Introduction
Oral squamous cell carcinoma (OSCC) is a lethal
intraoral malignancy associated with high morbidity and mortality;
the 5-year survival rate is less than 50% (1). There are no effective means to cure
this disease. In order to develop successfully molecular-targeted
therapies against this tumor, the first and most important
procedure is to reveal its pathogenesis and identify critical
oncogenes.
As a member of the bromodomain and extraterminal
(BET) family, bromodomain 4 (BRD4) plays a critical role in gene
regulation by facilitating the recruitment of the active form of
the positive transcription elongation factor b (P-TEFb) (2). Abnormal activation of the BRD4 gene is
associated with the tumorigenesis of many human malignancies.
Previous studies have reported that BRD4 is significantly
overexpressed in a range of malignant tumors including bladder
cancer, multiple myeloma, non-small cell lung cancer, leukemia and
hepatocellular carcinoma (3–7). BRD4
inhibition by small molecule inhibitors and siRNA has been
demonstrated to be a therapeutic strategy in many malignant tumors
(4,8).
Twist is a member of the basic helix-loop-helix
protein family and a key member of epithelial-to-mesenchymal
transition (EMT)-activating transcriptional factors (9–12). The
roles of Twist in tumor progression have been well investigated. It
has been reported that Twist is a potential oncogene that inhibits
apoptosis, and increases migration and invasion in several types of
tumors (12–14). Shi et al demonstrated that
BRD4 interacts with di-acetylated Twist which is critical for the
tumorigenicity of breast cancer, and they proposed that the
Twist-BRD4 complex may be a potential drug target for basal-like
breast cancer (9,15).
The C-Myc proto-oncogene belongs to the MYC family,
which also includes MYCN (N-Myc) and MYCL (L-Myc) (16). As a critical transcription
regulator, C-Myc plays a vital role in many physiological processes
of regulation, such as cell cycle control, protein synthesis, cell
adhesion and apoptosis (17).
Evidence shows that nearly half of human tumors, including leukemia
and many solid tumors are associated with the overexpression of the
C-Myc gene (18–21). C-Myc contributes to the pathogenesis
of a majority of human malignant tumors by promoting multiple
processes, including uncontrolled cell proliferation, cell growth
and genomic instability (16). It
was found that C-Myc regulates promoter-proximal pause of Pol II
through the recruitment of P-TEFb, which indicates that BRD4
inhibition is a therapeutic strategy in human tumors to target
C-Myc (4,22).
The above-mentioned studies demonstrate that BRD4,
C-Myc and Twist all play important roles in tumor development and
are the key treatment targets for a range of human malignant
tumors.
JQ1 is a BET-BRD inhibitor that has high binding
affinity for BRD4, and has been shown to be profoundly efficacious
against many malignant tumors, including hepatocellular carcinoma,
lung, gastric and colon cancer (4,7,23–26).
Previous studies have reported that JQ1 treatment significantly
down-regulated C-Myc expression in several tumors including
Kras-mutant non-small cell lung cancer and medulloblastoma
cells, and T cell acute lymphoblastic leukemia (24,27,28).
JQ1 also disrupts the interaction of BRD4 with Twist leading to
suppression of breast cancer (15).
All in all, JQ1, as a small molecule inhibitor of BRD4, can
suppress tumorigenesis in several human malignant tumors. While
C-Myc and Twist have been identified as important oncogenes in OSCC
(29,30), the effect of JQ1 on these gene
signals of OSCC has not been well investigated. Moreover, the
genetic status of BRD4 in OSCC is not yet defined.
In the present study, we hypothesized that the BRD4
inhibitor, JQ1, may inhibit the development and metastasis of OSCC
via the suppression of C-Myc and Twist. To test our hypothesis, we
investigated the effects of JQ1 at different concentrations on the
proliferation, apoptosis and invasion of Cal27 cells, as well as on
protein expression of BRD4, C-Myc and Twist.
Materials and methods
Cell culture
Cal27 cells (provided by Shanghai ninth People's
Hospital) were cultured in high-glucose Dulbecco's modified Eagle's
medium (DMEM) (HyClone, Logan, UT, USA), supplemented with 10%
fetal bovine serum (FBS; Gibco, Grand Island, NY, USA), 100 U/ml
penicillin and 100 µg/ml streptomycin (Invitrogen,
Camarillo, CA, USA) with 5% CO2 at 37°C. In the present
study, cells were maintained in a culture medium supplemented with
JQ1 (Selleck Chemicals, Houston, TX, USA) at concentrations of 0.1,
0.5 and 1 µM. Dimethyl sulfoxide (DMSO) was added in the
control group.
Immunocytochemical analysis
Cal27 cells were plated on coverslips at
2.5×104 cells/well into 24-well plates in 300 µl
of high-glucose DMEM in the presence of 10% FBS. After 24 h, the
cells were fixed in 4% (v/v) paraformaldehyde for 15 min,
permeabilized with 0.1% Triton X-100 for 3 min, and blocked with
10% donkey serum (2 h). The cells were then incubated with 1:200
primary rabbit anti-human BRD4 monoclonal (catalogue no. ab128874),
mouse anti-human C-Myc monoclonal (catalogue no. ab32) and rabbit
anti-human Twist polyclonal antibodies (catalogue no. ab50581) (all
from Abcam, Cambridge, MA, USA) overnight at 4°C. After washing,
the cells were incubated with 1:200 goat anti-rabbit (catalogue no.
SP-9000) and goat anti-mouse (catalogue no. SP-9002) (both from
ZSGB-BIO Origene, Beijing, China) secondary antibodies.
4′,6-Diamidino-2-phenylindole (DAPI) was used as the nuclear
counterstain. Images were collected by fluorescence microscopy.
Cell proliferation assay
The proliferation of cells was measured using Cell
Counting Kit-8 (CCK-8; Dojindo, Tokyo, Japan). Cal27 cells were
seeded in 96-well plates at a density of 2,000 cells/well and
cultured with high-glucose DMEM containing 10% FBS. After 24 h, the
groups were switched to high-glucose DMEM containing 2% FBS and
different concentrations of JQ1. After 1–5 days, 10 µl of
CCK-8 solution was added to each well, and the plates were
incubated for 2.5 h at 37°C. The optical density (OD) levels were
measured by a microplate reader scanning at 450 nm according to the
manufacturer's instructions.
Annexin V/PI assays for apoptosis
Cal27 cells were seeded into 6-well plates at a
density of 1×105 cells/well, and then maintained with
the aforementioned medium, which was supplemented with JQ1 at the
concentration of 0.5 µM. After 48 h, the apoptosis of cells
was detected by flow cytometry (FCM) with an Annexin
V-FITC/propidium iodide (PI) apoptosis detection kit (eBioscience,
Vienna, Austria) according to the manufacturer's instructions.
Briefly, Cal27 cells were washed once in a phosphate-buffered
saline (PBS) and once in a 1X binding buffer. The cells were then
resuspended in a 1X binding buffer, and 5 µl of
fluorochrome-conjugated Annexin V was added to each one. After
incubation for 10 min at room temperature, the cells were washed in
a 1X binding buffer. After adding 5 µl of PI staining
solution, the apoptotic cells were determined using a flow
cytometer (FACSCalibur; BD Biosciences, San Jose, CA, USA). Both
early and late apoptotic cells were included in cell death
determinations.
Cell cycle analysis
Cell cycle analysis was performed to evaluate the
influence of JQ1 on the Cal27 cell cycle using a Cell Cycle and
Apoptosis Analysis kit (Beyotime, Shanghai, China). After 24 h of
treatment with JQ1 at different concentrations, the cells were
washed once with PBS, and then resuspended in 1 ml of ice-cold 70%
ethanol and fixed for 12 h at 4°C. Then, the cells were washed with
PBS and stained with 500 µl staining solution (PI) for 30
min at 37°C. Cell cycle data were obtained using a flow cytometer.
The percentages of cells at the G1, G2 and S phases were
analyzed.
In vitro invasion assay
An in vitro cell invasion assay was performed
to evaluate the influence of JQ1 on the metastasis of Cal27 cells.
A Corning® Matrigel® Basement Membrane Matrix
(Becton-Dickinson and Co., Mountain View, CA, USA), which was used
to mimic the extracellular matrices underlying the cells in
vivo, was plated on the upper compartment of a 24-well
Transwell plate (8 µm; Costar, Cambridge, MA, USA). Cal27
cells (1×105) in high-glucose DMEM supplemented without
FBS and JQ1 at different concentrations were plated on the matrix.
As a chemoattractant, the lower compartment contained high-glucose
DMEM supplemented with 10% FBS. After 48 h of incubation, cells on
the upper surface of the filter were removed gently with a cotton
swab, while cells on the lower surface were washed with PBS, fixed
in 4% paraformaldehyde for 30 min at room temperature, and were
then stained with 0.1% crystal violet. The number of cells on the
lower surface was counted under a light microscope in five randomly
selected fields, and the mean number of cells was calculated per
field.
Quantitative RT-PCR (qRT-PCR) assay
The Cal27 cells were seeded into 6-well plates at a
density of 1×105 cells/well. After 24 h, the groups were
switched to high-glucose DMEM containing 10% FBS and JQ1 at
different concentrations. Total RnA was extracted using a
TRIzol® reagent (Takara Bio, Dalian, Japan) after 24 and
48 h according to the manufacturer's protocol, and
reverse-transcribed into cDNA using the Biometra Reverse
Transcription system (Biometra) at 42°C for 2 min, and 4°C for 30
min; the second step at 37°C for 15 min at 85°C for 5 sec, and 4°C
for 30 min with the reverse transcriptase kit (Takara Bio). qRT-PCR
was run in 20 µl of the reaction system containing 10
µl of 2X PCR Master Mix, 0.4 µl of each primer, 2
µl of cDNA and 7.2 µl of nuclease-free water on a
LightCycler Roche 480 with SYBR® Primix Ex Taq™ kit
(Takara Bio) under the following conditions: at 95°C for 30 sec; 45
cycles at 95°C for 5 sec, at 60°C for 35 sec and at 72°C for 1 min;
finally at 40°C for 30 sec, while GAPDH served as a reference gene.
Relative quantity of mRNA expression was calculated using the
2−ΔΔCt method. All experiments were repeated in
triplicate. The sequences of the primers for amplification of human
BRD4, C-Myc, Twist and GAPDH were as follows: BRD4,
5′-ACCTCCAACCCTAACAAGCC-3′ and 5′-TTTCCATAGTGTCTTGAGCACC-3′; C-Myc,
5′-GGCTCCTGGCAAAAGGTCA-3′ and 5′-CTGCGTAGTTGTGCTGATGT-3′; Twist,
5′-GTCCGCAGTCTTACGAGGAG-3′ and 5′-GCTTGAGGGTCTGAATCTTGCT-3′; GAPDH,
5′-GCACCGTCAAGGCTGAGAAC-3′ and 5′-TGGTGAAGACGCCAGTGGA-3′.
Western blot analysis
The Cal27 cells were seeded in 6-well plates at a
density of 1×105 cells/well, and were then maintained in
high-glucose DMEM with JQ1 at different concentrations. After 24
and 48 h, proteins were extracted from the cells using RIPA
containing 1% phenylmethylsulfonyl fluoride (PMSF) (both from
Beyotime) for 30 min. The protein concentration was determined
using the bicinchoninic acid (BCA) assay. An amount of 20 µg
of total protein was run on a 10% SDS-PAGE gel (Beyotime) and
electrotransferred to polyvinylidene fluoride (PVDF) membrane
(Invitrogen) for 1 h at 100 V in transfer buffer. The PVDF membrane
were then blocked with 5% non-fat milk for 1 h at room temperature,
and probed with 1:1,000 primary rabbit anti-human BRD4 monoclonal
(catalogue no. ab128874), mouse anti-human C-Myc monoclonal
(catalogue no. ab32), rabbit anti-human Twist polyclonal antibodies
(catalogue no. ab50581) (all from Abcam), and rabbit anti-human
cleaved-caspase 3 polyclonal antibody (catalogue no. 9661S; CST,
Danvers, MA, USA) overnight at 4°C on a gentle shaker. Following
washing with TBST (20 mmol/l Tris-HCl, 150 mmol/l NaCl and 0.05%
Tween-20; pH 7.4) three times, for 10 min each time, the membrane
was incubated in 1:5,000 HRP-labeled horse anti-mouse IgG
(catalogue no. 7076S) or goat anti-rabbit IgG (catalogue no. 7074S)
(both from CST). The proteins were visualized using the
Chemiluminescent HRP Substrate (Millipore, Billerica, MA, USA).
Statistical analysis
All data were analyzed and expressed as the mean ±
SEM from at least three replicates for each experiment. SPSS 16.0
software was used for data analysis. The significance of
differences between the experimental groups and the control group
was analyzed using one-way ANOVA. P-values <0.05 were considered
to indicate statistically significant results.
Results
BRD4, C-Myc and Twist are highly
expressed in the Cal27 cell line
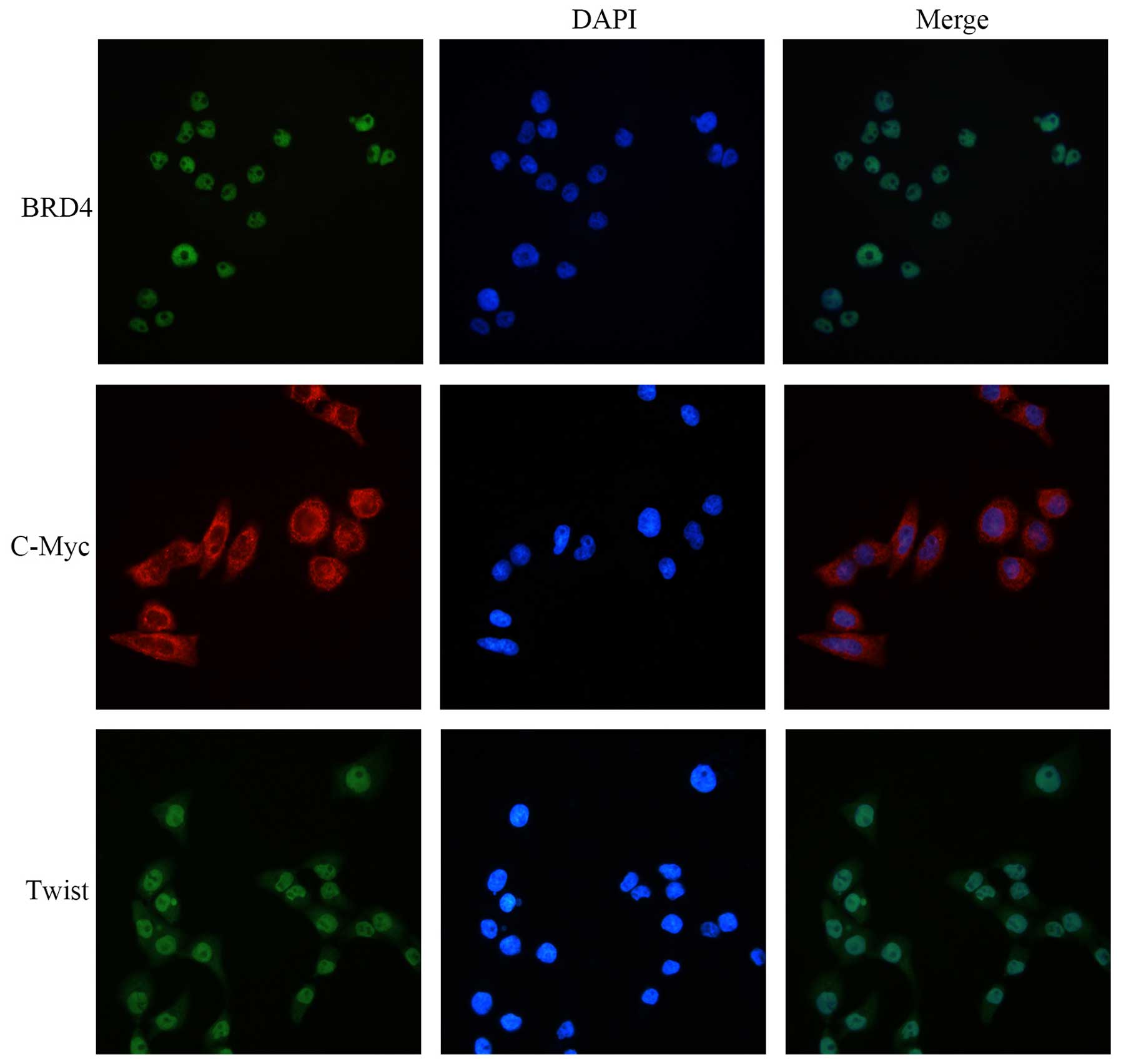
We first explored whether these proteins were
expressed in the Cal27 cell line. Immunofluorescence (IFC) staining
showed that BRD4, C-Myc and Twist were highly expressed in the
Cal27 cells. BRD4 was mainly located in the nucleus and C-Myc was
expressed in the cytoplasm. Twist was expressed mainly in the
nucleus and slightly in the cytoplasm (Fig. 1).
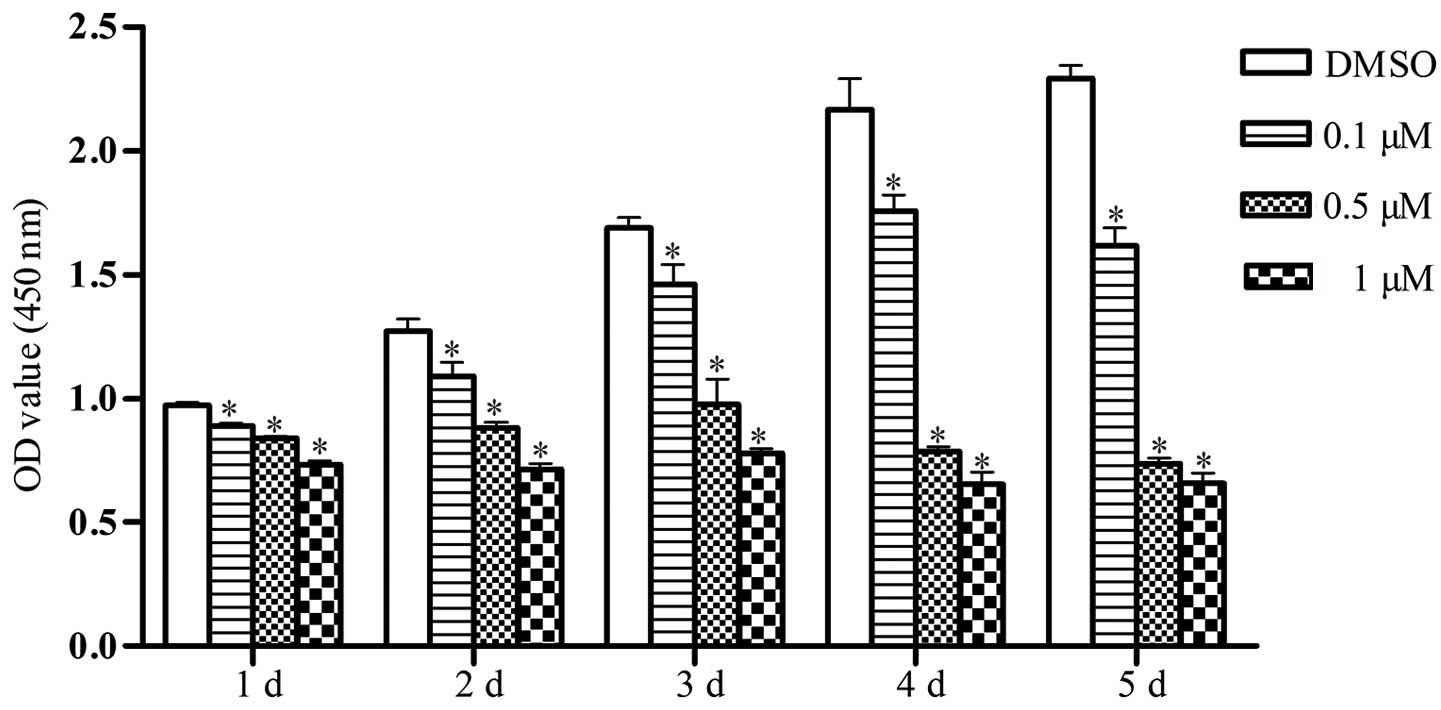
JQ1 reduces Cal27 cell proliferation
To evaluate the influence of JQ1 on the
proliferation of Cal27 cells, CCK-8 assays were performed by
treating cells with various concentrations of JQ1 for five days.
The results showed that, compared with the control group, cell
proliferation was significantly decreased following treatment with
JQ1 throughout the duration of the experiment, and the inhibitory
effect was dose-dependent (P<0.05) (Fig. 2).
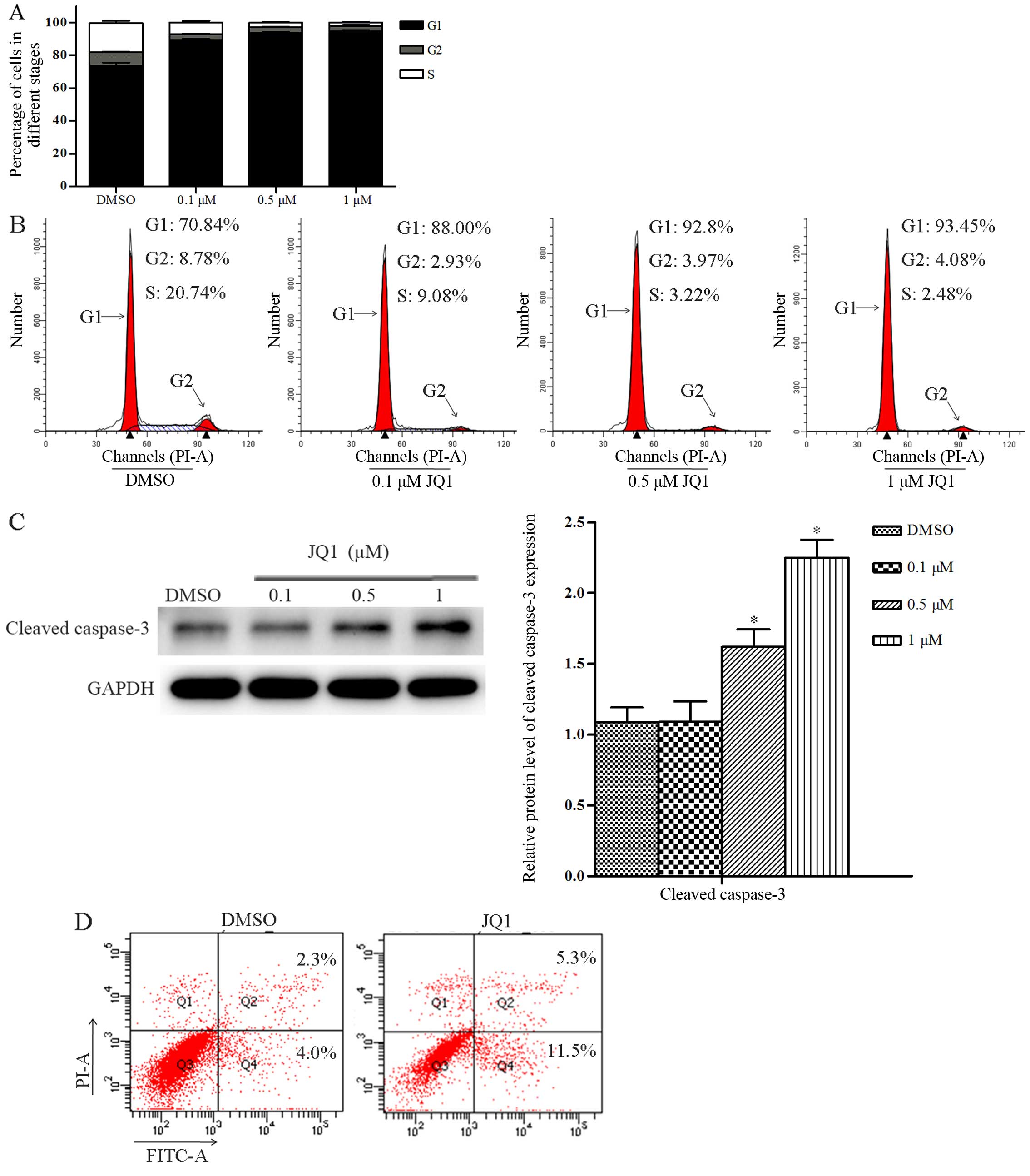
JQ1 induces cell cycle arrest and
apoptosis in Cal27 cells
To investigate the cellular mechanism underlying the
antiproliferative effects of JQ1 in Cal27 cells, we analyzed cell
cycle distribution using FCM. JQ1 treatment at the concentrations
of 0.1, 0.5 and 1 µM for 24 h led to a decreased percentage
of Cal27 cells in the S phase, and an increase in the percentage of
cells in the G1 phase (P<0.05) (Fig.
3A and B). Next, we evaluated apoptotic signaling pathways with
western blot assays. The results showed that a 24-h treatment with
JQ1 at the concentration of 0.1 µM had no effect on the
protein expression of cleaved caspase-3 in the Cal27 cells when
compared with the control group. Nevertheless, the protein
expression of cleaved caspase-3 was significantly upregulated after
a 24-h treatment with JQ1 at the concentrations of 0.5 and 1
µM (P<0.05) (Fig. 3C).
Then, we assessed apoptosis using FCM. JQ1 treatment at the
concentration of 0.5 µM for 48 h led to a significantly
increased percentage of early stage apoptosis in Cal27 cells when
compared with the control group (P<0.05) (Fig. 3D). JQ1 also induced Cal27 cells to
shrink, round and float, which are morphological changes
characteristic of apoptosis.
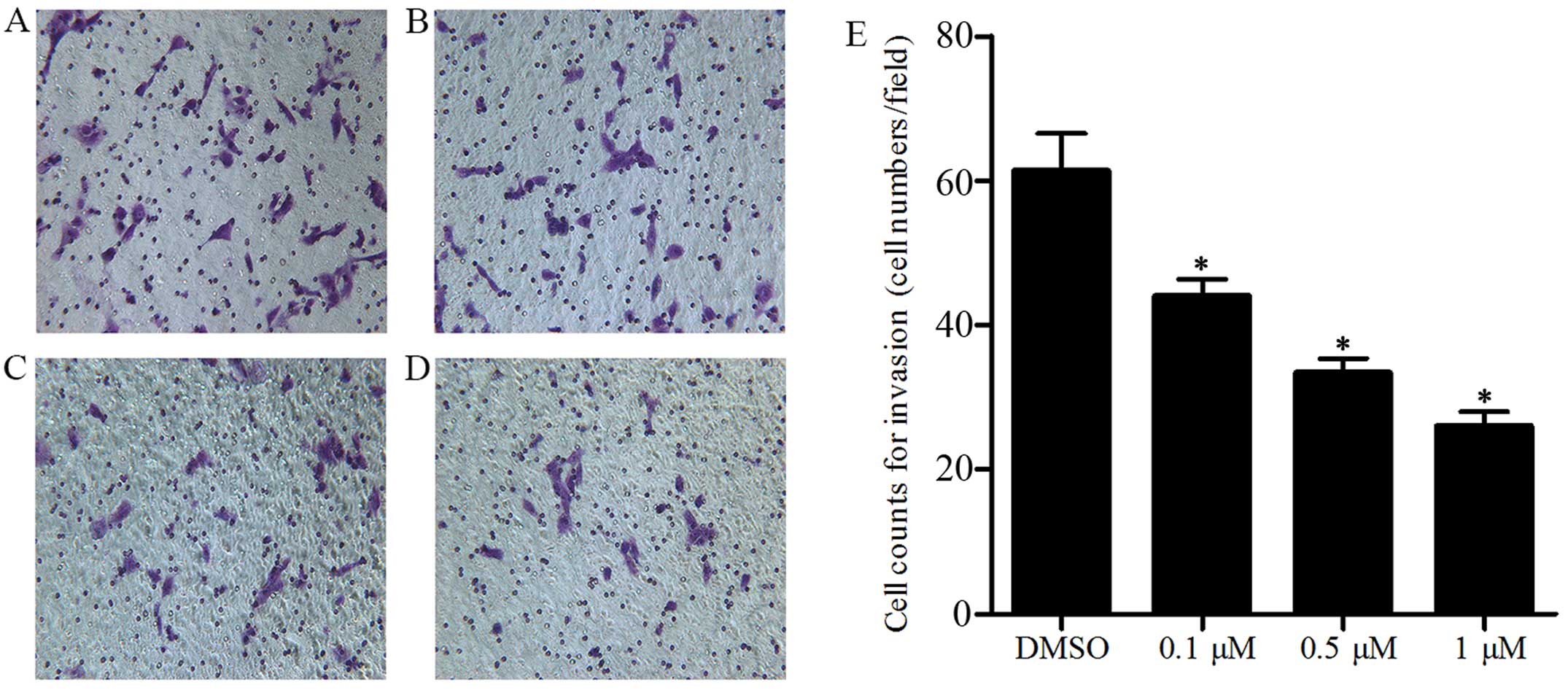
JQ1 reduces Cal27 cell invasion
Metastasis is a significant pathological process in
cancer. Consequently, an invasion assay was performed using a
Transwell to verify whether JQ1 attenuates the metastatic
capability of Cal27 cells. Following treatment for 48 h with JQ1 at
concentrations of 0.1, 0.5 and 1 µM, respectively, the cell
counts on the lower surface for the Cal27 cells were significantly
reduced when compared with the control group, and the decrease was
dose-dependent (P<0.05) (Fig.
4A–E). JQ1 reduced cell proliferation and induced apoptosis,
which may have influenced the cell counts, but there was no
evidence that the invasion was blocked by the cellular debris, and
the difference in cell counts was statistically significant
compared to the control. Therefore, the results revealed that BRD4
inhibition via JQ1 suppressed Cal27 cell invasion, and BRD4 is a
key player in this process.
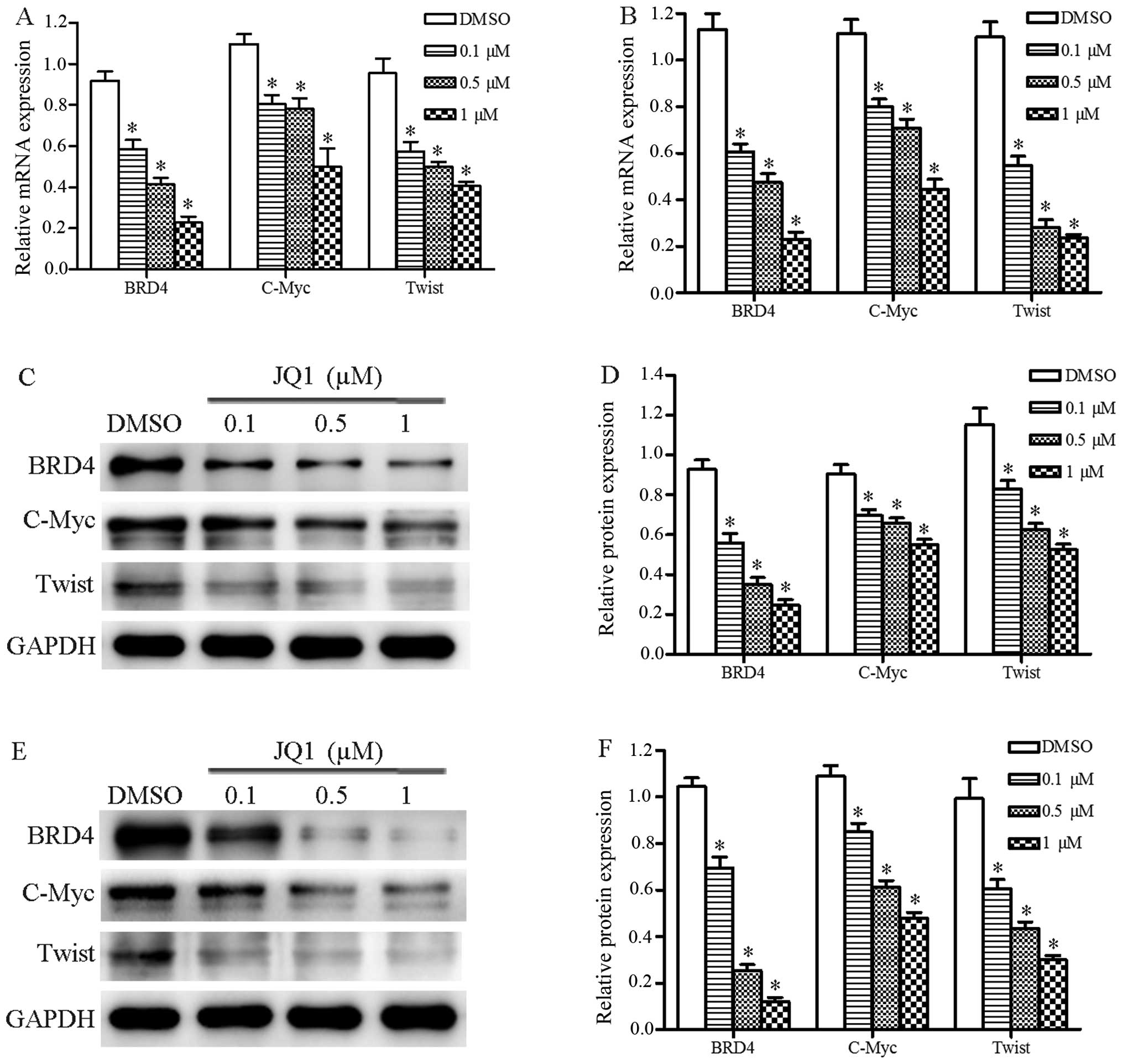
JQ1 represses BRD4, C-Myc, and Twist
expression in Cal27 cells
We evaluated whether JQ1 treatment suppressed
expression of BRD4, C-Myc and Twist in the Cal27 cells using
qRT-PCR and western blot assays. The results of qRT-PCR revealed
that JQ1 inhibited the mRNA expression of BRD4, C-Myc and Twistin
Cal27 cells after 24 and 48 h (P<0.05) (Fig. 5A and B). Western blot assays
consistently demonstrated that the protein expression levels of
BRD4, C-Myc and Twist were repressed in cells treated with JQ1
after 24 and 48 h (P<0.05) (Fig.
5C–F). These data demonstrated that JQ1 significantly
suppressed expression levels of several oncogenes and may be used
in OSCC treatment.
Discussion
BRD4 deregulation is important in the pathogenesis
of multiple human malignant tumors (5,31), and
BRD4 has been proven to be a therapeutic target for several
malignant tumors (7,32–34).
Studies have demonstrated that inhibition of BRD4 by JQ1 suppresses
the growth of many tumors such as thyroid cancer and hepatocellular
carcinoma by decreasing tumor cell viability, inducing cell
apoptosis and repressing expression of C-Myc (7,35).
However, the role of BRD4 in OSCC and the effect of BRD4 inhibitor
JQ1 on oral squamous cell carcinoma (OSCC) have not been well
investigated. In the present study, we evaluated the expression of
BRD4 in OSCC cell line, Cal27, and the effect of JQ1 on Cal27 cell
proliferation, apoptosis, invasion and expression of oncogenes to
analyze whether BRD4 could be a target for the treatment of
OSCC.
JQ1 is the first generation of BET-specific
inhibitors. It can displace BRD4 from chromatin by competitively
binding to the acetyl-lysine recognition pocket (23,36)
resulting in downregulated expression of a range of oncogenes
including C-Myc, Twist and many other oncogenes. To analyze the
effect of JQ1 on OSCC, we first investigated whether BRD4, C-Myc
and Twist were expressed in Cal27 cells by IFC staining. The result
showed that BRD4, C-Myc and Twist were highly expressed in Cal27
cells. BRD4 was mainly located in the nucleus, and C-Myc was
expressed in the cytoplasm, while Twist was detected both in the
nucleus and in the cytoplasm. These data demonstrated that BRD4
along with well known C-Myc and Twist are pivotal genes in OSCC
development.
Considering the important role of BRD4 in other
malignant tumor cells, we proposed that BRD4 could be a key player
in Cal27 cells and that BRD4 inhibitor JQ1 may suppress Cal27 cell
growth. In order to confirm our hypothesis, we investigated the
effect of JQ1 on Cal27 cell growth and invasion and found that JQ1
treatment at different concentrations effectively decreased cell
proliferation and inhibited cell invasion. To investigate the
cellular mechanism underlying the antiproliferative effects of JQ1
in Cal27 cells, cell cycle distribution and cell apoptosis after
JQ1 treatment were analyzed. The results found that JQ1 induced
early stage apoptosis, arrested cell cycle progression at the G1
phase and upregulated cleaved caspase-3 expression at low
concentration ranges in the Cal27 cells. These data suggest that
JQ1 suppresses OSCC cell survival and promotes cell apoptosis,
which are in agreement with the effect of JQ1 on many other
malignant cells (7,37).
Therefore, since JQ1 is a BRD4 inhibitor and BRD4 is
positively expressed in OSCC, the regulation of JQ1 of the
expression of BRD4 should be elucidated to better understand the
antitumor mechanisms of JQ1. Intriguingly, we found that JQ1
repressed expression of BRD4 in the Cal27 cells.
Decades of biological research have identified a
central role for C-Myc in the pathophysiology of cancer (38). Abnormal expression of C-Myc is
observed in a range of malignancies including breast, colon and
cervical cancer, small cell lung carcinoma, osteosarcomas,
glioblastomas and myeloid leukemias (39–41).
BET bromodomain inhibition by JQ1 was regarded as a therapeutic
strategy by which to target C-Myc (39). The present study found that mRNA and
protein expression of C-Myc in Cal27 cells treated with JQ1 at
different concentrations was significantly downregulated,
suggesting that JQ1 suppresses OSCC progression by repressing C-Myc
expression.
Twist is a key member of the EMT-activating
transcriptional factors which is closely associated with tumor
metastasis. Abnormal activation of Twist has been reported in many
types of human tumors (42). A
recent study demonstrated that overexpression of Twist is
associated with OSCC progression and may enhance OSCC cell invasion
(43). In addition, Zheng et
al reported that Twist was also identified in two types of OSCC
cell lines, SCC-4 and TCA8113 cells, and it enhanced cell invasion
(44), which provided new insight
into the role of Twist in OSCC progression. Shi et al
reported that the Twist-BRD4-Wnt5a axis is critical for
tumorigenicity in breast cancer, and disrupting the interaction of
BRD4 and Twist using JQ1 suppressed tumorigenesis of this malignant
cancer (15). Our results,
consistent with the above-mentioned data, revealed that both mRNA
and protein expression of Twist was significantly downregulated in
the Cal27 cells treated with JQ1 when compared with the control
group. These data implied that BRD4 inhibition by JQ1 inhibited
Cal27 cell invasion through regulation of Twist expression.
In summary, the inhibition of the growth and
invasion of OSCC cells by JQ1 was supported by our in vitro
results. The investigation of the effect of JQ1 on OSCC in
vivo is currently underway and may be explored in-depth in
future studies.
Acknowledgments
The present study was supported by the Science and
Technology Development Program of Shandong Province, (grant no.
2014GGH218038), and the Scholarship of Visiting Scholars of
Shandong Provincial Key Laboratory of Oral Tissue Regeneration
(grant no. SDKQ201404). The authors would also like to acknowledge
the grant support from Shandong Provincial Key Laboratory of Oral
Tissue Regeneration.
References
|
1
|
Omar E: Current concepts and future of
noninvasive procedures for diagnosing oral squamous cell carcinoma
- a systematic review. Head Face Med. 11:62015. View Article : Google Scholar :
|
|
2
|
Chiang CM: Brd4 engagement from chromatin
targeting to transcriptional regulation: Selective contact with
acetylated histone H3 and H4.F1000. Biol Rep. 1:982009.
|
|
3
|
Wu X, Liu D, Tao D, Xiang W, Xiao X, Wang
M, Wang L, Luo G, Li Y, Zeng F, et al: BRD4 regulates EZH2
transcription through up-regulation of C-MYC and represents a novel
therapeutic target in bladder cancer. Mol Cancer Ther.
15:1029–1042. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Delmore JE, Issa GC, Lemieux ME, Rahl PB,
Shi J, Jacobs HM, Kastritis E, Gilpatrick T, Paranal RM, Qi J, et
al: BET bromodomain inhibition as a therapeutic strategy to target
c-Myc. Cell. 146:904–917. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liao YF, Wu YB, Long X, Zhu SQ, Jin C, Xu
JJ and Ding JY: High level of BRD4 promotes non-small cell lung
cancer progression. Oncotarget. 7:9491–9500. 2016.PubMed/NCBI
|
|
6
|
Wedeh G, Cerny-Reiterer S, Eisenwort G,
Herrmann H, Blatt K, Hadzijusufovic E, Sadovnik I, Müllauer L,
Schwaab J, Hoffmann T, et al: Identification of
bromodomain-containing protein-4 as a novel marker and epigenetic
target in mast cell leukemia. Leukemia. 29:2230–2237. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li GQ, Guo WZ, Zhang Y, Seng JJ, Zhang HP,
Ma XX, Zhang G, Li J, Yan B, Tang HW, et al: Suppression of BRD4
inhibits human hepatocellular carcinoma by repressing MYC and
enhancing BIM expression. Oncotarget. 7:2462–2474. 2016.
|
|
8
|
Zuber J, Shi J, Wang E, Rappaport AR,
Herrmann H, Sison EA, Magoon D, Qi J, Blatt K, Wunderlich M, et al:
RNAi screen identifies Brd4 as a therapeutic target in acute
myeloid leukaemia. Nature. 478:524–528. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shi J, Cao J and Zhou BP: Twist-BRD4
complex: Potential drug target for basal-like breast cancer. Curr
Pharm Des. 21:1256–1261. 2015. View Article : Google Scholar
|
|
10
|
Wu T and Donohoe ME: The converging roles
of BRD4 and gene transcription in pluripotency and oncogenesis. RNA
Dis. 2:pii: e894. 2015.PubMed/NCBI
|
|
11
|
Leptin M: twist and snail as positive and
negative regulators during Drosophila mesoderm development. Genes
Dev. 5:1568–1576. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maestro R, Dei Tos AP, Hamamori Y,
Krasnokutsky S, Sartorelli V, Kedes L, Doglioni C, Beach DH and
Hannon GJ: twist is a potential oncogene that inhibits apoptosis.
Genes Dev. 13:2207–2217. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li QQ, Xu JD, Wang WJ, Cao XX, Chen Q,
Tang F, Chen ZQ, Liu XP and Xu ZD: Twist1-mediated
adriamycin-induced epithelial-mesenchymal transition relates to
multidrug resistance and invasive potential in breast cancer cells.
Clin Cancer Res. 15:2657–2665. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cheng GZ, Chan J, Wang Q, Zhang W, Sun CD
and Wang LH: Twist transcriptionally up-regulates AKT2 in breast
cancer cells leading to increased migration, invasion, and
resistance to paclitaxel. Cancer Res. 67:1979–1987. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi J, Wang Y, Zeng L, Wu Y, Deng J, Zhang
Q, Lin Y, Li J, Kang T, Tao M, et al: Disrupting the interaction of
BRD4 with diacetylated Twist suppresses tumorigenesis in basal-like
breast cancer. Cancer Cell. 25:210–225. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wahlström T and Arsenian Henriksson M:
Impact of MYC in regulation of tumor cell metabolism. Biochim
Biophys Acta. 1849:563–569. 2015. View Article : Google Scholar
|
|
17
|
Dang CV: MYC on the path to cancer. Cell.
149:22–35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vita M and Henriksson M: The Myc
oncoprotein as a therapeutic target for human cancer. Semin Cancer
Biol. 16:318–330. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nesbit CE, Tersak JM and Prochownik EV:
MYC oncogenes and human neoplastic disease. Oncogene. 18:3004–3016.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lüscher B and Vervoorts J: Regulation of
gene transcription by the oncoprotein MYC. Gene. 494:145–160. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Delgado MD and León J: Myc roles in
hematopoiesis and leukemia. Genes Cancer. 1:605–616. 2010.
View Article : Google Scholar
|
|
22
|
Rahl PB, Lin CY, Seila AC, Flynn RA,
McCuine S, Burge CB, Sharp PA and Young RA: c-Myc regulates
transcriptional pause release. Cell. 141:432–445. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Filippakopoulos P, Qi J, Picaud S, Shen Y,
Smith WB, Fedorov O, Morse EM, Keates T, Hickman TT, Felletar I, et
al: Selective inhibition of BET bromodomains. Nature.
468:1067–1073. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shimamura T, Chen Z, Soucheray M,
Carretero J, Kikuchi E, Tchaicha JH, Gao Y, Cheng KA, Cohoon TJ, Qi
J, et al: Efficacy of BET bromodomain inhibition in Kras-mutant
non-small cell lung cancer. Clin Cancer Res. 19:6183–6192. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lenhart R, Kirov S, Desilva H, Cao J, Lei
M, Johnston K, Peterson R, Schweizer L, Purandare A, Ross-Macdonald
P, et al: Sensitivity of small cell lung cancer to BET inhibition
is mediated by regulation of ASCL1 gene expression. Mol Cancer
Ther. 14:2167–2174. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang L, Tong Y, Zhang X, Pan M and Chen
S: Arsenic sulfide combined with JQ1, chemotherapy agents, or
celecoxib inhibit gastric and colon cancer cell growth. Drug Des
Devel Ther. 9:5851–5862. 2015.PubMed/NCBI
|
|
27
|
Henssen A, Thor T, Odersky A, Heukamp L,
El-Hindy N, Beckers A, Speleman F, Althoff K, Schäfers S, Schramm
A, et al: BET bromodomain protein inhibition is a therapeutic
option for medulloblastoma. Oncotarget. 4:2080–2095. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Loosveld M, Castellano R, Gon S, Goubard
A, Crouzet T, Pouyet L, Prebet T, Vey N, Nadel B, Collette Y, et
al: Therapeutic targeting of c-Myc in T-cell acute lymphoblastic
leukemia, T-ALL. Oncotarget. 5:3168–3172. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Georgy SR, Cangkrama M, Srivastava S,
Partridge D, Auden A, Dworkin S, McLean CA, Jane SM and Darido C:
Identification of a novel proto-oncogenic network in head and neck
squamous cell carcinoma. J Natl Cancer Inst. 107:pii: djv152. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu J, Xie F, Bao X, Chen W and Xu Q:
miR-300 inhibits epithelial to mesenchymal transition and
metastasis by targeting Twist in human epithelial cancer. Mol
Cancer. 13:1212014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang YH, Sui XM, Sui YN, Zhu QW, Yan K,
Wang LS, Wang F and Zhou JH: BRD4 induces cell migration and
invasion in HCC cells through MMP-2 and MMP-9 activation mediated
by the Sonic hedgehog signaling pathway. Oncol Lett. 10:2227–2232.
2015.PubMed/NCBI
|
|
32
|
Ambrosini G, Sawle AD, Musi E and Schwartz
GK: BRD4-targeted therapy induces Myc-independent cytotoxicity in
Gnaq/11-mutatant uveal melanoma cells. Oncotarget. 6:33397–33409.
2015.PubMed/NCBI
|
|
33
|
Wang YH, Sui YN, Yan K, Wang LS, Wang F
and Zhou JH: BRD4 promotes pancreatic ductal adenocarcinoma cell
proliferation and enhances gemcitabine resistance. Oncol Rep.
33:1699–1706. 2015.PubMed/NCBI
|
|
34
|
Zhang Z, Ma P, Jing Y, Yan Y, Cai MC,
Zhang M, Zhang S, Peng H, Ji ZL, Di W, et al: BET bromodomain
inhibition as a therapeutic strategy in ovarian cancer by
downregulating FoxM1. Theranostics. 6:219–230. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gao X, Wu X, Zhang X, Hua W and Zhang Y,
Maimaiti Y, Gao Z and Zhang Y: Inhibition of BRD4 suppresses tumor
growth and enhances iodine uptake in thyroid cancer. Biochem
Biophys Res Commun. 469:679–685. 2016. View Article : Google Scholar
|
|
36
|
Lochrin SE, Price DK and Figg WD: BET
bromodomain inhibitors - a novel epigenetic approach in
castration-resistant prostate cancer. Cancer Biol Ther.
15:1583–1585. 2014. View Article : Google Scholar
|
|
37
|
Baker EK, Taylor S, Gupte A, Sharp PP,
Walia M, Walsh NC, Zannettino AC, Chalk AM, Burns CJ and Walkley
CR: BET inhibitors induce apoptosis through a MYC independent
mechanism and synergise with CDK inhibitors to kill osteosarcoma
cells. Sci Rep. 5:101202015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
McKeown MR and Bradner JE: Therapeutic
strategies to inhibit MYC. Cold Spring Harb Perspect Med. 4:42014.
View Article : Google Scholar
|
|
39
|
Kandela I, Jin HY and Owen K:
Reproducibility Project: Cancer Biology: Registered report: BET
bromodomain inhibition as a therapeutic strategy to target c-Myc.
Elife. 4:e070722015. View Article : Google Scholar
|
|
40
|
Conacci-Sorrell M, McFerrin L and Eisenman
RN: An overview of MYC and its interactome. Cold Spring Harb
Perspect Med. 4:a0143572014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Meyer N and Penn LZ: Reflecting on 25
years with MYC. Nat Rev Cancer. 8:976–990. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Entz-Werlé N, Stoetzel C, Berard-Marec P,
Kalifa C, Brugiere L, Pacquement H, Schmitt C, Tabone MD, Gentet
JC, Quillet R, et al: Frequent genomic abnormalities at TWIST in
human pediatric osteosarcomas. Int J Cancer. 117:349–355. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
da Silva SD, Alaoui-Jamali MA, Soares FA,
Carraro DM, Brentani HP, Hier M, Rogatto SR and Kowalski LP: TWIST1
is a molecular marker for a poor prognosis in oral cancer and
represents a potential therapeutic target. Cancer. 120:352–362.
2014. View Article : Google Scholar
|
|
44
|
Zheng L, Li N, Guo F, Jian XC, Jiang CH,
Yin P, Min AJ and Huang L: Twist-related protein 1 enhances oral
tongue squamous cell carcinoma cell invasion through β-catenin
signaling. Mol Med Rep. 11:2255–2261. 2015.
|