Introduction
Despite the rapid development of diagnosis and
treatment in recent decades, breast cancer is still the second
leading cause of cancer death in women (1). Based on clinical, pathological, and
genetic findings, it is comprised of distinct subtypes (2), one of which is triple-negative breast
cancer (TNBC). TNBCs are distinguished by lack of estrogen receptor
(ER), progesterone receptor (PR), and amplification of the human
epidermal growth factor receptor 2 (HER2) (3), accounts for >15% of all breast
cancers (4) and are more prevalent
in young, African-American, and Latino women. Compared with other
breast cancer subtypes, TNBC patients have more aggressive clinical
course, higher rate of distant recurrence and poorer prognosis
(3,5,6). Thus,
it is essential to develop novel diagnostic and therapeutic methods
for TNBC patients.
Sentrin/SUMO-specific protease 1 (SENP1), a member
of SUMO-specific protease (SENP) family, is widely distributed in
the nuclei (7,8). SENP1 can deconjugate large numbers of
sumoylated proteins. Previously, many studies reported that SENP1
was overexpressed in human prostate cancer (CaP) cells (9), as well as lung cancer tissues
(10) and most of colon cancer
tissues (11). The expression of
SENP1 was reported to have strong prognostic impact on a
molecularly defined subset of CaPs (12). Another study revealed that SENP1
might be a prognostic marker and a therapeutic target for
metastasis in CaP patients, for its roles in regulating MMP2 and
MMP9 through the HIF-1α signaling pathway (13). Additionally, SENP1 3′-untranslated
region was demonstrated as a regulative target of miR-145, which
plays an important role in tumorigenesis of CaP (14). It was reported that the
miR-138/SENP1 cascade was relative to radiosensitization in lung
cancer cells (15). Silencing of
SENP1 expression results in upregulation of cyclin-dependent kinase
(CDK) inhibitors such as p16, p19, p21 and p27, further inhibits
cell growth and colony formation in colon cancer cell line,
suggesting that SENP1 is essential for cell growth in the colon
cancer cell line (11). Moreover,
clinical data showed that SENP1 was positively associated with
lymph node metastasis and TNM stage of pancreatic cancer (16). In Burkitt's lymphoma,
down-regulation of SENP1 expression can reduce the stability of
HIF-1α protein and induce the apoptosis of Daudi cells (17). Since the important roles of SENP1 in
various cancers, the expression level of SENP1 and whether it is
involved in TNBC still requires investigation.
In this study, we firstly investigated the
expression of SENP1 in the clinical samples of TNBC and non-TNBC
patients by using qRT-PCR, western blotting, and
immunohistochemical staining. Our results indicated that SENP1 was
highly expressed in TNBC tissues compared with normal breast
tissues, as well as non-TNBC tissues. To further investigate the
roles of SENP1 in TNBC, we constructed si-SENP1-transfected TNBC
cell lines, followed by biological function detection. Furthermore,
we explored the mechanisms behind the effects of SENP1 in TNBC
cells. Additionally, to explore the effects of SENP1 on TNBC
tumorigenesis and development, si-SENP1-transfected TNBC cells were
transplanted into nude mice to establish TNBC mouse xenograft
model. Our research demonstrated the high expression of SENP1 in
TNBC cell lines and the negative effect of si-SENP1 on
proliferation, invasion, migration, and colony formation ability of
TNBC cell lines via blocking of cell cycle and regulation of p21,
p27, and MMP9, as well as the suppression effect of SENP1
deficiency on tumor volume, proliferation, and lung metastasis in
TNBC tumor-bearing mice. The results of this study revealed the
inhibition effect of SENP1 deficiency on tumor proliferation and
migration both in vitro and in vivo, indicating the
potential roles of SENP1 to promote tumorigenesis and metastasis in
TNBC.
Materials and methods
Ethics statement
The protocols employed in this study and the use of
human tissues were approved by the Ethics Committee of Fudan
University Shanghai Cancer Center and conducted in full accordance
with ethical principles, including the World Medical Association
Declaration of Helsinki, and the local legislation. All of the
experiments were undertaken with the understanding and written
consent of each subject according to the above mentioned
principles.
Tissue specimens and
immunohistochemistry
Two independent cohorts totaling 82 cases of breast
cancer patients were obtained from 2014 to 2015 at Fudan University
Shanghai Cancer Center (non-TNBC, n=42; TNBC, n=40). The matched
adjacent tissues were obtained from the tissues that were located 5
cm away from the tumor margin. Immunohistochemical staining was
performed according to the manufacturer's instructions.
Paraffin-embedded TNBC and adjacent tissues were deparaffinized in
60°C, followed by treatment with xylene and graded alcohol. The
slides were blocked with 5% bovine serum albumin for 1 h, and then
incubated with rabbit anti-human SENP1 polyclonal antibody (cat.
no. ab58417) or rabbit anti-human Ki67 polyclonal antibody (both
from Abcam, Cambridge, MA, USA; cat. no. ab66155), then visualized
by the standard avidin-biotin-peroxidase complex method.
Hematoxylin was used for counterstaining. Morphologic images were
observed using Olympus fluorescence microscopy (Olympus Corp.,
Tokyo, Japan).
Cell culture
Normal breast cell lines (HMLE and MCF10A), non-TNBC
cell lines (MCF7, T47D, and ZR-75-1), and TNBC cell lines
(MDA-MB-231, MDA-MB-453, and MDA-MB-468) were purchased from ATCC
(Rockville, MD, USA) and all cell lines except HMLE were routinely
maintained in RPMI-1640 medium (Gibco, Carlsbad, CA, USA)
supplemented with 10% fetal bovine serum (FBS) (Invitrogen,
Carlsbad, CA, USA) and 50 U/ml penicillin - 50 µg/ml
streptomycin antibiotics (Gibco). HMLE cells were cultured in
DMEM/F12 containing growth factor, insulin, hydrocortisone, 100
U/ml penicillin, and 100 µg/ml streptomycin. Cell lines were
cultured in a 37°C incubator with humidified atmosphere of 5%
CO2.
Cell transfection
The retrovirus containing SENP1 siRNA or
non-specific siRNA as described previously (8) was transfected into TNBC cells to
generate si-SENP1-transfected TNBC cells (si-SENP1) or non-specific
siRNA-transfected cells (si-NS) via puromycin selection.
Real-time PCR
Total RNA was isolated by TRIzol reagent
(Invitrogen). The reverse transcription reactions were performed by
cDNA Synthesis Kit (Takara Biotechnology Co., Ltd., Dalian, China)
following the manufacturer's protocol. RT-PCR was performed using a
standard SYBR PrimeScript RT-PCR Kit (Takara Biotechnology Co.,
Ltd.) on ABI 7700 real-time PCR system (Applied Biosystems, Foster
City, CA, USA). Each sample was analyzed in triplicate and standard
deviations representing experimental errors were calculated. SENP1
forward, 5′ATCAGGCAGTGAAACGTTGGAC3′ and reverse,
5′GCAGGCTTCATTGTTTATCCCA3′; p21 forward, 5′ACCTCTCAGGGCCGAAAAC3′
and reverse, 5′TAGGGCTTCCTCTTGGAGAA3′; p27 forward,
5′CAGAGGACACACACTTGGTAGA3′ and reverse, 5′TCTTTTGTTTTGAGGAGAGGAA3′;
MMP9 forward, 5′AGTCCACCCTTGTGCTCTTCC3′ and reverse,
5′TGCCACCCGAGTGTAACCAT3′; β-actin forward,
5′CTTTTCCAGCCTTCCTTCTTGG3′ and reverse,
5′CAGCACTGTGTTGGCATAGAGG3′.
Western blotting
Cells were washed with PBS and lysed. Protein
concentration was measured by BCA assay with BCA Protein Assay Kit
(Takara Biotechnology Co., Ltd.; cat. no. T9300). The protein
extracts were equally loaded onto 10% SDS polyacrylamide gels,
electrophoresed and transferred to PVDF membranes. The membranes
were blocked in 5% fat-free milk in TBST buffer for 2 h at room
temperature. The rabbit anti-human polyclonal antibody was used in
conjunction with 0.4 µg/ml of anti-species-conjugated
horseradish peroxidase. Rabbit anti-human SENP1 polyclonal antibody
(cat. no. ab58417), rabbit anti-human p21 polyclonal antibody (cat.
no. ab7960), rabbit anti-human p27 polyclonal antibody (cat. no.
ab7961), rabbit anti-human MMP9 polyclonal antibody (cat. no.
ab38898) and rabbit anti-human β-actin polyclonal antibody (cat.
no. ab8227) were purchased from Abcam. The bands were detected by
chemiluminescent substrate kit (Millipore Corp.). The intensities
of the bands were quantified using the NIH ImageJ software
package.
Cell growth assay
Cells were seeded into 6-well plates at a density of
1×105 cells/well and cultured in the growth medium. Cell
growth was measured by MTT assay and recorded by a Benchmark
Microplate Reader (Bio-Rad Laboratories, Inc.) at 0, 2, 4 and 6
days.
Soft agar colony formation assay
Agarose (0.7%) was mixed with cells and placed above
the 1.2% agarose underlay. After 2 weeks, colonies were dyed with
crystal violet (0.01% solution). Then washed with PBS, the colonies
were imaged using a custom, automated plate imager with a digital
camera (Olympus SP-350; Olympus Corp.).
Cell cycle assay
Cells were collected and fixed using 75% ice-cold
ethanol at −20°C overnight, then cells were treated with Tris-HCl
buffer (pH 7.4) contained with 100 µg/ml RNase A and dyed
with propidium iodide (PI; 25 µg/ml). Then, cell cycle
distribution was monitored via flow cytometry. The data were
collected and analyzed using ModFit software.
Transwell assay
Cells in logarithmic growth phase were treated with
trypsin, washed with PBS and then resuspended in serum-free medium.
A total of 4×106 cells were added to the upper
compartment of the invasion chamber, and 500 µl of culture
medium were added to the lower compartment of the invasion chamber.
Each insert was precoated with 45 µg Matrigel (BD
Biosciences, San Jose, CA, USA). The Matrigel invasion chambers
were incubated at 37°C for 48 h in 5% CO2. The
non-invading cells were removed from the upper face of the membrane
and the invaded cells on the lower surface were stained with 0.05%
crystal violet for 30 min. After washed with PBS, the invaded cells
were subjected to microscopic inspection (DFC500; Leica
Microsystems GmbH, Wetzlar, Germany) according to the
manufacturer's instructions. Finally, the values for invasion were
obtained by counting three fields per membrane. Experiments were
independently repeated in triplicate.
Wound healing assay
Cells were seeded into the bottom of 6-well plates
marked a horizontal line on the back for targeting the same field
of vision. A line wound was made by scraping a 100-µl tip
across the confluent cell layer. Cells were washed three times to
remove detached cells and debris. Cells were incubated with
serum-free medium in a humidified atmosphere containing 5%
CO2 at 37°C. After cultured for 24 h, wound closure was
performed under a light microscope (DFC500; Leica Microsystems
GmbH) and measured using AxioVision version 4.7 software (Carl
Zeiss Meditec, Dublin, CA, USA). Experiments were independently
repeated in triplicate.
Xenograft tumor generation
A 50-µl suspension containing
5×106 tumor cells (si-SENP1 or si-NS cells) was injected
subcutaneously into one side of hind flanks of 6-week-old female
BALB/c nude mice. The mice were divided into two groups: si-SENP1
(n=8) and si-NS (n=8) group. After 7 days, when tumors had reached
a measurable size, tumor size was determined with a caliper and
calculated weekly on weeks 1–8. The experiment was repeated three
times. After 10 weeks, the mice were sacrificed, and the number of
nodules per lung was recorded.
Statistical analysis
The data are expressed as means ± SD and analyzed
using GraphPad Prism 5.0 software (GraphPad Software Inc.). Data
comparison between the two groups was carried out using the
two-tailed Student's t-test. For clinical data, p-value was
analyzed by Chi-square test using SPSS 17.0. P<0.05 was
considered statistically significant.
Results
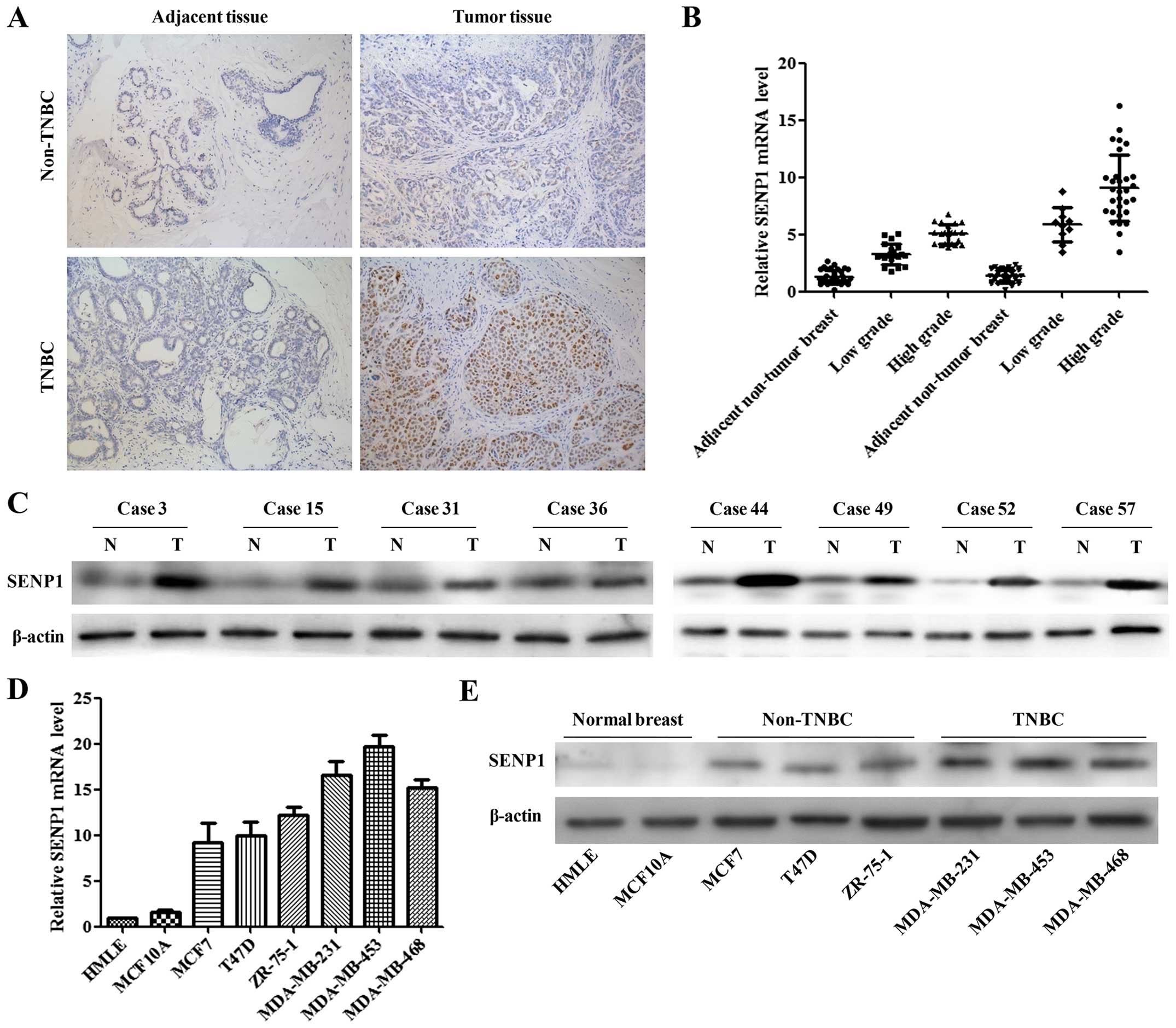
SENP1 was highly expressed in TNBC
Clinical data from TNBC and non-TNBC patients were
collected and compared. Statistical analysis of clinical data
indicated that the TNBC was significantly correlated with decreased
age (P<0.05), increased tumor differentiation grade
(P<0.001), and ductal histological type (P<0.001) (Table I). To investigate the expression
levels of SENP1 in TNBC patients, the tumor tissues and adjacent
normal breast tissues were collected from TNBC and non-TNBC
patients. Through immunohistochemical staining, qRT-PCR and western
blotting, we found that SENP1 was highly expressed in breast cancer
tissues compared with the adjacent normal tissues. Additionally, a
markedly higher level of SENP1 was observed in TNBC tissues,
compared to non-TNBC tissue (Fig.
1A–C). We then evaluated the level of SENP1 mRNA and protein
expression in normal mammary gland cell lines (HMLE and MCF10A),
breast cancer cell lines (MCF7, T47D, and ZR-75-1), as well as TNBC
cell lines (MDA-MB-231, MDA-MB-453, and MDA-MB-468). Consistent
with the tumor tissue results, both SENP1 mRNA and protein were
expressed significantly higher in MDA-MB-231 and MDA-MB-453 than
other cell lines (Fig. 1D and E).
These results suggested the potential carcinogenic roles of SENP1
in TNBC.
 | Table IRelationship between tumor
characteristics and non-triple-negative/triple-negative status. |
Table I
Relationship between tumor
characteristics and non-triple-negative/triple-negative status.
| Characteristics | Non-triple-negative n
(%) | Triple-negative n
(%) | P-value |
|---|
| Age at diagnosis
(years) | | | |
| ≤50 | 10 (24) | 16 (40) |
0.015 |
| >50 | 32 (76) | 24 (60) | |
| Tumor grade | | | |
| 1 | 2 (5) | 0 (0) | <0.001 |
| 2 | 19 (45) | 8
(20) | |
| 3 | 21 (50) | 32 (80) | |
| Histological
type | | | |
| Ductal | 29 (69) | 34 (85) | <0.001 |
| Lobular | 13 (31) | 6
(15) | |
| Tumor size
(cm) | | | |
| ≤2 | 21 (50) | 22 (55) | NS |
| >2 | 21 (50) | 18 (45) | |
| LN status | | | |
| Negative | 24 (57) | 19 (47) | NS |
| Positive | 18 (43) | 21 (53) | |
| LVI status | | | |
| Negative | 32 (76) | 26 (65) | NS |
| Positive | 10 (24) | 14 (35) | |
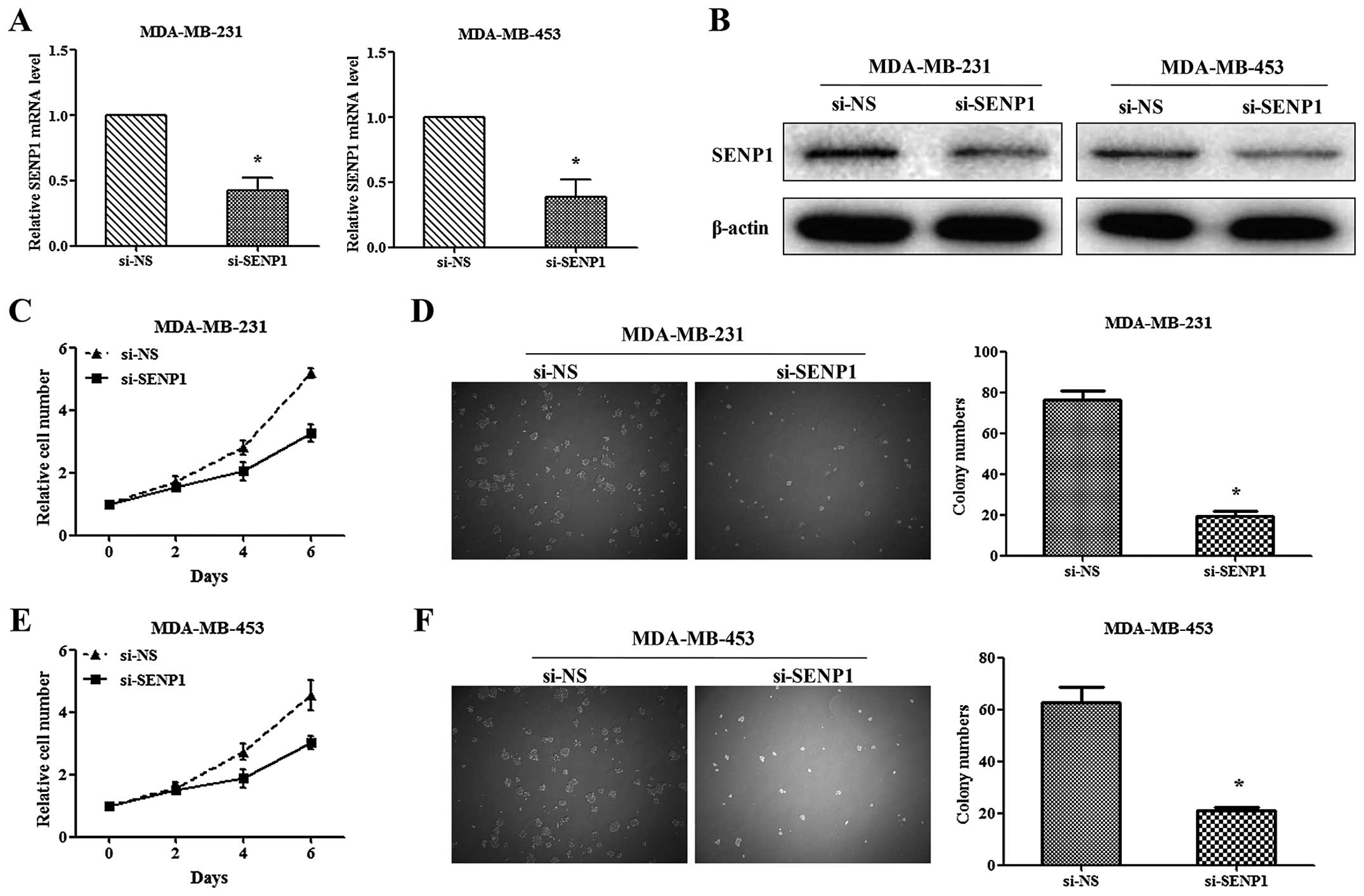
Depletion of SENP1 inhibited the
proliferation and colony formation of TNBC cells
Due to the high level of SENP1 in TNBC tumor
tissues, we subsequently investigated the function of SENP1 in TNBC
cell proliferation. Two TNBC cell lines, MDA-MB-231 and MDA-MB-453,
were transfected with si-SENP1 and non-specific RNA, as si-NS,
respectively. The transfection efficiency was evaluated via qRT-PCR
and western blotting (Fig. 2A and
B). The mRNA levels of SENP1 in si-SENP1-infected cells were
<40% compared with si-NS-infected cells. Through MTT assay, we
found that knockdown of SENP1 significantly inhibits the
proliferation of TNBC cells (Fig. 2C
and E) (5.194±0.162 vs. 3.283±0.278 in MDA-MB-231 and
4.546±0.482 vs. 3.048±0.207 in MDA-MB-453, respectively).
Consistent with the above, knockdown of SENP1 significantly
decreased TNBC cell colony numbers (Fig. 2D and F). These results indicated
that silencing of SENP1 could inhibit proliferation and colony
formation of TNBC cells in vitro.
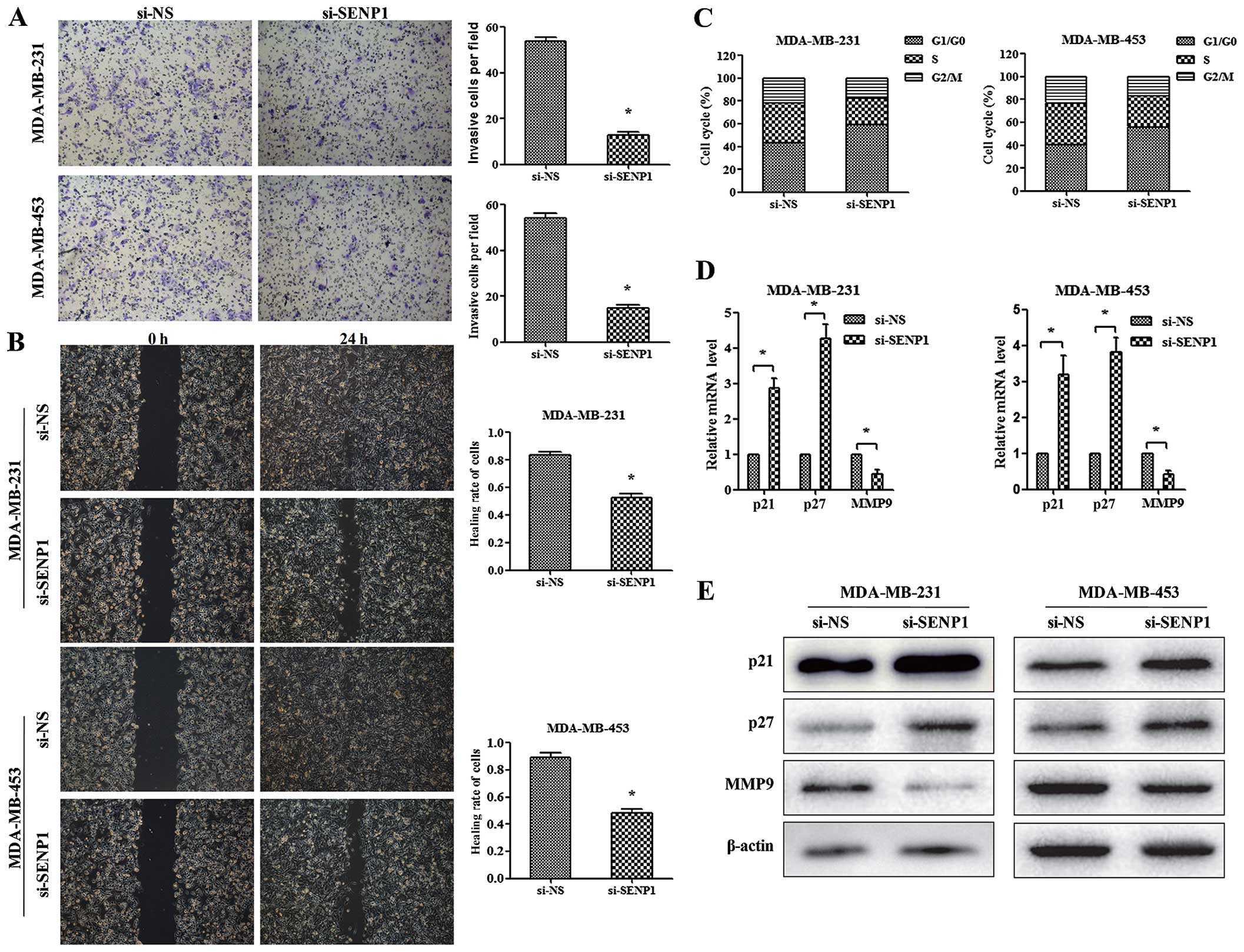
Depletion of SENP1 inhibits the invasion,
migration, and arrests TNBC cells in G1 phase of the cell
cycle
The effects of SENP1 silencing on cell invasion and
migration ability in TNBC cells were detected using Transwell and
wound healing assay. As shown in Fig.
3A, silencing of SENP1 significantly reduced the invasion
capacity of TNBC cells. Moreover, the results of wound healing
assay showed suppressed migration capacity of si-SENP1 TNBC cells
(Fig. 3B). Together, these results
revealed the impaired invasion and migration ability of TNBC cells
induced by SENP1 deficiency.
Additionally, to explore the mechanism of
SENP1-mediated functions in cell cycle control, we monitored cell
cycle synchronization of TNBC cells using FACS analysis. As shown
in Fig. 3C, si-SENP1 in both
MDA-MB-231 and MDA-MB-453 results in a higher percentage of cells
in G1/G0 phase and a concomitant lower percentage of cells in G2/M
stage and slight decrease in the percentage of cells in S phase.
The results indicated that reduction of SENP1 would give rise to
the inhibition of MDA-MB-231 and MDA-MB-453 cell proliferation
mainly through modulating cell cycle progression.
SENP1 is required for the expression of
p21, p27, and MMP9 in TNBC cells
Since that SENP-1 promotes metastasis through
upregulating MMP9 in prostate and pancreatic cancer (10,16),
as well as that p21 and p27 were important G1 CDK inhibitors in
breast cancer cell proliferation (18), the previous reports led us to
investigate the effect of SENP1 in regulating the expression of
p21, p27, and MMP9 in TNBC cells. As shown in Fig. 3D and E, the mRNA and protein levels
of p21 and p27 were significantly increased by SENP1 knockdown.
However, the expression of MMP9 was decreased in the
SENP1-depleting cells. These results indicated that SENP1 regulates
cell proliferation of TNBC through controlling the expression of
p21 and p27, and modulates cell invasion by altering the level of
MMP9.
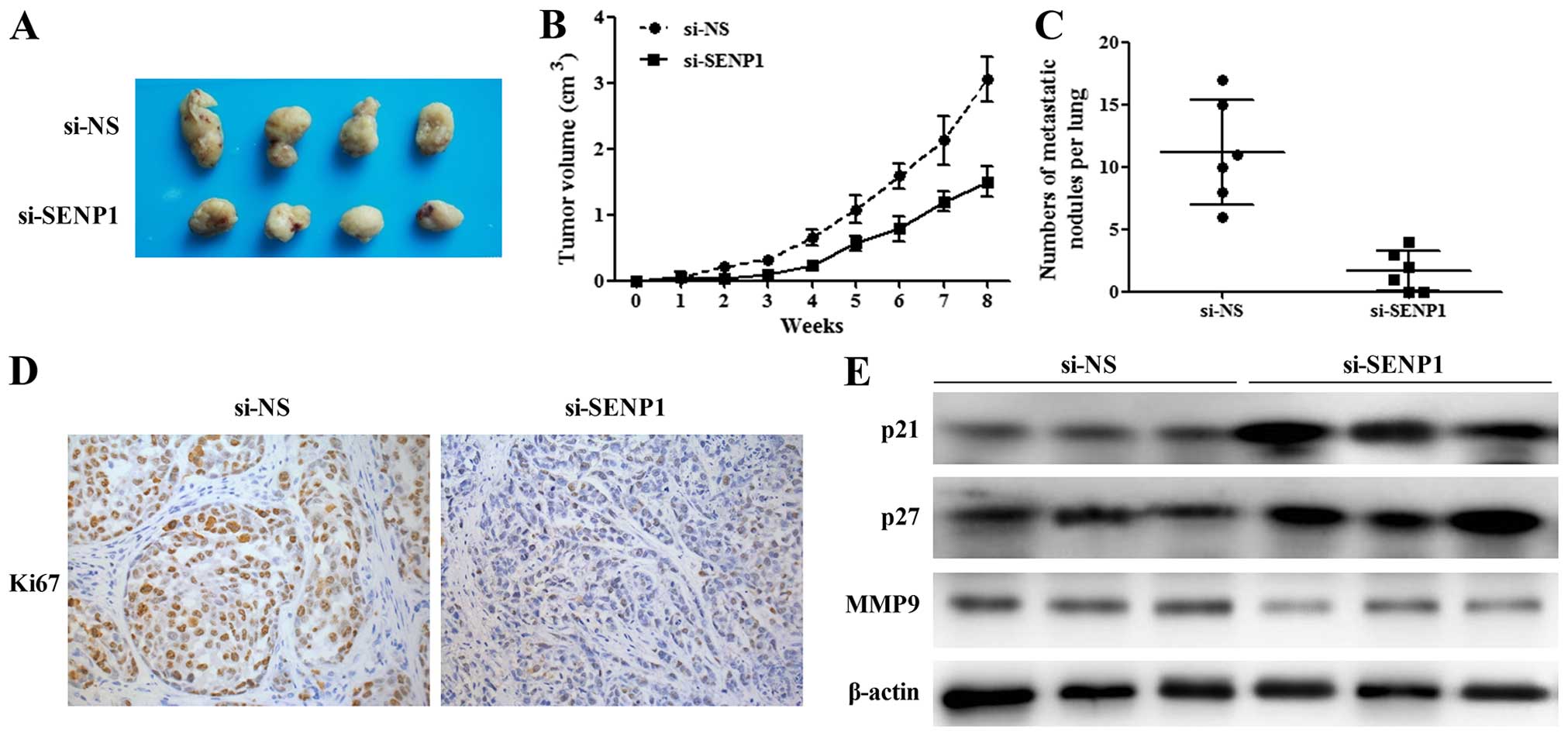
Silencing of SENP1 inhibits tumor growth
and lung metastases in vivo
To explore the effect of SENP1 on TNBC tumor growth
in vivo, cells transfected with si-NS and si-SENP1 were
subcutaneously injected into nude mice, respectively. The volume of
tumor was evaluated weekly from 1 to 8 weeks after transplantation.
As shown in Fig. 4A and B, the
tumor sizes in si-SENP1 group were significantly smaller than that
in the control group. Pulmonary metastasis detection revealed that
si-SENP1 cells had less lung metastases than si-NS control cells
(Fig. 4C). Further, we performed
IHC assay by using Ki67 antibody. We found that the expression
level of Ki67 in SENP1-depleting tumor cells is lower than that in
the control cells, which suggested that depletion of SENP1 would
inhibit TNBC cell proliferation in vivo (Fig. 4D). Furthermore, as shown in Fig. 4E, the protein levels of p21, p27,
and MMP9 were increased in tumor tissues from si-SENP1 TNBC cells
in comparison with those from si-NS TNBC cells. These results
elucidated the in vivo function of SENP1 in regulating TNBC
cell proliferation and tumor formation.
Discussion
The SENPs play a pivotal role in maintaining ordered
cellular physiology by modulating the balance between sumoylated
and unsumoylated proteins. In mammalian cells, there are six
identified SENP isoforms, namely SENP1, SENP2, SENP3, SENP5, SENP6,
and SENP7, respectively. Emerging studies have demonstrated that
various SENP isoforms were involved in the development of diverse
diseases, such as pancreatic (16),
colon (11), prostate (19,20),
bladder (21) and thyroid (22) cancer, as well as heart diseases
(23). SENP1 is the first
identified and most characterized SENP among the SENP family. Data
from previous studies showed abnormal expression of SENP1 in
several types of carcinomas. Wang et al reported that the
expression of SENP1 is directly associated with CaP aggressiveness
and recurrence. The absence of SENP1 restricted the tumor growth
and metastasis in CaP cells (10).
Moreover, SENP1 was increased in pancreatic cancer cells and
knockdown of SENP1 inhibited the proliferation, migration, and
invasion of pancreatic cancer cells (16). In this study, we found that SENP1
levels were markedly elevated in TNBC tissues. Through further
in vitro and in vivo assays, we elucidated that
knockdown of SENP1 modulated the proliferation, mobility, and cell
cycle of TNBC cells.
Cell cycle was regulated by the activation of CDKs,
which antagonized by the inhibitors, such as p21 and p27 (24,25).
The expression of p21 and p27 were reported to serve as the
molecular basis for cell proliferation blockage in breast cancer
cells (18,26,27).
For example, the signal transducer and activator of transcription 6
(STAT6) downregulates breast cancer cell proliferation by
increasing the expression of p21 and p27 (18). To further verify the ability of
SENP1 to mediate proliferation of TNBC cells, we investigated the
expression levels of p21 and p27 in si-SENP1 TNBC cells. We found
that silencing of SENP1 increased the expression of p21 and p27 in
the two TNBC cell lines, MDA-MB-231 and MDA-MB-453, indicating that
SENP1 regulates the proliferation of TNBC cells through repressing
the expression of CDK inhibitors, p21, and p27. However, the
mechanism of SENP1 in regulating the expression of p21 and p27
needs to be further investigated.
MMPs, the family of Zn-dependent endopeptidases, are
associated with the invasive properties of metastatic cancer cells
(28,29). It has been reported that MMP9 is
overexpressed in breast cancer cells and is correlated with the
invasion and metastasis of breast cancer (30,31).
Given that SENP1 affected the cancer invasion and metastasis, we
further investigated whether the expression of MMP9 could be
controlled by SENP1. Our data showed that knockdown of SENP1
resulted in MMP9 reduction both in mRNA and protein level. In
addition, it has been demonstrated that SENP1 regulates the
expression of MMP9 through HIF-1α signaling in CaP cells (20). Thus, it could be an interesting
point to confirm whether SENP1 regulates TNBC cell proliferation
via HIF-1α pathway.
In conclusion, we demonstrated that SENP1 was
upregulated in TNBC tumor tissues when compared to adjacent normal
and non-TNBC tumor tissues, respectively. Depletion of SENP1
attenuated TNBC cell proliferation, invasion, migration, and colony
formation ability, and arrested TNBC cells in G1/G0 cell cycle
progression in vitro, as well as the tumor size, tumor cell
proliferation, and lung metastases in vivo. Furthermore,
knockdown of SENP1 increased the expression of CKD inhibitors, p21
and p27, and decreased the expression of MMP9. Taken together, the
current study elucidated a critical role of SENP1 in TNBC
progression, which highlighted that SENP1 might be a potential
therapeutic target for TNBC.
Acknowledgments
This study was supported by the Shanghai Municipal
Science and Technology Commission Guidance Project, P.R. China
(contract no. 14411966000).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bauer KR, Brown M, Cress RD, Parise CA and
Caggiano V: Descriptive analysis of estrogen receptor
(ER)-negative, progesterone receptor (PR)-negative, and
HER2-negative invasive breast cancer, the so-called triple-negative
phenotype: A population-based study from the California Cancer
Registry. Cancer. 109:1721–1728. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Elias AD: Triple-negative breast cancer: A
short review. Am J Clin Oncol. 33:637–645. 2010. View Article : Google Scholar
|
|
5
|
Dent R, Trudeau M, Pritchard KI, Hanna WM,
Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P and Narod SA:
Triple-negative breast cancer: Clinical features and patterns of
recurrence. Clin Cancer Res. 13:4429–4434. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rakha EA, Elsheikh SE, Aleskandarany MA,
Habashi HO, Green AR, Powe DG, El-Sayed ME, Benhasouna A, Brunet
JS, Akslen LA, et al: Triple-negative breast cancer: Distinguishing
between basal and nonbasal subtypes. Clin Cancer Res. 15:2302–2310.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheng J, Kang X, Zhang S and Yeh ET:
SUMO-specific protease 1 is essential for stabilization of
HIF1alpha during hypoxia. Cell. 131:584–595. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li R, Wei J, Jiang C, Liu D, Deng L, Zhang
K and Wang P: Akt SUMOylation regulates cell proliferation and
tumorigenesis. Cancer Res. 73:5742–5753. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li T, Huang S, Dong M, Gui Y and Wu D:
Prognostic impact of SUMO-specific protease 1 (SENP1) in prostate
cancer patients undergoing radical prostatectomy. Urol Oncol.
31:1539–1545. 2013. View Article : Google Scholar
|
|
10
|
Wang RT, Zhi XY, Zhang Y and Zhang J:
Inhibition of SENP1 induces radiosensitization in lung cancer
cells. Exp Ther Med. 6:1054–1058. 2013.PubMed/NCBI
|
|
11
|
Xu Y, Li J, Zuo Y, Deng J, Wang LS and
Chen GQ: SUMO-specific protease 1 regulates the in vitro and in
vivo growth of colon cancer cells with the upregulated expression
of CDK inhibitors. Cancer Lett. 309:78–84. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Burdelski C, Menan D, Tsourlakis MC, Kluth
M, Hube-Magg C, Melling N, Minner S, Koop C, Graefen M, Heinzer H,
et al: The prognostic value of SUMO1/Sentrin specific peptidase 1
(SENP1) in prostate cancer is limited to ERG-fusion positive tumors
lacking PTEN deletion. BMC Cancer. 15:5382015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Q, Xia N, Li T, Xu Y, Zou Y, Zuo Y,
Fan Q, Bawa-Khalfe T, Yeh ET and Cheng J: SUMO-specific protease 1
promotes prostate cancer progression and metastasis. Oncogene.
32:2493–2498. 2013. View Article : Google Scholar
|
|
14
|
Wang C, Tao W, Ni S, Chen Q, Zhao Z, Ma L,
Fu Y and Jiao Z: Tumor-suppressive microRNA-145 induces growth
arrest by targeting SENP1 in human prostate cancer cells. Cancer
Sci. 106:375–382. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang H, Tang Y, Guo W, Du Y, Wang Y, Li P,
Zang W, Yin X, Wang H, Chu H, et al: Up-regulation of microRNA-138
induce radiosensitization in lung cancer cells. Tumour Biol.
35:6557–6565. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma C, Wu B, Huang X, et al: SUMO-specific
protease 1 regulates pancreatic cancer cell proliferation and
invasion by targeting MMP-9. Tumour Biol. 35:12729–12735. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang BB, Gao QM, Liang W, Xiu B, Zhang WJ
and Liang AB: Down-regulation of SENP1 expression increases
apoptosis of Burkitt lymphoma cells. Asian Pac J Cancer Prev.
13:2045–2049. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wei M, Liu B, Gu Q, Su L, Yu Y and Zhu Z:
Stat6 cooperates with Sp1 in controlling breast cancer cell
proliferation by modulating the expression of p21(Cip1/WAF1) and
p27 (Kip1). Cell Oncol (Dordr). 36:79–93. 2013. View Article : Google Scholar
|
|
19
|
Bawa-Khalfe T, Cheng J, Lin SH, Ittmann MM
and Yeh ET: SENP1 induces prostatic intraepithelial neoplasia
through multiple mechanisms. J Biol Chem. 285:25859–25866. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kaikkonen S, Jääskeläinen T, Karvonen U,
Rytinki MM, Makkonen H, Gioeli D, Paschal BM and JJ: SUMO-specific
protease 1 (SENP1) reverses the hormone-augmented SUMOylation of
androgen receptor and modulates gene responses in prostate cancer
cells. Mol Endocrinol. 23:292–307. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tan M, Gong H, Wang J, Tao L, Xu D, Bao E,
Liu Z and Qiu J: SENP2 regulates MMP13 expression in a bladder
cancer cell line through SUMOylation of TBL1/TBLR1. Sci Rep.
5:139962015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jacques C, Baris O, Prunier-Mirebeau D,
Savagner F, Rodien P, Rohmer V, Franc B, Guyetant S, Malthiery Y
and Reynier P: Two-step differential expression analysis reveals a
new set of genes involved in thyroid oncocytic tumors. J Clin
Endocrinol Metab. 90:2314–2320. 2005. View Article : Google Scholar
|
|
23
|
Kim EY, Chen L, Ma Y, Yu W, Chang J,
Moskowitz IP and Wang J: Enhanced desumoylation in murine hearts by
overexpressed SENP2 leads to congenital heart defects and cardiac
dysfunction. J Mol Cell Cardiol. 52:638–649. 2012. View Article : Google Scholar :
|
|
24
|
Malumbres M and Barbacid M: Cell cycle,
CDKs and cancer: A changing paradigm. Nat Rev Cancer. 9:153–166.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shackelford RE, Kaufmann WK and Paules RS:
Cell cycle control, checkpoint mechanisms, and genotoxic stress.
Environ Health Perspect. 107(Suppl 1): 5–24. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Groshong SD, Owen GI, Grimison B, Schauer
IE, Todd MC, Langan TA, Sclafani RA, Lange CA and Horwitz KB:
Biphasic regulation of breast cancer cell growth by progesterone:
Role of the cyclin-dependent kinase inhibitors, p21 and p27(Kip1).
Mol Endocrinol. 11:1593–1607. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang X, Gao P, Long M, Lin F, Wei JX, Ren
JH, Yan L, He T, Han Y and Zhang HZ: Essential role of cell cycle
regulatory genes p21 and p27 expression in inhibition of breast
cancer cells by arsenic trioxide. Med Oncol. 28:1225–1254. 2011.
View Article : Google Scholar
|
|
28
|
Akter H, Park M, Kwon OS, Song EJ, Park WS
and Kang MJ: Activation of matrix metalloproteinase-9 (MMP-9) by
neurotensin promotes cell invasion and migration through ERK
pathway in gastric cancer. Tumour Biol. 36:6053–6062. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shi M, Cao M, Song J, Liu Q, Li H, Meng F,
Pan Z, Bai J and Zheng J: PinX1 inhibits the invasion and
metastasis of human breast cancer via suppressing NF-κB/MMP-9
signaling pathway. Mol Cancer. 14:662015. View Article : Google Scholar
|
|
31
|
Sun YS, Zhao Z and Zhu HP: Hispolon
inhibits TPA-induced invasion by reducing MMP-9 expression through
the NF-κB signaling pathway in MDA-MB-231 human breast cancer
cells. Oncol Lett. 10:536–542. 2015.PubMed/NCBI
|