Introduction
Hepatocellular carcinoma (HCC) is the third most
common cause of cancer-related deaths worldwide. Approximately
70–80% of HCC patients are diagnosed at an advanced stage, 80% of
whom have underlying cirrhosis and only 20–30% of these were able
to undergo surgical resection (1).
Patients presenting with advanced or unresectable disease have a
very poor prognosis, with only 12% surviving for 5-years (2). An inability to diagnose during the
early stages and insufficient therapeutic intervention results in
most HCC patients progressing to metastasis, and the median
survival is only a few months (3).
Local ablation therapies are now deployed to treat advanced cases,
including percutaneous ethanol injection, radiofrequency ablation
(RFA), cryoablation, laser treatment, high-intensity focused
ultrasound and microwave treatment (4). Argon-helium cryotherapy is also an
effective local ablation therapy that has been used to treat HCC
(5). Compared with RFA and other
thermal ablation techniques, cryoablation can inflict greater
damage on tumour tissues and result in more clearly discernible
treatment areas, and can suppress ectopic tumours (6).
Recurrence following treatment in advanced HCC
patients cannot always be prevented, and while the treatments
listed above have decreased mortality, drug resistance and tumour
recurrence are common and remain to be addressed (7). The most important factor contributing
to poor prognosis is the inability to diagnose the disease early,
and identification of sensitive, robust circulating biomarkers is
critical. Circulating tumour cells (CTCs) are cancer cells that are
shed from either the primary tumour or its metastases and that
circulate in the peripheral blood. While metastases are directly
responsible for the majority of cancer deaths, CTCs may constitute
seeds for metastases and may indicate the spread of the disease
(8,9). CTCs are increasingly evaluated in
liquid biopsies, and their analysis holds great promise for
identification of patients at high-risk of relapse, for determining
specific adjuvant therapies for individual patients, and for
monitoring responses to treatments (10–12).
Counting the number of CTCs proved to be an independent prognostic
biomarker in small cell and non-small cell lung cancer patients
(13,14), and in other epithelial cell-derived
tumours such as breast (15,16),
colorectal (17), and prostate
cancer (18). CTCs are often
present in the blood of patients suffering metastasis, and
detection in peripheral blood is highly correlated with early
tumour metastases (19). CTCs can
also provide information on tumour biological activity and can
facilitate the real-time prediction of prognosis in patients
suffering distant metastases (17,18,20).
The purpose of the present study was to use immune magnetic bead
flow cytometry and real-time qPCR to measure the number of CTCs in
the peripheral blood of HCC patients before and after cryosurgery
and to correlate with disease prognosis.
Materials and methods
Patients
Patients with hepatocellular cancer were recruited
from the Fuda Cancer Hospital of Jinan University between June 2014
and June 2015, and all accepted cryoablation therapy. Inclusion and
exclusion criteria were as follows:
Inclusion criteria: examined by imaging and clinical
TNM stage III or IV; diagnosed by pathological examination as
malignant hepatocellular cancer; accepted cryosurgery in our
hospital to target local tumours, metastasis and tumour recurrence
in situ; voluntary consent was obtained; post-treatment
survival estimated at >3 months; age >18 and <85;
Karnofsky performance status (KPS) score >60 points; routine
blood, liver and kidney function.
Exclusion criteria: local and/or systemic
chemotherapy ongoing, or finished no more than 15 days before
experiments; blood coagulation disorders or severe anaemia; merging
into other primary tumours; concurrent venereal disease, leprosy,
AIDS or HIV infection, hepatitis, tuberculosis, blood parasites or
other infectious diseases.
In total, 47 patients with HCC met the above
criteria (Table I) and provided
written consent. The present study was approved by the Ethics
Committee of Fuda Cancer Hospital. Peripheral blood (17 ml) was
collected at 3 time points using ACD vacuum tubes (Becton-Dickinson
and Co., Franklin Lakes, NJ, USA) at 1 day before cryoablation, and
at 7 and 30 days after the operation.
 | Table IPatient information and baseline CTC
number. |
Table I
Patient information and baseline CTC
number.
| Group | N | No. of CTCs 1 day
before surgery |
|---|
| Age (years) |
| ≤60 | 23 | 17.04±4.22 |
| >60 | 24 | 18.33±5.73 |
|
Differentiation |
| High
differentiation | 13 | 16.62±6.87 |
| Medium/low
differentiation | 34 | 17.70±5.73 |
| Lymph node
metastasis |
| Yes | 31 | 17.65±5.86 |
| No | 16 | 17.81±5.65 |
| Clinical stage |
| III | 17 | 17.00±6.36 |
| IV | 30 | 18.10±5.40 |
Percutaneous cryoablation
Comprehensive cryoablation was performed on all 47
patients. Percutaneous cryoablation was performed under double-row
helical computed tomography (SOMATOM Emotion Duo; Siemens, Munich,
Germany) or color ultrasound (ALOKA-SSD-5500A; Aloka, Tokyo, Japan)
guidance. All cryosurgery was performed by Lizhi Niu and assistants
(Haibo Li and Feng Mu). Each procedure comprised 1–3 freeze/thaw
cycles accomplished using an argon gas-based cryosurgical unit
(Endocare Corp., Irvine, CA, USA) (21,22).
Depending on the location of the metastasis, probes were inserted
percutaneously under ultrasound or CT guidance; 2 or 5 mm probes or
rarely, 10 mm probes (Cryo-42; Endocare Corp.) were used according
to the size of the tumour. Two or more probes were simultaneously
used for large lesions. Individual tumours were frozen sequentially
on a tumour by tumour basis. The duration of freezing depended on
the formation of an 'ice ball' visible on ultrasonography as a
hypoechogenic area >1 cm larger than the diameter of the lesion.
Thawing was achieved by input of helium for a period of time equal
to the freezing time before the next freezing process was
begun.
Cell culture
HepG2 carcinoma cells obtained from Cell Resource
Center (Institute of Basic Medical Sciences, Chinese Academy of
Medical Sciences/Peking Union Medical College, Beijing, China) were
maintained in Dulbecco's modified Eagle's medium containing 10%
fetal calf serum at 37°C in a humidified atmosphere containing 5%
CO2.
Preparation of blood samples
Samples were stored at room temperature and
processed within 6 h after collection. Approximately 20 ml blood
was drawn via vein puncture from each of the 47 HCC patients and
from 10 healthy volunteers. Blood from healthy volunteers was used
to plot a standard curve for low cytometric experiments. To avoid
contamination with skin cells, 5 ml blood was discarded before
experimental samples were taken as previously described. Briefly,
mononucleocytes were separated from other blood components using
human peripheral blood lymphocyte separation liquid (Tianjin
Haoyang Biological Manufacture Co., Ltd., Tianjin, China) and
centrifugation at 1,800 × g for 20 min at 4°C. Interface cells were
removed and washed, and RBCs were removed using BD Pharm Lyse™
(Becton-Dickinson, San Jose, CA, USA). Following further washes,
mononuclear cells were counted and samples were divided into two
for RT-PCR and multiparameter flow cytometric experiments (each
sample contained at least 2–3×106 cells. Cell pellets
were resuspended in phosphate-buffered saline (PBS) (Life
Technologies, Shanghai, China) for multiparameter flow cytometry,
then in TRIzol reagent following counting using a TC10™ automatic
cell count meter (Bio-Rad, Hercules, CA, USA). Viable cells were
stained using trypan blue solution (Life Technologies, Carlsbad,
CA, USA) and stored at −70°C until needed for RNA extraction.
Flow cytometry
After separation of blood using human peripheral
blood lymphocyte separation liquid, mononucleocytes were washed
twice with sterile Hank's balanced salt solution (Life
Technologies). Isolated cells were enriched by binding to magnetic
CD326 (EpCAM) MicroBeads (Miltenyi Biotech Ltd., Bergisch Gladbach,
Germany) using magnetic-activated cell sorting (MACS). Enriched
isolated cells were then labelled with monoclonal antibodies
targeting epithelial cell antigens CD45, CD326 and cytokeratin 8,
18 and 19 (Miltenyi Biotech Ltd.) and incubated in the dark at room
temperature for 12 min. Antibodies specific for leukocytes (CD45)
labelled with phycoerythrin (PE) (10 µl), specific for
epithelial cells (cytokeratin 8, 18 and 19) labelled with
fluorescein isothiocyanate (FITC) (10 µl) and specific for
epithelial cells (CD326/Ep-CAM) labelled with allophycocyan (APC)
(10 µl) were added/7.5 ml whole blood. Cell pellets were
resuspended in 500 µl PBS and counted by flow cytometry
using a BD FACSCanto™ II apparatus (Becton-Dickinson). Cells that
were CD45-negative, CK- and CD326-positive were defined as
CTCs.
Real-time qPCR
Primers for GAPDH and tumour markers survivin,
MAGE-3 and CEA (Table II) have
been reported previously (23–26),
and were synthesized by the Shanghai Yingweijieji Corporation. RNA
was extracted from frozen samples using 1 ml TRIzol (Life
Technologies). After thawing, 0.2 ml chloroform (Guangzhou Chemical
Reagent Factory, Guangzhou, China) was added and samples were
centrifuged at 13,500 × g for 15 min at 4°C. Supernatants
containing intact RNA were placed into fresh tubes, and RNA was
precipitated with 500 µl isopropyl alcohol washed with 75%
ethanol (both from Tianjin Fuyu Fine Chemical Co., Ltd., Tianjin,
China), and dissolved in 50 µl RNase-free water. Using a
Thermo Scientific Multiskan Go (Thermo Fisher, Shanghai, China),
RNA concentration and purity were measured, and RNA was diluted to
the required concentration. Amplifications were performed in 8-tube
strips and subjected to one-step qPCR detection using SYBR-Green I
following an initial reverse transcription step. Reactions (20
µl) contained 10 µl of 2X One-Step SYBR RT-PCR buffer
4, 0.8 µl of PrimeScript Enzyme Mix 2 (both by Takara,
Dalian, China), 0.8 µl of 10 µM upstream and
downstream primers, 0.4 µl of 50X ROX Reference Dye II, 2
µl total RNA and 5.2 µl dH2O. The
reference dye was used to record the fluorescence signal reaching
the threshold cycle number (Ct) as defined in the manufacturer's
instructions (Life Technologies). Reactions were performed as
follows: reverse transcription, 42°C for 5 min, 95°C for 10 sec;
PCR, 40 cycles of 95°C for 5 sec and 60°C for 34 sec; melting
curve, 95°C for 15 sec, 60°C for 1 min, 95°C for 15 sec. PCR
experiments were stable, repeatable and did not suffer from
non-specific amplification.
 | Table IIPrimers used to amplify CTC marker
genes. |
Table II
Primers used to amplify CTC marker
genes.
| Primer name | Primer sequence
(5′–3′) | Product length
(bp) |
|---|
| MAGE-3-F | TGG AGG ACC AGA GGC
CCC C | 19 |
| MAGE-3-R | GGA CGA TTA TCA GGA
GGC CTG C | 22 |
| Survivin-F | TCC CTG GCT CCT CTA
CTG TT | 20 |
| Survivin-R | TGT CTC CTC ATC CAC
CTG AA | 20 |
| CEA-F | AAC TTC TCC TGG TCT
CTC AGC T | 22 |
| CEA-R | GCA AAT GCT TTA AGG
AAG AAG | 21 |
| GADPH-F | TGC ACC ACC AAC TGC
TTA GG | 20 |
| GADPH-R | GGA GGC AGG GAT GAT
GTT CT | 20 |
Statistical analysis
For PCR experiments, amplifications were performed
twice with each primer pair, averaged and analysis was performed on
triplicate data. PCR experiments yielded the threshold cycle number
(Ct) from the fluorescence signal based on the ΔCt method using the
equation ΔCt = CtTarget gene − CtGADPH.
Expression was expressed relative to the GADPH internal standard. A
lower ΔCt value indicates a higher level of expression. Gene
expression was measured before and after cryotherapy using the
2−ΔΔCt method as previously described (27) as follows: 2−ΔΔCt =
2− (ΔCt post-cryosurgery − ΔCt
pre-cryosurgery). After adjusting GADPH gene expression to
equal 1, 2−ΔΔCt gave the level of gene expression
relative to that before cryotherapy.
Data were analyzed using SPSS version 20.0 (IBM,
Armonk, NY, USA) and expressed as means ± SD. Random analysis of
variance was performed and P<0.05 was considered statistically
significant, whereas P<0.01 was considered statistically
significant for expression differences. GraphPad Prism version 6.0
(GraphPad Software, Inc., San Diego, CA, USA) was used to plot all
graphs.
Results
Flow cytometry
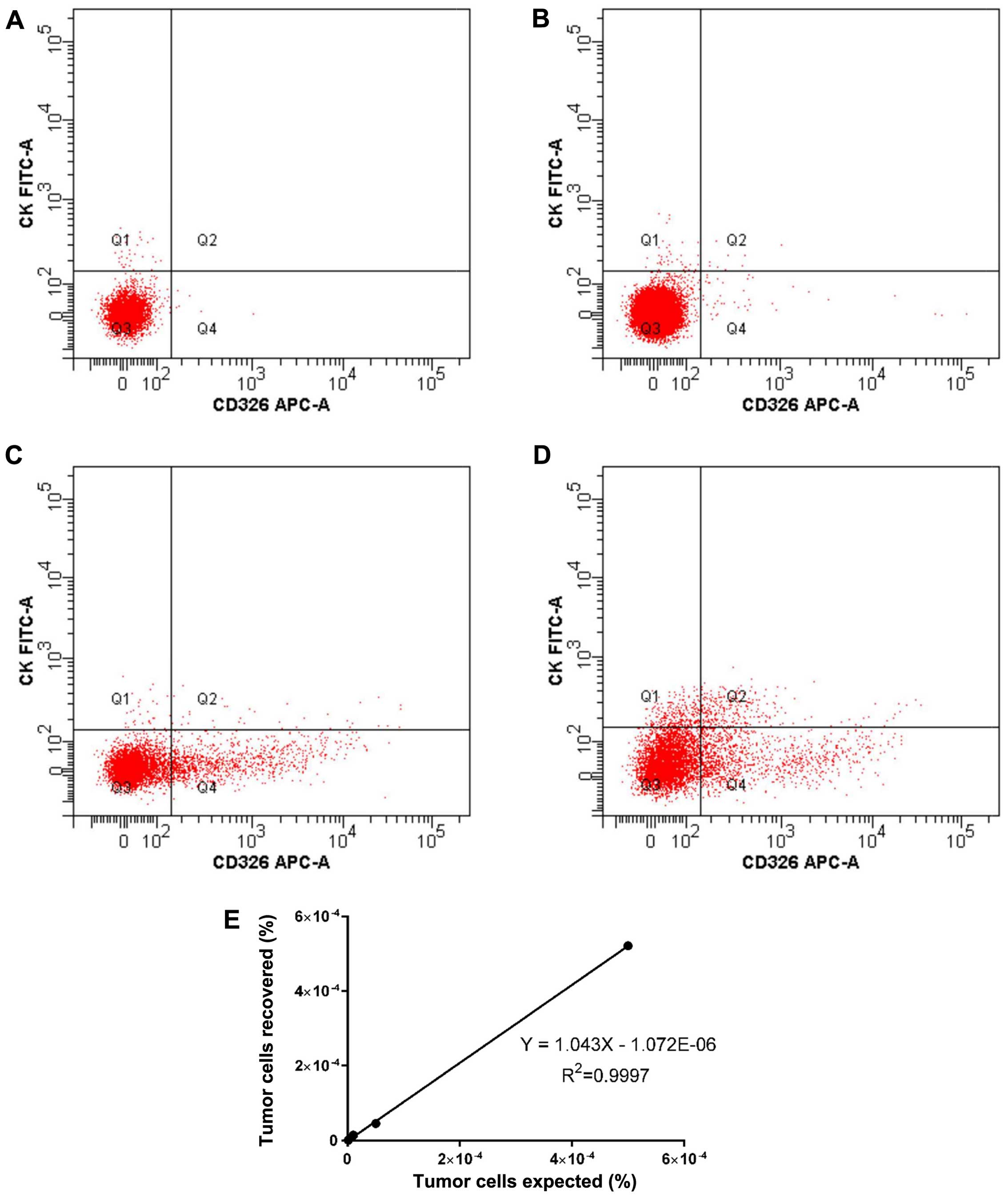
A standard curve was plotted using data from HepG2
cells from healthy volunteers, and serial dilution (0.0001, 0.001,
0.005 and 0.05%) of human HepG2 tumour cells in volunteers blood
established a lower detection limit of 0.001%, equivalent to one
cell/100,000 white blood cells (Fig.
1A–D). Below this level, background noise makes the signal
unreliable. Recovery and linearity were highly reproducible across
3 separate experiments (Fig. 1E),
and the number of tumour events recovered could be positively
correlated with the number of tumour events expected based on
serial dilution (R2=0.9998).
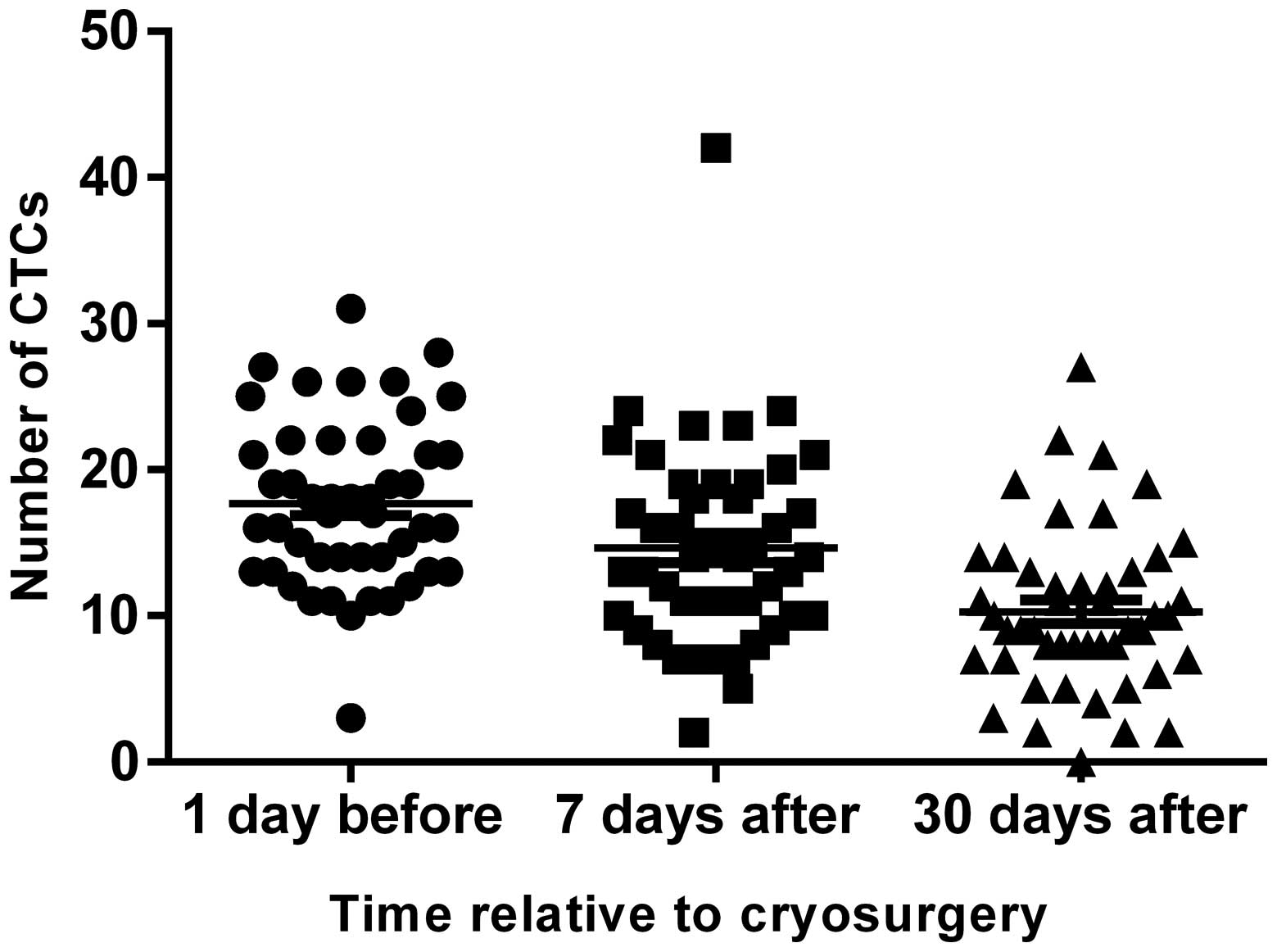
Peripheral blood CTCs from all the 47 patients was
tested at 1 day before HCC cryosurgery, and at 7 and 30 days after
surgery (Fig. 2). The number of
CTCs at 1 day before surgery was set as the baseline and was
17.70±5.725. The number of CTCs 7 and 30 days after surgery was
14.64±6.761 and 10.28±5.598, respectively. Random analysis of
variance was performed using SPSS version 20.0, which demonstrated
that the number of CTCs in peripheral blood decreased significantly
after cryosurgery (P<0.01; Table
III).
 | Table IIINumber of CTCs before and after
cryosurgery. |
Table III
Number of CTCs before and after
cryosurgery.
| Patient ID | No. of CTCs 1 day
before treatment | No. of CTCs 7 days
after treatment | No. of CTCs 30 days
after treatment |
|---|
| P1 | 11 | 8 | 4 |
| P2 | 13 | 15 | 9 |
| P3 | 12 | 13 | 8 |
| P4 | 22 | 14 | 11 |
| P5 | 19 | 7 | 3 |
| P6 | 11 | 10 | 9 |
| P7 | 22 | 17 | 12 |
| P8 | 10 | 5 | 2 |
| P9 | 18 | 7 | 7 |
| P10 | 15 | 19 | 11 |
| P11 | 22 | 12 | 8 |
| P12 | 15 | 9 | 5 |
| P13 | 17 | 22 | 15 |
| P14 | 18 | 21 | 9 |
| P15 | 28 | 16 | 8 |
| P16 | 16 | 13 | 19 |
| P17 | 12 | 9 | 11 |
| P18 | 14 | 16 | 13 |
| P19 | 21 | 17 | 22 |
| P20 | 13 | 15 | 8 |
| P21 | 14 | 11 | 7 |
| P22 | 26 | 18 | 21 |
| P23 | 16 | 14 | 12 |
| P24 | 25 | 23 | 27 |
| P25 | 21 | 14 | 17 |
| P26 | 21 | 11 | 9 |
| P27 | 27 | 12 | 10 |
| P28 | 16 | 8 | 11 |
| P29 | 13 | 11 | 14 |
| P30 | 14 | 7 | 5 |
| P31 | 18 | 15 | 13 |
| P32 | 26 | 16 | 17 |
| P33 | 24 | 18 | 10 |
| P34 | 17 | 19 | 8 |
| P35 | 25 | 13 | 10 |
| P36 | 14 | 10 | 9 |
| P37 | 13 | 19 | 6 |
| P38 | 16 | 10 | 12 |
| P39 | 19 | 11 | 8 |
| P40 | 11 | 7 | 2 |
| P41 | 18 | 21 | 14 |
| P42 | 31 | 42 | 19 |
| P43 | 19 | 24 | 14 |
| P44 | 3 | 2 | 0 |
| P45 | 26 | 20 | 5 |
| P46 | 11 | 23 | 2 |
| P47 | 19 | 24 | 7 |
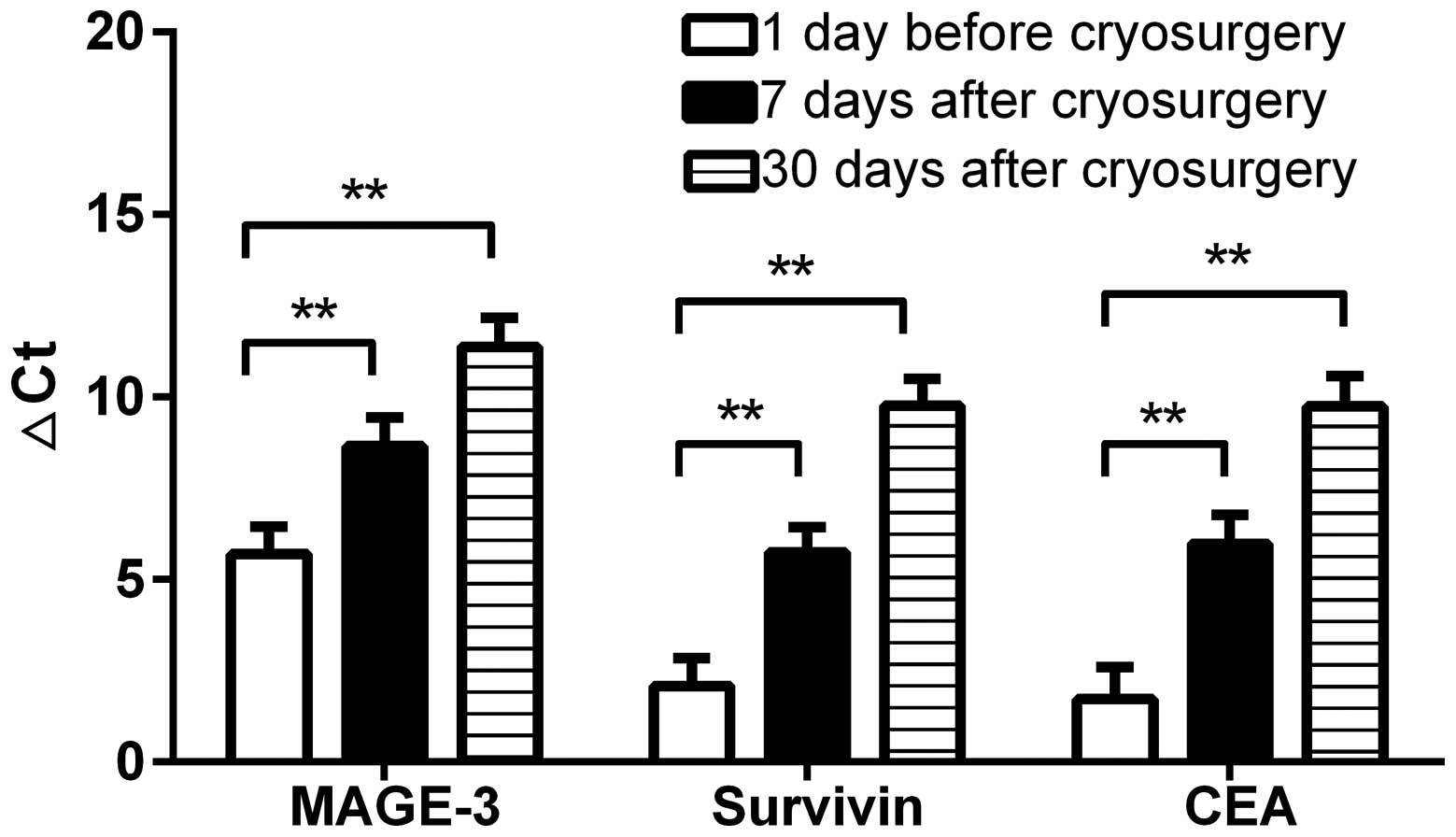
Real-time qPCR
Changes in ΔCt following
cryotherapy
In all 47 patients with locally advanced HCC, ΔCt
values of CTCs were elevated following cryotherapy, which
corresponded to a decrease in specific CTC tumour markers. This
suggests cryotherapy can reduce the number of peripheral blood CTCs
in HTC patients, which reduces the risk of tumour recurrence and
metastasis.
After cryotherapy, expression of different tumour
markers decreased by different amounts. The preoperative MAGE-3 ΔCt
value was 5.71±5.17, compared with a 7-day postoperative rise to
8.65±5.41, and a 30-day postoperative rise to 11.37±5.50. The
preoperative survivin ΔCt value was 2.09±5.16, compared with a 7-
and 30-day postoperative rise to 5.74±4.85 and 9.77±5.02,
respectively. The CEA ΔCt value increased from a preoperative value
of 1.73±5.99, to a 7-day postoperative value of 5.98±5.36, and a
postoperative 30-day value of 9.75±5.73. Random analysis of
variance using SPSS 17.0 showed that cryotherapy clearly increased
the ΔCt value of CTC markers (P<0.01; Fig. 3).
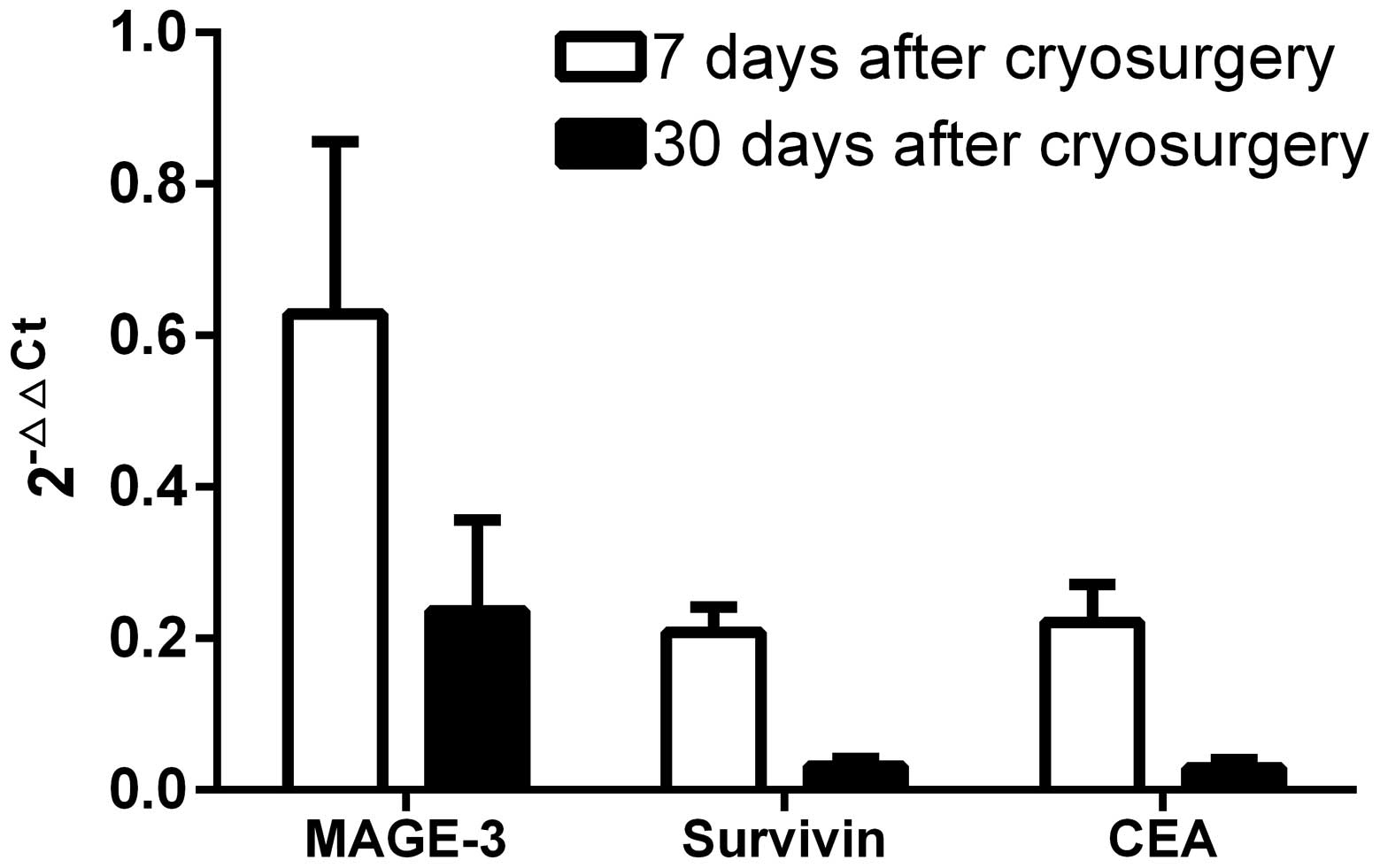
Changes in 2−ΔΔCt following
cryotherapy
Changes in 2−ΔΔCt values were assessed to
determine gene expression before and after cryotherapy. MAGE-3 was
0.63±1.56 and 0.24±0.82 at 7 and 30 days after cryosurgery,
respectively, compared with 0.21±0.22 and 0.03±0.07 for survivin,
and 0.22±0.34 and 0.02±0.08 for CEA (Fig. 4). 2−ΔΔCt values
correspond to the fold-change in relative gene expression, and
since all postoperative values were <1, cryosurgery clearly
decreased CTC markers, and the decrease was larger over time.
Discussion
Hepatocellular carcinoma (HCC) is the third most
common cause of cancer-related deaths worldwide. At present, tumour
resection and liver transplantation are the most effective
treatments (28,29), but unresectable lesions are treated
using ablation therapies such as percutaneous ethanol injection,
radiofrequency ablation (RFA), cryoablation, laser treatment,
high-intensity focused ultrasound and microwave treatment (4). Argon-helium cryotherapy has also been
tested on HCC (5) and was shown to
cause greater damage to tumour tissues (6). Additionally, treatment areas are more
easily discernible, and the therapy can successfully suppress
ectopic tumours. Although these treatments have decreased HCC
mortality, recurrence in advanced HCC patients is common and
difficult to prevent. Nowadays, serum AFP, a secretory protein, is
widely used for diagnosing HCC patients and monitoring disease
progression, but it has a sensitivity ranging from 39–97% and a
specificity ranging from 76–95%, even when used to screen high-risk
populations (30–32). A more reliable biomarker is ideally
needed in the clinic, a circulating tumour cells (CTCs) may provide
a more sensitive and robust circulating biomarker. CTCs are cancer
cells that have been shed from either the primary tumour or its
metastases and that circulate in the peripheral blood. While
metastases are directly responsible for the majority of cancer
deaths, CTCs may constitute seeds for metastases and may indicate
the spread of disease (8,9).
CTCs in liquid biopsies can provide information on
the risk of relapse, can help to determine which specific adjuvant
therapies may be appropriate, and can be used to monitor responses
to treatment (10–12). For advanced HCC patients with or
without other organ metastasis, conventional treatments generally
have little effect. Argon-helium knife cryoablation therapy is a
novel local treatment that is minimally invasive, thus
intraoperative complications such as bleeding and infection are
less prevalent than in conventional surgery. However, as with
conventional surgery, the risk of postoperative blood or lymphatic
metastasis is high, and tumour recurrence, metastasis and
ultimately death may result. Improving the survival and quality of
life for patients with locally advanced disease remains a priority,
and new methods for diagnosis and establishing prognosis are much
needed.
Counting the number of CTCs may aid cancer diagnosis
and help predict the likelihood of recurrence, and can also be used
to monitor the effectiveness of postoperative radiotherapy and
chemotherapy (33). Additionally,
dynamic detection of peripheral blood CTCs is likely to become a
reliable prognostic indicator for locally advanced hepatocellular
cancer patients, and may help to quickly identify those with a
high-risk of recurrence, thus improving survival rate and quality
of life. The isolation and identification of CTCs have developed
rapidly in recent years, and fluorescence-activated cell sorting
(FACS) combined with magnetic-activated cell sorting (MACS)
quantitatively analyzes and sort single cells and biological
particles at the functional level. This technique can analyze
thousands of cells at high speed, and can detect multiple
parameters of a single cell simultaneously, which is a big
advantage over conventional fluorescent approaches in terms of
speed and precision. Flow cytometric detection of peripheral blood
CTCs is dependent on the expression of tumour-specific markers such
as cytokeratins (CKs) on the surface of epithelial cells. CKs are
proteins that consist of keratin-containing intermediate filaments
that form the intracytoplasmic cytoskeleton, and their expression
primarily depends on the type of epithelia, the degree of terminal
differentiation and the stage of development (34). In many cases, cytokeratin expression
in tumours and peripheral blood has prognostic significance for
cancer patients, and CK8/18/19 expression has been used as a
biomarker for HCC histopathology (35,36).
In order to reduce the occurrence of false negatives, we used CD326
(EpCAM) as an additional specific marker for positive selection
(37), and used CD45 for negative
selection of leukocytes (38). Flow
cytometry can then be used to detect double-positive CTCs
(CD45−, CK+ and CD326+). In the
present study, we applied this method to detect peripheral blood
CTCs in 47 patients with locally advanced HCC. After cryoablation
therapy, the number of peripheral blood CTCs was markedly decreased
(P<0.01), indicating potential usefulness for prognostic
evaluation of cryosurgery. Although promising, at present there is
no effective method for evaluating the surgical success of
argon-helium knife cryoablation, and the results of the present
study may provide a breakthrough in this area.
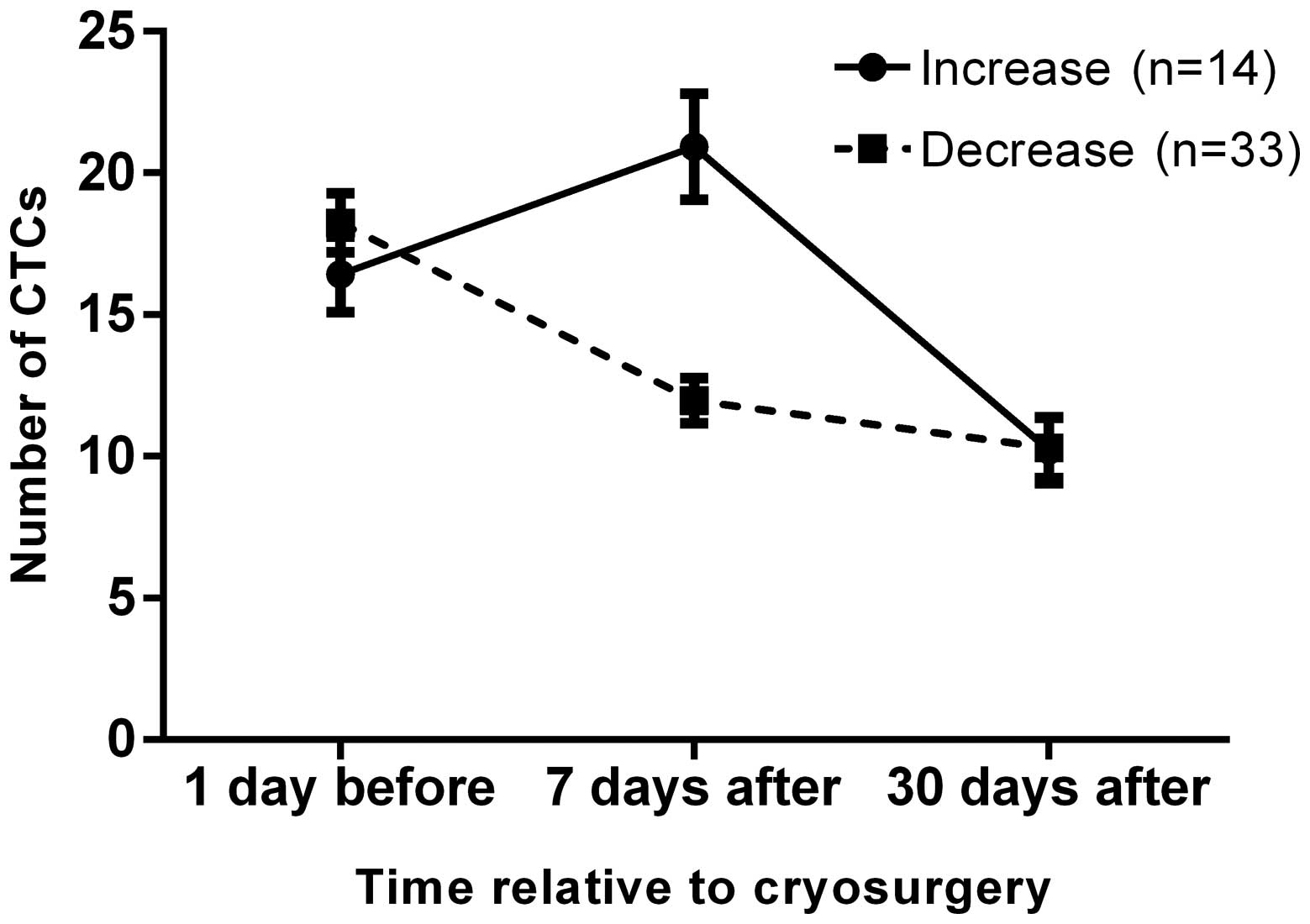
At 7 days after surgery, the number of CTCs in
peripheral blood increased in 14/47 patients (29.79%) compared with
preoperative numbers, but all patients exhibited a marked decrease
at 30 days after surgery (Fig. 5).
This may be due to the large number of CTCs released into the blood
during surgery and a delay in their removal by the immune system.
The initial postoperative rise may be associated with immunity
following cryoablation, since tumours release antigen that can lead
to 'high zonye tolerance' immunosuppression (39). This can reduce the ability of the
immune system to recognise tumour cells, but immune enhancement
could reverse this process to decrease CTCs by 30 days post-surgery
(40).
RT-qPCR is a commonly used and effective method for
measuring gene expression and detecting CTCs. This method is highly
sensitive, quantitative, rapid, non-polluting and facilitates
monitoring in real-time. RT-PCR also overcomes the high rate of
false positives that can be a problem for traditional PCR-based
methods. In the present study, we used an RT-qPCR method to measure
expression of the reference gene GADPH, along with the
metastasis-associated markers MAGE-3, survivin and CEA in CTCs from
47 locally advanced HCC patients before and after cryotherapy. The
results showed that CTCs in peripheral blood decreased following
cryosurgery (P<0.01), indicating a lower risk of tumour
recurrence and metastasis following this type of therapeutic
intervention. Patients expressing high levels of these CTC markers
are likely to have poor prognosis with increased risk of recurrence
and/or metastasis. Our method is therefore suitable for evaluation
of cryotherapy.
Numerous tumour treatments involve local surgical
excision of legions, and while initial results are often promising,
recurrence and/or metastasis can occur, and eradication of all
cancerous cells can be very difficult to achieve. Such as all
existing cancer treatments, cryosurgery is not perfect, and changes
in the immune system following surgery can influence the
therapeutic outcome. Our results indicate that detection of CTCs in
liquid biopsy experiments could help to determine whether
cryosurgery is likely to be successful. In the future, detection of
CTCs could conceivably replace radioscopy for early detection of
cancers and/or re-examination following surgery and postoperative
radiotherapy and chemotherapy.
Acknowledgments
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
Institutional and/or National Research Committee and with the 1964
Helsinki declaration and its later amendments or comparable ethical
standards.
References
|
1
|
Hu KQ: Advances in clinical application of
cryoablation therapy for hepatocellular carcinoma and metastatic
liver tumor. J Clin Gastroenterol. 48:830–836. 2014.PubMed/NCBI
|
|
2
|
Maluccio M and Covey A: Recent progress in
understanding, diagnosing, and treating hepatocellular carcinoma.
CA Cancer J Clin. 62:394–399. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Farazi PA and DePinho RA: The genetic and
environmental basis of hepatocellular carcinoma. Discov Med.
6:182–186. 2006.
|
|
4
|
Lencioni R: Loco-regional treatment of
hepatocellular carci noma. Hepatology. 52:762–773. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sheen AJ and Siriwardena AK: The end of
cryotherapy for the treatment of nonresectable hepatic tumors? Ann
Surg Oncol. 12:202–204. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hutchinson M, Shyn P and Silverman S:
Cryoablation of Liver Tumors. Image-Guided Cancer Therapy. Dupuy
DE, Fong Y and McMullen WN: Springer; New York: pp. 491–503. 2013,
View Article : Google Scholar
|
|
7
|
Ling S, Tian Y, Zhang H, Jia K, Feng T,
Sun D, Gao Z, Xu F, Hou Z, Li Y, et al: Metformin reverses
multidrug resistance in human hepatocellular carcinoma
Bel-7402/5-fluorouracil cells. Mol Med Rep. 10:2891–2897.
2014.PubMed/NCBI
|
|
8
|
Alix-Panabières C and Pantel K:
Circulating tumor cells: Liquid biopsy of cancer. Clin Chem.
59:110–118. 2013. View Article : Google Scholar
|
|
9
|
Nguyen DX, Bos PD and Massagué J:
Metastasis: From dissemination to organ-specific colonization. Nat
Rev Cancer. 9:274–284. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kang Y and Pantel K: Tumor cell
dissemination: Emerging biological insights from animal models and
cancer patients. Cancer Cell. 23:573–581. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gorges TM and Pantel K: Circulating tumor
cells as therapy-related biomarkers in cancer patients. Cancer
Immunol Immunother. 62:931–939. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lianidou ES, Markou A and Strati A:
Molecular characterization of circulating tumor cells in breast
cancer: Challenges and promises for individualized cancer
treatment. Cancer Metastasis Rev. 31:663–671. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hou JM, Greystoke A, Lancashire L,
Cummings J, Ward T, Board R, Amir E, Hughes S, Krebs M, Hughes A,
et al: Evaluation of circulating tumor cells and serological cell
death biomarkers in small cell lung cancer patients undergoing
chemotherapy. Am J Pathol. 175:808–816. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Krebs MG, Sloane R, Priest L, Lancashire
L, Hou JM, Greystoke A, Ward TH, Ferraldeschi R, Hughes A, Clack G,
et al: Evaluation and prognostic significance of circulating tumor
cells in patients with non-small-cell lung cancer. J Clin Oncol.
29:1556–1563. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cristofanilli M, Budd GT, Ellis MJ,
Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ,
Terstappen LW, et al: Circulating tumor cells, disease progression,
and survival in metastatic breast cancer. N Engl J Med.
351:781–791. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hayes DF, Cristofanilli M, Budd GT, Ellis
MJ, Stopeck A, Miller MC, Matera J, Allard WJ, Doyle GV and
Terstappen LW: Circulating tumor cells at each follow-up time point
during therapy of metastatic breast cancer patients predict
progression-free and overall survival. Clin Cancer Res.
12:4218–4224. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cohen SJ, Punt CJ, Iannotti N, Saidman BH,
Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC, et
al: Relationship of circulating tumor cells to tumor response,
progression-free survival, and overall survival in patients with
metastatic colorectal cancer. J Clin Oncol. 26:3213–3221. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
de Bono JS, Scher HI, Montgomery RB,
Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ
and Raghavan D: Circulating tumor cells predict survival benefit
from treatment in metastatic castration-resistant prostate cancer.
Clin Cancer Res. 14:6302–6309. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pantel K, Brakenhoff RH and Brandt B:
Detection, clinical relevance and specific biological properties of
disseminating tumour cells. Nat Rev Cancer. 8:329–340. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cristofanilli M: Circulating tumor cells,
disease progression, and survival in metastatic breast cancer.
Semin Oncol. 33(Suppl 9): S9–S14. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu KC, Niu LZ, He WB, Hu YZ and Zuo JS:
Percutaneous cryosurgery for the treatment of hepatic colorectal
metastases. World J Gastroenterol. 14:1430–1436. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Niu LZ, Li JL and Xu KC: Percutaneous
cryoablation for liver cancer. J Clin Transl Hepatol. 2:182–188.
2014.
|
|
23
|
Lin CH, Chao LK, Hung PH and Chen YJ: EGCG
inhibits the growth and tumorigenicity of nasopharyngeal
tumor-initiating cells through attenuation of STAT3 activation. Int
J Clin Exp Pathol. 7:2372–2381. 2014.PubMed/NCBI
|
|
24
|
Hussein YM, Ghareib AF, Mohamed RH, Radwan
MI and Elsawy WH: MAGE-3 and MAGE-4 genes as possible markers for
early detection of metastases in hepatitis C virus Egyptian
patients complicated by hepatocellular carcinoma. Med Oncol.
29:994–999. 2012. View Article : Google Scholar
|
|
25
|
Hu Y, Fan L, Zheng J, Cui R, Liu W, He Y,
Li X and Huang S: Detection of circulating tumor cells in breast
cancer patients utilizing multiparameter flow cytometry and
assessment of the prognosis of patients in different CTCs levels.
Cytometry A. 77:213–219. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kodera Y, Nakanishi H, Ito S, Yamamura Y,
Kanemitsu Y, Shimizu Y, Hirai T, Yasui K, Kato T and Tatematsu M:
Quantitative detection of disseminated free cancer cells in
peritoneal washes with real-time reverse transcriptase-polymerase
chain reaction: A sensitive predictor of outcome for patients with
gastric carcinoma. Ann Surg. 235:499–506. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
28
|
Poon RT and Fan ST: Hepatectomy for
hepatocellular carcinoma: Patient selection and postoperative
outcome. Liver Transpl. 10(Suppl 1): S39–S45. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ng KK, Lo CM, Chan SC, Chok KS, Cheung TT
and Fan ST: Liver transplantation for hepatocellular carcinoma: The
Hong Kong experience. J Hepatobiliary Pancreat Sci. 17:548–554.
2010. View Article : Google Scholar
|
|
30
|
Gutman S and Kessler LG: The US Food and
Drug Administration perspective on cancer biomarker development.
Nat Rev Cancer. 6:565–571. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Soresi M, Magliarisi C, Campagna P, Leto
G, Bonfissuto G, Riili A, Carroccio A, Sesti R, Tripi S and
Montalto G: Usefulness of alpha-fetoprotein in the diagnosis of
hepatocellular carcinoma. Anticancer Res. 23:1747–1753.
2003.PubMed/NCBI
|
|
32
|
Sanai FM, Sobki S, Bzeizi KI, Shaikh SA,
Alswat K, Al-Hamoudi W, Almadi M, Al Saif F and Abdo AA: Assessment
of alpha-fetoprotein in the diagnosis of hepatocellular carcinoma
in Middle Eastern patients. Dig Dis Sci. 55:3568–3575. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Saad F and Pantel K: The current role of
circulating tumor cells in the diagnosis and management of bone
metastases in advanced prostate cancer. Future Oncol. 8:321–331.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Franke WW, Schmid E, Osborn M and Weber K:
Intermediate-sized filaments of human endothelial cells. J Cell
Biol. 81:570–580. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kakehashi A, Kato A, Inoue M, Ishii N,
Okazaki E, Wei M, Tachibana T and Wanibuchi H: Cytokeratin 8/18 as
a new marker of mouse liver preneoplastic lesions. Toxicol Appl
Pharmacol. 242:47–55. 2010. View Article : Google Scholar
|
|
36
|
Tsuchiya K, Komuta M, Yasui Y, Tamaki N,
Hosokawa T, Ueda K, Kuzuya T, Itakura J, Nakanishi H, Takahashi Y,
et al: Expression of keratin 19 is related to high recurrence of
hepatocellular carcinoma after radiofrequency ablation. Oncology.
80:278–288. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Racila E, Euhus D, Weiss AJ, Rao C,
McConnell J, Terstappen LW and Uhr JW: Detection and
characterization of carcinoma cells in the blood. Proc Natl Acad
Sci USA. 95:4589–4594. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pachmann K, Heiss P, Demel U and Tilz G:
Detection and quantification of small numbers of circulating tumour
cells in peripheral blood using laser scanning cytometer (LSC).
Clin Chem Lab Med. 39:811–817. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Whiteside TL: What are regulatory T cells
(Treg) regulating in cancer and why? Semin Cancer Biol. 22:327–334.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Misao A, Sakata K, Saji S and Kunieda T:
Late appearance of resistance to tumor rechallenge following
cryosurgery. A study in an experimental mammary tumor of the rat.
Cryobiology. 18:386–389. 1981. View Article : Google Scholar : PubMed/NCBI
|