Introduction
Cervical cancer (CC) is one of the most frequent
gynecologic malignancies, accounting for significant morbidity and
mortality in females worldwide (1).
Tumor invasion and metastasis remain formidable obstacles to the
effective treatment of this disease. Thus, research on the
molecular mechanisms leading to invasion and metastatic
dissemination of carcinoma cells are urgently needed for a more
effective therapeutic strategy.
Astrocyte-elevated gene-1 (AEG-1), also known as
metadherin (MTDH) and lysine-rich CEACAM1 co-isolate protein
(LYRIC), is considered an important oncogene, whose expression is
involved in carcinogenesis, tumor progression and chemotherapeutic
resistance in several malignancies (2). Expression of AEG-1 is elevated in
glioblastoma multiforme and contributes to tumor cell survival,
proliferation and invasion (3).
Overexpression of AEG-1 contributes to the neoplastic phenotype of
bladder cancer cells by promoting survival, clonogenicity and
migration (4). It is reported that
AEG-1 is highly expressed in CC and associates with progression of
cervical intraepithelial neoplasia and unfavorable prognosis
(5,6). This suggests that AEG-1 plays a
crucial role in the progression of CC. However, the mechanism of
upregulation of AEG-1 in cervical cancer has not yet been fully
clarified. MicroRNAs (miRNAs or miR) are endogenous non-coding
small RNAs, many of which are abnormally expressed in various types
of cancers and involved in tumorigenesis and tumor development.
miR-124 is one of the most studied and best characterized miRNAs
with pivotal roles in several malignant processes, including tumor
proliferation, motility and angiogenesis (7). It has been reported that miR-124
promoter is heavily methylated in CC cell lines, thus contributing
to its slight expression in human CC specimens (8,9).
miR-124 was discovered to be involved in MALAT1-miR-124-RBG2 axis
where it affects growth and invasion of high-risk human
papillomavirus-positive CC cells (10). However, molecular mechanisms by
which miR-124 achieve its effects in CC are still being elucidated.
Recent studies revealed that several miRNAs may serve as potential
regulatory mechanisms of AEG-1 in human cancers, such as miR-26a
(11), miR-137 (12) and miR-375 (13). Intriguingly, bioinformatic analysis
showed that AEG-1 harbored a potential miR-124 binding site in its
3′-untranslated regions (3′-UTRs), suggesting that AEG-1 is a
putative target of miR-124. In the present study, the expression of
miR-124 was found to be inversely downregulated with the expression
of AEG-1 in cervical carcinoma. Furthermore, it was shown that
miR-124 can suppress CC cell proliferation, migration and invasion,
as well as inhibit epithelial-mesenchymal transition (EMT) in
cervical carcinoma through directly targeting AEG-1, suggesting
that miR-124/AEG-1 axis has a pivotal role in the progression of
CC.
Materials and methods
Ethics statements
The present study was conducted with the approval
from the Ethics Committee of the Second Affiliated Hospital of
Zhengzhou University. Written informed consent was obtained from
all patients before study initiation.
Tissue specimens
Pair-matched tumor and adjacent normal cervical
tissues from 18 CC patients were obtained from the Second
Affiliated Hospital of Zhengzhou University, China. None of the
patients had suffered from any other severe diseases that may
affect the study. None of them received radiotherapy or
chemotherapy before the tissues were obtained. All specimens were
obtained during routine resection surgery, then immediately
snap-frozen in liquid nitrogen, and stored at −80°C for further
analysis.
Cell culture
The human CC cell lines (HeLa, SiHa, CaSKi and C33A)
and normal cervical epithelial cells (End1/E6E7) were purchased
from the Chinese Academy of Sciences Cell Bank (Shanghai, China).
HeLa, SiHa and C33A cells were cultured in Dulbecco's modified
Eagle's medium (DMEM), CaSKi cells were cultured in RPMI-1640
medium (both from Gibco, Grand Island, NY, USA) supplemented with
10% heat-inactivated fetal bovine serum (FBS; Sigma-Aldrich, St.
Louis, MO, USA), 100 U/ml penicillin and 100 U/ml streptomycin
(Invitrogen, Carlsbad, CA, USA). End1/E6E7 cells were cultured in
KER-SFM medium (Gibco). All cells were fostered in a humidified
atmosphere chamber containing 5% CO2 at 37°C.
Cell transfection
HeLa and SiHa cells were respectively seeded into
24-well plates (1×106 cells/well) and grown to 70%
confluency before transfection. miR-124 mimics
(5′-GGCAUUCACCUCGUGCCUUA-3′) and negative control mimics (NC;
5′-ACUACUGAGUGACAGUAGA-3′) were synthesized by GenePharma
(Shanghai, China) and transfected into prepared cells at a final
concentration of 50 nM. For AEG-1 overexpression, the cDNA (without
the 3′-UTR) of AEG-1 was cloned into pcDNA3.1 (+) (Invitrogen) to
generate an AEG-1 overexpression vector. Cell transfections were
performed with Lipofectamine™ 2000 (Invitrogen) according to the
manufacturer's instructions. Total RNA and protein contents were
prepared 24 h post-transfection for further analysis.
Quantitative real-time PCR analysis
Total RNA was isolated from tissues or cultured
cells using TRIzol reagent and reverse transcribed by M-MLV reverse
transcriptase kit (both from Invitrogen) according to the
manufacturer's protocols. Quantitative real-time PCR (qRT-PCR) was
performed on an ABI 7900HT Fast Real-Time PCR system (Applied
Biosystems, Foster City, CA, USA). All PCRs were conducted in
triplicate and normalized to the internal control (GAPDH or U6).
The relative expression levels were evaluated using the
2−ΔΔCq method. The specific primers were designed as
follows: miR-124 RT primer,
5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGAGGCATTC-3′; miR-124 PCR
primers forward, 5′-ACACTCCAGCTGGGTAAGGCACGCGGTGA-3′ and reverse,
5′-TGGTGTCGTGGAGTCG-3′; U6 RT primer, 5′-TGGTGTCGTGGAGTCG-3′; U6
PCR primers forward, 5′-CTCGCTTCGGCAGCACA-3′ and reverse,
5′-AACGCTTCACGAATTTGCGT-3′; AEG-1 (MTDH) PCR primers forward,
5′-TGTTGAAGTGGCTCAGGG-3′ and reverse, 5′-CAGGAAATGATGCGGTTG-3′;
GAPDH PCR primers forward, 5′-CCACCCATGGCAAATTCCATG GCA-3′ and
reverse, 5′-TCTAGACGGCAGGTCAGGTCCACC-3′.
Cell proliferation assay
Transfected cells were seeded into 24-well plates
(3×103 cells/well) and cultured at 37°C. Cellular
proliferation was evaluated at the indicated time (24, 48, 72 or 96
h) using a Cell Counting Kit-8 (CCK-8; Sigma, St. Louis, MO, USA)
in accordance with the manufacturer's protocol. After incubation
with CCK-8 solution for another 4 h, the optical absorbance was
measured at the wavelength of 450 nm. The experiment was performed
in triplicate.
Cell apoptosis assay
HeLa and SiHa cells were transiently transfected
with miR-124 mimic or NC. Cells were harvested after 72 h by
trypsinization. Double staining with FITC-Annexin V and propidium
iodide (PI) was carried out using the FITC-Annexin V apoptosis
detection kit (BD Biosciences, Bedford, MA, USA). Cells were
analyzed by flow cytometry.
Cell invasion and migration assays
Cell invasion and migration abilities were
determined using an 8-µm-pore polycarbonate membrane Boyden
chamber insert in a Transwell apparatus (Costar, Cambridge, MA,
USA), with and without Matrigel (BD Biosciences), respectively.
Cells were harvested and resuspended in serum-free medium after 48
h transfection. For the invasion assays, 1×105 cells
were prepared in 200 µl of serum-free RPMI-1640 medium then
placed in the upper chamber coated with Matrigel. For the migration
assays, 3×104 cells were prepared in 200 µl of
serum-free RPMI-1640 medium and seeded into the upper chamber of
the Transwell. The lower chamber was filled with RPMI-1640 medium
containing 10% FBS to induce chemotaxis. After incubation for 24 h
at 37°C in a 5% CO2 incubator, the invaded or migrated
cells were fixed with 100% methanol, stained in 0.5% crystal violet
(Sigma), and counted under a microscope. The results were averaged
over three independent experiments.
Xenograft tumor model
To evaluate in vivo tumorigenesis, a CC
xenograf mouse model was used. Female BALB/c athymic nude mice
(4–6-weeks old) were purchased from the Laboratory Animal Center of
Chinese Academy of Sciences (Shanghai, China). All animal
experiments were performed with the guide and approval of the
Institutional Animal Care and Use Committee of the Second
Affiliated Hospital of Zhengzhou University. HeLa cells
(2×106) stably transfected with miR-124 mimic or NC were
injected subcutaneously into the flanks of nude mice (n=8). Once
palpable tumors developed, tumor volume was measured and calculated
using the formula: Volume = 0.5 × length × width2.
Thirty days after inoculation, the mice were euthanized and tumor
weights were assessed.
Western blot analysis
Total proteins were extracted from CC samples or
cells using RIPA buffer (Abcam, Cambridge, MA, USA). Protein
concentrations were determined by BCA protein assay kit (Takara Bio
Inc., Otsu, Japan). Twenty micrograms of protein was fractionated
by 10% SDS-PAGE. After electrophoresis, proteins were transferred
to nitrocellulose membranes (Millipore, Bedford, MA, USA). The
blots were probed with the corresponding primary antibodies
specific for either AEG-1, E-cadherin, β-catenin, N-cadherin,
vimentin, twist, matrix metalloproteinase-9 (MMP-9), aldo-keto
reductase family 1 member C2 (AKR1C2), neurofibromin 1 (NF1) or
GAPDH (Abcam). Thereafter, the membranes were incubated with
horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG or
HRP-conjugated goat anti-mouse IgG (Abcam). Signals were visualized
with an enhanced chemiluminescence system (Amersham Pharmacia
Biotech, San Francisco, CA, USA), and analyzed using ImageJ
software. Protein levels were determined by normalization to
GAPDH.
Plasmid construction and luciferase
assays
The wild-type 3′-UTR segment of AEG-1 was cloned
downstream of the luciferase coding region in the psi-CHECK™
Vectors (Promega, Madison, WI, USA) to generate luciferase reporter
vector AEG-1 3′-UTR-wt. The corresponding mutant constructs (AEG-1
3′-UTR-mut) were created by mutating the seed regions of the
miR-124-binding sites. Cells were plated into 24-well plates until
80% confluence before transfection. The wild-type or mutant AEG-1
3′-UTR plasmid was transiently co-transfected with miR-124 mimics
or NC into HeLa cells. Cell lysates were harvested 24 h
post-transfection and luciferase activities were measured by the
Dual-Luciferase Reporter Assay System (Promega). Luciferase
activity was normalized by Renilla luciferase activity.
Statistical analysis
All statistical calculations were performed using
SPSS software (version 19.0). Data were obtained from three
repeated experiments and expressed as mean ± standard deviation
(SD). Differences between groups were subjected to the Student's-t
test. Statistical significance was set at P<0.05.
Results
Downregulation of miR-124 in CC tissues
and cell lines
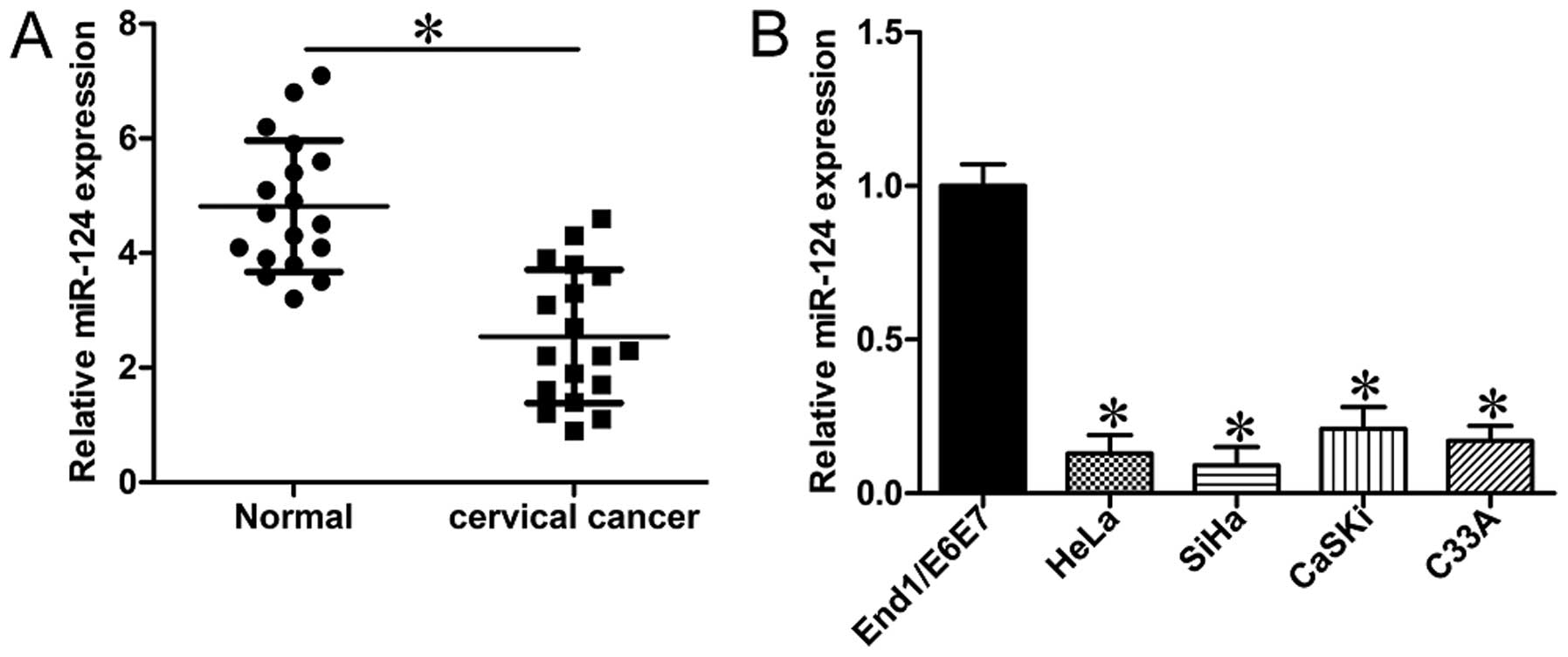
Expression of miR-124 in CC tissues was determined
and compared to that in the adjacent normal tissues. It was shown
that miR-124 expression levels were significantly reduced in CC
tissues (Fig. 1A; P<0.05). We
further evaluated the expression of miR-124 in CC cell lines. The
results showed that the expression levels of miR-124 in all four CC
cell lines (HeLa, SiHa, CaSKi and C33A) were much lower than that
in End1/E6E7, a normal cervical epithelial cell line (Fig. 1B; P<0.05). We next tested the
association between miR-124 and clinicopathological characteristics
of CC patients (Table I).
Consequently, low expression of miR-124 correlated with advanced
International Federation of Gynecology and Obstetrics (FIGO) stage,
vascular invasion and lymph node metastasis.
 | Table IAssociation between miR-124 expression
and clinicopathological parameters in study patients. |
Table I
Association between miR-124 expression
and clinicopathological parameters in study patients.
| | Expression of miR-124
| |
|---|
| Clinical
parameters | Cases | High
(n=10) | Low
(n=8) | P-value |
|---|
| Age (years) | (27–61) | | | 0.704 |
| <45 | 9 | 4 | 5 | |
| ≥45 | 9 | 6 | 3 | |
| Tumor size
(cm) | | | | 0.683 |
| <3 | 8 | 4 | 4 | |
| ≥3 | 10 | 6 | 4 | |
| HPV infection | | | | 0.537 |
| Positive | 10 | 6 | 4 | |
| Negative | 8 | 4 | 4 | |
| Pathological
type | | | | 0.725 |
| Squamous
carcinoma | 12 | 7 | 5 | |
|
Adenocarcinoma | 6 | 3 | 3 | |
| Tumor
differentiation | | | | 0.364 |
| Well/moderate | 9 | 6 | 3 | |
| Poor | 9 | 4 | 5 | |
| FIGO stage | | | | 0.042a |
| I+II | 11 | 5 | 6 | |
| III+IV | 7 | 5 | 2 | |
| Vascular
invasion | | | | 0.034a |
| Yes | 10 | 6 | 4 | |
| No | 8 | 4 | 4 | |
| Lymph node
metastasis | | | | 0.027a |
| Yes | 9 | 5 | 4 | |
| No | 9 | 5 | 4 | |
| AEG-1
expression | | | | 0.018a |
| High | 11 | 4 | 7 | |
| Low | 7 | 6 | 1 | |
These results showed that the expression of miR-124
was downregulated in human CC tissues and cells, and miR-124 may be
involved in the progression of cervical carcinoma.
Exogenous overexpression of miR-124
suppresses the proliferation, migration and invasion of CC
cells
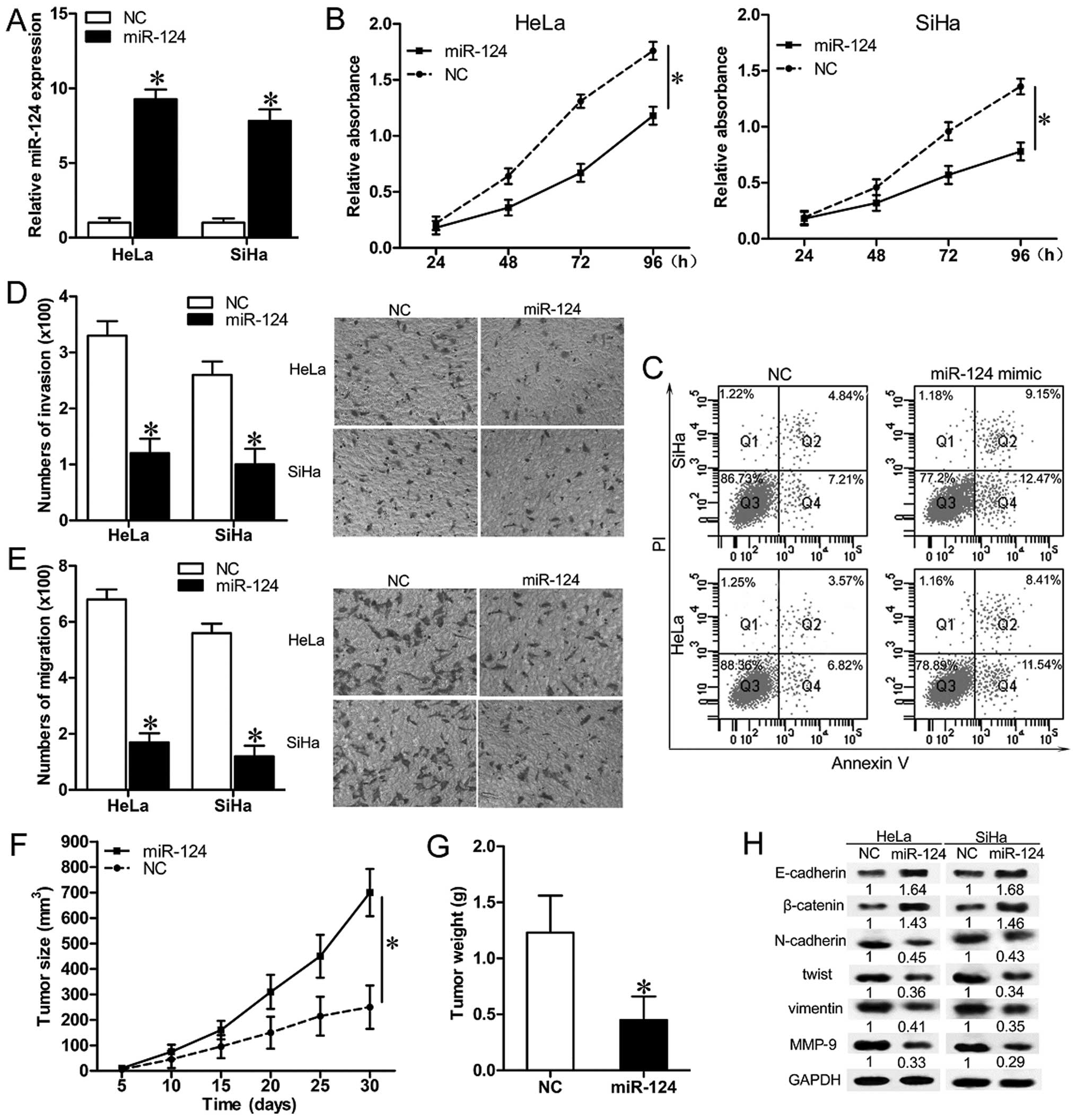
To investigate the role of miR-124 in cervical
carcinogenesis, miR-124 mimics or NC were transfected into HeLa and
SiHa cells (Fig. 2A), and the
biologic behavior of the cells were subsequently characterized.
Cell growth curves indicated that upregulation of miR-124
significantly suppressed cell proliferation (Fig. 2B; P<0.05). Transfection of
miR-124 leads to induction of apoptosis in both HeLa and SiHa cells
(Fig. 2C). Overexpression of
miR-124 significantly decreased the migratory and invasive
capacities of HeLa and SiHa cells (Fig.
2D and E; P<0.05). Xenografted tumors in mice inoculated
with miR-217 mimic infected HeLa cells grew much more slowly than
those in mice inoculated with NC (Fig.
2F and G; P<0.05). These results confirm that miR-124 acts
as a tumor-suppressor, and its upregulation inhibits cell
proliferation, migration and invasion in CC cell lines.
Exogenous overexpression of miR-124
inhibits EMT of CC cells
As epithelial-mesenchymal transition (EMT) is
thought to be critical for cancer cell invasion and metastasis, we
next examined the expression of several EMT-related molecules
(lower E-cadherin and β-catenin, higher N-cadherin, twist and
vimentin), and metastasis-related molecules (higher MMP-9) to
determine whether miR-124 is involved in regulation of EMT in
cervical carcinomas. In both HeLa and the SiHa cells, miR-124
overexpression increased the expression level of E-cadherin and
β-catenin. Simultaneously, protein levels of N-cadherin, twist,
vimentin and MMP-9 showed robust downregulation in both HeLa and
SiHa cells (Fig. 2H). These results
indicate that exogenous overexpression of miR-124 in CC cells
partially represses EMT in cervical carcinomas.
miR-124 targets AEG-1 3′-UTR and
downregulates its expression in cervical carcinomas
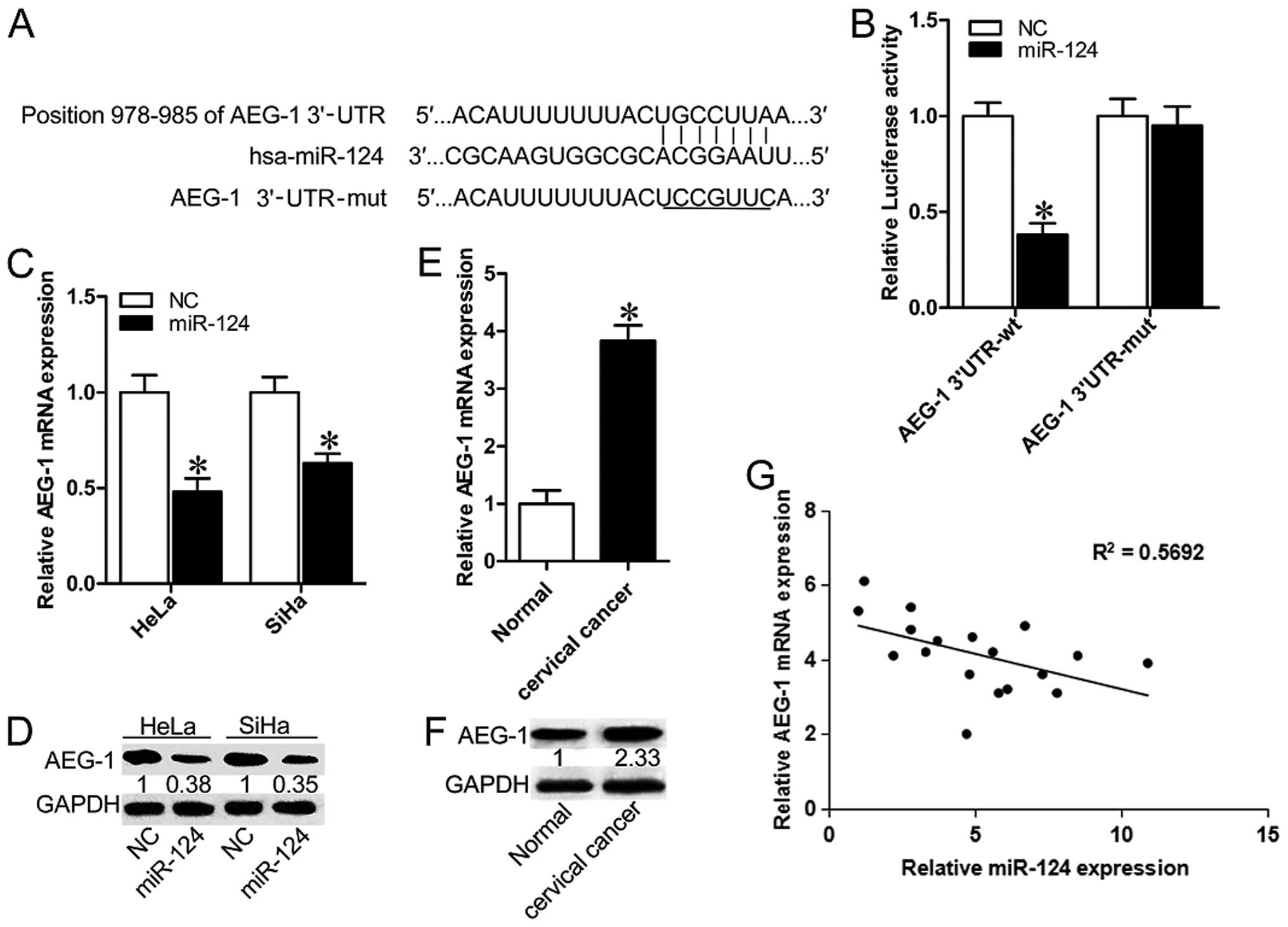
Bioinformatics analysis using miRTarBase (http://mirtarbase.mbc.nctu.edu.tw/index.php) and
TargetScan 7.0 (http://www.targetscan.org/vert_70/) identified that
AEG-1 harbors a potential miR-124 binding site (Fig. 3A). To verify whether AEG-1 is the
direct target of miR-124, luciferase report vectors that contain
the putative miR-124 binding sites within AEG-1 3′-UTR were
constructed. The overexpression of miR-124 significantly suppressed
the luciferase activity of AEG-1 containing a wild-type 3′-UTR, but
did not suppress activity of AEG-1 with a mutant 3′-UTR (Fig. 3B; P<0.05). Transfection of
miR-124 mimic led to a significant decrease of AEG-1 mRNA and
protein expression in HeLa and SiHa cells (Fig. 3C and D; P<0.05). To further
determine whether miR-124-mediated repression of AEG-1 is of
clinical relevance, we measured the expression of miR-124 and AEG-1
in CC specimens and pair-matched normal tissues. In contrast to the
significantly lower expression of miR-124, AEG-1 showed higher
levels in CC specimens when compared with the normal group
(Fig. 3E and F; P<0.05).
Furthermore, a negative correlation between mRNA levels of AEG-1
miR-124 and miR-124 was revealed by Spearman's correlation analysis
(Fig. 3G). Together, these results
suggest that AEG-1 is a target of miR-124 in cervical
carcinomas.
Exogenous overexpression of AEG-1
partially reverses the inhibitory effects of miR-124 on CC
cells
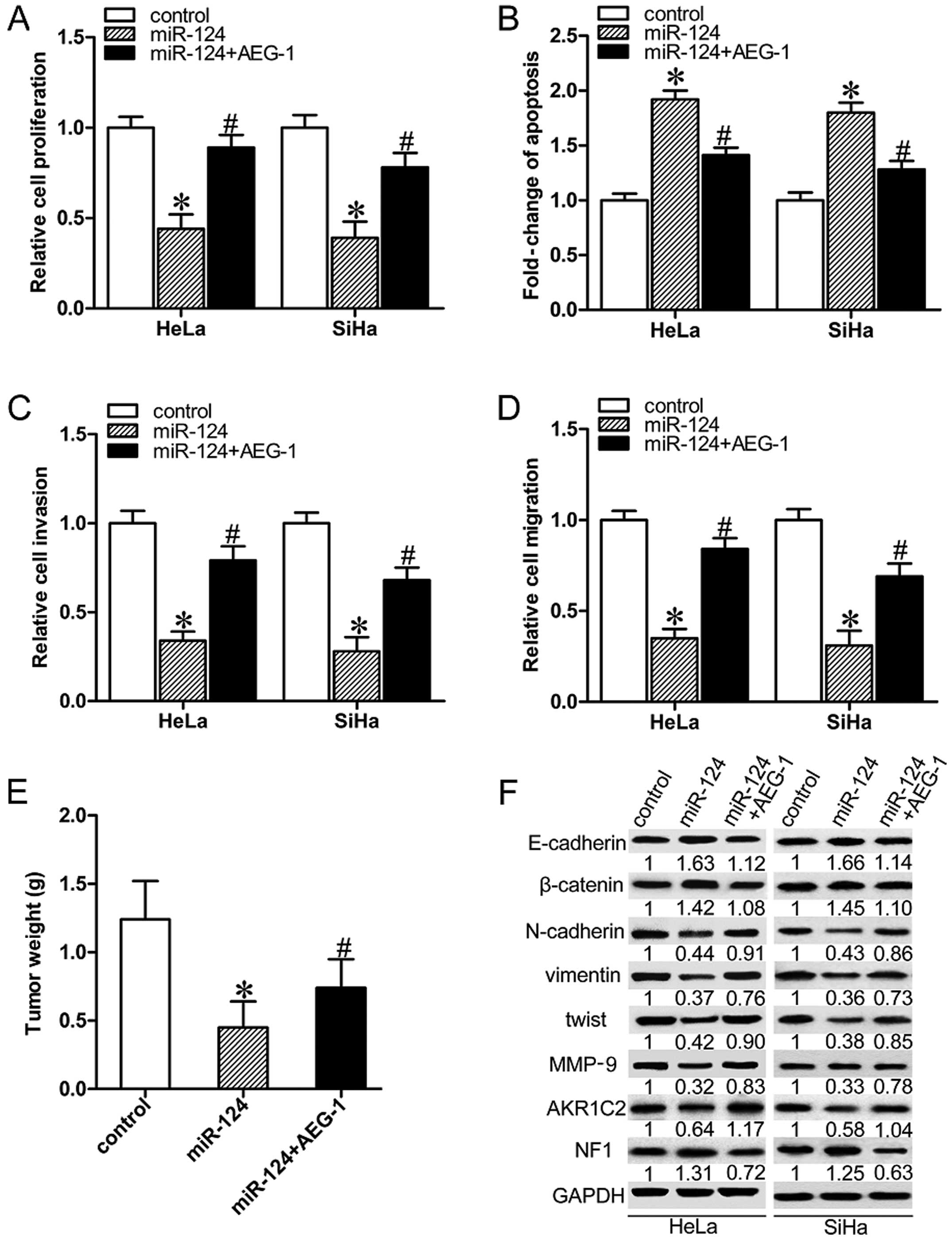
In order to investigate the contribution of AEG-1 to
migration and invasion of human CC cells, AEG-1 was force-expressed
along with miR-124 in HeLa and SiHa cells. Overexpression of AEG-1
partially nullified miR-124-mediated suppression of the
proliferation, migration and invasion, and reduced miR-124-induced
apoptosis in both HeLa and SiHa cells (Fig. 4A–D; P<0.05). The forced
expression of AEG-1 increased the average weight of the miR-124
overexpressing tumors (Fig. 3E;
P<0.05). Overexpression of AEG-1 partially restored
miR-124-induced downregulation of N-cadherin, twist, vimentin and
MMP-9, inhibited miR-124-induced upregulation of E-cadherin,
β-catenin in HeLa and SiHa cells (Fig.
4F). The tumor mass was harvested and weighed. We next
determined the expression levels of two novel AEG-1 downstream
factors AKR1C2 and NF1. As shown in Fig. 4F, miR-124 decreased the expression
of AKR1C2, but increased the expression of NF1. Collectively, our
results demonstrated that miR-124 exerts suppressive effects on the
progression of cervical carcinomas partly by targeting AEG-1.
Discussion
Numerous microRNAs (miRNAs) are known to play a key
role in tumorigenesis due to their ability to act as either
oncogenes or tumor suppressor genes. Various miRNAs, such as
miR-34a, are considered to be ideal targets for therapeutic
intervention in certain types of cancers (14). In the present study, we focused on
miR-124, which is abnormally expressed in a variety of cancer types
(7,15,16).
To date, evidence shows that miR-124 gene polymorphism is closely
related to susceptibility to cervical cancer (CC) (17). In the present study, qRT-PCR
analysis demonstrated that miR-124 was significantly down-regulated
in CC tissue samples and cancer cell lines. This finding was also
verified by a study suggesting that abnormal promoter methylation
and downregulation of miR-124 is a common event during cervical
carcinogenesis (9).
Recently, reports also indicated multiple functions
of miRNAs in cervical carcinomas. For example, miR-506 acts as a
tumor suppressor in cervical carcinomas (18). The present study revealed that
exogenous overexpression of miR-124 was able to markedly suppress
cell proliferation and induce cell apoptosis in two CC cell lines.
The introduction of miR-124 in HeLa and SiHa cells resulted in
markedly decreased migratory and invasive behavior, consistent with
previous studies showing that miR-124 inhibited the migration and
invasion of ovarian cancer cells (19), and suppressed tumor growth and
metastasis in nasopharyngeal carcinoma (20). Moreover, miR-124 overexpressed in CC
cells exhibited an increasing E-cadherin and β-catenin expression,
and decreasing N-cadherin, twist, vimentin and MMP-9 expression,
indicating that miR-124 inhibits EMT and metastasis in CC cases.
The above observations suggest that miR-124 acts as a
tumor-suppressor in CC, and ectopic expression of miR-124 may be
helpful for treatment of cervical carcinoma. However, the specific
regulatory mechanism and signal transduction network are still
poorly understood and need to be further explored.
Knockdown of AEG-1 inhibits migration and invasion
in colon cancer, suppresses metastasis in liver cancer (21), and decreases protective autophagy
and chemosensitization of cancer cells (22). Several miRNAs, such as miR-145 and
miR-217, were found to play an important role in tumor occurrence
and development by regulating the expression level of AEG-1 in
human cancers (23,24). The present study revealed, for the
first time, the involvement of miR-124 in tumorigenesis through
targeting AEG-1 in cervical carcinoma. miR-124 downregulated AEG-1
expression through interaction with its 3′-UTR, and overexpression
of miR-124 significantly suppressed AEG-1 expression in
vitro. Accumulating studies have reported that AEG-1 is highly
expressed in CC and is associated with cervical carcinoma
progression and angiogenesis (5,25). In
the present study, higher expression of AEG-1 was observed, and was
inversely correlated with miR-124 levels in CC specimens.
Furthermore, exogenous overexpression of AEG-1 reversed the
inhibitory effects of miR-124 on EMT and metastasis in CC cells. In
accordance with the effect of AEG-1 silencing (21), overexpression of miR-124 also
results in downregulation of AKR1C2 and upregulation of NF1. Both
AKR1C2 and NF1 were involved in the process of proliferation and
migration in vitro and in vivo (26,27).
Thus, the abnormal expression of AEG-1/AKR1C2/NF1 may contribute to
the molecular mechanisms of inhibition of miR-124 on cell
proliferation and migration in CC.
However, the present study did not clarify the
specific mechanism by which AEG-1 affects EMT in CC. Both miR-124
and AEG-1 are involved in the EMT process though various signaling
networks and effector targets, such as PI3K/Akt, NF-κB,
Wnt/β-catenin and TGF-β pathways (28,29).
According to existing research, PI3K/Akt and Wnt/β-catenin seems to
be more critical in the EMT process in CC. A previous study showed
that AEG-1 mediates CCL20/CCR6-induced EMT development via both
ERK1/2 and Akt signaling pathways in CC (30). Another study demonstrated that AEG-1
promotes EMT via the Wnt signaling pathway in cervical squamous
cell carcinoma (31). Previously it
was suggested that overexpression of miR-124 significantly
suppressed migration and invasion and the activity of non-canonical
Wnt signaling in osteosarcoma cells (32), while AEG-1 can activate Wnt
signaling to promote metastasis in tongue squamous cell carcinoma.
Thus, miR-124 mediated downregulation of AEG-1 may regulate the EMT
process mainly via the Wnt signaling in CC. The specific mechanism
in this process remains to be elucidated in the future.
Taken together, our results indicated that miR-124
is significantly downregulated in cervical carcinoma. miR-124 may
suppress CC cell proliferation, migration and invasion, as well as
inhibit EMT and metastasis in cervical carcinoma through directly
targeting AEG-1. Our findings suggest that the miR-124/AEG-1 link
has considerable value as a potential therapeutic target in
cervical carcinoma cases.
Abbreviations:
|
CC
|
cervical cancer
|
|
miR-124
|
microRNA-124
|
|
EMT
|
epithelial-mesenchymal transition
|
|
AEG-1
|
astrocyte-elevated gene-1
|
|
MTDH
|
metadherin
|
|
LYRIC
|
lysine-rich CEACAM-1-associated
protein
|
|
3′-UTR
|
3′-untranslated region
|
|
MMP-9
|
matrix metallo-proteinase-9
|
|
AKR1C2
|
aldo-keto reductase family 1 member
C2
|
|
NF1
|
neurofibromin 1
|
References
|
1
|
Bhat S, Kabekkodu SP, Noronha A and
Satyamoorthy K: Biological implications and therapeutic
significance of DNA methylation regulated genes in cervical cancer.
Biochimie. 121:298–311. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shi X and Wang X: The role of MTDH/AEG-1
in the progression of cancer. Int J Clin Exp Med. 8:4795–4807.
2015.PubMed/NCBI
|
|
3
|
Hu B, Emdad L, Bacolod MD, Kegelman TP,
Shen XN, Alzubi MA, Das SK, Sarkar D and Fisher PB: Astrocyte
elevated gene-1 interacts with Akt isoform 2 to control glioma
growth, survival, and pathogenesis. Cancer Res. 74:7321–7332. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nikpour M, Emadi-Baygi M, Fischer U,
Niegisch G, Schulz WA and Nikpour P: MTDH/AEG-1 contributes to
central features of the neoplastic phenotype in bladder cancer.
Urol Oncol. 32:670–677. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Long M, Dong K, Gao P, Wang X, Liu L, Yang
S, Lin F, Wei J and Zhang H: Overexpression of astrocyte-elevated
gene-1 is associated with cervical carcinoma progression and
angiogenesis. Oncol Rep. 30:1414–1422. 2013.PubMed/NCBI
|
|
6
|
Huang K, Li LA, Meng Y, You Y, Fu X and
Song L: High expression of astrocyte elevated gene-1 (AEG-1) is
associated with progression of cervical intraepithelial neoplasia
and unfavorable prognosis in cervical cancer. World J Surg Oncol.
11:2972013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang X, Wu Q, Xu B, Wang P, Fan W, Cai Y,
Gu X and Meng F: MiR-124 exerts tumor suppressive functions on the
cell proliferation, motility and angiogenesis of bladder cancer by
fine-tuning UHRF1. FEBS J. 282:4376–4388. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wilting SM, van Boerdonk RA, Henken FE,
Meijer CJ, Diosdado B, Meijer GA, le Sage C, Agami R, Snijders PJ
and Steenbergen RD: Methylation-mediated silencing and tumour
suppressive function of hsa-miR-124 in cervical cancer. Mol Cancer.
9:1672010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiménez-Wences H, Martínez-Carrillo DN,
Peralta-Zaragoza O, Campos-Viguri GE, Hernández-Sotelo D,
Jiménez-López MA, Muñoz-Camacho JG, Garzón-Barrientos VH,
Illades-Aguiar B and Fernández-Tilapa G: Methylation and expression
of miRNAs in precancerous lesions and cervical cancer with HPV16
infection. Oncol Rep. 35:2297–2305. 2016.PubMed/NCBI
|
|
10
|
Liu S, Song L, Zeng S and Zhang L:
MALAT1-miR-124-RBG2 axis is involved in growth and invasion of
HR-HPV-positive cervical cancer cells. Tumour Biol. 37:633–640.
2016. View Article : Google Scholar
|
|
11
|
Liu P, Tang H, Chen B, He Z, Deng M, Wu M,
Liu X, Yang L, Ye F and Xie X: miR-26a suppresses tumour
proliferation and metastasis by targeting metadherin in triple
negative breast cancer. Cancer Lett. 357:384–392. 2015. View Article : Google Scholar
|
|
12
|
Guo J, Xia B, Meng F and Lou G: miR-137
suppresses cell growth in ovarian cancer by targeting AEG-1.
Biochem Biophys Res Commun. 441:357–363. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He XX, Chang Y, Meng FY, Wang MY, Xie QH,
Tang F, Li PY, Song YH and Lin JS: MicroRNA-375 targets AEG-1 in
hepatocellular carcinoma and suppresses liver cancer cell growth in
vitro and in vivo. Oncogene. 31:3357–3369. 2012. View Article : Google Scholar
|
|
14
|
Di Martino MT, Leone E, Amodio N, Foresta
U, Lionetti M, Pitari MR, Cantafio ME, Gullà A, Conforti F, Morelli
E, et al: Synthetic miR-34a mimics as a novel therapeutic agent for
multiple myeloma: In vitro and in vivo evidence. Clin Cancer Res.
18:6260–6270. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cai JJ, Qi ZX, Chen LC, Yao Y, Gong Y and
Mao Y: miR-124 suppresses the migration and invasion of glioma
cells in vitro via Capn4. Oncol Rep. 35:284–290. 2016.
|
|
16
|
Jiang L, Lin T, Xu C, Hu S, Pan Y and Jin
R: miR-124 interacts with the Notch1 signalling pathway and has
therapeutic potential against gastric cancer. J Cell Mol Med.
20:313–322. 2016. View Article : Google Scholar
|
|
17
|
Wu H and Zhang J: miR-124 rs531564
polymorphism influences genetic susceptibility to cervical cancer.
Int J Clin Exp Med. 7:5847–5851. 2014.
|
|
18
|
Wen SY, Lin Y, Yu YQ, Cao SJ, Zhang R,
Yang XM, Li J, Zhang YL, Wang YH, Ma MZ, et al: miR-506 acts as a
tumor suppressor by directly targeting the hedgehog pathway
transcription factor Gli3 in human cervical cancer. Oncogene.
34:717–725. 2015. View Article : Google Scholar
|
|
19
|
Zhang H, Wang Q, Zhao Q and Di W: MiR-124
inhibits the migration and invasion of ovarian cancer cells by
targeting SphK1. J Ovarian Res. 6:842013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Peng XH, Huang HR, Lu J, Liu X, Zhao FP,
Zhang B, Lin SX, Wang L, Chen HH, Xu X, et al: MiR-124 suppresses
tumor growth and metastasis by targeting Foxq1 in nasopharyngeal
carcinoma. Mol Cancer. 13:1862014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li C, Wu X, Zhang W, Li J, Liu H, Hao M,
Wang J, Zhang H, Yang G, Hao M, et al: AEG-1 promotes metastasis
through downstream AKR1C2 and NF1 in liver cancer. Oncol Res.
22:203–211. 2014. View Article : Google Scholar
|
|
22
|
Huang Y and Li LP: Progress of cancer
research on astrocyte elevated gene-1/Metadherin (Review). Oncol
Lett. 8:493–501. 2014.PubMed/NCBI
|
|
23
|
Wang M, Wang J, Deng J, Li X, Long W and
Chang Y: MiR-145 acts as a metastasis suppressor by targeting
metadherin in lung cancer. Med Oncol. 32:3442015. View Article : Google Scholar
|
|
24
|
Wang B, Shen ZL, Jiang KW, Zhao G, Wang
CY, Yan YC, Yang Y, Zhang JZ, Shen C, Gao ZD, et al: MicroRNA-217
functions as a prognosis predictor and inhibits colorectal cancer
cell proliferation and invasion via an AEG-1 dependent mechanism.
BMC Cancer. 15:4372015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu JQ, Zhou Q, Zhu H, Zheng FY and Chen
ZW: Overexpression of astrocyte elevated gene-1 (AEG-1) in cervical
cancer and its correlation with angiogenesis. Asian Pac J Cancer
Prev. 16:2277–2281. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ji Q, Chang L, Stanczyk FZ, Ookhtens M,
Sherrod A and Stolz A: Impaired dihydrotestosterone catabolism in
human prostate cancer: Critical role of AKR1C2 as a pre-receptor
regulator of androgen receptor signaling. Cancer Res. 67:1361–1369.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Baek ST and Tallquist MD: Nf1 limits
epicardial derivative expansion by regulating epithelial to
mesenchymal transition and proliferation. Development.
139:2040–2049. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zoni E, van der Pluijm G, Gray PC and
Kruithof-de Julio M: Epithelial plasticity in cancer: Unmasking a
microRNA network for TGF-β-, Notch-, and Wnt-mediated EMT. J Oncol.
2015:1989672015. View Article : Google Scholar
|
|
29
|
Lee SG, Kang DC, DeSalle R, Sarkar D and
Fisher PB: AEG-1/MTDH/LYRIC, the beginning: Initial cloning,
structure, expression profile, and regulation of expression. Adv
Cancer Res. 120:1–38. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang J, Zhu D, Lv Q, Yi Y, Li F and Zhang
W: The key role of astrocyte elevated gene-1 in CCR6-induced EMT in
cervical cancer. Tumour Biol. 36:9763–9767. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Song E, Yu W and Xiong X, Kuang X, Ai Y
and Xiong X: Astrocyte elevated gene-1 promotes progression of
cervical squamous cell carcinoma by inducing epithelial-mesenchymal
transition via Wnt signaling. Int J Gynecol Cancer. 25:345–355.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang C, Hu Y, Wan J and He H:
MicroRNA-124 suppresses the migration and invasion of osteosarcoma
cells via targeting ROR2-mediated non-canonical Wnt signaling.
Oncol Rep. 34:2195–2201. 2015.PubMed/NCBI
|