Introduction
Epithelial ovarian cancer (EOC) is one of the most
malignant gynecological cancers worldwide (1,2). In
the United States alone, there were more than 20,000 estimated new
cases, and more than 14,000 deaths due to EOC (3). Both genetic and epigenetic factors may
contribute significantly to the origin of EOC (2). Despite numerous efforts, the exact
mechanisms of EOC pathogenesis are fundamentally unknown, and
methods for early diagnosis and novel therapies are largely
lacking. Thus, it is critical to explore the molecular mechanisms
underlying EOC carcinogenesis, development and metastasis to seek
novel therapeutic targets for patients with EOC.
MicroRNAs (miRNAs) are a group of small-length
(18–22 nucleotides long) non-coding RNAs that bind the
complementary sites of the 3′-untranslated region (3′-UTR) of
target genes to induce gene inhibition and protein degradation
(4,5). miRNAs have been shown to play critical
roles in human cancer, acting as either oncogenes or tumor
suppressors (6,7). In human EOC, both upregulated and
downregulated miRNAs have been discovered (8), suggesting that the regulation of
miRNAs in EOC may be complex, as well as their associated signaling
pathways. MicroRNA-127-3p (miR-127-3p), also referred to as
miR-127, was often found to be downregulated in human tumors and
acts as a tumor suppressor in breast and gastric cancer (9,10).
miR-127-3p was also found to be downregulated in clinical samples
from EOC patients (11,12). Yet, the functional mechanisms of
miR-127-3p have never been elucidated in EOC.
In the present study, we evaluated the expression
pattern of miR-127-3p in both EOC cell lines and EOC tumor samples.
Then, we hypothesized that miR-127-3p may act as a tumor suppressor
in EOC as in other human cancers, and tested this hypothesis by
endogenously overexpressing miR-127-3p in EOC cell lines, OVCAR-3
and Caov-3, through lentiviral transduction. The functional effects
of miR-127-3p overexpression on EOC proliferation, drug (bufalin)
sensitivity, invasion and in vivo tumorigenicity were then
carefully evaluated. Furthermore, we hypothesized that the
Bcl-2-associated athanogene 5 (BAG5) gene is the downstream target
of miR-127-3p in EOC. We then tested this hypothesis through
dual-luciferase reporter assay and qRT-PCR. Subsequently, BAG5 was
upregulated in OVCAR-3 and Caov-3 cells, and its interaction with
miR-127-3p overexpression on regulating EOC was further
investigated.
Materials and methods
Statement of ethics
All experimental protocols were approved by the
ethics committees at the participating hospitals. All procedures
were conducted in accordance to the principles of the Declaration
of Helsinki, and Local and National Medical Practice Laws. Consent
forms were signed by all participating patients.
Cell lines and patients
In this study, five EOC cell lines, SKOV-3, OVCAR-3,
Caov-3, ES-2, PA-1 and a non-tumorigenic human-derived ovarian cell
line, HS-832, were obtained from the American Type Culture
Collection (ATCC; Manassas, VA, USA). Four EOC cell lines, MCAS,
OVCA432, OVCA429 and PEO4 were obtained from the Cell Bank of the
Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China). All cells were maintained in RPMI-1640 medium
supplemented with 10% fetal bovine serum (FBS) (both from Thermo
Fisher Scientific, USA) in a tissue culture chamber supplied with
95% CO2 and 5% CO2 at 37°C. The clinical
samples of paired EOC tumors (tumor) and their adjacent normal
ovarian epithelial tissues (normal) were surgically obtained from
13 patients between June 2012 to December 2015. Clinical samples
were snap-frozen in liquid nitrogen and stored in an −80°C
bio-freezer (Forma Scientific, USA) until processed.
RNA isolation and quantitative
RT-PCR
Total RNA was isolated from the EOC cell lines or
EOC patient clinical samples using an RNeasy Mini kit (Qiagen, USA)
according to the manufacturer's protocol. Using 100 ng RNA from
each sample, cDNA was reversely synthesized with a TaqMan Reverse
Transcription kit (Applied Biosystems, USA). Quantitative RT-PCR
(qRT-PCR) was conducted on an ABI Prism 7900 Sequence detection
system (Applied Biosystems). For BAG5 gene detection, a SYBR Green
PCR Master Mix kit (Applied Biosystems) was conducted with the
following reaction conditions, 95°C for 15 min; 38 cycles of 95°C
for 30 sec, 58°C for 40 sec, and 70°C for 30 sec. 18S was used as a
loading control. For miR-127-3p detection, a TaqMan miRNA assay
(Applied Biosystems) was conducted with the following reaction
conditions, 95°C for 15 min; and 38 cycles of 95°C for 30 sec and
62°C for 40 sec. U6 snRNA was used as a loading control. All
reactions were conducted in biological triplicates. Relative gene
expression levels were measured as fold changes using the
2−∆∆Ct method.
miR-127-3p overexpression
A lentiviral vector expressing miR-127-3p mimics
(L-miR127-Mimic), and a control lentivirus (L-C) were obtained from
RiboBio Biotech (China). To overexpress hsa-miR-127-3p in EOC cell
lines, 5×105 OVCAR-3 or Caov-3 cells were transduced
with L-miR127-Mimic, along with a multiplicity of infection of 10
and 5 µg/ml Polybrene for 4 h. The control EOC cells were
transduced with L-C. After 3 washes (5 min each time), the EOC
cells were replenished with fresh culture medium and maintained for
48 h. After that, floating cells were aspirated. Healthy cells were
suspended, re-seeded and readied for the next experiments.
Cancer proliferation assay
Five hundred OVCAR-3 or Caov-3 cells were seeded in
a 96-well plate. The growth of EOC cells was measured by a
CellTiter 96® Aqueous One cell proliferation assay
(Promega, Madison, WI, USA) according the manufacturer's protocol.
Briefly, proliferation solution (20 µl) was added into the
cell culture for 1 h at 0, 1, 2, 3 and 4 days. The absorbance was
measured at 490 nm.
Bufalin sensitivity assay
OVCAR-3 or Caov-3 cells were incubated with bufalin
at various concentrations (0, 0.1, 0.5, 1, 10 and 100 ng/ml) for 48
h. The sensitivity of the EOC cells to bufalin was estimated by
relative cell viability, which was measured by proliferation assay
and then normalizing the absorbance values to the ones under
control conditions (no bufalin treatment).
Invasion assay
In vitro invasive capability was measured by
a wound closure assay. Briefly, 5×103 OVCAR-3 or Caov-3
cells were seeded in 96-well plates. Once confluent, the cells were
incubated with 10 µg/ml mitomycin (Sigma-Aldrich, USA) for 2
h to stop proliferation. Then, a 96-pin wound-maker (Essen
Biosciences, UK) was used to create defined wound areas.
Phase-contrast images were captured immediately (0 h) and 24 h
after wound creation. The invasive capability was determined by
measuring the reduction on the wound area between 0 and 24 h using
ImageJ software (NIH, Bethesda, MA, USA).
Tumorigenicity assay
OVCAR-3 cells transduced with L-miR127-Mimic or L-C
were subcutaneously injected into both flanks of four adult athymic
nude mice (1×106/injection in 8-week old mice). Tumor
volume (mm3) was monitored weekly for 5 weeks, using the
formula L × W2/2, in which L and W are the longest and
shortest diameters respectively of the tumor. After 5 weeks, the
mice were sacrificed. Tumors from both flanks were extracted from 4
mice (M1–M4) for visual examination.
Luciferase reporter assay
Wild-type and mutant 3′-UTRs of the human BAG5 gene
were cloned into the psiCHECK2 luciferase vector (Promega). HEK293T
cells were seeded in 6-well plates and co-transfected with
BAG5-WildType, BAG5-Mutant or Renilla luciferase vectors,
and L-C or L-miR-Mimic lentiviruses. Forty-eight hours after
co-transfection, a dual-luciferase reporter assay (Promega) was
conducted according to the manufacturer's protocol. Relative
luciferase activities (against Renilla activity) were
normalized to the activity with L-C transfection.
BAG5 upregulation assay
The whole sequence of the human BAG5 gene was cloned
into a eukaryotic expression vector pcDNA3.1 (Life Technologies,
USA) to generate a BAG5 overexpressing vector (pcDNA/BAG5). An
empty pcDNA3.1 vector (pcDNA/+) was used as the control. OVCAR-3
and Caov-3 cells were then transfected with pcDNA/BAG5 or pcDNA/+
using Lipofectamine 2000 reagent (Thermo Fisher Scientific).
Forty-eight hours after transfection, qRT-PCR was conducted to
verify upregulation efficiency.
Statistical analysis
All assays were repeated at least three times.
Results are presented as means ± standard errors. Statistic
analyses were conducted using the Student's t-test on Windows-based
SPSS software (SPSS 11.0; SPSS, Inc., USA). Statistical differences
were significant at P<0.05.
Results
miR-127-3p is downregulated in EOC cell
lines and EOC tumors
The expression of miR-127-3p was compared among nine
EOC cell lines, SK-OV-3, OVCAR-3, Caov-3, ES-2, PA-1, MCAS,
OVCA432, OVCA429, PEO4 and a non-tumorigenic human-derived ovarian
cell line, HS-832 by qRT-PCR. It showed that miR-127-3p was
markedly downregulated in all examined EOC cell lines, when
compared with its level in the HS-832 cells (Fig. 1A, P<0.05). The expression of
miR-127-3p was also compared, by qRT-PCR, between paired EOC tumors
(T) and adjacent normal ovarian epithelial tissues (normal) in 13
patients with EOC. The result demonstrated that miR-127-3p was
downregulated in the EOC tumors compared to the level in the normal
ovarian tissues (Fig. 1B,
P<0.05).
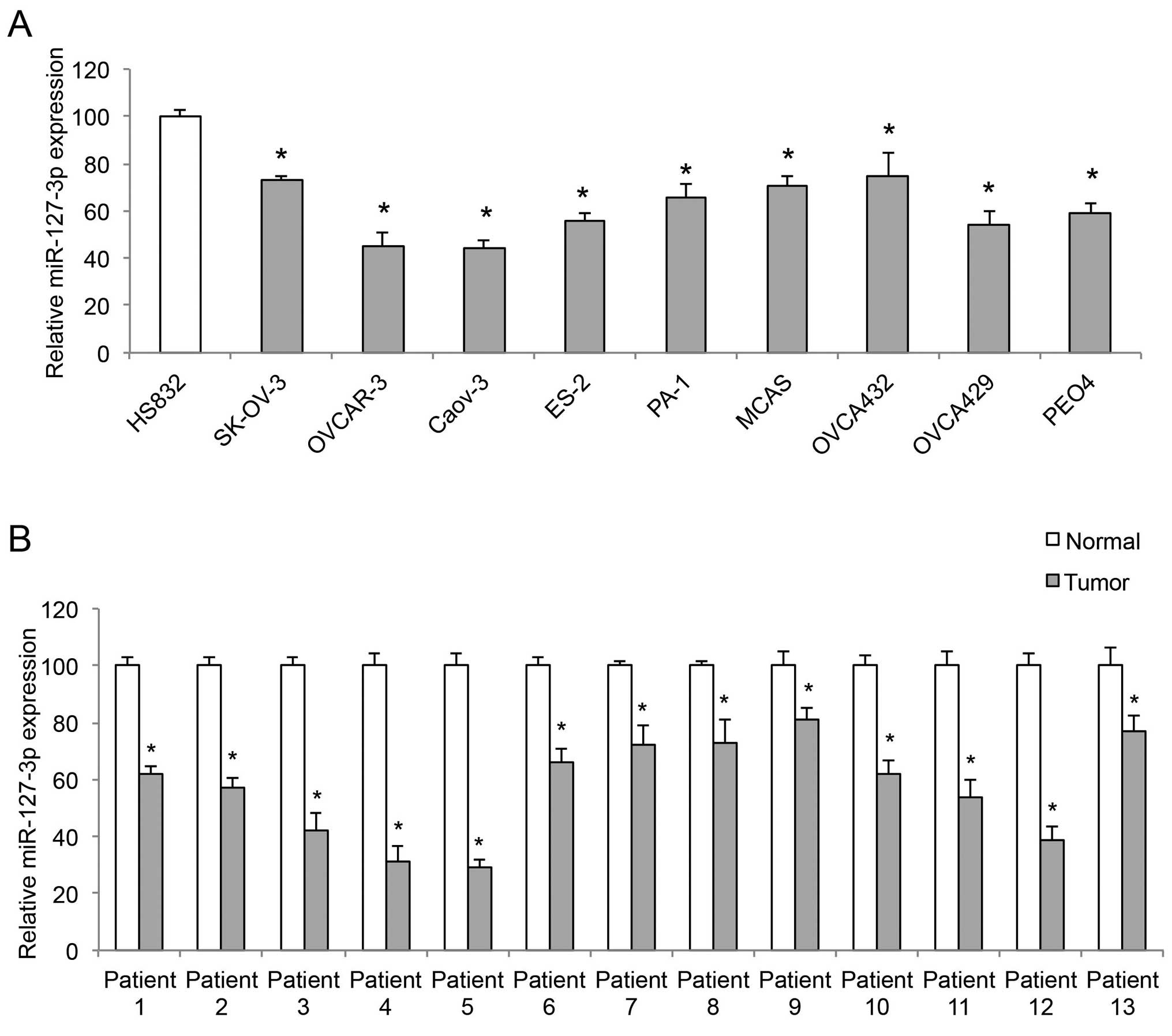 | Figure 1miR-127-3p is downregulated in EOC.
(A) Quantitative RT-PCR (qRT-PCR) was conducted to compare the gene
levels of miR-127-3p among 9 EOC cell lines, SK-OV-3, OVCAR-3,
Caov-3, ES-2, PA-1, MCAS, OVCA432, OVCA429, PEO4, and a
non-tumorigenic human-derived ovarian cell line, HS-832
(*P<0.05). (B) qRT-PCR was conducted to compare
paired clinical samples, EOC tumors (tumor) vs. adjacent normal
ovarian tissues (normal), from 13 EOC patients
(*P<0.05). |
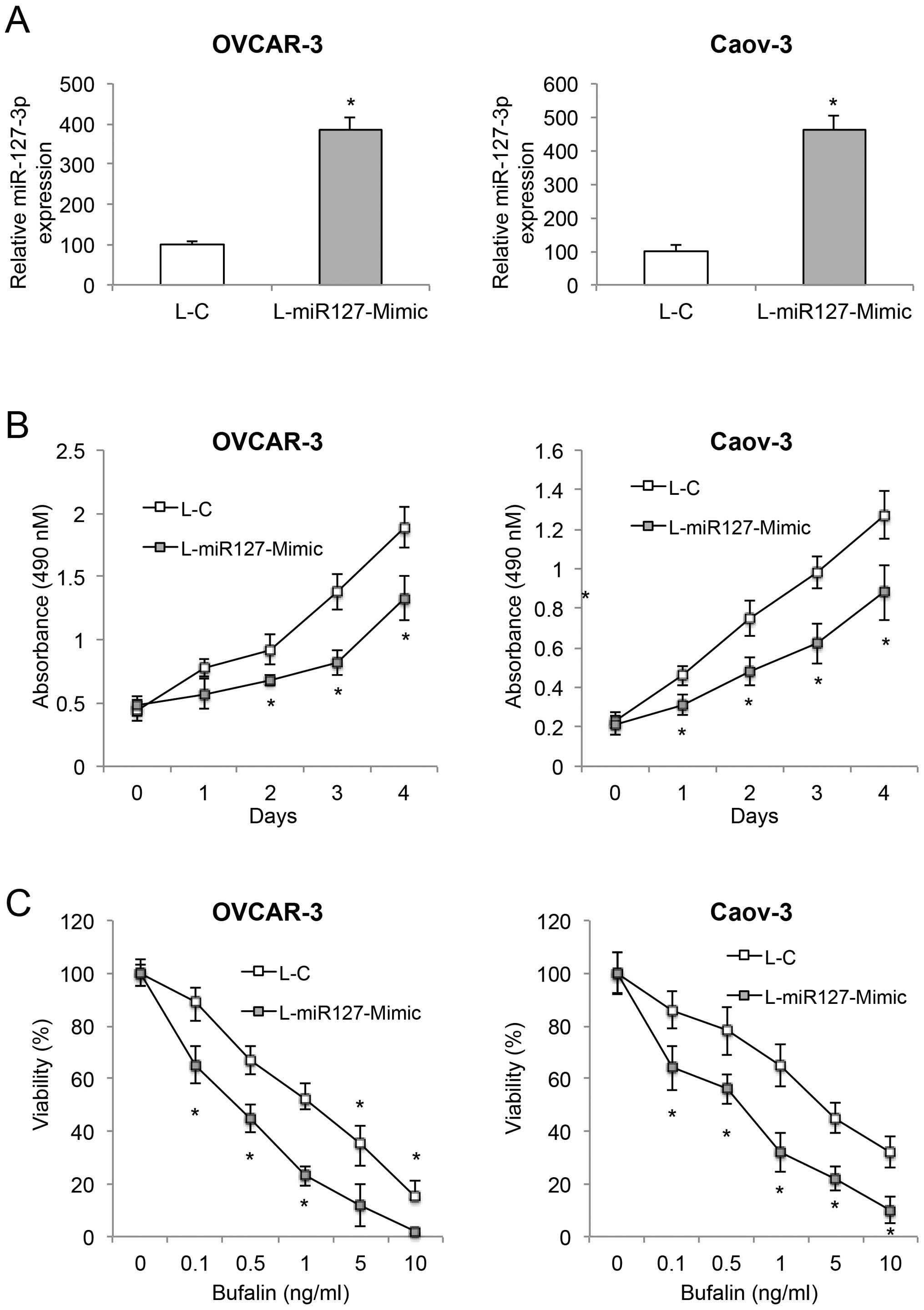
miR-127-3p overexpression inhibits cancer
growth and increases bufalin sensitivity in EOC
Two of the EOC cell lines, OVCAR-3 and Caov-3, were
transduced with L-miR127-Mimic to overexpress endogenous
miR-127-3p. The control EOC cells were transduced with an empty
control lentivirus, L-C. After transduction, qRT-PCR was conducted
to compare miR-127-3p expression between the EOC cells transduced
with L-miR127-mimic or L-C. Transduction of L-miR127-Mimic, as
compared to L-C, markedly upregulated endogenous miR-127-3p in both
the OVCAR-3 and Caov-3 cells (Fig.
2A, P<0.05).
We then compared cancer cell growth in the
lentiviral-transduced EOC cells. The proliferation assay showed
that miR-127-3p overexpression significantly inhibited cancer
growth in the OVCAR-3 cells 2 days after the onset of the
proliferation assay, and in Caov-3 cells 24 h after the onset of
the proliferation assay (Fig. 2B,
P<0.05). We also assessed the effect of miR-127-3p on EOC
bufalin sensitivity. miR-127-3p overexpression significantly
increased bufalin sensitivity by reducing cell viability in both
the OVCAR-3 and Caov-3 cells (Fig.
2C, P<0.05).
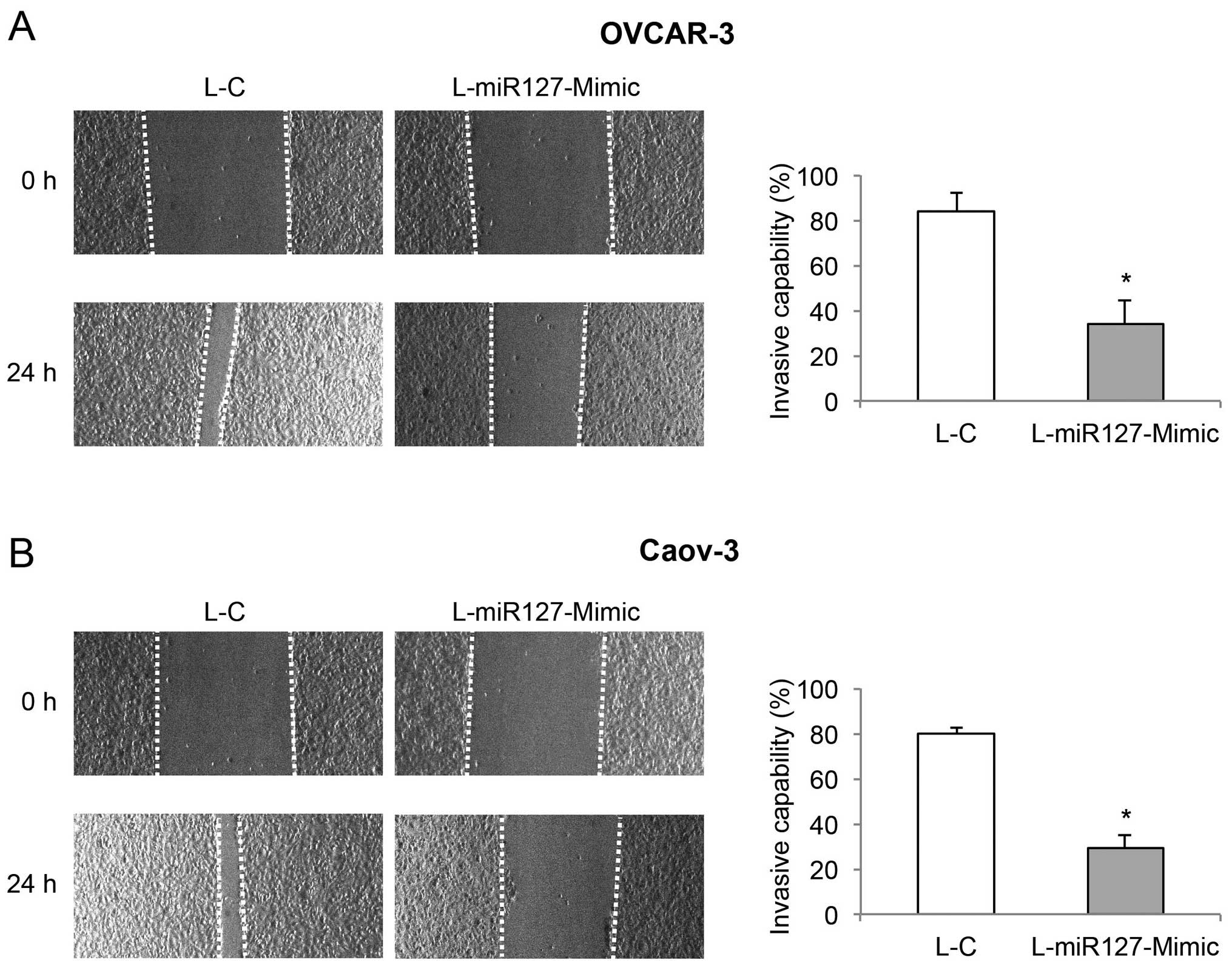
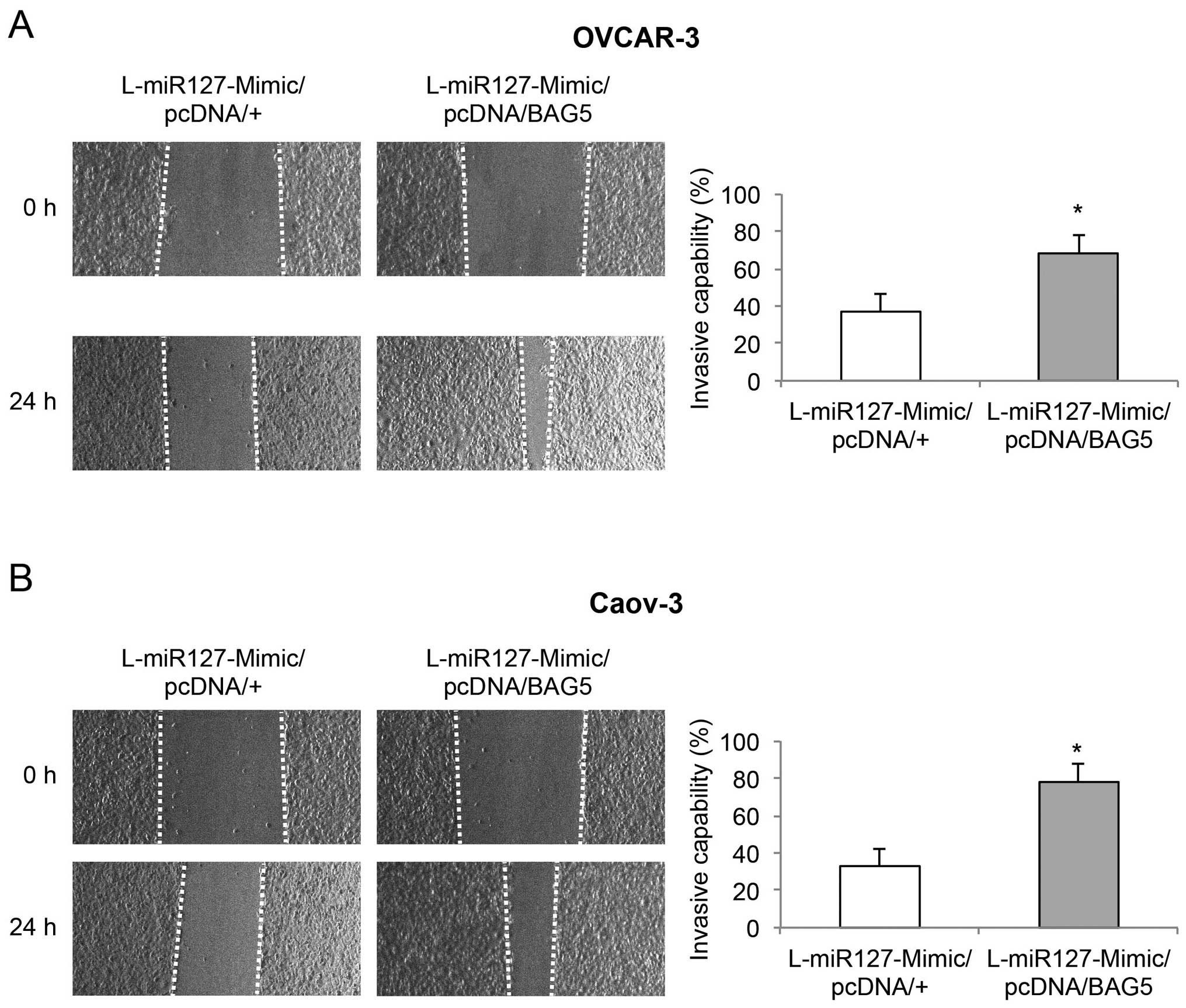
miR-127-3p overexpression reduces cancer
cell invasive capability in EOC
In the lentiviral-transduced OVCAR-3 and Caov-3
cells, a wound-closure assay was conducted to assess the effect of
miR-127-3p overexpression on EOC invasion. Images are shown for the
EOC cells immediately after wound creation (0 h), and 24 h later. A
reduced degree of wound closure was observed in the OVCAR-3 and
Caov-3 cells transduced with L-miR127-Mimic, than that in the cells
transduced with L-C (Fig. 3, left).
Subsequent quantification confirmed that miR-127-3p overexpression
markedly reduced the invasive capabilities in both the OVCAR-3 and
Caov-3 cells (Fig. 3, right,
P<0.05).
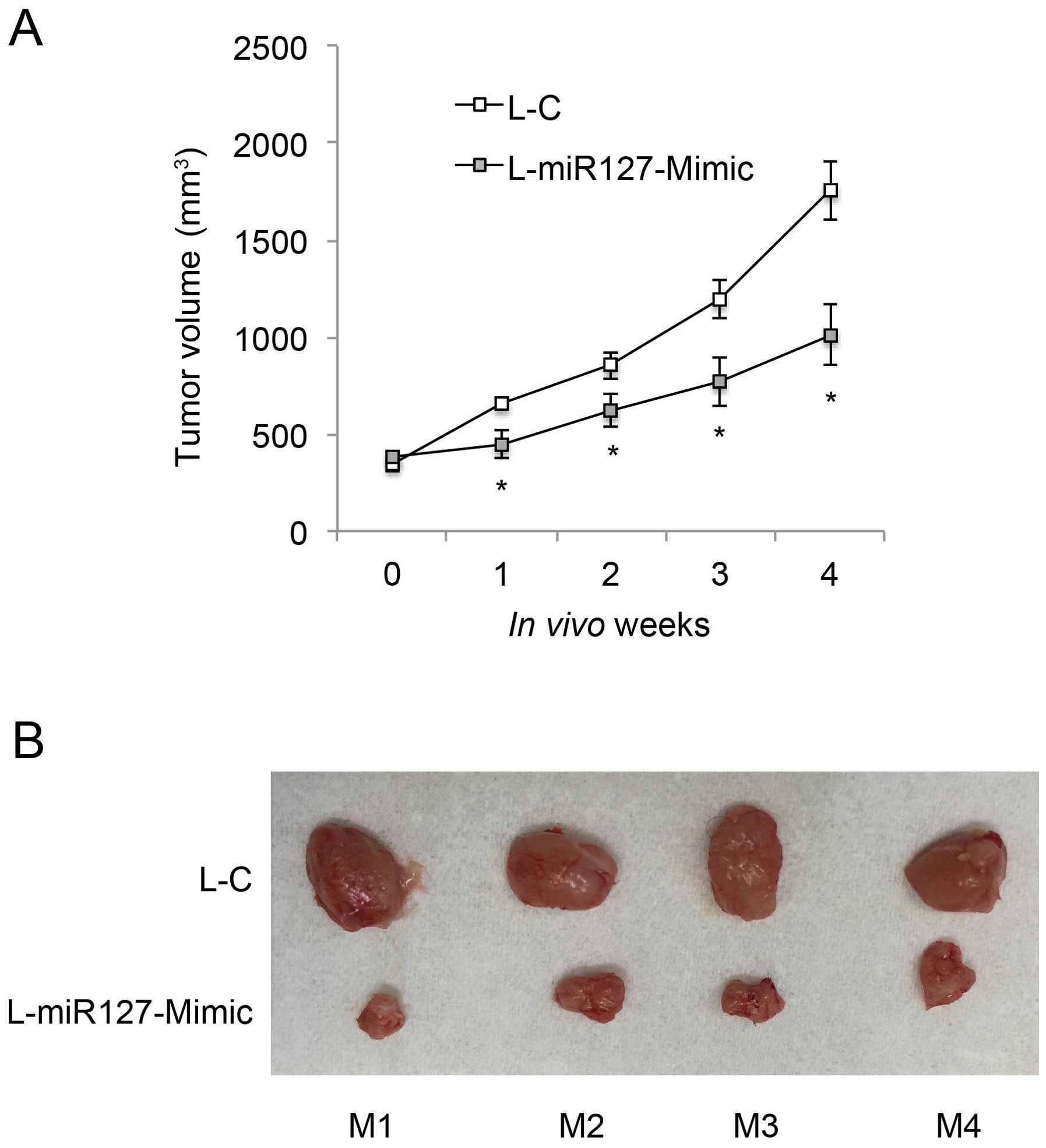
miR-127-3p overexpression inhibits in
vivo tumorigenicity of EOC
We then investigated whether miR-127-3p
overexpression had an effect on the in vivo growth of EOC.
L-miR127-Mimic-transduced OVCAR-3 cells (1×106) were
subcutaneously injected into the left flanks of 8-week-old athymic
nude mice (n=4). L-C-transduced OVCAR-3 cells (1×106)
were subcutaneously injected into the right flanks of the nude
mice. The in vivo growth curves were monitored weekly, for 5
consecutive weeks, by measuring tumor volume (mm3). The
result showed that miR-127-3p overexpression markedly inhibited
in vivo EOC tumor growth (Fig.
4A, P<0.05). At the end of the tumorigenicity assay, tumors
were extracted from both flanks of the mice, and compared.
L-miR-127-Mimic-transduced EOC tumors were significantly smaller
than the L-C-transduced tumors in all 4 mice (Fig. 4B).
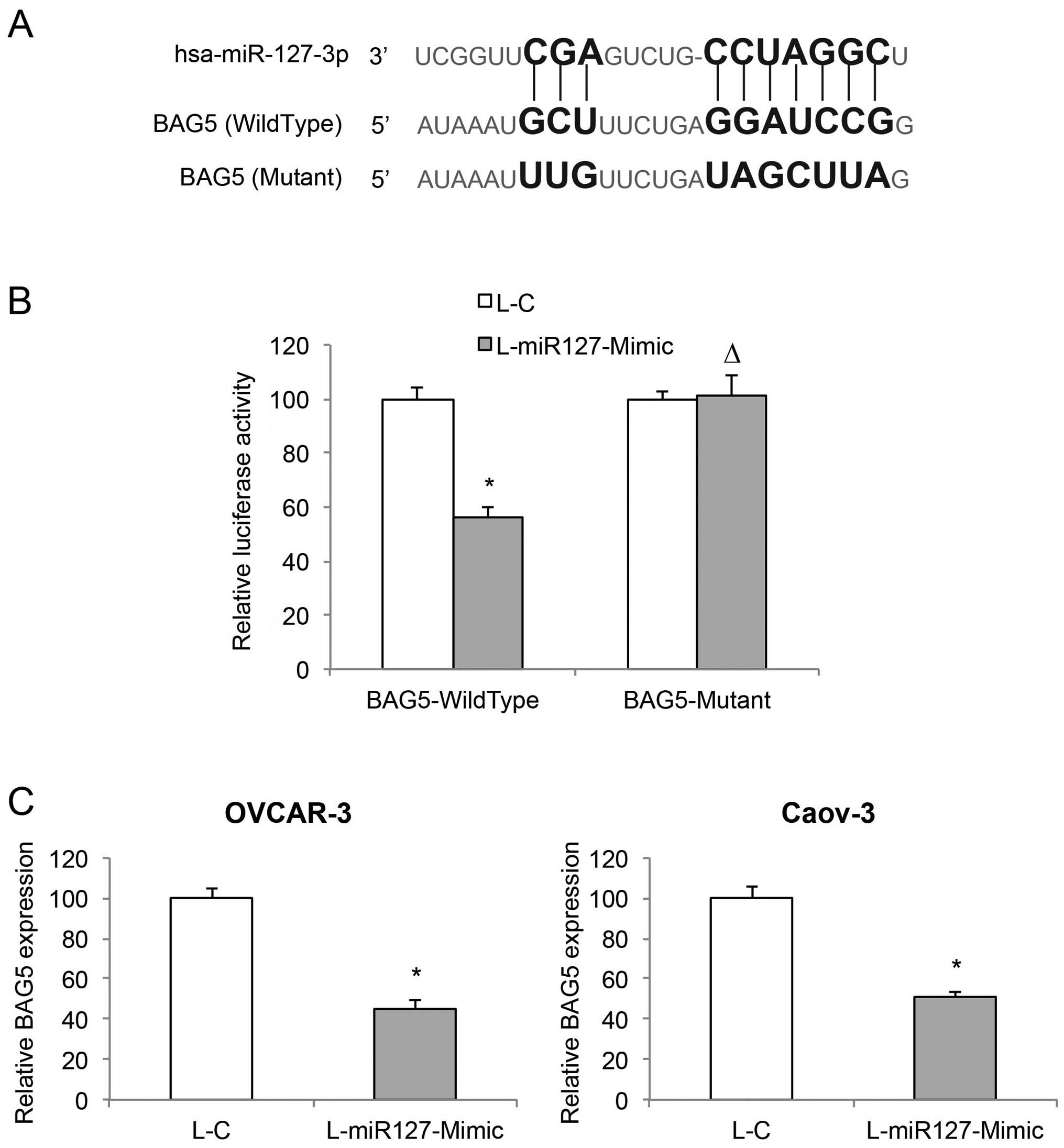
BAG5 is directly associated with
miR-127-3p overexpression in EOC
Since we demonstrated that miR-127-3p overexpression
has a tumor-suppressive effect on EOC, we then explored the
molecular target of miR-127-3p. Through web-search on miRNA
targets, including miRDB (www.mirdb.org)
and TargetScan Human (www.targetscan.org), we found that the BAG5 gene is a
candidate downstream target gene of miR-127-3p (Fig. 5A). We then used a dual-luciferase
reporter assay to verify this hypothesis. HEK293T cells were
co-transfected with BAG5-Wild-type, BAG5-Mutant or Renilla
luciferase vectors, and L-C or L-miR127-Mimic lentiviruses.
Forty-eight hours after co-transfection, quantification
demonstrated that the relative luciferase activities between L-C
and L-miR127-Mimic transfections significantly differed in cells
transfected with BAG5-WildType luciferase reporter, but in cells
transfected with BAG5-Mutant luciferase reporter (Fig. 5B, P<0.05; P>0.05), thus
confirming that BAG5 is the target gene of miR-127-3p. In addition,
we examined the mRNA levels of BAG5 in miR-127-3p overexpressed
OVCAR-3 and Caov-3 cells. The result of qRT-PCR demonstrated that
miR-127-3p overexpression significantly downregulated endogenous
BAG5 in the EOC cells (Fig. 5C,
P<0.05).
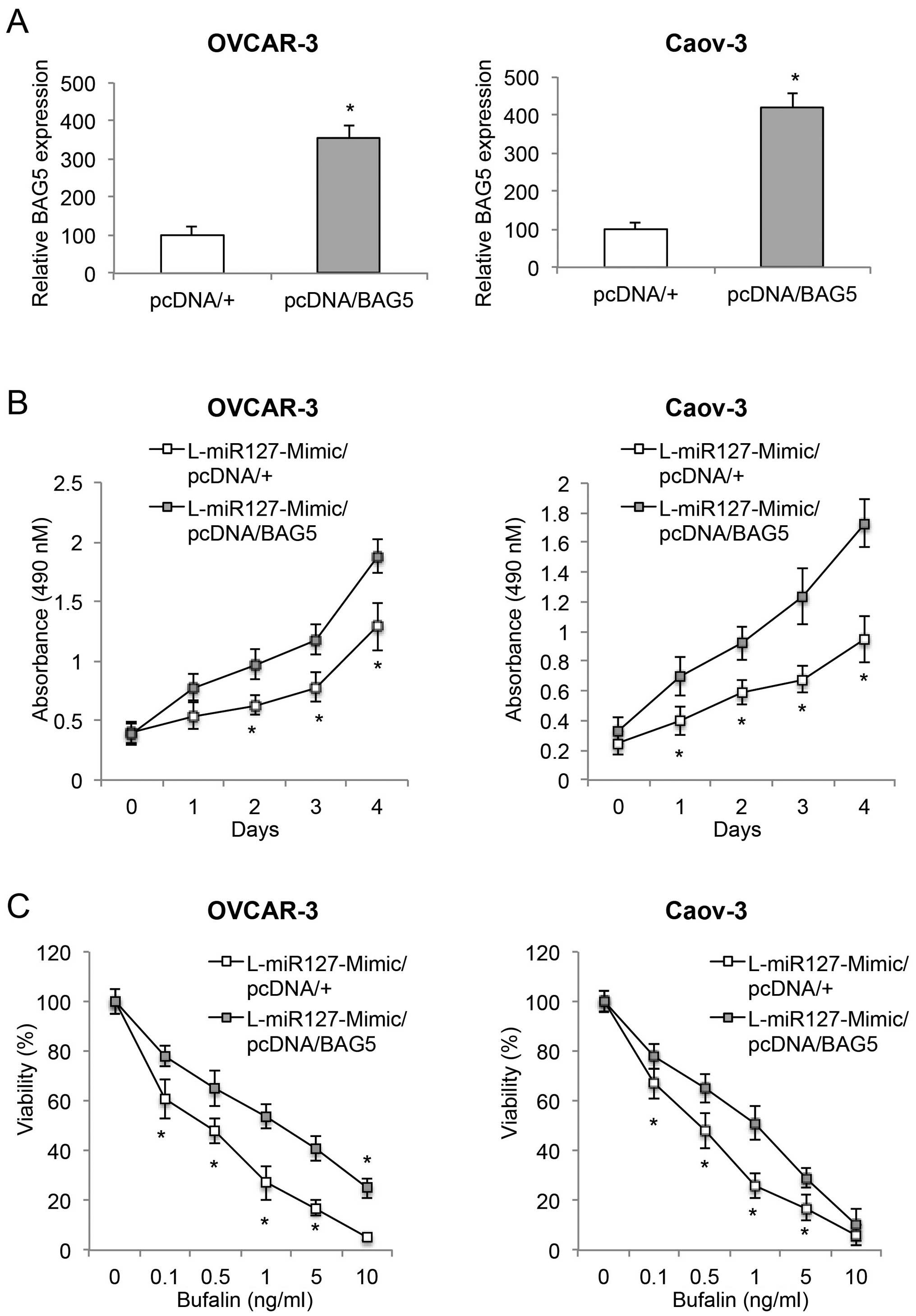
BAG5 upregulation induces opposite
effects opposite to those of miR-127-3p overexpression in EOC
In order to elucidate the function of the BAG5 gene
in miR-127-3p-mediated EOC regulation, we transfected
L-miR127-Mimic-transduced OVCAR-3 and Caov-3 cells with a
BAG5-overexpressing vector pcDNA/BAG5, or a control vector pcDNA/+.
Forty-eight hours after transfection, qRT-PCR demonstrated that
endogenous BAG5 mRNAs were significantly upregulated in the EOC
cells transfected with pcDNA/BAG5 than the expression level in the
EOC cells transfected with pcDNA/+ (Fig. 6A, P<0.05).
We then examined the effect of BAG5 upregulation on
cancer growth, bufalin sensitivity and invasive capabilities in
those double-transfected EOC cells. The proliferation assay showed
that BAG5 upregulation revitalized cancer growth in the
miR-127-3p-overexpressing EOC cells (Fig. 6B, P<0.05). The bufalin
sensitivity assay demonstrated that BAG5 upregulation ameliorated
bufalin sensitivity in the miR-127-3p-overexpressing EOC cells
(Fig. 6C, P<0.05). Furthermore,
wound-closure assay showed that BAG5 upregulation also
significantly restored invasive capability in the
miR-127-3p-overexpressing EOC cells (Fig. 7, P<0.05). Thus, our experiments
using the double-transfected EOC cells clearly demonstrated that
BAG5 upregulation induced effects opposite those of miR-127-3p
overexpression on EOC growth, bufalin sensitivity and invasion.
Discussion
MicroRNAs have been shown to play important roles in
EOC regulation, acting as either oncogenes or tumor suppressors
(8,12). MicroRNA-127-3p has been identified
as a tumor suppressor in other human cancers (9,10,13),
and has been shown to be downregulated in EOC tumors (11,12).
Yet, the exact functional roles of miR-127-3p in EOC are elusive.
In our study, we first used quantitative method, qRT-PCR, to assess
the expression pattern of miR-127-3p. We verified that miR-127-3p
was downregulated in EOC cell lines in vitro, as well as in
in vivo clinical samples of EOC tumors.
Since all evidence points to a downregulated
expression pattern of miR-127-3p in EOC, we took further step to
explore the functional mechanism of miR-127-3p. We thus created two
EOC cell lines, OVCAR-3 and Caov-3, with stable miR-127-3p
overexpression. Subsequent functional experiments demonstrated that
miR-127-3p acted as a tumor suppressor in EOC, as overexpression of
miR-127-3p inhibited EOC proliferation, drug (bufalin) sensitivity,
invasion and in vivo tumor development. Therefore,
collectively (9,10,13),
it may well demonstrate that miR-127-3p predominantly acts as a
tumor suppressor across various cancer types.
Through investigations on breast and bladder cancer,
it was found that the major molecular target of tumor-suppressive
miR-127-3p was a zinc-finger repressor gene BCL6 (9,14).
However, the results of our study revealed that the BAG5 gene, a
novel candidate, was likely the target gene of miR-127-3p in
ovarian cancer. Our dual-luciferase reporter assay demonstrated
that has-miR-127-3p did bind to the complimentary sites on the
human BAG5 gene. Our qRT-PCR analysis also showed that the BAG5
gene was directly downregulated by miR-127-3p overexpression in the
EOC cell lines, OVCAR-3 and Caov-3. Moreover, our functional
experiments provided unambiguous evidence showing that upregulation
of the BAG5 gene inversely regulated the tumor-suppressive effects
of miR-127-3p overexpression in EOC. Notably, BAG5 belongs to the
gene family of BCL2, which was reported to be involved in
chromosomal translocations of the BCL6 gene (15,16).
Therefore, future investigations focusing on trans-locational
evidence of BCL2/BCL6 in patients with EOC may help to identify
whether BAG5 and BCL6 are interactively involved in the microRNA
regulation in EOC.
In conclusion, we discovered novel regulatory
mechanism of miR-27-3p acting as a tumor suppressor in EOC.
Moreover, we discovered a novel molecular target, BAG5 gene, to be
the downstream target of miR-127-3p in regulating EOC development.
Our results further the knowledge of epigenetic regulations in EOC,
as well as help to development new targets for early diagnosis and
optimal therapies for patients with EOC.
References
|
1
|
Jazaeri AA: Molecular profiles of
hereditary epithelial ovarian cancers and their implications for
the biology of this disease. Mol Oncol. 3:151–156. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Al Bakir M and Gabra H: The molecular
genetics of hereditary and sporadic ovarian cancer: Implications
for the future. Br Med Bull. 112:57–69. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alvarez-Garcia I and Miska EA: MicroRNA
functions in animal development and human disease. Development.
132:4653–4662. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang B, Wang Q and Pan X: MicroRNAs and
their regulatory roles in animals and plants. J Cell Physiol.
210:279–289. 2007. View Article : Google Scholar
|
|
6
|
Pichler M and Calin GA: MicroRNAs in
cancer: From developmental genes in worms to their clinical
application in patients. Br J Cancer. 113:569–573. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sassen S, Miska EA and Caldas C: MicroRNA:
Implications for cancer. Virchows Arch. 452:1–10. 2008. View Article : Google Scholar
|
|
8
|
Iorio MV, Visone R, Di Leva G, Donati V,
Petrocca F, Casalini P, Taccioli C, Volinia S, Liu CG, Alder H, et
al: MicroRNA signatures in human ovarian cancer. Cancer Res.
67:8699–8707. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen J, Wang M, Guo M, Xie Y and Cong YS:
miR-127 regulates cell proliferation and senescence by targeting
BCL6. PLoS One. 8:e802662013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo LH, Li H, Wang F, Yu J and He JS: The
tumor suppressor roles of miR-433 and miR-127 in gastric cancer.
Int J Mol Sci. 14:14171–14184. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Resnick KE, Alder H, Hagan JP, Richardson
DL, Croce CM and Cohn DE: The detection of differentially expressed
microRNAs from the serum of ovarian cancer patients using a novel
real-time PCR platform. Gynecol Oncol. 112:55–59. 2009. View Article : Google Scholar
|
|
12
|
Zhang L, Volinia S, Bonome T, Calin GA,
Greshock J, Yang N, Liu CG, Giannakakis A, Alexiou P, Hasegawa K,
et al: Genomic and epigenetic alterations deregulate microRNA
expression in human epithelial ovarian cancer. Proc Natl Acad Sci
USA. 105:7004–7009. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Saito Y, Liang G, Egger G, Friedman JM,
Chuang JC, Coetzee GA and Jones PA: Specific activation of
microRNA-127 with downregulation of the proto-oncogene BCL6 by
chromatin-modifying drugs in human cancer cells. Cancer Cell.
9:435–443. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Horn H, Ziepert M, Becher C, Barth TF,
Bernd HW, Feller AC, Klapper W, Hummel M, Stein H, Hansmann ML, et
al German High-Grade Non-Hodgkin Lymphoma Study Group: MYC status
in concert with BCL2 and BCL6 expression predicts outcome in
diffuse large B-cell lymphoma. Blood. 121:2253–2263. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kramer MH, Hermans J, Wijburg E, Philippo
K, Geelen E, van Krieken JH, de Jong D, Maartense E, Schuuring E
and Kluin PM: Clinical relevance of BCL2, BCL6, and MYC
rearrangements in diffuse large B-cell lymphoma. Blood.
92:3152–3162. 1998.PubMed/NCBI
|