Introduction
Angiogenesis is the formation of new blood vessels
sprouting from pre-existing vasculature and is also associated with
both physiological processes (wound healing, tissue remodeling and
developmental progress) and pathological processes (tumor growth,
metastasis and coronary artery disease) (1,2). It
includes primary existing capillary sprouting, branching and
remodeling into a mature blood vessel network (3). In adulthood, angiogenesis is
stimulated at sites of tissue repair and in diseases constituting
enhanced angiogenesis (4).
Moreover, angiogenesis plays an important role in solid tumors and
is regulated by protease-mediated degradation of matrix proteins
releasing angiogenic factors such as vascular endothelial growth
factor (VEGF), epidermal growth factor (EGF), and fibroblast growth
factor (FGF), followed by the activation and proliferation of
endothelial cells to sprout new vessels (5,6).
EphrinB2 is a membrane-bound ligand that is
expressed on arterial endothelial cells (7). It has an intracellular domain and
possesses an intrinsic signaling capacity called 'reverse
signaling' (8,9). EphrinB2 'reverse signaling' is
triggered by its intracellular domain, which comprises several
sites for tyrosine, serine phosphorylation and a C-terminal
PDZ-binding motif (10). The
'reverse' signal transduction pathway mediated by PDZ-binding motif
is an important factor for the formation of the vascular system
(11). Thus, inhibition of
phosphorylation- or PDZ-dependent signaling downstream of EphrinB
ligands prevents endothelial cell sprouting. EphB and EphrinB not
only undergo internalization of themselves but promote the
internalization of the surrounding membrane and other proteins
(12). For example, EphrinB2
signaling has been reported to promote the internalization of
VEGFR2 and VEGFR3, and the PDZ-binding motif is critical in this
process. Hence, inhibition of EphrinB2 may be useful to
simultaneously interfere with the function of VEGFR2 and VEGFR3
which act together during angiogenesis (13). EphrinB2 stimulation can also
regulate VEGF/VEGFR signaling in the context of cancer, resulting
in the activation of phosphatidylinositol 3-kinase (PI3K)/AKT and
extracellular signal-regulated kinase-mitogen-activated protein
kinase (ERK-MAPK) pathways. These signaling pathways regulate
important cellular functions including endothelial cell
proliferation, migration and angiogenesis (14,15).
EphrinB2 ligand expressed in various tumor cells is
related to its high vascularity. In the present study, we
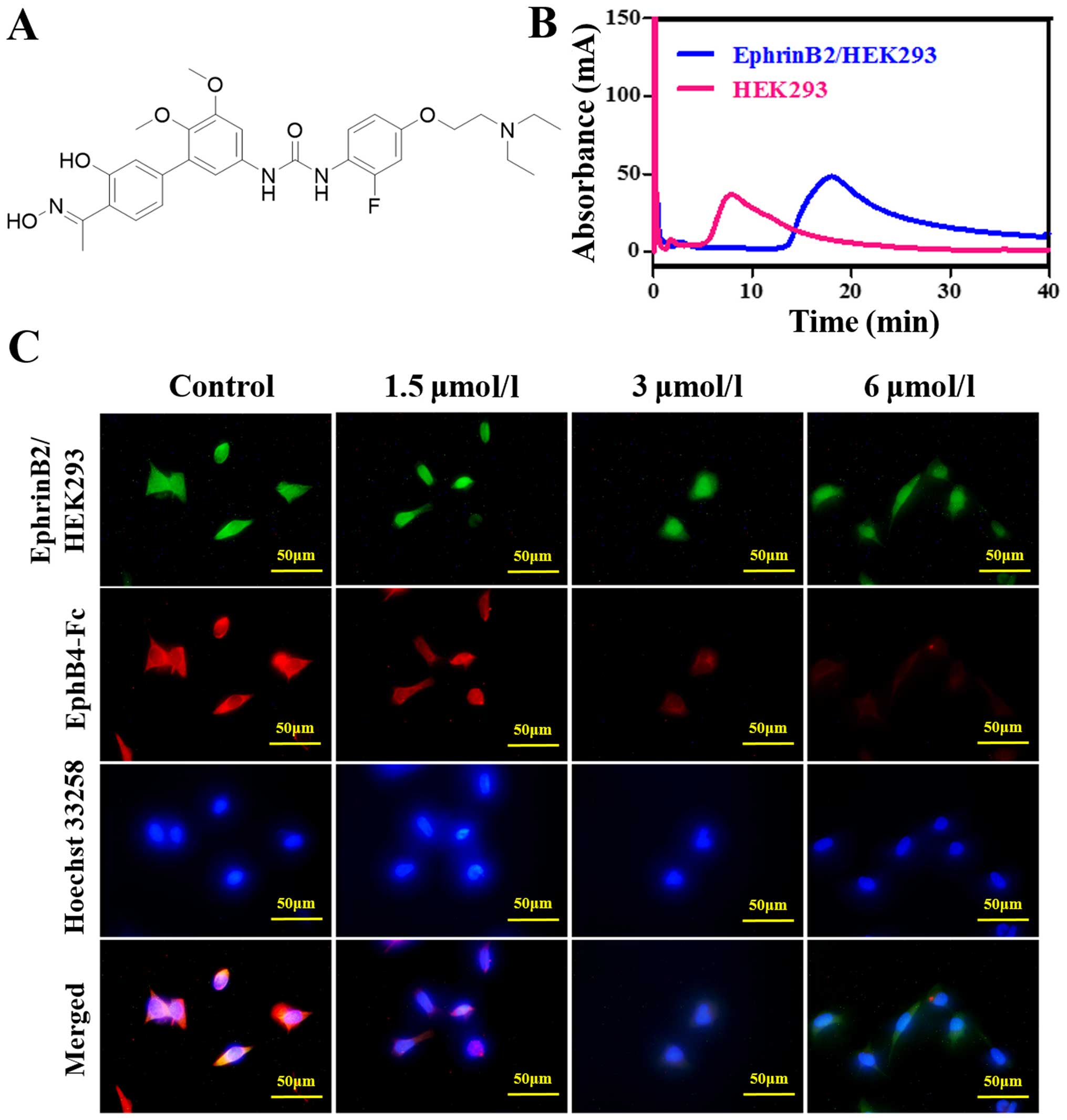
investigated the effect of taspine derivate 12k (Fig. 1A) which was synthesized with taspine
as a lead compound bearing a biphenyl scaffold on colorectal cancer
cells Caco-2 for high expression of EphrinB2 (16). Previously, 12k has been reported to
have potent anticancer activity. Based on the promising results, we
further investigated the antitumor activity of 12k on the tumor
growth and migration of human colorectal cancer cells Caco-2 and
the related mechanism.
Materials and methods
Chemicals and reagents
Taspine derivate 12k (purity >98%) was
synthesized in the Research and Engineering Center for Natural
Medicine, Xi'an Jiaotong University. Dulbecco's modified Eagle's
medium (DMEM), methyl thiazolyl tetrazolium (MTT),
dimethylsulphoxide (DMSO) and fibrinogen from bovine plasma were
obtained from Sigma-Aldrich (St. Louis, MO, USA). Fetal bovine
serum (FBS) was purchased from HyClone (Logan, UT, USA). Trypsin
was obtained from Amresco (Solon, OH, USA). Penicillin was
purchased from Harbin General Pharmaceutical Factory (Harbin,
China), and streptomycin was purchased from North China
Pharmaceutical (Shijiazhuang, China). Thrombin was obtained from
Guoao Pharmaceutical (Changchun, China). G418 was obtained from
Gibco (Carlsbad, CA, USA). Crystal violet was purchased from
Beijing Chemical Plant (Beijing, China). PI3K p110α rabbit mAb,
PI3K p110β rabbit mAb, PI3K p110γ rabbit mAb, PI3K class III rabbit
mAb, p-PI3K p85/p55 rabbit mAb, PI3K p85 rabbit mAb, p44/42 MAPK
(ERK1/2) rabbit mAb, phospho-p44/42 MAPK (p-ERK1/2) rabbit mAb and
phospho-mTOR rabbit mAb were all purchased from Cell Signaling
Technology (Boston, MA, USA). Phospho-EphrinB2 rabbit polyclonal
antibody was purchased from Abcam (Cambridge, UK). PTEN rabbit mAb,
RAC1 rabbit mAb, CD34 rabbit polyclonal antibody, PICK1 rabbit
polyclonal antibody, syntenin rabbit polyclonal antibody, VEGFR3
rabbit polyclonal antibody, CD45 rabbit polyclonal antibody and
mTOR polyclonal antibody were obtained from Proteintech Group, Inc.
(Chicago, IL, USA). AKT rabbit mAb, phospho-AKT rabbit mAb, HIF-1α
rabbit mAb and EphrinB2 rabbit mAb were purchased from Epitomics,
Inc. (Burlingame, CA, USA). Rabbit anti-GAPDH, goat anti-rabbit
IgG, BCA protein assay reagent kit and Enhanced Chemiluminescent
(ECL) Plus Reagent kit were obtained from Pierce Biotechnology
(Rockford, IL, USA). Protease inhibitor cocktail and phosphatase
inhibitor cocktail were purchased from Roche Technology (Basel,
Switzerland).
Cell culture and animals
Human colon cancer cell lines LoVo and Caco-2 were
purchased from the Shanghai Institute of Biochemistry and Cell
Biology of the Chinese Academy of Sciences (Shanghai, China).
HEK293 cells were obtained from Professor Xu Li (School of
Medicine, Xi'an Jiaotong University). The EphrinB2/HEK293 cell line
which over-expresses EphrinB2 was constructed at the Research and
Engineering Center for Natural Medicine, Xi'an Jiaotong University.
LoVo, Caco-2 and HEK293 cells were cultured in DMEM supplemented
with 10% FBS. The EphrinB2/HEK293 cells were maintained in DMEM
supplemented with 10% FBS and 200 mg/ml G418. All cell lines were
incubated in a humidified atmosphere of 5% CO2 at
37°C.
Mice (4–6 weeks old, body weight 15–18 g) were
purchased from the Animal Experimental Center of Xi'an Center of
Xi'an Jiaotong University. The mice were maintained under laminar
air flow conditions with a 12 h light/12 h dark cycle. Laboratory
food and water were freely available. Animal care was in accordance
with the National Institute of Health guidelines and the Animal
Experimental Committee of Xi'an Jiaotong University (SYXK shaan
2015-002).
Preparation of CM SP
Exponentially growing EphrinB2/HEK293 and HEK293
cells were harvested and washed with 5 mM PBS three times, and the
precipitate was suspended with 50 mM Tris-HCl (pH 7.4), followed by
ultrasonic destruction for 30 min. The homogenate was centrifuged
at 1,000 × g for 10 min, the supernatant was removed and the
homogenate was next centrifuged at 12,000 × g for 10 min. The
precipitate was then suspended with 5 mM PBS. The CMSP was prepared
by adsorption of the cell membrane suspension (5 ml) on activated
silica (0.05 g) under vacuum and with gentle agitation. The CMSP
was incubated overnight and then washed with 5 mM PBS five times.
Finally, the mixture obtained was packed into a column (10×2.0 mm
i.d.) using a wet packing method (10 MPa, 5 min). All the
procedures were performed at 4°C. CMC analysis was performed on a
Shimadzu LC-20A apparatus that consisted of two LC-20AD pumps, a
DGU-20A3 degasser, an SIL-20A autosampler, a CTO-20A column oven,
and an SPD-M20A diode array detector (Shimadzu, Kyoto, Japan). The
data were acquired using LC solution software (Shimadzu). The
detection wavelength was 250.4 nm. The chromatographic conditions
were as follows: CMC column, 10.0×2.0 mm; flow rate, 0.6 ml/min;
column temperature, 37°C; mobile phase, 50 mM phosphate-buffered
saline, pH 7.4.
Fluorescence localization competitive
antagonism assay
Exponentially growing EphrinB2/HEK293 cells were
plated into a 96-well plate at a density of 5,000 cells/well and
cultivated overnight. Then 12k at different concentrations (1.5, 3,
6 µmol/l) and EphB4-Fc (0.04 mg/l) were added for 4–8 h at
37°C. Cells were washed with PBS, fixed with 4% paraformaldehyde
for 15 min and treated with 1% Triton X-100 for 8 min at room
temperature. Finally, the cells were stained with Hoechst 33258 for
15–20 min. All images were recorded under a inverted fluorescence
microscope.
Cell viability assay
Exponentially growing EphrinB2/HEK293, HEK293, LoVo
and Caco-2 cells were seeded into a 96-well plate and cultivated
overnight. Then various concentrations of 12k were added for 48 h.
The medium was replaced with 180 µl serum-free DMEM and 20
µl MTT solution (5 mg/ml). After a 4-h incubation, the
supernatants were removed, and the formazan crystals were dissolved
with 150 µl DMSO. After being shaken thoroughly for 15 min,
the absorbance was measured at 490 nm on a microplate reader
(Bio-Rad, USA). Results are expressed as a percentage of the cell
viability ratio. Percentage of cell viability ratio = [1 −
(ODtreatment group − ODblank
group)/(ODcontrol group − ODblank
group)] × 100%. The experiment was performed in
triplicate.
Colony survival assay
Exponentially growing Caco-2 cells were seeded into
a 12-well plate (200 cells/well) overnight. Then the cells were
treated with 12k at 0, 1.5, 3 and 6 µmol/l and the plate was
incubated in a CO2 incubator until the colonies were
clearly visible and countable. The colonies were fixed with
methanol for 15 min and stained with crystal violet for 15 min.
After being washed sufficiently, images were captured using
enhanced chemiluminescence reagent and the inverted fluorescence
microscope. Survival was plotted as the percentage of the surviving
cells to the untreated control.
Wound healing assay
Exponentially growing Caco-2 cells were seeded into
a 12-well plate and cultivated to grow until ~80% confluency
overnight. Wounds were made by scratching the cells with pipette
tips (100–200 µl) on the following day. Then 12k at 0, 1.5,
3 and 6 µmol/l was added to allow cells to migrate into the
scratched area at different times. The migration of cells was
visualized at time 0 h (after the wound was scratched), 24 and 48 h
after 12k treatment. The distances of the wound at different
concentrations were measured and the migration distances were
calculated. Results are expressed as the percentage of the
migration rate. Migration rate = (Migration distancetreatment
group/Migration distancecontrol group) × 100%.
Tissue model for angiogenesis
The model was prepared and analyzed using a
published method (17). Briefly,
mice were sacrificed by cervical dislocation and the lung tissue
was separated. After being washed by PBS, lung tissue was cut into
pieces ~0.5–1 mm3 and cultured into a 48-well plate
which was coated with lypolymerized fibrinogen with thrombin. After
consolidation, additional lypolymerized fibrinogen with thrombin
was placed on the lung tissue and different concentrations of 12k
were added. Sprouting vessels were observed under a
stereomicroscope on day 5. Neovessel outgrowth was monitored
throughout the experiment and imaged using phase microscopy. All
aforementioned experiments were conducted on three separate mice
and repeated three times.
Western blotting
Caco-2 cells exposed to 12k (0, 1.5, 3, 6
µmol/l) for 48 h were lysed with cell RIPA buffer containing
protease inhibitor and phosphatase inhibitor on ice for 30 min. The
insoluble protein lysate was harvested followed by ultrasonic
destruction for 30 min. Then the lysate was centrifuged at 12,000
rpm for 10 min at 4°C. Protein concentration was determined by the
BCA protein quantification kit according to the manufacturer's
instructions. The cell lysates were denatured by boiling with 5X
reducing sample buffer for 5 min and run on SDS-PAGE gel. After
electrophoresis, the separated proteins were transferred to PVDF
membranes and blocked with 5% non-fat milk in TBST buffer for 2 h
at room temperature with continuous agitation. The membranes were
then incubated with specific primary antibodies overnight at 4°C
followed by washing and incubation with secondary antibodies at a
dilution of 1:20,000 in TBST buffer for 2 h at 37°C. The membranes
were then developed with enhanced chemiluminescence (ECL) kit. The
Image-Pro Plus software (Image-Pro Plus 5.1; Media Cybernetics,
Inc., Rockville, MD, USA) was used to quantify the protein.
Statistical analysis
Data are expressed as the means ± SEM. Statistical
analysis was performed using the statistical software SPSS 18.0 and
ANOVA was used to analyze statistical differences between groups
under different conditions. P<0.05 was considered statistically
significant.
Results
Effect of 12k on EphrinB2
The elution profiles of 12k for the EphrinB2/HEK293
and HEK293 cell CMC columns are shown in Fig. 1B. The immobilized receptors EphrinB2
at the surface of the stationary phase 12k in the effluent could
combine with EphrinB2. The retention behavior indicated that 12k
could bind to EphrinB2. Fluorescent competition study was used to
determine whether 12k could competitively bind to the site on
EphrinB2 occupied by EphB4, as EphB4 is a well-known ligand. A
decrease in the expression of EphB4 was observed (Fig. 1C). This indicated that 12k could
compete with the EphB4 binding of EphrinB2.
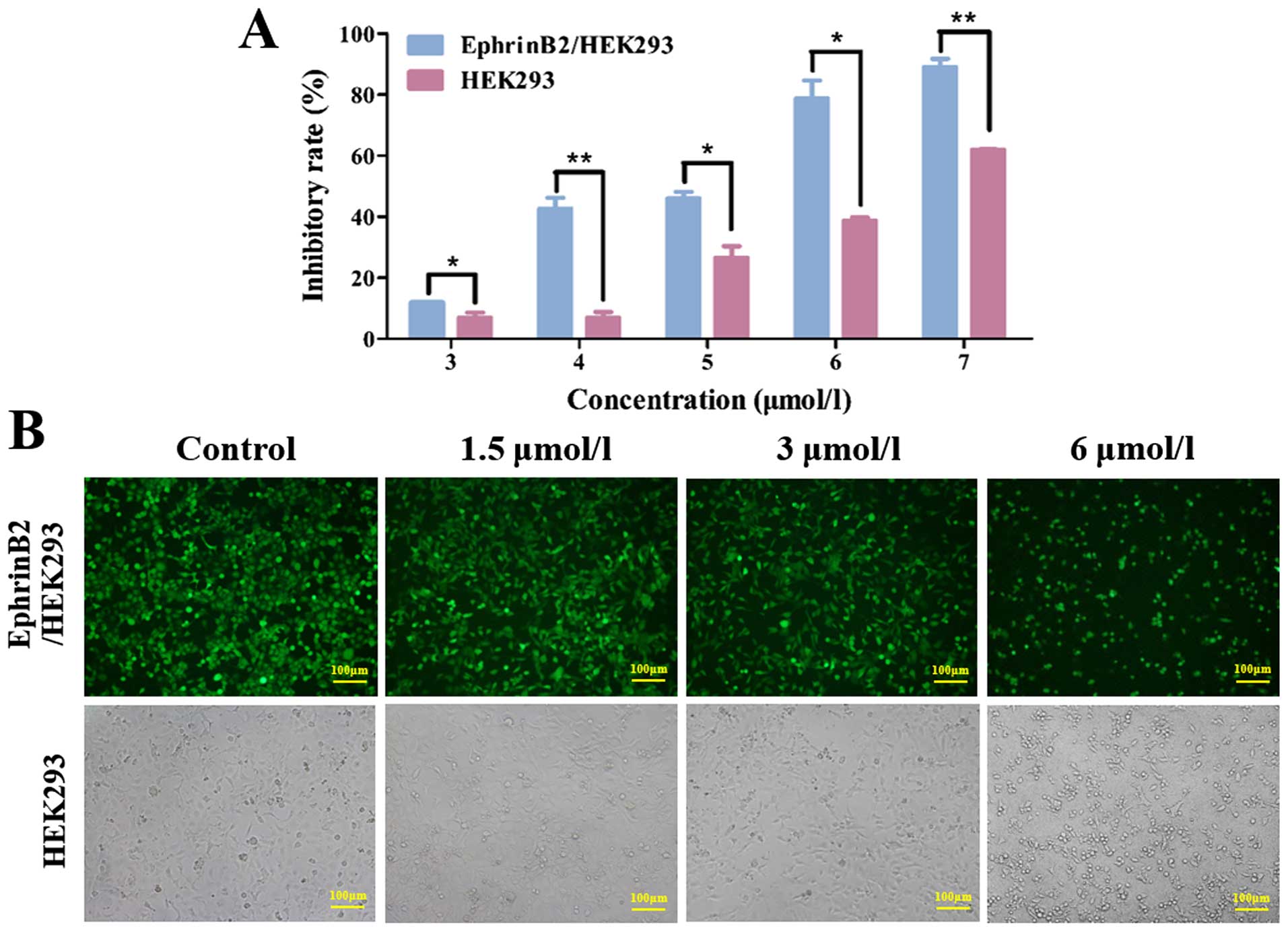
We investigated the effect of 12k on the growth of
EphrinB2/HEK293 and HEK293 cells using MTT assay. The results
indicated that 12k inhibited the growth of EphrinB2/HEK293 and
HEK293 cells in a dose-dependent manner (Fig. 2A). Furthermore, 12k was more
effective in suppressing EphrinB2/HEK293 cell growth than HEK293
cells. The IC50 values of 12k for EphrinB2/HEK293 and
HEK293 cells at 48 h were 4.36 and 7.00 µmol/l respectively.
Meanwhile, the morphology of the EphrinB2/HEK293 and HEK293 cells
treated with 12k were observed under an inverted microscope
(Fig. 2B). Collectively, the CMC
assay, fluorescent competition assay and MTT assay showed that 12k
acts mainly on EphrinB2.
12k inhibits colon cancer cell
proliferation and Caco-2 cell colony formation
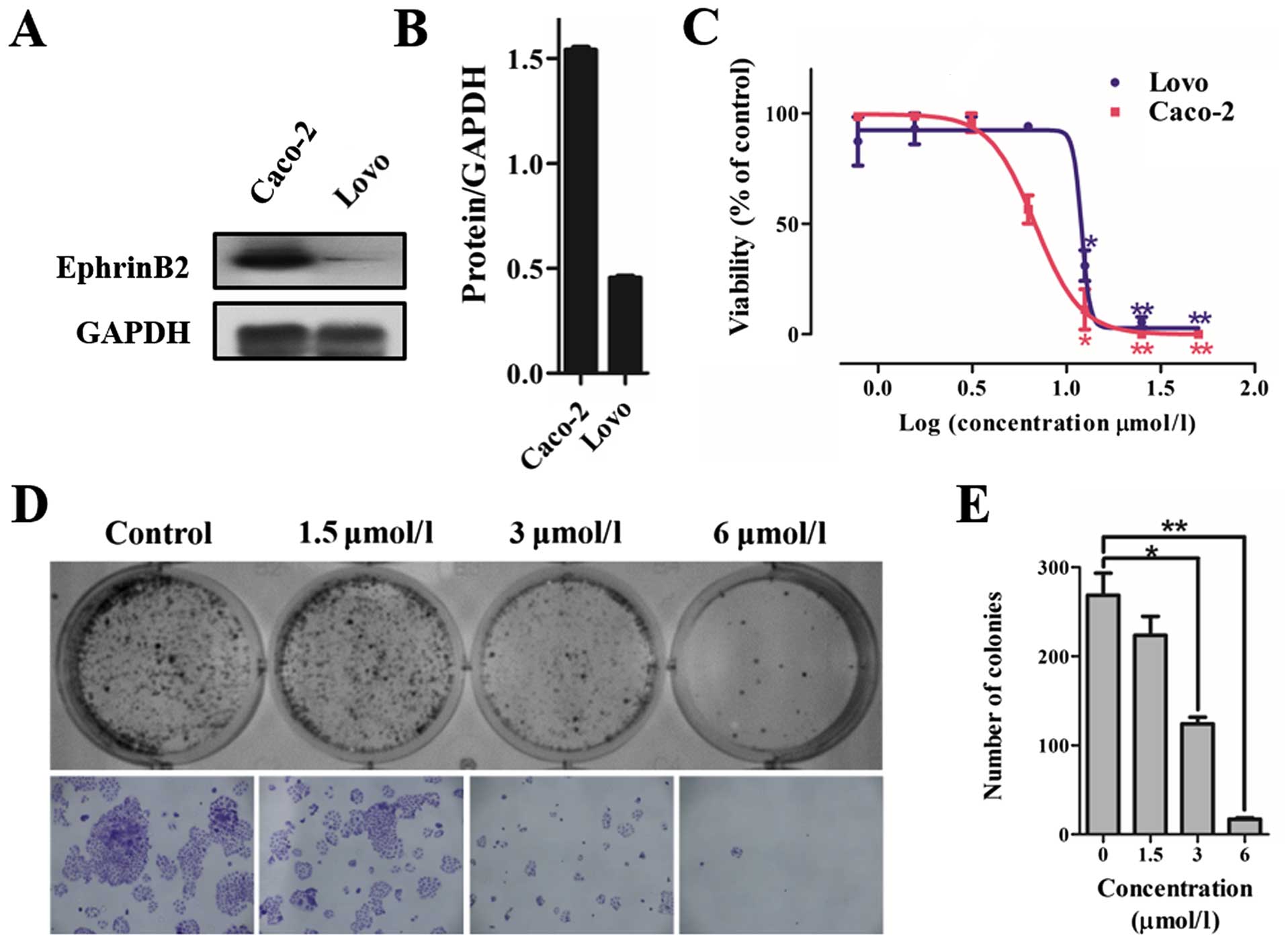
Colorectal cancer cell lines were screened for high
expression of EphrinB2. We examined the levels of EphrinB2 in
colorectal cancer cells including Caco-2 and LoVo cells. Western
blotting results indicated that the expression of EphrinB2 in the
Caco-2 cells was significantly higher than that noted in the LoVo
cells (Fig. 3A and B).
We further assessed the effect of 12k on colon
cancer cell growth. The results showed that 12k significantly
inhibited cell proliferation and the IC50 values of 12k
for the LoVo and Caco-2 cells at 48 h were 12.01 and 6.73
µmol/l respectively. 12k showed a higher suppressive effect
on the Caco-2 cells than on the LoVo cells (Fig. 3C). Consequently, Caco-2 cells were
used for the subsequent experiments.
We also investigated the action of 12k on Caco-2
cell colony formation. The results showed that upon 10–15 days of
continuous culture, 12k significantly inhibited the colony
formation of the Caco-2 cells in a dose-dependent manner (Fig. 3D and E). These findings indicate
that 12k has potential antitumor properties in colon cancer
cells.
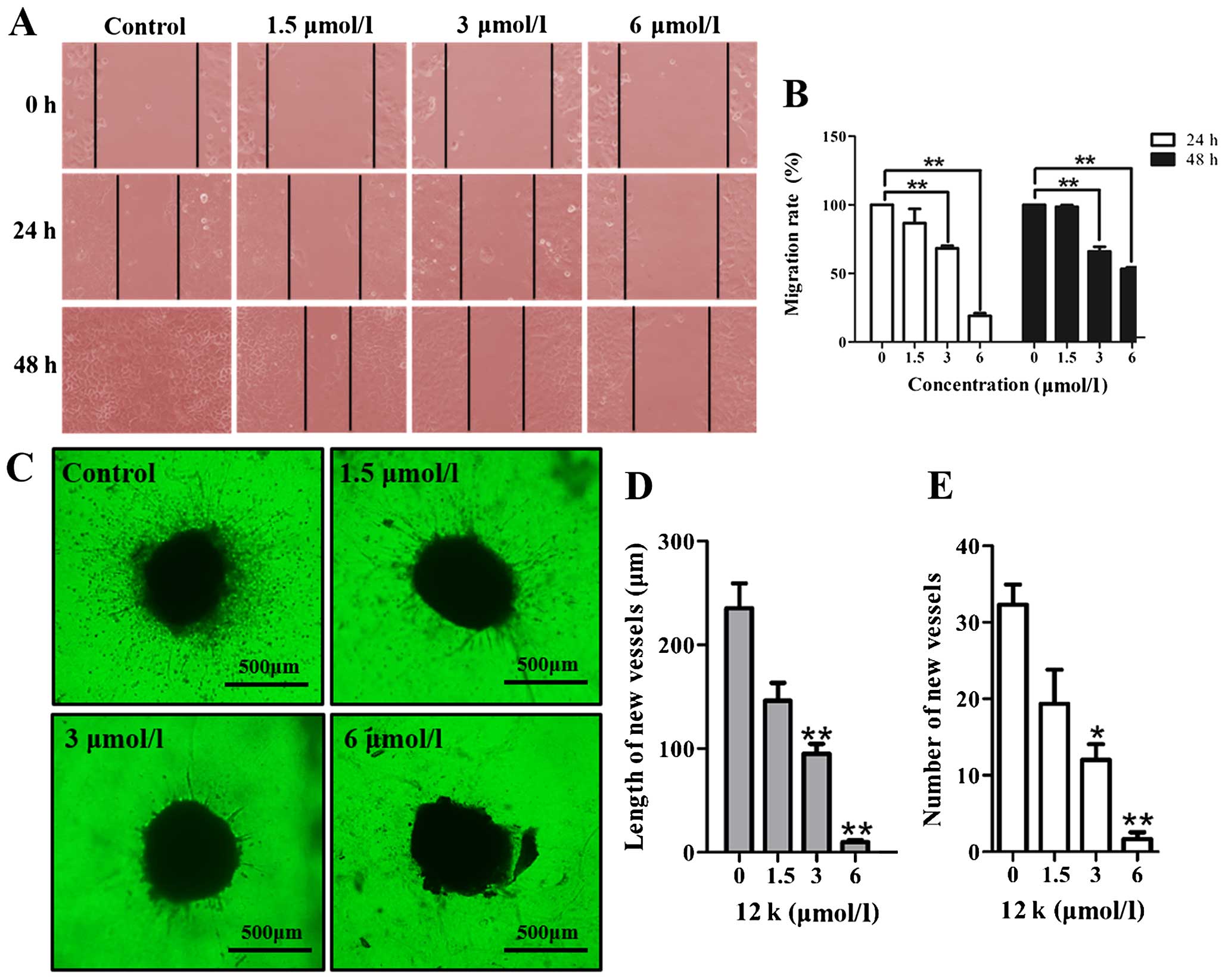
12k inhibits Caco-2 cell migration
Meanwhile, the ability of 12k to inhibit the
migration of the Caco-2 cells was determined. The results showed
that in the absence of 12k, the cells migrated within 48 h to fill
the scratched area, while the treatment of 12k significantly
prevented the migration of the Caco-2 cells in a dose-dependent
manner at 24 and 48 h (Fig. 4A and
B). The wound healing assay validated that 12k impaired Caco-2
cell migration.
12k inhibits angiogenesis in a tissue
model of angiogenesis
To evaluate the influence of 12k on angiogenesis, we
used a lung tissue model of angiogenesis as established previously
(17). 12k at different
concentrations was incubated with the lung tissues. The results
showed that 12k at 1.5, 3 and 6 µmol/l disrupted the
formation of vessels outgrown from the periphery of the lung
tissues (Fig. 4C). The number and
length of the vessels following treatment with 12k at different
concentrations were reduced in a dose-dependent manner (Fig. 4D and E). The findings indicate that
12k inhibited vessel development in an animal model.
12k regulates EphrinB2 and PDZ in Caco-2
cells
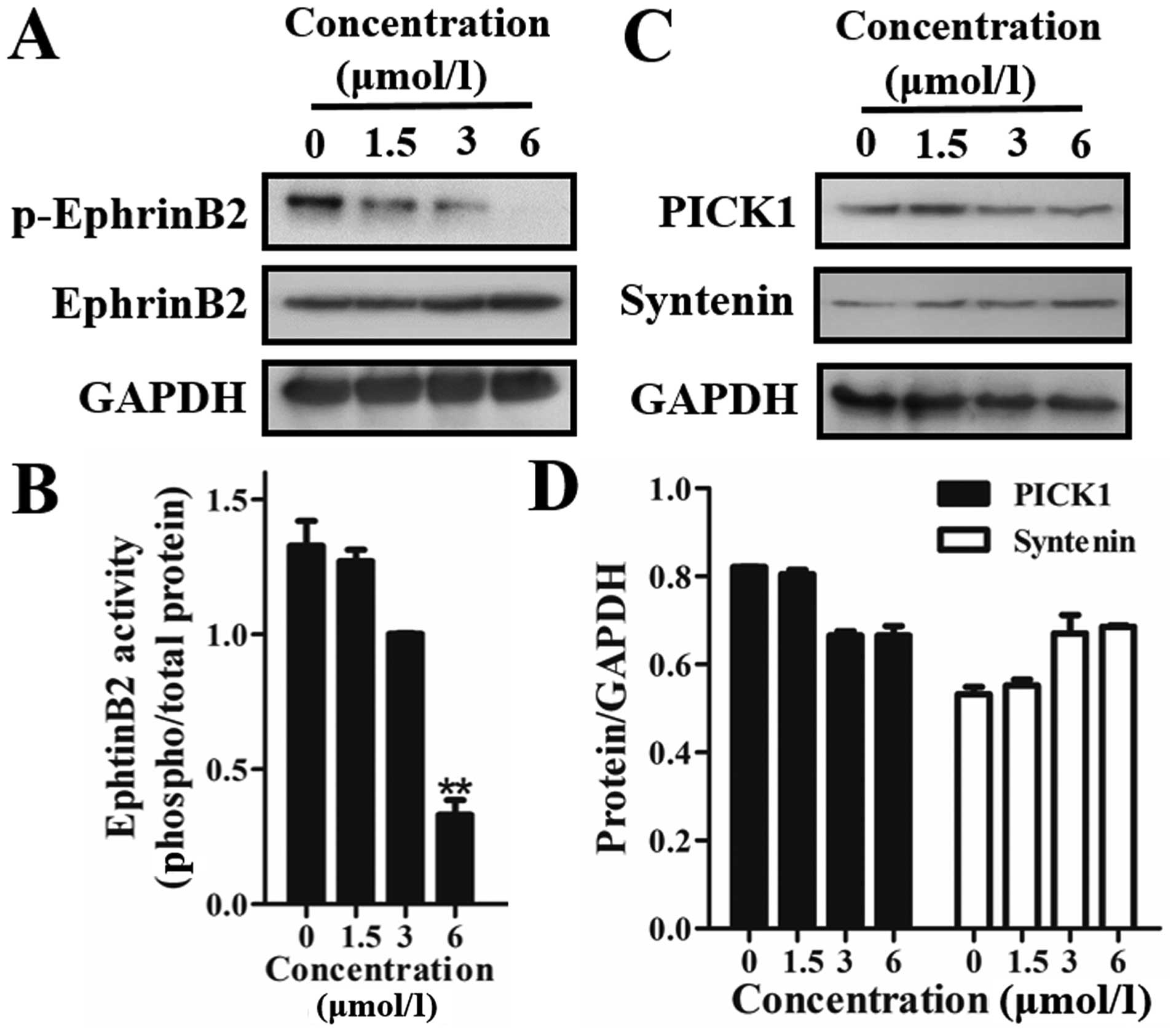
Firstly, we detected the effect of 12k on EphrinB2.
The results showed that 12k effectively downregulated the
phosphorylation of EphrinB2 in the Caco-2 cells (Fig. 5A and B). This indicated that 12k
inhibited migration and angiogenesis through regulation of EphrinB2
regulation.
It has been reported that PDZ-binding motif proteins
such as syntenin and PICK1 interact with EphrinB2 (18,19).
The results showed that treatment of 12k markedly suppressed the
expression of PICK1 in a dose-dependent manner, but had a minimal
effect on syntenin (Fig. 5C and D).
All things considered, these results demonstrated that 12k
inhibited the phosphorylation of EphrinB2 by mediating the
cytoplasmic PDZ-binding motif of PICK1, but had no effect on
syntenin.
12k inhibits VEGFR2 downstream signaling
pathways
PDZ-dependent EphrinB2 signaling regulates VEGFR2
activity. VEGFR downstream signaling pathways include both
PI3K/AKT/mTOR and MAPK signaling cascades which play an important
role in proliferation, survival, angiogenesis, and metastasis of
tumor cells by promoting endothelial cell proliferation and
migration. In the present study, we further investigated the effect
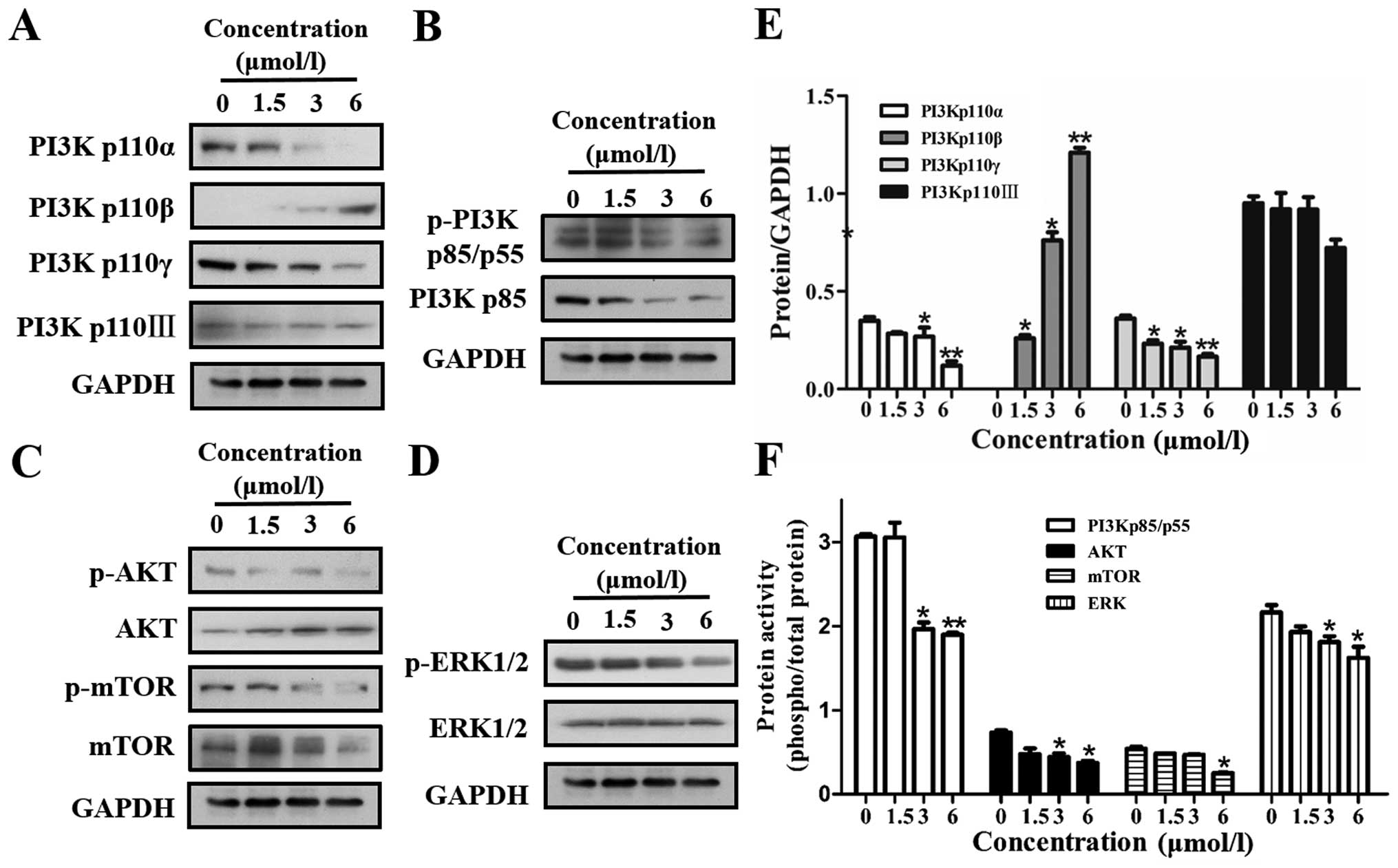
of 12k on the PI3K/AKT/mTOR and MAPK pathways. As shown in Fig. 6A and E, 12k inhibited the PI3Kp110α,
PI3Kp110γ and PI3Kp110III subunit, but upregulated the PI3Kp110β
subunit. Meanwhile, phosphorylation of PI3Kp85/p55 was inhibited by
12k in a concentration-dependent manner (Fig. 6B and F). In addition, activation of
the serine/threonine protein kinase AKT and mTOR were also
suppressed upon 12k treatment in the Caco-2 cells (Fig. 6C and F). We then performed western
blotting to determine whether 12k could inhibit the activation of
MAPK cascades. As shown in Fig. 6D and
F, 12k significantly downregulated the phosphorylation of ERK
in the Caco-2 cells. Our data clearly indicated that 12k not only
inhibits the constitutive activation of the PI3K/AKT/mTOR signaling
cascade; but it also inhibits the activation of ERK in Caco-2
cells.
12k regulates VEGFR3 regulatory
molecules
EphrinB2 signaling has been reported to promote the
internalization of VEGFR2 and VEGFR3. Thus, inhibition of EphrinB2
may simultaneously interfere with the function of VEGFR2 and
VEGFR3. Next, we explored the potential role of 12k in VEGFR3
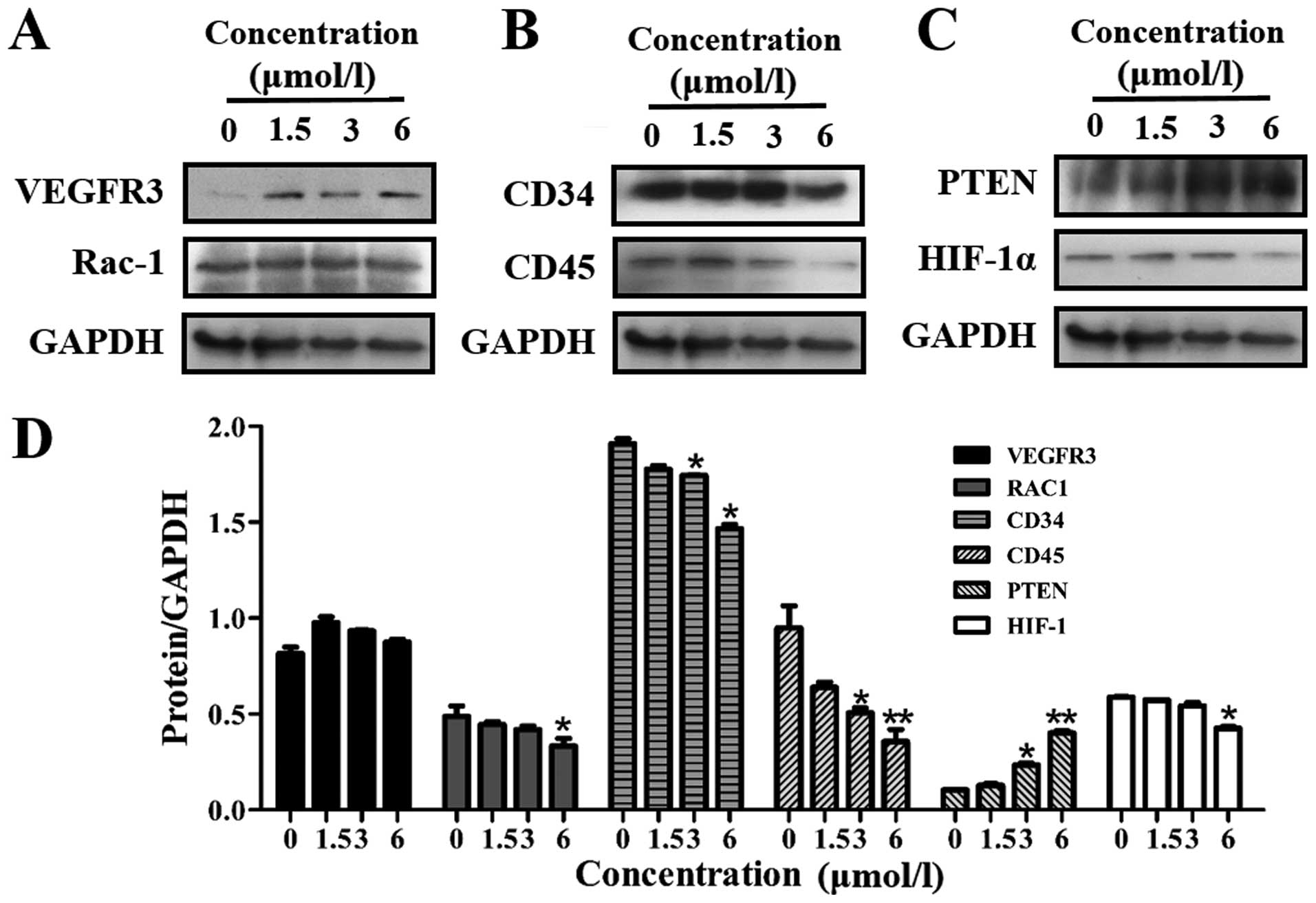
activation in Caco-2 cells. As shown in Fig. 7A and D, 12k had no effect on the
expression of VEGFR3 and Rac-1.
Effects of 12k on angiogenesis regulatory
molecules
The related angiogenesis proteins CD34 and CD45 were
down-regulated following treatment with 12k (Fig. 7B and D). PTEN, a negative regulator
of the PI3K signaling pathway, was upregulated while HIF-1α,
overexpressed in many human cancers, was reduced by 12k (Fig. 7C and D).
Discussion
Colorectal cancer is the most common type of cancer,
accounting for ~10% of all cancer cases (20). Treatments for colorectal cancer
include surgery, radiation therapy, chemotherapy and targeted
therapy. Recently, drugs targeting key pathways have generated new
perspectives for the treatment of colorectal cancer. The EphrinB2
signaling pathway plays a key role in development and postnatal
angiogenesis in physiology and disease. Thus, EphrinB2 appears to
be a promising prognostic indicator and target to modulate
angiogenesis in cancer therapies (8).
In this study, we demonstrated that a novel taspine
derivative, 12k, played a significant role in inhibiting
proliferation via binding to EphrinB2 and regulating the EphrinB2
signaling pathway. The CMC assay with EphrinB2/HEK293 and HEK293
cells indicated that 12k could bind to EphrinB2. Also fluorescent
competition binding assay further validated that 12k may have
direct competition at a single common binding site on EphrinB2.
Meanwhile, 12k had a more significant suppressive effect on high
EphrinB2-expressing EphrinB2/HEK293 cells than on HEK293 cells. Our
experimental findings suggest that 12k may be effective against
EphrinB2.
To explore 12k as a potential chemotherapeutic agent
for the treatment of colorectal cancers, we investigated the
expression of EphrinB2 in Caco-2 and LoVo cells, as well as
explored the inhibitory effect of 12k on cell growth. The results
demonstrated that 12k treatment resulted in a reduction in
colorectal cell viability and showed a higher suppressive effect on
high EphrinB2-expressing Caco-2 cells. Meanwhile, we found that 12k
inhibited Caco-2 cell colony formation. Moreover, cell migration is
important during early invasion and the subsequent metastasis and
angiogenesis of a tumor and is responsible for most cancer deaths
(21). In the present study, it was
confirmed by our experiments that 12k had a significant inhibitory
effect on Caco-2 cell migration. The results also showed that 12k
inhibited the development of neovessels sprouting from the edge of
the lung tissues in the tissue model of angiogenesis in
vitro.
We subsequently investigated the anti-proliferation
mechanism of 12k. Our results initially indicated that 12k
downregulated the phosphorylation of EphrinB2 at the protein level.
Various reports suggest that EphrinB2 reverse signaling dependent
on the C-terminal PDZ-binding motif regulates angiogenic sprouting
and branching in pathological angiogenesis. Our results indicated
that 12k suppressed the PDZ-binding motif protein, PICK1, but had a
minimal effect on syntenin. Previous findings indicate that
EphrinB2 signaling simultaneously induces the internalization of
VEGFR2 and VEGFR3. Furthermore, the PI3K/AKT/mTOR and the MAPK
signaling pathways, which contribute to the metastasis and
angiogenesis of tumor cells, are downstream targets of
VEGFR2-mediated signaling. Rac-1 binds to a variety of effector
proteins to regulate cellular responses such as epithelial cell
polarization. Hence, we further explored the effect of 12k on the
VEGFR2 and VEGFR3 signaling pathways, which act together during
angiogenesis. Our results showed that 12k exhibited inhibitory
activity on the expression levels of various isoforms of PI3K
catalytic subunits (p110α, p110β and p110γ) and regulatory subunits
(p110α and p110β and p85). At the same time, its downstream
effector, Akt, and mTOR activation were also downregulated. In
addition, ERK activation in the MAPK/ERK pathway was also
suppressed by 12k. Simultaneously, we noted that 12k had no effect
on VEGFR3 and Rac-1. Collectively, 12k reduced the phosphorylation
of EphrinB2 by suppressing its PDZ-binding motif protein, PICK1,
and also affected the VEGFR2 signaling pathway.
Cluster of differentiation 34 (CD34) is a cell
surface glycoprotein, and it functions as a cell-cell adhesion
factor (22). CD34 is selectively
expressed on capillary endothelial cells and is considered to be an
important marker of tissue vascularization. CD45 is correlated with
CRC disease stage and outcome (23). Thus, CD34 and CD45 that are related
to CRC angiogenesis were also analyzed. Our results showed that 12k
suppressed the expression of CD34 and CD45. PI3K catalyzes the
production of phosphatidylinositol-3,4,5-triphosphate by
phosphorylating phosphatidylinositol (PI),
phosphatidylinositol-4-phosphate (PIP) and
phosphati-dylinositol-4,5-bisphosphate (PIP2). Growth factors and
hormones trigger this phosphorylation event, which in turn
coordinates cell growth, cell cycle entry, cell migration and cell
survival (24). PTEN reverses this
process, and the PI3K signaling pathway is constitutively activated
in human cancers that have loss of function of PTEN (25). HIF-1α is overexpressed in many human
cancers (26). HIF-1α
overexpression is heavily implicated in the promotion of tumor
growth and metastasis through its role in initiating angiogenesis
and regulating cellular metabolism to overcome hypoxia (27). Western blot assay showed that PTEN
activity was strongly increased and HIF-1α activity was
decreased.
In conclusion, the results presented in this study
demonstrated that 12k inhibited CRC growth by angiogenesis
responses in vitro. Its mechanism involved the
downregulation of the activation of EphrinB2 and the PDZ-binding
motif protein. Meanwhile, 12k functioned by reducing the
phosphorylation of PI3K, Akt, mTOR and ERK. Furthermore, 12k
downregulated the expression of CD34, CD45 and HIF-1α. These
results suggest that 12k may constitute a novel anti-angiogenic
drug that acts by targeting EphrinB2 signaling.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant nos. 81370088 and 81503101), the
Fundamental Research Funds for the Central Universities of
Zhuizong, the Project of Shaanxi Star of Science and Technology
(grant no. 2012Kjxx-06), the National Science Foundation for
Post-doctoral Scientists of China (grant no. 2015M570843) and the
Supporting Plan for New Century Excellent Talents of the Ministry
of Education (grant no. NCET-13-0467).
Abbreviations:
|
CRC
|
colorectal cancer
|
|
EGF
|
epidermal growth factor
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
FGF
|
fibroblast growth factor
|
|
MAPK
|
mitogen-activated protein kinase
|
|
PI3K
|
phosphotidylinositol 3-kinase
|
|
VEGF
|
vascular endothelial growth factor
|
References
|
1
|
Folkman J: Tumor angiogenesis: Therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Folkman J: Angiogenesis in cancer,
vascular, rheumatoid and other disease. Nat Med. 1:27–31. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Folkman J: Tumor angiogenesis: A possible
control point in tumor growth. Ann Intern Med. 82:96–100. 1975.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carmeliet P: Mechanisms of angiogenesis
and arteriogenesis. Nat Med. 6:389–395. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ferrara N and Kerbel RS: Angiogenesis as a
therapeutic target. Nature. 438:967–974. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Salvucci O and Tosato G: Essential roles
of EphB receptors and EphrinB ligands in endothelial cell function
and angiogenesis. Adv Cancer Res. 114:21–57. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pitulescu ME and Adams RH: Eph/ephrin
molecules - a hub for signaling and endocytosis. Genes Dev.
24:2480–2492. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pasquale EB: Eph-ephrin bidirectional
signaling in physiology and disease. Cell. 133:38–52. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chrencik JE, Brooun A, Recht MI, Kraus ML,
Koolpe M, Kolatkar AR, Bruce RH, Martiny-Baron G, Widmer H,
Pasquale EB, et al: Structure and thermodynamic characterization of
the EphB4/Ephrin-B2 antagonist peptide complex reveals the
determinants for receptor specificity. Structure. 14:321–330. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sawamiphak S, Seidel S, Essmann CL,
Wilkinson GA, Pitulescu ME, Acker T and Acker-Palmer A: Ephrin-B2
regulates VEGFR2 function in developmental and tumour angiogenesis.
Nature. 465:487–491. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Y, Nakayama M, Pitulescu ME, Schmidt
TS, Bochenek ML, Sakakibara A, Adams S, Davy A, Deutsch U, Lüthi U,
et al: Ephrin-B2 controls VEGF-induced angiogenesis and
lymphangiogenesis. Nature. 465:483–486. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gomez MC, Bravo GB, Caceres CG, Gongora A
and Baldi A: The eph/ephrin system, an example of versatility and
functional diversity in molecular biology of cancer and
angiogenesis. Acta Bioquim Clin Latinoam. 4:675–679. 2011.
|
|
14
|
Steinle JJ, Meininger CJ, Chowdhury U, Wu
G and Granger HJ: Role of ephrin B2 in human retinal endothelial
cell proliferation and migration. Cell Signal. 15:1011–1017. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Maekawa H, Oike Y, Kanda S, Ito Y, Yamada
Y, Kurihara H, Nagai R and Suda T: Ephrin-B2 induces migration of
endothelial cells through the phosphatidylinositol-3 kinase pathway
and promotes angiogenesis in adult vasculature. Arterioscler Thromb
Vasc Biol. 23:2008–2014. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao H, Su P, Shi Y, Shen X, Zhang Y, Dong
J and Zhang J: Discovery of novel VEGFR-2 inhibitors. Part II:
Biphenyl urea incorporated with salicylaldoxime. Eur J Med Chem.
90:232–240. 2015. View Article : Google Scholar
|
|
17
|
Dai B, Zhang Y, Zhan Y, Zhang D, Wang N
and He L: A novel tissue model for angiogenesis: Evaluation of
inhibitors or promoters in tissue level. Sci Rep. 4:36932014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Grootjans JJ, Reekmans G, Ceulemans H and
David G: Syntenin-syndecan binding requires syndecan-synteny and
the co-operation of both PDZ domains of syntenin. J Biol Chem.
275:19933–19941. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dev KK: PDZ domain protein-protein
interactions: A case study with PICK1. Curr Top Med Chem. 7:3–20.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Thorat MA and Cuzick J: Role of aspirin in
cancer prevention. Curr Oncol Rep. 15:533–540. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lou L, Ye W, Chen Y, Wu S, Jin L, He J,
Tao X, Zhu J, Chen X, Deng A, et al: Ardipusilloside inhibits
survival, invasion and metastasis of human hepatocellular carcinoma
cells. Phytomedicine. 19:603–608. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nielsen JS and McNagny KM: Novel functions
of the CD34 family. J Cell Sci. 121:3683–3692. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chew A, Salama P, Robbshaw A, Klopcic B,
Zeps N, Platell C and Lawrance IC: SPARC, FOXP3, CD8 and CD45
correlation with disease recurrence and long-term disease-free
survival in colorectal cancer. PLoS One. 6:e220472011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cantley LC: The phosphoinositide 3-kinase
pathway. Science. 296:1655–1657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Simpson L and Parsons R: PTEN: Life as a
tumor suppressor. Exp Cell Res. 264:29–41. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhong H, De Marzo AM, Laughner E, Lim M,
Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL and Simons
JW: Overexpression of hypoxia-inducible factor 1alpha in common
human cancers and their metastases. Cancer Res. 59:5830–5835.
1999.PubMed/NCBI
|
|
27
|
Bos R, van der Groep P, Greijer AE,
Shvarts A, Meijer S, Pinedo HM, Semenza GL, van Diest PJ and van
der Wall E: Levels of hypoxia-inducible factor-1alpha independently
predict prognosis in patients with lymph node negative breast
carcinoma. Cancer. 97:1573–1581. 2003. View Article : Google Scholar : PubMed/NCBI
|