Introduction
Esophageal cancer is a common aggressive malignancy
characterized by late diagnosis and early metastasis (1,2).
Esophageal cancer is the eighth most common cancer and sixth most
common cause of cancer-related deaths worldwide, with an estimated
455,800 new cases and 400,200 deaths occurring in 2012 alone
(3,4). In 2015, an estimated 16,980
individuals were diagnosed with esophageal cancer in the USA, and
15,590 individuals succumbed to the disease, making it the ninth
leading cause of cancer-related deaths in that country (5). Esophageal cancers are histologically
classified as squamous cell carcinoma (SCC) or adenocarcinoma
(3). Esophageal squamous cell
carcinoma (ESCC) represents a predominant type of esophageal
cancer, accounting for approximately 90% of all esophageal cancers
(6). Despite improvements in
surgical approaches combined with chemotherapy and/or radiotherapy,
the prognosis of ESCC patients remains unsatisfactory, with 5-year
survival rates of patients with stage III disease ranging from 10
to 15% (7). Therefore, effective
treatments against ESCC are needed, such as treatments targeting
molecules and pathways specifically activated in this disease.
T-cell immunoglobulin and mucin domain-containing
protein-3 (TIM-3), also known as hepatitis A virus cellular
receptor 2, is one of the members of the TIM family (8). TIM-3 is selectively expressed on
IFN-γ-producing CD4+ T helper 1 (Th1) and
CD8+ T cytotoxic 1 (Tc1) T cells, but not on Th2 cells
(9). Numerous studies have verified
that TIM-3 is involved in immune regulation of tumors. Interaction
between TIM-3 and its ligand, galectin-9, triggers cell death in
Th1 cells and induces peripheral tolerance (10). Indeed, the TIM-3/galectin-9
signaling pathway mediates T-cell senescence and predicts poor
survival in patients with hepatitis B virus-associated
hepatocellular carcinoma (11).
Furthermore, previous studies have revealed an important role of
TIM-3 in T-cell exhaustion in tumors. In patients with advanced
melanoma, TIM-3+ PD-1+ NY-ESO-1-specific
CD8+ T cells exhibit a highly exhausted phenotype, and
co-blockade of TIM-3 and PD-1 signaling pathways contributes to
restore production of effector cytokines and proliferation of
NY-ESO-1-specific CD8+ T cells (12). Recently, several studies have found
that TIM-3 is overexpressed in several types of human tumors, such
as lung (13), prostate (14), gastric (15) and bladder urothelial (16) cancer, and its overexpression is
associated with poor prognosis in these cancers.
Although TIM-3 plays an important role in a variety
of tumors, the expression and biological functions of TIM-3 in ESCC
remain unknown. In the present study, we sought to detect the
expression of TIM-3 in human ESCC tissues and evaluate the
clinicopathological significance of TIM-3 expression in ESCC.
Furthermore, we explored the effects of TIM-3 on the proliferation,
apoptosis, migration and invasion of ESCC cells, and the possible
signaling pathways involved.
Materials and methods
Patients and tissue specimens
A total of 64 patients who underwent surgical
resection at the Fourth Hospital of Hebei Medical University
between January 2006 and January 2008 were enrolled in this study.
All patients were diagnosed with ESCC based on pathology, and none
of the patients received radiotherapy, chemotherapy or biological
therapy before surgery. Complete clinical data and follow-up
examinations were available for all patients. The study included 48
men and 16 women (median age, 57.5 years; range, 37–78 years). The
median follow-up was 31 months, with a range from 7 to 105 months.
Tumor-node-metastasis (TNM) stage was determined using criteria of
the World Health Organization (WHO) and the International Union
Against Cancer (UICC). Tumor masses as well as adjacent normal
tissues, which were at least 5 cm distal to tumor margins, were
snap-frozen in liquid nitrogen for quantitative real-time reverse
transcriptase-PCR (qRT-PCR) assay or fixed in 10% neutral formalin
solution for immunohistochemistry. This study protocol was approved
by the Ethics Department of the Fourth Hospital of Hebei Medical
University (Shijiazhuang, China), and informed consent was obtained
from patients or their families.
Cell lines and culture
Human esophageal squamous cell lines Eca109, TE-1,
TE-13, KYSE30, and KYSE170 were purchased from the Cell Bank of the
Chinese Academy of Sciences (Shanghai, China). Cell lines were
cultured in RPMI-1640 (Gibco, Carlsbad, CA, USA) supplemented with
25 mM HEPES, 2 mM L-glutamine, 10% fetal bovine serum (FBS;
Sijiqing, Hangzhou, China), 100 U/ml penicillin and 100 g/ml
streptomycin. All cells were incubated at 37°C in a humidified
atmosphere with 5% CO2.
qRT-PCR
Total RNA from tissue specimens and cultured cells
was extracted using TRIzol (Invitrogen, Carlsbad, CA, USA)
according to the manufacturer's instructions. RNA was
reverse-transcribed into cDNA using the RevertAid First Strand cDNA
Synthesis kit (Thermo Fisher Scientific, Shanghai, China) according
to the manufacturer's instructions. Amplification of the generated
cDNA was performed on Mx3005P Real-Time PCR Cycler (Stratagene
Corp.; Agilent Technologies, Inc., USA) using SYBR-Green PCR Master
Mix (Promega, Madison, WI, USA). Levels of mRNA were normalized to
those of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The
primer sequences used in this study are shown in Table I. The PCR conditions were 95°C for
10 min, followed by 40 cycles of 95°C for 15 sec, 60°C for 30 sec,
and 72°C for 30 sec. Verification of specific product amplification
was determined by melting curve analysis. Relative expression of
TIM-3 mRNA in carcinoma tissues and matched adjacent normal tissues
was calculated using the 2-ΔCt method (17). The fold change of each mRNA was
evaluated using the 2−ΔΔCt method (18).
 | Table ISequences of the qRT-PCR primers used
for mRNA analysis. |
Table I
Sequences of the qRT-PCR primers used
for mRNA analysis.
| Genes | Primer
sequences | AT (°C) | Product size
(bp) |
|---|
| TIM-3 | F |
5′CGCTGAGTACGTCGTGGAGTC-3′ | 60 | 172 |
| R |
5′-CTGATGATCTTGAGGCTGTTGTC-3′ | | |
| MMP-9 | F |
5′-GGGCACTCCCAATAA-3′ | 60 | 93 |
| R |
5′-GATGCTAGGCTTCCTG-3′ | | |
| TIMP-1 | F |
5′-ACCAGACCACCTTATACCAGCG-3′ | 60 | 395 |
| R |
5′-GGACTGGAAGCCCTTTTCAGAG-3′ | | |
| E-cadherin | F |
5′-CCGCCATCGCTTACA-3′ | 60 | 220 |
| R |
5′-GGCACCTGACCCTTGTA-3′ | | |
| N-cadherin | F |
5′-GAAAGACCCATCCACG-3′ | 60 | 217 |
| R |
5′-CCTGCTCACCACCACTA-3′ | | |
| Vimentin | F |
5′-AAGGCGAGGAGAGCAGGATT-3′ | 60 | 180 |
| R |
5′-GGTCATCGTGATGCTGAGAAG-3′ | | |
| GAPDH | F |
5′-CAGATACTGGCTAAATGGGGAT-3′ | 60 | 162 |
| R |
5′-ACCTTGGCTGGTTTGATGAC-3′ | | |
Immunohistochemistry
Formalin-fixed tissues were embedded in paraffin,
and sections (4 µm thick) were cut from each paraffin block.
Paraffin sections were dewaxed in xylene twice for 15 min each and
rehydrated through a graduated series of alcohol solutions (5 min
per step). Antigen was retrieved by boiling sections in citrate
buffer (pH 6.0) for 5 min in a pressure cooker, and endogenous
peroxidase activity was blocked by incubation in 3%
H2O2 for 20 min. Sections were incubated at
37°C for 60 min in 10% normal goat serum to block non-specific
background staining, and incubated overnight at 4°C with rabbit
anti-human TIM-3 polyclonal antibody (1:100 dilution; Abcam,
Southampton, UK). Subsequently, the sections were incubated with a
biotinylated secondary antibody, followed by the
streptavidin-peroxidase complex. Chromogen [3,3′-diaminobenzidine
(DAB)] was added, after which sections were counterstained with
Mayer's hematoxylin. Negative control sections were treated as
described, except that primary antibody was omitted.
Evaluation of TIM-3 protein
expression
Two pathologists blinded to clinical information
independently assessed the immunohistochemistry results. Five
visual fields were randomly selected in each section, and 200 tumor
cells were counted in each visual field at ×200 magnification.
Cells were regarded as positive if the cell membrane and/or
cytoplasm stained brown. The percentage of positive cells and the
intensity of staining were evaluated in all sections, and the final
score for each section was derived from the multiplication of the
two. The scoring system for the percentage of positive cells was as
follows: 1 point, ≤33%; 2 points, >33 to ≤66%; 3 points,
>66%. The scoring system for staining intensity was as follows:
1 point, absent/weak staining; 2 points, moderate staining; 3
points, strong staining. Sections with a final overall score of ≤3
were classified as the TIM-3 low expression group; other sections
were classified as the TIM-3 high expression group (19).
Western blotting
To extract total protein, the cells were collected
and lysed in RIPA buffer supplemented with PMSF (Beyotime,
Shanghai, China) at 4°C for 30 min. Total protein concentrations
were measured using the BCA Protein Assay kit (Beyotime). Equal
amounts of protein were separated by 10% SDS-PAGE and transferred
to polyvinylidene fluoride (PVDF) membranes. The membranes were
blocked with 5% non-fat dry milk at room temperature for 1 h and
incubated at 4°C overnight with the respective antibodies: rabbit
anti-GAPDH, TIM-3, matrix metalloproteinase (MMP)-9, tissue
inhibitor of metalloproteinase (TIMP)-1, E-cadherin, N-cadherin,
vimentin, Snail, Akt, GSK-3β, p-Akt, and p-GSK-3β (Abcam). The
membranes were washed with TBST three times for 5 min each, and
incubated with horseradish peroxidase-conjugated secondary antibody
for 1 h at room temperature. The bands were quantitated in
grayscale using Image-Pro Plus software 6.0 (Media Cybernetics,
Silver Spring, MD, USA).
Plasmid transfection and establishment of
stable cell lines
To knock down TIM-3 expression in the Eca109 and
TE-1 cells, shRNA targeting TIM-3 or negative control shRNA
(Applied BioProbes; GeneCopoeia Co.) was transfected into Eca109
and TE-1 cells using Lipofectamine 2000 (Invitrogen) according to
the manufacturer's instructions. The sequences of the specific
shRNA against TIM-3 were as follows: forward,
5′-GGGACTCTAGATTGGCCAATG-3′ and reverse, 5′-TAAGACTACGGCGCGAAGC-3′.
Stable trans fected clones were selected for 2 weeks using 3
µg/ml puromycin (Sigma, St. Louis, MO, USA), and monoclonal
cells were picked up with tips. Subsequently, the resistant clones
were amplified in conventional medium with 3 µg/ml
puromycin.
MTS assay
Cell viability was evaluated using the
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulphophenyl)-2H-tetrazolium
(MTS) assay. Cells were seeded into 96-well plates at a density of
4×103 cells/well for 24, 48 or 72 h. At each time point,
20 µl of MTS solution (Promega) was added to each well and
incubated at 37°C for 3 h in the dark. The absorbance of each well
was measured at 492 nm using a spectrophotometer (Thermo Fisher
Scientific). Each experiment included six replications and was
repeated three times.
Colony formation assay
Cells were seeded at a density of 3×103
cells/well in 6-well culture plates and cultured at 37°C for 1
week. Colonies were washed with phosphate-buffered saline (PBS),
fixed with 4% neutral formalin and stained with 0.1% crystal violet
(Sigma). Colonies containing >50 cells were counted. Each
experiment was performed in triplicate.
Migration and invasion assays
Cell migration and invasion assays were performed in
24-well Transwell cell culture chambers (Corning, Cambridge, MA,
USA) containing a polycarbonate membrane filter with 8.0-µm
pores. For the cell invasion assay, 30 µl of Matrigel (1:7
dilution; Becton-Dickinson, San Jose, CA, USA) was added to the
upper chamber and dried overnight at 37°C in a 5% CO2
incubator. A total of 5×104 cells were resuspended in
200 µl serum-free RPMI-1640 medium and seeded into the upper
chamber. The lower chamber was filled with 600 µl of
RPMI-1640 medium containing 20% FBS. After incubation at 37°C with
5% CO2 for 24 h, non-invading cells still on the upper
side of the chamber were wiped with a cotton swab. Invaded cells on
the bottom of the polycarbonate membrane were fixed for 20 min with
4% neutral formalin, and stained for 30 min with 0.1% crystal
violet. For the cell migration assay, we performed a similar
procedure as described above, except that the upper chamber was not
coated with Matrigel. Each experiment was performed in triplicate.
The number of cells that migrated or invaded through the
polycarbonate membrane was counted in five randomly selected visual
fields at ×200 magnification using a high-resolution inverted
microscope.
Detection of apoptosis by flow
cytometry
Cells were collected and resuspended in 100
µl 1X binding buffer at a density of 1×106
cells/ml. Cell apoptosis was detected using the Annexin V-PE/7-AAD
Apoptosis Detection kit (Becton-Dickinson) according to the
manufacturer's instructions. The Annexin V-PE and 7-AAD stained
cells were examined by flow cytometry on a BD FACScalibur (BD
Biosciences).
Statistical analysis
All statistical analyses were analyzed using SPSS
17.0 (IBM, Chicago, IL, USA), and figures were generated using
GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA).
Differences in mean values between groups were assessed for
significance using the Student's t-test or one-way analysis of
variance (ANOVA). Differences in TIM-3 expression between ESCC and
matched adjacent normal tissues were assessed using the
χ2 test. The relationships between TIM-3 expression and
clinicopathological features were analyzed using the χ2
test or Fisher's exact test. Overall survival was calculated using
the Kaplan-Meier method, and the differences between groups were
assessed for significance using the log-rank test. Data are
presented as means ± SD from at least three independent
experiments. P<0.05 was considered statistically
significant.
Results
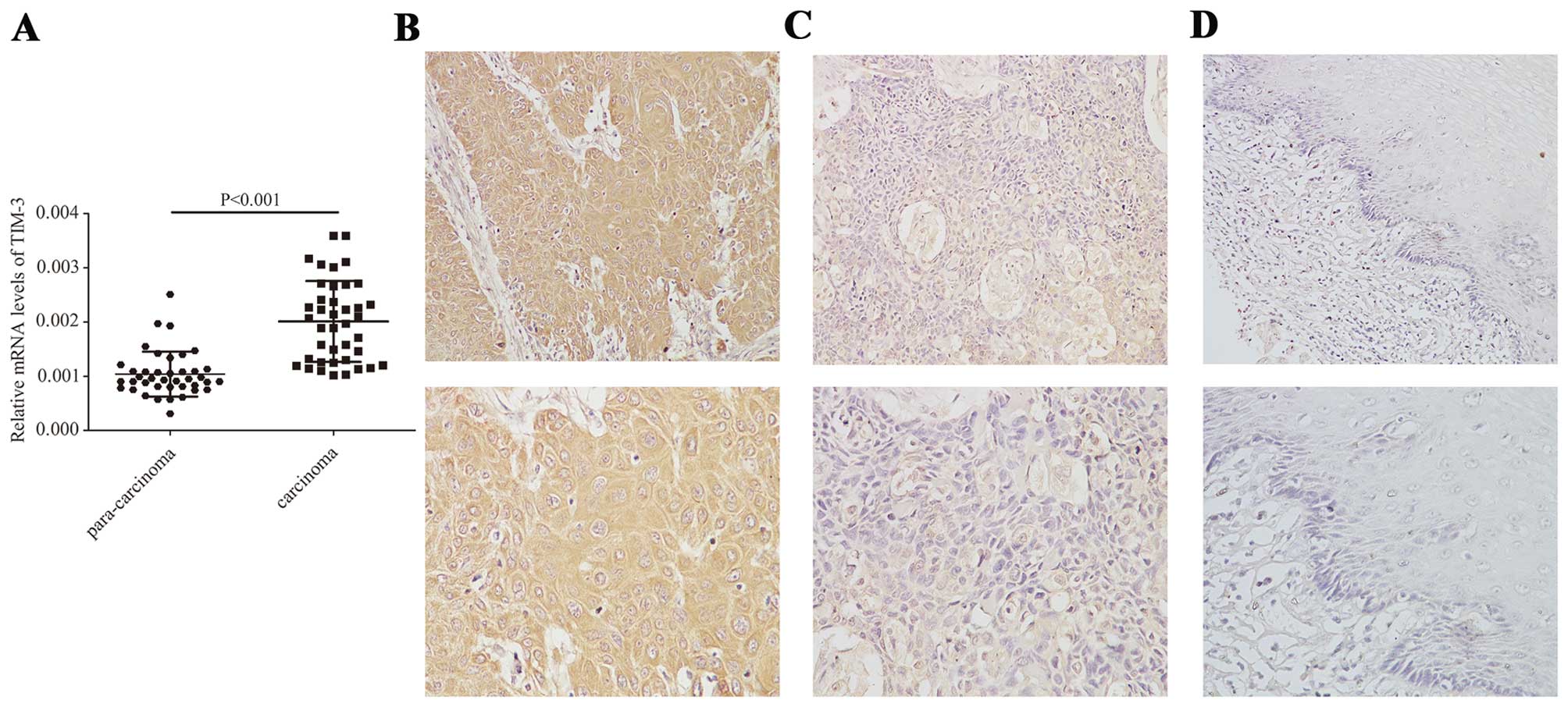
TIM-3 is upregulated in ESCC tissues
TIM-3 mRNA levels were analyzed by qRT-PCR in 40
pairs of ESCC and matched adjacent normal tissues. The relative
mRNA expression of TIM-3 was significantly higher in the tumor
tissues than that in the adjacent normal tissues (0.002±0.0007 vs.
0.001±0.0004; P<0.001, Fig.
1A).
TIM-3 protein levels were analyzed by
immunohistochemistry in 64 pairs of ESCC and adjacent normal tissue
specimens. TIM-3 protein was localized predominantly in the
cellular membrane and the cytoplasm of tumor cells (Fig. 1B–D). TIM-3-positive staining
(overall score >1) was present in 55 of the 64 ESCC tissue
sections (85.9%), compared to only 7 of the 64 normal tissue
sections (10.9%). Of the 55 TIM-3-positive ESCC samples, 19 (34.5%)
showed low TIM-3 expression (overall score ≤3), and 36 (65.5%)
showed high TIM-3 expression (overall score >3), whereas all 7
of the TIM-3-positive normal tissue samples showed low TIM-3
expression. Statistical analysis of these results indicated that
TIM-3 protein expression was significantly higher in the ESCC
tissues than that in the adjacent normal tissues (P<0.001,
Table II).
 | Table IIExpression of TIM-3 in ESCC and
matched adjacent normal tissues. |
Table II
Expression of TIM-3 in ESCC and
matched adjacent normal tissues.
| Tissue types | Cases | TIM-3 expression
| P-valuea |
|---|
| Negative | Positive |
|---|
| Tumor tissues | 64 | 9 | 55 | <0.001 |
| Matched normal
tissues | 64 | 57 | 7 | |
Association between TIM-3 expression and
clinicopatho-logical parameters
We analyzed the correlations between TIM-3 protein
expression and numerous clinicopathological parameters, including
age, gender, tumor size, TNM stage, lymphatic metastasis, and depth
of tumor invasion (Table III).
Our results revealed that the expression of TIM-3 protein was
significantly associated with TNM stage (P=0.008), lymph node
metastasis (P=0.042) and depth of tumor invasion (P=0.042).
However, no significant differences were observed between the TIM-3
protein expression and the other clinico-pathological
parameters.
 | Table IIICorrelation of TIM-3 expression and
clinicopathological parameters of the ESCC cases. |
Table III
Correlation of TIM-3 expression and
clinicopathological parameters of the ESCC cases.
|
Characteristics | Cases | TIM-3 expression
| χ2 | P-value |
|---|
| Low | High |
|---|
| Gender | | | | 1.354 | 0.244 |
| Male | 48 | 23 | 25 | | |
| Female | 16 | 5 | 11 | | |
| Age (years) | | | | 0.301 | 0.583 |
| ≤55 | 25 | 12 | 13 | | |
| >55 | 39 | 16 | 23 | | |
| Tumor size
(cm) | | | | 0.000 | 1.000 |
| ≤5 | 32 | 14 | 18 | | |
| >5 | 32 | 14 | 18 | | |
| TNM stage | | | | 10.653 | 0.008a,b |
| I | 10 | 9 | 1 | | |
| II | 32 | 11 | 21 | | |
| III | 20 | 7 | 13 | | |
| IV | 2 | 1 | 1 | | |
| Lymph node
metastasis | | | | 4.135 | 0.042c |
| Negative | 39 | 21 | 18 | | |
| Positive | 25 | 7 | 18 | | |
| Depth of tumor
invasion | | | | 4.136 | 0.042c |
|
pT1-pT2 | 19 | 12 | 7 | | |
|
pT3-pT4 | 45 | 16 | 29 | | |
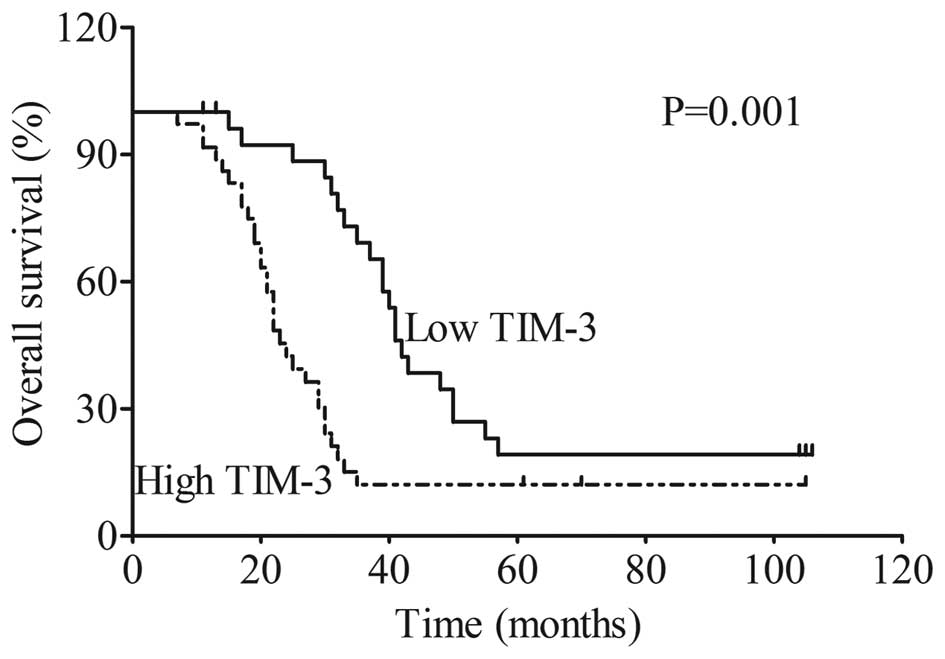
TIM-3 expression is associated with poor
prognosis in ESCC patients
Patients were divided into two groups (TIM-3 low
expression group or TIM-3 high expression group) as described in
the 'Materials and methods' section. Kaplan-Meier survival analysis
indicated that patients in the TIM-3 high expression group had a
significantly shorter overall survival than patients in the TIM-3
low expression group (P=0.001, Fig.
2). The overall 5-year survival rate for patients in the TIM-3
high expression group was 9% with a median survival time of 22
months, while the overall 5-year survival rate for patients in the
TIM-3 low expression group was 19.2% with a median survival time of
41 months.
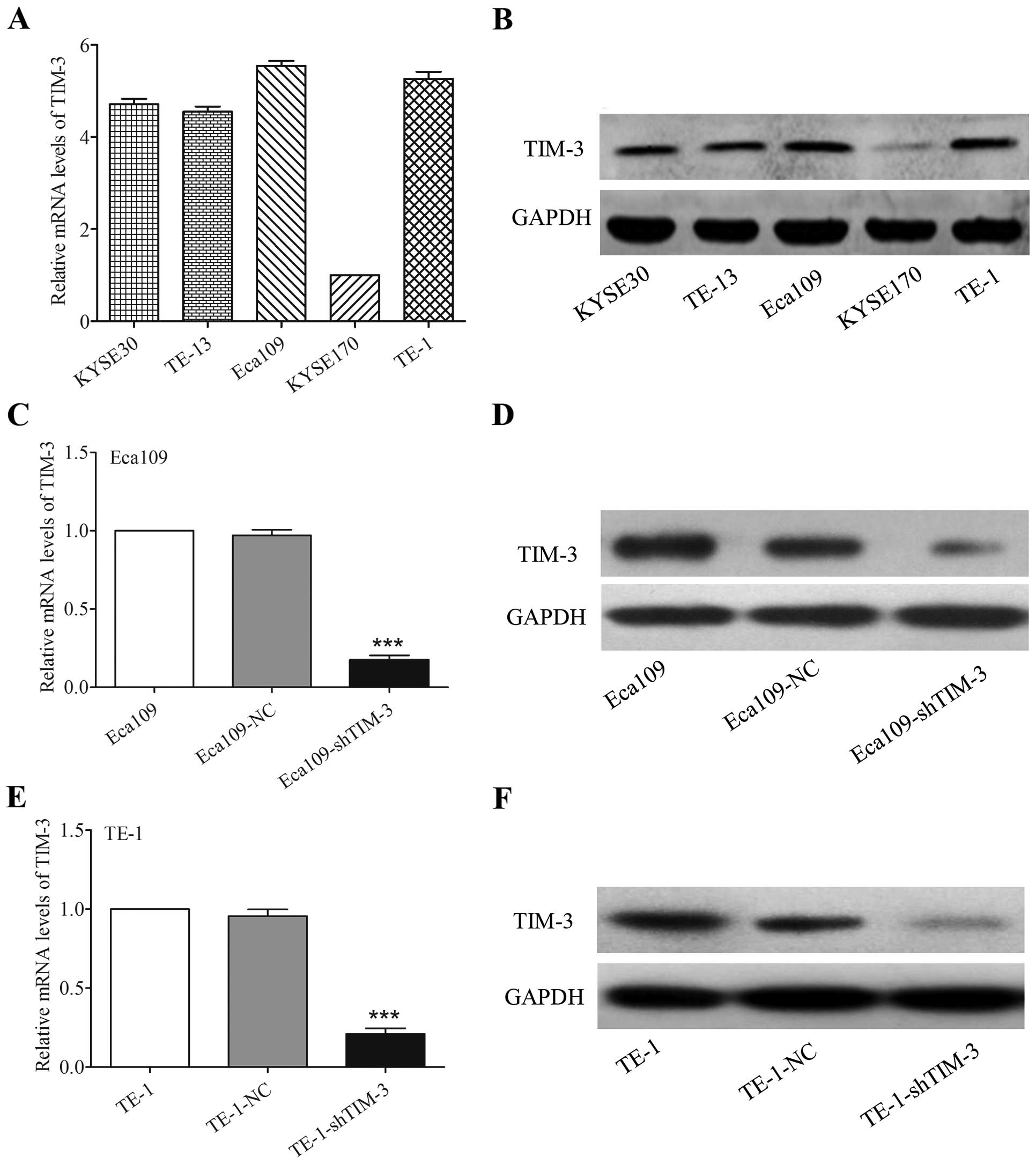
shRNA effectively inhibits TIM-3
expression in ESCC cell lines
The expression levels of TIM-3 mRNA and protein in
various ESCC cell lines, including KYSE30, TE-13, Eca109, KYSE170
and TE-1, were evaluated by qRT-PCR and western blotting,
respectively. These results showed that TIM-3 expression was
relatively highest in the Eca109 and TE-1 cell lines (Fig. 3A and B). Therefore, we selected
these two cell lines to knock down TIM-3 expression and explore the
effects on cancer cell function. We established shRNA-mediated
stable Eca109-shTIM-3 and TE-1-shTIM-3 cells, and their respective
negative controls (Eca109-NC and TE-1-NC cells). Transfection
efficiency was evaluated by qRT-PCR and western blotting. The mRNA
and protein levels of TIM-3 were markedly decreased in the
Eca109-shTIM-3 and TE-1-shTIM-3 cells than levels in their
corresponding parental or negative control cells (P<0.001,
Fig. 3C–F).
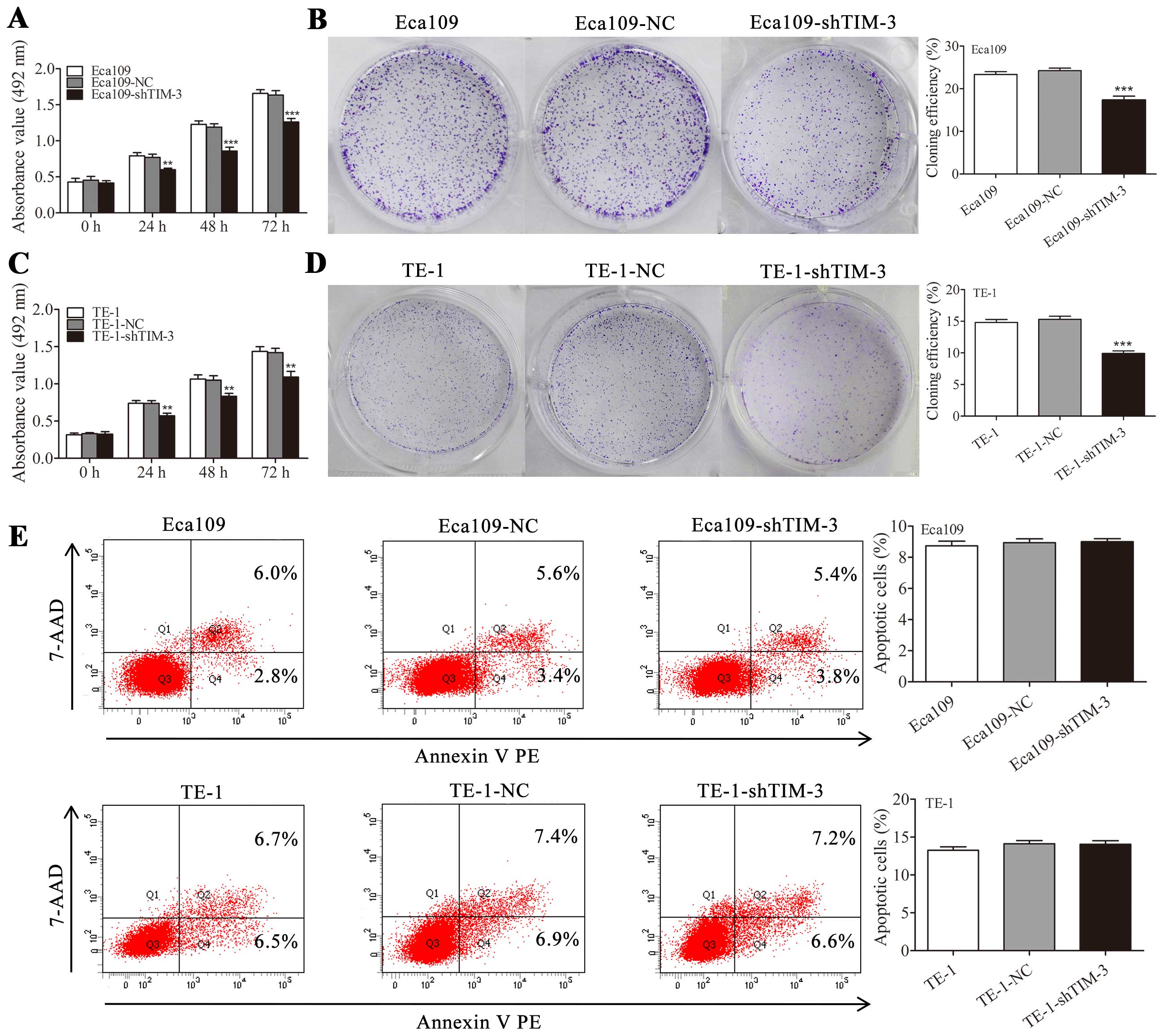
Downregulation of TIM-3 inhibits cell
proliferation without affecting apoptosis
Effects of TIM-3 knockdown on ESCC proliferation
were examined by MTS and colony formation assays. In the MTS assay,
the absorbance at 492 nm was significantly lower in the
Eca109-shTIM-3 and TE-1-shTIM-3 cells than those in their
corresponding parental or negative control cells at 24, 48 and 72 h
(P<0.01, Fig. 4A and C).
Consistent with these results, colony formation potential was
significantly decreased in the Eca109-shTIM-3 and TE-1-shTIM-3
cells (P<0.001, Fig. 4B and D).
In contrast to its effects on proliferation, TIM-3 knockdown did
not significantly affect cell apoptosis based on an Annexin
V-PE/7-AAD staining assay (Fig.
4E).
Downregulation of TIM-3 inhibits the
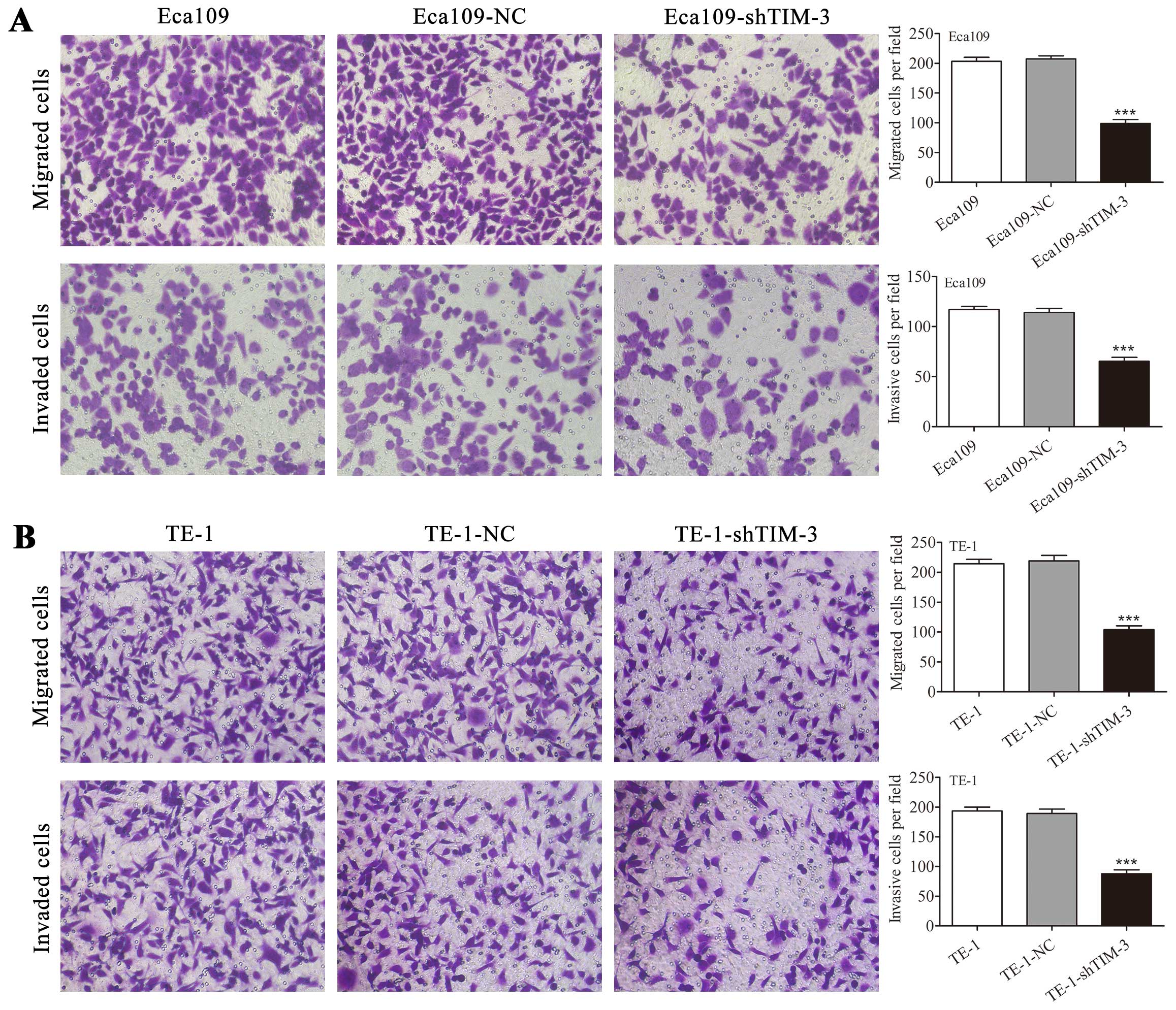
migratory and invasive potential of ESCC cells
To characterize the effects of TIM-3 knockdown on
the migration and invasion of ESCC cells, we performed Transwell
migration and Matrigel invasion assays. In the Transwell migration
assay, the number of Eca109-shTIM-3 cells which migrated through
the Transwell was dramatically decreased compared with the number
of Eca109-NC and untransfected Eca109 cells which migrated
(P<0.001, Fig. 5A). Similarly,
in a Transwell invasion assay, the number of Eca109-shTIM-3 cells
which invaded through the Transwell was significantly decreased
compared with the number of Eca109-NC and untransfected Eca109
cells which invaded (P<0.001, Fig.
5A). Similar results were obtained in the TE-1 cells
(P<0.001, Fig. 5B).
Downregulation of TIM-3 regulates the
expression of metastasis-related molecules in the ESCC cells
MMPs and their inhibitors, termed TIMPs, have been
reported to be closely associated with tumor growth, invasion and
metastasis (20,21). Among the MMP members, in particular
MMP-9 plays a prominent role in ESCC cell invasion and metastasis
(22,23). Therefore, we investigated whether
TIM-3 knockdown affects the expression of MMP-9 and TIMP-1 in
Eca109 and TE-1 cells. qRT-PCR and western blotting revealed that
TIM-3 depletion significantly downregulated MMP-9 and upregulated
TIMP-1 in both cell lines (Fig.
6A–C).
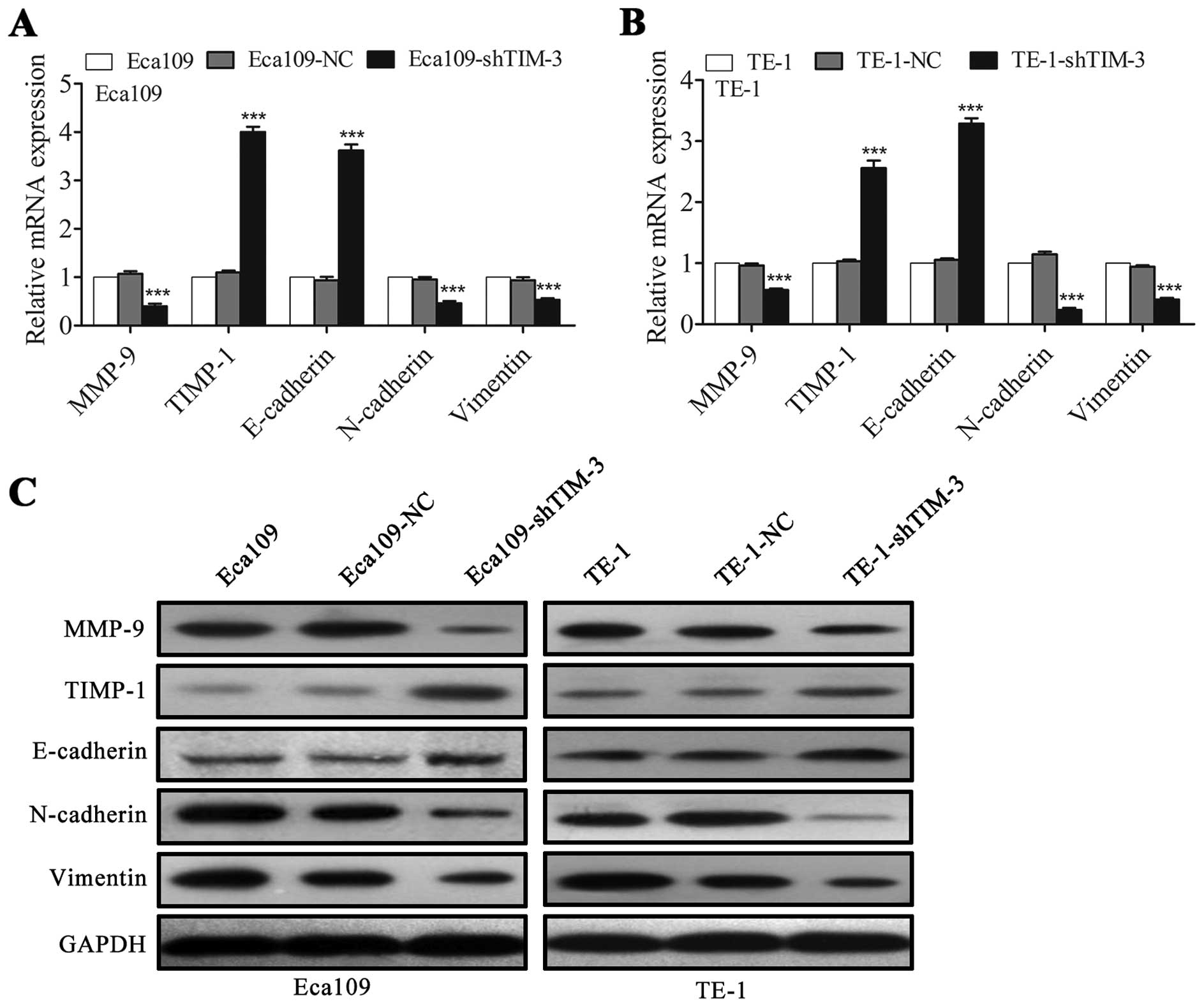 | Figure 6Downregulation of TIM-3 regulates the
expression of metastasis-related molecules in ESCC cells. (A and B)
The mRNA expression of the indicated genes MMP-9, TIMP-1,
E-cadherin, N-cadherin, and vimentin were determined using qRT-PCR
in (A) Eca109 and (B) TE-1 cells. (C) Levels of the corresponding
proteins were evaluated using western blotting. GAPDH was used for
normalization. Data are shown as means ± SD.
***P<0.001 vs. the NC group. TIM-3, T-cell
immunoglobulin and mucin domain-containing protein-3; ESCC,
esophageal squamous cell carcinoma; MMP, matrix metalloproteinase;
TIMP, tissue inhibitor of metalloproteinase; GAPDH,
glyceraldehyde-3-phosphate dehydrogenase; NC, negative control. |
Epithelial-mesenchymal transition (EMT) is a key
step in the metastatic process of many solid tumors and represents
a hallmark of this event (24). We
aimed to ascertain whether TIM-3 depletion would inhibit the
metastatic potential of ESCC cells in part by inhibiting EMT.
Indeed, we found that TIM-3 depletion upregulated the epithelial
marker E-cadherin and downregulated the mesenchymal markers
N-cadherin and vimentin in both the Eca109 and TE-1 cells (Fig. 6A–C).
Downregulation of TIM-3 reverses EMT by
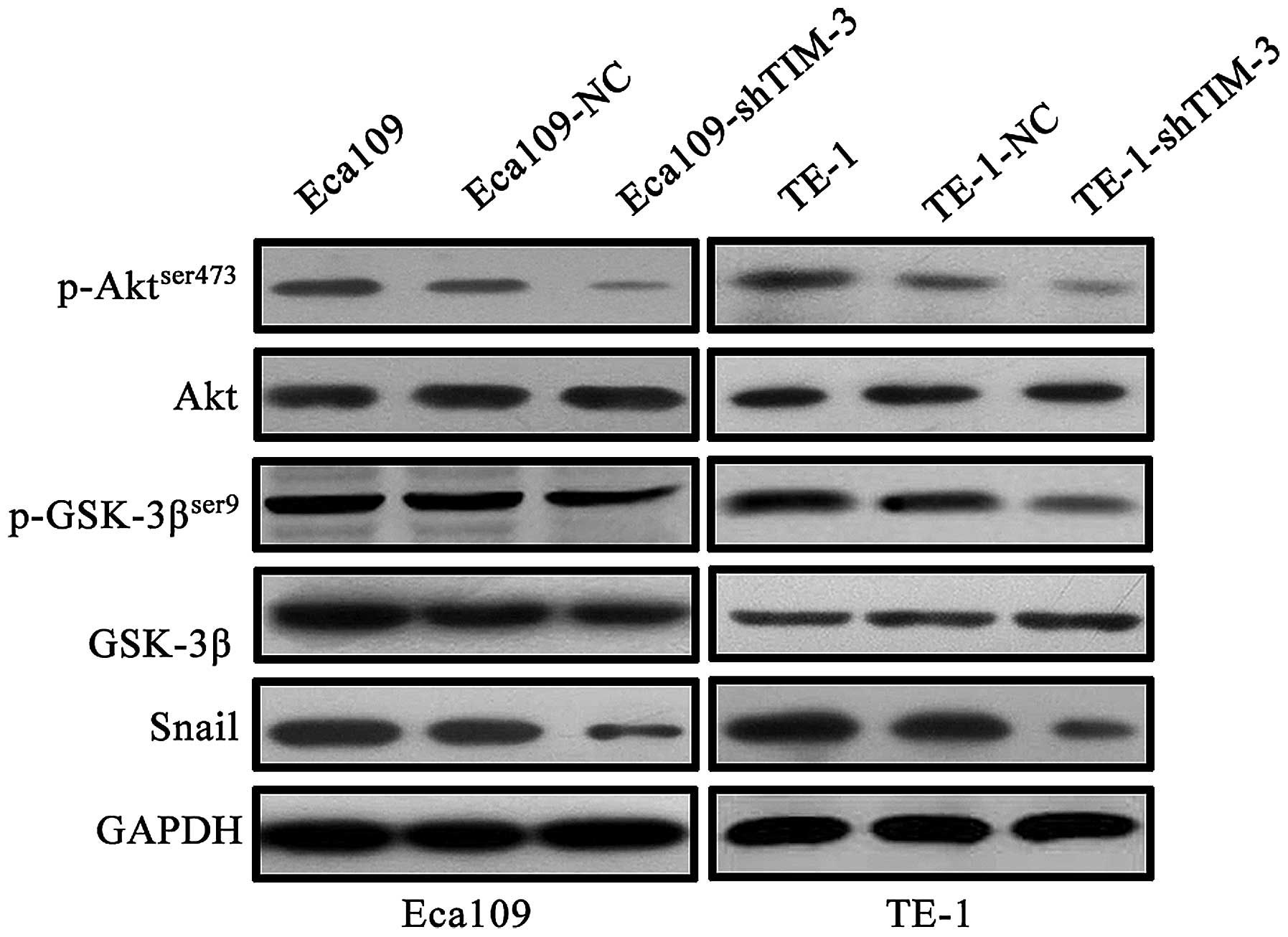
suppressing Akt/GSK-3β/Snail signaling in the ESCC cells
Accumulating evidence has demonstrated that the
Akt/GSK-3β/Snail signaling pathway can drive EMT to facilitate
tumor progression (25,26). To elucidate the potential signaling
pathways involved in TIM-3-mediated EMT, we investigated the
effects of TIM-3 knockdown on the Akt/GSK-3β/Snail signaling
pathway. Western blotting revealed that the protein level of the
active form of Akt (phosphorylated on Ser473), but not total Akt,
the active form of GSK-3β (phosphorylated on Ser9), but not total
GSK-3β were markedly decreased by knockdown of TIM-3 (Fig. 7). In addition, EMT-related
transcription factor Snail was downregulated after TIM-3 knockdown
(Fig. 7). These findings suggest
that TIM-3 depletion acts via the Akt/GSK-3β/Snail signaling
pathway to inhibit the EMT in ESCC.
Discussion
ESCC is a highly aggressive and fatal malignancy
characterized by early local invasion and systemic metastasis
(27). Despite aggressive treatment
strategies, the overall survival rate of ESCC patients with
metastasis and recurrence has seen no significant improvement
(28,29). TIM-3, a negative immune regulator,
has been shown to have prognostic and functional significance in
several types of human cancers (30,31).
Therefore, we sought to investigate whether TIM-3 may play a role
in human ESCC. Our results with the patient tissues suggested that
TIM-3 was upregulated in the ESCC tumors, and our study on the ESCC
cell lines showed that downregulation of TIM-3 inhibited cell
motility and invasion and reversed EMT by suppressing the
Akt/GSK-3β/Snail signaling pathway. These findings suggest that
TIM-3 may serve as a potential target of molecular therapies to
inhibit ESCC aggression and metastasis.
In the present study, we report, for the first time,
that TIM-3 was expressed in both ESCC tissue sections and ESCC cell
lines. In line with our study, two recent studies identified that
TIM-3 was expressed on leukemic stem cells (LSCs) in patients with
acute myeloid leukemia (32,33).
Meanwhile, Wiener et al (34) found that TIM-3 was expressed not
only on mast cells around melanomas, but also on tumor cells in
tissue sections and human melanoma cell lines WM35 and HT168-M1. We
further found that the expression level of TIM-3 was significantly
higher in ESCC tissues than that in the matched adjacent normal
tissues at both the mRNA and protein levels, which suggests that
TIM-3 overexpression might correlate with the carcinogenesis of
ESCC. In addition, further analysis demonstrated that TIM-3
expression was clearly associated with lymph node metastasis, TNM
stage, and depth of tumor invasion, suggesting that TIM-3 is
involved in ESCC progression and metastasis. Since we found a
significantly worse overall survival rate for patients with high
TIM-3 expression, our results also raise the possibility that high
TIM-3 expression may serve as a marker of poor prognosis for
patients with ESCC. Consistently, similar results have already been
reported for cervical (31), lung
(35) and clear cell renal cancer
(36,37).
To probe the potential biological functions of
upregulated TIM-3 in ESCC, we generated stable Eca109-shTIM-3 and
TE-1-shTIM-3 cell lines in which endogenous TIM-3 was knocked down
by shRNA. The current in vitro experiments provided evidence
that TIM-3 knockdown inhibited the proliferation, migration and
invasion of both Eca109 and TE-1 cell lines. Consistent with our
results, Yuan et al (36)
demonstrated that knockdown of TIM-3 suppressed the proliferation
and invasion of clear cell renal carcinoma (ccRCC) cell lines A498
and 769P. Meanwhile, Cao et al (31) also suggested that downregulation of
TIM-3 significantly decreased the migration and invasion of HeLa
cells. Our findings suggest that TIM-3 promotes the invasion and
metastasis of ESCC. To test this more directly, we examined the
effects of TIM-3 knockdown on expression of metastasis-related
molecules. One was MMP-9, which helps degrade the extracellular
matrix to allow tumor cell invasion and metastasis (38,39).
TIM-3 depletion significantly decreased levels of MMP-9 mRNA and
protein in the Eca109 and TE-1 cells, which at least partly might
explain why TIM-3 knockdown reduced the invasive potential of ESCC
cells in our experiments. MMP activity is negatively controlled by
TIMPs (40), and imbalance between
MMPs and TIMPs can facilitate cancer progression (41). Consistent with our hypothesis that
TIM-3 helps drive ESCC metastasis, we found that knockdown of TIM-3
upregulated TIMP-1 expression, presumably reducing MMP-9
activity.
EMT is a key event during cancer metastasis, which
confers an aggressive phenotype to tumor cells (42). A variety of molecular events have
been identified to participate in EMT, but whether TIM-3 is
involved in EMT and cancer metastasis remains unclear. We
hypothesized that TIM-3 could promote EMT and ESCC metastasis. To
test this hypothesis, we examined the effects of TIM-3 knockdown on
expression of EMT-associated molecules. At the molecular level, EMT
involves downregulation of the intercellular adhesion molecule
E-cadherin and upregulation of various mesenchymal markers,
including N-cadherin, vimentin and fibronectin (43). Here, we showed that TIM-3 depletion
upregulated E-cadherin and downregulated N-cadherin and vimentin.
In further support of this hypothesis, we found that TIM-3
depletion downregulated Snail, a nuclear transcription factor
involved in EMT. Our findings are consistent with a study
suggesting that TIM-3 may trigger the process of EMT to promote
tumor development in human osteosarcoma (44). These results indicate that TIM-3
promoted ESCC metastasis in part by regulating EMT. The ability of
Snail to trigger EMT appears to depend on phosphorylation of Akt,
which in turn phosphorylates GSK-3β (25,45).
Indeed, the activated Akt/GSK-3β/Snail signaling pathway has been
shown to trigger EMT and contribute to an aggressive phenotype in
several human malignant tumors (46–49).
We showed that TIM-3 depletion significantly reduced levels of
p-AKT, p-GSK-3β and Snail, further supporting the hypothesis that
TIM-3 helps drive EMT in ESCC and suggesting that TIM-3 acts via
the Akt/GSK-3β/Snail signaling pathway to do so.
In conclusion, this study revealed that TIM-3 plays
a crucial role in ESCC metastasis by inducing EMT via, at least
partially, the Akt/GSK-3β/Snail signaling pathway. Moreover, the
characterization of TIM-3 may contribute to the identification of a
potential therapeutic target and valuable prognostic biomarker for
patients with ESCC.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (no. 81173611).
References
|
1
|
Vay C, Hosch SB, Stoecklein NH, Klein CA,
Vallböhmer D, Link BC, Yekebas EF, Izbicki JR, Knoefel WT and
Scheunemann P: Integrin expression in esophageal squamous cell
carcinoma: Loss of the physiological integrin expression pattern
correlates with disease progression. PLoS One. 9:e1090262014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pennathur A, Gibson MK, Jobe BA and
Luketich JD: Oesophageal carcinoma. Lancet. 381:400–412. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
5
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen T, Wang C, Wu F, Zhang X, Yang H,
Deng X, He Q, Li W and Li G: Altered localization of p120 catenin
in the cytoplasm rather than the membrane correlates with poor
prognosis in esophageal squamous cell carcinoma. PLoS One.
10:e01186452015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu Y, Wang J, Qiu M and Xu L, Li M, Jiang
F, Yin R and Xu L: Upregulation of the long noncoding RNA TUG1
promotes proliferation and migration of esophageal squamous cell
carcinoma. Tumour Biol. 36:1643–1651. 2015. View Article : Google Scholar
|
|
8
|
McIntire JJ, Umetsu SE, Akbari O, Potter
M, Kuchroo VK, Barsh GS, Freeman GJ, Umetsu DT and DeKruyff RH:
Identification of Tapr (an airway hyperreactivity regulatory locus)
and the linked Tim gene family. Nat Immunol. 2:1109–1116. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Monney L, Sabatos CA, Gaglia JL, Ryu A,
Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel
RA, et al: Th1-specific cell surface protein Tim-3 regulates
macrophage activation and severity of an autoimmune disease.
Nature. 415:536–541. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu C, Anderson AC, Schubart A, Xiong H,
Imitola J, Khoury SJ, Zheng XX, Strom TB and Kuchroo VK: The Tim-3
ligand galectin-9 negatively regulates T helper type 1 immunity.
Nat Immunol. 6:1245–1252. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li H, Wu K, Tao K, Chen L, Zheng Q, Lu X,
Liu J, Shi L, Liu C, Wang G, et al: Tim-3/galectin-9 signaling
pathway mediates T-cell dysfunction and predicts poor prognosis in
patients with hepatitis B virus-associated hepatocellular
carcinoma. Hepatology. 56:1342–1351. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fourcade J, Sun Z, Benallaoua M, Guillaume
P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V and Zarour HM:
Upregulation of Tim-3 and PD-1 expression is associated with tumor
antigen-specific CD8+ T cell dysfunction in melanoma
patients. J Exp Med. 207:2175–2186. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gao X, Zhu Y, Li G, Huang H, Zhang G, Wang
F, Sun J, Yang Q, Zhang X and Lu B: TIM-3 expression characterizes
regulatory T cells in tumor tissues and is associated with lung
cancer progression. PLoS One. 7:e306762012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Piao YR, Jin ZH, Yuan KC and Jin XS:
Analysis of Tim-3 as a therapeutic target in prostate cancer.
Tumour Biol. 35:11409–11414. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheng G, Li M, Wu J, Ji M, Fang C, Shi H,
Zhu D, Chen L, Zhao J, Shi L, et al: Expression of Tim-3 in gastric
cancer tissue and its relationship with prognosis. Int J Clin Exp
Pathol. 8:9452–9457. 2015.PubMed/NCBI
|
|
16
|
Yang M, Yu Q, Liu J, Fu W, Cao Y, Yu L,
Shao S, Wang X, Niu H and Wang Y: T-cell immunoglobulin mucin-3
expression in bladder urothelial carcinoma: Clinicopathologic
correlations and association with survival. J Surg Oncol.
112:430–435. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ling XL, Zhang T, Hou XM and Zhao D:
Clinicopathological significance of fascin-1 expression in patients
with non-small cell lung cancer. Onco Targets Ther. 8:1589–1595.
2015.PubMed/NCBI
|
|
18
|
Li J, Huang H, Sun L, Yang M, Pan C, Chen
W, Wu D, Lin Z, Zeng C, Yao Y, et al: MiR-21 indicates poor
prognosis in tongue squamous cell carcinomas as an apoptosis
inhibitor. Clin Cancer Res. 15:3998–4008. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Loos M, Hedderich DM, Ottenhausen M, Giese
NA, Laschinger M, Esposito I, Kleeff J and Friess H: Expression of
the costimulatory molecule B7-H3 is associated with prolonged
survival in human pancreatic cancer. BMC Cancer. 9:4632009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Herszényi L, Hritz I, Lakatos G, Varga MZ
and Tulassay Z: The behavior of matrix metalloproteinases and their
inhibitors in colorectal cancer. Int J Mol Sci. 13:13240–13263.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen Y, Jiang T, Mao A and Xu J:
Esophageal cancer stem cells express PLGF to increase cancer
invasion through MMP9 activation. Tumour Biol. 35:12749–12755.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao G, Kang J, Jiao K, Xu G, Yang L, Tang
S, Zhang H, Wang Y, Nie Y, Wu K, et al: High expression of GRP78
promotes invasion and metastases in patients with esophageal
squamous cell carcinoma. Dig Dis Sci. 60:2690–2699. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Shi J, Chai K, Ying X and Zhou BP:
The role of snail in EMT and tumorigenesis. Curr Cancer Drug
Targets. 13:963–972. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu W, Yang Z and Lu N: A new role for the
PI3K/Akt signaling pathway in the epithelial-mesenchymal
transition. Cell Adhes Migr. 9:317–324. 2015. View Article : Google Scholar
|
|
26
|
Wen W, Ding J, Sun W, Fu J, Chen Y, Wu K,
Ning B, Han T, Huang L, Chen C, et al: Cyclin G1-mediated
epithelial-mesenchymal transition via phosphoinositide 3-kinase/Akt
signaling facilitates liver cancer progression. Hepatology.
55:1787–1798. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu M, Xu Y, Mao X, Gao Y, Shao L and Yan
F: Overexpression of metastasis-associated in colon cancer-1
associated with poor prognosis in patients with esophageal cancer.
Pathol Oncol Res. 19:749–753. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mohamed A, El-Rayes B, Khuri FR and Saba
NF: Targeted therapies in metastatic esophageal cancer: Advances
over the past decade. Crit Rev Oncol Hematol. 91:186–196. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li LY, Jiang H, Xie YM, Liao LD, Cao HH,
Xu XE, Chen B, Zeng FM, Zhang YL, Du ZP, et al: Macrolide analog
F806 suppresses esophageal squamous cell carcinoma (ESCC) by
blocking β1 integrin activation. Oncotarget. 6:15940–15952. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Anderson AC: Tim-3: An emerging target in
the cancer immunotherapy landscape. Cancer Immunol Res. 2:393–398.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cao Y, Zhou X, Huang X, Li Q, Gao L, Jiang
L, Huang M and Zhou J: Tim-3 expression in cervical cancer promotes
tumor metastasis. PLoS One. 8:e538342013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kikushige Y, Shima T, Takayanagi S, Urata
S, Miyamoto T, Iwasaki H, Takenaka K, Teshima T, Tanaka T, Inagaki
Y, et al: TIM-3 is a promising target to selectively kill acute
myeloid leukemia stem cells. Cell Stem Cell. 7:708–717. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jan M, Chao MP, Cha AC, Alizadeh AA,
Gentles AJ, Weissman IL and Majeti R: Prospective separation of
normal and leukemic stem cells based on differential expression of
TIM3, a human acute myeloid leukemia stem cell marker. Proc Natl
Acad Sci USA. 108:5009–5014. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wiener Z, Kohalmi B, Pocza P, Jeager J,
Tolgyesi G, Toth S, Gorbe E, Papp Z and Falus A: TIM-3 is expressed
in melanoma cells and is upregulated in TGF-beta stimulated mast
cells. J Invest Dermatol. 127:906–914. 2007. View Article : Google Scholar
|
|
35
|
Zhuang X, Zhang X, Xia X, Zhang C, Liang
X, Gao L, Zhang X and Ma C: Ectopic expression of TIM-3 in lung
cancers: A potential independent prognostic factor for patients
with NSCLC. Am J Clin Pathol. 137:978–985. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yuan J, Jiang B, Zhao H and Huang Q:
Prognostic implication of TIM-3 in clear cell renal cell carcinoma.
Neoplasma. 61:35–40. 2014. View Article : Google Scholar
|
|
37
|
Komohara Y, Morita T, Annan DA, Horlad H,
Ohnishi K, Yamada S, Nakayama T, Kitada S, Suzu S, Kinoshita I, et
al: The coordinated actions of TIM-3 on cancer and myeloid cells in
the regulation of tumorigenicity and clinical prognosis in clear
cell renal cell carcinomas. Cancer Immunol Res. 3:999–1007. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hadler-Olsen E, Winberg JO and
Uhlin-Hansen L: Matrix metalloproteinases in cancer: Their value as
diagnostic and prognostic markers and therapeutic targets. Tumour
Biol. 34:2041–2051. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang X, Wang Y, Yamamoto G and Tachikawa
T: Expression of matrix metalloproteinases MMP-2, MMP-9 and their
tissue inhibitors TIMP-1 and TIMP-2 in the epithelium and stroma of
salivary gland pleomorphic adenomas. Histopathology. 55:250–260.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jinga DC, Blidaru A, Condrea I, Ardeleanu
C, Dragomir C, Szegli G, Stefanescu M and Matache C: MMP-9 and
MMP-2 gelatinases and TIMP-1 and TIMP-2 inhibitors in breast
cancer: Correlations with prognostic factors. J Cell Mol Med.
10:499–510. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Groblewska M, Siewko M, Mroczko B and
Szmitkowski M: The role of matrix metalloproteinases (MMPs) and
their inhibitors (TIMPs) in the development of esophageal cancer.
Folia Histochem Cytobiol. 50:12–19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Davis FM, Stewart TA, Thompson EW and
Monteith GR: Targeting EMT in cancer: Opportunities for
pharmacological intervention. Trends Pharmacol Sci. 35:479–488.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wu WL, Wang WY, Yao WQ and Li GD:
Suppressive effects of microRNA-16 on the proliferation, invasion
and metastasis of hepatocellular carcinoma cells. Int J Mol Med.
36:1713–1719. 2015.PubMed/NCBI
|
|
44
|
Shang Y, Li Z, Li H, Xia H and Lin Z:
TIM-3 expression in human osteosarcoma: Correlation with the
expression of epithelial-mesenchymal transition-specific
biomarkers. Oncol Lett. 6:490–494. 2013.PubMed/NCBI
|
|
45
|
Zhou BP, Deng J, Xia W, Xu J, Li YM,
Gunduz M and Hung MC: Dual regulation of Snail by GSK-3β-mediated
phosphorylation in control of epithelial-mesenchymal transition.
Nat Cell Biol. 6:931–940. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu A, Shao C, Jin G, Liu R, Hao J, Song
B, Ouyang L and Hu X: miR-208-induced epithelial to mesenchymal
transition of pancreatic cancer cells promotes cell metastasis and
invasion. Cell Biochem Biophys. 69:341–346. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu L, Dai Y, Chen J, Zeng T, Li Y, Chen
L, Zhu YH, Li J, Li Y, Ma S, et al: Maelstrom promotes
hepatocellular carcinoma metastasis by inducing
epithelial-mesenchymal transition by way of Akt/GSK-3β/Snail
signaling. Hepatology. 59:531–543. 2014. View Article : Google Scholar
|
|
48
|
Zhang J, Wei J, Lu J, Tong Z, Liao B, Yu
B, Zheng F, Huang X, Chen Z, Fang Y, et al: Overexpression of Rab25
contributes to metastasis of bladder cancer through induction of
epithelial-mesenchymal transition and activation of
Akt/GSK-3β/Snail signaling. Carcinogenesis. 34:2401–2408. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li Q, Wu J, Wei P, Xu Y, Zhuo C, Wang Y,
Li D and Cai S: Overexpression of forkhead Box C2 promotes tumor
metastasis and indicates poor prognosis in colon cancer via
regulating epithelial-mesenchymal transition. Am J Cancer Res.
5:2022–2034. 2015.PubMed/NCBI
|