Introduction
Gastric cancer (GC) is one of the most common
malignancies and a leading cause of cancer-related deaths worldwide
(1) which remains prevalent in
Eastern Asia, South America and Eastern Europe (2,3).
Common treatments for advanced GC include surgery, chemotherapy and
radiotherapy. Although chemotherapy is effective for suppressing
cancer progression and prolonging survival to some extent,
chemoresistance represents a predominant obstacle towards the
chemotherapeutic treatment of GC (4). It is worth noting that an extremely
efficient mechanism of tumor resistance to drugs is the proton
pump-mediated acidification of the tumor microenvironment (5).
GC cells often exist in an ischemic microenvironment
with acidic conditions which is a consequence of the production of
acidic by-products from explosive glycolysis in vivo
(6,7). Maintenance of cellular pH homeostasis
is vital for the survival and function of cancer cells (8,9).
Hydrogen/potassium adenosine triphosphatase
(H+/K+-ATPase) plays a vital role in the
maintenance of cellular pH homeostasis by exchanging luminal
K+ for cytoplasmic H+. This proton pump also
participates in the formation of abnormal pH gradients that are
typical of GC cells during tumorigenesis (10). Proton pump inhibitors (PPIs) inhibit
gastric H+/K+-ATPase irreversibly which
prevents intracellular proton extrusions in GC cells consequently
reducing cancer cell survival under acidic conditions (11). It was reported that rabeprazole
attenuates the cell viability of human GC cells through
inactivation of the ERK1/2 signaling pathway (12). Moreover, pantoprazole (PPZ) could be
used to suppress the invasiveness of SGC-7901/ADR cells by
targeting epithelial-mesenchymal transition (EMT) (13).
Cancer stem cells (CSCs) play pivotal roles in
cancer initiation, progression, recurrence and chemoresistance
(14–17). CSCs give rise to tumors through
self-renewal and are able to differentiate into multiple cell types
(18–21). Chemotherapies that kill the bulk of
cancer cells, may ultimately fail as they do not eliminate CSCs
that then cause the relapse of tumors (22). Recently, it has been established
that CSCs are linked to EMT, metastasis, drug resistance,
progression and relapse of GC (23–26).
As a result, exploitation of specific therapies targeting CSCs has
been a crucial issue in the chemotherapeutic treatment of GC.
In the present study, we found that the PPI PPZ
suppressed the proliferation, sphere formation and GC stem
cell-mediated 5-fluorouracil (5-FU) chemoresistance of SGC-7901 and
HGC-27 cells by targeting EMT and β-catenin signaling for the first
time and our findings suggest that PPIs may serve as a promising
CSC-oriented novel antineoplastic agent.
Materials and methods
Cell culture, sphere formation culture
and reagents
Human GC cell lines SGC-7901 and HGC-27 were
purchased from Auragene Bioscience Co. (Changsha, China). Under
standard conditions, both cell lines were cultured in normal
Dulbeccos modified Eagles medium (DMEM) (Gibco-BRL, Grand Island,
NY, USA) with 10% fetal bovine serum and 100 U/ml penicillin at
37°C in a humidified atmosphere of 95% air and 5% CO2.
Under sphere culture conditions, parental cell lines or the
floating spheres obtained from transfections were plated in
serum-free defined media (SFDM): low-glucose (1 g/l) DMEM
supplemented with L-glutamine, sodium pyruvate,
penicillin/streptomycin (Wisent, Inc.), 20 ng/ml basic FGF, 20
ng/ml EGF and B27 (Invitrogen, Grand Island, NY, USA) using 24-well
ultra-low attachment plates (Corning, Inc., Tewksbury, MA, USA).
Spheres were dissociated with trypsin every 5–7 days and split to
1:3 ratio for next sphere passage if it was necessary. PPI PPZ
(H20010032) was obtained from Hangzhou Meidong Pharmaceutical Co.,
Ltd. (Hangzhou, China). SGC-7901 and HGC-27 parental cells or
spheres were treated with 100 µg/ml PPZ to generate cell models of
interest.
Flow cytometric analysis
Cells (1×106) were labeled with
PE-conjugated anti-CD44 (BioLegend, Inc., San Diego, CA, USA) and
FITC-conjugated CD24 (BD Pharmingen, Mississauga, ON, Canada) for
20 min, washed twice, resuspended in PBS and analyzed on a BD
FACSCalibur™ platform (BD Biosciences). Data were analyzed by FCS
Express software (BD Biosciences) using floating quadrants to
enumerate negative, single- and double-positive populations.
Drug resistance assay
Parental cells and spheres of P4 were planted at
2,000 cells/well in 96-well plates and then divided into three
groups as follows: treatment with 5-FU (20 µg/ml), treatment with
pantoprazol (PPZ; 100 µg/ml) and co-treatment with 5-FU and PPZ
(5-FU + PPZ). Cell viability was examined. Drug resistance was
determined after treatment for 96 h by MTT assay.
Self-renewal assay
Sphere formation assay was performed to detect the
self-renewal capacity. A total of 4,000 cells/well were seeded in
ultra-low attachment 6-well plate (Corning, Inc.) in SFDM medium
for 2 weeks, after which sphere formation was assessed by counting
the number of spheres (≥3 cells) under a microscope.
Cell growth assay of parental cells
and spheres
A total of 2,000 cells were plated in 96-well plates
and cultured in a CO2 incubator. The cells were
harvested at 24, 48, 72 and 96 h. The optical density at 570 nm
(OD570) of each well was measured with an ELISA reader (ELx800;
BioTek Instruments, Inc.).
Real-time RT-PCR
Total RNA was extracted from the cells with TRIzol
reagent (Invitrogen) following the manufacturer's instructions. The
relative mRNA levels of CSC markers of CD44, CD24, ABCG2, EpCAM,
Lgr5 and drug-resistance markers BMI1, ALDH1, Tcf4 were detected by
real-time RT-PCR using the standard SYBR-Green RT-PCR kit (Takara
Bio, Inc., Otsu, Japan) following the manufacturer's instructions
and β-actin was used as an internal control. The specific primer
pairs are listed in Table I. The
relative expression of target genes was quantified using GraphPad
Prism 4.0 software (GraphPad Software, San Diego, CA, USA) and the
2−ΔΔCt method (27).
 | Table I.The primer sequences used in real-time
RT-PCR. |
Table I.
The primer sequences used in real-time
RT-PCR.
| Gene |
| Primer sequences |
|---|
| CD44 | Sense |
CATCCCAGACGAAGACAGTCC |
|
| Antisense |
TGATCAGCCATTCTGGAATTTG |
| CD24 | Sense |
GACATGGGCAGAGCAATGGTGGC |
|
| Antisense |
GAGTGAGACCACGAAGAGACTGGC |
| ABCG2 | Sense |
CTGAGATCCTGAGCCTTTGG |
|
| Antisense |
TGCCCATCACAACATCATCT |
| EpCAM | Sense |
CGCCATATGCAGGAAGAATGTGT |
|
| Antisense |
CGCCTCGAGTTATTTTAGACCCTGCATTG |
| Lgr5 | Sense |
CCCGGGTTTCAGAGACAACTTC |
|
| Antisense |
TCCACATGCTTTATTCCAGCAATC |
| BMI1 | Sense |
ACGATGCCCAGCAGCAATGACT |
|
| Antisense |
AAGTGGACCATTCCTTCTCCAGGT |
| ALDH1 | Sense |
GATGAAGCTGCGGAATTTG |
|
| Antisense |
TCTTTGCTCGTTCAATGCTC |
| Tcf4 | Sense |
TGCGATGTTTTCACCTCCTG |
|
| Antisense |
TGCCAAAGAAGTTGGTCCATT |
| β-actin | Sense |
AGGGGCCGGACTCGTCATACT |
|
| Antisense |
GGCGGCACCACCATGTACCCT |
Western blot analysis
Cells were solubilized in cold RIPA lysis buffer.
Subsequently, the proteins were separated with 8% SDS-PAGE and then
transferred to a PVDF membrane. The membranes were blocked in 5%
non-fat dried milk in PBST for 3 h and then incubated overnight
with specific primary antibodies with β-actin as a control. After
incubation with the goat anti-rabbit or anti-mouse secondary
antibody, immune complexes were detected using an ECL kit (Auragene
Bioscience Co.). The primary antibodies against CD44 (1:1,000,
ab54037), CD24 (1:1,000, ab113289), ABCG2 (1:1,000, ab63907), EpCAM
(1:1,000, ab32392), Lgr5 (1:1,000, ab75732), BMI1 (1:1,000,
ab126783), ALDH1 (1:1,000, ab6192), Tcf4 (1:1,000, ab60727),
E-cadherin (1:1,000, ab15148), N-cadherin (1:1,000, ab18203),
vimentin (1:1,000, ab133260) and Snail (1:1,000, ab82846) were all
obtained from Abcam (USA), and total β-catenin (1:1,000, #9562) and
active β-catenin (1:1,000, #4270) were purchased from Cell
Signaling Technology, Inc. (CST; USA) The images were captured
using GeneSnap software from SynGene (Cambridge, UK). The protein
levels were normalized to β-actin.
Statistical analysis
The data are shown as the mean ± SD. The Student's
t-test was used to analyze the differences between the experimental
and control groups. Statistical analyses were performed using SPSS
11.0 software (SPSS, Inc., Chicago, IL, USA), and P<0.05 was
considered to indicate a statistically significant difference.
Results
PPZ enhances 5-FU chemosensitivity and
suppresses sphere formation and the expression of stem cell markers
in GC cell lines
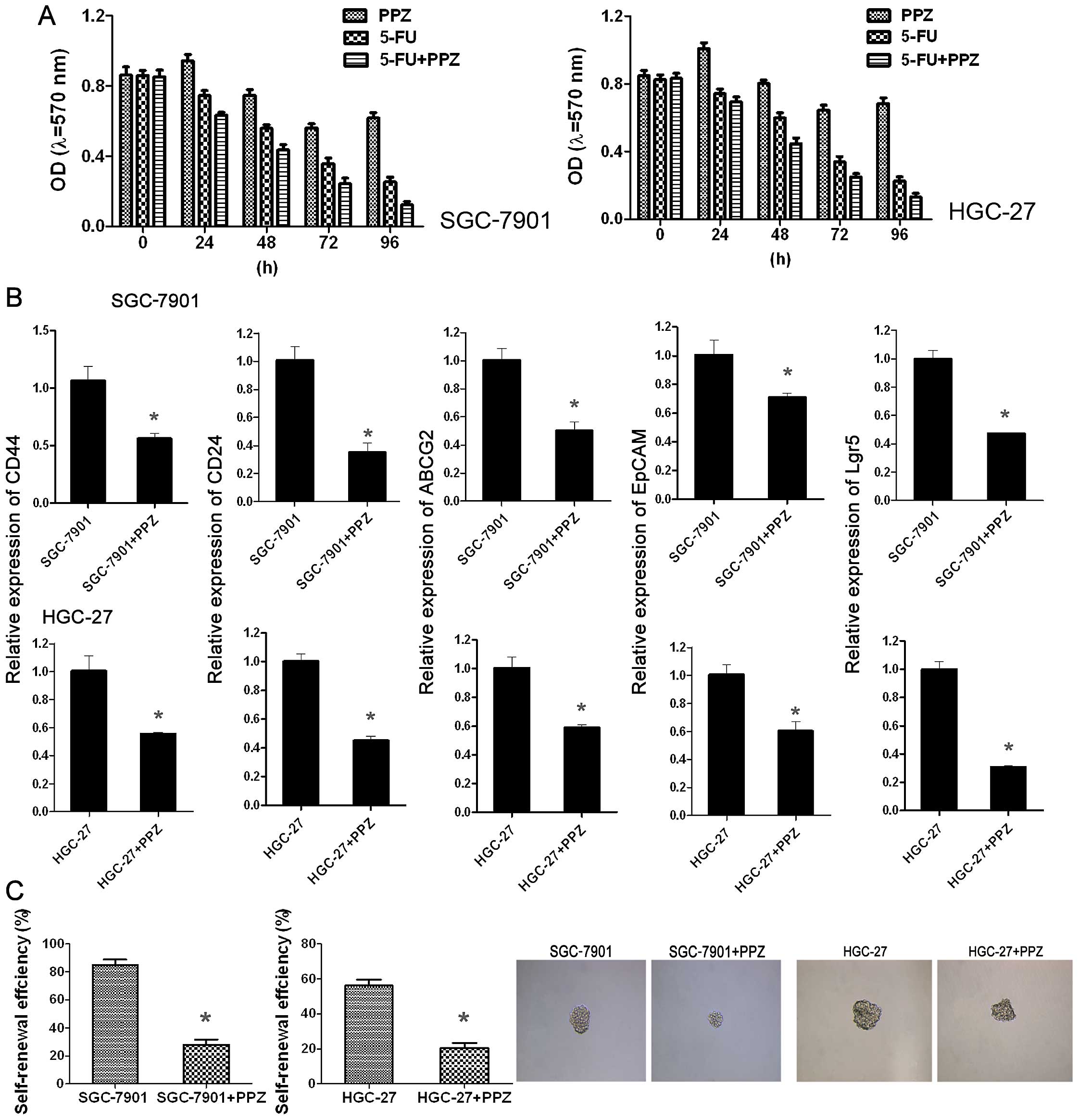
In order to ascertain whether PPZ exerts synergistic
action with 5-FU to inhibit cell proliferation, SGC-7901 and HGC-27
cells were divided into three groups: 5-FU-treated, PPZ-treated and
5-FU combined with PPZ-treated. The results showed that the
inhibition of proliferation by PPZ appeared after 48 h and until 96
h, inhibition was still slight. After addition of 5-FU (24 h),
inhibition of proliferation appeared; at 48 until 96 h, it became
significant. When cells were co-treated with 5-FU + PPZ, the
inhibition of proliferation was most obvious. This suggested that
PPZ enhanced 5-FU chemosensitivity of both the SGC-7901 and HGC-27
cells (Fig. 1A). Then the sphere
formation of SGC-7901 and HGC-27 cells was assessed and the results
showed that after PPZ treatment, the self-renewal efficiency of
both cell lines declined obviously, not only the sphere numbers but
also the size (Fig. 1C). CSCs play
a role in chemoresistance, thus real-time RT-PCR and western blot
analysis were performed to detect the relative expression of stem
cell markers. In both cell lines, after PPZ treatment, the mRNA
levels and protein expression of CD44, CD24, ABCG2, EpCAM and Lgr5
were reduced significantly (Fig.
1B). All of these results suggest that the antitumor effect of
PPZ may be related to the mediation of GC cell stemness.
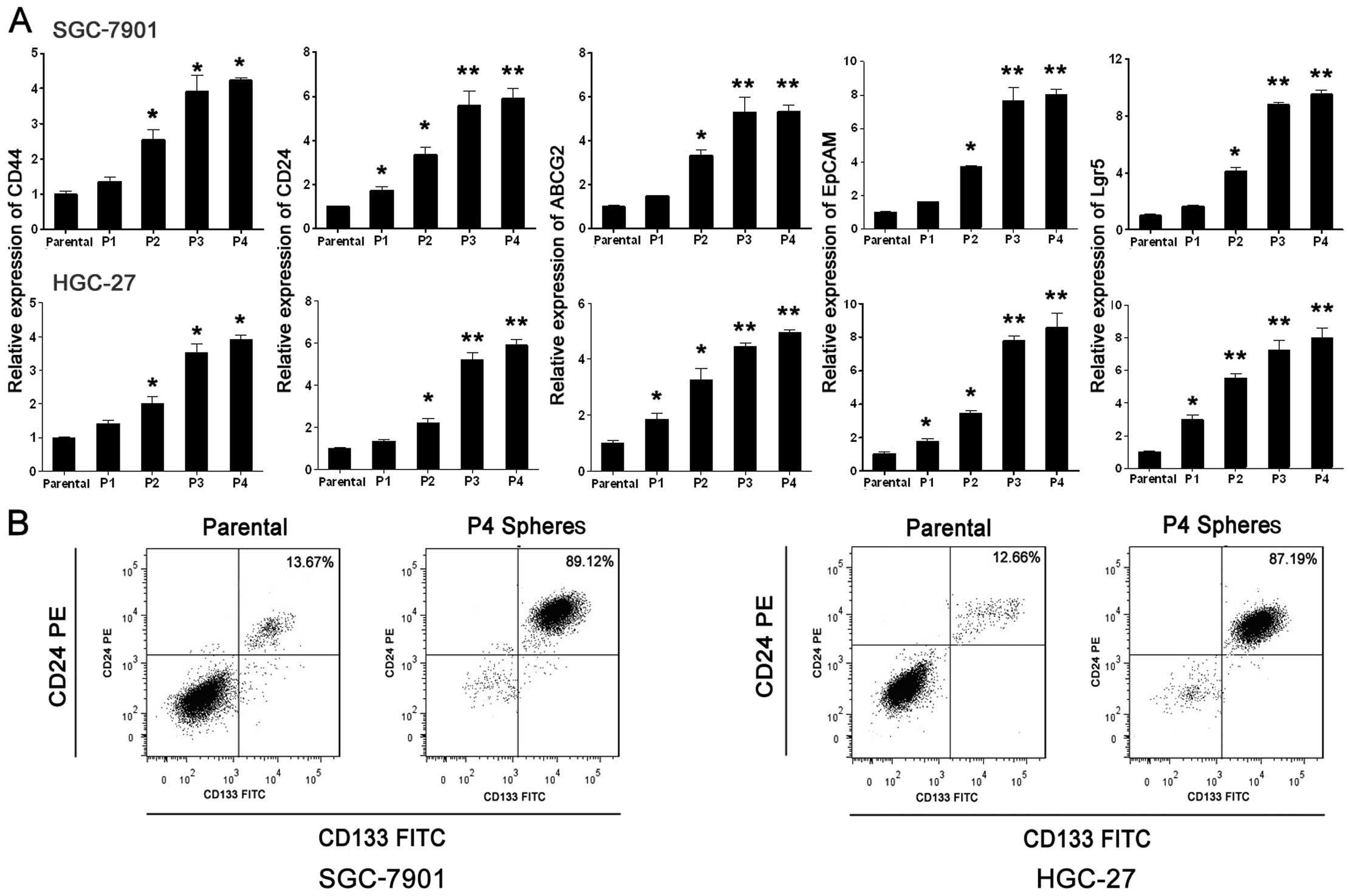
The establishment of GC CSC
models
Successive sphere formation culture was performed to
establish the CSC models using SGC-7901 and HGC-27 cell lines. The
relative mRNA levels of classic CSC marker genes were used as a
measurement of the CSC proportion. It was found that the relative
mRNA levels of classic CSC markers CD44, CD24, ABCG2, EpCAM and
Lgr5 were significantly increased from P1 to P3, but there was no
significant difference between P3 to P4 (Fig. 2A). These results suggest that when
spheres were cultured to P4, the CSC proportion reached a relative
peak amplitude using these methods. Flow cytometric analysis was
used to detect the CD133- and CD24-positive cell ratio of parental
cells and P4 spheres and the results showed that compared with the
parental groups the ratio of CD133+/CD24+
cells was enhanced obviously in the P4 sphere groups (Fig. 2B). It was concluded that during the
successive sphere formation culture to P4, the GC CSCs were
effectively enriched and the P4 spheres were able to be used as the
GC CSC models in the following experiments.
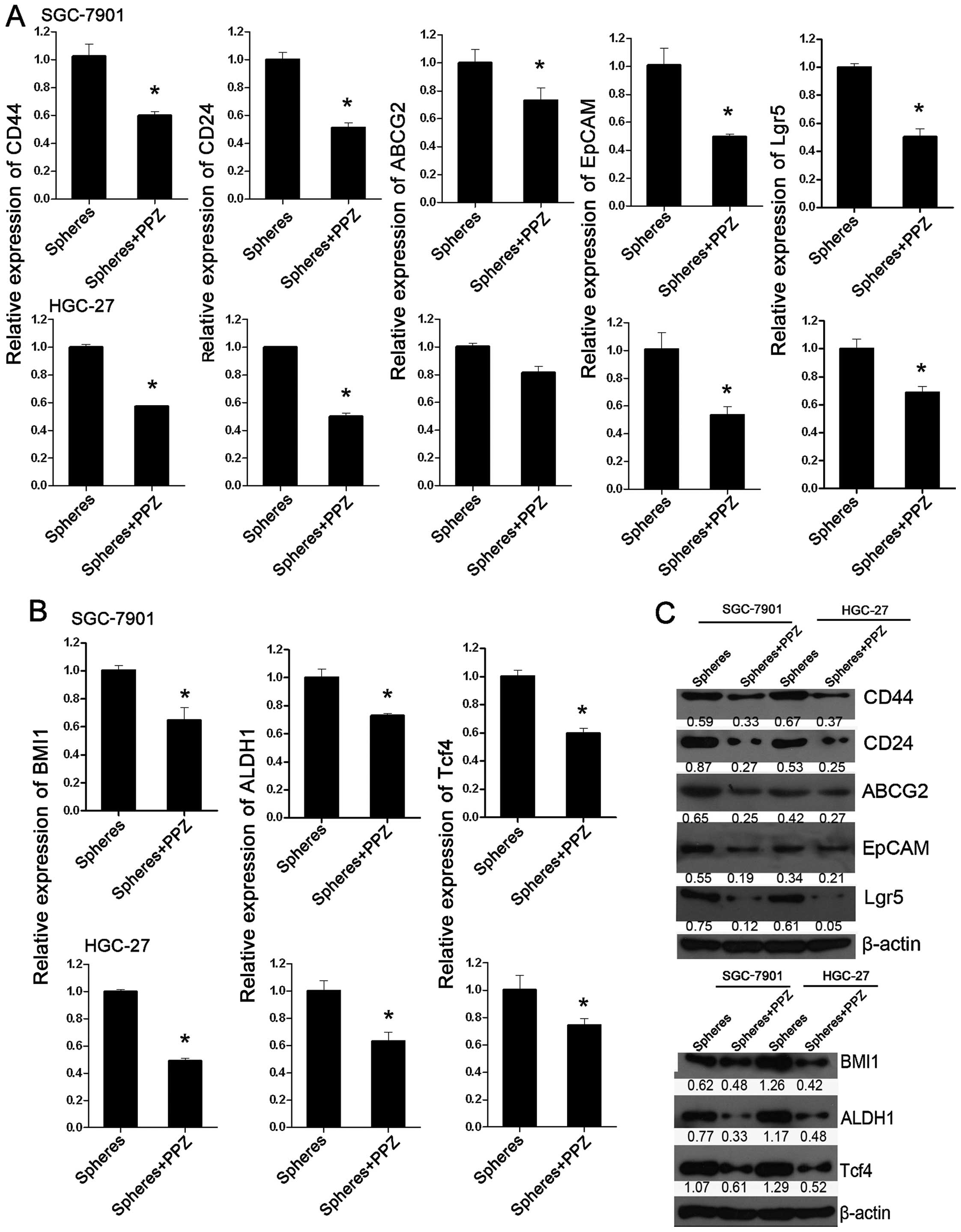
PPZ inhibits the expression of CSC
markers and drug-resistance markers
P4 spheres of both SGC-7901 and HGC-27 cells were
treated with PPZ (100 µg/ml), and the expression of CSC markers
CD44, CD24, ABCG2, EpCAM, Lgr5 and drug-resistance markers BMI1,
ALDH1 and Trf4 was detected. Not only CSC marker genes but also
anti-drug genes were all significantly inhibited at both the mRNA
and protein levels when compared with the spheres without PPZ
treatment (Fig. 3). The results
suggest that PPZ exerts its tumor inhibition effect by reducing the
stemness of GC cells.
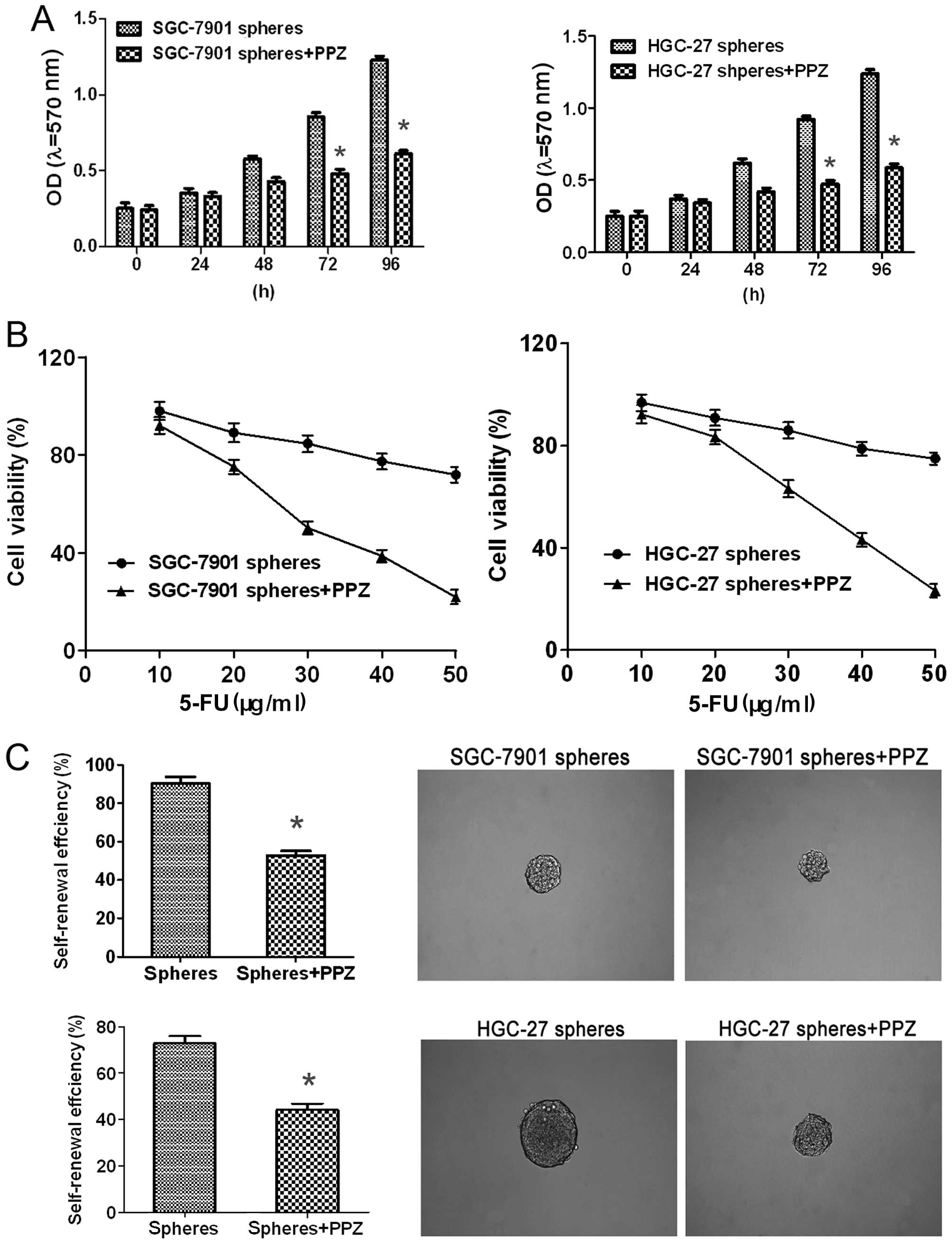
PPZ enhances 5-FU chemosensitivity,
and suppresses cell proliferation and sphere formation of
GC-initiating cells
P4 spheres of both SGC-7901 and HGC-27 cells were
used as GC initiation cell models which were treated with PPZ (100
µg/ml). Then the cell proliferation was measured by MTT assay.
Compared with the P4 spheres without PPZ, the cell proliferation
capacity was obviously decreased after 48 h and became even more
significant after 72 h in both cell lines (Fig. 4A). P4 spheres of both cell lines
were treated with 10–50 µg/ml 5-FU combined with or without PPZ
under normal culture conditions. The cell viability assay showed
that the cell viability was significantly inhibited when cells were
co-treated with 5-FU + PPZ which indicated that PPZ enhanced the
5-FU sensitivity of the GC-initiating cells at least to some extent
(Fig. 4B). Then the sphere
formation of both P4 spheres was measured. After PPZ treatment, the
self-renewal efficiency of both sphere types was obviously
declined, not only sphere numbers but also the size (Fig. 4C). These results demonstrated that
PPZ could exert its tumor inhibitory effect by reducing the
stemness of GC cells at the cell functional level.
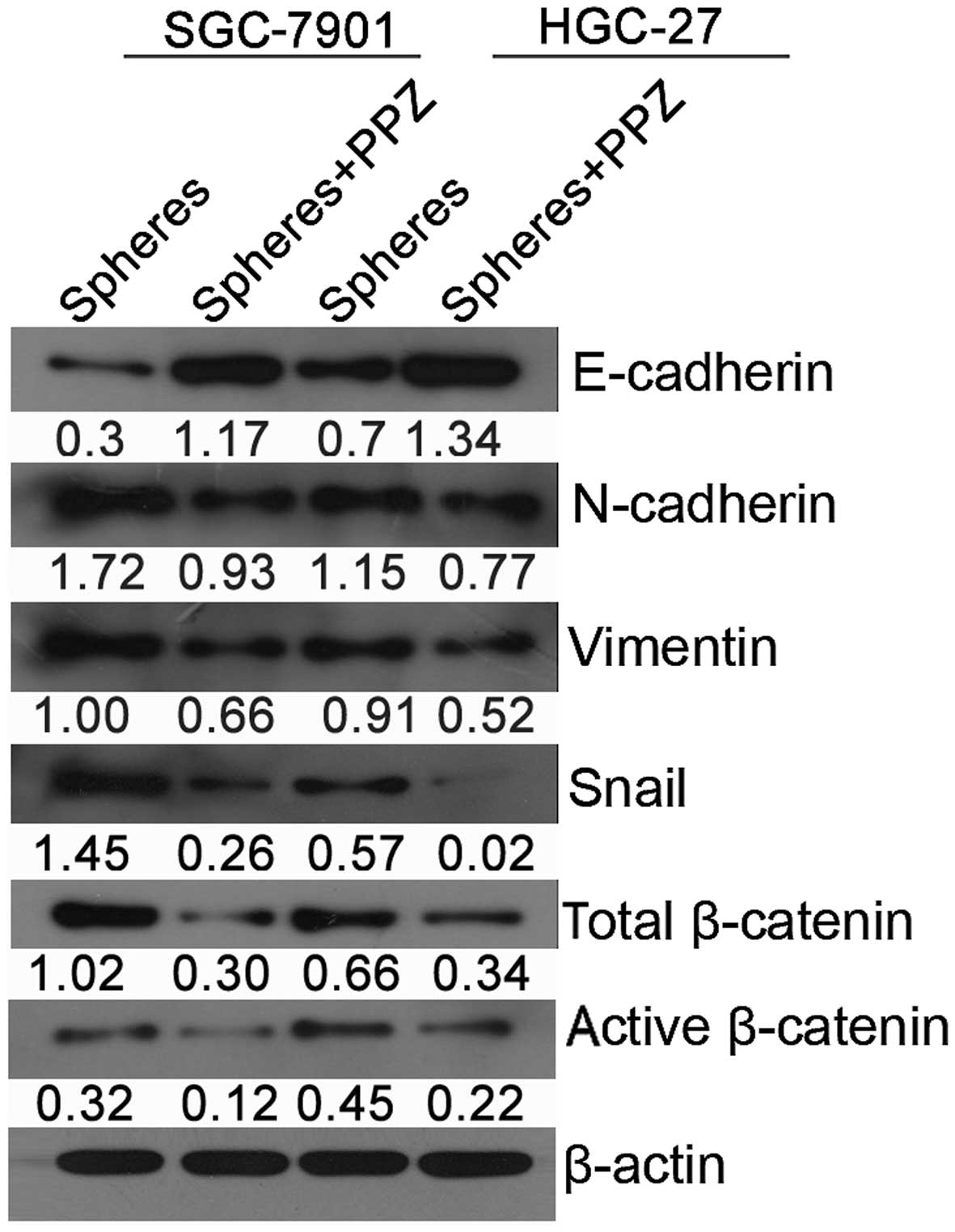
PPZ suppresses expression of
EMT-related genes and the activation of β-catenin signaling
To explore the potential downstream molecular
pathways underlying the targeting of stemness inhibition by PPZ, we
assessed the expression of genes involved in EMT and classic
CSC-related signaling pathways including E-cadherin, N-cadherin,
vimentin, Snail and total/active β-catenin by western blot analysis
in both types of P4 spheres with or without PPZ treatment. A
significant reduction in the expression of N-cadherin, vimentin,
Snail and total/active β-catenin proteins and upregulation of
E-cadherin were observed in the spheres treated with PPZ in both
types of GC spheres (Fig. 5). It
was demonstrated that the inhibitory effect associated with the PPZ
targeting of GC CSCs was mediated by EMT and activation of the
β-catenin signaling at least partly.
Discussion
Current antineoplastic strategies are aimed at
promoting the efficiency and specificity of therapies for GC.
Intracellular proton extrusion in GC cells has been reported to
promote cancer cell survival under acidic conditions via
H+/K+-ATPase. PPZ is a frequently used
second-generation PPI that irreversibly inactivates gastric
H+/K+-ATPase. Early as 2004, relative
research found that PPI selectively induced in vivo and
in vitro apoptotic cell death in GC and could be used for a
selective anticancer effect (28).
In regards to GC chemotherapy, the results are not
satisfactory, and among the tested chemotherapeutic agents, only a
limited number of compounds [5-FU, adriamycin (doxorubicin) and
cisplatin] have demonstrated response rates ranging from 15 to 50%
selectively. Recent studies have elucidated the presence of CSCs
that have the exclusive ability to regenerate tumors. These CSCs
share many characteristics with normal stem cells, including
self-renewal, differentiation and drug-resistance (29). The presence of CSCs has already been
associated with chemotherapeutic failure in a variety of solid
tumors including GC. Thus, revealing the underlying molecular
mechanisms responsible for maintenance and chemoresistance of CSCs
has become a crucial issue in the clinical treatment of GC. PPZ was
reported to suppress proliferation and restore the chemosensitivity
of GC SGC-7901 cells by inhibiting the STAT3 signaling pathway
(30). It was found that PPZ can
effectively reverse the aggressiveness and EMT marker expression of
SGC-7901/ADR (adriamycin-resistant) cells and EMT was a typical
feature of CSCs. Our research showed that associated with the
proliferation, and 5-FU resistance inhibition, administration of
PPZ decreased the expression of GC CSC markers. Thus, we
hypothesized that PPZ could play a role in targeting CSCs.
The CSC model suggests that a small subset of cancer
cells possesses stem cell properties and plays a crucial role in
tumor initiation, metastasis and resistance to anticancer therapy
(31). The characteristic features
of CSCs include: i) self-renewing (tested by sphere formation in
SFDM); ⅱ) high tumorigenicity in xenograft-based model; and ⅲ)
ability to differentiate to the cell types of the tumor of origin
(32,33). To date, although the purity is not
100%, there are three relatively satisfactory methods by which to
establish CSC models in tumor cells: side population isolation,
sphere culture and immunomagnetic bead isolation. No matter what
isolation method is used, the relative expression levels of stem
cell surface markers are favourable indices to measure the
abundance of CSC models. In our study, we adopted a successive
sphere culture method to establish our GC CSC models. Using qPCR,
we found that the relative mRNA levels of stem cell markers were
significantly upregulated from P1 to P3, but were not further
upregulated from P3 to P4. The results suggested that, using the
successive sphere culture method, we received a comparatively high
proportion of GC CSCs from P4. Thus, in our following experiments,
the P4 spheres were used as our CSC models.
Upon pre-treatment with PPI, ERK1/2 phosphorylation
was completely inhibited; the inhibitory action on the
phosphorylation of ERK1/2 might contribute to the induction of
apoptosis in GC cells by PPI (34).
Furthermore, PPZ treatment resulted in a profound reduction in both
total and phosphorylated forms of Akt and GSK-3β, which in turn
suppressed the adriamycin-induced Wnt/β-catenin signaling in
SGC-7901/ADR cells. It is possible to suppress the invasiveness of
SGC-7901/ADR cells by PPZ which targets the EMT and
Akt/GSK-3β/β-catenin signaling (13). It is worth mentioning that EMT and
Wnt/β-catenin signaling are classic relative signaling pathways
that mediate the stemness of CSCs. EMT is an important way to
induce CSC formation in a number of solid tumors. Moreover, EMT is
associated with increased expression of stem cell-related
transcription factors and with increased tumorigenic ability
(31). In our experiment, when
spheres were treated with PPZ, the EMT course and the activation of
β-catenin were both inhibited to some extent which demonstrated
that PPZ exerts its antitumor effect by targeting CSCs via the
EMT/β-catenin pathways.
Our research for the first time demonstrated that
PPZ could be used to promote a selective anticancer effect
targeting GC CSC inhibition, but the underlying molecular mechanism
should be further explored. PPZ may be a promising breakthrough in
GC therapy.
Acknowledgements
This study was supported by the Science and
Technology Program of Haicang District of Xiamen City (grant no.
350205Z20144010-05).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Center MM, DeSantis C and Ward
EM: Global patterns of cancer incidence and mortality rates and
trends. Cancer Epidemiol Biomarkers Prev. 19:1893–1907. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Smith MG, Hold GL, Tahara E and El-Omar
EM: Cellular and molecular aspects of gastric cancer. World J
Gastroenterol. 12:2979–2990. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wöhrer SS, Raderer M and Hejna M:
Palliative chemotherapy for advanced gastric cancer. Ann Oncol.
15:1585–1595. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Spugnini EP, Buglioni S, Carocci F,
Francesco M, Vincenzi B, Fanciulli M and Fais S: High dose
lansoprazole combined with metronomic chemotherapy: A phase I/II
study in companion animals with spontaneously occurring tumors. J
Transl Med. 12:2252014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Holm E, Hagmüller E, Staedt U,
Schlickeiser G, Günther HJ, Leweling H, Tokus M and Kollmar HB:
Substrate balances across colonic carcinomas in humans. Cancer Res.
55:1373–1378. 1995.PubMed/NCBI
|
|
7
|
Vaupel P, Kallinowski F and Okunieff P:
Blood flow, oxygen and nutrient supply, and metabolic
microenvironment of human tumors: A review. Cancer Res.
49:6449–6465. 1989.PubMed/NCBI
|
|
8
|
Stubbs M, McSheehy PM and Griffiths JR:
Causes and consequences of acidic pH in tumors: A magnetic
resonance study. Adv Enzyme Regul. 39:13–30. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stubbs M, Rodrigues L, Howe FA, Wang J,
Jeong KS, Veech RL and Griffiths JR: Metabolic consequences of a
reversed pH gradient in rat tumors. Cancer Res. 54:4011–4016.
1994.PubMed/NCBI
|
|
10
|
Fais S: Proton pump inhibitor-induced
tumour cell death by inhibition of a detoxification mechanism. J
Intern Med. 267:515–525. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sachs G, Shin JM and Howden CW: Review
article: The clinical pharmacology of proton pump inhibitors.
Aliment Pharmacol Ther. 23:(Suppl 2). 2–8. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gu M, Zhang Y, Zhou X, Ma H, Yao H and Ji
F: Rabeprazole exhibits antiproliferative effects on human gastric
cancer cell lines. Oncol Lett. 8:1739–1744. 2014.PubMed/NCBI
|
|
13
|
Zhang B, Yang Y, Shi X, Liao W, Chen M,
Cheng AS, Yan H, Fang C, Zhang S, Xu G, et al: Proton pump
inhibitor pantoprazole abrogates adriamycin-resistant gastric
cancer cell invasiveness via suppression of Akt/GSK-β/β-catenin
signaling and epithelial-mesenchymal transition. Cancer Lett 356 (2
Pt B). 704–712. 2015. View Article : Google Scholar
|
|
14
|
Bitarte N, Bandres E, Boni V, Zarate R,
Rodriguez J, Gonzalez-Huarriz M, Lopez I, Sola J Javier, Alonso MM,
Fortes P, et al: MicroRNA-451 is involved in the self-renewal,
tumorigenicity, and chemoresistance of colorectal cancer stem
cells. Stem Cells. 29:1661–1671. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Levina V, Marrangoni A, Wang T, Parikh S,
Su Y, Herberman R, Lokshin A and Gorelik E: Elimination of human
lung cancer stem cells through targeting of the stem cell
factor-c-kit autocrine signaling loop. Cancer Res. 70:338–346.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Van den Broeck A, Vankelecom H, Van Delm
W, Gremeaux L, Wouters J, Allemeersch J, Govaere O, Roskams T and
Topal B: Human pancreatic cancer contains a side population
expressing cancer stem cell-associated and prognostic genes. PLoS
One. 8:e739682013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamashita T, Honda M, Nakamoto Y, Baba M,
Nio K, Hara Y, Zeng SS, Hayashi T, Kondo M, Takatori H, et al:
Discrete nature of EpCAM+ and CD90+ cancer
stem cells in human hepatocellular carcinoma. Hepatology.
57:1484–1497. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Beck B, Driessens G, Goossens S, Youssef
KK, Kuchnio A, Caauwe A, Sotiropoulou PA, Loges S, Lapouge G, Candi
A, et al: A vascular niche and a VEGF-Nrp1 loop regulate the
initiation and stemness of skin tumours. Nature. 478:399–403. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bu P, Chen KY, Chen JH, Wang L, Walters J,
Shin YJ, Goerger JP, Sun J, Witherspoon M, Rakhilin N, et al: A
microRNA miR-34a-regulated bimodal switch targets Notch in colon
cancer stem cells. Cell Stem Cell. 12:602–615. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chaffer CL, Marjanovic ND, Lee T, Bell G,
Kleer CG, Reinhardt F, D'Alessio AC, Young RA and Weinberg RA:
Poised chromatin at the ZEB1 promoter enables breast cancer cell
plasticity and enhances tumorigenicity. Cell. 154:61–74. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Scaffidi P and Misteli T: In vitro
generation of human cells with cancer stem cell properties. Nat
Cell Biol. 13:1051–1061. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gupta PB, Onder TT, Jiang G, Tao K,
Kuperwasser C, Weinberg RA and Lander ES: Identification of
selective inhibitors of cancer stem cells by high-throughput
screening. Cell. 138:645–659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fagoonee S, Li H, Zhang H, Altruda F and
Pellicano R: Gastric cancer as a stem-cell disease: Data and
hypotheses. Panminerva Med. 56:289–300. 2014.PubMed/NCBI
|
|
24
|
Gao G, Sun Z, Wenyong L, Dongxia Y, Zhao R
and Zhang X: A preliminary study of side population cells in human
gastric cancer cell line HGC-27. Ann Transplant. 20:147–153. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu ZY, Tang JN, Xie HX, Du YA, Huang L, Yu
PF and Cheng XD: 5-Fluorouracil chemotherapy of gastric cancer
generates residual cells with properties of cancer stem cells. Int
J Biol Sci. 11:284–294. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang Z, Guo L, Liu D, Sun L, Chen H, Deng
Q, Liu Y, Yu M, Ma Y, Guo N, et al: Acquisition of resistance to
trastuzumab in gastric cancer cells is associated with activation
of IL-6/STAT3/Jagged-1/Notch positive feedback loop. Oncotarget.
6:5072–5087. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Arocho A, Chen B, Ladanyi M and Pan Q:
Validation of the 2-DeltaDeltaCt calculation as an alternate method
of data analysis for quantitative PCR of BCR-ABL P210 transcripts.
Diagn Mol Pathol. 15:56–61. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yeo M, Kim DK, Kim YB, Oh TY, Lee JE, Cho
SW, Kim HC and Hahm KB: Selective induction of apoptosis with
proton pump inhibitor in gastric cancer cells. Clin Cancer Res.
10:8687–8696. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lobo NA, Shimono Y, Qian D and Clarke MF:
The biology of cancer stem cells. Annu Rev Cell Dev Biol.
23:675–699. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang S, Chen M, Ding X, Zhang X and Zou
X: Proton pump inhibitor selectively suppresses proliferation and
restores the chemosensitivity of gastric cancer cells by inhibiting
STAT3 signaling pathway. Int Immunopharmacol. 17:585–592. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lichner Z, Saleh C, Subramaniam V,
Seivwright A, Prud'homme GJ and Yousef GM: miR-17 inhibition
enhances the formation of kidney cancer spheres with stem
cell/tumor initiating cell properties. Oncotarget. 6:5567–5581.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Prud'homme GJ: Cancer stem cells and novel
targets for antitumor strategies. Curr Pharm Des. 18:2838–2849.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yeo M, Kim DK, Park HJ, Cho SW, Cheong JY
and Lee KJ: Blockage of intracellular proton extrusion with proton
extrusions with proton pump inhibitor induces apoptosis in gastric
cancer. Cancer Sci. 99:1852008.
|