Introduction
Normal growth and differentiation of the breast are
largely known to be under endocrine control. The sex steroid
hormone, estrogen, participates in the growth of normal breast
tissue (1). Estrogen exerts its
biological effects through two different pathways. On the one hand,
estrogen could combine to the classical estrogen receptors (ERα and
ERβ) to regulate target gene expression for the downstream cascades
(2). In addition, estrogen could
bind to the G protein-coupled estrogen receptor 30 (GPER1/GPR30) on
the membrane with high affinity to mediate non-genomic responses
(3). The imbalance of estrogen
results in the occurrence of breast cancer (4). Breast cancer is one of the most common
female cancers (5), and several
studies have shown the close relationship between
estrogen-activated non-genomic signaling and breast cancer. The
membrane receptor GPR30 is widely expressed in breast cancer lines
and breast primary tumors (6), and
it is involved in estrogen-induced cell proliferation and migration
(7).
Estrogen-induced non-genomic responses include
cyclic-AMP (cAMP) generation, intracellular calcium mobilization
and mitogen-activated protein kinase (MAPK). Ca2+
homeostasis is linked with the modulation of Ca2+,
Ca2+ channels and Ca2+-related proteins. In
vascular smooth muscle cells, L-type Ca2+ channel
blocker reduced intracellular Ca2+ induced by
17β-estradiol through GPR30 (8).
17β-estradiol-induced Ca2+ influx via L-type calcium
channels activates the Src/ERK/cAMP signal pathway in rat neurons
(9). These findings show that
Ca2+ channels have attracted interest, particularly
L-type Ca2+ channels in estrogen signaling pathway. The
L-type Ca2+ channel α 1D subunit Cav1.3 was identified
and belongs to voltage-gated Ca2+ channels. Cav1.3 is
highly expressed in prostate cancer tissues, and induces
androgen-mediated cell growth mediated (10). In endometrial cancer cells, estrogen
regulates Cav1.3 expression through GPR30, and initiates calcium
influx via Cav1.3 to promote cell proliferation and migration by
activating the ERK1/2/CREB pathway (10). However, the function of Cav1.3 in
breast cancer is not clear. In the present study, we investigated
the mechanism by which estrogen regulates Cav1.3 expression and
indicated the roles of Cav1.3 in breast cancer.
Ca2+ channels are important therapeutic
targets. Gene therapy using RNA interference can be directed
against tumors, however, the enhancement of the transfection
efficiency and specificity would be an important step for small
interfering RNA (siRNA) delivery. To date ultrasound-targeted
microbubble destruction (UTMD) is shown to be a valid method for
gene transfection (11). In the
present study, we investigated the effect of UTMD of Cav1.3 siRNA
on tumor size and survival rate in breast cancer.
In the present study, we found that estrogen
upregulated Cav1.3 expression in dosage- and time-dependent manner.
Estrogen induced the calcium influx through GPR30 and Cav1.3. The
increase of intracellular Ca2+ promoted the expression
of p-ERK1/2 to enhance cell proliferation. UTMD of Cav1.3 siRNA
resulted in the inhibition of tumor volume and the increase of
survival rate.
Materials and methods
Cell culture and transfections
Human breast cancer cell line MCF-7 was obtained
from laboratory stocks and cultured in Dulbecco's modified Eagle's
medium (DMEM), supplemented with 10% fetal bovine serum (both from
Gibco-Invitrogen, Carlsbad, CA, USA) at 37°C in humidified
incubator with 5% CO2. The siRNA sequences against
Cav1.3, GPR30 and GFP were commercially obtained (HanBio, Shanghai,
China).
MCF-7 cells were seeded into 6-well plates at 50%
confluency. Cav1.3 siRNA, GPR30 siRNA or GFP siRNA (Santa Cruz
Biotechnology, Santa Cruz, CA, USA) was diluted into 250 µl
Opti-MEM medium at the concentration of 50 nM, and 5 µl
Lipofectamine 2000 (Invitrogen) was added into 250 µl
Opti-MEM medium at room temperature. After 10 min, diluted siRNA
and Lipofectamine 2000 were mixed well for another 10 min, and then
dispensed into plates. Fresh medium was added 6 h after
transfection, and experiments were conducted for 48 h after
transfection.
Western blotting
Proteins were isolated from the cells administered
different treatments. Thirty micrograms protein was subjected to
SDS-PAGE and transfected into polyvinylidene fluoride (PVDF)
membranes (Millipore, Bedford, MA, USA). The membranes were blocked
in 2% non-fat milk in Trisbuffered saline (TBS) (20 mM Tris and 140
mM NaCl; pH 7.5) at room temperature, rinsed three times with TBS +
0.2% Tween-20 (TBST), then incubated with Cav1.3 (1:1,000 dilution)
or phospho-ERK1/2 (1:1,000 dilution) antibody overnight at 4°C,
followed by horseradish peroxidase-conjugated secondary antibody.
The ECL substrate was used to detect their expression. The band
intensities were determined using the Bio-Rad imaging system
(Hercules, CA, USA).
Calcium measurements
MCF-7 cells were seeded in glass bottom fluorescence
measurement dishes (Corning, Corning, NY, USA), and then pretreated
with Cav1.3 siRNA, GPR30 siRNA or GFP siRNA for 48 h, respectively.
After being loaded with 10 µM Fluo-3/AM (Invitrogen), cells
were bathed in medium until treatment with E2 for 15 min. The
concentration of intracellular calcium was measured using a
confocal laser scanning microscope Carl Zeiss LSM 700 (Thornwood,
NY, USA). Data were analyzed using the Image-Pro Plus software.
5-Ethynyl-2′-deoxyuridine (EdU)
detection
Cell proliferation was measured by EdU assay kit
(Invitrogen). Cells were seeded into 96-well plates and transfected
with Cav1.3 siRNA or GFP siRNA, respectively. After 48 h, cells
were exposed to 50 µM of EdU for 4 h at 37°C and fixed in 4%
paraformaldehyde for 10 min at room temperature. After being washed
with a phosphate-buffered saline (PBS; 140 mM NaCl, 2.7 mM KCl, 10
mM Na2HPO4 and 1.8 mM
KH2PO4) and permeabilized with 0.2% Triton
X-100 in PBS at 37°C for 30 min at room temperature. After washing
with PBS twice for 5 min, cells were reacted with 100 µl of
1X Apollo reaction cocktail for 30 min, and were stained with 100
µl of Hoechst 33342 (5 µg/ml) for 30 min. The nuclear
was stained with 4′,6-diamidino-2-phenylindole (DAPI) (10 mg/ml)
and visualized under a fluorescent microscope.
siRNA-microbubble preparation
Cationic lipid microbubbles were prepared by
sonicating an aqueous dispersion of 1 mg/ml polyethylene glycol
2000 stearate (PEG2000), 2 mg/ml distearoylphophatidyl choline
(DSPC) and 0.4 mg/ml 1,2-distearoyl-3-trimethyl ammonium propane
(DOTAP) (all from Avanti, Germany) with perfluoropropane gas
(12). Cav1.3 siRNA was added into
cationic lipid microbubbles, and the mixture was incubated on a
flat rocker to facilitate siRNA-microbubble interaction for 30 min.
MCF-7 cells were transfected with Cav1.3 siRNA-microbubbles in
combination with ultrasound.
Tumor xenografting and ultrasound
Female athymic BALB/c nude mice (4–6 weeks old) were
purchased from Shanghai Experimental Animal Centre, Chinese Academy
of Science. For investigation in vivo, breast tumor
xenografts were obtained by subcutaneously injecting
4×106 MCF-7 cells suspended in 0.2 ml 0.9% NaCl into the
nude mice. Once palpable tumors were established and reached 190
mm3, the mice were divided into groups for different
treatments. G1, the group injected with 0.2 ml 0.9% NaCl; G2, the
group injected with GFP siRNA; G3, the group injected with Cav1.3
siRNA; G4, the group injected with Cav1.3 siRNA-microbubbles. After
being injected, mice were treated with ultrasound for 20 min. A
single-element transducer with a 1/2-inch diameter aperture with 1
MHz ultrasound was used in the experiments. An acoustic pressure of
1 MPa at the focus with a 50% duty cycle and a sonication intensity
of 0.9w/cm2 were employed. The animal study was approved
by the Ethics Committee of Shandong University.
Statistical analysis
The results are expressed as mean ± SD. Means of
different treatment groups were tested for statistical difference
compared to the untreated control group using a Student's t-test
and considered significantly different at p<0.05. Statistical
analysis was performed using Prism 5 (GraphPad Software, La Jolla,
CA, USA).
Results
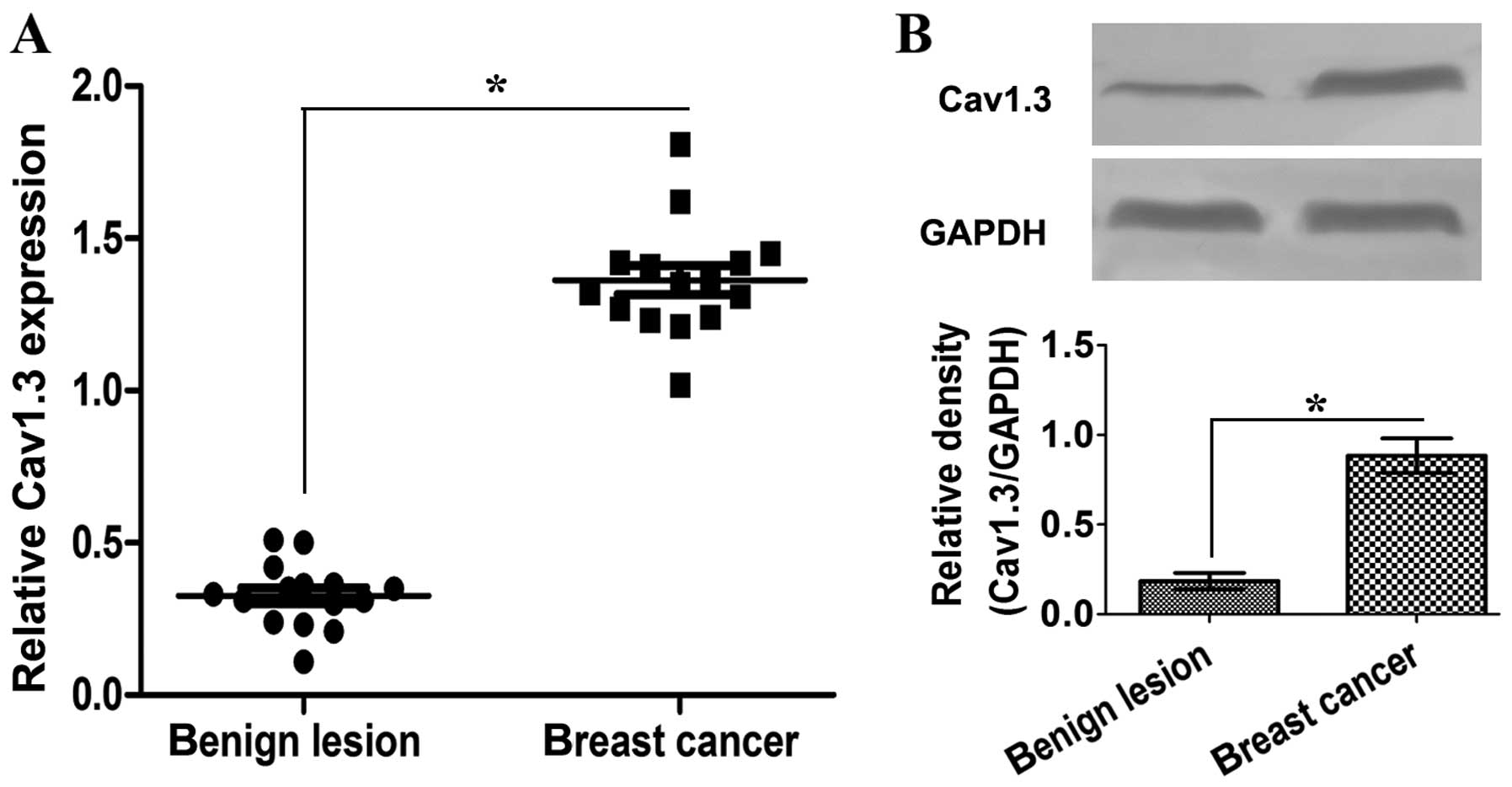
Cav1.3 is highly expressed in breast
cancer tissues
It has been reported that Cav1.3 functions in tumor
development (10,13). We analyzed the expression of Cav1.3
in benign lesion breast and breast cancer tissues by qRT-PCR and
western blotting. The mRNA level of Cav1.3 in breast cancer tissues
was significantly higher than that in benign lesion breast tissues
(Fig. 1A). Similar results were
seen in the protein level (Fig.
1B). These results show that Cav1.3 may play a role in breast
cancer development.
E2 upregulates Cav1.3 expression through
GPR30 in MCF-7 cells
The progression of breast cancer is reported to be
associated with estrogen (14). In
order to examine the effect of estrogen on Cav1.3 expression, we
treated MCF-7 cells at different concentration of E2 and analyzed
the protein level of Cav1.3. E2 upregulated Cav1.3 expression
gradually in dosage-dependent manner (Fig. 2A). A time-course experiment was
performed for 0–60 min with 1 µM E2 chosen for subsequent
analysis. Concerning the expression of Cav1.3, a distinct response
was observed within 30 min (Fig.
2B). These results revealed that E2 may upregulate Cav1.3
expression through the membrane non-genomic pathway. We found GPCR
antagonist PTX, but not ER antagonist ICI 182780 inhibited Cav1.3
expression in MCF-7 cells (Fig.
2C), showing that some GPCR is involved in estrogen-induced
Cav1.3 expression. In endometrial cancer HEC-1A cells, estrogen
modulated Cav1.3 expression rapidly through GPR30 (13). To confirm the function of GPR30, we
knocked down GPR30 and examined the protein level of Cav1.3 after
E2 induction. The silencing of GPR30 suppressed the expression of
Cav1.3 (Fig. 2D). These results
show that E2 upregulates Cav1.3 expression through GPR30 on the
membrane in MCF-7 cells.
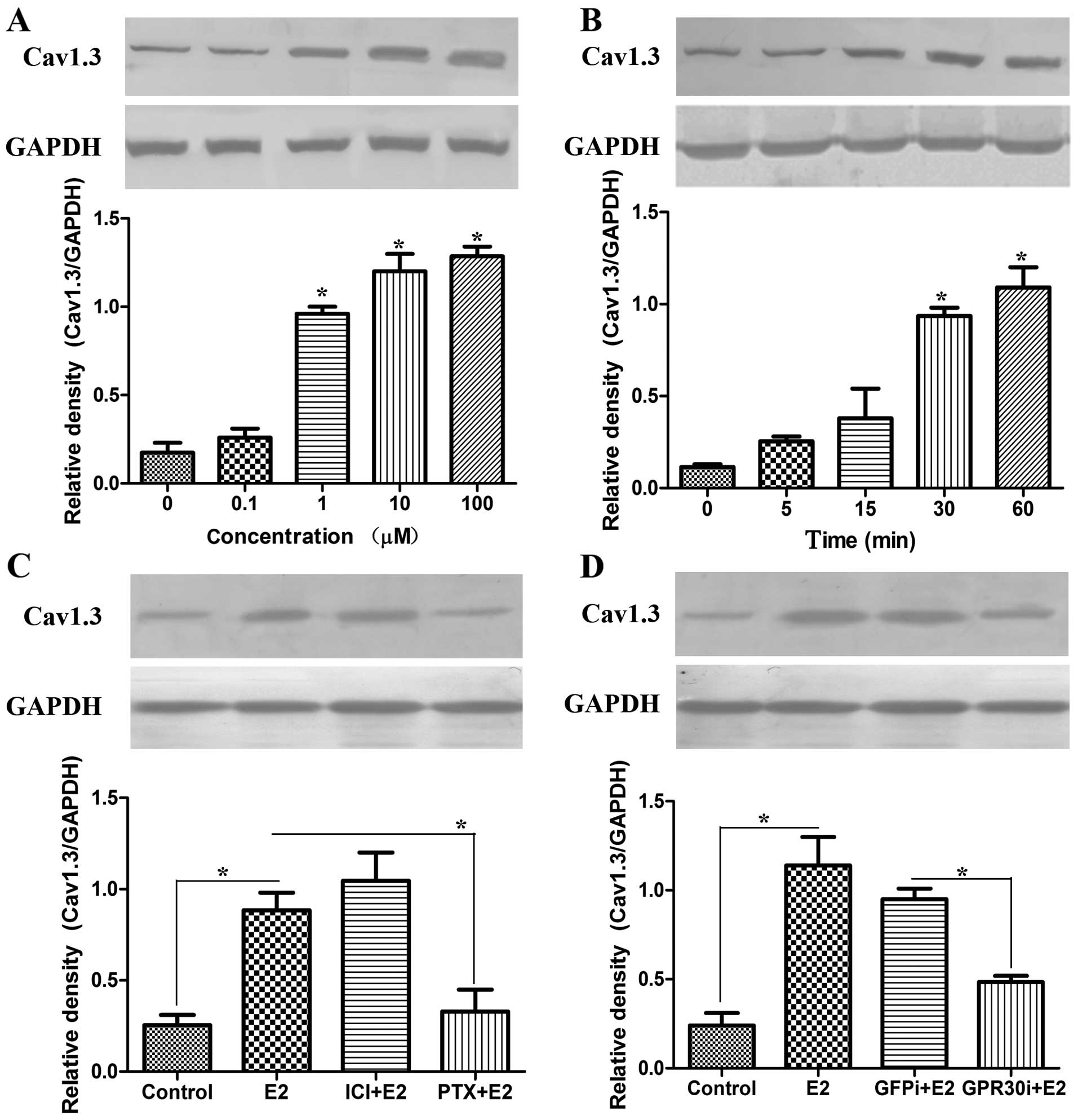 | Figure 2E2 upregulates the expression of
Cav1.3 through GPR30 in MCF-7 cells. (A) E2 upregulated Cav1.3
expression in dosage-dependent manner analyzed by western blotting.
MCF-7 cells were treated with E2 at the concentration of 0, 0.1, 1,
10 and 100 µM for 1 h, respectively. (B) Cav1.3 expression
rapidly responded to E2 within 30 min. MCF-7 cells were incubated
with 1 µM E2 for 0, 5, 15, 30 and 60 min, respectively. The
proteins were extracted for western blotting with Cav1.3 antibody.
GAPDH was used as the internal control; *p<0.05. (C)
The GPCR antagonist PTX suppressed the expression of Cav1.3
upregulated by E2. MCF-7 cells were pretreated with ER antagonist
ICI 182780 (1 µM) or GPCR antagonist PTX (200 ng/ml),
respectively. After 30 min, cells were incubated with E2 at 1
µM for 30 min. Proteins were extracted for western blotting.
(D) GPR30 siRNA inhibited the induction of Cav1.3 by E2. MCF-7
cells were transfected with GFP siRNA or GPR30 siRNA for 48 h,
respectively. After 1 µM E2 incubation, proteins of
different treatments were extracted for western blotting. GAPDH was
used as the interval control; *p<0.05. |
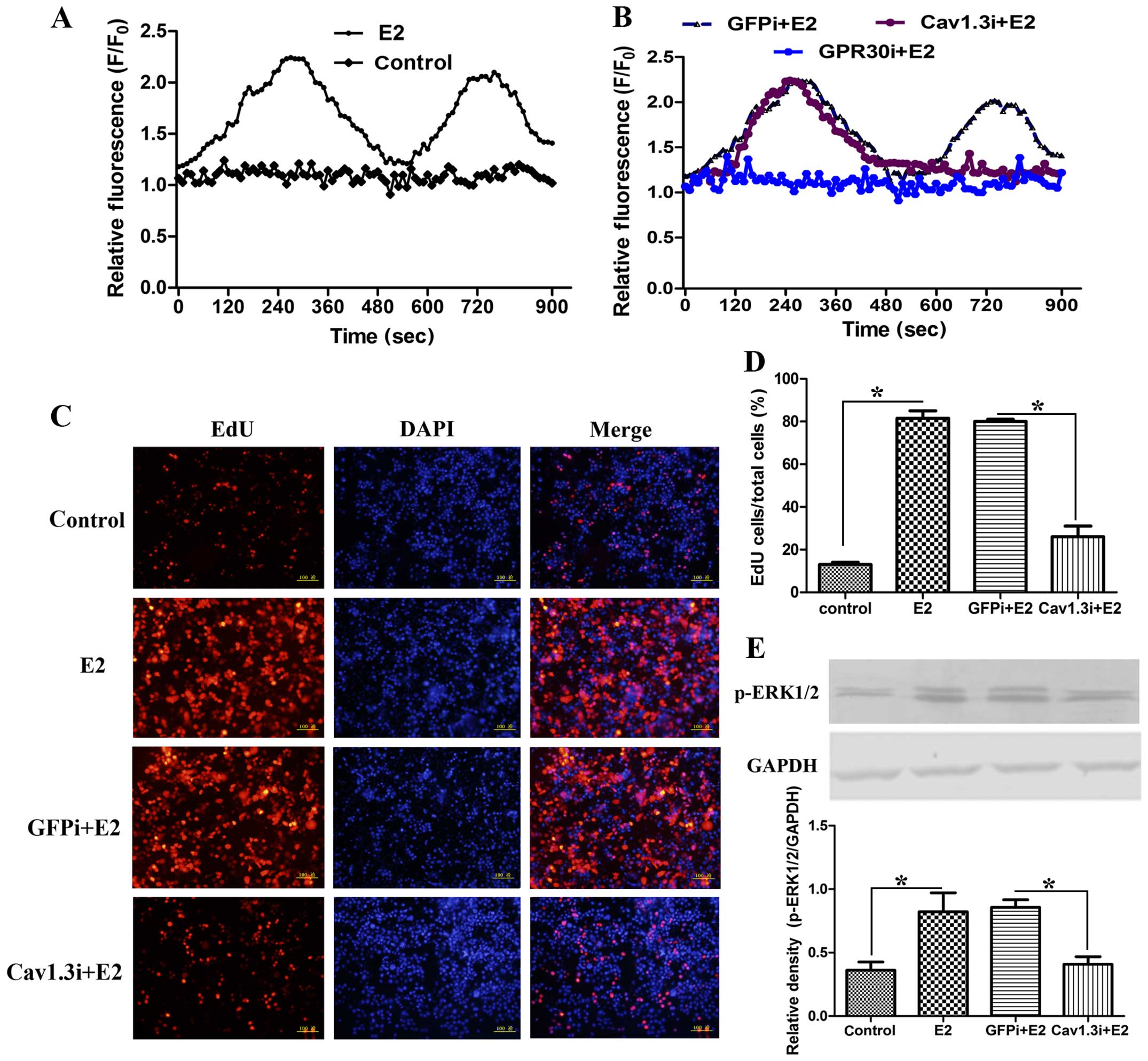
Estrogen modulates calcium mobilization
and activated p-ERK1/2 expression via Cav1.3 to promote cell
proliferation
In MCF-7 cells, we found estrogen stimulated the
increase of intracellular Ca2+ signal. After E2
induction, the Ca2+ level showed two peaks. The first
peak appeared at 280 sec, and the second at 720 sec (Fig. 3A). When GPR30 was blocked, the two
waves were inhibited (Fig. 3B). In
order to investigate the effect of Cav1.3 on Ca2+
signal, we knocked down Cav1.3. The silencing of Cav1.3
significantly affected E2-induced second peak (Fig. 3B), showing that Cav1.3 is involved
in E2-stimulated Ca2+ influx. Various studies have shown
that the Ca2+ signal is closely related to cell
proliferation (15). Cell
proliferation was detected by EdU staining to examine the role of
Cav1.3. The silencing of Cav1.3 significantly blocked cell
proliferation (Fig. 3C and D) and
the expression of p-ERK1/2 (Fig.
3E). These results revealed that E2 induces Ca2+
influx via Cav1.3 to increase the expression of p-ERK1/2 and
promote cell proliferation.
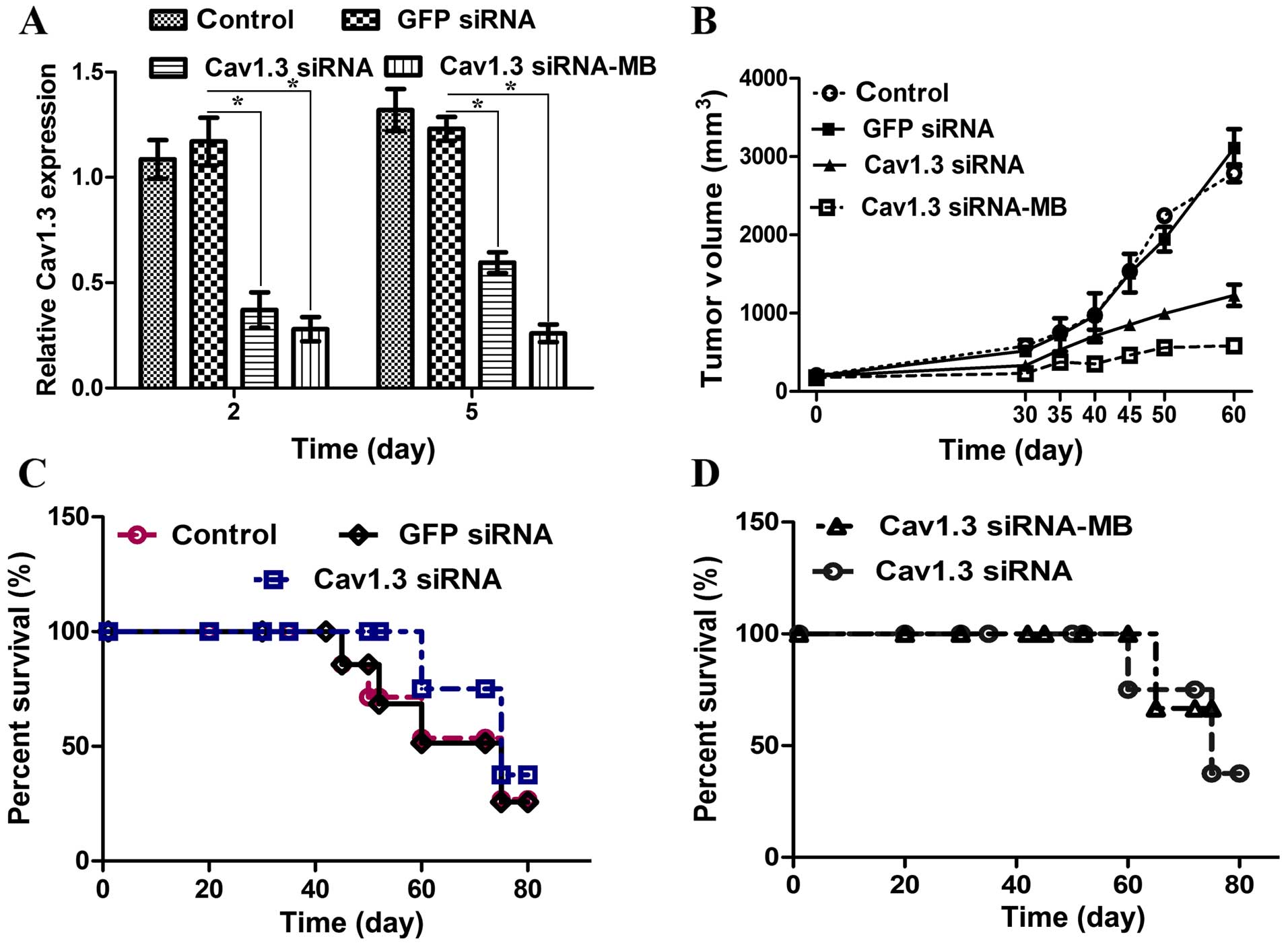
Cav1.3 siRNA-microbubble with ultrasound
improves the interference efficiency
In order to determine whether or not UTMD is a good
method for siRNA delivery, UTMD of Cav1.3 siRNA was used in MCF-7
cells in vitro and in mice in vivo. The time of
Cav1.3 silencing by UTMD of Cav1.3 siRNA was longer than that only
by Cav1.3 siRNA tranfection (Fig.
4A). Cav1.3 siRNA injection diminished the tumor volume.
Moreover, the tumor size in the mice treated with Cav1.3
siRNA-microbubbles was smaller than that in the Cav1.3 siRNA
injected mice (Fig. 4B). The Cav1.3
siRNA delivery increased the survival rate and delayed the day of
first death (Fig. 4C). Compared
with the Cav1.3 siRNA injection, the effect of Cav1.3
siRNA-microbubble delivery on tumor growth and survival rate was
more significant (Fig. 4D). These
results reveal that UTMD of Cav1.3 enhances the efficiency of RNA
interference. The signaling pathway initiated by estrogen in MCF-7
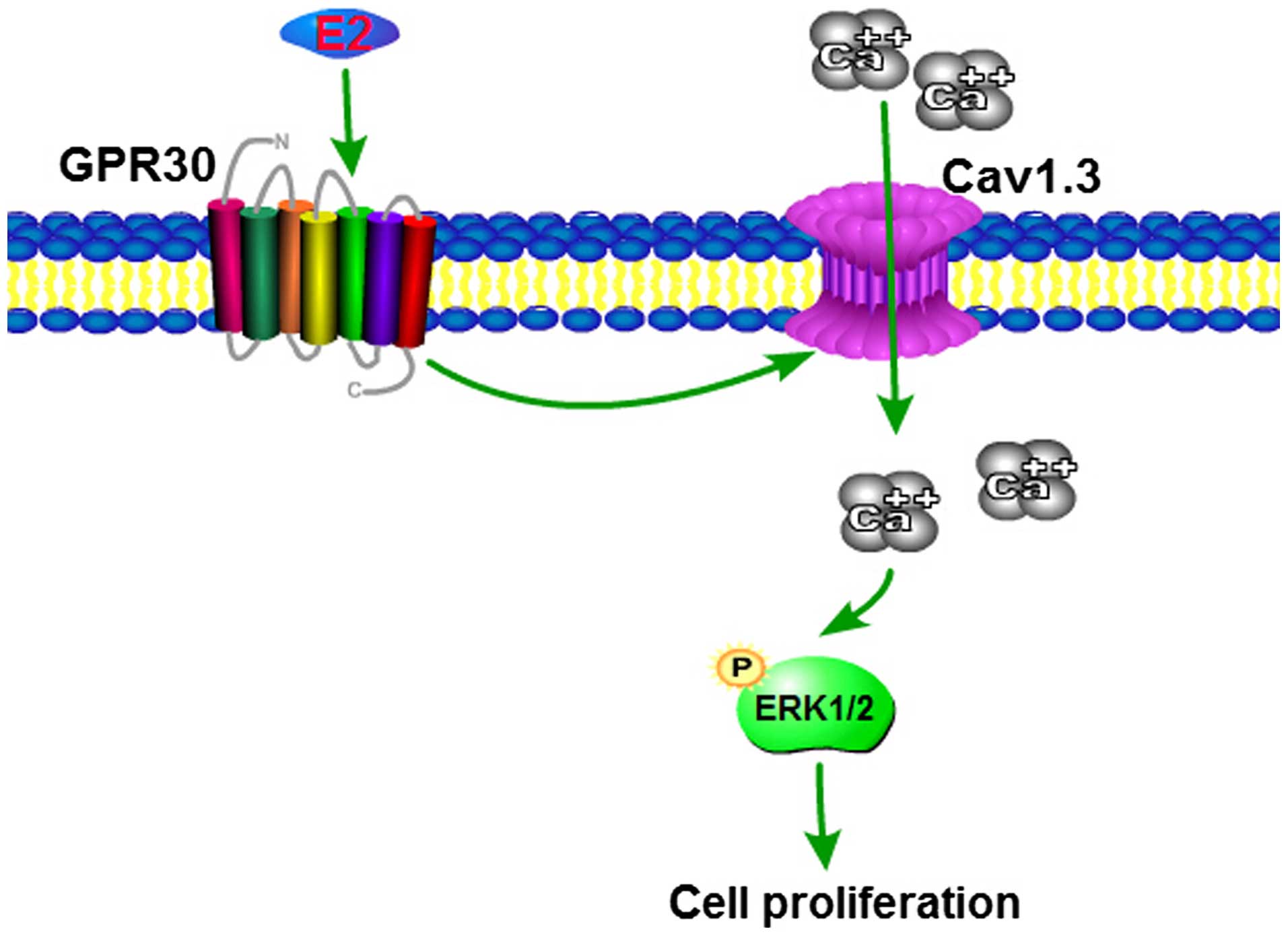
cells is shown in Fig. 5.
Discussion
E2 rapidly upregulates Cav1.3 expression
through GPR30 in MCF-7 cells
Cav1.3, as a type of L-type Ca2+ channel,
exists in many tissues, including brain, nervous system and kidney.
Although Cav1.3 is overexpressed in endometrial (13) and prostate cancers (10), the relationship between Cav1.3 and
breast cancer is not clear. In the present study, Cav1.3 was highly
expressed in the breast cancer tissues, revealing Cav1.3 functions
in tumor development. E2 rapidly stimulated Cav1.3 expression
within 30 min in MCF-7 cells. The ER antagonist ICI 182780 did not
inhibit the upregulation of Cav1.3 induced by E2, but GPCR
antagonist PTX did, indicating that E2 modulated the Cav1.3
expression ER-independently. It has been shown that E2-induced
non-genomic pathway via the membrane receptor GPR30 is associated
with tumor development (16).
17β-estradiol induced migration, adhesion and invasion of breast
cancer through GPR30 (17). The
expression of GPR30 is prognostic in primary breast cancer
(18). When GPR30 was knocked down,
the activation of Cav1.3 was blocked, showing that E2 upregulated
Cav1.3 expression via GPR30. These results indicated the function
of Cav1.3 in breast cancer and showed a new mechanism by which
estrogen regulated Cav1.3 expression.
Ca2+-mediated signaling plays roles in
various cellular processes, including cancer initiation, tumor
progression and invasion (19). E2
could either activate (20) or
inhibit (21) Ca2+
influx via Ca2+ channels (22). In the present study, E2 induced two
peak of Ca2+ at 280 and 720 sec in MCF-7 cells. When
Cav1.3 was silenced, the second wave was blocked, revealing that E2
induced Ca2+ flux through Cav1.3. Transfection of Cav1.3
siRNA suppressed the cell proliferation of MCF-7 cells and the
expression of p-ERK1/2. It has been shown that E2 activated the
ERK1/2/CREB signaling by the rapid Ca2+ influx via
L-type Ca2+ channel (9),
which provided a link between Ca2+ flux and gene
transcription. In endometrial cancer, Cav1.3 was necessary for the
expression of p-ERK1/2 and CREB (13). In addition, in MCF-7 cells, E2
induced Ca2+ influx via Cav1.3 to activate the
expression of p-EKR1/2 to promote the cell proliferation. Taken
together, these findings showed the mechanism of Cav1.3 involving
in the progression of breast cancer.
Cav1.3 siRNA-MB delivered by ultrasound
suppresses the tumor growth and improves the survival rate
The above results have revealed that Cav1.3 may be a
new target for the treatment of breast cancer. SiRNAs have become
useful tools to inactivate target gene expression, however the
efforts on the delivery efficacy and specificity in the clinic need
further attention (23). Various
studies have shown that UTMD could be a powerful technology for
gene therapy (24), including
plasmid (25), siRNA (26) and different drugs delivery (27). In the present study, we found that
the UTMD of Cav1.3 siRNA prolonged the time of Cav1.3 silencing
than Cav1.3 siRNA, revealing that the microbubble of Cav1.3 siRNA
maintained the stability of siRNA. In vitro, the tumor
volume of the mice treated with the UTMD of Cav1.3 siRNA was
significantly reduced, and the first death day of these mice was
obviously delayed. Therefore, Cav1.3 siRNA in combination with UTMD
enhanced the efficacy of gene therapy, and UTMD is a promising
method for breast cancer therapy.
In conclusion, in the present study, Cav1.3 was
highly expressed in breast cancer. E2 activated the expression of
Cav1.3 via the membrane receptor GPR30 in MCF-7 cells. Moreover, E2
induced Ca2+ influx through Cav1.3 to activate the
expression of p-ERK1/2 for cell proliferation. These results
revealed the biological basis of E2-inducing Cav1.3 expression and
the function of Cav1.3 in breast cancer. We employed the UTMD
method to deliver Cav1.3 siRNA into cells and mice to show that
UTMD of Cav1.3 siRNA is a useful tool for breast cancer
therapy.
References
|
1
|
Pelekanou V and Leclercq G: Recent
insights into the effect of natural and environmental estrogens on
mammary development and carcinogenesis. Int J Dev Biol. 55:869–878.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gibson DA and Saunders PT: Estrogen
dependent signaling in reproductive tissues - a role for estrogen
receptors and estrogen related receptors. Mol Cell Endocrinol.
348:361–372. 2012. View Article : Google Scholar
|
|
3
|
Revankar CM, Cimino DF, Sklar LA,
Arterburn JB and Prossnitz ER: A transmembrane intracellular
estrogen receptor mediates rapid cell signaling. Science.
307:1625–1630. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gompel A and Santen RJ: Hormone therapy
and breast cancer risk 10 years after the WHI. Climacteric.
15:241–249. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: Defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Scaling AL, Prossnitz ER and Hathaway HJ:
GPER mediates estrogen-induced signaling and proliferation in human
breast epithelial cells and normal and malignant breast. Horm
Cancer. 5:146–160. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pandey DP, Lappano R, Albanito L, Madeo A,
Maggiolini M and Picard D: Estrogenic GPR30 signalling induces
proliferation and migration of breast cancer cells through CTGF.
EMBO J. 28:523–532. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Holm A, Hellstrand P, Olde B, Svensson D,
Leeb-Lundberg LM and Nilsson BO: The G protein-coupled estrogen
receptor 1 (GPER1/GPR30) agonist G-1 regulates vascular smooth
muscle cell Ca2+ handling. J Vasc Res.
50:421–429. 2013. View Article : Google Scholar
|
|
9
|
Wu TW, Wang JM, Chen S and Brinton RD:
7β-estradiol induced Ca2+ influx via L-type calcium
channels activates the Src/ERK/cyclic-AMP response element binding
protein signal pathway and BCL-2 expression in rat hippocampal
neurons: a potential initiation mechanism for estrogen-induced
neuroprotection. Neuroscience. 135:59–72. 2005. View Article : Google Scholar
|
|
10
|
Chen R, Zeng X, Zhang R, Huang J, Kuang X,
Yang J, Liu J, Tawfik O, Thrasher JB and Li B: Cav 1.3 channel α1D
protein is overexpressed and modulates androgen receptor
transactivation in prostate cancers. Urologic Oncology: Seminars
and Original Investigations. 32(5)Elsevier; pp. 524–536. 2014,
View Article : Google Scholar
|
|
11
|
Carson AR, McTiernan CF, Lavery L, Grata
M, Leng X, Wang J, Chen X and Villanueva FS: Ultrasound-targeted
microbubble destruction to deliver siRNA cancer therapy. Cancer
Res. 72:6191–6199. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Leong-Poi H, Kuliszewski MA, Lekas M,
Sibbald M, Teichert-Kuliszewska K, Klibanov AL, Stewart DJ and
Lindner JR: Therapeutic arteriogenesis by ultrasound-mediated VEGF
plasmid gene delivery to chronically ischemic skeletal muscle. Circ
Res. 101:295–303. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hao J, Bao X, Jin B, Wang X, Mao Z, Li X,
Wei L, Shen D and Wang JL: Ca2+ channel subunit α 1D
promotes proliferation and migration of endometrial cancer cells
mediated by 17β-estradiol via the G protein-coupled estrogen
receptor. FASEB J. 29:2883–2893. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Clemons M and Goss P: Estrogen and the
risk of breast cancer. N Engl J Med. 344:276–285. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hao B, Webb SE, Miller AL and Yue J: The
role of Ca2+ signaling on the self-renewal and neural
differentiation of embryonic stem cells (ESCs). Cell Calcium.
59:67–74. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lappano R, Pisano A and Maggiolini M: GPER
function in breast cancer: An overview. Front Endocrinol. 5:662014.
View Article : Google Scholar
|
|
17
|
Shang D, Li Z, Zhu Z, Chen H, Zhao L, Wang
X and Chen Y: Baicalein suppresses 17-β-estradiol-induced
migration, adhesion and invasion of breast cancer cells via the G
protein-coupled receptor 30 signaling pathway. Oncol Rep.
33:2077–2085. 2015.PubMed/NCBI
|
|
18
|
Wanajo A, Sasaki A, Nagasaki H, Shimada S,
Otsubo T, Owaki S, Shimizu Y, Eishi Y, Kojima K, Nakajima Y, et al:
Methylation of the calcium channel-related gene, CACNA2D3, is
frequent and a poor prognostic factor in gastric cancer.
Gastroenterology. 135:580–590. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Prevarskaya N, Skryma R and Shuba Y:
Calcium in tumour metastasis: New roles for known actors. Nat Rev
Cancer. 11:609–618. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sarkar SN, Huang RQ, Logan SM, Yi KD,
Dillon GH and Simpkins JW: Estrogens directly potentiate neuronal
L-type Ca2+ channels. Proc Natl Acad Sci USA.
105:15148–15153. 2008. View Article : Google Scholar
|
|
21
|
Boulware MI, Weick JP, Becklund BR, Kuo
SP, Groth RD and Mermelstein PG: Estradiol activates group I and II
metabotropic glutamate receptor signaling, leading to opposing
influences on cAMP response element-binding protein. J Neurosci.
25:5066–5078. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Improta-Brears T, Whorton AR, Codazzi F,
York JD, Meyer T and McDonnell DP: Estrogen-induced activation of
mitogen-activated protein kinase requires mobilization of
intracellular calcium. Proc Natl Acad Sci USA. 96:4686–4691. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Castanotto D and Rossi JJ: The promises
and pitfalls of RNA-interference-based therapeutics. Nature.
457:426–433. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cao WJ, Rosenblat JD, Roth NC, Kuliszewski
MA, Matkar PN, Rudenko D, Liao C, Lee PJ and Leong-Poi H:
Therapeutic angiogenesis by ultrasound-mediated microRNA-126-3p
delivery. Arterioscler Thromb Vasc Biol. 35:2401–2411. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Taniyama Y, Tachibana K, Hiraoka K, Namba
T, Yamasaki K, Hashiya N, Aoki M, Ogihara T, Yasufumi K and
Morishita R: Local delivery of plasmid DNA into rat carotid artery
using ultrasound. Circulation. 105:1233–1239. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu Q, Sun T, Tian H, Wang C and Zhou H:
Ultrasound-mediated vascular endothelial growth factor C (VEGF-C)
gene microbubble transfection inhibits growth of MCF-7 breast
cancer cells. Oncol Res. 20:297–301. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Frenkel V: Ultrasound mediated delivery of
drugs and genes to solid tumors. Adv Drug Deliv Rev. 60:1193–1208.
2008. View Article : Google Scholar : PubMed/NCBI
|