Introduction
As one of the three malignant tumors of the
gynecologic reproductive system, the morbidity of ovarian cancer is
ranked only second to cervical and endometrial cancer. Moreover,
its mortality rate is highest among all female reproductive tract
malignant tumors (1). The majority
(up to 85–90%) of ovarian malignant carcinomas originate from the
coelomic epithelium (2,3). Since the ovary is deeply located in
the pelvic cavity and there is no specificity for incipient
symptoms of ovarian cancer, epithelial ovarian carcinoma develops
rapidly, and is prone to metastasis and wide dissemination
(2); 70–80% of patients are
diagnosed at stage III and IV while seeking medical services for
ascites or pelvic mass (4).
Ovarian cancer is a multifactor disease with genetic
susceptibility (3). Gene mutations
in patients lead to differences in ovarian cancer susceptibility.
Ovarian cancer is difficult to be definitely diagnosed at an early
stage, and thus patients are at an advanced stage when diagnosed
(5). Studying molecular markers
with specificity is of great significance in diagnosing ovarian
cancer early and improving the patient survival rate (6). DNA damage epigenetics, such as DNA
abnormal demethylation and hyperphosphorylation, has become a new
‘hot’ research topic in recent years (7). DNA damage is closely associated with
cancer-suppressor genes aroused by abnormal DNA transfer to
daughter cells, therefore it is an essential mechanism for tumors
to inhibit gene inactivation (7).
In addition, DNA damage response is extremely important for
monitoring the organism and rehabilitation mechanism (8).
DNA (cytosine-5)-methyltransferase 3α (DNMT3a)
encoding DNA transmethylase 3A has been investigated in recent
years, and the DNA methylation process may have catalytic action
(9). In general, the DNA
methylation level is believed to be highly correlated to tumor
occurrence. Gene mutations in AML patients reach 20%. DNMT3a and b
are de novo methylation enzymes, and also contribute to
maintaining DNA methylation patterns in embryonic stem (ES) and
somatic cells. The inactivation of DNMT3a and b results in the
gradual loss of DNA methylation. De novo methylation
activities are coordinated to permit the faithful inheritance of
DNA methylation and would tip the balance of DNA methylation
inheritance to increase DNA methylation.
As a type of non-coding small RNAs, microRNAs
(miRNAs) inhibit the function of target mRNAs by inhibiting
translation or inducing degradation (10), which regulates cell differentiation,
proliferation and apoptosis. Research shows that various tumor
tissues have abnormal expression or mutation of miRNAs, which have
an effect on tumor growth, invasion and metastasis (10,11).
miR-182 is an important miRNA molecule (12), which promotes benign and malignant
tumor cell invasion and transformation, indicating the significance
of miR-182 in ovarian cancer. It is conducive to ovarian cancer
prevention and treatment through effective regulation.
Materials and methods
Patients and tissue samples
The present study was approved by the Ethics Review
Committee of Liaocheng People's Hospital and informed consent for
the use of tissues was obtained for all individuals. Twelve freshly
resected ovarian cancer and tissues from 8 ovariectomized patients
were collected from Liaocheng People's Hospital, Shandong, China.
Adjacent and cancer tissue samples were collected from 12 freshly
resected ovarian cancer patients, and normal ovarian tissue samples
were collected from 8 ovariectomized patients, in accordance with
the institutional guidelines and immediately frozen in liquid
nitrogen for further analysis.
Cell culture
Human ovarian cancer Caov-3, OV-1063, OVCAR-3 and
Caov-4 cell lines were cultured in Dulbecco's modified Eagles
medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS)
(both from HyClone, Thermo Scientific, Waltham, MA, USA) and 100
units penicillin-streptomycin at 37°C in a 5% CO2
atmosphere in a humidified incubator.
Quantitative RT-PCR assay
RNA was extracted from frozen tissues or cell
samples using TRIzol reagent (Life Technologies) according to the
manufacturer's instructions. RNA (1 µg) was used to compound cDNA
using a SuperScript™ One-Step RT-PCR kit (Toyobo, Tokyo, Japan)
according to the manufacturer's instructions. qRT-PCR was performed
using SYBR-Green Mix reagent (Takara, Dalian, China) and the primer
sequences of miR-182 are listed in Table I.
 | Table I.Characteristics of the primers used
for qRT-PCR. |
Table I.
Characteristics of the primers used
for qRT-PCR.
| Primer | Sequence (5′-3′)
(sense) | Sequence (5′-3′)
(antisense) |
|---|
| DNMT3a |
TATGAACAGGCCGTTGGCATC |
AAGAGGTGGCGGATGACTGG |
| β-actin |
CACGAAACTACCTTCAACTCC |
CATACTCCTGCTTGCTGATC |
| miR-182 |
TGCGGTTTGGCAATGGTAGAAC |
TGCGGTTTGGCAATGGTAGAAC |
| U6 |
GCTTGCTTCGGCAGCACATATAC |
TGCATGTCATCCTTGCTCAGGG |
Transfection
Human ovarian cancer Caov-3 cells (2×105)
were plated onto 6-well plates and were transfected with the
miR-182 plasmid (miR-182 overexpression), the DNMT3 plasmid (DNMT3a
overexpression), DNMT3a siRNA (downregulation of DNMT3a expression)
or the negative control using transfection reagent Lipofectamine
2000 (Invitrogen, Carlsbad, CA, USA).
Flow cytometric analysis of
apoptosis
Human ovarian cancer Caov-3 cells (2×105)
transfected with the different plasmids were plated onto 6-well
plates. The apoptosis rate was assessed to detect early stage
apoptosis by analysis of Annexin V-FITC binding (Becton-Dickinson,
San Jose, CA, USA). Annexin V-FITC (10 µl) and 5 µl of propidium
iodide (PI) were added into every well and the cells were cultured
for 30 min. The apoptosis rate was detected using a flow cytometer
(BD Biosciences, Oxford, UK).
Western blotting
Caov-3 cells transfected with the different plasmids
were directly lysed in Laemmli's sample buffer and boiled for 30
min on ice. After removal of the insoluble fraction by
centrifugation, total protein expression was quantitated using the
BCA method (Life Technologies Co., Shanghai, China). Total protein
extracts (50 µg) were resolved on 10% SDS-PAGE and transferred to
nitrocellulose membranes for western blotting. The membranes were
incubated with the primary antibodies: anti-DNMT3a, anti-p53,
anti-c-Myc (all from Bethyl Laboratories, Cambridge Biosciences,
Cambridge, UK) and β-actin (Sangon Biotech) at a dilution of
1:1,000, at room temperature for 1 h. After washing with 5% bovine
serum albumin (BSA) dissolved in Tris-buffered saline (TBS),
containing 0.05% Tween-20 (TBST), the membranes were incubated with
goat anti-rabbit secondary antibody conjugated to horseradish
peroxidase (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) at
a 1:2,000 dilution at room temperature for 1 h. Positive band
intensities were detected using a gel documentation system
(LAS-3000; Fujifilm, Tokyo, Japan).
Caspase-3 and −9 activity
Human ovarian cancer Caov-3 cells (1×103)
transfected with the different plasmids were plated onto 96-well
plates. Ac-DEVD-pNA (caspase-3) and Ac-LEHD-pNA (caspase-9) were
added into every well and the cells were cultured for 30 min.
Caspase-3 and −9 activity was detected using a BioTek Synergy plate
reader (BioTek, Potton, UK) at 450 nm.
Statistical analysis
The data are expressed as the mean ± SD. The
Students t-test was used to determine the statistically significant
differences in numbers with two significant levels (p<0.05).
Results
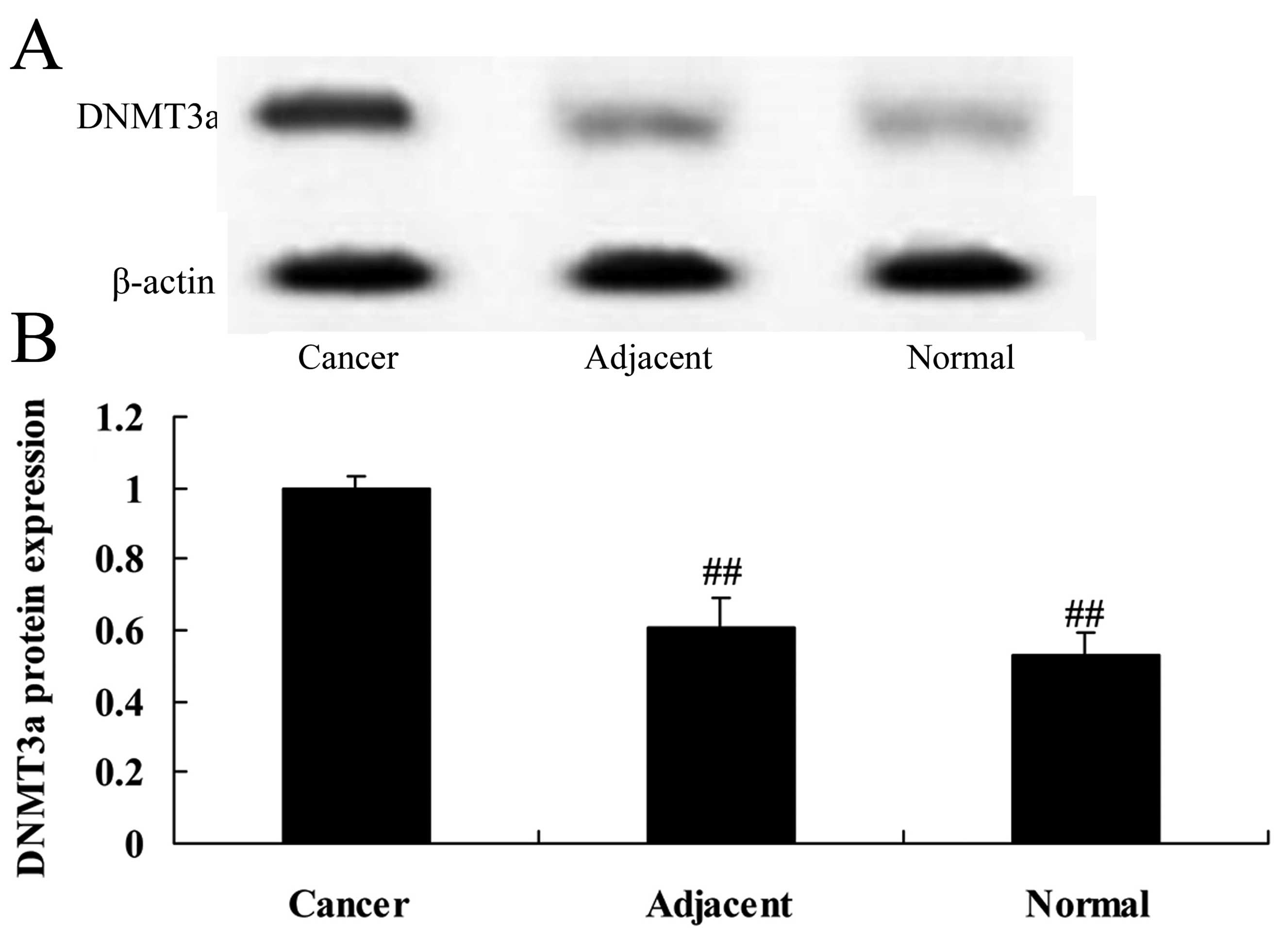
The protein expression of DNMT3a in
normal, adjacent cancer and cancer tissues
Firstly, we assessed the DNMT3a protein expression
in 12 freshly resected ovarian cancer and 8 ovariectomized patient
tissues. We found that the protein expression of DNMT3a in the
cancer tissues was much higher than that noted in the adjacent
cancer or normal tissues (Fig. 1).
However, the protein expression of DNMT3a in the adjacent cancer
tissues was very similar to that noted in the normal tissues
(Fig. 1).
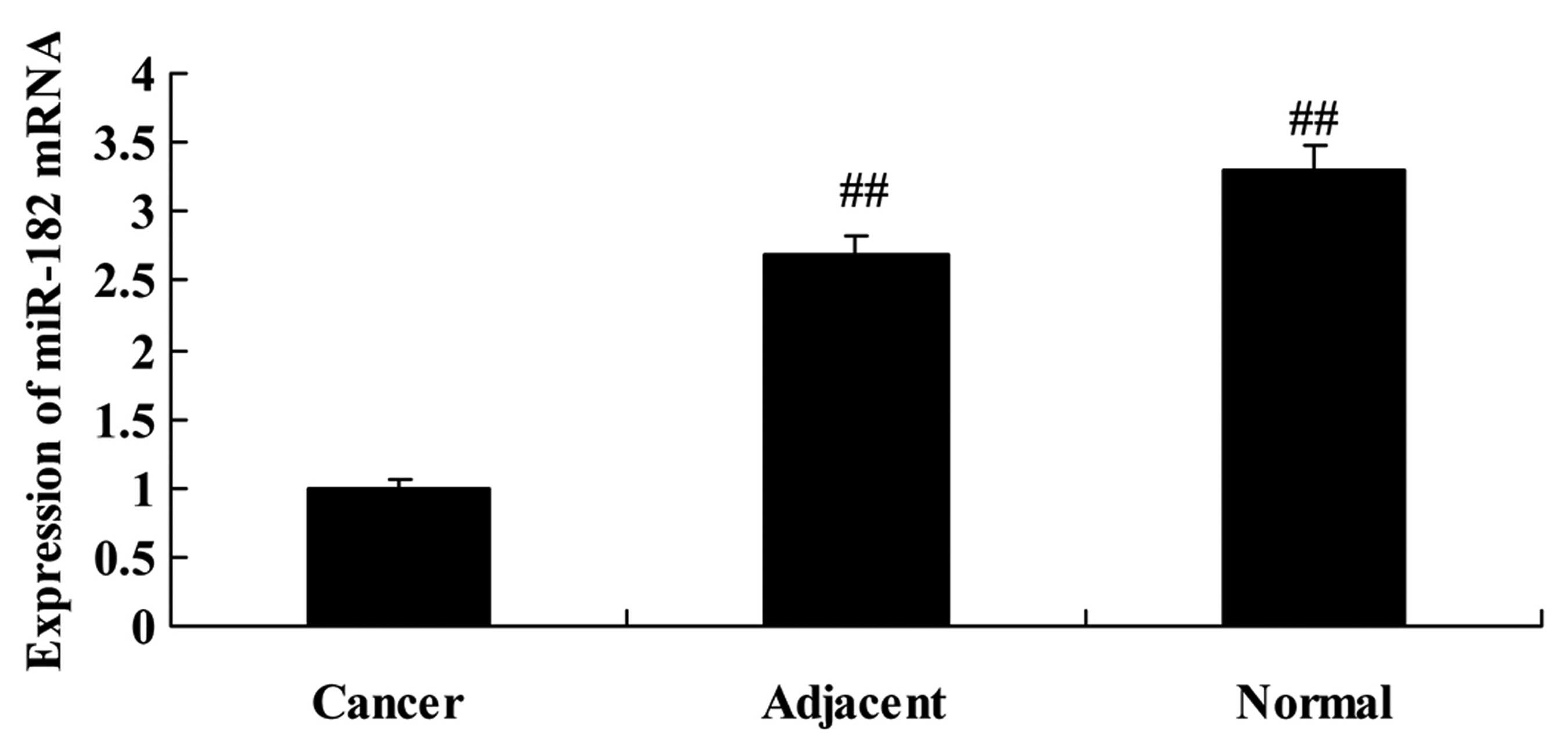
RT-PCR analysis of miR-182 in normal,
adjacent cancer and cancer tissues
Next, we found that the expression of miR-182 in
cancer tissues was lower than that noted in the adjacent cancer or
normal tissues (Fig. 2). However,
the expression of miR-182 in the adjacent cancer tissues was very
similar to that noted in the normal tissues (Fig. 2).
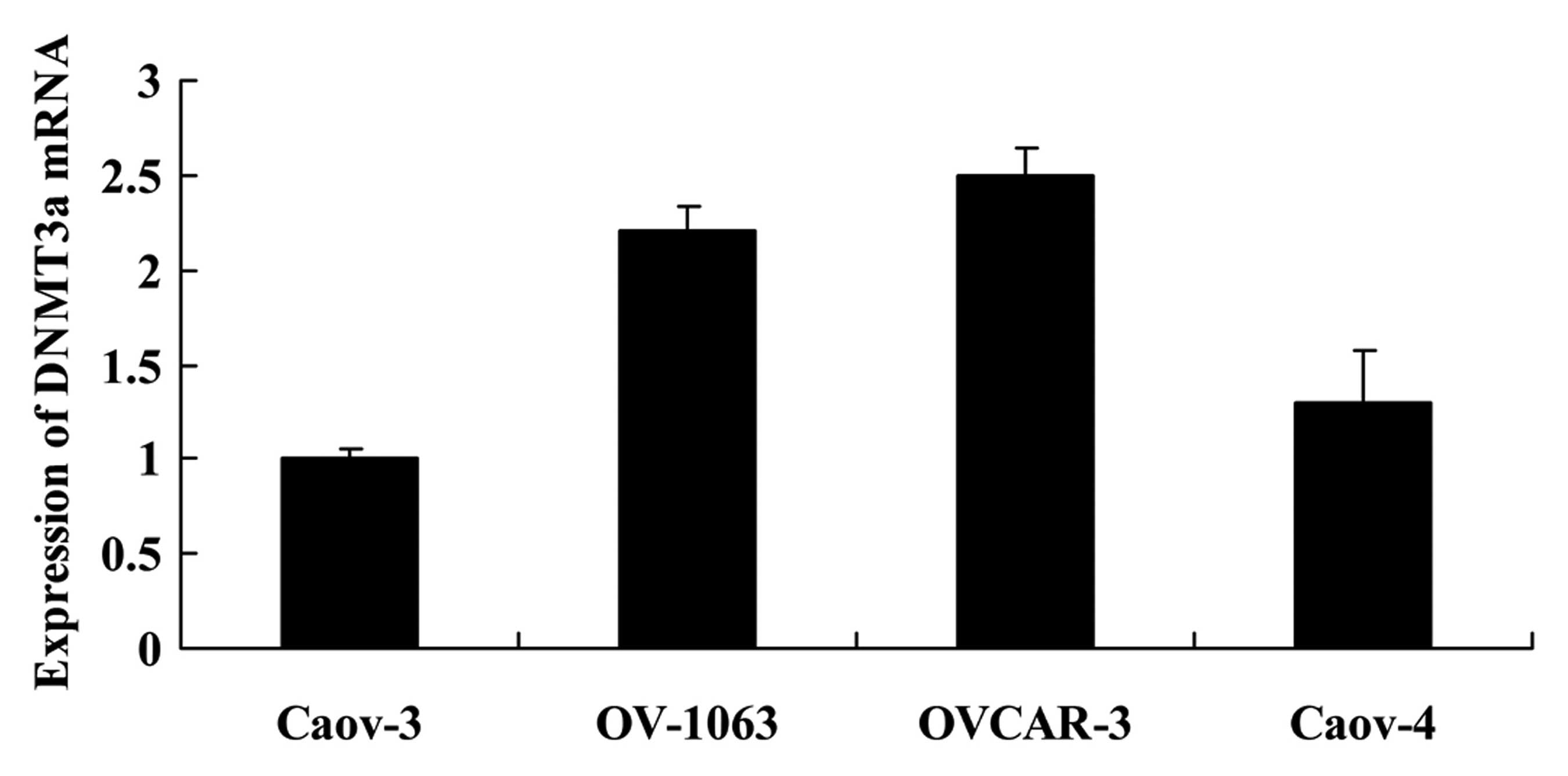
Expression of DNMT3a in human ovarian
cancer cells
We used Caov-3, OV-1063, OVCAR-3 and Caov-4 cells to
analyze the expression of DNMT3a mRNA. As shown in Fig. 3, the expression of DNMT3a in the
Caov-3 cells was the lowest. Thus, we selected Caov-3 cells to
serve as the main cells used in the present study.
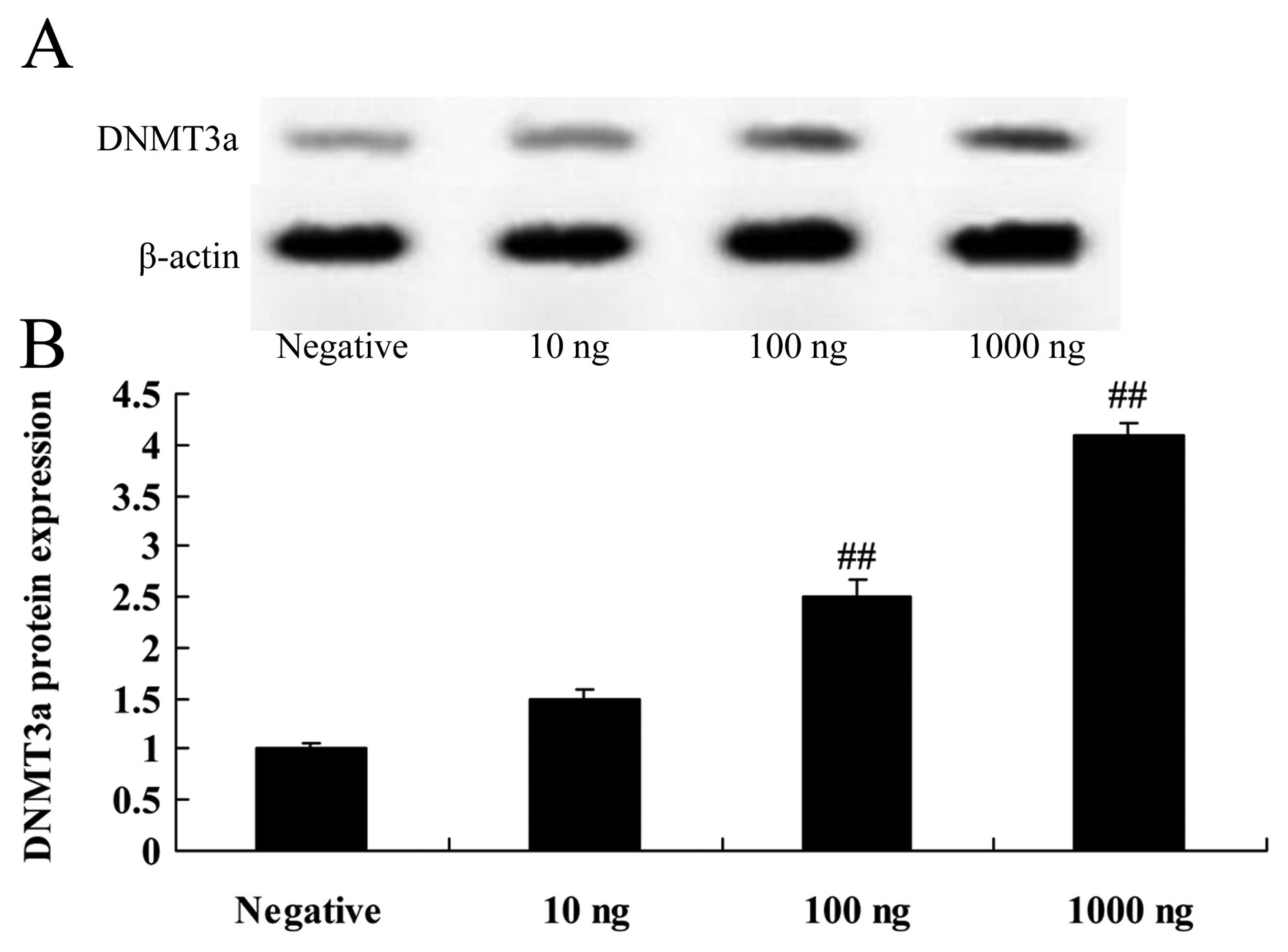
Overexpression of DNMT3a affects the
DNMT3a protein in human ovarian cancer
The DNMT3 plasmid was transfected into Caov-3 cells,
in which we induced expression of DNMT3a. As shown in Fig. 4, the DNMT3 plasmid induced the
DNMT3a protein expression in the Caov-3 cells in a dose-dependent
manner. Particularly, 100 or 1,000 ng DNMT3 plasmid significantly
activated the protein expression of DNMT3a in the Caov-3 cells,
compared with the negative control group (Fig. 4).
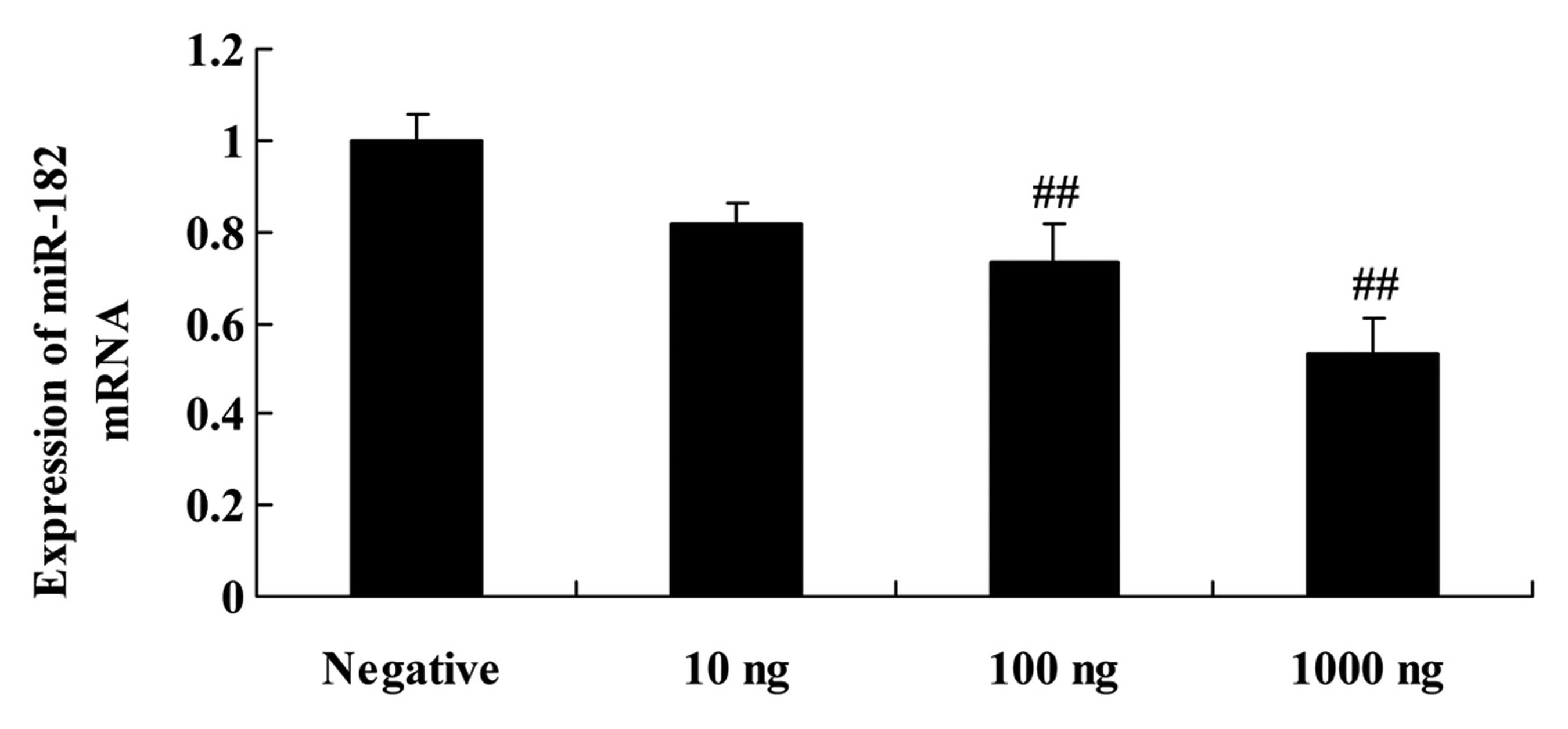
Overexpression of DNMT3a affects
miR-182 in human ovarian cancer
When the DNMT3 plasmid was transfected into the
Caov-3 cells, miR-182 expression was assessed using quantitative
RT-PCR assay. As shown in Fig. 5,
the DNMT3 plasmid suppressed miR-182 expression in the Caov-3 cells
in a dose-dependent manner. The DNMT3 plasmid (100 or 1,000 ng)
significantly suppressed miR-182 expression in the Caov-3 cells,
compared with that in the negative control group (Fig. 5).
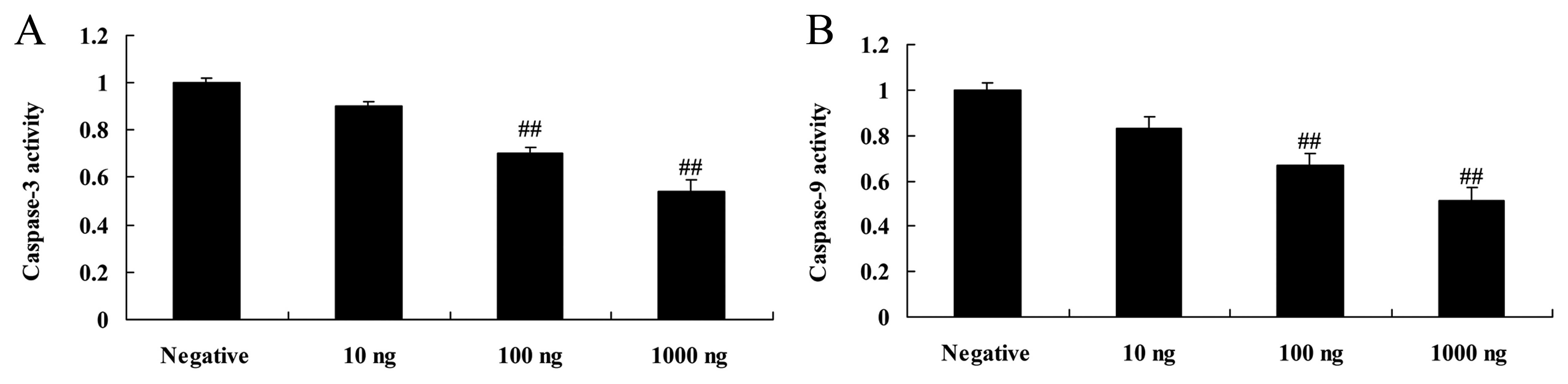
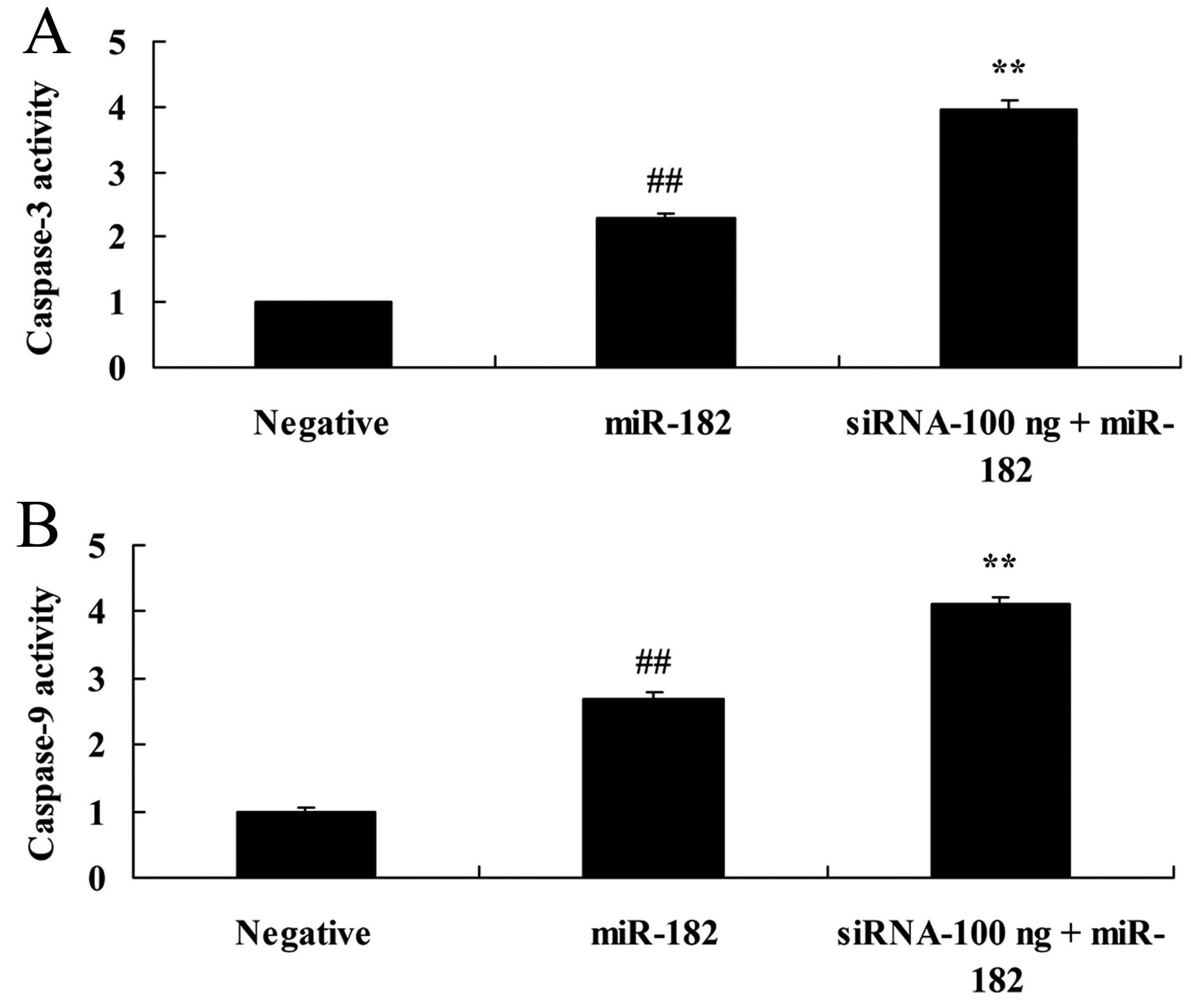
Overexpression of DNMT3a affects
caspase-3 and −9 activity in human ovarian cancer
Overexpression of DNMT3a inhibited caspase-3 and −9
activity in the Caov-3 cells in a dose-dependent manner. As shown
in Fig. 6, the DNMT3 plasmid (100
or 1,000 ng) significantly inhibited caspase-3 and −9 activity in
the Caov-3 cells, as compared with the negative control group.
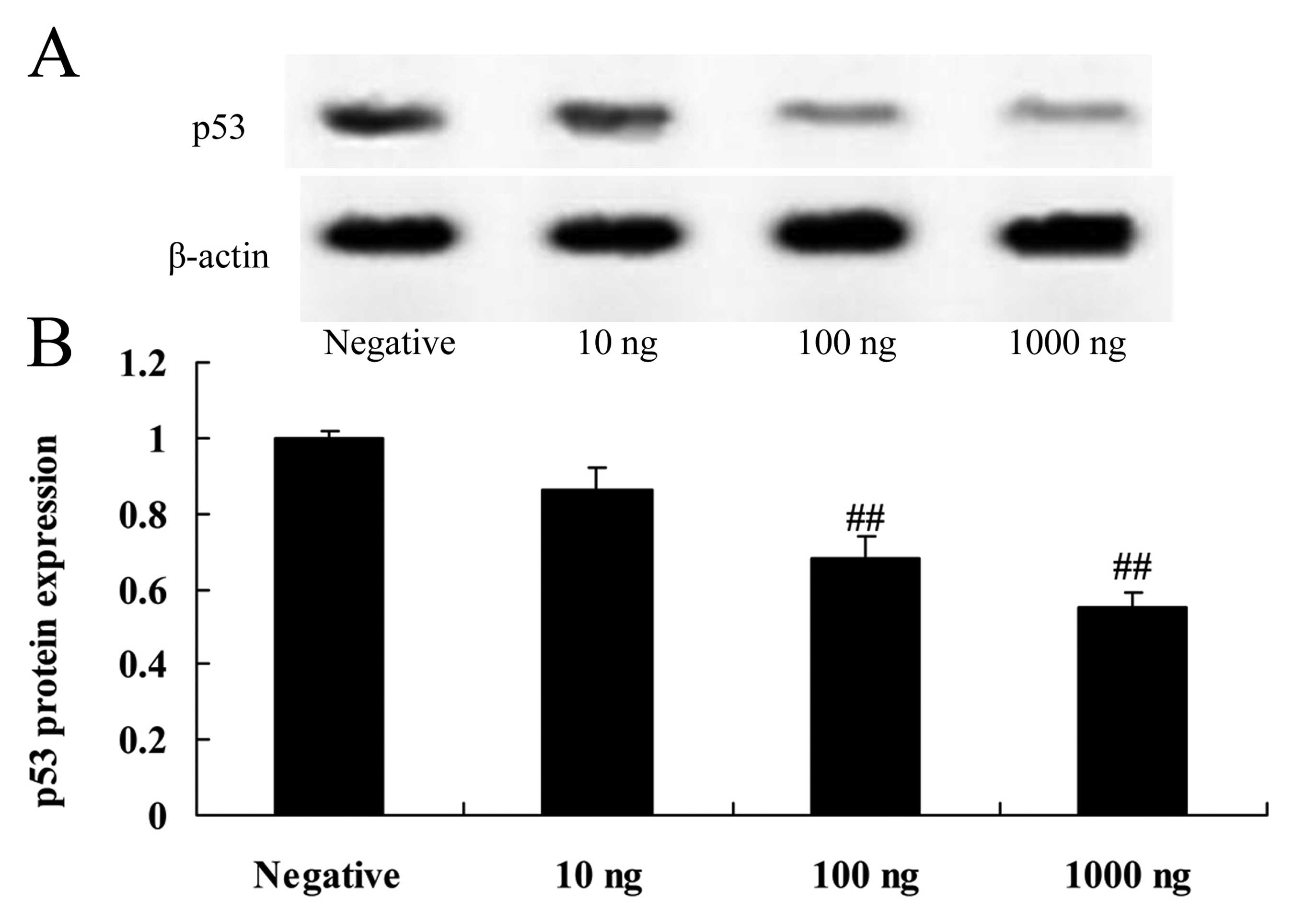
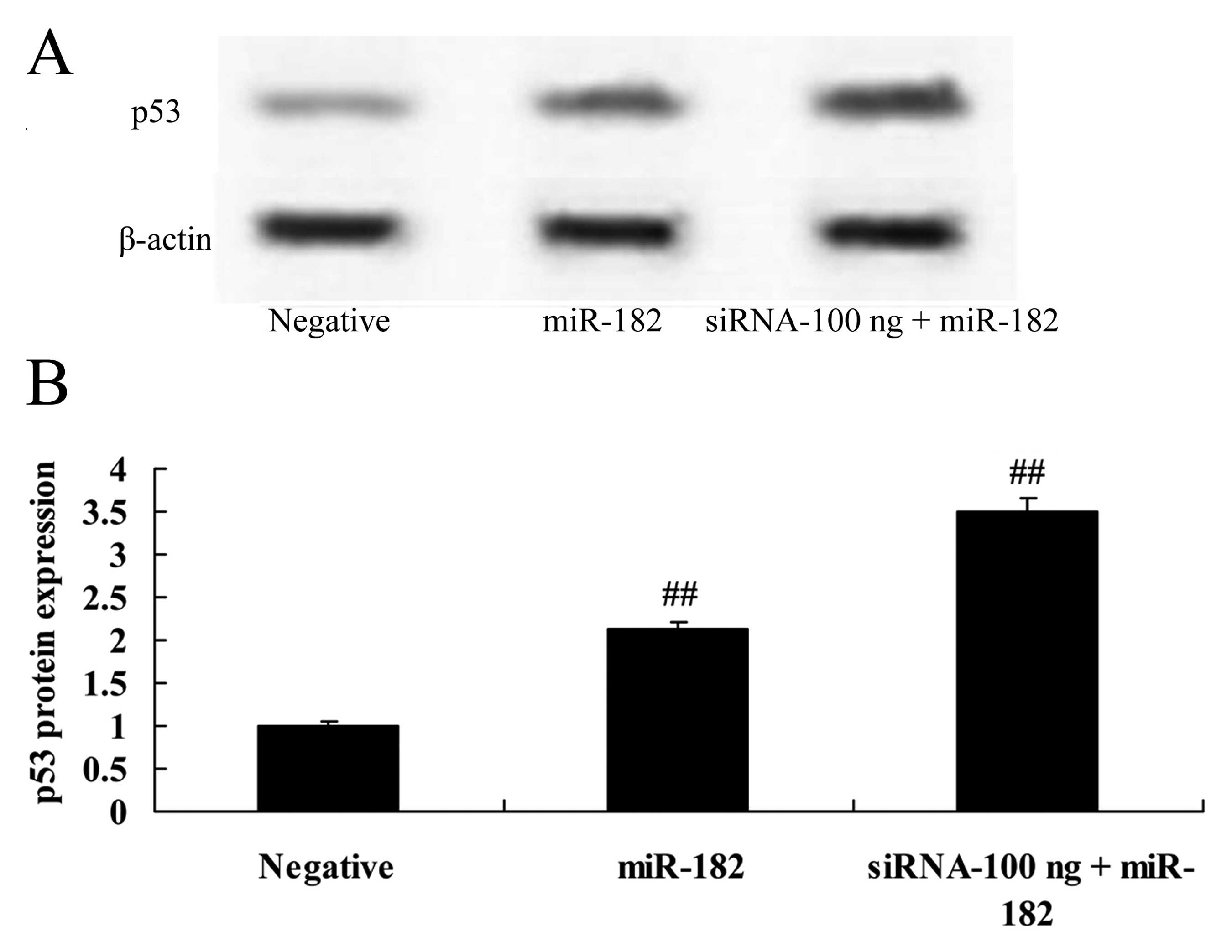
Overexpression of DNMT3a affects p53
protein expression in human ovarian cancer
Overexpression of DNMT3a suppressed p53 protein
expression in the Caov-3 cells in a dose-dependent manner. As shown
in Fig. 7, 100 or 1,000 ng of the
DNMT3 plasmid significantly suppressed p53 protein expression in
the Caov-3 cells, as compared with the negative control group.
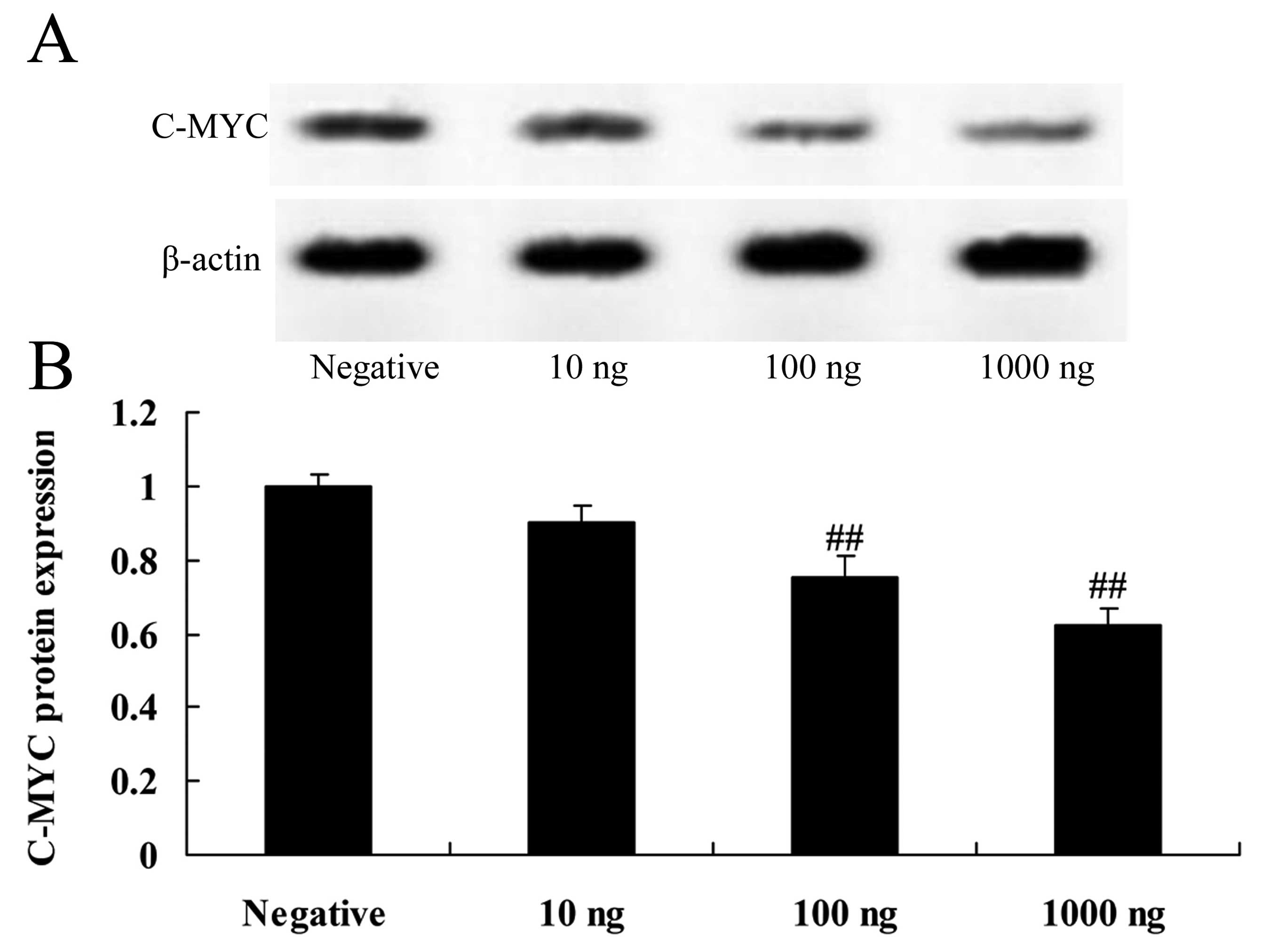
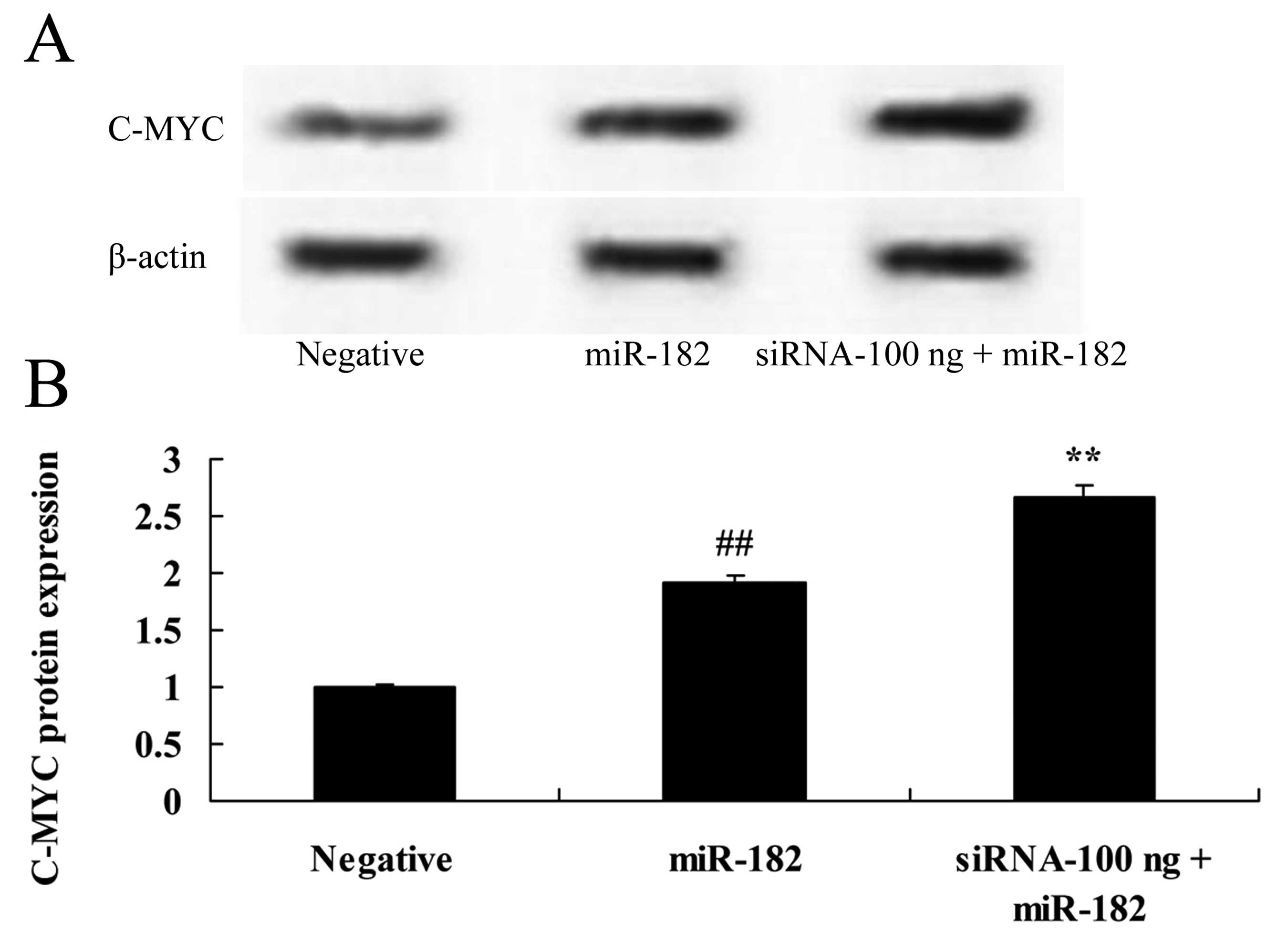
Overexpression of DNMT3a affects c-Myc
protein expression in human ovarian cancer
Overexpression of DNMT3a inhibited c-Myc protein
expression in the Caov-3 cells in a dose-dependent manner (Fig. 8). As shown in Fig. 8, 100 or 1,000 ng of the DNMT3
plasmid significantly inhibited c-Myc protein expression in the
Caov-3 cells, as compared with the negative control group.
DNMT3a regulates the effect of miR-182
on the apoptotic rate of human ovarian cancer
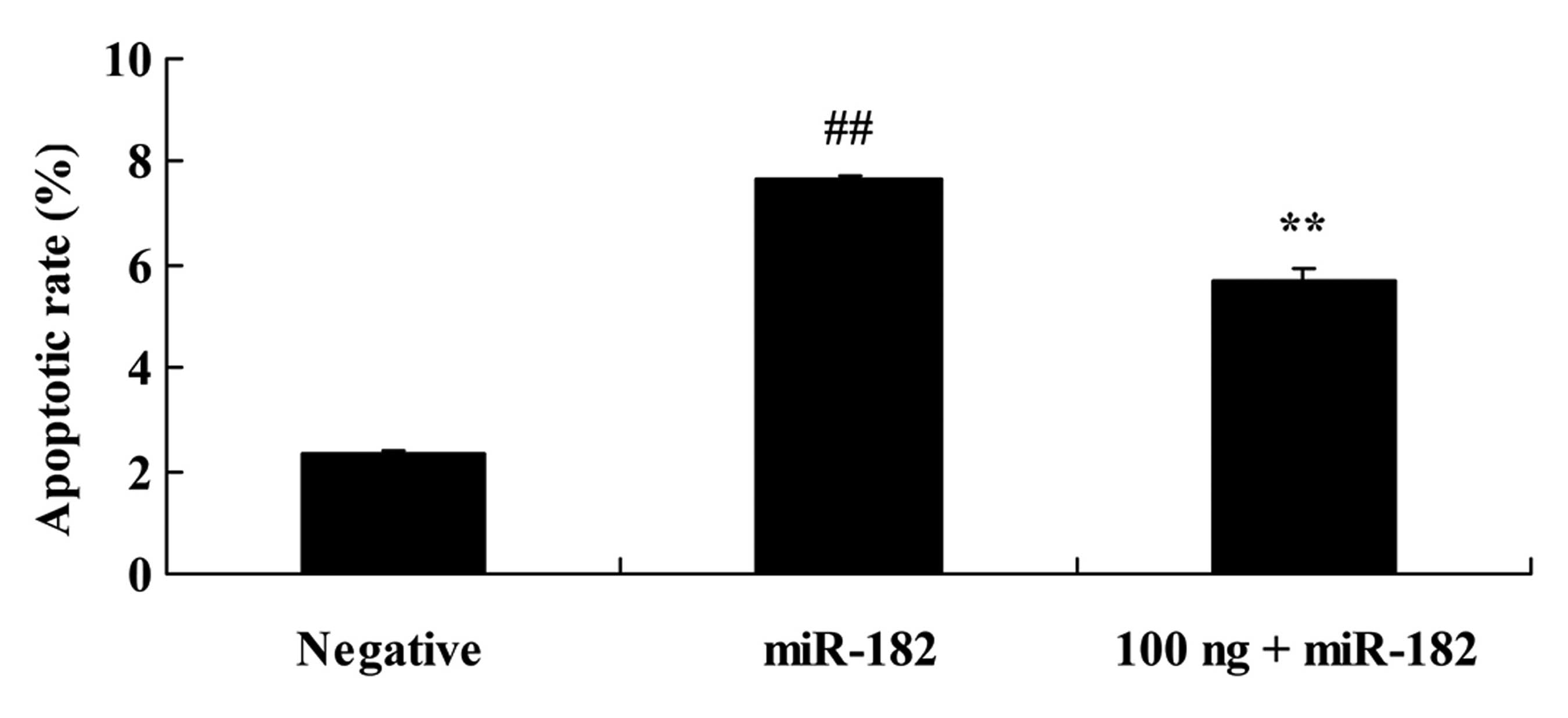
We analyzed the DNMT3a-regulated miR-182 effects on
the apoptotic rate of human ovarian cancer. As shown in Fig. 9, overexpression of miR-182
significantly increased the apoptotic rate in the Caov-3 cells
compared with the negative control group. The DNMT3 plasmid (100
ng) significantly reduced the apoptotic rate of the Caov-3 cells
induced by the miR-182 plasmid, compared with the miR-182 plasmid
group.
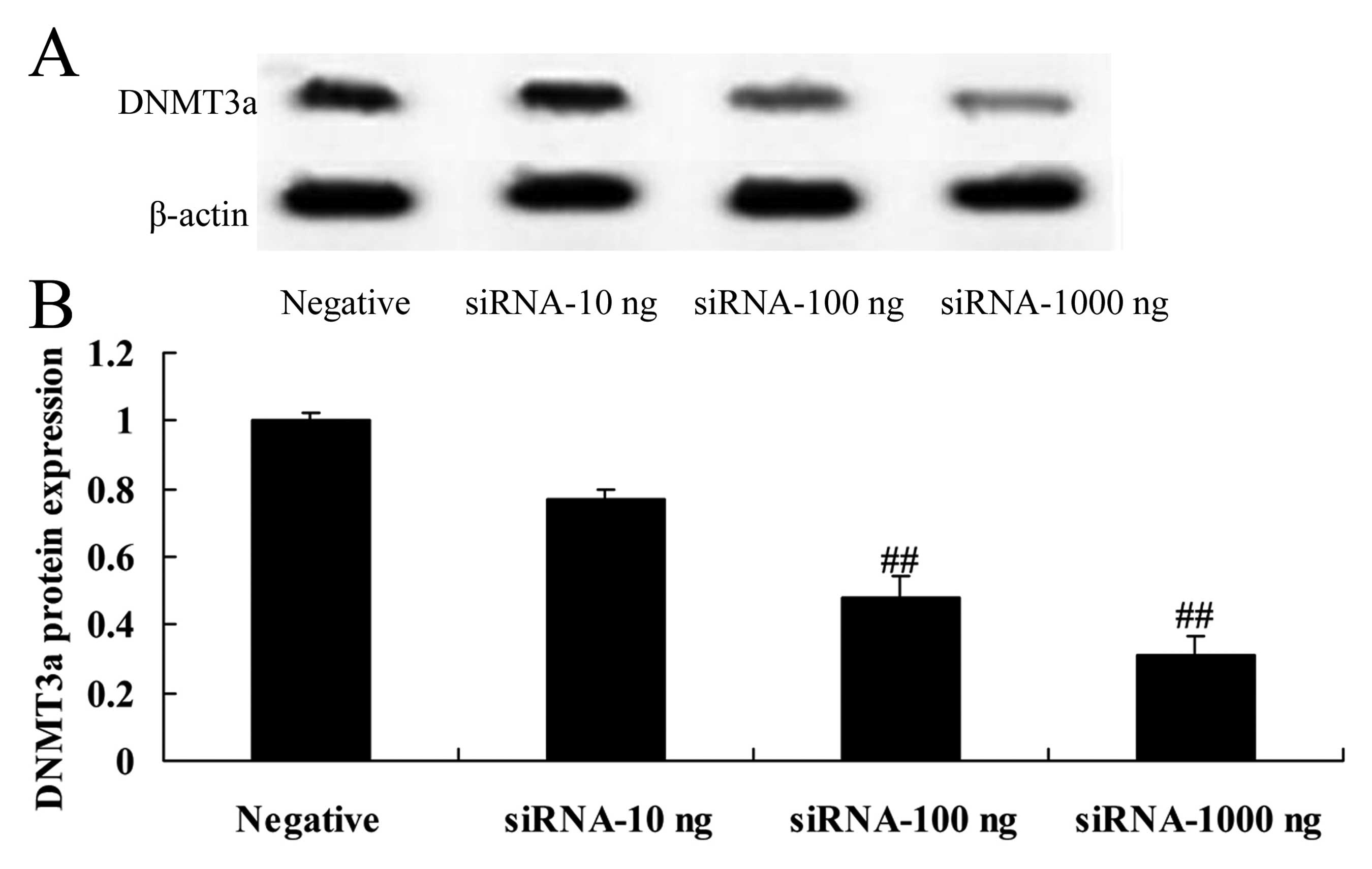
Downregulation of DNMT3a expression
affects the DNMT3a protein in human ovarian cancer
To research the effects of the downregulation of
DNMT3a expression on the DNMT3a protein in human ovarian cancer,
DNMT3a protein expression was assessed using western blotting. As
shown in Fig. 10, downregulation
of DNMT3a expression inhibited the protein expression of DNMT3a in
the Caov-3 cells. Particularly, 100 or 1,000 ng DNMT3 siRNA
significantly inhibited the protein expression of DNMT3a in the
Caov-3 cells, compared with the negative control group (Fig. 10).
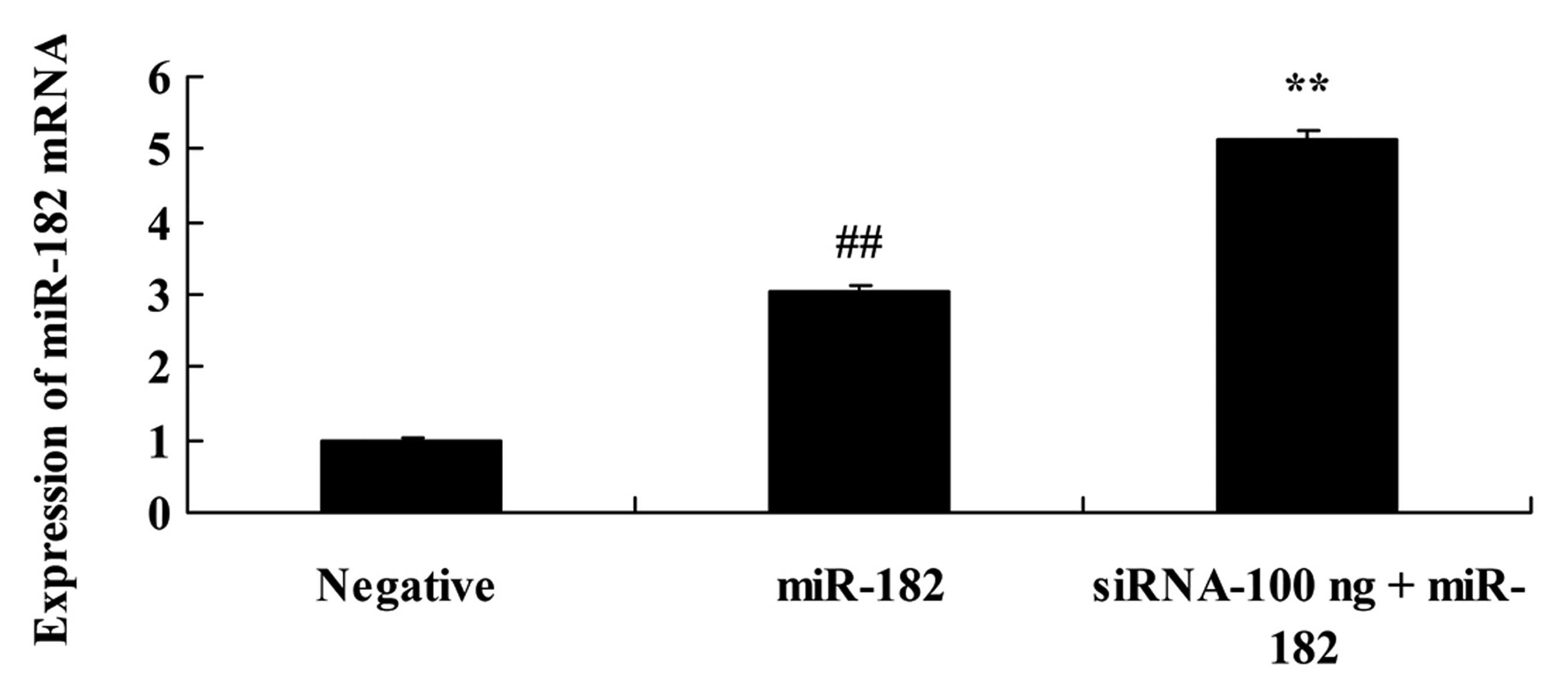
Downregulation of DNMT3a expression
affects miR-182 in human ovarian cancer
To further research the effects of the
downregulation of DNMT3a expression on miR-182 expression in human
ovarian cancer, miR-182 expression was assessed using quantitative
RT-PCR assay. As shown in Fig. 11,
100 ng of DNMT3 siRNA significantly increased the miR-182
expression in the Caov-3 cells, compared with the miR-182 plasmid
group.
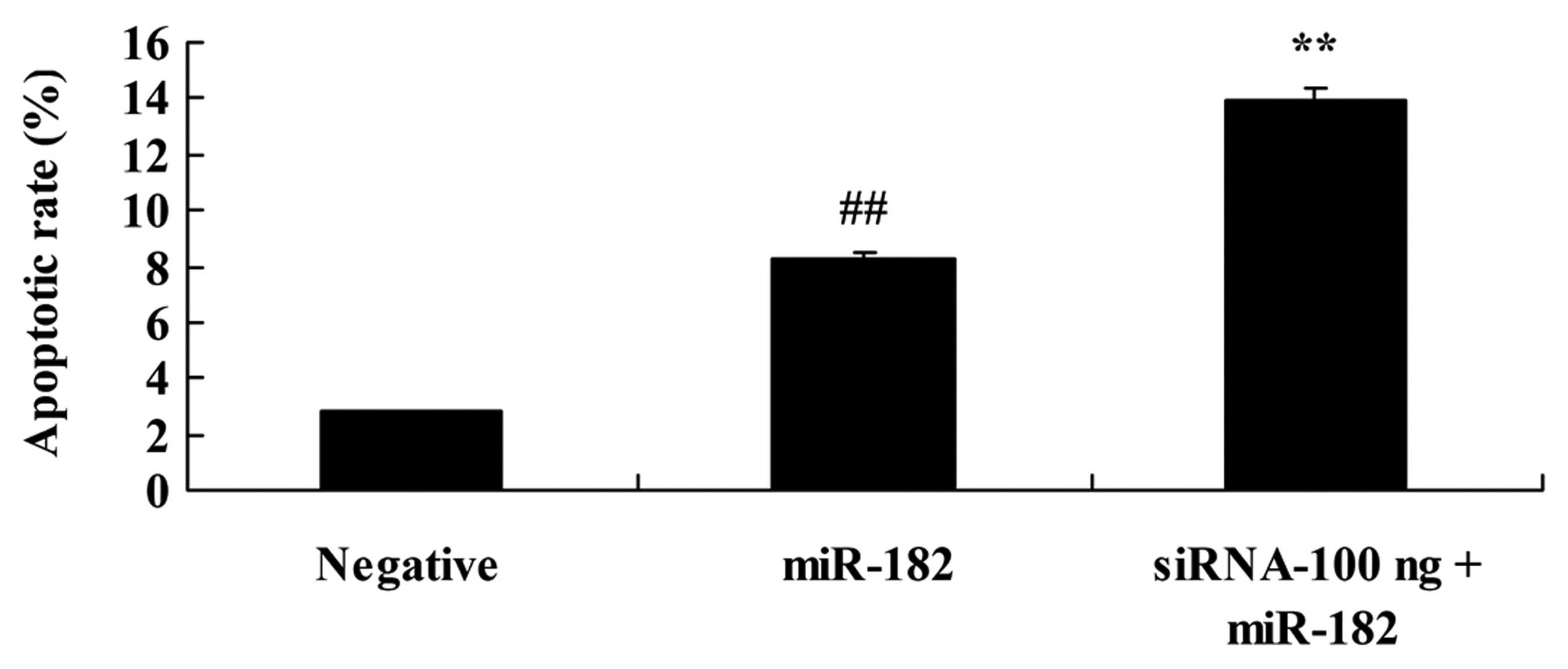
Downregulation of DNMT3a expression
affects the apoptotic rate of human ovarian cancer cells
We explored the effect of the downregulation of
DNMT3a on the apoptotic rate of human ovarian cancer. As shown in
Fig. 12, 100 ng of DNMT3 siRNA
significantly increased the apoptotic rate in the Caov-3 cells,
compared with the miR-182 plasmid group.
Downregulation of DNMT3a expression
affects caspase-3 and −9 activity in human ovarian cancer
We further explored the affect of the downregulation
of DNMT3a expression on caspase-3 and −9 activity in human ovarian
cancer. As shown in Fig. 13, 100
ng of DNMT3 siRNA significantly increased caspase-3 and −9 activity
in the Caov-3 cells, compared with the miR-182 plasmid group.
Downregulation of DNMT3a expression
affects p53 protein expression in human ovarian cancer
We investigated the affects of the downregulation of
DNMT3a expression on p53 protein expression in human ovarian
cancer. Compared with miR-182 plasmid group, 100 ng of siRNA DNMT3
plasmid significantly enhanced p53 protein expression in the Caov-3
cells (Fig. 14).
Downregulation of DNMT3a expression
affects c-Myc protein expression in human ovarian cancer
We investigated the affects of the downregulation of
DNMT3a expression on c-Myc protein expression in human ovarian
cancer. As shown in Fig. 15, 100
ng of siRNA DNMT3 plasmid significantly increased c-Myc protein
expression in the Caov-3 cells, compared with the miR-182 plasmid
group.
Discussion
Ovarian cancer is the fifth leading gynecologic
malignant tumor resulting in the death of American women (3). The main reason for the high lethality
is that ovarian cancer is frequently at the advanced stage at
initial diagnosis (5). After
surgery, only 30% of patients gain a 5-year survival rate after
initial diagnosis, although good results can be achieved by
chemotherapy (8). At present, the
major issue associated with ovarian cancer is the lack of early
diagnosis and distant metastasis at the advanced stage, accompanied
by chemotherapy resistance (6).
Therefore, accurate and early diagnosis, and effective and safe
therapeutic strategies are key factors in ovarian cancer treatment
(7). In the present study, DNMT3a
protein expression was identified in the cancer tissue group, which
was higher than that in the adjacent cancer or normal group.
However, miR-182 expression in the adjacent cancer group was very
similar to that of the normal group.
The expression of DNMT3a in ovarian cancer ascites
tumor cell strain SKOV3 was found to be high (9), while the expression of DNMT3b was
weak. During the developmental process, the main function of DNMT1
is to maintain DNA methylation mode of the body. However, the main
function of DNMT3a and 3b is to establish a new methylation mode.
Generally, it has been recognized that DNMT1 mainly participates in
and maintains methylation (13),
whereas DNMT3a and 3b mainly participate in de novo
methylation. However, recent research has shown that DNMT3a and 3b
participate in and maintain methylation as well (13,14).
In addition, they can enhance the fidelity of methylation
reproduction mediated by DNMT1 which can participate in de
novo methylation in vitro with lower activity (9). mRNAs encoding three types of DNA
methyltransferase genes and the protein expressed by them are
highly expressed (15).
Furthermore, a large number of studies indicate that the three can
lead to abnormal DNA methylation of suppressor genes in cancer
cells and silencing through direct or synergistic effects (15,16).
Consequently, DNA transmethylase plays an important role in
methylation start-up and maintenance in tumor cells. DNMT3a
upregulation was observed in human ovarian cancer Caov-3, OV-1063,
OVCAR-3 and Caov-4 cells.
miRNAs are non-coding single-stranded RNAs, 17–28 bp
in length, which play a vital role in the regulation of genetic
transcription, and participate in the genesis and development of
various tumors (17). The abnormal
expression of miR-182 has been found in numerous tumors and is
closely related to tumor metastasis (12,18).
In ovarian cancer, melanoma, hepatocellular carcinoma and glioma,
miR-182 expression has been shown to be upregulated, and to promote
the genesis and development of tumors as an oncogene (10).
Our results of western blotting and quantitative
RT-PCR assay showed that DNMT3a was highly expressed and miR-182
expression was downregulated in cancer tissues. Downregulation of
DNMT3a expression significantly activated miR-182 expression in the
Caov-3 cells. Our data indicate a role for DNMT3a in the regulation
of miR-182 and elucidate the anticancer mechanism in ovarian
cancer. Moreover, Sun et al reported that miR-182 induced
cervical cancer cell apoptosis by suppressing DNMT3a expression
(19).
COMT mRNA and protein expression has been shown to
be increased in numerous cancer cells, and to lead to DNA
methylation of suppressor genes in cancer cells through direct or
synergistic effects (20).
Consequently, DNA transmethylase plays a very significant role in
methylation start-up and maintenance in tumor cells (21). In the present study, DNMT3a
overexpression significantly inhibited caspase-3 and −9 activity in
the Caov-3 cells, which was reversed by DNMT3a downregulaton.
The p53 gene is a cancer-suppressor gene and is
associated with the incidence of human tumors (22). Known as a ‘gene guard’, p53 protein
plays a vital role in the cell response to the environment and all
types of stress, such as DNA damage, proto-oncogene activation and
oxygen deprivation (23,24). Located at the center of such stress
signals (23), p53 transfers
signals to cells and promotes downstream gene transcription, so as
to function as a transcription factor, which includes short or long
term cell retardation, DNA duplication, rehabilitation and cell
apoptosis thus maintaining the integrity of the cellular genome or
preventing the early proliferation of cancer cells (25). In addition, our results showed that
DNMT3a overexpression significantly suppressed Caov-3 cell p53
protein expression, which was reversed by DNMT3a downregulation. Ma
et al indicated that p53 inhibits epigenetic reprogramming
through HDAC1 and DNMT3a (26).
The c-Myc gene is located on the long arm of #8
chromosome (8q24), and belongs to protooncogenes of nuclear
transcription factor type (27).
The encoding protein is intranuclear combining on DNA chain, which
is implementing regulation at transcription process (28). Presenting as a large number of gene
amplification, the formation of c-Myc induction affects cell
proliferation and transformation (28,29).
It can directly regulate transcription of other genes through other
genes and bypass systems. In the present study, the core finding is
that DNMT3a overexpression significantly suppressed c-Myc protein
expression in Caov-3 cells, which was altered by the downregulation
of DNMT3a expression. Stewart et al suggested that DNMT3a
mutates leukemia cells and induces apoptosis through p53 and
c-Myc-independent manner (30).
In summary, further elucidation of the
DNMT3a-miR-182 regulatory mechanism was presented. DNMT3a regulates
miR-182 via caspase-3 and −9-mediated apoptosis and DNA damage
response may contribute to the induction of apoptosis in ovarian
cancer.
References
|
1
|
von Gruenigen VE, Frasure HE, Kavanagh MB,
Lerner E, Waggoner SE and Courneya KS: Feasibility of a lifestyle
intervention for ovarian cancer patients receiving adjuvant
chemotherapy. Gynecol Oncol. 122:328–333. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shikany JM, Flood AP, Kitahara CM, Hsing
AW, Meyer TE, Willcox BJ, Redden DT and Ziegler RG: Dietary
carbohydrate, glycemic index, glycemic load, and risk of prostate
cancer in the Prostate, Lung, Colorectal, and Ovarian Cancer
Screening Trial (PLCO) cohort. Cancer Causes Control. 22:995–1002.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Markman M, Moon J, Wilczynski S, Lopez AM,
Rowland KM Jr, Michelin DP, Lanzotti VJ, Anderson GL and Alberts
DS: Single agent carboplatin versus carboplatin plus pegylated
liposomal doxorubicin in recurrent ovarian cancer: Final survival
results of a SWOG (S0200) phase 3 randomized trial. Gynecol Oncol.
116:323–325. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang J, Sharma A, Ghamande SA, Bush S,
Ferris D, Zhi W, He M, Wang M, Wang X, Miller E, et al: Serum
protein profile at remission can accurately assess therapeutic
outcomes and survival for serous ovarian cancer. PLoS One.
8:e783932013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Havrilesky LJ, Pokrzywinski R, Revicki D,
Higgins RV, Nycum LR, Kohler MF, Berchuck A, Myers ER and Secord
AA: Cost-effectiveness of combination versus sequential docetaxel
and carboplatin for the treatment of platinum-sensitive, recurrent
ovarian cancer. Cancer. 118:386–391. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Darcy KM, Tian C and Reed E: A Gynecologic
Oncology Group study of platinum-DNA adducts and excision repair
cross-complementation group 1 expression in optimal, stage III
epithelial ovarian cancer treated with platinum-taxane
chemotherapy. Cancer Res. 67:4474–4481. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
van de Vaart PJ, van der Vange N,
Zoetmulder FA, van Goethem AR, van Tellingen O, ten Bokkel Huinink
WW, Beijnen JH, Bartelink H and Begg AC: Intraperitoneal cisplatin
with regional hyperthermia in advanced ovarian cancer:
Pharmacokinetics and cisplatin-DNA adduct formation in patients and
ovarian cancer cell lines. Eur J Cancer. 34:148–154. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stefanou DT, Bamias A, Episkopou H,
Kyrtopoulos SA, Likka M, Kalampokas T, Photiou S, Gavalas N,
Sfikakis PP, Dimopoulos MA, et al: Aberrant DNA damage response
pathways may predict the outcome of platinum chemotherapy in
ovarian cancer. PLoS One. 10:e01176542015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ahluwalia A, Hurteau JA, Bigsby RM and
Nephew KP: DNA methylation in ovarian cancer. II. Expression of DNA
methyltransferases in ovarian cancer cell lines and normal ovarian
epithelial cells. Gynecol Oncol. 82:299–304. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu H, Fang J, Zhang J, Zhao Z, Liu L,
Wang J, Xi Q and Gu M: miR-182 targets CHL1 and controls tumor
growth and invasion in papillary thyroid carcinoma. Biochem Biophys
Res Commun. 450:857–862. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu H, Liu Y, Bai L, Kijlstra A and Yang P:
Predisposition to Behçet's disease and VKH syndrome by genetic
variants of miR-182. J Mol Med Berl. 92:961–967. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tang H, Wang Z, Liu Q, Liu X, Wu M and Li
G: Disturbing miR-182 and −381 inhibits BRD7 transcription and
glioma growth by directly targeting LRRC4. PLoS One. 9:e841462014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen T, Ueda Y, Xie S and Li E: A novel
DNMT3a isoform produced from an alternative promoter localizes to
euchromatin and its expression correlates with active de novo
methylation. J Biol Chem. 277:38746–38754. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bai X, Song Z, Fu Y, Yu Z, Zhao L, Zhao H,
Yao W, Huang D, Mi X, Wang E, et al: Clinicopathological
significance and prognostic value of DNA methyltransferase 1, 3a,
and 3b expressions in sporadic epithelial ovarian cancer. PLoS One.
7:e400242012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mostowska A, Sajdak S, Pawlik P, Lianeri M
and Jagodzinski PP: DNMT1, DNMT3a and DNMT3B gene variants in
relation to ovarian cancer risk in the Polish population. Mol Biol
Rep. 40:4893–4899. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zou Y, Huang MZ, Liu FY, Yang BC, Wang LQ,
Wang F, Yu XH, Wan L, Wan XD, Xu XY, et al: Absence of DICER1,
CTCF, RPL22, DNMT3a, TRRAP, IDH1 and IDH2 hotspot mutations in
patients with various subtypes of ovarian carcinomas. Biomed Rep.
3:33–37. 2015.PubMed/NCBI
|
|
17
|
Olivieri F, Ahtiainen M, Lazzarini R,
Pöllänen E, Capri M, Lorenzi M, Fulgenzi G, Albertini MC, Salvioli
S, Alen MJ, et al: Hormone replacement therapy enhances IGF-1
signaling in skeletal muscle by diminishing miR-182 and miR-223
expressions: A study on postmenopausal monozygotic twin pairs.
Aging Cell. 13:850–861. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wallis CJ, Gordanpour A, Bendavid JS,
Sugar L, Nam RK and Seth A: MiR-182 is associated with growth,
migration and invasion in prostate cancer via suppression of FOXO1.
J Cancer. 6:1295–1305. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun J, Ji J, Huo G, Song Q and Zhang X:
miR-182 induces cervical cancer cell apoptosis through inhibiting
the expression of DNMT3a. Int J Clin Exp Pathol. 8:4755–4763.
2015.PubMed/NCBI
|
|
20
|
Mirza S, Sharma G, Parshad R, Gupta SD,
Pandya P and Ralhan R: Expression of DNA methyltransferases in
breast cancer patients and to analyze the effect of natural
compounds on DNA methyltransferases and associated proteins. J
Breast Cancer. 16:23–31. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Endo S, Amano M, Nishimura N, Ueno N, Ueno
S, Yuki H, Fujiwara S, Wada N, Hirata S, Hata H, et al:
Immunomodulatory drugs act as inhibitors of DNA methyltransferases
and induce PU.1 up-regulation in myeloma cells. Biochem Biophys Res
Commun. 469:236–242. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu C, Xu P, Chen D, Fan X, Xu Y, Li M,
Yang X and Wang C: Roles of autophagy-related genes Beclin-1 and
LC3 in the development and progression of prostate cancer and
benign prostatic hyperplasia. Biomed Rep. 1:855–860.
2013.PubMed/NCBI
|
|
23
|
Pappano WN, Zhang Q, Tucker LA, Tse C and
Wang J: Genetic inhibition of the atypical kinase Wee1 selectively
drives apoptosis of p53 inactive tumor cells. BMC Cancer.
14:4302014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Marjanović M, Sánchez-Huertas C, Terré B,
Gómez R, Scheel JF, Pacheco S, Knobel PA, Martínez-Marchal A, Aivio
S, Palenzuela L, et al: CEP63 deficiency promotes p53-dependent
microcephaly and reveals a role for the centrosome in meiotic
recombination. Nat Commun. 6:76762015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Korch C, Spillman MA, Jackson TA, Jacobsen
BM, Murphy SK, Lessey BA, Jordan VC and Bradford AP: DNA profiling
analysis of endometrial and ovarian cell lines reveals
misidentification, redundancy and contamination. Gynecol Oncol.
127:241–248. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ma PJ, Zhang H, Li R, Wang YS, Zhang Y and
Hua S: P53-mediated repression of the reprogramming in cloned
bovine embryos through direct interaction with HDAC1 and indirect
interaction with DNMT3a. Reprod Domest Anim. 50:400–409. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tang LJ, Li Y, Liu YL, Wang JM, Liu DW and
Tian QB: USP12 regulates cell cycle progression by involving c-Myc,
cyclin D2 and BMI-1. Gene. 578:92–99. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Perez EA, Jenkins RB, Dueck AC, Wiktor AE,
Bedroske PP, Anderson SK, Ketterling RP, Sukov WR, Kanehira K, Chen
B, et al: C-MYC alterations and association with patient outcome in
early-stage HER2-positive breast cancer from the north central
cancer treatment group N9831 adjuvant trastuzumab trial. J Clin
Oncol. 29:651–659. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nguyen JC, Kubik MJ, Broome HE, Curtin PT,
Dell'Aquila ML and Wang HY: Successful treatment of both double
minute of C-MYC and BCL-2 rearrangement containing large B-cell
lymphoma with subsequent unfortunate development of therapy-related
acute myeloid leukemia with t(3;3)(q26.2;q21). Pathol Res Pract.
211:883–891. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stewart HJ, Horne GA, Bastow S and
Chevassut TJ: BRD4 associates with p53 in DNMT3a-mutated leukemia
cells and is implicated in apoptosis by the bromodomain inhibitor
JQ1. Cancer Med. 2:826–835. 2013. View
Article : Google Scholar : PubMed/NCBI
|