 0.108 | 0.133 | 0.201 |
| miRNA-375 | 0.193 | 0.178 | 0.227 |
| miRNA-183 | 0.236 | 0.288 | 0.112 |
| let-7e | 0.293 | 0.211 | 0.172 |
miR-27a-3p directly targets YAP1 in OSCC
cell lines
To investigate the bio-function of miR-27a-3p in
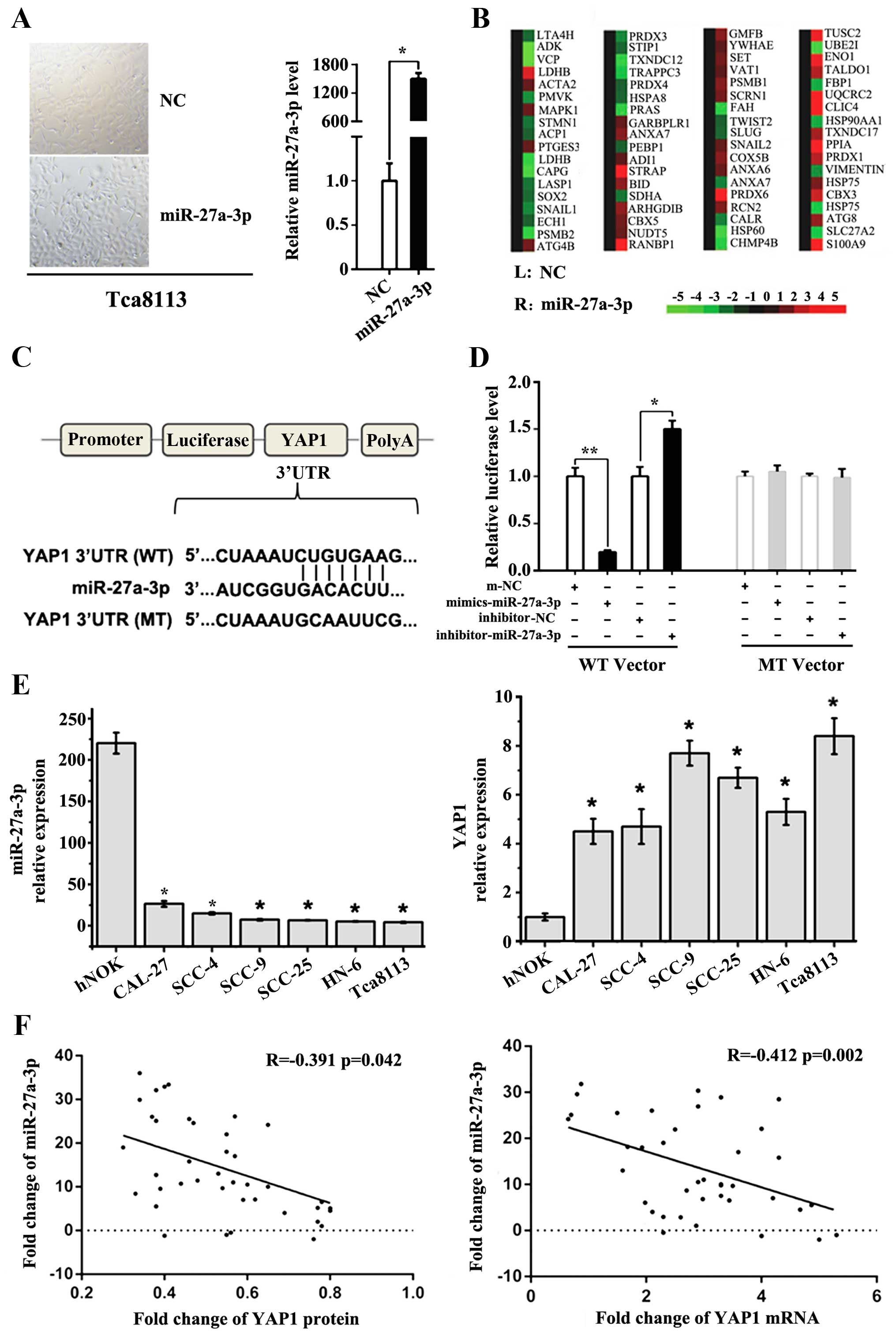
OSCC, Tca8113 cell lines transfected with miR-27a-3p mimics were
successfully established (Fig. 2A).
Then, SYBR Green-Based Gene expression array analysis of cancer
related genes was employed to identify the related genes expression
changes between the above cell groups (Fig. 2B). Through the data analysis and
miRNA target prediction, YAP1 (Yes-associated protein-1) was
finally identified as a new potential target of miR-27a-3p
(Fig. 2C). Results of
dual-luciferase reporter assays confirmed that miR-27a-3p directly
target YAP1 mRNA 3′UTR region in OSCC cell lines (Fig. 2D). YAP1 is a potent oncogene, which
is amplified in various human cancers. An inverse correlation was
also observed between the expression levels of miR-27a-3p and YAP1
in both OSCC cell lines and tumor samples (Fig. 2E and F). However, intriguingly, SYBR
Green-based gene expression array also showed that overexpression
of miR-27a-3p in Tca8113 significantly decreased several epithelial
to mesenchymal transition (EMT)-activating transcription factors,
including Sox2, vimentin (10),
Snail (11) and Twist (12). Therefore, Further studies were
needed to reveal the regulatory network between miR-27a-3p and EMT
in human OSCC cells.
miR-27a-3p inhibits EMT-activating
transcription factors through the YAP1-OCT4-Sox2 signal axis
Recent studies have shown YAP1 could regulate OCT4
activity and Sox2 expression to facilitate self-renewal in lung
cancer stem-like cells (13) and
Sox2 promoted tumor metastasis by stimulating
epithelial-to-mesenchymal transition (EMT)-transcription factors in
various tumor tissues (14), which
provide some clues as to how miR-27a-3p acts on EMT process in OSCC
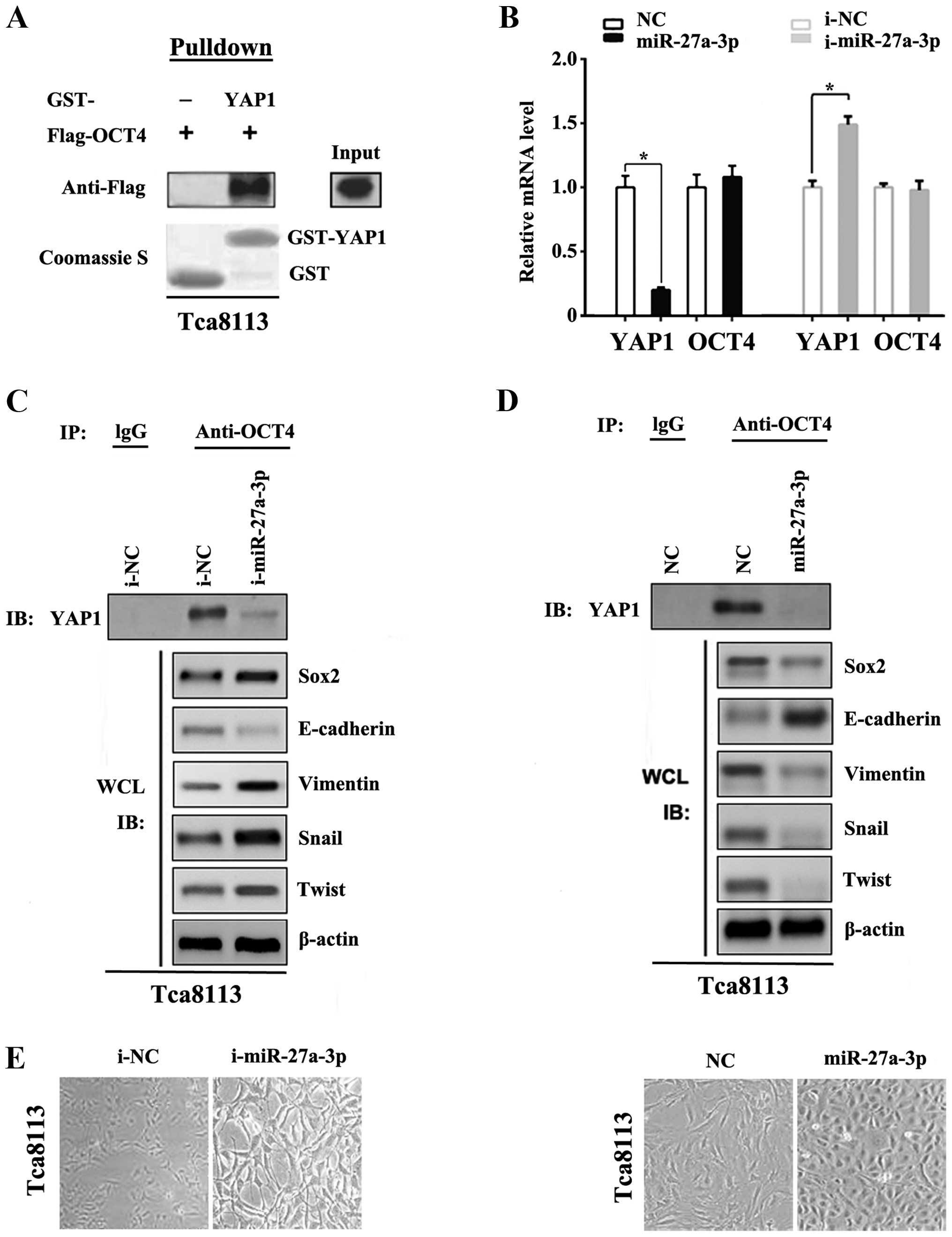
cells. To verify the interaction between YAP1 and OCT4 in Tca8113,
GST-pull down assay was used in this study. Results confirmed that
YAP1 could bind to OCT4 directly in Tca8113 cells (Fig. 3A). Then, we detected the mRNA
expression level of YAP1 and OCT4 by the real-time qPCR in the
presence or absence of miR-27a-3p or miR-27a-3p inhibitor
transfection. As the results show in Fig. 3B, miR-27a-3p decreased the YAP1 mRNA
level significantly, while the transfection of miR-27a-3p inhibitor
derepressed YAP1 mRNA level reversely. The OCT4 expression level
was not affected in either treatment group. Subsequently,
co-immunoprecipitation and western blot assays were both employed
to reveal the inner relationship of miR-27a-3p, YAP1 and
transcription factors of EMT. In the EMT process, loss of the
epithelial marker E-cadherin, and increased expression of
mesenchymal markers vimentin are regarded as markers of EMT
activation. Additionally, Snail1/2 and Twist1/2 are also the main
upstream inducers of EMT. As shown in Fig. 3C and D, miR-27a-3p was able to
decrease the YAP1 expression and influence EMT-related inducers
Snail and Twist. miR-27a-3p transfection also induced morphological
changes from an extended, fibroblast-like morphology to more
epithelial-like cells in Tca8113 (Fig.
3E). Taken together, our findings demonstrated miR-27a-3p acted
as one of the vital upstream molecules that mediate the EMT via
interruption of YAP1-OCT4/Sox2 signal axis through targeting YAP1
in OSCC cells.
miR-27a-3p transfection effectively
decreases the invasive ability of OSCC cell lines
Description of small aggregates of tumor cells
extending from the tumor mass into the adjacent stroma provided
morphological evidence of EMT at invasive fronts of human tumors
(15). Through prior experimental
results and data analysis, we identified miR-27a-3p as one of vital
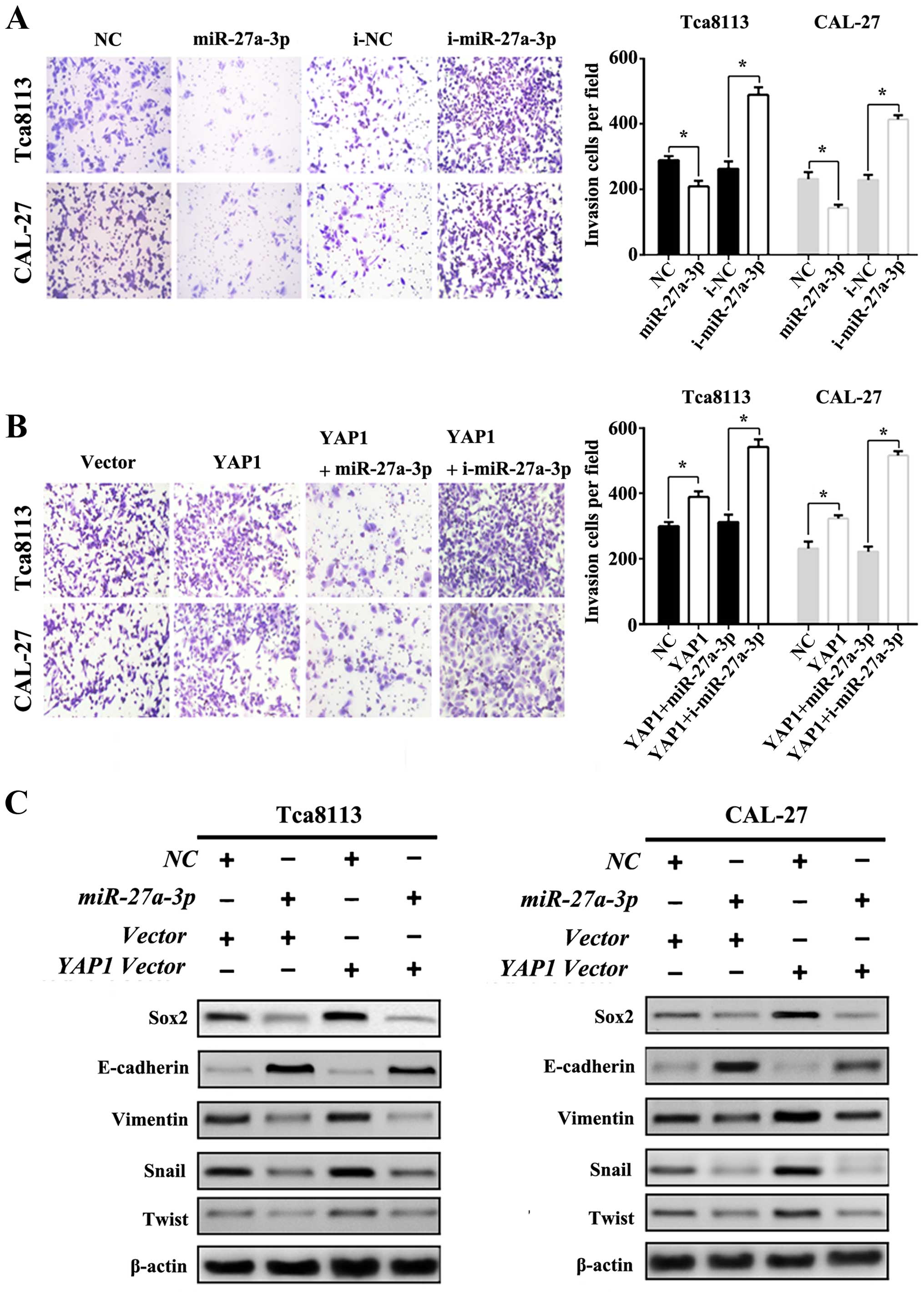
upstream molecules that mediate the EMT in OSCC cell lines. Cell
matrix adhesion assay was used to explore the cell invasive ability
in the presence or absence of miR-27a-3p transfection in the OSCC
cell lines Tca8113 and CAL-27. As shown in Fig. 4A, miR-27a-3p decreased the invasive
ability of OSCC cell lines significantly, in contrast, the presence
of miR-27a-3p inhibitor reversed miR-27a-3p-induced inhibition of
cell invasive ability. Furthermore, recombinant plasmid pVAX-YAP1
(referred as YAP1 vector) was constructed and used in the
subsequent experiments. As shown in the Fig. 4B, increased expression of YAP1 could
promote the invasive ability of OSCC cell lines. However,
miR-27a-3p expression inhibited the invasive effect of YAP1 while
miR-27a-3p inhibitor enhanced the invasive effect of YAP1
significantly. Then, western blot assays also confirmed that
miR-27a-3p could decrease the expression level of EMT-transcription
factors in OSCC cell lines Tca8113 and CAL-27 (as shown in Fig. 4C), suggesting that miR-27a-3p might
play pivotal roles in effectively manipulating invasion through the
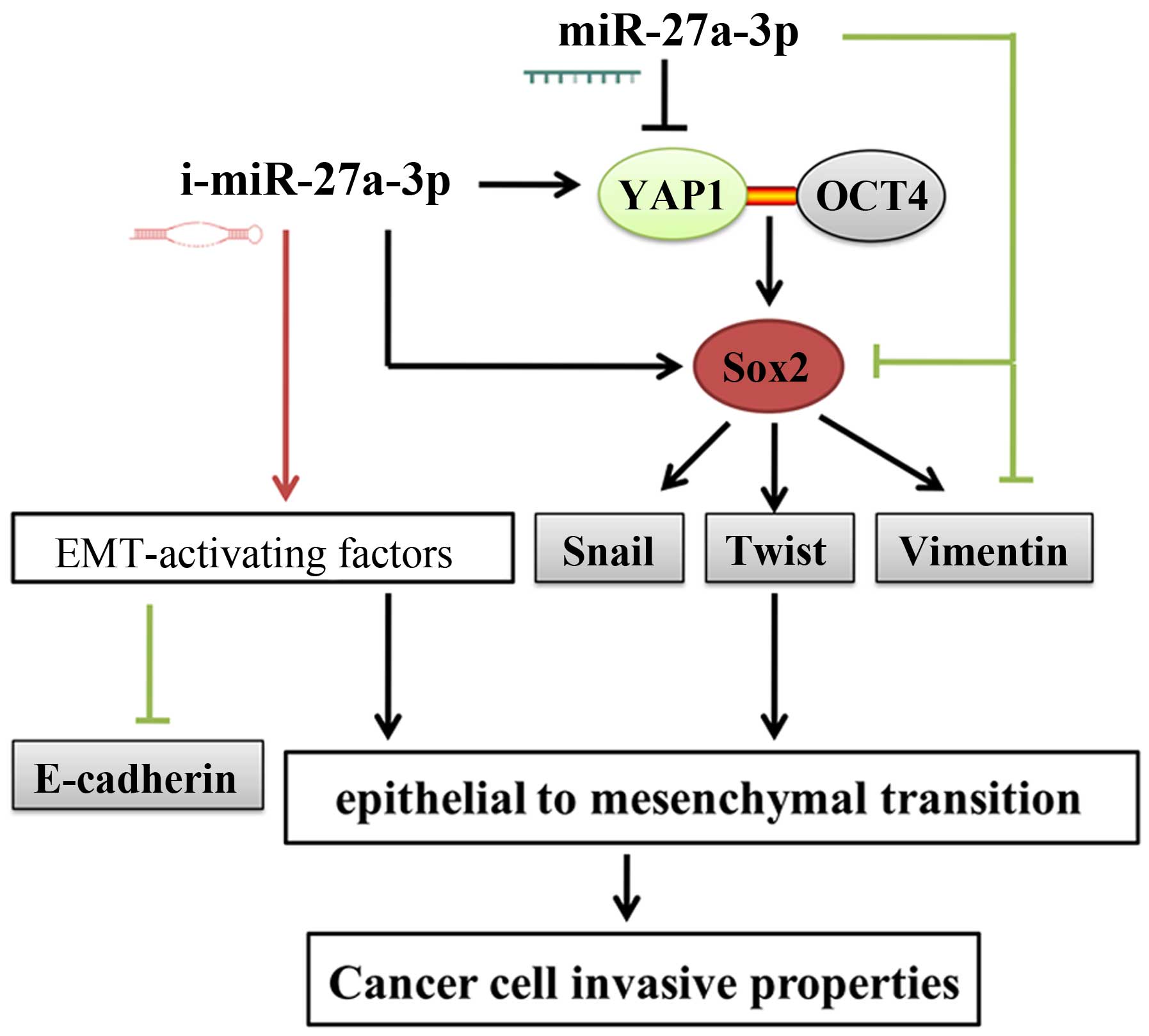
inhibition of EMT in OSCC cells. To better understand the
miR-27a-3p function related to the EMT process, graphical
illustration of this study is shown in Fig. 5.
Discussion
MicroRNAs (miRNAs) are endogenous small non-coding
RNAs that play crucial roles in numerous biological processes
(16–18). However, the role of miRNAs in OSCC
has not been fully determined. In this study, we profiled isolated
cells from freshly resected tumors from OSCC patients and OSCC cell
lines using a SYBR-Green-based qPCR miRNA array to identify the
expression change of miRNAs. Based on the databases and clinical
analysis related to the clincopathological factors in 50 OSCC
cases, miR-27a-3p was identified as a potential oncogene in OSCC.
Furthermore, SYBR-Green-based gene expression array analysis was
employed to identify the related gene expression changes after
Tca8113 transfected with miR-27a-3p mimics or the miR-27a-3p
negative control. Intriguingly, the status of expression of several
epithelial to mesenchymal transition (EMT)-activating transcription
factors, including Sox2, Snail and Twist, were significantly
decreased in the miR-27a-3p group when compared with the NC
group.
The epithelial-mesenchymal transition (EMT) is a
process by which epithelial cells lose their cell polarity and
cell-cell adhesion, which have been regarded as a key developmental
program that is often activated during the initiation of metastasis
for cancer progression. Many oncogenes have been reported to be
involved in tumor growth and metastasis by inducing EMT in various
cancers, including OSCC (19).
Preliminary data indicate that the main EMT drivers, such as Snail
and Twist, could repress the expression of the miR-200 family, but
whether miRNAs can also control their expression still need further
investigation (20,21). In our study, we identified that YAP1
(Yes-associated protein-1) was the direct target gene of miR-27a-3p
through dual-luciferase reporter assay. YAP1 is a oncogenic
component of the Hippo signaling pathway that has become the new
target of antitumor therapy (22,23).
Recently, it has already been reported that YAP1 might act as a
transcriptional co-activator for Oct4 and the disruption of this
interaction could abrogate the induction of Sox2 (13). Sox2 is a key upregulated factor in
lung squamous cell carcinoma, directing many genes involved in
tumor progression. Previous studies also proved that Sox2 improves
invasiveness of breast cancer cells by promoting EMT which is
dependent on Twist1 and the status of Sox2 transcription activity
(24). To verify the interaction
between miR-27a-3p and EMT process, GST-pull down in combination
with co-immunoprecipitation and western blot assays were employed
to verify our hypothesis. As expected, miR-27a-3p could decrease
the YAP1 expression level and EMT-activating transcription factors,
which might attribute to the interruption of YAP1-OCT4/Sox2 signal
axis.
By prior experimental results and analysis, we
identify miR-27a-3p as one of vital upstream molecules that could
mediate the EMT-activating transcription factors through directly
target 3′UTR region of YAP1 in OSCC cell lines. With the presence
of miR-27a-3p transfection, YAP1 expression was inhibited and
induction of Sox2 was also abrogated since the interaction of
YAP1-OCT4 was disrupted. In conclusion, this study supports a
possible new mechanism that miR-27a-3p could suppress tumor
progression by inhibiting EMT through the YAP1-OCT4/Sox2 signal
axis. Our results might provide scientific foundation of clinical
diagnostics and biomedical research on miR-27a-3p and EMT factors
in human cancers.
Acknowledgments
pVAX-YAP1 and the blank vector pVAX plasmid were
gifts from Professor Wu Shi (Tsinghua University).
References
|
1
|
Leusink FK, van Es RJ, de Bree R,
Baatenburg de Jong RJ, van Hooff SR, Holstege FC, Slootweg PJ,
Brakenhoff RH and Takes RP: Novel diagnostic modalities for
assessment of the clinically node-negative neck in oral
squamous-cell carcinoma. Lancet Oncol. 13:e554–e561. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Benfey PN: Molecular biology: microRNA is
here to stay. Nature. 425:244–245. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Park K and Kim KB: miRTar Hunter: A
prediction system for identifying human microRNA target sites. Mol
Cells. 35:195–201. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sudol M: YAP1 oncogene and its eight
isoforms. Oncogene. 32:39222013. View Article : Google Scholar
|
|
5
|
Sleeman JP and Thiery JP: SnapShot: The
epithelial-mesenchymal transition. Cell. 145:162.e12011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sánchez-Tilló E, Liu Y, de Barrios O,
Siles L, Fanlo L, Cuatrecasas M, Darling DS, Dean DC, Castells A
and Postigo A: EMT-activating transcription factors in cancer:
Beyond EMT and tumor invasiveness. Cell Mol Life Sci. 69:3429–3456.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li J, Wang Q, Wen R, Liang J, Zhong X,
Yang W, Su D and Tang J: miR-138 inhibits cell proliferation and
reverses epithelial-mesenchymal transition in non-small cell lung
cancer cells by targeting GIT1 and SEMA4C. J Cell Mol Med.
19:2793–2805. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang L, Yang J, Li J, Shen X, Le Y, Zhou
C, Wang S, Zhang S, Xu D and Gong Z: MircoRNA-33a inhibits
epithelial-to-mesenchymal transition and metastasis and could be a
prognostic marker in non-small cell lung cancer. Sci Rep.
5:136772015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qu MH, Han C, Srivastava AK, Cui T, Zou N,
Gao ZQ and Wang QE: miR-93 promotes TGF-β-induced
epithelial-to-mesenchymal transition through downregulation of
NEDD4L in lung cancer cells. Tumour Biol. Nov 18–2015.Epub ahead of
print.
|
|
10
|
Vuoriluoto K, Haugen H, Kiviluoto S,
Mpindi JP, Nevo J, Gjerdrum C, Tiron C, Lorens JB and Ivaska J:
Vimentin regulates EMT induction by Slug and oncogenic H-Ras and
migration by governing Axl expression in breast cancer. Oncogene.
30:1436–1448. 2011. View Article : Google Scholar
|
|
11
|
Naber HP, Drabsch Y, Snaar-Jagalska BE,
ten Dijke P and van Laar T: Snail and Slug, key regulators of
TGF-β-induced EMT, are sufficient for the induction of single-cell
invasion. Biochem Biophys Res Commun. 435:58–63. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Smit MA and Peeper DS: Deregulating EMT
and senescence: Double impact by a single twist. Cancer Cell.
14:5–7. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bora-Singhal N, Nguyen J, Schaal C,
Perumal D, Singh S, Coppola D and Chellappan S: YAP1 regulates OCT4
activity and SOX2 expression to facilitate self-renewal and
vascular mimicry of stem-like cells. Stem Cells. 33:1705–1718.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li X, Xu Y, Chen Y, Chen S, Jia X, Sun T,
Liu Y, Li X, Xiang R and Li N: SOX2 promotes tumor metastasis by
stimulating epithelial-to-mesenchymal transition via regulation of
WNT/β-catenin signal network. Cancer Lett. 336:379–389. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Choi HJ, Park KJ, Shin JS, Roh MS, Kwon HC
and Lee HS: Tumor budding as a prognostic marker in stage-III
rectal carcinoma. Int J Colorectal Dis. 22:863–868. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu P, Li Y, Yang S, Yang H, Tang J and Li
M: Micro-ribonucleic acid 143 (miR-143) inhibits oral squamous cell
carcinoma (OSCC) cell migration and invasion by downregulation of
phospho-c-Met through targeting CD44 v3. Oral Surg Oral Med Oral
Pathol Oral Radiol. 120:43–51. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang HP, Wang YH, Cao CJ, Yang XM, Ma SC,
Han XB, Yang XL, Yang AN, Tian J, Xu H, et al: A regulatory circuit
involving miR-143 and DNMT3a mediates vascular smooth muscle cell
proliferation induced by homocysteine. Mol Med Rep. 13:483–490.
2016.
|
|
18
|
Zhang X, Zheng L, Sun Y, Wang T and Wang
B: Tangeretin enhances radiosensitivity and inhibits the
radiation-induced epithelial-mesenchymal transition of gastric
cancer cells. Oncol Rep. 34:302–310. 2015.PubMed/NCBI
|
|
19
|
Chaw SY, Majeed AA, Dalley AJ, Chan A,
Stein S and Farah CS: Epithelial to mesenchymal transition (EMT)
biomarkers -E-cadherin, beta-catenin, APC and vimentin - in oral
squamous cell carcinogenesis and transformation. Oral Oncol.
48:997–1006. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gill JG, Langer EM, Lindsley RC, Cai M,
Murphy TL, Kyba M and Murphy KM: Snail and the microRNA-200 family
act in opposition to regulate epithelial-to-mesenchymal transition
and germ layer fate restriction in differentiating ESCs. Stem
Cells. 29:764–776. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kong D, Li Y, Wang Z, Banerjee S, Ahmad A,
Kim HR and Sarkar FH: miR-200 regulates PDGF-D-mediated
epithelial-mesenchymal transition, adhesion, and invasion of
prostate cancer cells. Stem Cells. 27:1712–1721. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Keren-Paz A, Emmanuel R and Samuels Y: YAP
and the drug resistance highway. Nat Genet. 47:193–194. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cottini F, Hideshima T, Xu C, Sattler M,
Dori M, Agnelli L, ten Hacken E, Bertilaccio MT, Antonini E, Neri
A, et al: Rescue of Hippo coactivator YAP1 triggers DNA
damage-induced apoptosis in hematological cancers. Nat Med.
20:599–606. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu F, Ye X, Wang P, Jung K, Wu C, Douglas
D, Kneteman N, Bigras G, Ma Y and Lai R: Sox2 suppresses the
invasiveness of breast cancer cells via a mechanism that is
dependent on Twist1 and the status of Sox2 transcription activity.
BMC Cancer. 13:3172013. View Article : Google Scholar : PubMed/NCBI
|