Introduction
Cervical cancer is the second most commonly
diagnosed type of cancer among women and is a serious threat to
womens health worldwide. Approximately 20 million women die of
cervical cancer each year (1). In
recent years, significant advances have been made in understanding
the mechanism of cervical carcinogenesis (2). However, the detailed mechanism of
cervical carcinogenesis is still unknown to date. Therefore,
identifying key factors in cervical cancer development may provide
potential therapeutic targets for the prevention and treatment of
cervical cancer. MicroRNAs (miRNAs) are a group of non-coding RNAs
~21–23 nt in length that negatively regulate gene expression by
imprecisely binding to complementary sequences in the 3′
untranslated region (3 UTR) of their target mRNAs (3,4).
Increasing evidence shows that miRNAs are involved in many
important biological processes, including development, cell
proliferation, metabolism and signal transduction (5,6). In
recent years, researchers have focused on the relationship between
miRNAs and cancer. Altered expression of miRNAs has been found to
be associated with the initiation and progression of various
cancers (7–9). However, the potential involvement of
miRNAs in cervical cancer remains to be fully elucidated.
As the earliest discovered miRNA in C.
elegans, let-7 has been widely studied in a variety of tumors.
It can regulate a variety of oncogenes, such as K-Ras
(9), EZH2 (10) and HMGA (11). Our previous study showed that let-7a
suppresses the proliferation of lung cancer by inhibiting the
K-Ras and c-Myc gene (12),
and let-7a was found to participate in the regulation of diabetic
nephropathy through the promoter regulation area rs1143770
polymorphism (13). These results
suggest that let-7a is an active regulatory factor and plays an
important role in a variety of diseases. In chip analysis of
miRNAs, various miRNAs which displayed abnormal expression in
cervical cancer included let-7 (14). This suggests that let-7 may
participate in the development of cervical cancer. However, its
mechanism is still unclear.
To the best of our knowledge, the TGF-β/SMAD
signaling pathway plays a role in numerous types of cancers,
including cervical cancer (15–17).
The TGF-β/SMAD signaling pathway mediated by TGF-β1 is thought to
be a key signaling pathway in the regulation of cell proliferation
and induction of apoptosis (18).
Previous research has revealed that the proliferation and invasion
of cervical cancer may be inhibited by TGF-β1 (17). TGF-β binds to TGFBR1 resulting in
phosphorylation of the carboxy-terminal serine residue of the SMAD
intracellular messenger proteins, SMAD2 and SMAD3 (19). This phosphorylation results in
oligomerization of SMAD2 and SMAD3 with SMAD4, a necessary step for
nuclear translocation (20).
Whether or not let-7a regulates the proliferation of cervical
cancer by the TGF-β/SMAD signaling pathway requires further
investigation.
In the present study, let-7a was decreased in
cervical cancer as detected by real-time PCR. In addition,
overexpression of let-7a inhibited the proliferation of cervical
cancer cells and promoted cell apoptosis. Moreover, TGFBR1 was
confirmed to be a target of let-7a by bioinformatic analysis,
dual-luciferase reporter assay and western blotting. Furthermore,
data showed that TGF-β1 and SMAD4 were increased in cervical
squamous carcinoma and adenocarcinoma patient tissues.
Additionally, the expression levels of TGF-β1 and SMAD4 were
reduced in the cervical cancer cells when let-7a was overexpressed.
In addition, the expression of let-7a was increased when TGF-β1 was
silenced in the cervical cancer cells. Taken together, our results
indicate that let-7a may play an important regulatory role in
cervical cancer through the TGF-β/SMAD signaling pathway.
Materials and methods
A total of 50 peripheral blood samples were
collected and analyzed, including 25 incident cervical cancer cases
and 25 healthy women. The patients were diagnosed as having
cervical cancer according to pathologic findings. The age range of
the patients was beween 30 and 70 years. The control group was
comprised of married females, 26.0–69.0 years of age, randomly
selected during the same time period as the cases studied among
healthy individuals without any history of cancer.
Paraffin-embedded tissues were obtained from
patients with squamous carcinoma of the cervix (SCC) (n=20),
adenocarcinoma of the uterine cervix (AUC) (n=15) and cervical
intraepithelial neoplasia (CIN) (n=15). All patients were female
Han Chinese from Chongqing Province, 30.0–67.0 years of age, from
the First Affiliated Hospital of Chongqing Medical University.
Cell culture and transfection
The human cervical cancer cell lines (HeLa, SiHa and
CaSki) and normal human immortalization keratinocye cells (HaCaT)
were grown in Dulbeccos modified Eagles medium (DMEM) with 10%
fetal bovine serum (FBS) (both from Gibco, Carlsbad, CA, USA) in a
humidified atmosphere containing 5% CO2 at 37°C. HeLa,
SiHa and CaSki cells were transfected with let-7a mimics (Shanghai
GenePharma Corp., Shanghai, China) or a mimic control, short
double-scrambled RNAs (dsRNAs) similar to Dicer-processed miRNAs,
using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). Then, the
HeLa, SiHa and CaSki cells were divided into the let-7a group
(transfected with let-7a mimics), control group (transfected with
let-7a mimic control) and the untreated group (untransfected). HeLa
cells were also transfected with TGF-β1 siRNA (Shanghai GenePharma
Corp.) or siRNA control by Lipofectamine 2000, as previously
described (21).
Real-time reverse transcription
(RT)-PCR
Total RNA was extracted using TRIzol reagent
(Invitrogen) according to the manufacturer's instructions. TaqMan
miRNA assays (Applied Biosystems, Foster City, CA, USA) were used
to semi-quantify miRNAs according to the manufacturer's
instructions. Briefly, 10 ng of total RNA was reverse transcribed
using miRNA-specific looped RT primer, MultiScribe reverse
transcriptase, RT buffer, dNTPs and RNase inhibitor in the GeneAmp
9700 PCR system (both from Applied Biosystems) under the following
conditions: 16°C for 30 min, 42°C for 30 min, 85°C for 5 min.
Real-time PCR was performed on the resulting complementary DNA
(cDNA) using miRNA-specific forward PCR primers, specific reverse
PCR primer, miRNA-specific TaqMan probe and TaqMan Universal PCR
Master Mix in a 7500 Real-Time PCR system (Applied Biosystems) as
follows: 95°C for 10 min followed by 40 cycles of 95°C for 15 sec
and 60°C for 1 min. U6 served as an internal control (Applied
Biosystems). Relative fold-change in let-7a expression was
calculated using the ΔΔCT method (18) and the values are expressed as
2−ΔΔCt.
MTT assay
HeLa cells were seeded at a density of
2×103 cells/well in 96-well plates. After 1, 2, 3 or 4
days of incubation, 20 µl MTT (5 mg/ml) was added to each well and
cultured for 4 h. The supernatant was removed, 150 µl of dimethyl
sulfoxide (DMSO) was added and shaken for 5 min until the crystals
were dissolved. The optical density (OD) 490 nm value was measured
using an enzyme-linked immunosorbent assay reader. The data are
expressed as the percentage of growth relative to that of cells in
the untreated group. The inhibition rate of HeLa was calculated
using the following formula: Inhibition rate of cells (%) = (1 -
A490 value of let-7a group/A490 value of HeLa group × 100%. All
experiments were performed in triplicate.
Flow cytometric assay
HeLa cells were harvested by trypsinization, washed
in ice-cold phosphate-buffered saline (PBS), and fixed in 80%
ice-cold ethanol in PBS. Before staining, the cells were pelleted
using a chilled centrifuge and resuspended in cold PBS. Bovine
pancreatic RNase (Sigma-Aldrich, St. Louis, MO, USA) was added to a
final concentration of 2 µg/ml and cells were incubated at 37°C for
30 min, followed by incubation with 20 µg/ml propidium iodide (PI;
Sigma-Aldrich) for 20 min at room temperature to analyze the cell
cycle, or incubation with 61 µl FITC-Annexin V and 20 µl PI with
300 µl binding buffer for 15 min at room temperature to analyze the
apoptotic rate. The profiles of 1×104 cells were
analyzed using a FACSCalibur flow cytometer (BD Biosciences,
Bedford, MA, USA).
Cloning and identification
To construct a plasmid containing the TGFBR1 3′ UTR
fused to pmiR-RB-REPORT™ dual-luciferase vector (Guangzhou RuiBio
Corp., Guangzhou, China), the 3′ UTR of the TGFBR1 gene was
amplified using the following primers: forward,
5′-GCGCTCGAGGGGTGTTTAGGAGGCTGGT-3′ and reverse,
5′-AATGCGGCCGCCATACAACTTTTCCTTCGG-3′, and then cloned into a
pmiR-RB-REPORT™ vector with restriction endonucleases XhoI
and NotI to generate a pmiR-RB-REPORT™-TGFBR1-3′ UTR
wild-type. The potential binding sequences of let-7a on the TGFBR1
3′ UTR were mutated by the QuikChange™ Site-Directed Mutagenesis
kit (Stratagene). The recombinant plasmids were confirmed by
restriction enzyme digestion and DNA sequencing.
Dual-luciferase reporter assay
Luciferase assays were performed according to the
manufacturer's protocol. 293T cells seeded into 96-well plates at a
density of 4×103/well, and then transfected with
TGFBR1-3′ UTR-wt (200 ng/ml), TGFBR1-3′ UTR-mut (200 ng/ml) and
let-7a mimics (50 nmol/l) with Lipofectamine 2000. After 48 h
post-transfection, the cells were lysed and assayed for luciferase
activities using the Dual-Glo Luciferase Assay System (Promega,
Madison, WI, USA). The data recorded on the luminometer were
normalized by dividing firefly luciferase activity with that of
Renilla luciferase, and were analyzed and graphed.
Western blot analysis
After the above-mentioned treatment, cells were
washed three times in ice-cold PBS (pH 7.4), and lysed with
ice-cold RIPA buffer (Shanghai Beyotime Corp., Shanghai, China).
The lysate was clarified by centrifugation at 15,000 × g for 30 min
at 4°C. The resulting supernatant was stored at −80°C until further
analysis. In addition, the protein content was determined using the
BCA protein assay kit with BSA as the standard. Proteins (20 µg)
were denatured at 100°C in loading buffer for 10 min and separated
by SDS-PAGE. After electrotransfer, the membrane was probed with
antibodies against p53 (Abcam, Cambridge, UK), TGF-β1, SMAD4 (both
from ProteinTech, Chicago, IL, USA) or GAPDH (Abcam). Then, the
blots were incubated with a secondary antibody (Abcam). After
washing, the bound antibody was visualized using an enhanced
chemiluminescence assay kit (Tiangen Biotechnology, Corp. Beijing,
China) according to the manufacturer's instructions.
Immunohistochemistry
Cervical cancer tissues were embedded in paraffin
and sectioned at a thickness of 5 µm. After deparaffinization of
the sections, antigen retrieval was carried out by boiling the
samples in citrate buffer for 15 min at 92–98°C. To be naturally
cooled to room temperature, the sections were washed thrice with
PBS. Then, 10% normal goat serum was added for 1 h at 37°C, and
subsequently incubation was carried out with human anti-rabbit
TGF-β1 antibody (1:200 dilution) and human anti-rabbit SMAD4
antibody (1:200) (both from ProteinTech) in PBS at 4°C for
overnight. The sections were then incubated with goat anti-rabbit
secondary antibodies (IgG/HRP) for 15 min at 37°C using Non-Bio
Two-Step Histostain™ Plus kits and DAB staining (Zhongshan Golden
Bridge Biotechnology, Corp. Beijing, China). The nuclei were
counterstained with hematoxylin. The remaining procedures were
performed in accordance with the manufacturer's instructions. The
immunohistologic staining for TGF-β1 and SMAD4 was observed by
light microscopy.
Immunofluorescence
Cervical cancer tissues were embedded in paraffin
and sectioned at a thickness of 5 µm. After deparaffinization of
the sections, antigen retrieval was carried out by boiling the
samples in citrate buffer for 15 min at 92–98°C. In order to be
naturally cooled to room temperature, the sections were washed
thrice with PBS. Then 10% normal goat serum was added for 1 h at
37°C. After that incubation was carried out with human anti-rabbit
TGF-β1 antibody (1:200 dilution) and human anti-rabbit SMAD4
antibody (1:200) (both from ProteinTech) in PBS at 4°C for
overnight. After washing twice with PBS, the sections were
incubated with TRITC-conjugated anti-rabbit IgG secondary antibody
(1:50 dilution) (Zhongshan Goldenbridge Biotechnology, Corp.
Beijing, China) for 90 min at 37°C in the dark, washed thrice with
PBS for 5 min and finally sealed with 50% glycerin. Observations
were performed using confocal laser scanning microscopy (CLSM;
Leica, Wetzlar, Germany).
Statistical analysis
All statistical tests were performed using SPSS
software (SPSS, Inc., Chicago, IL, USA). Data for multiple variable
comparisons were analyzed by one-way analysis of variance (ANOVA).
p-values at <0.05 were considered statistically significant. All
data are presented as mean ± standard deviation (SD).
Results
Expression of let-7a is decreased in
cervical cancer patient serum and cells
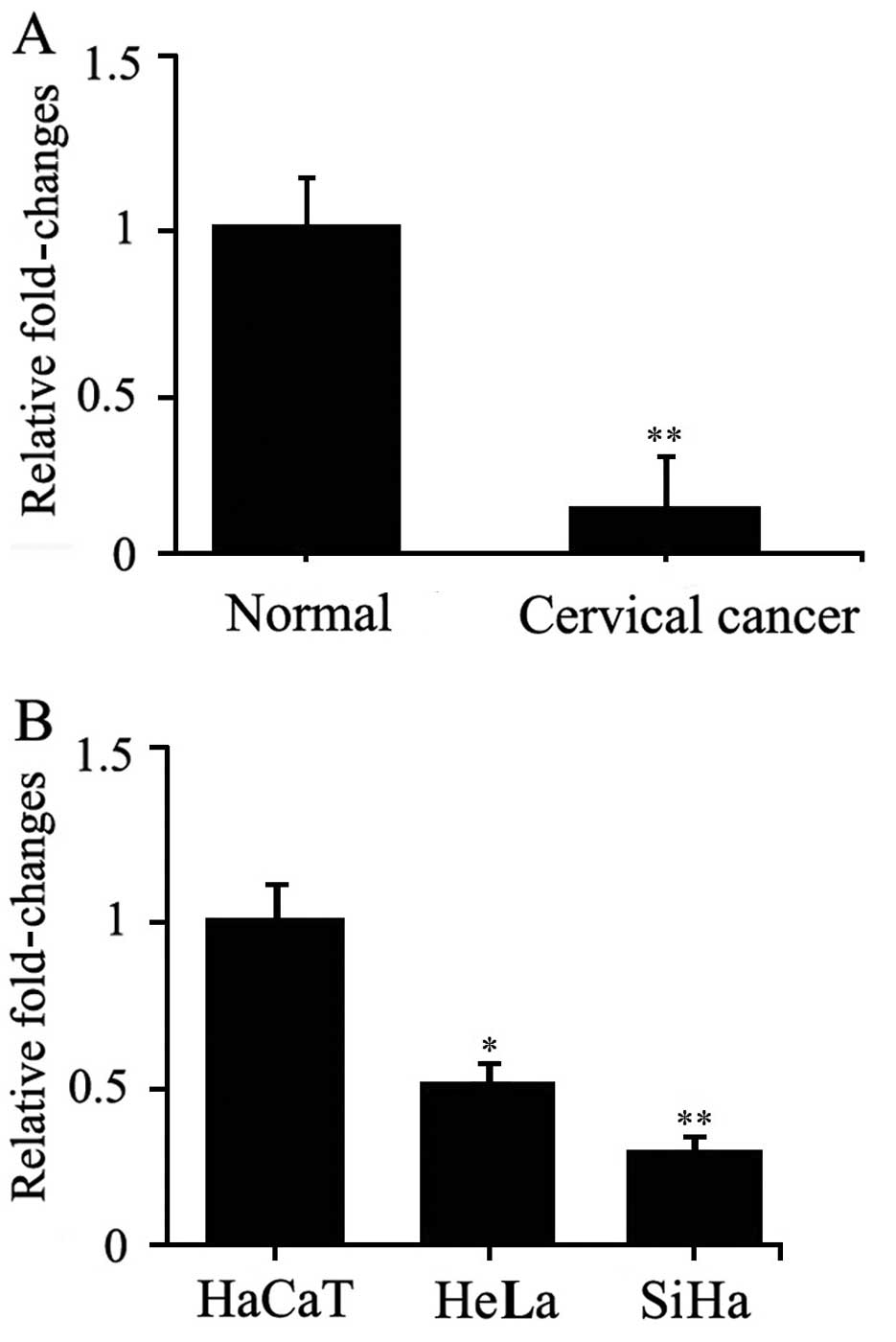
To validate the alteration in expression of let-7a
in cervical cancer, the expression of let-7a was assessed in the
serum of 25 cervical cancer patients and 25 normal controls by
quantitative real-time PCR. The results showed that let-7a was
markedly decreased in the serum of cervical cancer patients
compared with that noted in the normal controls (Fig. 1A). These findings corroborated the
results of Shishodia et al (22), who found that let-7a expression was
downregulated in cervical cancer tissues. These findings show that
let-7a is consistently decreased in blood and cancer tissues of
cervical cancer patients. Meanwhile, the expression of let-7a was
examined in two cervical cancer cell lines. The results showed that
let-7a was also decreased in the SiHa and HeLa cells compared with
that in normal HaCaT cells (Fig.
1B). These data suggest that let-7a participates in the
occurrence and development of cervical cancer.
Overexpression of let-7a inhibits cell
proliferation and promotes cell apoptosis in HeLa cells
Let-7a was overexpressed in the HeLa cells using the
commercially available let-7a mimics. MTT assay showed that the
inhibition rates of the let-7a group were 31.58, 34.78, 44.64 and
31.16% at 24, 48, 72 and 96 h, respectively (Table I). In addition, the cell
proliferation was significantly inhibited in the let-7a group of
HeLa cells compared with that in the control and untreated groups
(all, p<0.05). This indicated that let-7a significantly
inhibited the proliferative ability of the HeLa cells.
 | Table I.Effects of let-7a on the proliferation
of HeLa cells. |
Table I.
Effects of let-7a on the proliferation
of HeLa cells.
| Absorbance | Untreated group | Control group | let-7a group | Inhibition rate
(%)e |
|---|
| A490 (24 h) | 0.19±0.02 | 0.23±0.02 |
0.13±0.02a,d | 31.58 |
| A490 (48 h) | 0.46±0.04 | 0.47±0.05 |
0.30±0.03b,d | 34.78 |
| A490 (72 h) | 1.12±0.15 | 1.07±0.09 |
0.62±0.05b,d | 44.64 |
| A490 (96 h) | 1.38±0.16 | 1.46±0.21 |
0.95±0.12a,c | 31.16 |
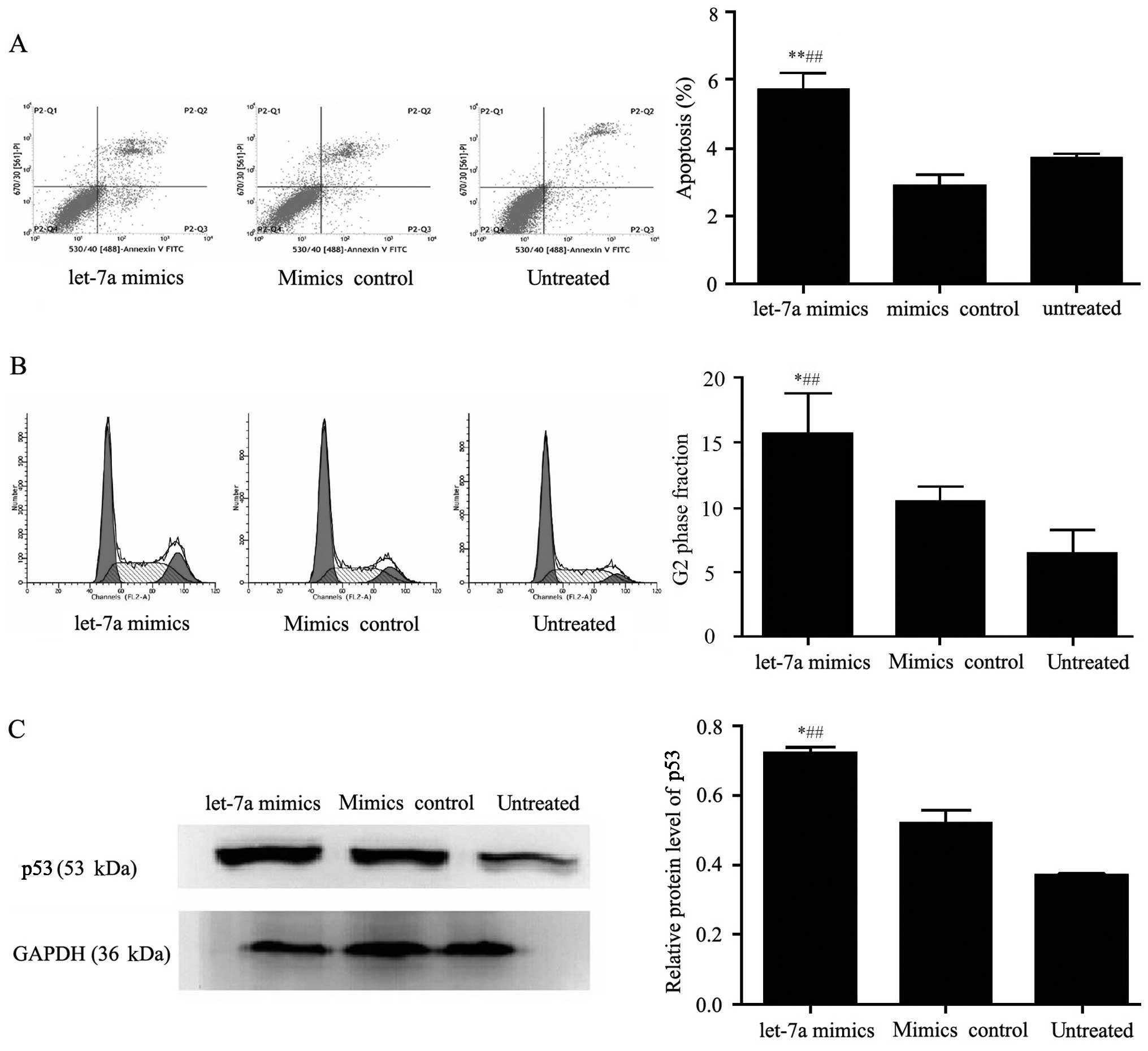
Furthermore, the cell apoptosis rates were tested by
flow cytometry and were 3.71±0.21, 2.90±0.54 and 5.71±0.86% in the
untreated, mimic control and let-7a group, respectively. The
results showed that overexpression of let-7a significantly
inhibited the cell apoptosis of HeLa cells compared with that in
the control and untreated groups (p<0.01) (Fig. 2A). These results demonstrated that
let-7a had an effect on the cell apoptosis of HeLa cells.
Additionally, cell cycle analysis by flow cytometry
showed that cells in the let-7a group were arrested in the G2 phase
when compared with cells in the control and untreated groups
(p<0.05). In addition, the percentage of cells in the let-7a
group which remained in the G2 phase was increased by 9.33%
compared with that in the untreated group, and increased by 5.28%
compared with that in the control group (Fig. 2B). Meanwhile, western blot results
showed the key cell cycle factor p53 was increased in the let-7a
group compared with that in the control and untreated groups
(Fig. 2C). These data indicated
that let-7a inhibited the cell proliferation of HeLa cells by
affecting the cell cycle.
Let-7a negatively regulates TGFBR1
expression
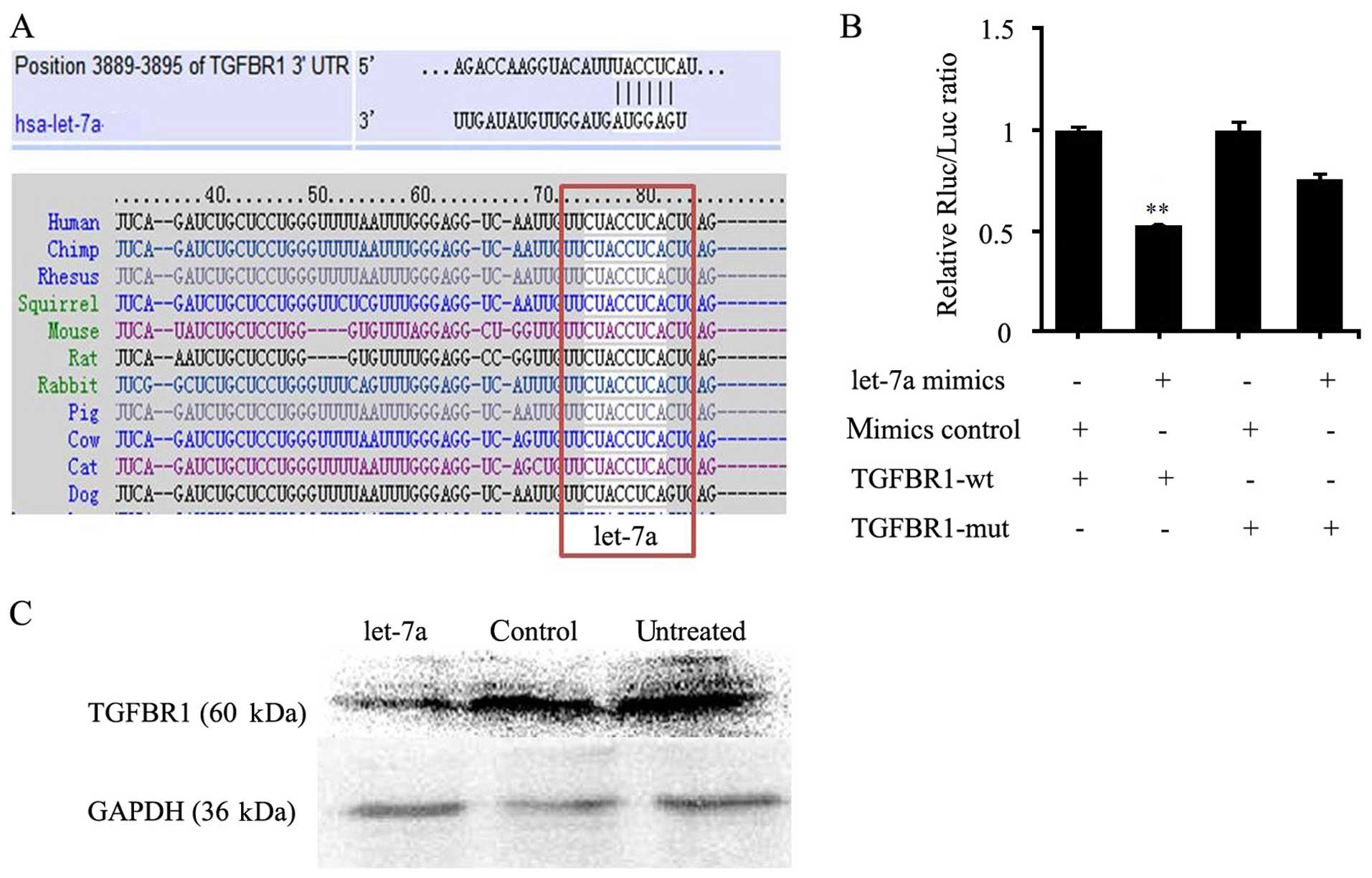
To explore the mechanism of let-7a in cervical
cancer, we aimed to ascertain the target genes. By bioinformatic
analysis, a 7-nt match (nucleotides 2–8) to the seed region at the
5′-end of mature let-7a was present in the TGFBR1 3′ UTR. By
analyzing homology, the putative let-7a target site was highly
conserved (Fig. 3A). Western
blotting showed that the expression level of TGFBR1 was reduced
significantly in the let-7a mimic group, compared with that in the
control group in the HeLa cells (Fig.
3C). In addition, dual-luciferase reporter assay results showed
that transcripts carrying the TGFBR1 3′ UTR exhibited a significant
reduction in luciferase activity in the let-7a mimic group. In
contrast, the mimic control had no significant effect on the
luciferase activity (Fig. 3B).
Therefore, TGFBR1 was a target of let-7a.
let-7a regulates the TGF-β/SMAD
signaling pathway in cervical cancer cells
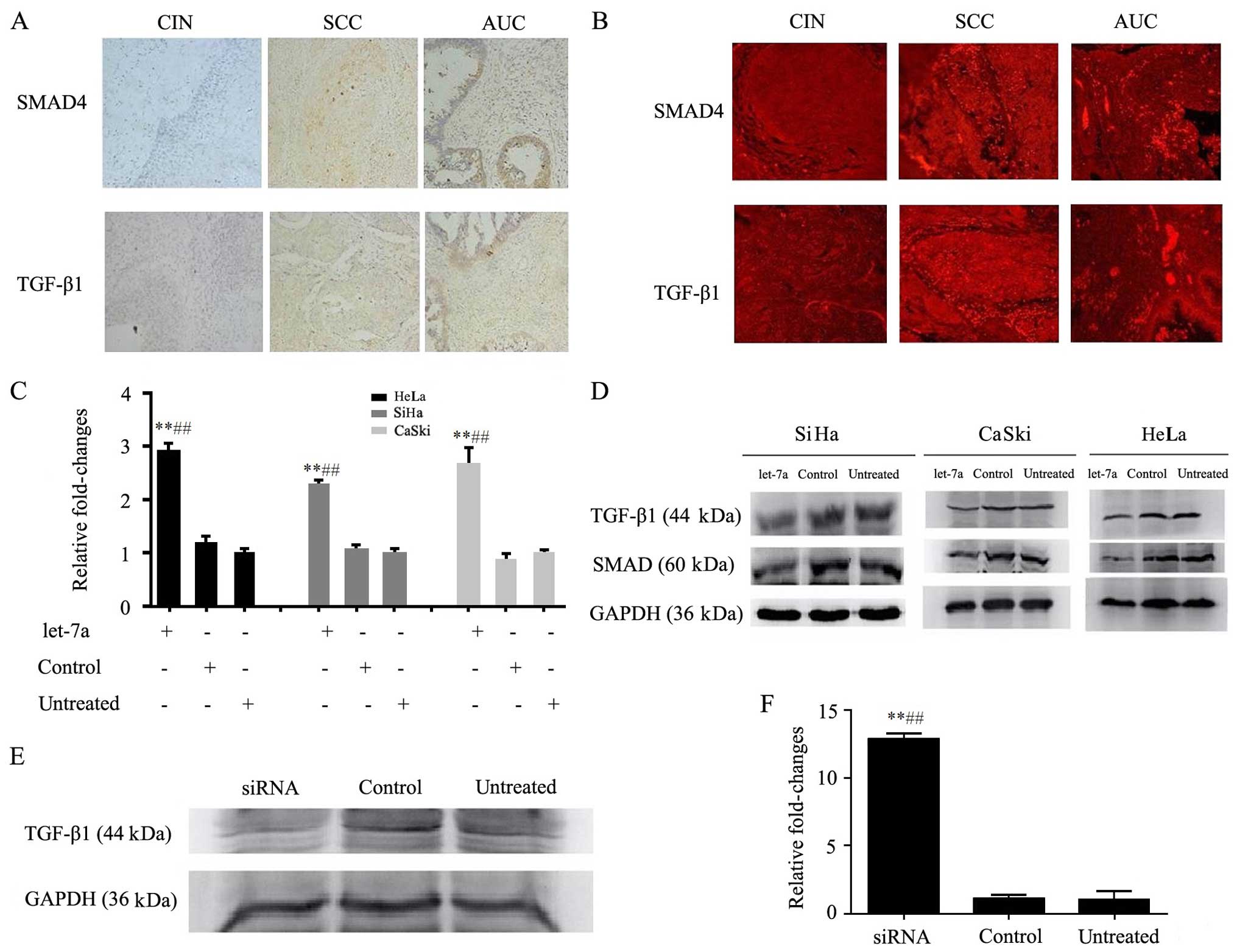
The expression levels of TGF-β1 and SMAD4 were
detected by immunohistochemistry in cervical squamous carcinoma and
adenocarcinoma tissues. The results showed that TGF-β1 and SMAD4
protein levels were positive in the cervical squamous carcinoma and
adenocarcinoma compared with levels in the adjacent mucosa
(Fig. 4A). Meanwhile, tissue
specimens were immunofluorescence stained for protein presence with
antibodies of TGF-β1 and SMAD4. The cervical cancer tissues showed
a stronger TGF-β1 and SMAD4 presence than those of normal cervical
tissues as demonstrated by the red fluorescence signals due to
immunofluorescence (Fig. 4B).
let-7a mimics or control was transfected into
cervical cancer cells (SiHa, HeLa and CaSki) by Lipofectamine 2000.
Real-time RT-PCR displayed that let-7a was significantly enhanced
in the let-7a group compared with that in the control and untreated
groups (p<0.05) (Fig. 4C). This
suggests that let-7a was successfully transfected into the
cells.
TGF-β1 and SMAD4 are important factors in the
TGF-β/SMAD signaling pathway. Thus, the expression levels of TGF-β1
and SMAD4 were assessed in the HeLa, SiHa and CaSki cells by
western blotting. The results showed that TGF-β1 and SMAD4 proteins
were decreased in the let-7a group, compared with that in the
control and untreated groups (p<0.05) (Fig. 4D). This indicated that
overexpression of let-7a decreased the expression of TGF-β1 and
SMAD4 proteins in the cervical cancer cells.
Moreover, TGF-β1 siRNA was transfected into the HeLa
cells and its expression was assessed by western blotting. The
results showed that the expression of TGF-β1 was reduced in the
TGF-β1 siRNA group compared with that in the control and untreated
groups (Fig. 4E). No significant
differences in the levels of TGF-β1 protein were observed between
the control and untreated groups. This indicated that the
expression of TGF-β1 was successfully suppressed in the HeLa cells.
As expected, knockdown of TGF-β1 markedly increased the expression
of let-7a in the HeLa cells as detected by real-time PCR
(p<0.05) (Fig. 4F). These data
further confirmed that the expression level of TGF-β1 protein was
inversely correlated with let-7a.
Discussion
In humans, the let-7 family consists of 13 members,
all sharing a common seed sequence. let-7 miRNA is involved in many
physiological, as well as pathological processes. As known, let-7
target genes, such as HMGA2, RAS and LIN28 are oncogenes involved
in cell cycle progression (23,24).
The let-7 level was found to be low in a variety of primary and
metastatic tumors, and its loss or downregulation was associated
with increased cancer aggressiveness and poor clinical outcome
(25). In the present study, we
explore the expression level of let-7a in cervical cancer and
studied the molecular mechanism of let-7a in regards to the
proliferation of cervical cancer cells.
In the present study, real-time PCR was used to
detect the expression of circulating let-7a in patient serum. The
results showed that let-7a was decreased in the serum of cervical
cancer patients. In addition, the expression of let-7a was reduced
in three human cervical cancer cells. Additionally, it was reported
that let-7a was decreased in the cancer tissues of cervical cancer
patients (22). Collectively, the
results displayed the downregulation of the expression of let-7a in
patients and in cervical cancer cell lines.
To investigate the biological function of let-7a in
cervical cancer, we assessed the cell proliferation, cell apoptosis
and cell cycle in the HeLa cells. MTT assay showed that the
inhibition rates of the let-7a group were 31.58, 34.78, 44.64 and
31.16%; at 24, 48, 72 and 96 h, respectively. In addition, the cell
proliferation was significantly inhibited in the let-7a group in
the HeLa cells compared with that in the control and untreated
groups. This suggests that overexpression of let-7a inhibits the
cell proliferation of cervical cancer. In addition, previous
studies have shown that let-7a inhibited the cell proliferation in
prostate and lung cancer (26,27).
Therefore, let-7a may play a role in cell proliferation in various
diseases. Our results also found that the cell apoptosis rates were
3.71±0.21, 2.90±0.54 and 5.71±0.86% in the untreated, control and
let-7a groups. Moreover, it was reported that downregulation of the
expression of let-7a markedly suppressed cell cycle progression and
induced apoptosis in gastric cancer cells (28). This suggests that let-7a promotes
cell apoptosis. Furthermore, cell cycle analysis showed that cells
were arrested in the G2 phase in the let-7a group compared with
that in the control and untreated groups. In addition, the
percentage of cells arrested in the G2 phase in the let-7a group
increased by 9.33% compared with that in the untreated group. This
suggests that let-7a may inhibit cell proliferation of HeLa cells
by G2 phase arrest. In addition, the key cell cycle factor p53 was
increased in the let-7a group compared with that in the control and
untreated group. The tumor suppressor p53 is a transcription factor
that responds to various types of cellular stress, including DNA
damage and oncogene activation. p53 regulates the expression of
genes involved in a variety of cellular functions, including cell
cycle arrest and apoptosis (29).
These findings demonstrated that overexpression of let-7a
suppressed the biological behavior of cervical cancer cells.
To explore the mechanism of let-7a in cervical
cancer, we focused on the target gene for let-7a, TGFBR1. Our
results showed that a highly conserved 7-nt match of mature let-7a
was present in the TGFBR1 3′ UTR by bioinformatic analysis. In
addition, dual-luciferase reporter assay showed that TGFBR1
exhibited a significantly reduction in the presence of let-7a
mimics. In addition, western blot results showed that
overexpression of let-7a decreased the expression of TGFBR1.
Therefore, TGFBR1 is a target of let-7a. As known, TGFBR1 is an
important element of the TGF-β/SMAD signaling pathway which has
emerged as a central mediator of cancer progression due to its
capability to regulate cell growth, differentiation and migration
(30). Studies have also shown that
repression of TGFBR1 enhanced the cell proliferation of lung cancer
and cell migration and invasion of breast cancer (31,32).
Moreover, our data showed that TGF-β1 and SMAD4 were both increased
in cervical squamous carcinoma and adenocarcinoma patient tissues.
In addition, western blot results showed that overexpression of
let-7a reduced the expression of TGF-β1 and SMAD4. In addition,
silencing of TGF-β1 protein increased the expression of let-7a.
Therefore, let-7a had an effect on the TGF-β/SMAD signaling pathway
in cervical cancer.
In conclusion, our results indicated that let-7a
expression was downregulated in cervical cancer, and overexpression
of let-7a affected cell proliferation, cell apoptosis and cell
cycle through the TGF-β/SMAD signaling pathway in cervical
cancer.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (81270912), and the Project of
the Science and Technology Commission Foundation of Chongqing
Province (cstc2013jcyjA10044).
References
|
1
|
Haie-Meder C, Morice P and Castiglione M:
ESMO Guidelines Working Group: Cervical cancer: ESMO Clinical
Practice Guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 21:(Suppl 5). v37–v40. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Castellsagué X, Díaz M, de Sanjosé S,
Muñoz N, Herrero R, Franceschi S, Peeling RW, Ashley R, Smith JS,
Snijders PJ, et al: International Agency for Research on Cancer
Multicenter Cervical Cancer Study Group: Worldwide human
papillomavirus etiology of cervical adenocarcinoma and its
cofactors: Implications for screening and prevention. J Natl Cancer
Inst. 98:303–315. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aranha MM, Santos DM, Xavier JM, Low WC,
Steer CJ, Solá S and Rodrigues CM: Apoptosis-associated microRNAs
are modulated in mouse, rat and human neural differentiation. BMC
Genomics. 11:5142010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schwarzenbacher D, Balic M and Pichler M:
The role of microRNAs in breast cancer stem cells. Int J Mol Sci.
14:14712–14723. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lawrie CH, Gal S, Dunlop HM, Pushkaran B,
Liggins AP, Pulford K, Banham AH, Pezzella F, Boultwood J,
Wainscoat JS, et al: Detection of elevated levels of
tumour-associated microRNAs in serum of patients with diffuse large
B-cell lymphoma. Br J Haematol. 141:672–675. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mitchell PS, Parkin RK, Kroh EM, Fritz BR,
Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant
KC, Allen A, et al: Circulating microRNAs as stable blood-based
markers for cancer detection. Proc Natl Acad Sci USA.
105:10513–10518. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ho AS, Huang X, Cao H, Christman-Skieller
C, Bennewith K, Le QT and Koong AC: Circulating miR-210 as a novel
hypoxia marker in pancreatic cancer. Transl Oncol. 3:109–113. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsujiura M, Ichikawa D, Komatsu S,
Shiozaki A, Takeshita H, Kosuga T, Konishi H, Morimura R, Deguchi
K, Fujiwara H, et al: Circulating microRNAs in plasma of patients
with gastric cancers. Br J Cancer. 102:1174–1179. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xia XM, Jin WY, Shi RZ, Zhang YF and Chen
J: Clinical significance and the correlation of expression between
Let-7 and K-ras in non-small cell lung cancer. Oncol Lett.
1:1045–1047. 2010.PubMed/NCBI
|
|
10
|
Kong D, Heath E, Chen W, Cher ML, Powell
I, Heilbrun L, Li Y, Ali S, Sethi S, Hassan O, et al: Loss of let-7
up-regulates EZH2 in prostate cancer consistent with the
acquisition of cancer stem cell signatures that are attenuated by
BR-DIM. PLoS One. 7:e337292012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mayr C, Hemann MT and Bartel DP:
Disrupting the pairing between let-7 and Hmga2 enhances oncogenic
transformation. Science. 315:1576–1579. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
He XY, Chen JX, Zhang Z, Li CL, Peng QL
and Peng HM: The let-7a microRNA protects from growth of lung
carcinoma by suppression of k-Ras and c-Myc in nude mice. J Cancer
Res Clin Oncol. 136:1023–1028. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou J, Peng R, Li T, Luo X, Peng H, Zha
H, Yin P, Wen L and Zhang Z: A potentially functional polymorphism
in the regulatory region of let-7a-2 is associated with an
increased risk for diabetic nephropathy. Gene. 527:456–461. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee JW, Choi CH, Choi JJ, Park YA, Kim SJ,
Hwang SY, Kim WY, Kim TJ, Lee JH, Kim BG, et al: Altered MicroRNA
expression in cervical carcinomas. Clin Cancer Res. 14:2535–2542.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fan DM, Tian XY, Wang RF and Yu JJ: The
prognosis significance of TGF-β1 and ER protein in cervical
adenocarcinoma patients with stage Ib~IIa. Tumour Biol.
35:11237–11242. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chu GC, Dunn NR, Anderson DC, Oxburgh L
and Robertson EJ: Differential requirements for Smad4 in
TGFbeta-dependent patterning of the early mouse embryo.
Development. 131:3501–3512. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
López-Muñoz H, Escobar-Sánchez ML,
López-Marure R, Lascurain-Ledesma R, Zenteno E, Hernández-Vazquez
JM, Weiss-Steider B and Sánchez-Sánchez L: Cervical cancer cells
induce apoptosis in TCD4+ lymphocytes through the
secretion of TGF-β. Arch Gynecol Obstet. 287:755–763. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bai X, He T, Liu J, Wang Y, Fan L, Tao K,
Shi J, Tang C, Su L and Hu D: Loureirin B inhibits fibroblast
proliferation and extracellular matrix deposition in hypertrophic
scar via TGF-β/Smad pathway. Exp Dermatol. 24:355–360. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Feng XH and Derynck R: Specificity and
versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev
Biol. 21:659–693. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pasche B, Pennison MJ, Jimenez H and Wang
M: TGFBR1 and cancer susceptibility. Trans Am Clin Climatol Assoc.
125:300–312. 2014.PubMed/NCBI
|
|
21
|
Sun Y, Peng R, Peng H, Liu H, Wen L, Wu T,
Yi H, Li A and Zhang Z: miR-451 suppresses the NF-kappaB-mediated
proinflammatory molecules expression through inhibiting LMP7 in
diabetic nephropathy. Mol Cel Endocrinol. 433:75–86. 2016.
View Article : Google Scholar
|
|
22
|
Shishodia G, Shukla S, Srivastava Y,
Masaldan S, Mehta S, Bhambhani S, Sharma S, Mehrotra R, Das BC and
Bharti AC: Alterations in microRNAs miR-21 and let-7a correlate
with aberrant STAT3 signaling and downstream effects during
cervical carcinogenesis. Mol Cancer. 14:1162015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nair VS, Maeda LS and Ioannidis JP:
Clinical outcome prediction by microRNAs in human cancer: A
systematic review. J Natl Cancer Inst. 104:528–540. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shell S, Park SM, Radjabi AR, Schickel R,
Kistner EO, Jewell DA, Feig C, Lengyel E and Peter ME: Let-7
expression defines two differentiation stages of cancer. Proc Natl
Acad Sci USA. 104:11400–11405. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Serguienko A, Grad I, Wennerstrøm AB,
Meza-Zepeda LA, Thiede B, Stratford EW, Myklebost O and Munthe E:
Metabolic reprogramming of metastatic breast cancer and melanoma by
let-7a microRNA. Oncotarget. 10:2451–2465. 2015. View Article : Google Scholar
|
|
26
|
Dong Q, Meng P, Wang T, Qin W, Qin W, Wang
F, Yuan J, Chen Z, Yang A and Wang H: MicroRNA let-7a inhibits
proliferation of human prostate cancer cells in vitro and in vivo
by targeting E2F2 and CCND2. PLoS One. 5:e101472010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhong Z, Dong Z, Yang L, Chen X and Gong
Z: Inhibition of proliferation of human lung cancer cells by green
tea catechins is mediated by upregulation of let-7. Exp Ther Med.
4:267–272. 2014.
|
|
28
|
Song H, Xu W, Song J, Liang Y, Fu W, Zhu
XC, Li C, Peng JS and Zheng JN: Overexpression of Lin28 inhibits
the proliferation, migration and cell cycle progression and induces
apoptosis of BGC-823 gastric cancer cells. Oncol Rep. 33:997–1003.
2015.PubMed/NCBI
|
|
29
|
Lee JY, Kim HJ, Yoon NA, Lee WH, Min YJ,
Ko BK, Lee BJ, Lee A, Cha HJ, Cho WJ, et al: Tumor suppressor p53
plays a key role in induction of both tristetraprolin and let-7 in
human cancer cells. Nucleic Acids Res. 41:5614–5625. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Akhurst RJ and Hata A: Targeting the TGFβ
signalling pathway in disease. Nat Rev Drug Discov. 11:790–811.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lei Z, Xu G, Wang L, Yang H, Liu X, Zhao J
and Zhang HT: MiR-142-3p represses TGF-β-induced growth inhibition
through repression of TGFβR1 in non-small cell lung cancer. FASEB
J. 28:2696–2704. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fang Y, Chen Y, Yu L, Zheng C, Qi Y, Li Z,
Yang Z, Zhang Y, Shi T, Luo J, et al: Inhibition of breast cancer
metastases by a novel inhibitor of TGFβ receptor 1. J Natl Cancer
Inst. 105:47–58. 2013. View Article : Google Scholar : PubMed/NCBI
|