Introduction
Breast cancer is one of the most common malignancies
among women, in both developed and developing countries, accounting
for 23% (1.38 million) of the total new cancer cases and 14%
(458,400) of the total cancer-related deaths in 2008 (1,2). There
are conventional strategies for breast cancer treatment, including
surgery, radiotherapy, chemotherapy, hormone therapy and targeted
biological therapy (3). Although
recent years have seen tremendous progress in the treatment of
breast cancers (4), controversies
remain, patients continue to die, and a cure remains elusive
(5). Therefore, the development of
new anticancer agents for breast cancer is important to reduce the
mortality caused by this disease.
Natural products are an important source of
promising leads for the development of novel cancer therapeutics,
because of their potential anticancer properties and few side
effects (6–8). It is well known that vinblastine,
vincristine, paclitaxel, and camptothecin have been widely applied
as typical examples of plant-derived anticancer drugs in the clinic
(9–11). Therefore, it is very important to
develop new anticancer drugs from natural products.
Eupatorium lindleyanum DC.
(Compositae), called 'Ye-Ma-Zhui' by local residents, is a
perennial herbaceous plant. It has been used to treat cough and
tracheitis due to its antimicrobial, antihistamine and
anti-inflammatory activities (12–14).
Many compounds have been isolated from this plant, including
alkaloids, flavonoids, esters and sesquiterpenes (12,15,16).
Pharmacological studies have demonstrated a variety of biological
activities of this plant (12,15,17).
However, there is little information concerning its anticancer
activity.
In this study, the authors investigated the
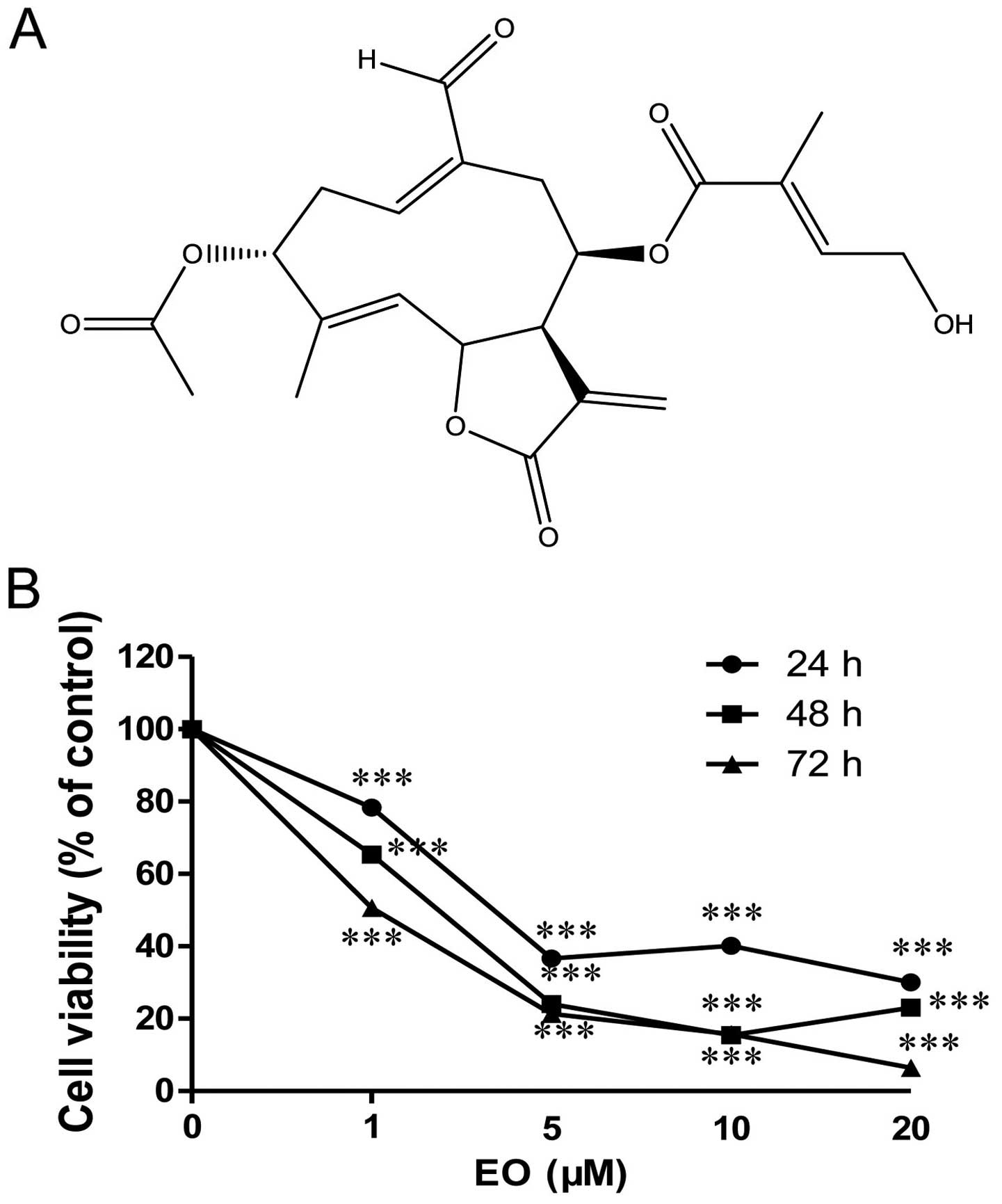
anticancer effect of Eupalinolide O (EO) (Fig. 1A), a novel sesquiterpene lactone
isolated from the Eupatorium lindleyanum DC., on human
breast cancer in vitro. Notably, EO showed significant
anticancer activity against human breast cancer cells. EO
suppressed cell proliferation, induced apoptosis and arrested the
cell cycle at the G2/M phase in MDA-MB-468 cells. Furthermore, the
authors found that the induction of apoptosis by EO was performed
in a caspase-dependent manner.
Materials and methods
Cell culture and reagents
The human breast cancer cell line MDA-MB-468 was
purchased from the Cell Bank of the Institute of Biochemistry and
Cell Biology, Chinese Academy of Sciences (Shanghai, China) and
stored in liquid nitrogen. Cells were cultured in Dulbecco's
modified Eagle's medium (DMEM) culture medium containing 10% fetal
bovine serum (FBS; both from Gibco, USA), 100 U/ml penicillin G,
2.5 μg/ml amphotericin B and 100 μg/ml streptomycin
(complete medium) at 37°C with 5% CO2 in a humidified
atmosphere.
3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide
(MTT) and dimethyl sulfoxide (DMSO) were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Propidium iodide (PI)/RNase
staining kit and Annexin V-FITC/7AAD kit were purchased from BD
Pharmingen (San Diego, CA, USA). Antibodies against caspase-3
(#9662), cleaved caspase-3 (Asp175, #9664), cdc2 (#9116),
phospho-Akt (Ser473/Thr308, #9275), Akt (pan, #4691), phospho-cdc2
(Tyr15, #4539), PARP (#9532), cyclin B1 (#12231), Bcl-2 (#2870),
Bcl-xL (#2764), caspase-8 (#4790), caspase-9 (#9508), cleaved
caspase-9 (Asp330, #9501), β-tubulin (#2128), and horseradish
peroxidase-conjugated secondary antibodies were purchased from Cell
Signaling Technologies (Beverly, MA, USA). Bad (N-term, 1541-1) was
purchased from Abcam (Cambridge, UK).
Cell viability assay
The viability of cells treated with or without EO
was measured by MTT assay. The MDA-MB-468 cells were placed into
96-well plates at a final concentration of 5×103
cells/well in complete medium, and allowed to attach for 24 h.
Subsequently the cells were treated with a range of concentrations
of EO for 24, 48 and 72 h, then 20 μl MTT solution (5 mg/ml)
was added, and the cells were incubated for another 4 h at 37°C in
the dark. Formed formazan crystals were dissolved in 100 μl
DMSO and the absorbance was measured at 570 nm on a microplate
reader (Bio-Rad, USA). The IC50 value was calculated by
GraphPad Prism 5.0 software (GraphPad Software, Inc., San Diego,
CA, USA).
Flow cytometric analysis of cell cycle
arrest and apoptosis
Cells were plated in 6-well plates (3×105
cells/well) and treated with varying concentrations of EO for 72 h.
For cell cycle analysis, cells were harvested, washed twice with
ice-cold PBS and fixed in 70% ethanol at 4°C overnight, and stained
with PI/RNase (0.5 ml/test, 1×106 cells) for 15 min at
room temperature before analysis. To quantify the apoptotic cells,
the treated cells were washed twice with ice-cold PBS and stained
with Annexin-V-FITC/7AAD according to the manufacturer's
instructions. Samples were subsequently analyzed by flow cytometer
(Guava Technologies; Merck KGaA, Darmstadt, Germany) and DNA
content was quantified using ModFit software.
Evaluation of mitochondrial membrane
potential (Δψm)
The MDA-MB-468 cells (3×105) were seeded
in 6-well plates for 24 h before the experiment. After the
treatment with 2, 4, and 8 μM EO for 24 h, cells were
harvested, washed twice with ice-cold PBS, and incubated with JC-1
(10 μg/ml) in the dark for 15 min at 37°C. Cells were washed
three times with ice-cold PBS and analyzed by flow cytometry using
emission wavelengths of 590 nm and 529 nm.
Western blot analysis
Cells were harvested, washed twice with ice-cold PBS
after treatment with EO, lysed by incubation in RIPA buffer
containing a protease inhibitors cocktail (1 mM
phenylmethanesulfonyl fluoride and 1 μg/ml leupeptin) and a
phosphatase inhibitors cocktail (1 mM sodium fluoride and 1 mM
sodium orthovanadate) for 30 min on ice, and then centrifuged at
12,000 rpm for 15 min at 4°C. Supernatants were collected, and
equal amounts of denatured proteins (heated samples at 100°C for 10
min) were separated by SDS-PAGE and transferred to PVDF membranes
(Millipore, Bedford, MA, USA), blocked with 5% nonfat milk at room
temperature for 1 h, and incubated with the respective specific
primary antibodies overnight at 4°C. The membranes were washed
three times with Tris-buffered saline-5% Tween-20 (TBST) solution
and incubated with a horseradish peroxidase-conjugated secondary
antibody at room temperature for 2 h. Chemiluminescent detection
was performed by ECL (Bio-Rad, USA).
Statistical analysis
All data are expressed as the mean ± SD of three
independent experiments. Statistical significance was analyzed
using a Student's t-test. The criterion of statistical significance
was **p<0.01; ***p<0.001.
Results
EO shows significant cytotoxicity against
the MDA-MB-468 cells
To evaluate the anti-proliferative effect of EO in
MDA-MB-468 human breast cancer cells, the cells were treated with
indicated concentrations of EO for 24, 48 and 72 h. The cytotoxic
effect was measured by MTT assay. As shown in Fig. 1B, EO induced cytotoxicity in the
MDA-MB-468 cells in a concentration- and time-dependent manner.
IC50 at 72 h was 1.04 μM. The effects of EO on
the MDA-MB-468 cells may be the result of the induction of
apoptosis and/or inhibition of growth. Therefore, the authors
investigated whether EO induces apoptosis in human breast cancer
cells.
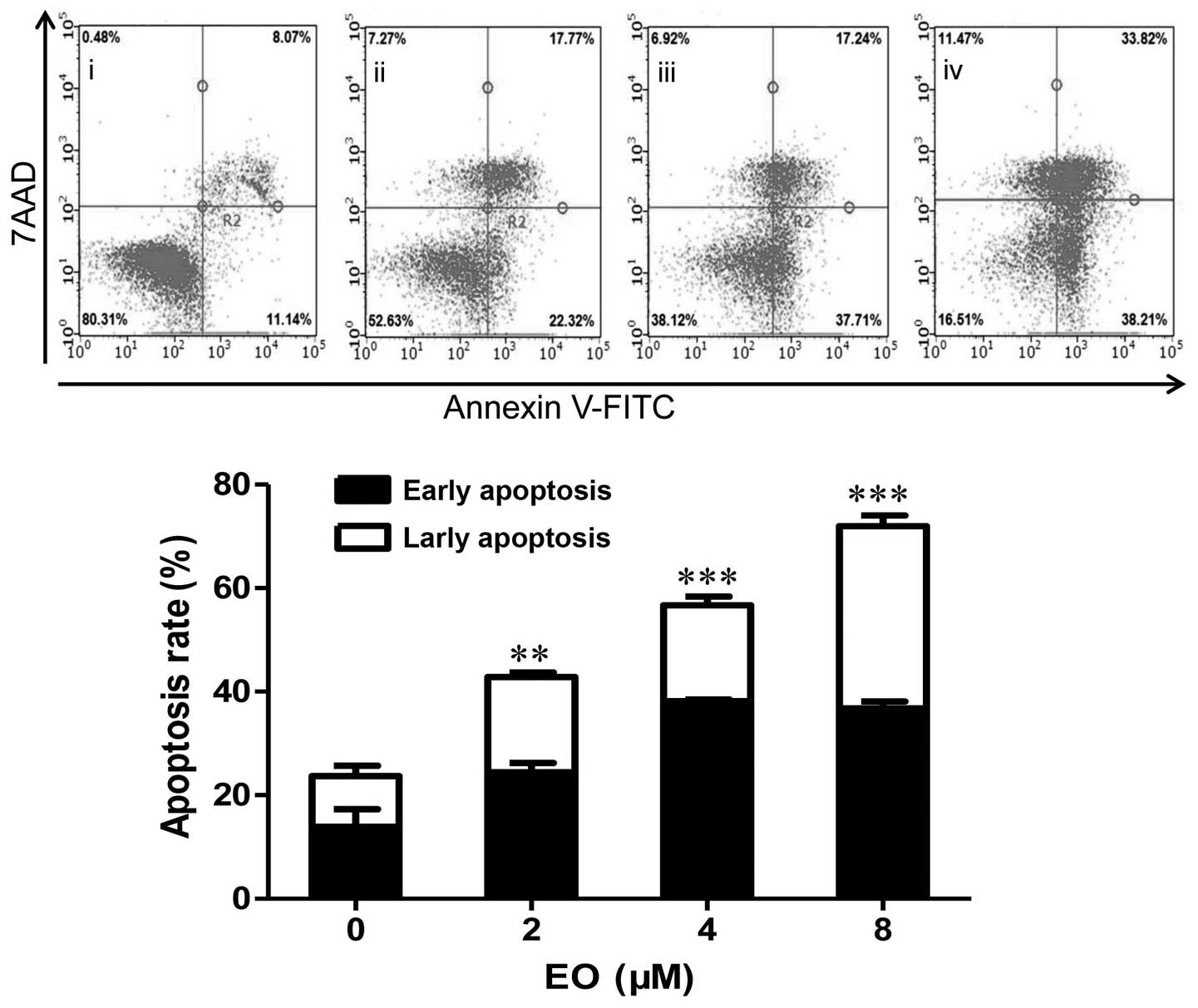
EO induces apoptosis in the MDA-MB-468
cells
To further determine whether the growth-inhibitory
effect of EO was related to the induction of apoptosis, treated
cells were analyzed using Annexin V-FITC/7AAD staining by flow
cytometric analysis. As shown in Fig.
2, after incubation with EO, the percentage of apoptotic cells
was significantly increased. These results indicated that the
induction of apoptotic cell death can be a potential mechanism of
the anticancer effect of EO against human breast cancer cells.
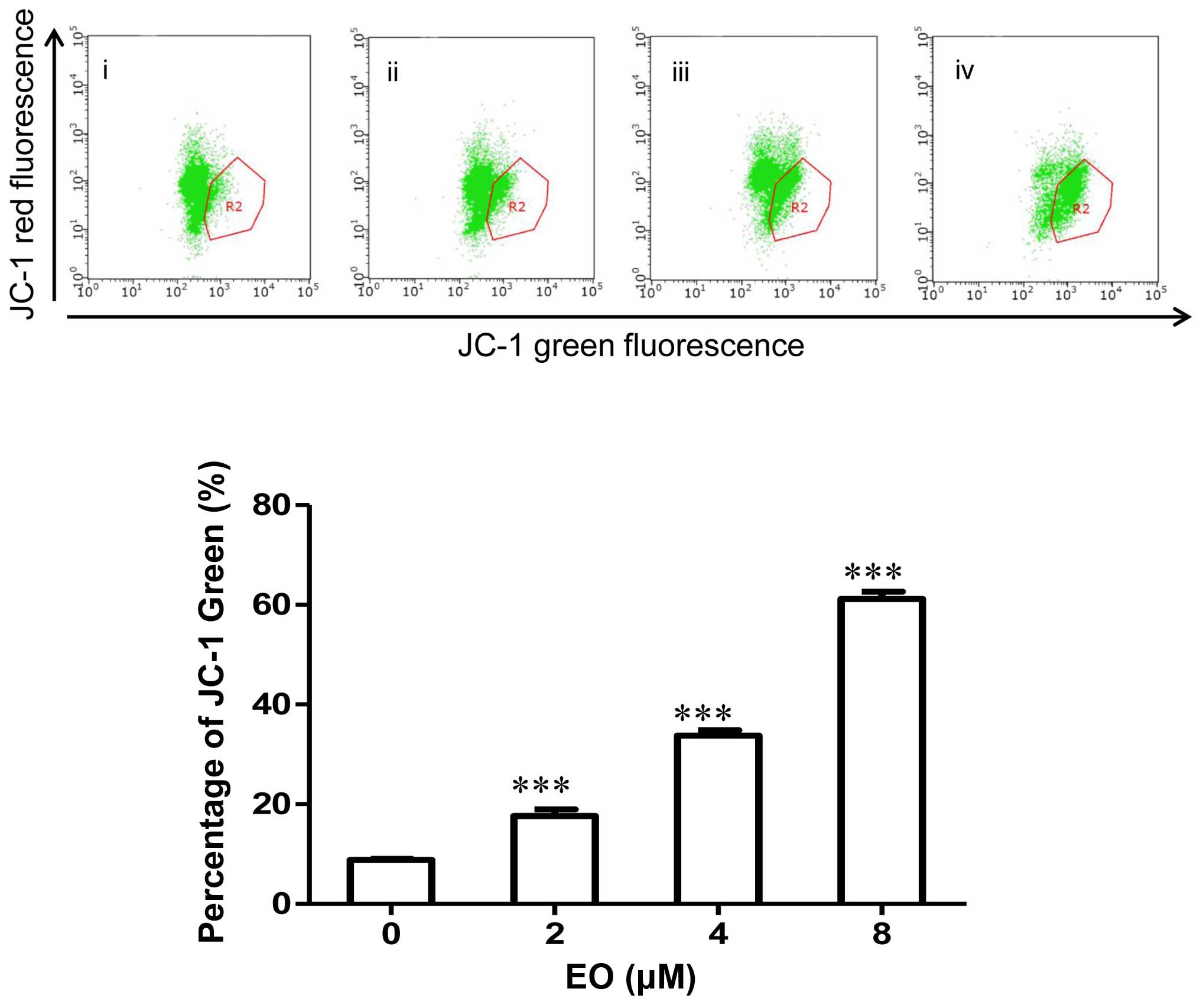
Treatment of EO results in loss of
mitochondrial membrane potential (Δψm)
The Δψm is an early event preceding caspase
activation, and is regarded as a hallmark of apoptosis (18). Induction of apoptosis via
mitochondrial pathways results in the loss of mitochondrial
membrane potential. Therefore, we measured Δψm in EO-treated
MDA-MB-468 cells using the membrane-permeable JC-1 dye. In
apoptotic cells with low Δψm, JC-1 remains in the monomeric form,
which has green fluorescence (19).
As shown in Fig. 3, a marked
increase in green fluorescence could be seen in the MDA-MB-468
cells treated with 2, 4 and 8 μM of EO. These results
demonstrated that EO induced Δψm disruption in breast cancer
cells.
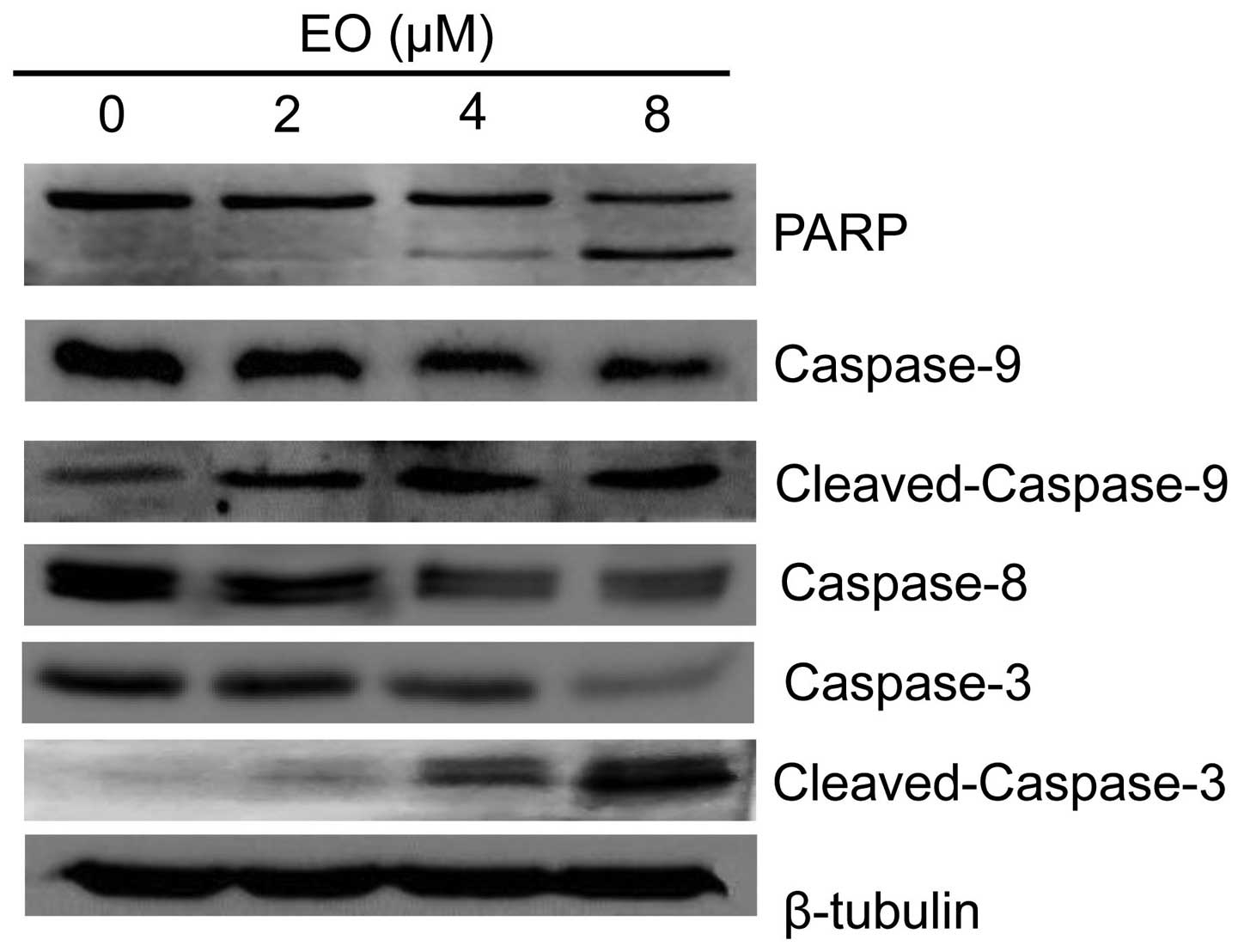
EO induces apoptosis in the MDA-MB-468
cells by activation of caspases
As known the activation of caspases plays important
roles in cancer cell death. To confirm whether caspases are
involved in EO-induced MDA-MB-468 cell death, we next examined the
activation of caspases and the expression of poly(ADP-ribose)
polymerase (PARP) by western blot analysis. As shown in Fig. 4, EO treatment significantly
decreased the protein levels of pro-caspase-3, pro-caspase-8 and
pro-caspase-9, while increasing the protein levels of cleaved
caspase-3 and caspase-9. To further investigate the enzymatic
activation of caspase-3, we measured cleaved PARP which is a
substrate of caspase-3 and an enzyme that protects DNA. The
formation of the fragment of PARP was detected in cells treated
with EO (Fig. 4). These results
demonstrated that EO induced apoptosis in the MDA-MB-468 cells by
the activation of caspases.
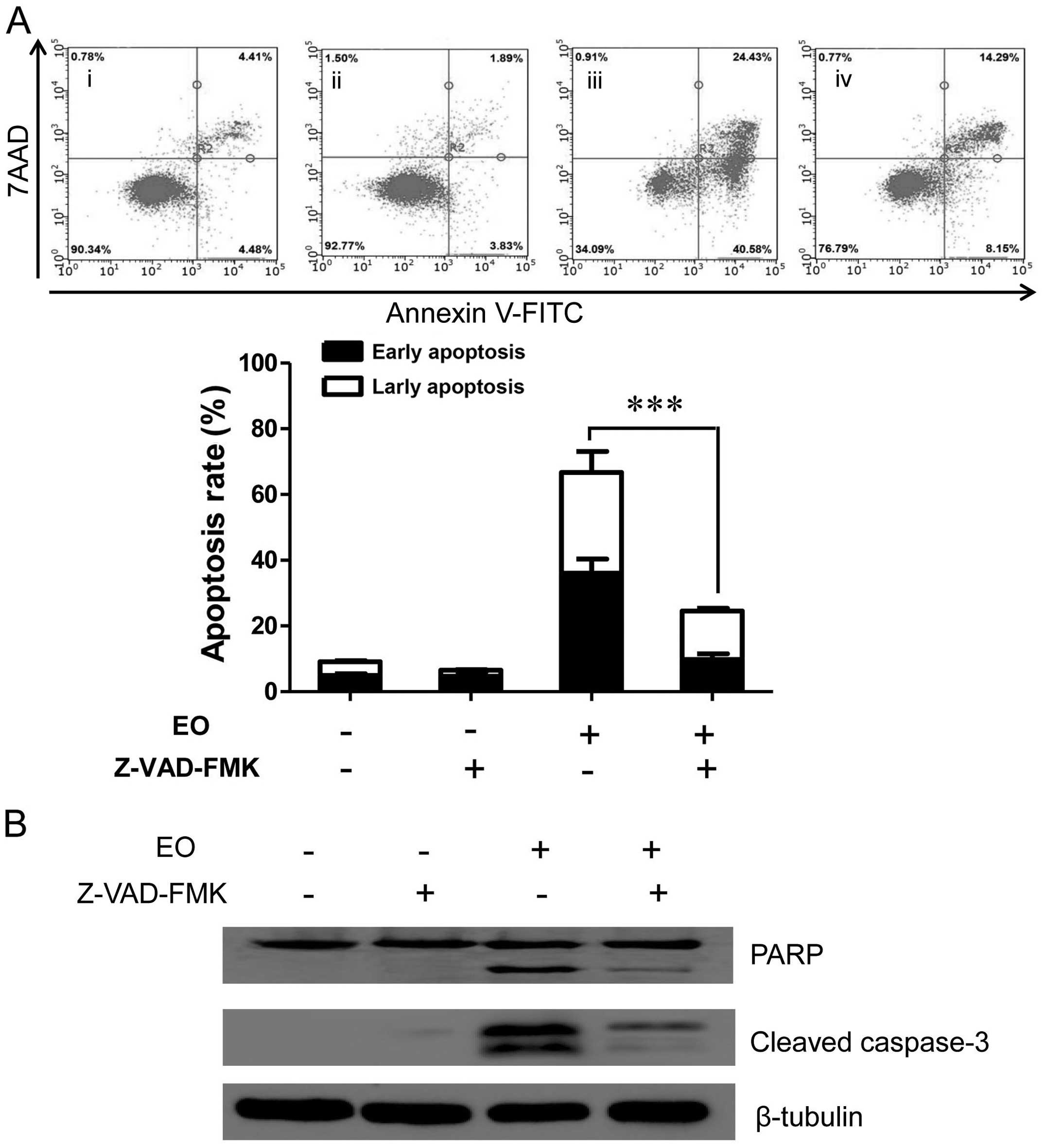
EO induces apoptosis in a
caspase-dependent manner
To further investigate the role of caspase
activation in EO-induced apoptosis, the effect of the pan-caspase
inhibitor Z-VAD-FMK in preventing EO-induced cell death was
examined. As shown in Fig. 5A, when
cells were treated with 8 μM of EO, the percentage of
apoptotic cells reached 65.01% at 24 h. However, when cells were
pre-treated with caspase inhibitor Z-VAD-FMK for 2 h, the
percentage of apoptotic cells was reduced to 22.44% after 24 h.
Moreover, western blot analysis showed that cleavage of caspase-3
and PARP were inhibited (Fig. 5B).
These results indicated that EO induced apoptosis mainly by
caspase-dependent mechanisms in the MDA-MB-468 cells.
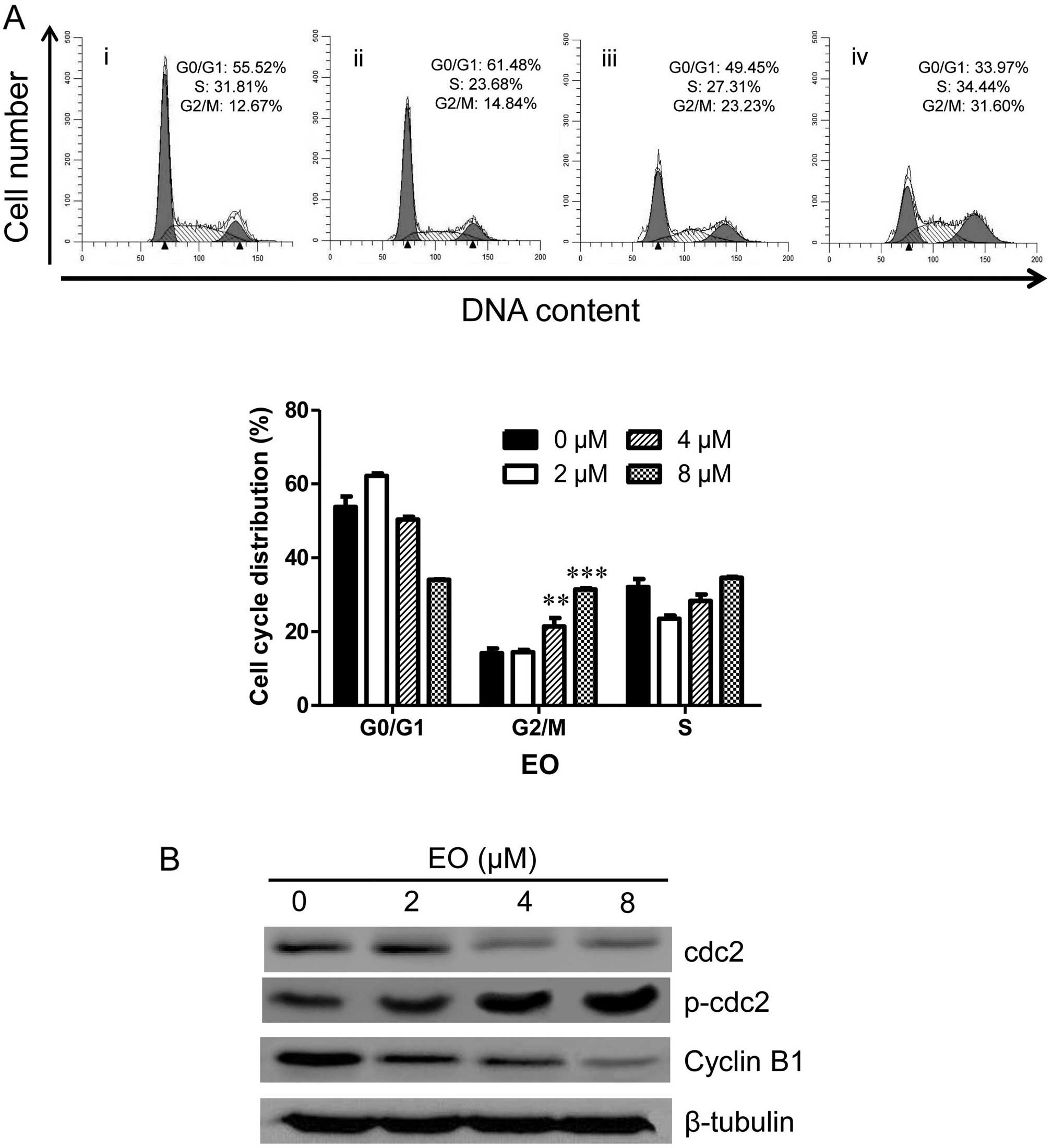
EO induces cell cycle arrest in the
MDA-MB-468 cells
To investigate whether EO modulates the cell cycle
of the MDA-MB-468 cells, the cells were treated with the indicated
concentrations (0, 2, 4 and 8 μM) of EO for 24 h. The
detection of cell cycle distribution was performed by flow
cytometry. As shown in Fig. 6A, the
G2/M phase distribution was increased from 12.67% in the vehicle
control group to 31.60% in a concentration-dependent manner after 8
μM of EO treatment. To elucidate the molecular mechanism, we
next detected the expression of key molecules (cyclin B1, cdc2 and
p-cdc2) which regulate the G2/M phase transition in the EO-treated
MDA-MB-468 cells. As shown in Fig.
6B, treatment with EO decreased the protein levels of cyclin B1
and cdc2 while increasing the protein level of p-cdc2 (Tyr15) in a
dose-dependent manner. These results suggested that the cell cycle
arrest in G2/M phase may be associated with the anticancer effect
of EO on human breast cancer cells.
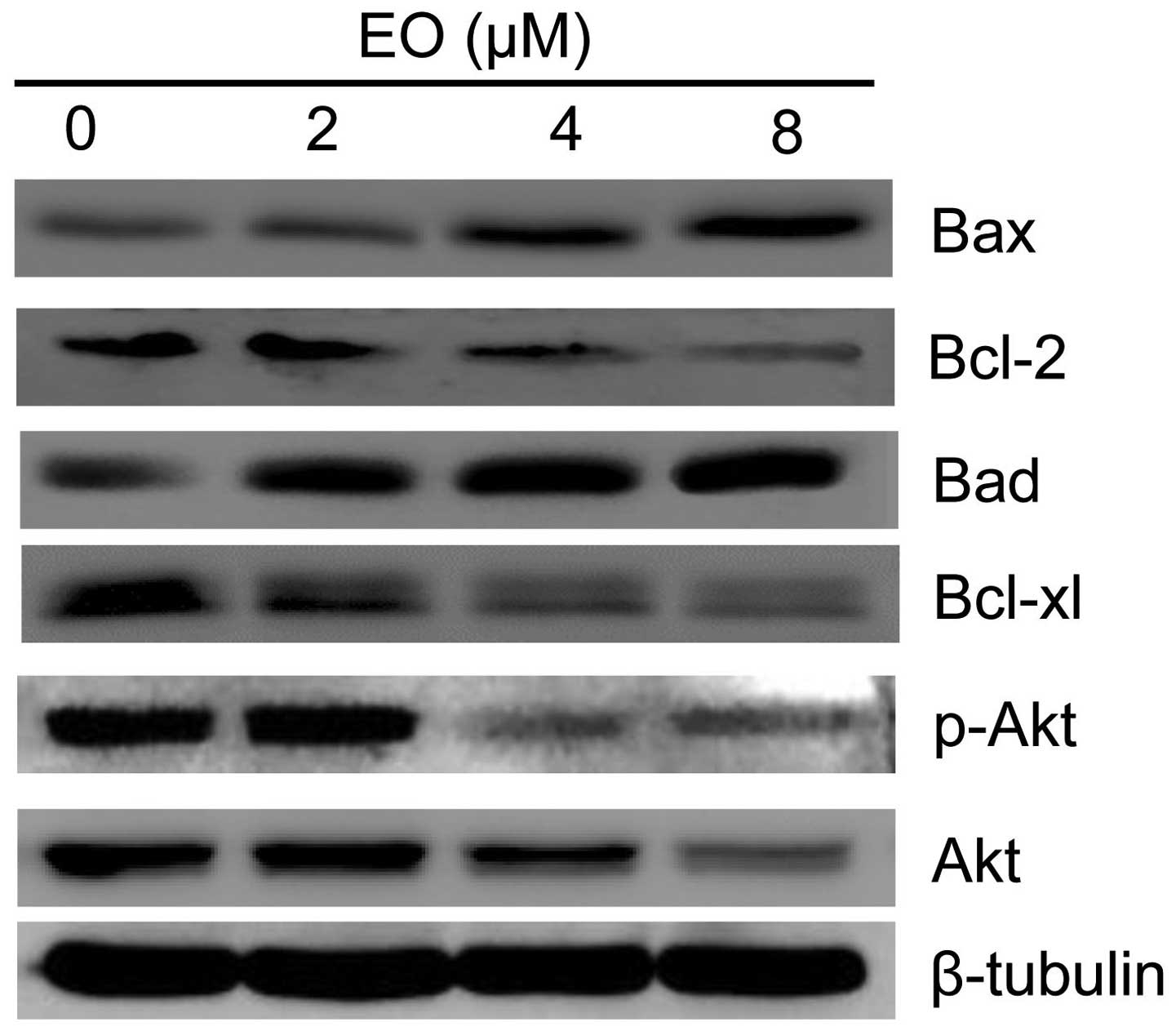
EO regulates the Bcl-2 family of proteins
and the Akt signaling pathways in the MDA-MB-468 cells
The Bcl-2 family of proteins, such as Bcl-2 and Bax,
are upstream signals of caspase activation and play important roles
in regulating mitochondrial-related apoptosis (20). The Bcl-2 family includes
anti-apoptotic and pro-apoptotic proteins. The release of proteins
from the inner-membrane space of mitochondria is one of the central
events in the apoptotic process, which can lead to the activation
of caspases and the ultimate demise of the cell (21). To elucidate further the anticancer
mechanism, the role of the Bcl-2 family of proteins in EO-induced
apoptosis was investigated. As shown in Fig. 7, after treatment with EO, the
expression of anti-apoptotic Bcl-xl and Bcl-2 was significantly
decreased, and the expression of pro-apoptotic Bax and Bad was
increased. Collectively, these results suggest that EO may induce
apoptosis of the MDA-MB-468 cells through Bcl-2 degradation.
To further explore the possible mechanism of EO on
the induction of apoptosis in breast cancer cells, we assessed its
effect on the Akt signaling pathways. The Akt signaling pathways
have been shown to be critical regulators of cell growth,
differentiation and survival (22,23).
Sesquiterpene lactones can promote cancer cell survival by
activating the PI3K/Akt pathway (24). The Akt pathway is a major
anti-apoptotic signaling pathway, and previous studies have shown
that chemotherapy drugs induce apoptosis of tumor cells by
inhibiting the Akt pathway (25–27).
Thus, we determined the protein expression of Akt in the MDA-MB-468
cells and found that EO significantly reduced the expression of
both Akt and p-Akt which is an active form of Akt in a
dose-dependent manner (Fig. 7).
These data suggest that the Akt pathway may be an effective target
for EO in the MDA-MB-468 cells.
Discussion
In the present study, the authors investigated the
anticancer effects of EO against the MDA-MB-468 breast cancer
cells. EO induced apoptotic cell death in the MDA-MB-468 cells
through cell cycle arrest in the G2/M phase (Fig. 6), activation of caspases (Fig. 4), and disruption of the
mitochondrial membrane potential (Δψm) (Fig. 3). Notably, the effect of EO in the
induction of apoptosis was in a caspase-dependent manner (Fig. 5). Furthermore, the Akt signaling
pathway in the MDA-MB-468 cells was suppressed which may play a
critical role in EO-induced apoptosis (Fig. 7).
The induction of cell cycle arrest and apoptosis are
common mechanisms proposed for the cytotoxic effects of anticancer
drugs (28). Cell cycle arrest can
trigger proliferation inhibition and apoptosis in cancer cells
(29,30). During the cell cycle, the G2/M
checkpoint is a potential target for cancer therapy, and
cdc2/cyclin B1 are critical and complex regulators at the G2/M
phase (31). Some anticancer drugs
induce G2/M arrest through the downregulation of the expression of
cyclin B1 and cdc2 (32). In our
study, the results showed that EO treatment resulted in G2/M arrest
and decreased expression of cdc2 and cyclin B1, suggesting that a
decrease of cyclin B1 and cdc2 expression may be the molecular
mechanism through which EO induced G2/M arrest.
Apoptosis regulates biological processes that play
an important role in homeostasis, development and elimination of
damaged cells. Apoptosis in cancer cells can be a promising
treatment method in cancer therapy. In general, drug-induced
apoptosis is one major mechanism of action for the treatment of
cancer, and various signaling pathways are involved in the process.
It is well known that the two main signaling pathways that induce
apoptosis are the mitochondrial-mediated intrinsic and death
receptor-mediated extrinsic pathways (33). The activation of caspase protease is
the basis of cell apoptosis. In some cells, these cysteine
proteases can directly cleave and activate caspase-3 to induce
apoptosis in the executive phase (34). After the activation of caspase-3,
several specific substrates including PARP cleavage, eventually
lead to apoptosis (35). Consistent
with the above notion, in this study, EO treatment significantly
decreased the protein levels of pro-caspase-3, pro-caspase-8,
pro-caspase-9 and PARP while increasing the protein levels of
cleaved caspase-3, caspase-9 and PARP (Fig. 4). These results demonstrated that EO
induced apoptosis by triggering the intrinsic and extrinsic
apoptotic pathways in the MDA-MB-468 cells. Notably, the effect on
the induction of apoptosis may be prevented by the treatment of the
pan-caspase inhibitor Z-VAD-FMK.
The Δψm is an early event preceding caspase
activation, and is regarded as a hallmark of apoptosis (18). Induction of apoptosis via the
mitochondrial pathways results in the loss of mitochondrial
membrane potential. In addition, the mitochondrial-mediated
intrinsic apoptotic pathway is regulated by the proteins of the
Bcl-2 family (36). Indeed, we
found that EO decreased Δψm in the MDA-MB-468 cells (Fig. 3) and downregulated the expression of
anti-apoptotic Bcl-xl and Bcl-2 while upregulating that of
pro-apoptotic Bax and Bad (Fig. 7),
indicating that the loss of Δψm plays an important role in
EO-induced apoptosis in breast cancer cells. These results indicate
that EO induces apoptosis of breast cancer cells through the
induction of mitochondrial dysfunction caused by deregulation of
the Bcl-2 family proteins.
The PI3K/Akt signaling pathway is a critical
transduction pathway which plays an important role in the
regulation of cell proliferation, cell cycle and apoptosis
(37). Recent studies have
demonstrated that various anticancer drugs induce G2/M arrest
accompanied by downregulation of Akt (38,39).
Furthermore, it is reported that the PI3K/Akt pathway also
participates in the regulation of the Bcl-2 family proteins, which
are key regulators of the apoptotic pathway (40). In our study, the expression of the
Akt pathway was significantly suppressed in the MDA-MB-468 cells
following treatment with EO.
In conclusion, this study has shown for the first
time that EO, a novel sesquiterpene lactone, caused the inhibition
of the proliferation of breast cancer MDA-MB-468 cells through cell
cycle arrest and induction of apoptosis. The apoptotic cell death
response was found to be caspase-dependent. Furthermore, the
inhibition of the Akt signaling pathway may play a vital role in
EO-induced apoptosis. These results clearly indicate that EO is a
promising anticancer agent against breast cancer.
Acknowledgments
This study was financially supported by the Zhejiang
Provincial Natural Science Fund (no. LZ15H310001), the Zhejiang
Province Chinese Medicine Scientific Research Fund Project (no.
2016ZA050), the Young Scholar Program of Students' Scientific and
Technological Innovation Activities in Zhejiang's Universities (no.
2015R410025) and the Zhejiang Chinese Medical University Research
Fund Project (no. 2015ZR06). We extremely thank our team members
Rui Zhu, Sha-Sha Tian and Zhi-Hui Zhu from Zhejiang Chinese Medical
University for their technical support.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yasemi M, Ahmadi MRH, Khajavikhan J,
Peyman H, Asadollahi KH, Yasemi MR and Hemati K: An 8 years
retrospective study of breast cancer incidence in Ilam province,
Western Iran. J Clin Diagn Res. 7:2923–2925. 2013.
|
|
3
|
Howard JH and Bland KI: Current management
and treatment strategies for breast cancer. Curr Opin Obstet
Gynecol. 24:44–48. 2012. View Article : Google Scholar
|
|
4
|
Are C, Rajaram S, Are M, Raj H, Anderson
BO, Chaluvarya Swamy R, Vijayakumar M, Song T, Pandey M, Edney JA,
et al: A review of global cancer burden: Trends, challenges,
strategies, and a role for surgeons. J Surg Oncol. 107:221–226.
2013. View Article : Google Scholar
|
|
5
|
Head J and Johnston SR: New targets for
therapy in breast cancer: Farnesyltransferase inhibitors. Breast
Cancer Res. 6:262–268. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mann J: Natural products in cancer
chemotherapy: Past, present and future. Nat Rev Cancer. 2:143–148.
2002. View
Article : Google Scholar
|
|
7
|
Koehn FE and Carter GT: The evolving role
of natural products in drug discovery. Nat Rev Drug Discov.
4:206–220. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs over the 30 years from 1981 to 2010. J Nat
Prod. 75:311–335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cragg GM and Newman DJ: Antineoplastic
agents from natural sources: Achievements and future directions.
Expert Opin Investig Drugs. 9:2783–2797. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Butler MS: The role of natural product
chemistry in drug discovery. J Nat Prod. 67:2141–2153. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kaczirek K, Schindl M, Weinhäusel A,
Scheuba C, Passler C, Prager G, Raderer M, Hamilton G, Mittlböck M,
Siegl V, et al: Cytotoxic activity of camptothecin and paclitaxel
in newly established continuous human medullary thyroid carcinoma
cell lines. J Clin Endocrinol Metab. 89:2397–2401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huo J, Yang SP, Ding J and Yue JM: Two new
cytotoxic sesquiterpenoids from Eupatorium lindleyanum DC. J Integr
Plant Biol. 48:473–477. 2006. View Article : Google Scholar
|
|
13
|
Ji LL, Luo YM and Yan GL: Studies on the
antimicrobial activities of extracts from Eupatorium lindleyanum DC
against food spoilage and food-borne pathogens. Food Contr.
19:995–1001. 2008. View Article : Google Scholar
|
|
14
|
Ye G, Huang XY, Li ZX, Fan MS and Huang
CG: A new cadinane type sesquiterpene from Eupatorium lindleyanum
(Compositae). Biochem Syst Ecol. 36:741–744. 2008. View Article : Google Scholar
|
|
15
|
Wu SQ, Xu NY, Sun Q, Han HY and Zhang J:
Six New sesquiterpenes from Eupatorium lindleyanum. Helv Chim Acta.
95:1637–1644. 2012. View Article : Google Scholar
|
|
16
|
Wu SQ, Xu NY, Zhang J, Yao S and Chu CJ:
Three new acyclic diterpenoids from Eupatorium lindleyanum DC. J
Asian Nat Prod Res. 14:652–656. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ito K, Sakakibara Y, Haruna M and Lee KH:
Four new germacranolides from Eupatorium lindleyanum DC. Chem Lett.
8:1469–1472. 1979. View Article : Google Scholar
|
|
18
|
Charlot JF, Prétet JL, Haughey C and
Mougin C: Mitochondrial translocation of p53 and mitochondrial
membrane potential (ΔΨm) dissipation are early events in
staurosporine-induced apoptosis of wild type and mutated p53
epithelial cells. Apoptosis. 9:333–343. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reers M, Smiley ST, Mottola-Hartshorn C,
Chen A, Lin M and Chen LB: Mitochondrial membrane potential
monitored by JC-1 dye. Methods Enzymol. 260:406–417. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kajiwara T, Takeuchi T, Ueki T, Moriyama
N, Ueki K, Kakizoe T and Kawabe K: Effect of Bcl-2 overexpression
in human prostate cancer cells in vitro and in vivo. Int J Urol.
6:520–525. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Henry-Mowatt J, Dive C, Martinou JC and
James D: Role of mitochondrial membrane permeabilization in
apoptosis and cancer. Oncogene. 23:2850–2860. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Takeda K and Akira S: STAT family of
transcription factors in cytokine-mediated biological responses.
Cytokine Growth Factor Rev. 11:199–207. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gottlieb TM, Leal JF, Seger R, Taya Y and
Oren M: Cross-talk between Akt, p53 and Mdm2: Possible implications
for the regulation of apoptosis. Oncogene. 21:1299–1303. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Farha AK, Dhanya SR, Mangalam SN, Geetha
BS, Latha PG and Remani P: Deoxyelephantopin impairs growth of
cervical carcinoma SiHa cells and induces apoptosis by targeting
multiple molecular signaling pathways. Cell Biol Toxicol.
30:331–343. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li MH, Cha YN and Surh YJ: Peroxynitrite
induces HO-1 expression via PI3K/Akt-dependent activation of
NF-E2-related factor 2 in PC12 cells. Free Radic Biol Med.
41:1079–1091. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sheppard K, Kinross KM, Solomon B, Pearson
RB and Phillips WA: Targeting PI3 kinase/AKT/mTOR signaling in
cancer. Crit Rev Oncog. 17:69–95. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gao L, Wang Y, Xu Z, Li X, Wu J, Liu S,
Chu P, Sun Z, Sun B, Lin Y, et al: SZC017, a novel oleanolic acid
derivative, induces apoptosis and autophagy in human breast cancer
cells. Apoptosis. 20:1636–1650. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xavier CP, Lima CF, Preto A, Seruca R,
Fernandes-Ferreira M and Pereira-Wilson C: Luteolin, quercetin and
ursolic acid are potent inhibitors of proliferation and inducers of
apoptosis in both KRAS and BRAF mutated human colorectal cancer
cells. Cancer Lett. 281:162–170. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pu L, Amoscato AA, Bier ME and Lazo JS:
Dual G1 and G2 phase inhibition by a novel, selective Cdc25
inhibitor
7-chloro-6-(2-morpholin-4-ylethylamino)-quinoline-5,8-dione. J Biol
Chem. 277:46877–46885. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chao JI, Kuo PC and Hsu TS:
Down-regulation of survivin in nitric oxide-induced cell growth
inhibition and apoptosis of the human lung carcinoma cells. J Biol
Chem. 279:20267–20276. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Su M, Chung HY and Li Y:
6-O-Angeloylenolin induced cell-cycle arrest and apoptosis in human
nasopharyngeal cancer cells. Chem Biol Interact. 189:167–176. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang CJ, Wang CS, Hung JY, Huang HW, Chia
YC, Wang PH, Weng CF and Huang MS: Pyrogallol induces G2-M arrest
in human lung cancer cells and inhibits tumor growth in an animal
model. Lung Cancer. 66:162–168. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Denault JB and Boatright K: Apoptosis in
Biochemistry and Structural Biology. 3–8 February 2004, Keystone,
CO, USA. IDrugs. 7:315–317. 2004.PubMed/NCBI
|
|
34
|
Scaffidi C, Fulda S, Srinivasan A, Friesen
C, Li F, Tomaselli KJ, Debatin KM, Krammer PH and Peter ME: Two
CD95 (APO-1/Fas) signaling pathways. EMBO J. 17:1675–1687. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lazebnik YA, Kaufmann SH, Desnoyers S,
Poirier GG and Earnshaw WC: Cleavage of poly(ADP-ribose) polymerase
by a proteinase with properties like ICE. Nature. 371:346–347.
1994. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schwarz M, Andrade-Navarro MA and Gross A:
Mitochondrial carriers and pores: Key regulators of the
mitochondrial apoptotic program? Apoptosis. 12:869–876. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kauffmann-Zeh A, Rodriguez-Viciana P,
Ulrich E, Gilbert C, Coffer P, Downward J and Evan G: Suppression
of c-Myc-induced apoptosis by Ras signalling through PI(3)K and
PKB. Nature. 385:544–548. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Katayama K, Fujita N and Tsuruo T:
Akt/protein kinase B-dependent phosphorylation and inactivation of
WEE1Hu promote cell cycle progression at G2/M
transition. Mol Cell Biol. 25:5725–5737. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Weir NM, Selvendiran K, Kutala VK, Tong L,
Vishwanath S, Rajaram M, Tridandapani S, Anant S and Kuppusamy P:
Curcumin induces G2/M arrest and apoptosis in
cisplatin-resistant human ovarian cancer cells by modulating Akt
and p38 MAPK. Cancer Biol Ther. 6:178–184. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Asnaghi L, Calastretti A, Bevilacqua A,
D'Agnano I, Gatti G, Canti G, Delia D, Capaccioli S and Nicolin A:
Bcl-2 phosphory-lation and apoptosis activated by damaged
microtubules require mTOR and are regulated by Akt. Oncogene.
23:5781–5791. 2004. View Article : Google Scholar : PubMed/NCBI
|