Introduction
Ovarian carcinoma is the fifth leading cause of
death among the female population, which accounts for approximate
3% of cancers among women, and it is one of the most lethal
gynecologic malignancies (1).
Although the strategies for cancer therapy have been developed for
the last two decades, ovarian cancer mortality has not improved
(1). Thus, estimating the risk of
future progression and develop early diagnosis biomarker may have
significant impact on ovarian carcinoma prognosis. For the last 10
years, intensive research efforts have been made toward the
diagnostic and treatment of ovarian carcinoma to promote the
long-term survival rate of ovarian cancer patient. However, without
real improvement (2). Therefore,
there is an urgent need to identify potential indicators for more
effective therapies for human ovarian cancer.
MicroRNAs (miRNAs) are a class of non-coding, small
endogenous RNAs with 18–22 nucleotides in length playing important
roles in post-transcriptional regulation (3). miRNAs usually imperfectly bind to the
3′-untranslated region (3′UTR) of complementary target mRNA
resulting in mRNA degradation or prevention translation (4,5).
miRNAs play important roles in various normal cellular and
biological processes, such as cell proliferation, differentiation,
and apoptosis (6–8). Increasing focus on the roles of miRNAs
in human cancer have been reported. Previous studies have shown
that 98 miRNAs locate at genomic regions involved in cancers,
indicating that miRNAs are tightly interrelated with occurrence and
development of cancers (9).
MicroRNAs also play critical roles in carcinogenesis and
progression (10), and can act as
either oncogenes or tumor suppressors (11). miR-497 was first identified in
neuroblastoma and acts as a predictive indicator for neuroblastoma
patient survival (12). The
expression of miR-497 was significantly downregulated in a variety
of human cancers and acted as a tumor suppressor (13–15).
However, the expression level of miR-497 and its exact role in
evolution and progression of human ovarian cancer is still
unclear.
In our present study, which aims to investigate the
functions of dysregulated miR-497 in human ovarian cancer, we
confirmed that the expression of miR-497 was significantly
decreased in human ovarian cancer. In addition, we also evaluated
the effect of overexpression of miR-497 on human ovarian cancer
cell proliferation, invasion, migration and cell apoptosis.
Moreover, we further confirmed that paired box 2 (PAX2) is a
directly target gene of miR-497. Our findings suggest that miR-497
acts as a tumor suppressor and may represent novel diagnostic and
therapeutic biomarker for human ovarian cancer, and miR-497-PAX2
may represent a potential therapeutic target for diagnosis and
therapy of ovarian cancer.
Materials and methods
Tissue collection
Between 2014 and 2015, 26 ovarian cancer tissues and
para-carcinoma tissues were obtained from patients through surgery
at Hepatobiliary and Enteric Surgery Research Center, Xiangya
Hospital, and were diagnosed with ovarian cancer based on
histopathological evaluation according to the International
Federation of Obstetrics and Gynecology (FIGO) criteria. All
patients were operated without any local or systemic treatment. All
samples were collected and frozen in liquid nitrogen immediately,
and then stored at −80°C for RNA or protein extraction. All
participants provided consent for the use of their specimens in
this research, and the study was approved by the Institute Research
Ethics Committee of Xiangya Hospital. All procedures performed in
studies involving human participants were in accordance with the
Ethical Standards of the Institutional and/or National Research
Committee and with the 1964 Helsinki Declaration and its later
amendments or comparable ethical standards.
Cell lines
Human ovarian surface immortalized epithelial cells
(IOSE25) were cultured as described (16). Human ovarian cancer cell line SKOV3
was purchased from the American Type Culture Collection (ATCC;
Manassas, VA, USA). The ovarian cancer cell SKOV3 was cultured
according to the vendor's instructions. In brief, cells were
cultured in McCoy's 5A medium modified (ATCC) with 10% fetal bovine
serum (FBS; Gibco, Carlsbad, CA, USA), and the medium contained 1%
penicillin-streptomycin (100 U/ml penicillin and 100 µg/ml
streptomycin). All cells were cultured and maintained under
standard conditions.
Plasmid construction
PAX2 3′UTR containing the putative binding sites for
miR-497 was cloned from the genome DNA and constructed into
downstream of psi-CHECK2 vector (Promega), named PAX2-3′UTR-WT.
PAX2 mutant 3′UTR recombinant plasmid was produced by QuikChange
Site-Directed Mutagenesis kit (Stratagene, USA), which generated a
mutation of 7 bps from UGCUGCU to ACGACGG in the predicted miR-497
target binding sites, named as PAX2-3′UTR-MUT. All plasmids were
confirmed by DNA sequencing.
Cell transfection
The miR-497 mimics and mock were purchased from a
commercial manufacturer (Ribobio, China). PAX2-siRNA
(5′-GAAGUCAAGUCGAGUCUAU-3′) and negative control siRNA (NC-siRNA)
(5′-AGGUAGUGUAAU CGCCUUGTT-3′) were obtained from Genechem Biotech
(Shanghai, China). Cells (1×104) were seeded in a
24-well plate and incubated for 24 h, then ovarian cancer cells
were transfected with mimics (100 mM), siRNA (100 mM) or vectors (2
µg) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA)
in serum-free medium in accordance with the manufacturer's
instructions.
Quantitative real-time PCR (qRT-PCR)
analysis
Total RNA from tissues or cultured cells was
extracted using TRIzol reagent (Invitrogen Inc.). Two micrograms of
total RNA was used for cDNA synthesis by using M-MLV Reverse
Transcriptase (Promega, USA). The relative expression of miRNA-497
was analyzed using a SYBR PrimeScript miRNA RT-PCR kit (Takara,
China) and results were normalized to U6. SYBR Green Real-Time
Master Mix (Toyobo, Japan) was used to perform quantitative
real-time PCR to detect the relative expression of PAX2, and GAPDH
was used as an internal control. The gene-specific primers were
synthesized by Sangon (Shanghai, China) (Table I). qRT-PCR and data collection were
performed on Applied Biosystems 7500 Sequence detection system
(ABI, USA). The miR-497 and PAX2 expression levels were calculated
using the 2−ΔΔCt method.
 | Table IPrimer sequences used for miRNA and
mRNA expression analysis. |
Table I
Primer sequences used for miRNA and
mRNA expression analysis.
| Name | Primer sequence
(5′-3′) |
|---|
| miR-497-RT |
CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGACAAACCA |
| U6-RT |
CGCTTCACGAATTTGCGTGTCAT |
| U6-F |
CTCGCTTCGGCAGCACA |
| U6-R |
AACGCTTCACGAATTTGCGT |
| miR-497-F |
ACACTCCAGCTGGGCAGCAGCACACT |
| Universal-R |
TGGTGTCGTGGAGTCG |
| PAX2-F |
CCTCGCTCCAATGGTGAGAA |
| PAX2-R |
TGCTGCTGGGTGAAGGTGTC |
| GAPDH-F |
ACACCCACTCCTCCACCTTT |
| GAPDH-R |
TTACTCCTTGGAGGCCATGT |
Western blot analysis
Total proteins from tissues or cultured cells were
extracted with SDS lysis buffer (Beyotime, China) and BCA protein
assay kit (Pierce, USA) was used to detect the protein
concentration. Equal amounts of total proteins were boiled using
loading buffer for 10 min and separated in 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred to polyvinylidene difluoride membranes (PVDF)
(Millipore, USA). The membranes were blocked in Tris-buffered
saline (TBS) containing 5% nonfat-dried milk at 37°C for 2 h and
incubated with PAX2 antibodies (1:800; Abcam) for 1 h at room
temperature, then membranes were incubated with goat anti-rabbit
IgG-HRP (1:2,000; Santa Cruz Biotechnology, USA) at 37°C for 1 h.
Proteins were detected by enhanced chemiluminescence (ECL;
Beyotime). GAPDH (1:2,000; Abcam), a constitutive expression gene,
was used as an internal control.
Dual-luciferase assays
Cells (100 µl) (1×106/ml) were
cultured in 24-well plates and incubated for 24 h. miR-497 mimics
or Mock (100 nM) were co-transfected with 20 ng PAX2-3′UTR-WT or
-MUT psi-CKECK2 vectors into cells using Lipofectamine 2000
reagent. Dual-luciferase reporter assay system (Promega) was used
to detect luciferase activities after 48 h of transfection. Firefly
luciferase activity was normalized to Renilla luciferase
activity.
CCK-8 assay
The cell proliferation was analyzed using Cell
Counting Kit-8 (Dojindo, Japan). Ovarian cancer cells
(1×104) were seeded in 96-well plate. After transfected
with mimics or siRNA, cells were cultured with 10% FBS for 24, 48
and 72 h. Then, the cells were treated with 10 µl CCK-8
solution at 37°C for 2 h, The absorbency at 450 nm was measured
using a microplate reader Thermo Plate (Rayto Life and Analytical
Science Co., Ltd., Germany).
Cell migration and invasion assays
Transwell insert (24-well) with 8-µm pore
size (Corning Costar Corporation) was used to perform migration
assay and invasion assay. For migration assay, 1×104
SKOV3 cells were plated in 100 µl medium without FBS into
upper chamber of Transwell insert with non-coated membrane. For
invasion assay, 1×105 SKOV3 cells suspended in 100
µl serum-free medium were loaded in the upper
Matrigel-coated chamber instead. In both assays, 500 µl of
culture medium containing 15% FBS was added to the lower chamber
for culture. Then, cells were allowed to migrate or invade for 24 h
at 37°C. The migrated or invaded SKOV3 cells were fixed with 100%
methanol for 30 min at room temperature and stained with 0.5%
crystal violet (Sigma, USA) for 15 min, and the permeating cells
were counted under a phase-contrast microscope (Olympus, Tokyo,
Japan).
Flow cytometry
Annexin V-FITC/PI apoptosis detection kit (Sigma)
was used to detect cell apoptosis by flow cytometry. In brief,
after transfected with mimics or siRNA for 48 h, 1×106
SKOV3 cells were harvested and stained using the Annexin V-FITC/PI
apoptosis detection kit according to the manufacturer's
instructions. Cells were analyzed using Becton-Dickinson flow
cytometer. Annexin V-FITC(+)/PI(−) and Annexin V-FITC(+)/PI(+)
represent the early cell apoptosis and late apoptosis/necrosis,
respectively.
Statistical analysis
All results are expressed as the mean ± SD from
three independent experiments. Statistical analysis was performed
using SPSS 20.0 software (SPSS Inc., USA). Statistical significance
between groups was determined using a Student's t-test or one-way
analysis of variance (ANOVA) test. P-value of <0.05 was
considered to be statistically significant.
Results
miRNA-497 is decreased in ovarian cancer
tissues and cells
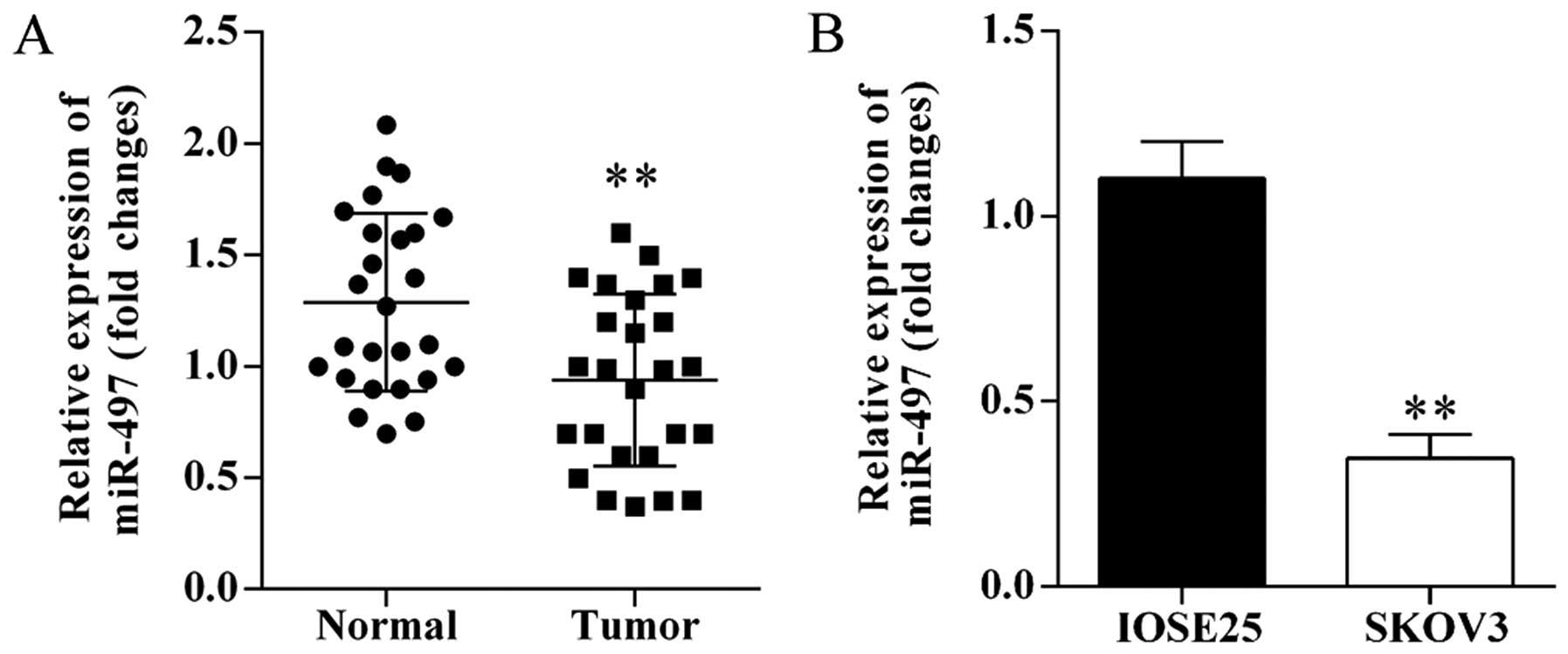
The expression levels of miR-497 in human ovarian
cancer tissues and cells were investigated using qRT-PCR. miRNA-497
was significantly decreased in ovarian cancer tissues compared with
matched adjacent normal tissues (Fig.
1A). Furthermore, the expression of miR-497 was significantly
downregulated in SKOV3 cells compared to IOSE25 cells (Fig. 1B).
Overexpression of miR-497 suppresses
SKOV3 cell proliferation, migration and invasion
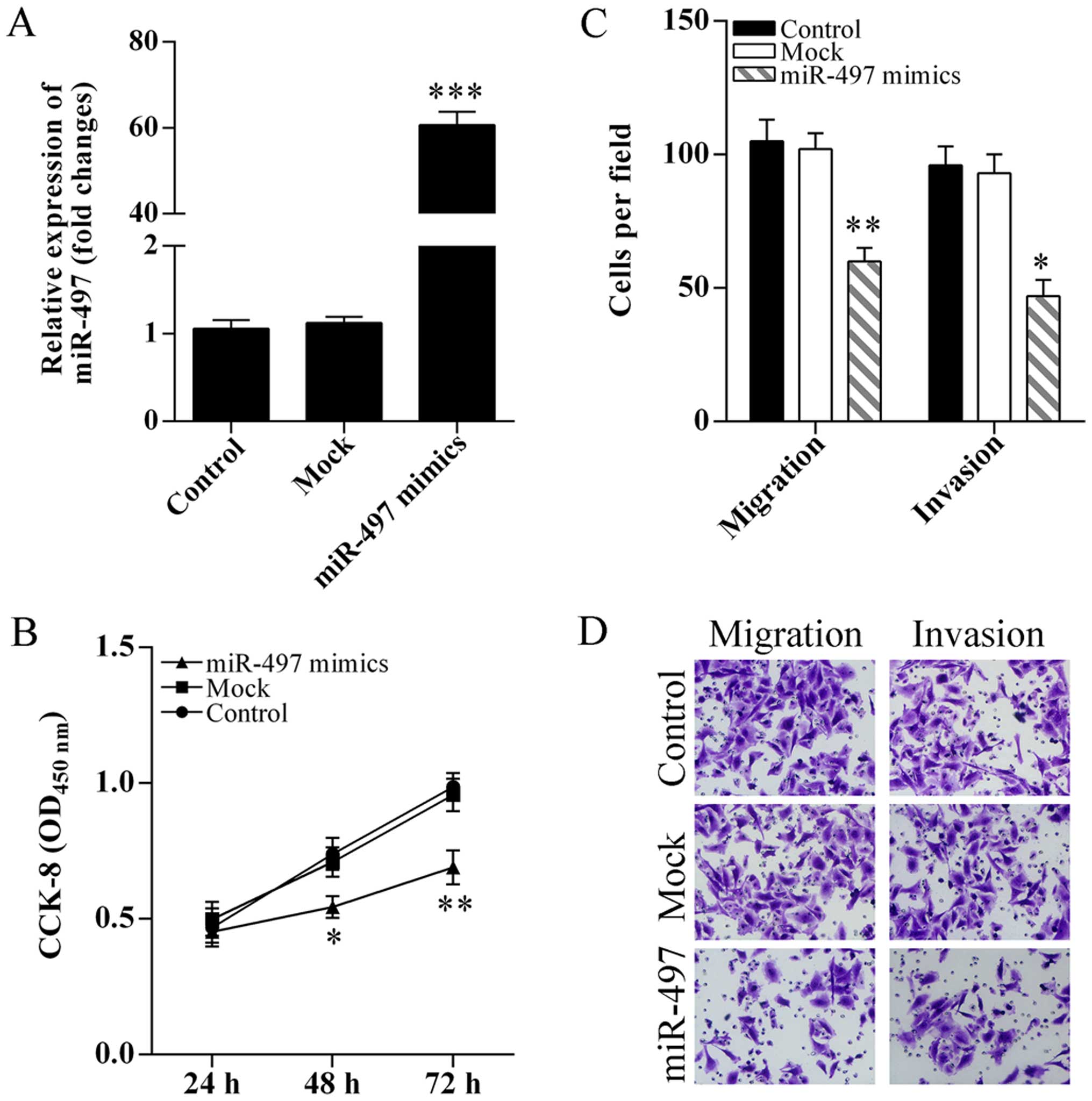
To determine the role of miR-497 in cell
proliferation, migration and invasion, SKOV3 cells were transfected
with miR-497 mimics (Fig. 2A). The
SKOV3 cell proliferation was significantly inhibited after
transfection of miR-497 mimics (Fig.
2B). Migration and invasion abilities of SKOV3 cells after
overexpression of miR-497 were analyzed using Transwell assay. The
SKOV3 cell migration and invasion were significantly suppressed
after transfection of miR-497 mimics (Fig. 2C and D).
Overexpression of miR-497 induces cell
apoptosis
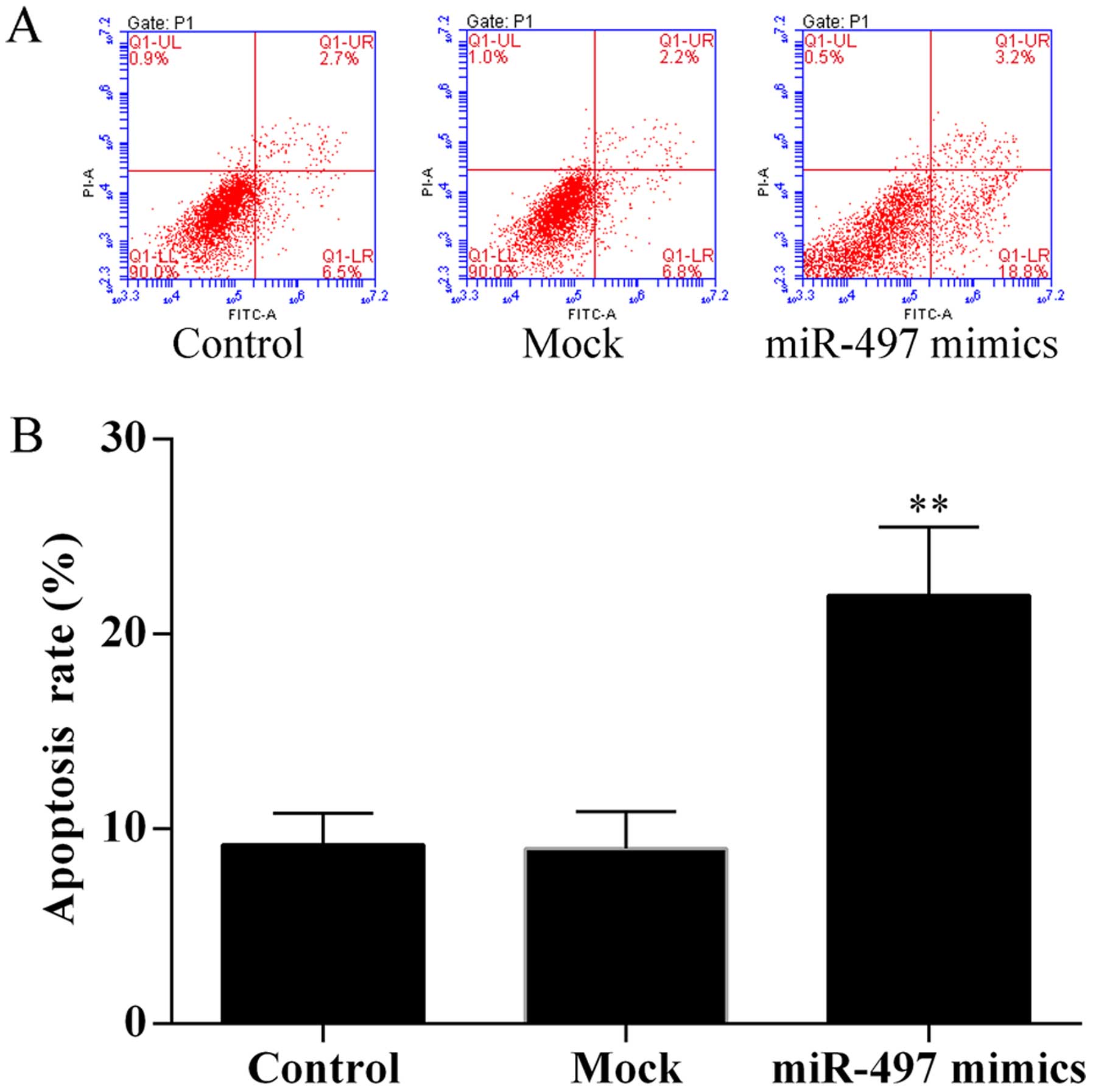
To evaluate the effect of miR-497 on SKOV3 cell
apoptosis, cell apoptosis assay was performed using the Annexin
V-FITC/PI staining method by flow cytometry. The results showed
that overexpression of miR-497 induced SKOV3 cell apoptosis
(Fig. 3).
Paired box 2 (PAX2) is a target gene of
miR-497
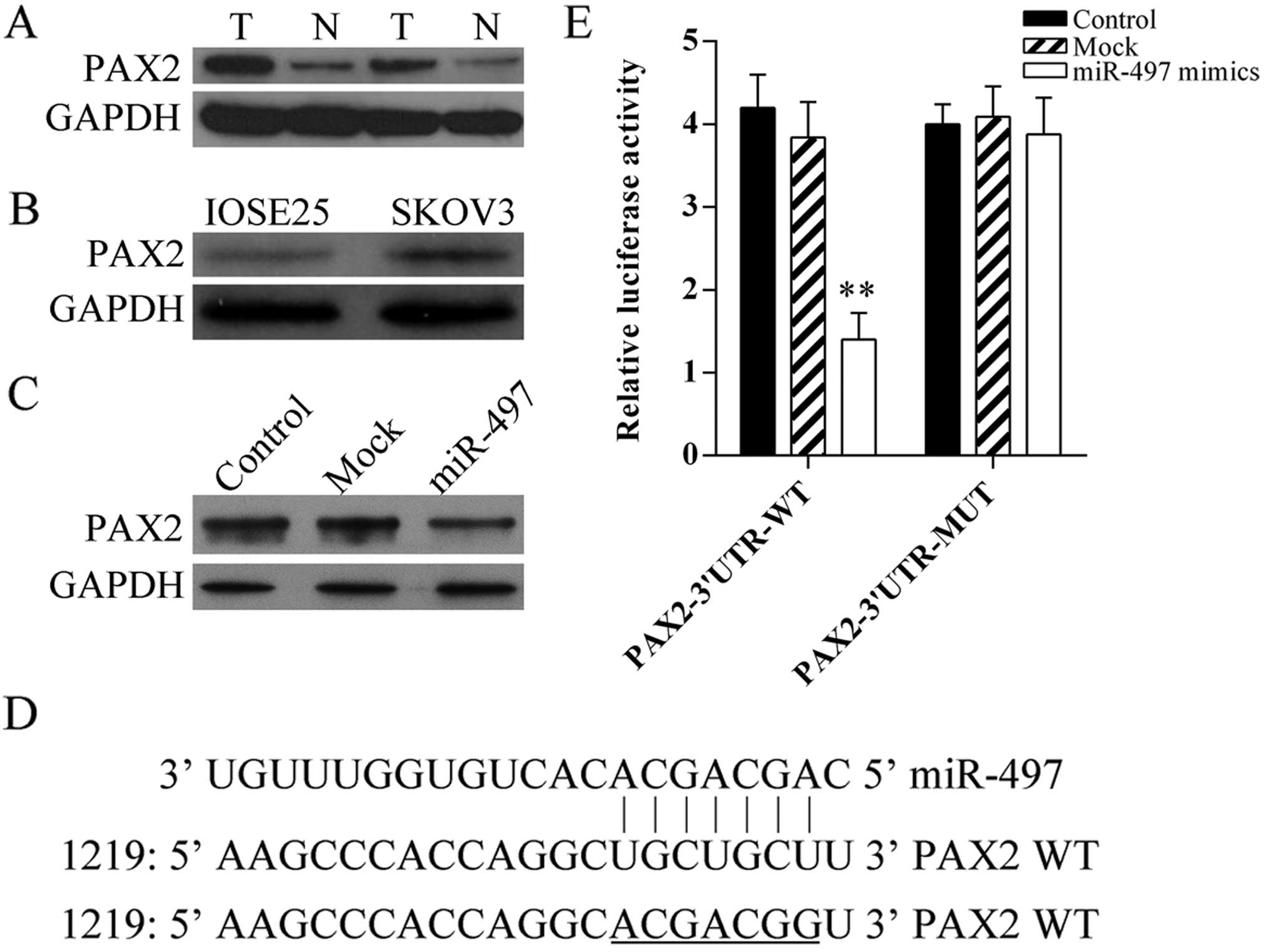
Firstly, the protein expression of PAX2 in human
ovarian cancer tissues and cells was detected using western blot
analysis, the result showed PAX2 was increased in tumor tissues
(Fig. 4A) and SKOV3 cells (Fig. 4B). In addition, to determine that
miR-497 regulates PAX2 expression directly in ovarian cancer, the
expression of PAX2 in miR-497 mimic-transfected SKOV3 cells was
analyzed using western blot analysis (Fig. 4C). Furthermore, miRNA target
predication databases (TargetScan: www.targetscan.org and MICRORNA.ORG:
www.microrna.org) were used for computational
analyses. miR-497 has one predictive target site in the human PAX2
3′UTR (Fig. 4D). Moreover,
dual-luciferase assay was performed to verify whether PAX2 is a
direct target gene of miR-497. The results showed that miR-497
mimics suppressed the luciferase activity of the reporter (Fig. 4E). Our results indicated that this
site of PAX2 3′UTR was the exact regulation site for miR-497.
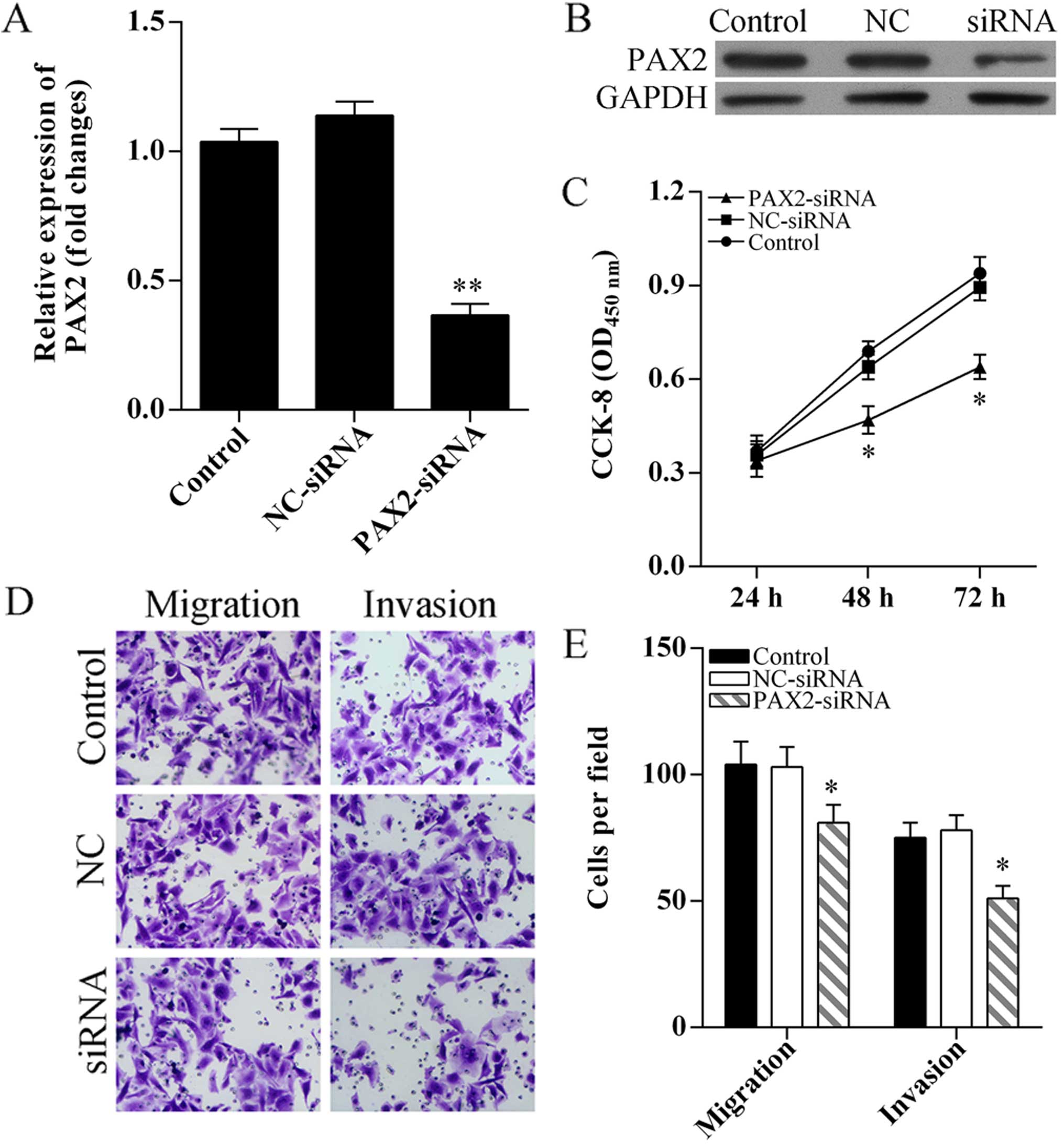
Knockdown of PAX2 inhibits SKOV3 cell
proliferation, migration and invasion
To determine the role of PAX2 in cell proliferation,
migration and invasion, PAX2 was knocked down using small
interference RNA (Fig. 5A and B).
CCK-8 assay results showed SKOV3 cell proliferation was
significantly suppressed after PAX2-siRNA transfection (Fig. 5C). Transwell assay was performed to
analyzed cell migration and invasion, the results showed knockdown
of PAX2 inhibited SKOV3 cell migration and invasion (Fig. 5D and E).
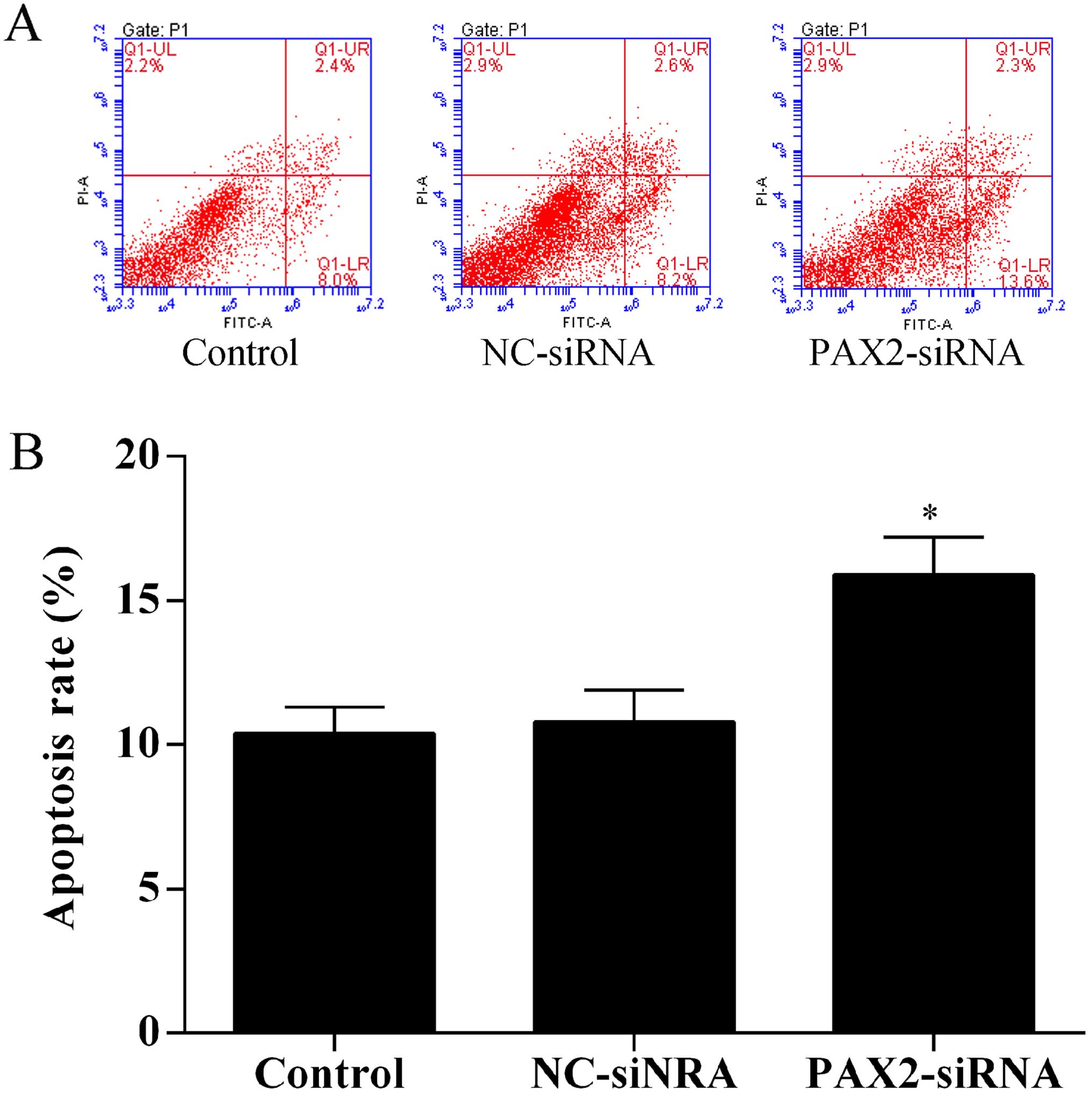
Knockdown of PAX2 induces SKOV3 cell
apoptosis
To investigate the effect of PAX2 on ovarian cancer
cell apoptosis, the Annexin V-FITC/PI staining method was used to
perform apoptosis assays by flow cytometry. The results showed that
knockdown of PAX2 promoted SKOV3 cell apoptosis (Fig. 6).
Discussion
In recent years, increased number of studies have
reported that miRNA regulates gene expression involved in variety
of biological processes including cell growth, differentiation,
migration and invasion, and tumorigenesis (4,17).
Many miRNAs are aberrantly expressed in human cancers and
unconventional expression of miRNAs result in uncontrolled tumor
growth (18,19). Therefore, increased understanding of
how miRNA regulates cell proliferation and survival may provide
better strategies for the diagnosis and treatment of human cancer,
including ovarian cancer.
MicroRNA-497 is located on chromosome 17p13.120 and
is lost in 93% of human small-cell lung cancers (21). Previous studies indicated that
miR-497 acts as a tumor suppressor in human cancers. The expression
of miR-497 has been found decreased in colorectal cancer (22), breast cancer (23), pancreatic cancer (24), human osteosarcoma (25), cervical cancer (26) and human lung cancer (27). In addition, downregulation of
miR-497 was associated with malignancy of colorectal cancer
(22) and breast cancer (23). Furthermore, overexpression of
miR-497 can inhibit pancreatic cancer and breast cancer cell growth
both in vitro and in vivo (15,23).
In our present study, the expression level of miR-497 in human
ovarian cancer tissues and cells was confirmed and the role of
miR-497 in ovarian cancer cell proliferation and apoptosis was
evaluated. Our results showed that miR-497 was significantly
decreased in human ovarian cancer tissues and cells. In addition,
overexpression of miR-497 inhibited SKOV3 cell proliferation,
suppressed cell migration and invasion, and promoted cell
apoptosis. Furthermore, overexpression of miR-497 by
mimic-transfection reduced the PAX2 protein expression, and
dual-luciferase assay confirmed that the PAX2 is a target gene of
miR-497. Moreover, knockdown of PAX2 by RNA interference suppressed
SKOV3 cell growth, inhibited cell migration and invasion, and
induced cell apoptosis. All the above indicated that miR-497 acts
as a tumor suppressor by regulating cell proliferation, metastasis
and apoptosis in human ovarian cancer, and miR-497-PAX2 may
represent a new potential diagnosis and therapeutic target for the
treatment of human ovarian cancer.
Paired box (PAX) genes are named for the
conserved DNA sequence motif which comprises a 128-amino acid
domain and encodes a group of transcription factors (28). Nine PAX genes have been
identified in mammals (29).
PAX2, one of the PAX genes, is a critical regulator
of embryogenesis (30–32). Embryonic PAX2 gene expression
is largely attenuated in adult tissue, such as breast (33). Loss of PAX2 is associated
with kidney hypoplasia and vesicoureteral reflux (34). On the contrary, upregulation of PAX2
is associated with human cancers (35,36).
PAX2 was significantly increased in human prostate cancer (37) and breast cancer (38). Inhibit the expression of PAX2 in
renal cell carcinoma cells (39)
and knock-down of PAX2 in bladder cancer cells (40) suppressed the cell growth and induced
cell apoptosis. Similarly, silencing of PAX2 led to Kaposi sarcoma
cell death and inhibited cell invasion and migration (41). However, the function of PAX2
in the evolution and progression of ovarian cancer is still
unclear. In our present study, PAX2 was increased in human ovarian
cancer tissues and cells. Silencing of PAX2 markedly suppressed
SKOV3 cell proliferation, blocked cell migration and invasion, and
induced cell apoptosis. We confirmed that the expression of PAX2
was regulated by miR-497, at least partly. Taken together, our
findings indicate that decreasing of miR-497 may promote the
proliferation capacity of human ovarian cancer through the
PAX2-mediated signal pathway.
In conclusion, we demonstrated that miR-497 is
decreased in human ovarian cancer tissues and cells, and acts as a
tumor suppressor. We also confirmed PAX2 is a target gene of
miR-497. Overexpression of miR-497 or silencing of PAX2 suppressed
the proliferative and induced apoptosis of ovarian cancer cells.
Based on the above, our study present that miR-497 has the
potential role as a useful diagnostic and therapeutic biomarker for
human ovarian cancer.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Breuer EK and Murph MM: The role of
proteomics in the diagnosis and treatment of women's cancers:
Current trends in technology and future opportunities. Int J
Proteomics. 2011:pii: 373584. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Doench JG and Sharp PA: Specificity of
microRNA target selection in translational repression. Genes Dev.
18:504–511. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Filipowicz W, Bhattacharyya SN and
Sonenberg N: Mechanisms of post-transcriptional regulation by
microRNAs: Are the answers in sight? Nat Rev Genet. 9:102–114.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Meyer SU, Thirion C, Polesskaya A,
Bauersachs S, Kaiser S, Krause S and Pfaffl MW: TNF-α and IGF1
modify the microRNA signature in skeletal muscle cell
differentiation. Cell Commun Signal. 13:42015. View Article : Google Scholar
|
|
7
|
Tian L, Fang YX, Xue JL and Chen JZ: Four
microRNAs promote prostate cell proliferation with regulation of
PTEN and its downstream signals in vitro. PLoS One. 8:e758852013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ambros V: MicroRNA pathways in flies and
worms: Growth, death, fat, stress, and timing. Cell. 113:673–676.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Calin GA, Sevignani C, Dumitru CD, Hyslop
T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M,
et al: Human microRNA genes are frequently located at fragile sites
and genomic regions involved in cancers. Proc Natl Acad Sci USA.
101:2999–3004. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Farazi TA, Hoell JI, Morozov P and Tuschl
T: MicroRNAs in human cancer. Adv Exp Med Biol. 774:1–20. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lages E, Ipas H, Guttin A, Nesr H, Berger
F and Issartel JP: MicroRNAs: Molecular features and role in
cancer. Front Biosci (Landmark Ed). 17:2508–2540. 2012. View Article : Google Scholar
|
|
12
|
Bray I, Bryan K, Prenter S, Buckley PG,
Foley NH, Murphy DM, Alcock L, Mestdagh P, Vandesompele J, Speleman
F, et al: Widespread dysregulation of miRNAs by MYCN amplification
and chromosomal imbalances in neuroblastoma: Association of miRNA
expression with survival. PLoS One. 4:e78502009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao X, Zhao Z, Xu W, Hou J and Du X:
Down-regulation of miR-497 is associated with poor prognosis in
renal cancer. Int J Clin Exp Pathol. 8:758–764. 2015.PubMed/NCBI
|
|
14
|
Itesako T, Seki N, Yoshino H, Chiyomaru T,
Yamasaki T, Hidaka H, Yonezawa T, Nohata N, Kinoshita T, Nakagawa
M, et al: The microRNA expression signature of bladder cancer by
deep sequencing: The functional significance of the miR-195/497
cluster. PLoS One. 9:e843112014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu J, Wang T, Cao Z, Huang H, Li J, Liu W,
Liu S, You L, Zhou L, Zhang T, et al: miR-497 downregulation
contributes to the malignancy of pancreatic cancer and associates
with a poor prognosis. Oncotarget. 5:6983–6993. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li NF, Broad S, Lu YJ, Yang JS, Watson R,
Hagemann T, Wilbanks G, Jacobs I, Balkwill F, Dafou D, et al: Human
ovarian surface epithelial cells immortalized with hTERT maintain
functional pRb and p53 expression. Cell Prolif. 40:780–794. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lagos-Quintana M, Rauhut R, Lendeckel W
and Tuschl T: Identification of novel genes coding for small
expressed RNAs. Science. 294:853–858. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu H, Li W, Chen C, Pei Y and Long X:
miR-335 acts as a potential tumor suppressor miRNA via
downregulating ROCK1 expression in hepatocellular carcinoma. Tumour
Biol. 36:6313–6319. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tu Y, Liu L, Zhao D, Liu Y, Ma X, Fan Y,
Wan L, Huang T, Cheng Z and Shen B: Overexpression of miRNA-497
inhibits tumor angiogenesis by targeting VEGFR2. Sci Rep.
5:138272015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bailey-Wilson JE, Amos CI, Pinney SM,
Petersen GM, de Andrade M, Wiest JS, Fain P, Schwartz AG, You M,
Franklin W, et al: A major lung cancer susceptibility locus maps to
chromosome 6q23–25. Am J Hum Genet. 75:460–474. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Girard L, Zöchbauer-Müller S, Virmani AK,
Gazdar AF and Minna JD: Genome-wide allelotyping of lung cancer
identifies new regions of allelic loss, differences between small
cell lung cancer and non-small cell lung cancer, and loci
clustering. Cancer Res. 60:4894–4906. 2000.PubMed/NCBI
|
|
22
|
Guo ST, Jiang CC, Wang GP, Li YP, Wang CY,
Guo XY, Yang RH, Feng Y, Wang FH, Tseng HY, et al: MicroRNA-497
targets insulin-like growth factor 1 receptor and has a tumour
suppressive role in human colorectal cancer. Oncogene.
32:1910–1920. 2013. View Article : Google Scholar :
|
|
23
|
Li D, Zhao Y, Liu C, Chen X, Qi Y, Jiang
Y, Zou C, Zhang X, Liu S, Wang X, et al: Analysis of miR-195 and
miR-497 expression, regulation and role in breast cancer. Clin
Cancer Res. 17:1722–1730. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu JW, Wang TX, You L, Zheng LF, Shu H,
Zhang TP and Zhao YP: Insulin-like growth factor 1 receptor
(IGF-1R) as a target of miR-497 and plasma IGF-1R levels associated
with TNM stage of pancreatic cancer. PLoS One. 9:e928472014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ruan WD, Wang P, Feng S, Xue Y and Zhang
B: MicroRNA-497 inhibits cell proliferation, migration, and
invasion by targeting AMOT in human osteosarcoma cells. Onco
Targets Ther. 9:303–313. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Luo M, Shen D, Zhou X, Chen X and Wang W:
MicroRNA-497 is a potential prognostic marker in human cervical
cancer and functions as a tumor suppressor by targeting the
insulin-like growth factor 1 receptor. Surgery. 153:836–847. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu S, Fu G-B, Tao Z, OuYang J, Kong F,
Jiang BH, Wan X and Chen K: miR-497 decreases cisplatin resistance
in ovarian cancer cells by targeting mTOR/P70S6K1. Oncotarget.
6:26457–26471. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Eccles MR, He S, Legge M, Kumar R, Fox J,
Zhou C, French M and Tsai RW: PAX genes in development and disease:
The role of PAX2 in urogenital tract development. Int J Dev Biol.
46:535–544. 2002.PubMed/NCBI
|
|
29
|
Robson EJ, He SJ and Eccles MR: A PANorama
of PAX genes in cancer and development. Nat Rev Cancer. 6:52–62.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Luu VD, Boysen G, Struckmann K, Casagrande
S, von Teichman A, Wild PJ, Sulser T, Schraml P and Moch H: Loss of
VHL and hypoxia provokes PAX2 up-regulation in clear cell renal
cell carcinoma. Clin Cancer Res. 15:3297–3304. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chan-Ling T, Chu Y, Baxter L, Weible Ii M
and Hughes S: In vivo characterization of astrocyte precursor cells
(APCs) and astrocytes in developing rat retinae: Differentiation,
proliferation, and apoptosis. Glia. 57:39–53. 2009. View Article : Google Scholar
|
|
32
|
Sims-Lucas S, Cusack B, Baust J,
Eswarakumar VP, Masatoshi H, Takeuchi A and Bates CM: Fgfr1 and the
IIIc isoform of Fgfr2 play critical roles in the metanephric
mesenchyme mediating early inductive events in kidney development.
Dev Dyn. 240:240–249. 2011. View Article : Google Scholar :
|
|
33
|
Li CG and Eccles MR: PAX genes in cancer;
Friends or foes? Front Genet. 3:62012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Weber S, Taylor JC, Winyard P, Baker KF,
Sullivan-Brown J, Schild R, Knüppel T, Zurowska AM, Caldas-Alfonso
A, Litwin M, et al: SIX2 and BMP4 mutations associate with
anomalous kidney development. J Am Soc Nephrol. 19:891–903. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tong GX, Chiriboga L, Hamele-Bena D and
Borczuk AC: Expression of PAX2 in papillary serous carcinoma of the
ovary: immunohistochemical evidence of fallopian tube or secondary
Müllerian system origin? Mod Pathol. 20:856–863. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tung CS, Mok SC, Tsang YT, Zu Z, Song H,
Liu J, Deavers MT, Malpica A, Wolf JK, Lu KH, et al: PAX2
expression in low malignant potential ovarian tumors and low-grade
ovarian serous carcinomas. Mod Pathol. 22:1243–1250. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Khoubehi B, Kessling AM, Adshead JM, Smith
GL, Smith RD and Ogden CW: Expression of the developmental and
oncogenic PAX2 gene in human prostate cancer. J Urol.
165:2115–2120. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Silberstein GB, Dressler GR and Van Horn
K: Expression of the PAX2 oncogene in human breast cancer and its
role in progesterone-dependent mammary growth. Oncogene.
21:1009–1016. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gnarra JR and Dressler GR: Expression of
Pax-2 in human renal cell carcinoma and growth inhibition by
antisense oligonucleotides. Cancer Res. 55:4092–4098.
1995.PubMed/NCBI
|
|
40
|
Muratovska A, Zhou C, He S, Goodyer P and
Eccles MR: Paired-box genes are frequently expressed in cancer and
often required for cancer cell survival. Oncogene. 22:7989–7997.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Buttiglieri S, Deregibus MC, Bravo S,
Cassoni P, Chiarle R, Bussolati B and Camussi G: Role of Pax2 in
apoptosis resistance and proinvasive phenotype of Kaposi's sarcoma
cells. J Biol Chem. 279:4136–4143. 2004. View Article : Google Scholar
|