Introduction
Melanoma is a highly malignant tumor derived from
melanin cells with an incidence rate of 1–3% among all tumors. Most
melanoma patients have metastasis by the time of treatment. This
disease is extremely resistant to radiotherapy and chemotherapy.
The 5-year survival rate is <16% (1,2). Many
new and effective treatment methods have emerged in recent years,
such as tyrosine kinase inhibitors and immune checkpoint blockades
(3). However, the vast majority of
patients, even in developed countries, cannot afford these
treatments.
Dendritic cell (DC)-based vaccine therapy is the
earliest and most effective method among cellular immunotherapies
for the treatment of melanoma. DCs are the most effective
antigen-presenting cells (APCs) in the body and considered to be a
primary part of the immune response. DCs play an extremely
important role in the immune response because they activate
cytotoxic T lymphocytes (4–6). In the last decade, DC-loaded tumor
antigens to prepare cancer vaccines for treating melanoma have
shown benefits in patients with melanoma.
The treatment effect of DC-based vaccine therapy is
attributed to the loaded antigenic peptides. Access to effective
tumor antigenic peptides is fundamental in determining the effect
of treatment (7,8). Autologous tumor antigenic peptides
from patients are undoubtedly the best choice. Most melanoma
patients in China are unable to access fresh tumor tissue after
surgery, making it impossible to obtain autologous tumor antigen
peptides, which seriously affects treatment. Thus, finding an
alternative for such patients has become a research focus.
Heat shock protein 70 (HSP70) has an important role
as a molecular chaperone that binds to tumor antigen peptides in
tumor cells (9–11). In our previous study, we purified
HSP70-HER-2-peptide complexes (PCs) from the human breast cancer
cell line SKBR-3, and showed that HSP70-HER-2-PCs contain more
comprehensive tumor antigen peptides and induce stronger immune
activity (12). Numerous studies
show that human melanoma cell lines also contain a number of
effective tumor antigen peptides (13,14).
The aim of this study was to determine whether HSP70-PCs purified
from human melanoma cell lines induce sufficient immune activity
compared with autologous tumor antigen peptides.
In the current study, we purified HSP70-PCs using
our method from human melanoma cell lines A375, A875, M21, M14,
WM-35, and SK-HEL-1. The obtained antigens were named M-HSP70-PCs.
Their immunological activities were determined by pulsing DCs and
inducing specific CD8+ T cells. Autologous HSP70-PCs
purified from primary cells of melanoma patients were used as
controls.
Materials and methods
Cell culture
Human melanoma cell lines A375, A875, M21, M14,
WM-35, and SK-HEL-1 were purchased from Peking Union Medical
College (Beijing, China). The cells were cultured in RPMI-1640
medium or Dulbecco's modified Eagle's medium (both from Gibco,
Invitrogen Co., Carlsbad, CA, USA) according to the manufacturer's
instructions. Media were supplemented with 10% heat-inactivated
fetal calf serum (FCS) (HyClone, Logan, UT, USA), 2 mol/l
L-glutamine (Gibco, Invitrogen Co.), 100 U/ml penicillin G, and 100
µg/ml streptomycin. Cells were cultured at 37°C in a
humidified atmosphere with 5% CO2.
Purification of M-HSP70-PCs
The methods used to purify HSP70-PCs have been
described previously (12). Human
melanoma cell lines were heated in a water bath at 42°C for 12 h,
followed by recovery for 2 h at 37°C in an atmosphere containing 5%
CO2. After heat shock, the tumor cells were digested by
0.02% trypsin, and then 5×106 cells of each cell line
were homogenized for 15 min on ice in a hypotonic buffer consisting
of 50 mM Tris-HCl, 150 mM NaCl, 1 mM phenylmethylsulfonyl fluoride,
1 mM sodium fluoride, and 5 mM
3-[(3-cholamidopropyl)dimethylammonio]-1-propane-sulfonate (CHAPS),
pH 7.2. After ultrasonication at 0°C for 30 min, the homogenate was
centrifuged at 10,000 × g for 90 min at 4°C. The supernatant was
dialyzed against buffer A (20 mM Tris-HCl, 150 mM NaCl, 1 mM
CaCl2, 1 mM MnCl2, 0.5 mM
phenylmethylsulfonyl fluoride, and 15 mM β-mercaptoethanol, pH 7.4)
overnight at 4°C. The sample was then loaded onto a Con A Sepharose
column (Sigma Chemical Co.). Unbound proteins were collected at a
flow rate of 12 ml/h. The fraction was dialyzed against buffer B
(20 mM Tris-HCl, 20 mM NaCl, 3 mM MgCl2, 1 mM
MnCl2, 0.5 mM phenylmethylsulfonyl fluoride and 15 mM
β-mercaptoethanol, pH 7.4) overnight at 4°C. The sample was then
applied to an ADP-agarose column (Sigma Chemical Co.) equilibrated
with buffer B at a flow rate of 12 ml/h. The column was eluted with
buffer B containing 0.5 M NaCl until proteins were not detected by
the Bradford method. The target protein was eluted with buffer B
containing 3 mM ADP (Sigma Chemical Co.). The endotoxin level in
the preparations was determined by the Limulus Amebocyte Lysate
(LAL) assay (Ocean Biologicals Co., China).
Preliminary characterization of
M-HSP70-PCs
M-HSP70-PCs were subjected to sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Coomassie
Brilliant Blue staining. For western blot analysis, separated
proteins were blotted onto a polyvinylidene fluoride membrane
(Immobilon-P; Millipore Corp., Billerica, MA, USA). Primary
monoclonal antibodies included rabbit anti-Melan-A (ab51061;
Abcam), mouse anti-NY-ESO-1 (sc-52869; Santa Cruz Biotechnology).
Goat anti-rabbit and anti-mouse horseradish peroxidase-conjugated
secondary antibodies were purchased from Santa Cruz Biotechnology
(sc-2030 and sc-2031). After secondary antibody incubation,
proteins were visualized by autoradiography.
Collection of fresh melanoma tumor
tissue
Surgical specimens were obtained from 15 patients
who underwent resection of skin melanoma tumors at the Oncology
Department, Affiliated Beijing Shi Ji Tan Hospital of Capital
Medical University in 2011. No patients had received preoperative
chemotherapy or radiotherapy. The age of the patients ranged from
35 to 61 years. Of the 15 patients, 10 were males and 5 females.
All pathological types were malignant melanoma of the skin. The
study was approved by the Human Research Ethics Committee of
Beijing Shijitan Hospital. All patients provided informed consent
for the collection of tissue samples. After the success of primary
tumor cell culture, peripheral blood mononuclear cells (PBMCs) were
isolated from peripheral blood of patients.
Primary melanoma cell culture and
purification of autologous HSP70-PCs
The methods of melanoma primary cell culture have
been described previously with some modifications (15). The resected skin melanoma tissues
were immediately placed in ice-cold RPMI-1640 medium containing 100
U/ml penicillin G and 100 µg/ml streptomycin, and
transported to the laboratory within 10 min. After removal of
necrotic tissues, the samples were rinsed with phosphate-buffered
saline (PBS) twice and cut into small fragments. The fragments were
incubated with 1% collagenase type II (Sigma Chemical Co.) in a
gently shaking water bath for 1 h at 37°C. After passed through a
38-µm mesh sieve, the resulting cell suspension was washed
twice and centrifugated at 300 × g for 10 min. The cells were
diluted to 5×105 cells/ml and incubated in RPMI-1640
supplemented with 10% heat-inactivated FCS at 37°C with 5%
CO2. To remove fibroblasts, the concentration of FCS in
the medium was changed to 5% in the second week of culture, and
then returned to 10% in the third week. Cells were passaged at 75%
confluency.
The autologous HSP70-PCs of patients were purified
from 5×106 primary cells according to the method
described in 'Purification of M-HSP70-PCs'.
Preparation of DCs and CD8+ T
cells
DCs were generated as described previously (16). Briefly, PBMCs were isolated from
heparinized venous blood of the patients by density gradient
centrifugation using Ficoll-Hypaque (1.077 g; Beijing Zhongshan
Jinqiao Biotechnology Co., Ltd., China) and cultured in RPMI-1640
medium containing 10% FCS for 2 h. Non-adherent cells were
collected to generate CD8+ T cells, and the adherent
cells were cultured for 7 days in RPMI-1640 medium containing 10%
FCS, 800 U/ml recombinant human granulocyte-macrophage
colony-stimulating factor (GM-CSF), and 500 U/ml recombinant human
interleukin (IL)-4 (both from R&D Systems, Inc., USA) to
generate DCs. Half medium volumes were replaced every other day,
and 50 U/ml tumor necrosis factor-α (R&D Systems, Inc.) was
added to the culture medium on the sixth day. The surface
phenotype, include CD80, CD83, CD86 and HLA-DR, of all the DCs in
this research were detected by flow cytometry, respectively.
CD8+ T cells were harvested from the
non-adherent fraction. Briefly, non-adherent cells were resuspended
in RPMI-1640 medium containing 10% FCS, 100 U/ml penicillin G, and
100 µg/ml streptomycin. Recombinant human interferon (IFN)-γ
(1,000 IU/ml) (PeproTech, Inc., USA) was added on day 0. After 24 h
of incubation, 50 ng/ml mouse anti-human monoclonal antibody
against CD3 (Becton, Dickinson and Co., USA), 100 U/ml recombinant
human IL-1β (R&D Systems, Inc.), and 300 U/ml recombinant IL-2
(PeproTech, Inc., USA) were added. Cells were incubated at 37°C in
a humidified atmosphere with 5% CO2 and subcultured
every third day in fresh complete medium with 300 U/ml IL-2 at
2×106 cells/ml.
Immunofluorescence staining
DCs (1×105) were pulsed with 10 µg
M-HSP70-PCs purified from the six melanoma cell lines at 37°C for
12 h. After culture, cytospin slides were prepared by
centrifugation (100 × g for 5 min) using 100 µl of cell
suspension containing about 1×106 cells/ml. The cells
were air-dried, immediately fixed with 4% paraformaldehyde,
permeabilized with 0.5% Triton X-100, and blocked in 3% bovine
serum albumin (BSA) at 4°C for 1 h. The cells were then incubated
with monoclonal rabbit anti-Melan-A and monoclonal mouse
anti-NY-ESO-1 antibodies at a 1:100 dilution in PBS for 1 h at
37°C. After three washes in PBS, the cells were incubated with
Texas Red-conjugated goat anti-rabbit IgG (ab6719) and
FITC-conjugated goat anti-mouse IgG (ab6785; both from Abcam) at a
1:50 dilution in PBS for 30 min in the dark at 37°C. The cells were
washed again three times in PBS and smeared onto the slides. Images
were acquired using a Olympus DP71 microscope (Olympus) and
analyzed by the included software.
Enzyme-linked immunospot (ELISPOT)
assay
An ELISPOT assay was performed to assess the IFN-γ
production of autologous T cells using an IFN-γ ELISPOT kit
(R&D Systems, Inc.). DCs were divided into three groups (A-C)
with 1×105 cells in each and pulsed for 12 h. Group A
received GM-CSF and IL-4 only, group B received 10 µg
M-HSP70-PCs purified from the six melanoma cell lines, and group C
received 10 µg autologous HSP70-PCs purified from primary
cells of each melanoma patient. After washing with PBS, the three
groups of DCs were co-cultured with autologous T cells isolated
using a nylon wool column at a 1:10 ratio in a 96-well culture
plate (Nunc, Roskilde, Denmark) in the presence of 20 U/ml IL-2 for
7 days. Stimulated T cells (1×104 cells/well) as
effector cells and primary melanoma cells (5×103
cells/well) as target cells were transferred to the ELISPOT plate,
followed by incubation at 37°C for 18 h. The level of IFN-γ was
detected as described in the IFN-γ ELISPOT kit manual with an
automated ELISPOT reader system (Biosys Co., Germany).
Induction of CD8+ T cells by
DCs pulsed with M-HSP70-PCs and in vitro cytotoxicity testing
An LDH release assay was used to determine the
specific cytolytic activities of CD8+ T cells as
described previously. Autologous DCs obtained using the method
described above and autologous CD8+ T cells of each
melanoma patient were used in the following experiment. Cells were
divided into three groups with 1×106 cells in each.
Then, the three groups of CD8+ T cells were co-cultured
with the three groups of autologous DCs pulsed under the conditions
mentioned above at a 10:1 ratio. The co-cultures were treated with
300 U/ml IL-2 in a 96-well plate for 1 week and named groups A, B
and C correspondingly. Then, the three groups of CD8+ T
cells were used as effector cells in the assay using an LDH
Cytotoxicity Detection kit (BioVision, Inc., USA). The
corresponding primary melanoma cells were used as target cells in
this assay. Briefly, target and effector cells were resuspended in
assay medium (RPMI-1640 with 1% BSA), and then target cells
(1×104 cells/well) were co-cultured with effector cells
at various ratios (1:5, 1:10, and 1:20) in a 96-well round-bottomed
culture plate at 37°C. After incubation for 4 h, the cells were
centrifuged at 50 × g for 10 min, and the supernatant was collected
and transferred to another 96-well for the LDH assay. LDH detection
mixture (100 µl/well) was added, followed by incubation in
the dark for 30 min at room temperature. After addition of 50
µl stop solution per well, the absorbance of the samples was
measured by a microplate reader at 490 nm as the reference
wavelength.
Treatment of a patient with melanoma of
the nasal skin and lung metastasis by M-HSP70-PCs
A 47-year-old woman was admitted to the Department
of Otolaryngology of Beijing Cancer Hospital for diagnosis of
melanoma in her nasal skin in November 2011. Extensive resection of
the tumor was performed in the same month. Postoperative
pathological diagnosis confirmed the original diagnosis. One week
after the operation, positron emission tomography and computed
tomography (CT) showed no abnormalities, and the patient began
chemotherapy (temozolomide + cisplatin + endostar). After four
cycles of chemotherapy, the patient had a severe gastrointestinal
reaction and some lung metastases were detected by enhanced chest
CT in June 2012. Chemotherapy was stopped, and a review examination
in July 2012 found that the lung metastases had become larger and
increased. The patient came to our hospital for cellular
immunotherapy, and we collected peripheral blood to culture
autologous DCs and CD8+ T cells. Because the patient
could not obtain autologous tumor antigen peptides, after informed
consent, she began M-HSP70-PC-loaded autologous DC and
M-HSP70-PC-loaded autologous co-cultured DC-CD8+ T-cell
treatment in September 2012. The therapy was applied once every 6
months, and each cycle included upper arm subcutaneous injection of
M-HSP70-PC (50 µg)-pulsed DCs (5×107) and
intravenous reinfusion with co-cultured M-HSP70-PC (50
µg)-pulsed DCs-CD8+ T cells
(4×1010).
Statistical analysis
Values are expressed as the means ± standard
deviation (SD) or a percentage. All analyses were conducted using
SPSS 17.0 software. The results were considered statistically
significant at P<0.05.
Results
Preliminary characterization of
M-HSP70-PCs
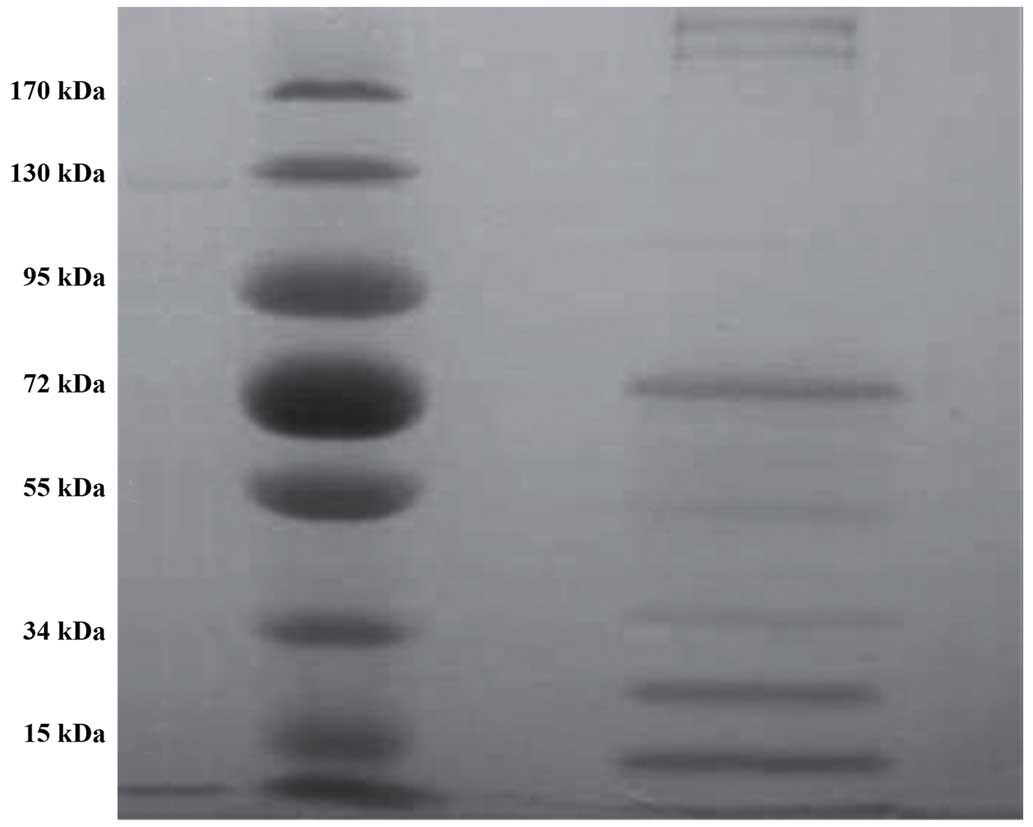
M-HSP70- PCs were purified from six melanoma cell
lines and analyzed by SDS-PAGE. The purified products contained
various proteins according to the different molecular weights
(Fig. 1). The 13- and 21-KDa
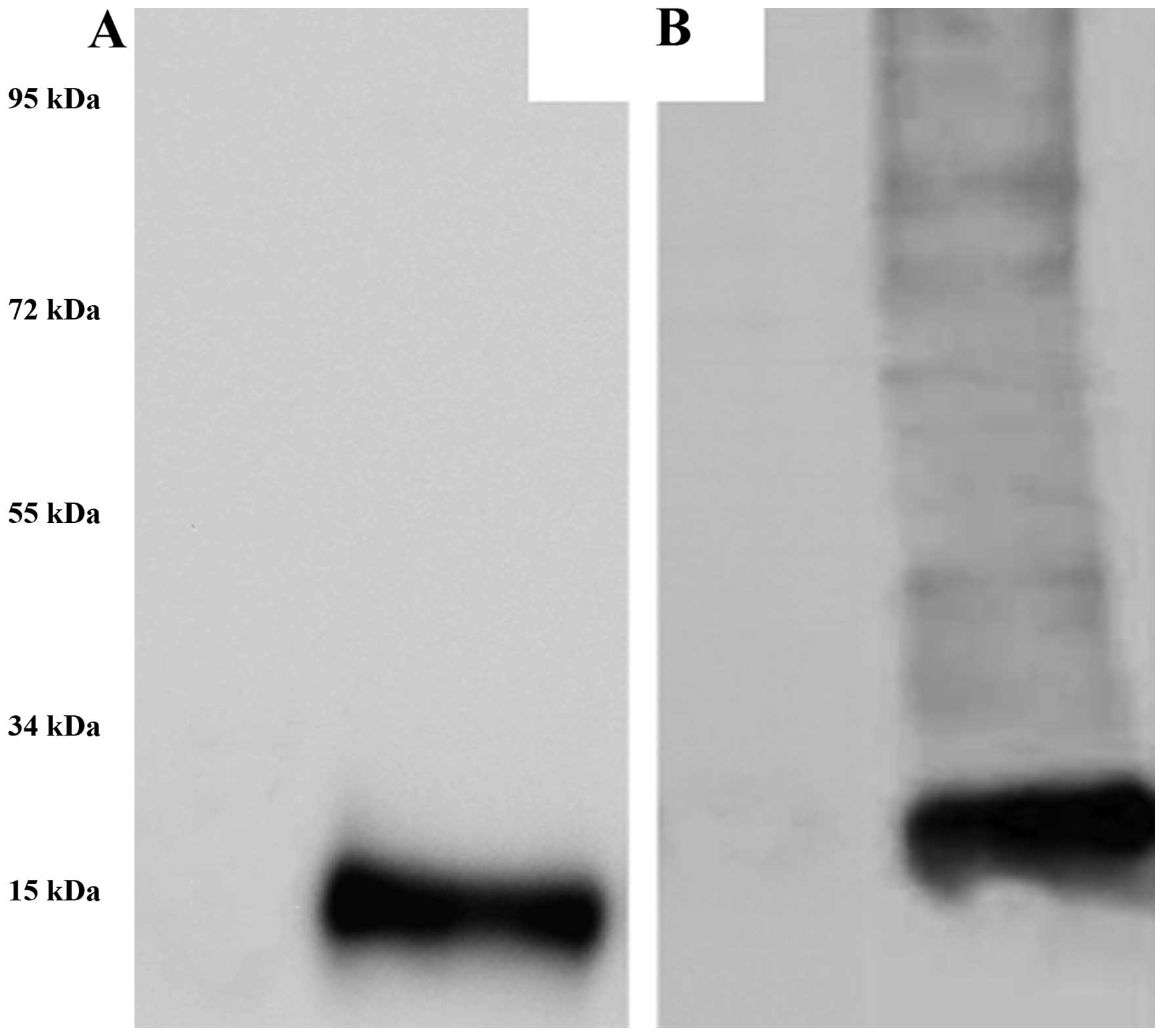
proteins were especially striking. In western blot analysis,
Melan-A- and NY-ESO-1-specific antibodies bound to M-HSP70-PCs
(Fig. 2), demonstrating that the
obtained complex contained Melan-A and NY-ESO-1 proteins that are
the most important melanoma antigen peptides. Other proteins may be
melanoma antigen peptides bound by HSP70.
Quantitative detection was performed using the
Bradford standard curve method. The protein content of M-HSP70-PCs
purified from the six melanoma cell lines (3×107 cells)
was 917.03 µg. The endotoxin levels in the preparations were
lower than 0.03 EU/mg as determined by the LAL assay.
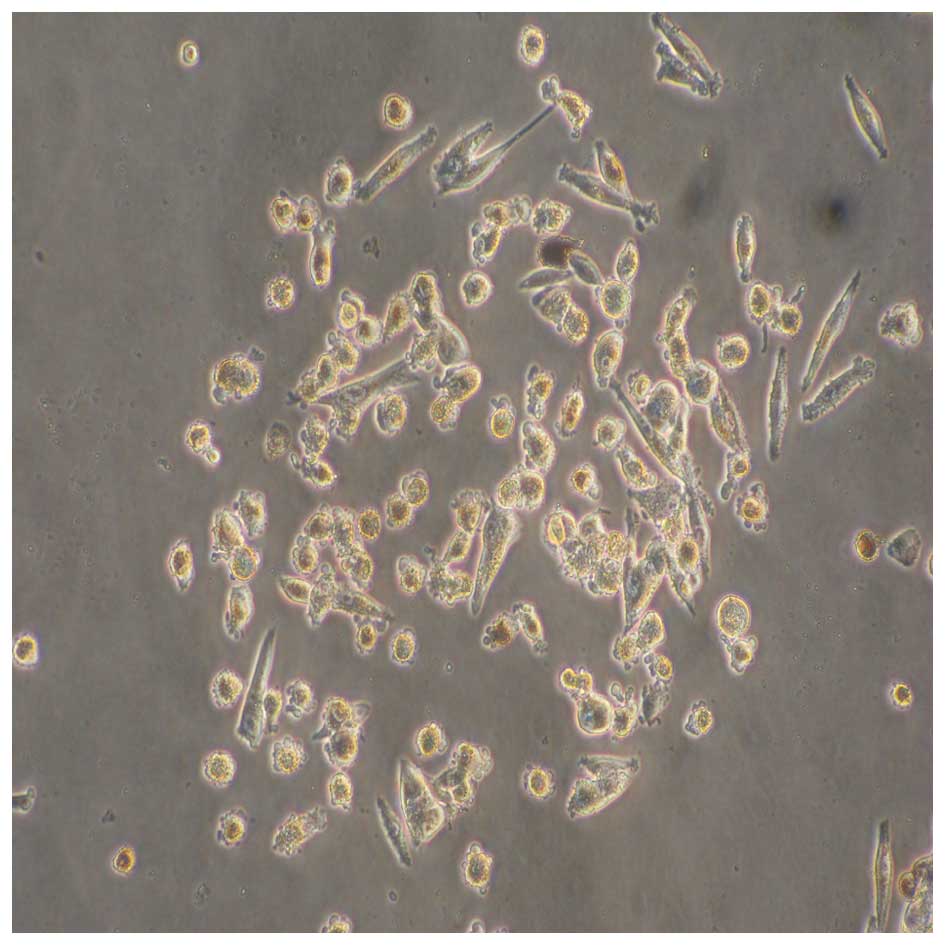
Primary melanoma cell culture
Of the 15 primary melanoma cell cultures obtained
from surgical specimens, four became contaminated during culture
and two underwent senescence. The remaining cultures (9 cases) were
passaged 4–5 times, and the number of cells reached
1×107 usable for preparation of autologous HSP70-PCs,
and as target cells for further experiments (Fig. 3). Detailed information on the 9
cases of melanoma patients are shown in Table I.
 | Table IInformation on malignant melanoma
patients (9 cases). |
Table I
Information on malignant melanoma
patients (9 cases).
| Patient no. | Age | Gender | Tumor site | Tumor size
(mm) |
|---|
| 1 | 51 | Male | Abdominal skin | 52×44 |
| 2 | 37 | Male | Upper extremity
skin | 35×30 |
| 3 | 59 | Female | Abdominal skin | 32×28 |
| 4 | 53 | Male | Facial skin | 63×46 |
| 5 | 37 | Male | Abdominal skin | 41×30 |
| 6 | 40 | Male | Lower limb
skin | 57×33 |
| 7 | 35 | Female | Upper extremity
skin | 50×25 |
| 8 | 61 | Male | Upper extremity
skin | 31×26 |
| 9 | 42 | Female | Back skin | 44×31 |
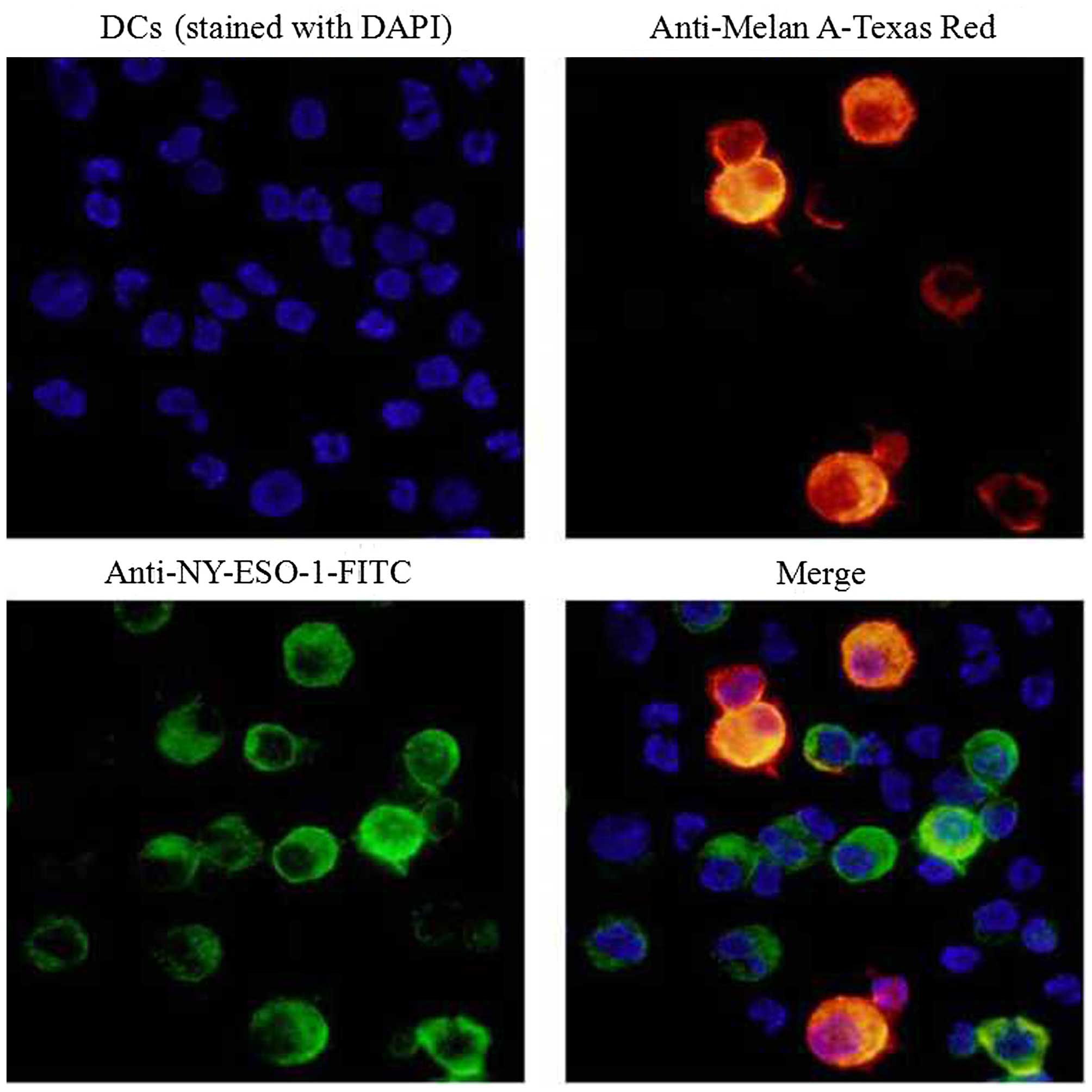
Immunofluorescence staining
Next, we determined the ability of DCs to take up
M-HSP70-PCs purified from melanoma cell lines. DCs were pulsed with
M-HSP70-PCs at 37°C for 12 h. After the numerous aforementioned
treatments and extensive washing to remove unbound proteins, the
DCs were analyzed under an Olympus DP71 microscope. Fig. 4 shows that DCs contained Melan-A
protein (red) and NY-ESO-1 protein (green). Evidently, the
M-HSP70-PCs were taken up by the DCs.
Antigen-specific IFN-γ production induced
by DCs pulsed with M-HSP70-PCs
Cell-mediated immunity, which is particularly
important for tumor suppression, is characterized by the production
of type I cytokines. Therefore, we explored the ability of DCs
pulsed with M-HSP70-PCs to stimulate autologous T cells and induce
IFN-γ secretion. Autologous T cells co-cultured with the three DC
groups were used as effector cells, and primary melanoma cells,
which were used to obtain the autologous HSP70-PCs, were applied as
target cells. The cells were then analyzed by an IFN-γ ELISPOT.
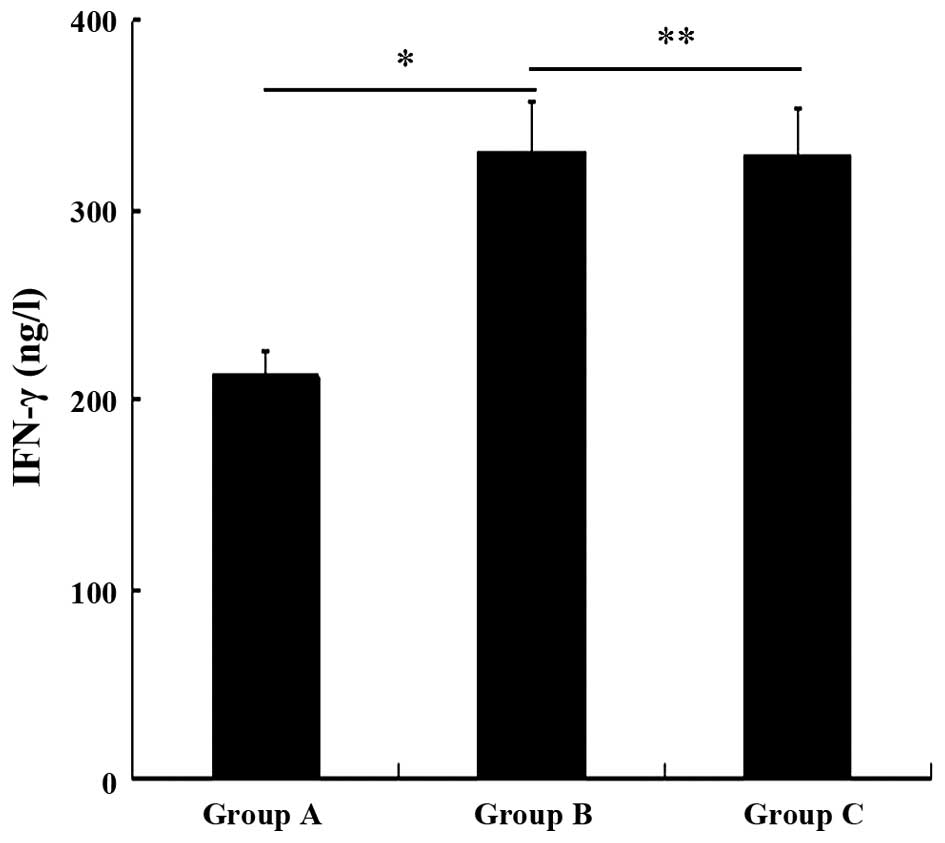
Fig. 5 shows a significantly higher
IFN-γ levels in groups B and C than in group A (P<0.05). There
was no significant difference between groups B and C (P>0.05).
This result confirmed that the DCs pulsed with M-HSP70-PCs
stimulated T cells to secrete the same levels of type I cytokines
compared with DCs pulsed with autologous HSP70-PCs.
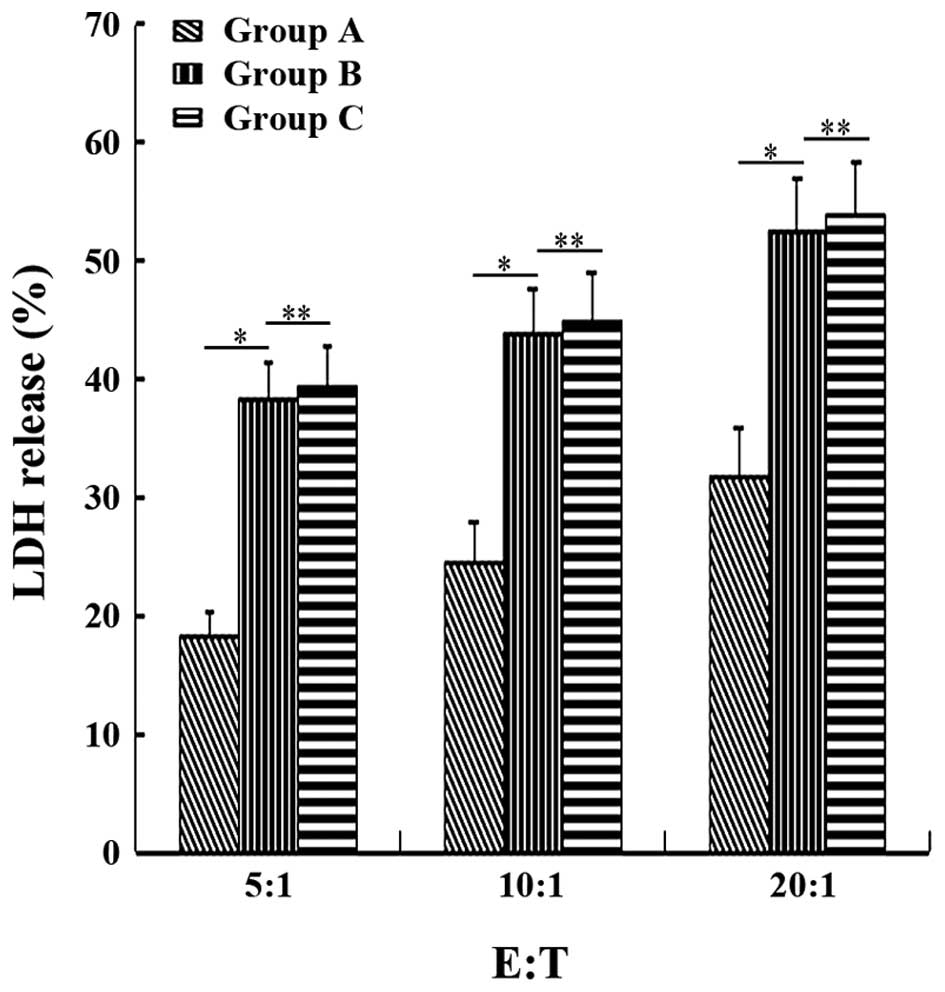
Cytotoxicity in vitro
The three groups of autologous CD8+ T
cells were induced by co-culturing with the three groups of
autologous DCs. The specific cytolytic activities of
CD8+ T cells against primary melanoma cells was examined
by detecting the level of LDH release after 4 h of co-culturing
effector cells (CD8+ T cells) with target cells
(autologous primary melanoma cells) at 5:1, 10:1, and 20:1. The LDH
release level was not significantly different between groups B and
C (P>0.05), but significantly higher in group A (P<0.05)
(Fig. 6). This result indicated
that M-HSP70-PCs had an equivalent ability as autologous HSP70-PCs
to induce autologous CD8+ T cells to kill the melanoma
cells of patients. Therefore, M-HSP70-PCs may be an efficient and
general melanoma antigen complex.
M-HSP70-PC-induced specific DCs and
CD8+ T-cell treatment of a patient with melanoma of the
nasal skin and lung metastasis
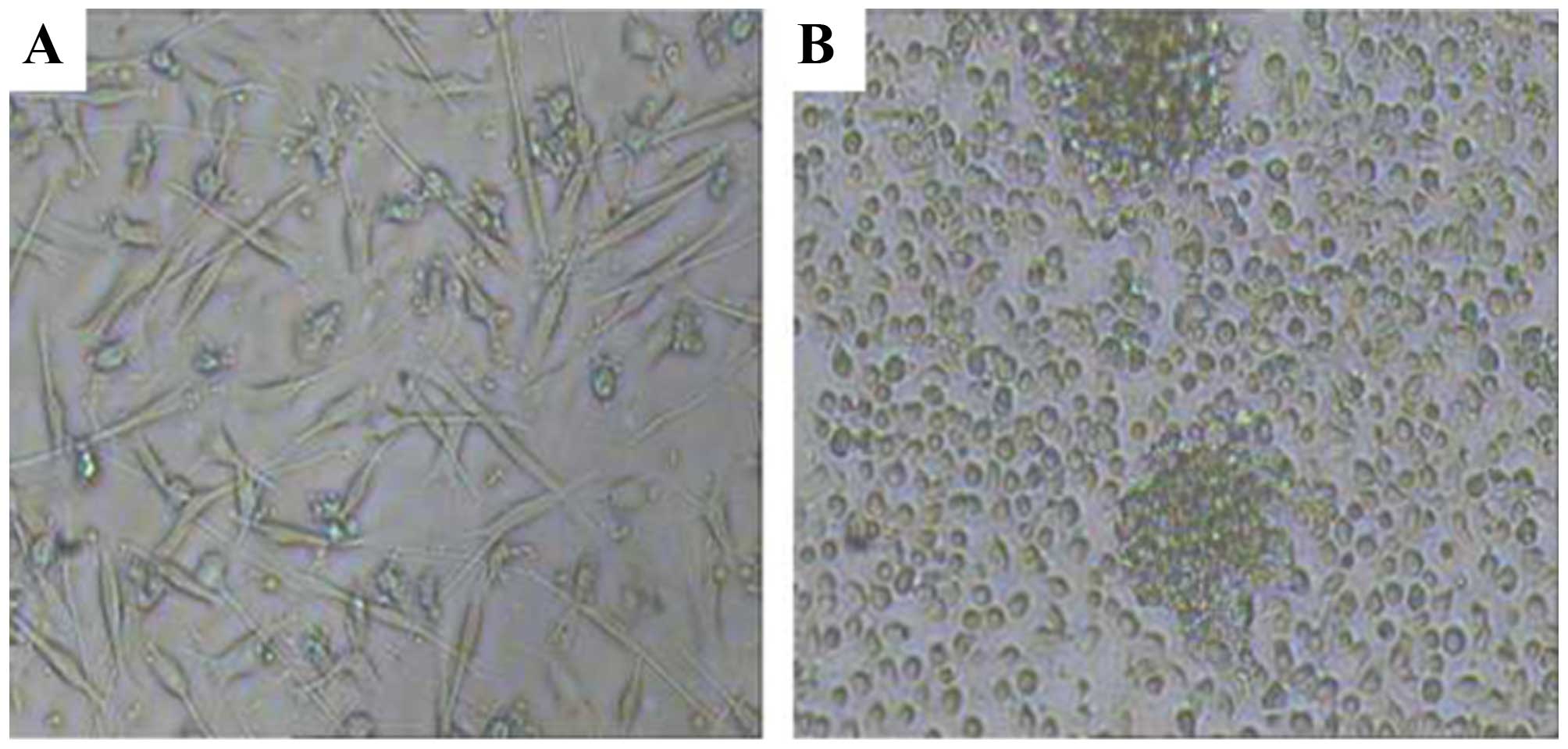
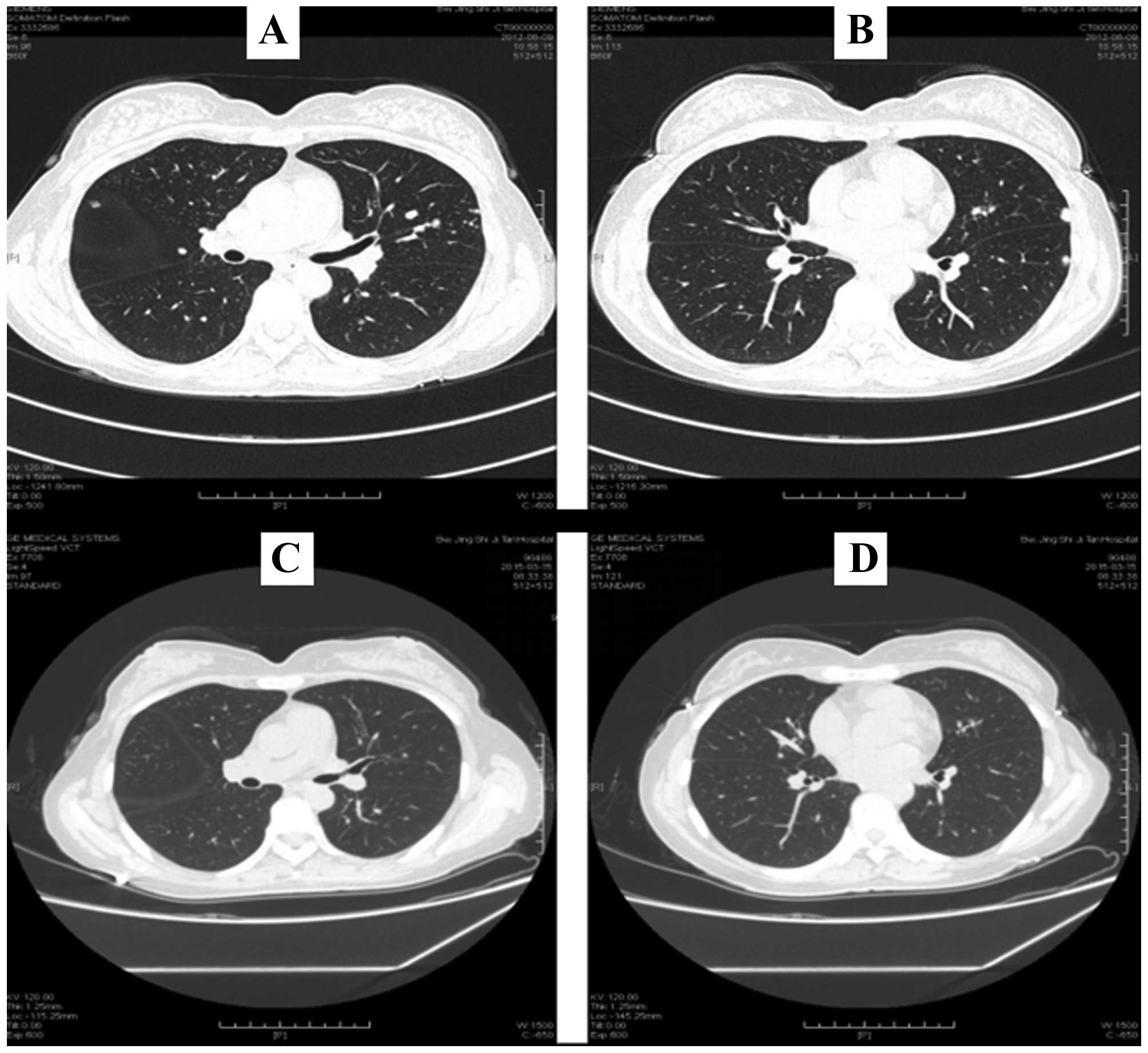
A patient with melanoma of the nasal skin and lung
metastasis began treatment with autologous DCs and CD8+
T cells induced by M-HSP70-PCs. The patient had not received any
other therapy since September 2012. The DCs and CD8+ T
cells were induced from autologous peripheral blood (Fig. 7A and B), and the treatment was
performed once every 6 months. After six cycles of treatment, the
primary tumor of the nasal skin had not relapsed and no new
metastases had appeared. An enhanced chest CT in March 2015 showed
that some lung metastases had shrunk and others had disappeared
(Fig. 8).
Discussion
In recent years, with the rapid development of
modern tumor immunology, immunotherapy of melanoma has achieved
great success. Many new treatments, such as cytotoxic T
lymphocyte-associated antigens, B-type Raf kinase inhibitors, and
anti-programmed cell death 1/ligand antibodies, have greatly
extended progress-free and overall survival of melanoma patients
(3,17–19).
However, there are huge costs associated with these treatments.
Cellular immune therapy such as DC-based
immunotherapy has been recognized worldwide in recent years. DCs
are professional APCs bridging innate and adaptive immunities.
Their role in the periphery is to take up antigenic proteins that
are processed and presented to T lymphocytes at lymphoid organs in
combination with major histocompatibility molecules (4,20,21).
Many clinical trials of immunotherapy have been performed using
DC-based vaccines against melanoma since Nestle et al
(22) reported the efficacy of a
melanoma lysate or peptide-treated DC vaccine in 1998 (23,24).
These studies have confirmed that DC-based vaccines are an
efficient, safe and inexpensive method of melanoma treatment.
Therefore, DC therapy has become one of the most widely used and
effective treatments for melanoma in China as well as
worldwide.
The type of antigens pulsed into DCs determines the
effect of DC-based vaccines, and purified autologous tumor antigens
are no doubt the best choice. However, for various reasons, some
patients cannot obtain autologous tumor antigens. Early studies
revealed many important antigens in human tumor cell lines. They
used lysates of human tumor cell lines to pulse DCs for treatment
of the same kind of tumor and achieved a certain therapeutic effect
(4,12,13,24,25).
HSP70 is overexpressed in many tumors and acts at a
crossroad of key intracellular processes in its role as a molecular
chaperone. It associates with numerous tumor antigenic peptides and
forms immunogenic complexes called HSP70-PCs (9,10,26).
Our previous study revealed that HSP70-PCs purified from tumor
cells have better immunocompetence than lysates of the same cells.
Moreover, we established a new method using the detergent CHAPS to
purify HSP70-PCs containing more efficient tumor peptides from
human cancer cells (12).
We believe that a different cell line may contain
different tumor antigen peptides which could be combined with
HSP70. The six human melanoma lines we used were the total we were
able to obtain at that time. In this study, we purified M-HSP70-PCs
from human melanoma cell lines using the new method. To determine
their immune activity, autologous HSP70-PCs purified from primary
tumor cells of melanoma patients were used as a control. The
M-HSP70-PCs had the same immunocompetence to induce DCs and
CD8+ T cells to specifically kill tumor cells from which
the autologous HSP70-PCs were purified. This result suggests that
M-HSP70-PCs may be an effective and general antigen complex of
melanoma. In SDS-PAGE and western blot analyses, Melan-A and
NY-ESO-1 were detected in M-HSP70-PCs.
We applied M-HSP70-PCs as antigens to treat one
patient with melanoma and lung metastasis, and achieved a good
therapeutic effect. The success of the treatment verified the
safety and effectiveness of M-HSP70-PCs. It is worth mentioning
that we used both autologous DCs and co-cultured
DCs-CD8+ T cells as the therapeutic cells, because we
believe there is some immune suppression in cancer patients and
intravenous reinfusion of a large number of CD8+ T cells
co-cultured with DCs in vitro might enhance the treatment
effect.
In summary, we prepared an effective and general
antigen complex for melanoma treatment. The product, M-HSP70-PCs,
may contain comprehensive and efficient melanoma antigens with the
same immunocompetence against human melanoma cells as autologous
antigens. We will explore the identity of other antigens in
M-HSP70-PCs in further studies to reveal new melanoma-associated
antigen peptides. Simultaneously, we will continue to conduct
clinical trials to confirm the treatment effect of this therapy.
The findings of this study provide a better therapeutic approach
for DC-based cellular immuno-therapy of melanoma patients who
cannot access autologous tumor antigens.
Acknowledgments
This research is supported by grants from the
National Natural Science Foundation of China (81260392).
References
|
1
|
Kim JS, Kim YG, Pyo M, Lee HK, Hong JT,
Kim Y and Han SB: Adoptive cell therapy of melanoma with
cytokine-induced killer cells. Immune Netw. 15:58–65. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ko JM and Fisher DE: A new era: Melanoma
genetics and therapeutics. J Pathol. 223:241–250. 2011. View Article : Google Scholar
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oshita C, Takikawa M, Kume A, Miyata H,
Ashizawa T, Iizuka A, Kiyohara Y, Yoshikawa S, Tanosaki R, Yamazaki
N, et al: Dendritic cell-based vaccination in metastatic melanoma
patients: Phase II clinical trial. Oncol Rep. 28:1131–1138.
2012.PubMed/NCBI
|
|
5
|
Nakai N, Hartmann G, Kishimoto S and Katoh
N: Dendritic cell vaccination in human melanoma: Relationships
between clinical effects and vaccine parameters. Pigment Cell
Melanoma Res. 23:607–619. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hussein MR: Dendritic cells and melanoma
tumorigenesis: An insight. Cancer Biol Ther. 4:501–505. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tagliamonte M, Petrizzo A, Tornesello ML,
Buonaguro FM and Buonaguro L: Antigen-specific vaccines for cancer
treatment. Hum Vaccin Immunother. 10:3332–3346. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ranieri E, Kierstead LS, Zarour H,
Kirkwood JM, Lotze MT, Whiteside T and Storkus WJ: Dendritic
cell/peptide cancer vaccines: Clinical responsiveness and epitope
spreading. Immunol Invest. 29:121–125. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hightower LE: Heat shock, stress proteins,
chaperones, and proteotoxicity. Cell. 66:191–197. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Srivastava PK: Peptide-binding heat shock
proteins in the endoplasmic reticulum: Role in immune response to
cancer and in antigen presentation. Adv Cancer Res. 62:153–177.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sherman M and Multhoff G: Heat shock
proteins in cancer. Ann NY Acad Sci. 1113:192–201. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao Y, Chen X, Gao W, Yang Y, Ma H and Ren
X: A new purification method for enhancing the immunogenicity of
heat shock protein 70-peptide complexes. Oncol Rep. 28:1977–1983.
2012.PubMed/NCBI
|
|
13
|
Li Y, Zhang L, Wang S, Shi P and Qu W:
Optimizing dendritic cell preparation for fusion with melanoma
cells. Iran J Immunol. 11:166–176. 2014.PubMed/NCBI
|
|
14
|
Powell KL, Stephens AS and Ralph SJ:
Development of a potent melanoma vaccine capable of stimulating
CD8(+) T-cells independently of dendritic cells in a mouse model.
Cancer Immunol Immunother. 64:861–872. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Soo JK, Ross AD and Bennett DC: Isolation
and culture of melanoma and naevus cells and cell lines. Methods
Mol Biol. 731:141–150. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thurner B, Röder C, Dieckmann D, Heuer M,
Kruse M, Glaser A, Keikavoussi P, Kämpgen E, Bender A and Schuler
G: Generation of large numbers of fully mature and stable dendritic
cells from leukapheresis products for clinical application. J
Immunol Methods. 223:1–15. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Grossmann KF and Margolin K: Long-term
survival as a treatment benchmark in melanoma: Latest results and
clinical implications. Ther Adv Med Oncol. 7:181–191. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Min L and Hodi FS: Anti-PD1 following
ipilimumab for mucosal melanoma: Durable tumor response associated
with severe hypothyroidism and rhabdomyolysis. Cancer Immunol Res.
2:15–18. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Merelli B, Massi D, Cattaneo L and Mandalà
M: Targeting the PD1/PD-L1 axis in melanoma: Biological rationale,
clinical challenges and opportunities. Crit Rev Oncol Hematol.
89:140–165. 2014. View Article : Google Scholar
|
|
20
|
Kurtz DM and Gambhir SS: Tracking cellular
and immune therapies in cancer. Adv Cancer Res. 124:257–296. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chiang CL, Coukos G and Kandalaft LE:
Whole tumor antigen vaccines: Where are we? Vaccines (Basel).
3:344–372. 2015. View Article : Google Scholar
|
|
22
|
Nestle FO, Alijagic S, Gilliet M, Sun Y,
Grabbe S, Dummer R, Burg G and Schadendorf D: Vaccination of
melanoma patients with peptide- or tumor lysate-pulsed dendritic
cells. Nat Med. 4:328–332. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thurner B, Haendle I, Röder C, Dieckmann
D, Keikavoussi P, Jonuleit H, Bender A, Maczek C, Schreiner D, von
den Driesch P, et al: Vaccination with mage-3A1 peptide-pulsed
mature, monocyte-derived dendritic cells expands specific cytotoxic
T cells and induces regression of some metastases in advanced stage
IV melanoma. J Exp Med. 190:1669–1678. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schneble EJ, Yu X, Wagner TE and Peoples
GE: Novel dendritic cell-based vaccination in late stage melanoma.
Hum Vaccin Immunother. 10:3132–3138. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gong J, Nikrui N, Chen D, Koido S, Wu Z,
Tanaka Y, Cannistra S, Avigan D and Kufe D: Fusions of human
ovarian carcinoma cells with autologous or allogeneic dendritic
cells induce antitumor immunity. J Immunol. 165:1705–1711. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tamura Y, Peng P, Liu K, Daou M and
Srivastava PK: Immunotherapy of tumors with autologous
tumor-derived heat shock protein preparations. Science.
278:117–120. 1997. View Article : Google Scholar : PubMed/NCBI
|