Introduction
Lung cancer is one of the most common malignant
tumors and is a major cause of global morbidity and mortality. Even
with recent advances in diagnosis and clinical treatment, the
five-year survival rate is only 15% (1). Non-small cell lung cancer (NSCLC),
such as the A549 cell line, accounts for 85% of all lung cancer
cases (2). Notably, most patients
with NSCLC are diagnosed after having reached a terminal stage and
developed metastases in adjacent or distant organs (3). Systemic pharmacotherapy is the primary
treatment for these patients (4).
Recently, drugs targeting key pathways involved in NSCLC have
generated new approaches for treating this condition. However,
efficacious, curative drug therapies for NSCLC and its metastases
remain elusive (5). There is an
urgent need for more effective agents for the clinical treatment of
NSCLC.
Tumor development involves abnormal cell
proliferation and migration (6).
Additionally, cell proliferation is closely related to the cell
cycle; therefore, inducing apoptosis, arresting the cell cycle and
inhibiting metastasis are effective methods of controlling tumor
cell growth (7). Many cytokines and
signaling pathways, including Bax, Bcl-2, matrix metallo-
proteinases (MMPs), cyclins, AKT, mitogen-activated protein kinase
(MAPK), c-Jun NH2-terminal protein kinase (JNK) and
extracellular signal-regulated kinase (ERK), play influential roles
in regulating the abnormal proliferation and migration of tumor
cells (8–10). These molecular players and signaling
cascades are involved in regulating apoptosis, migration and the
cell cycle.
Angelicin is a traditional Chinese medicine and a
well-known furocoumarin that has been a common treatment for a long
time (11). Recently, it has been
used to treat various skin diseases (12,13).
Moreover, previous studies have demonstrated that angelicin has
potential for curing leukemia by inhibiting tumor cell
proliferation (14). Furthermore,
angelicin is reportedly a potential candidate for treating
neuroblastoma by inducing cell apoptosis (11). However, there have been few studies
on the effect of angelicin on NSCLC (15).
In this study, we aimed to assess the abrogation of
A549 cell growth resulting from angelicin inducing apoptosis,
arresting the cell cycle and inhibiting metastasis. To gain insight
into the potential anticancer mechanism of angelicin, we further
investigated its effects on growth and related metastasis signaling
pathways. Our results suggest that angelicin inhibits A549 cell
activity by regulating ERK and JNK signaling as well as related
metastasis signaling. All the results suggest that angelicin could
be an effective therapeutic candidate for NSCLC intervention.
Materials and methods
Ethics statement
All experiments were approved by the Huazhong
University of Science and Technology Committee and the Tongji
Medical College Ethics Committee, Tongji Hospital (Wuhan,
China).
Reagents
Dimethyl sulfoxide (DMSO), angelicin and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
were purchased from Sigma-Aldrich, Invitrogen Life Technologies
(Carlsbad, CA, USA). Polyclonal antibodies against Bax, Bcl-2,
caspase-3, caspase-9, cyclin B1, cyclin E1, Cdc2, E-cadherin, MMP2,
MMP9, p-ERK1/2, ERK1/2, p-JNK1, JNK1, P-p38 MAPK, p38 MAPK, Akt and
p-Akt were purchased from Cell Signaling Technology, Inc. (Danvers,
MA USA). GAPDH, goat anti-mouse IgG and goat anti-rabbit IgG were
purchased from Proteintech Group, Inc. (Rosemont, IL, USA).
Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum
(FBS), penicillin and streptomycin were purchased from Gibco,
Invitrogen Life Technologies (Carlsbad, CA, USA). A protease
inhibitor cocktail was purchased from Roche Diagnostics (Basel,
Switzerland). A BCA protein assay reagent kit and an enhanced
chemiluminescence (ECL) plus reagent kit were obtained from Pierce
Biotechnology, Inc. (Rockford, IL, USA).
Cell lines and culture
Human lung cancer A549 cells were purchased from the
Chinese Academy of Sciences Cell Bank (CBP60084; Shanghai, China)
and were maintained in RPMI-1640 with 10% FBS and antibiotics
(penicillin and streptomycin) in an incubator with a humidified
atmosphere (5% CO2, 37°C).
MTT assay
An MTT colorimetric assay was performed according to
the manufacturer's instructions. In brief, exponentially growing
cells were seeded in 96-well plates at a density of
5×103 cells/well. Following an overnight incubation, the
cells were treated with various doses of angelicin for 24 h at
37°C. Then, the medium was discarded, and MTT (0.5 mg/ml) was added
into each well and incubated for 4 h at 37°C. Subsequently, the
MTT-containing medium was removed and replaced with 150 µl of DMSO.
The absorbance at 570 nm was then determined using a Bio-Rad Model
680 microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). The IC50 was calculated from the MTT dose-response
curves of cell viability against drug concentration. Three
replicate wells were used for each analysis.
TdT-mediated dUTP nick end labeling
(TUNEL) assay
A TUNEL assay was utilized to analyze the
pro-apoptotic effect of angelicin in A549 cells. After being
appropriately treated with angelicin, cells were fixed in 4%
paraformaldehyde for 30 min and then permeabilized with 0.05 %
Triton X-100 on ice for 5 min. The cells were subjected to TUNEL
while being incubated in a humidified chamber at 37°C for 60 min in
the dark. The cells were washed 3 times with phosphate-buffered
saline (PBS; pH 7.4); anti-fluorescence quenching solution was then
added and allowed to react for 5 min. Finally, the cells were
examined using a confocal laser scanning microscope.
Flow cytometric apoptosis assay
To detect the apoptotic effects of angelicin on A549
cells, an Annexin V-FITC/propidium iodide (PI) apoptosis detection
kit was used. In brief, A549 cells were seeded in a 6-well plate
and incubated for 24 h; the cells were then treated with the DMSO
control or angelicin (10, 25 or 50 µmol) for 24 h. Next, the cells
were collected, washed with PBS, and resuspended in 100 µl of 1X
binding buffer. Annexin V-FITC/PI were added to each group, and the
cells were incubated for 15 min at room temperature in the dark.
The cells were then analyzed by flow cytometry (BD Calibur; BD
Biosciences, San Jose, CA, USA). Each experiment was performed 3
times.
Flow cytometric cell cycle
analysis
Flow cytometry used to examine the effect of
angelicin on the cell cycle. After being treated with the DMSO
control or angelicin (10, 25 or 50 µmol) for 24 h, A549 cells were
harvested, washed twice with PBS (pH 7.4) and fixed with 70%
ethanol for 20 min. Then, the cells were centrifuged (300 × g, 5
min) to eliminate the ethanol, washed twice with PBS (pH 7.4) and
stained with PI in the dark for 30 min. Finally, the cell cycle
distribution was assessed by flow cytometry (BD Calibur; BD
Biosciences). Each experiment was performed 3 times.
Wound-healing assay
The anti-migratory effects of angelicin on A549
cells were examined through a wound-healing assay. After attached
cells had grown to 90% confluence, a wound in the monolayer was
created using a pipette tip, and the cells were washed twice with
PBS (pH 7.4). Then, the cells were treated with the DMSO control or
angelicin (10, 25 or 50 µmol) for 24 h. The number of migrated
cells was determined using an inverted microscope. Five randomly
chosen fields were analyzed in each well.
Transwell migration assay
Transwell chambers (Corning Costar, Cambridge, MA,
USA) were used for the cell migration assays. A549 cells
(1×105) were seeded in the top chamber with FBS-free
medium. Culture medium containing the DMSO control or angelicin
(10, 25 or 50 µmol) was added to the bottom chamber. After the
cells were incubated for 24 h at 37°C, the upper side of the
membrane was removed, and the cells in the lower chamber were fixed
in 4% paraformaldehyde for 15 min. The fixed cells were washed with
PBS (pH 7.4) 3 times and then stained with 0.25% crystal violet for
5 min. Cell migration was evaluated using an inverted microscope
(×200). Six randomly chosen fields were analyzed in each group and
presented as the mean of 3 independent experiments.
Western blotting
After the cells were treated with the DMSO control
or angelicin, proteins from the A549 cell lysates were extracted
and 12% SDS-PAGE was used to separate the protein samples. Then,
the proteins were transferred to a PVDF membrane, which was blocked
with 5% skim milk and incubated with different antibodies overnight
at 4°C. The antibodies were diluted to the following
concentrations: Bax (1:1,000), Bcl-2 (1:2,000), caspase-3
(1:1,000), caspase-9 (1:800), cyclin B1 (1:1,000), cyclin E1
(1:1,000), Cdc2 (1:2,000), E-cadherin (1:2,000), MMP2 (1:800), MMP9
(1:800), p-ERK1/2 (1:2,000), ERK1/2 (1:2,000), p-JNK1 (1:1,000),
JNK1 (1:1,000), P-p38 MAPK (1:1,000), p38 MAPK (1:1,000), Akt
(1:2,000) and p-Akt (1:1,000). After being washed 3 times in TBST,
the membrane was incubated with HRP-conjugated secondary antibodies
for 1 h at room temperature. An ECL kit was used to develop the
immuno-reactive bands.
Animal groups and in vivo xenograft
study
For the A549 xenograft studies, female nude mice
aged 5 weeks were used. A549 cells (5×106) were
subcutaneously implanted in the right flank of the mice. The
tumor-bearing mice were randomly divided into two groups: the PBS
(pH 7.4) control group and the angelicin group. Mice in the
angelicin group were treated with angelicin for 4 consecutive weeks
by oral gavage (100 mg/kg), while mice in the control group
received PBS (pH 7.4). Tumor volume and body weight were monitored
once every 2 days. At the end of 4 weeks, the mice were sacrificed
and the tumor xenografts were removed and measured.
Experimental lung metastasis
experiments
Male nude mice (SIPPR-BK Laboratory Animal Co.,
Ltd., Shanghai, China) aged 5 weeks were used. A549 cells
(5×106) were injected via the tail vein. Two weeks
later, the mice were randomly divided into two groups: the PBS (pH
7.4) control group and the angelicin (100 mg/kg/day) group. Mice in
the angelicin group received an intragastric administration of
angelicin dissolved in PBS (pH 7.4) every day for 4 weeks. The
animals were sacrificed after this period. The lungs were then
removed and fixed in Bouin's solution for 4 h, and the number of
metastatic lesions was determined macroscopically.
Immunohistochemistry
Sections of the lung specimens were deparaffinized
and rehydrated. Then, the sections were rinsed in PBS (pH 7.4) 3
times and incubated in 3% hydrogen peroxide for 15 min at room
temperature. After being blocked in 10% goat serum for 30 min, the
tissue sections were incubated overnight at 4°C with polyclonal
antibodies (MMP2, 1:100; MMP9, 1:80; and E-cadherin, 1:50). The
sections were subsequently incubated with peroxidase-conjugated
goat anti-rabbit secondary antibody (1:100) for 1 h at room
temperature, washed 5 times with PBS (pH 7.4), and visualized with
the peroxidase substrate diaminobenzidine (DAB) under a
microscope.
Statistical analysis
The data shown in this study were obtained from at
least 3 independent experiments and analyzed using SPSS 16.0 (SPSS,
Inc., Chicago, IL, USA). Statistical significance was determined
using an unpaired Student's t-test. All results are presented as
the mean ± standard deviation (SD). P<0.05 was considered
statistically significant vs. the control group.
Results
Angelicin inhibits the growth of A549
cells
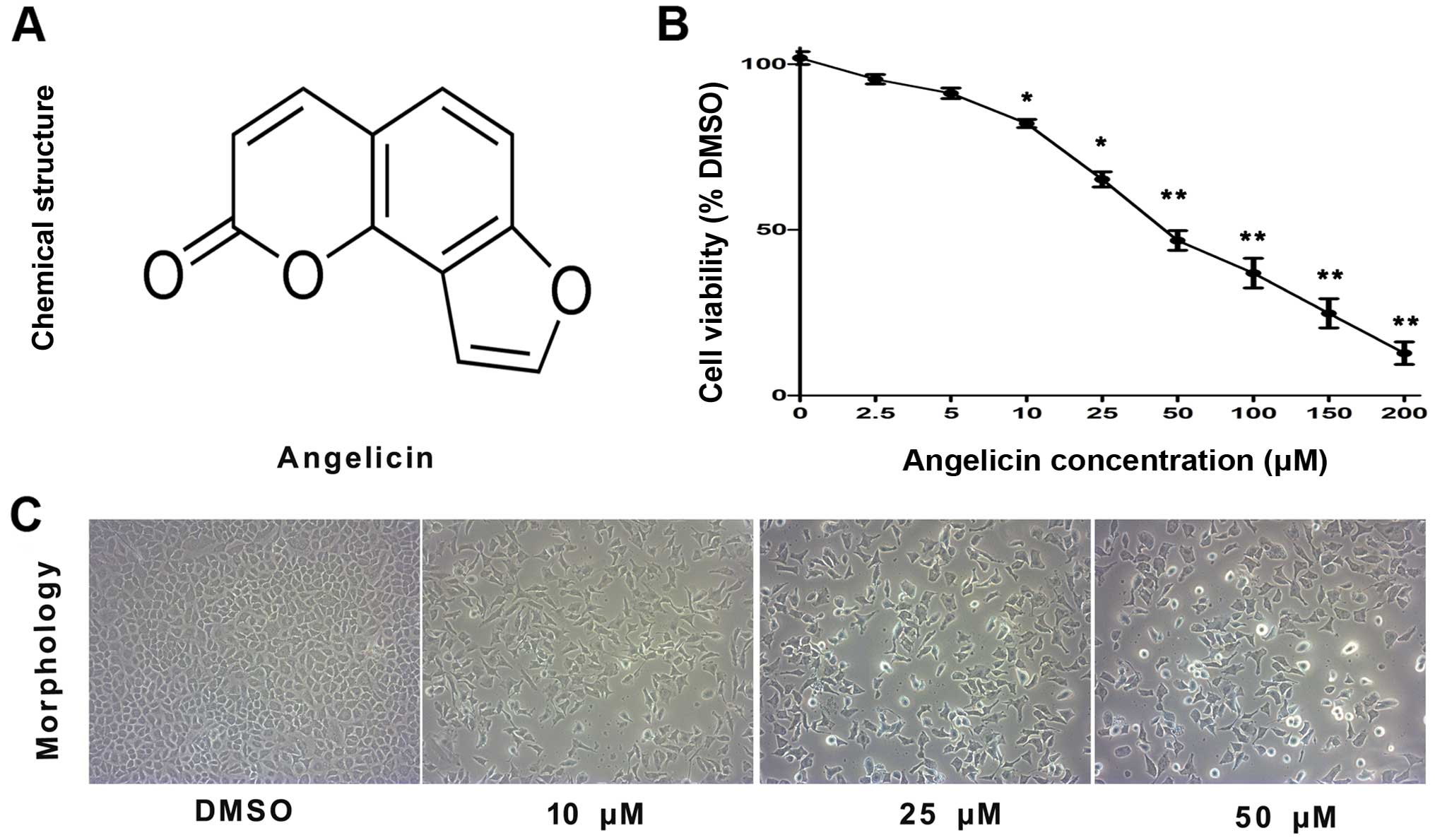
The chemical structure of angelicin is shown in
Fig. 1A. To examine the cytotoxic
effects of angelicin on A549 cells, an MTT assay was performed.
A549 cells were treated with different concentrations of angelicin
(0, 2.5, 5, 10, 25, 50, 100, 150 and 200 µmol/l) for 24 h. As shown
in Fig. 1B, the proliferation of
angelicin-treated A549 cells was markedly suppressed at 24 h
compared with that of cells in the control group. These results
showed that the angelicin treatment suppressed A549 cell
proliferation in a dose-dependent manner. As the IC50
value for angelicin was 50.14 µmol, doses of 10, 25 and 50 µmol
angelicin were selected for use in subsequent experiments to assess
A549 cell growth suppression.
Additionally, the morphological changes of
angelicin-treated cells were assessed using an inverted microscope
(Fig. 1C). The results showed that
the angelicin treatment caused marked morphological alterations,
including the adherence of cells in poor condition, reduced cell
volume, chromatin condensation, karyopyknosis and nuclear
fragmentation. Furthermore, as the dose of angelicin increased, the
morphological alterations became more apparent.
Angelicin induces apoptosis and
regulates the expression of apoptosis-associated proteins in A549
cells
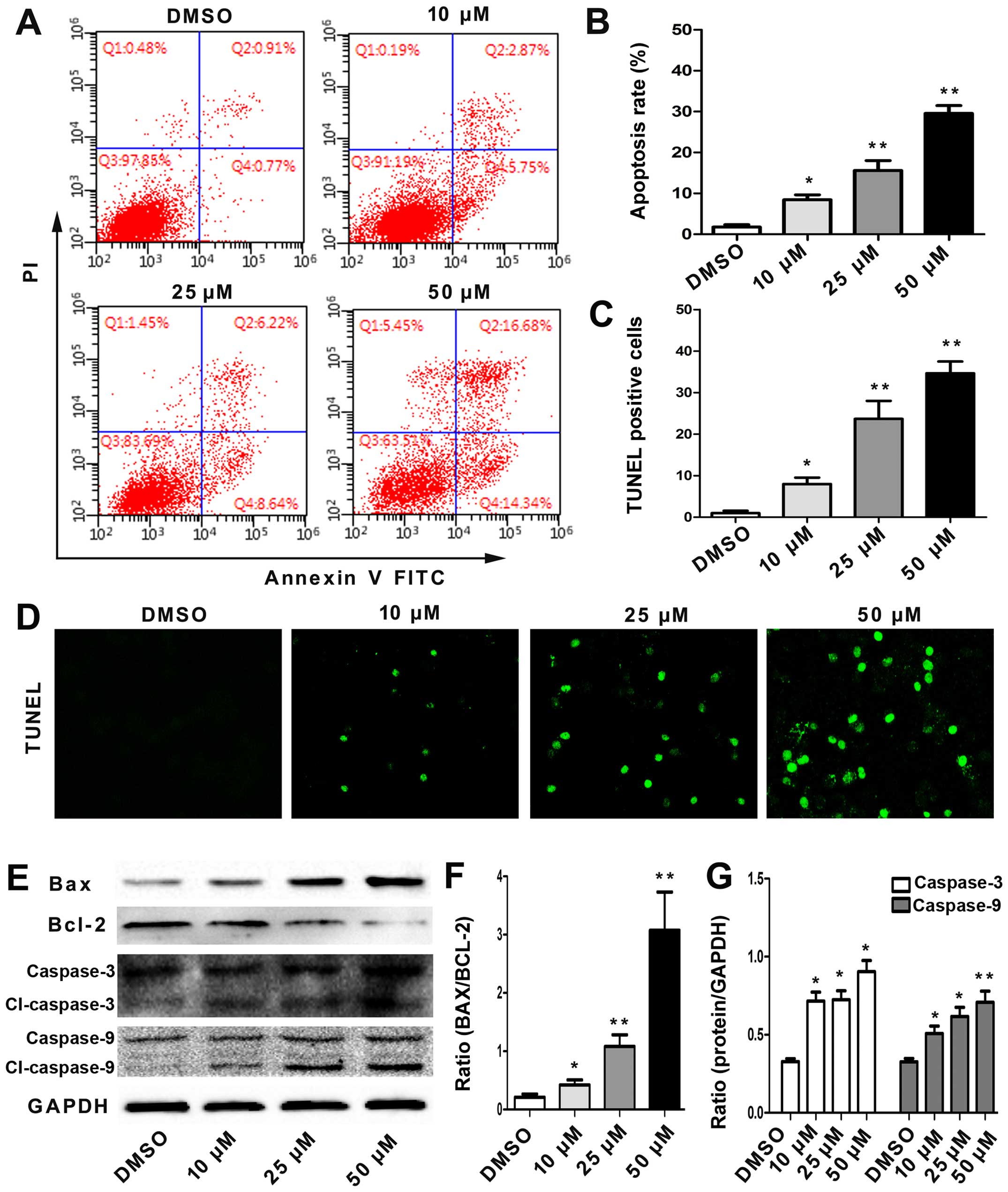
To assess whether angelicin-induced cell growth
suppression was associated with cell apoptosis, the effects of
angelicin on apoptosis were evaluated by flow cytometry using
Annexin V-FITC/PI double staining. As shown in Fig. 2A and B, the percentage of early- and
late-stage apoptotic cells increased in a dose-dependent manner.
This result showed that the angelicin treatment caused a
significant increase in apoptosis.
Moreover, the extent of A549 cell apoptosis was
examined using TUNEL staining. The TUNEL assay revealed only 2±2%
TUNEL-positive nuclei in the DMSO control cells, and in accordance
with the flow cytometric analysis, a significantly increased
percentage of TUNEL-positive nuclei was observed in the cells
incubated with angelicin (Fig. 2C and
D). In addition, angelicin-induced apoptosis occurred in a
dose-dependent manner.
Furthermore, to gain insight into the potential
pro-apoptotic mechanisms of angelicin, the protein expression
levels of Bax, Bcl-2, caspase-3 and caspase-9, which are important
apoptosis-associated proteins, were detected by western blotting.
As shown in Fig. 2E-G, the
angelicin treatment markedly induced Bax expression to a level
comparable to that of the control cells and significantly reduced
Bcl-2 expression in a dose-dependent manner. In addition, the
angelicin treatment promoted the expression of cleaved caspase-3
and caspase-9 in a dose-dependent manner, demonstrating the
pro-apoptotic effect of angelicin. All the results demonstrated
that the pro-apoptotic effect of angelicin may be associated with
the regulation of these proteins.
Angelicin arrested the cell cycle and
regulated related proteins
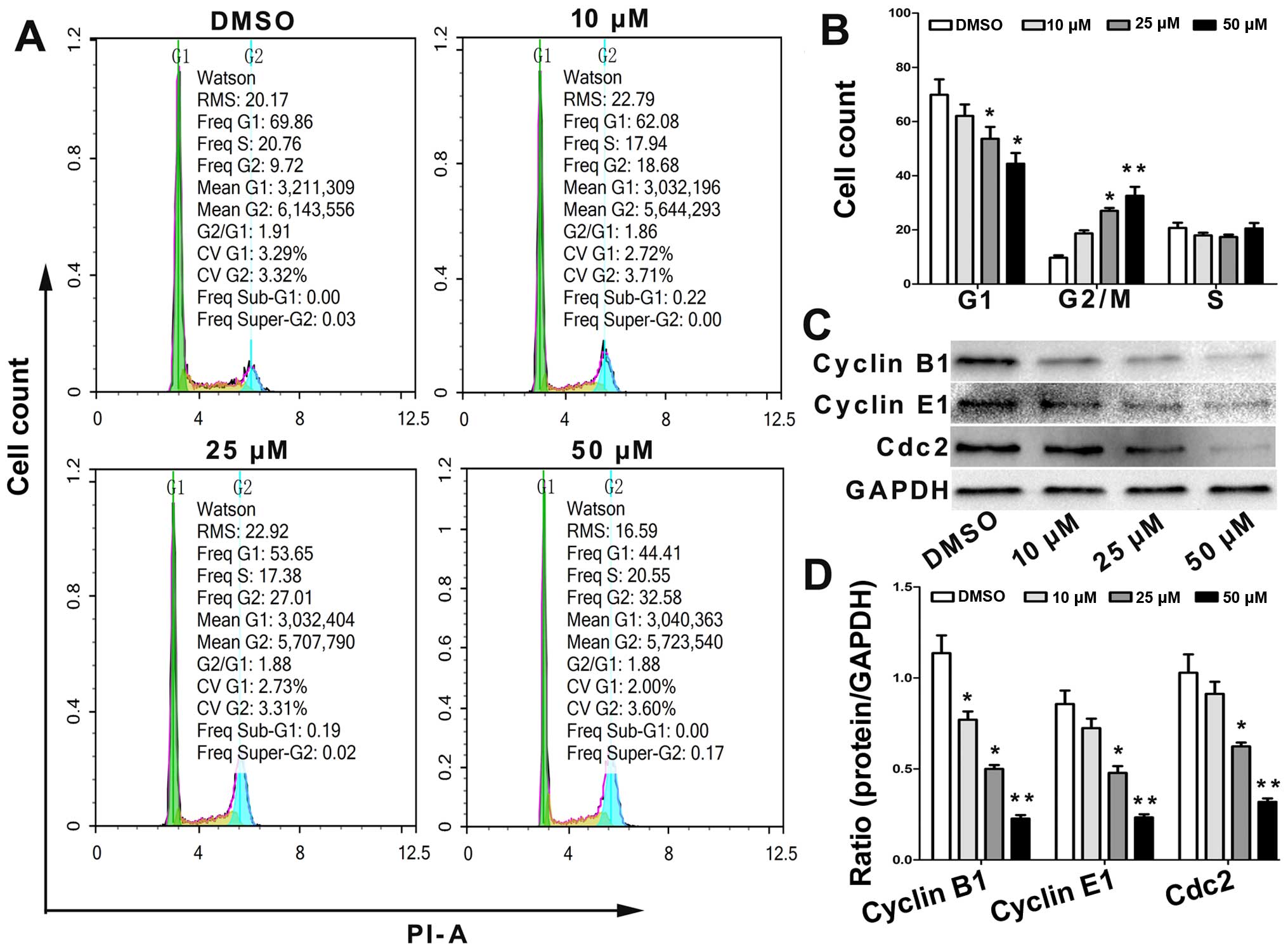
The effect of angelicin on the cell cycle
distribution was determined using flow cytometry. Cells were or
were not treated with angelicin at 10, 25 and 50 µmol for 24 h and
were then stained with PI. The results showed that the proportion
of cells in the G2/M and G0/G1 phase increased and decreased,
respectively, in a dose-dependent manner (Fig. 3A and B).
To further elucidate the specific regulatory
proteins responsible for the cell cycle arrest, we explored the
effect of angelicin on the regulatory proteins cyclin B1, cyclin E1
and Cdc2. As shown in Fig. 3C and
D, the levels of cyclin B1, cyclin E1 and Cdc2 were
significantly downregulated in the angelicin-treated cells. These
data revealed that alterations in the expression levels of cell
cycle regulatory proteins and the arrest of cell growth in the G2/M
phase are involved in angelicin-induced changes in cell cycle
progression.
Angelicin inhibits A549 cell migration
and invasion
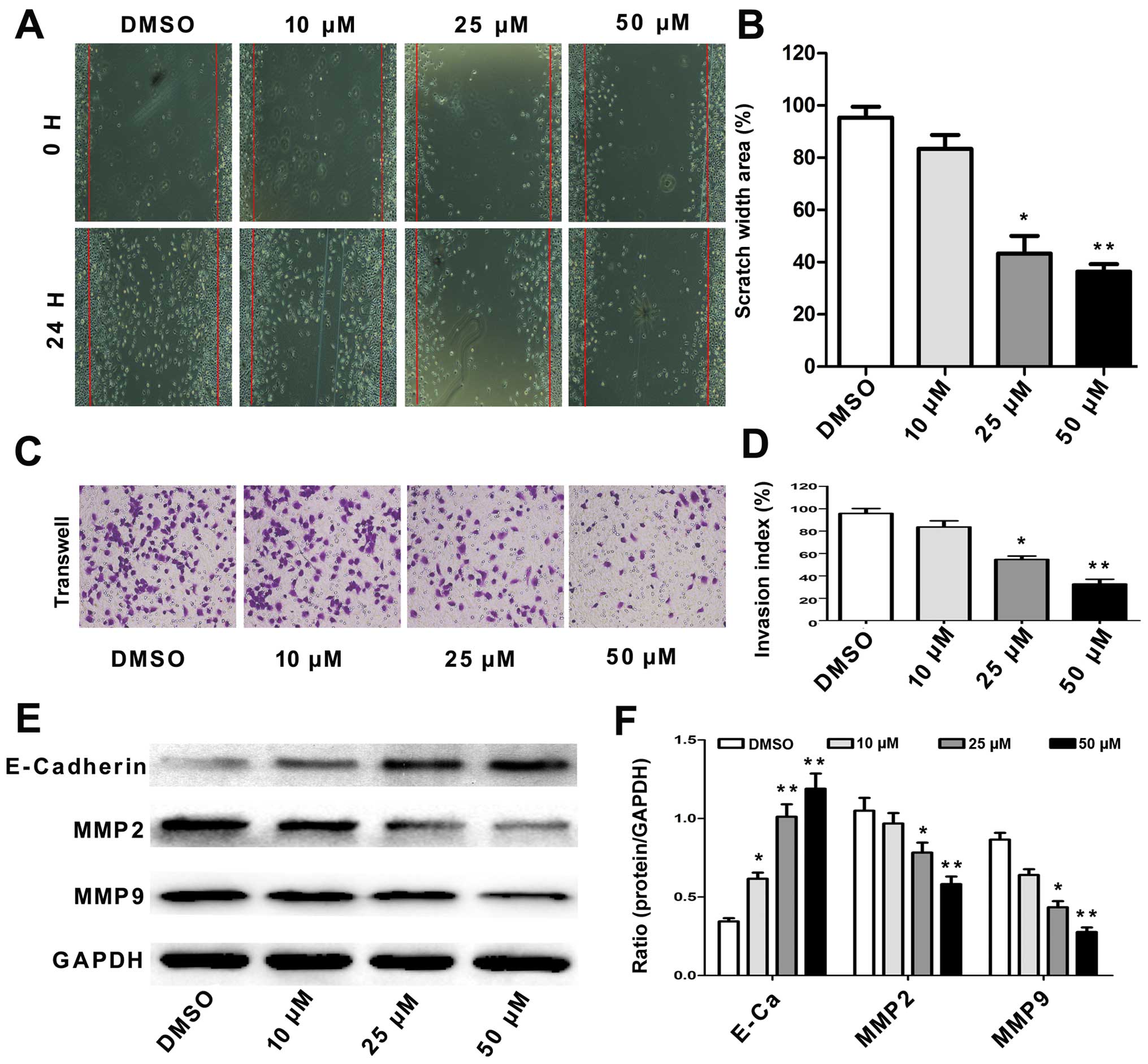
Tumor cell migration and invasion are key steps in
cancer metastasis (16). To
evaluate the potential effect of angelicin on A549 cell migration,
we assessed alterations in cell mobility using a scratch test and a
Transwell assay. The results showed that the angelicin treatment
significantly decreased A549 cell migration capability in a
dose-dependent manner (Fig. 4A and
B). The Transwell assay results showed that the migration and
invasion of the angelicin-treated A549 cells were significantly
inhibited in a dose-dependent manner compared with that of the
control cells (Fig. 4C and D).
E-cadherin, MMP2 and MMP9 are responsible for cell
migration, invasion and cell-matrix adhesion, particularly in the
process of cancer metastasis (17).
Therefore, we detected the effects of angelicin on the expression
of these proteins using western blotting. As shown in Fig. 4E and F, we observed that angelicin
strongly increased E-cadherin expression but reduced MMP2 and MMP9
expression in A549 cells in a dose-dependent manner compared with
that of the control cells. These results indicate that angelicin
directly inhibits the migration and invasion of A549 cells.
Angelicin inhibits A549 cell growth
and migration via ERK and JNK pathways
The MAPK and Akt signaling pathways have important
roles in regulating tumor cell apoptosis, cell cycle progression
and metastasis (17). To further
explore the mechanism underlying angelicin-induced apoptosis, cell
cycle arrest and migration inhibition in A549 cells, we evaluated
whether angelicin modulates the MAPK and Akt signaling pathways
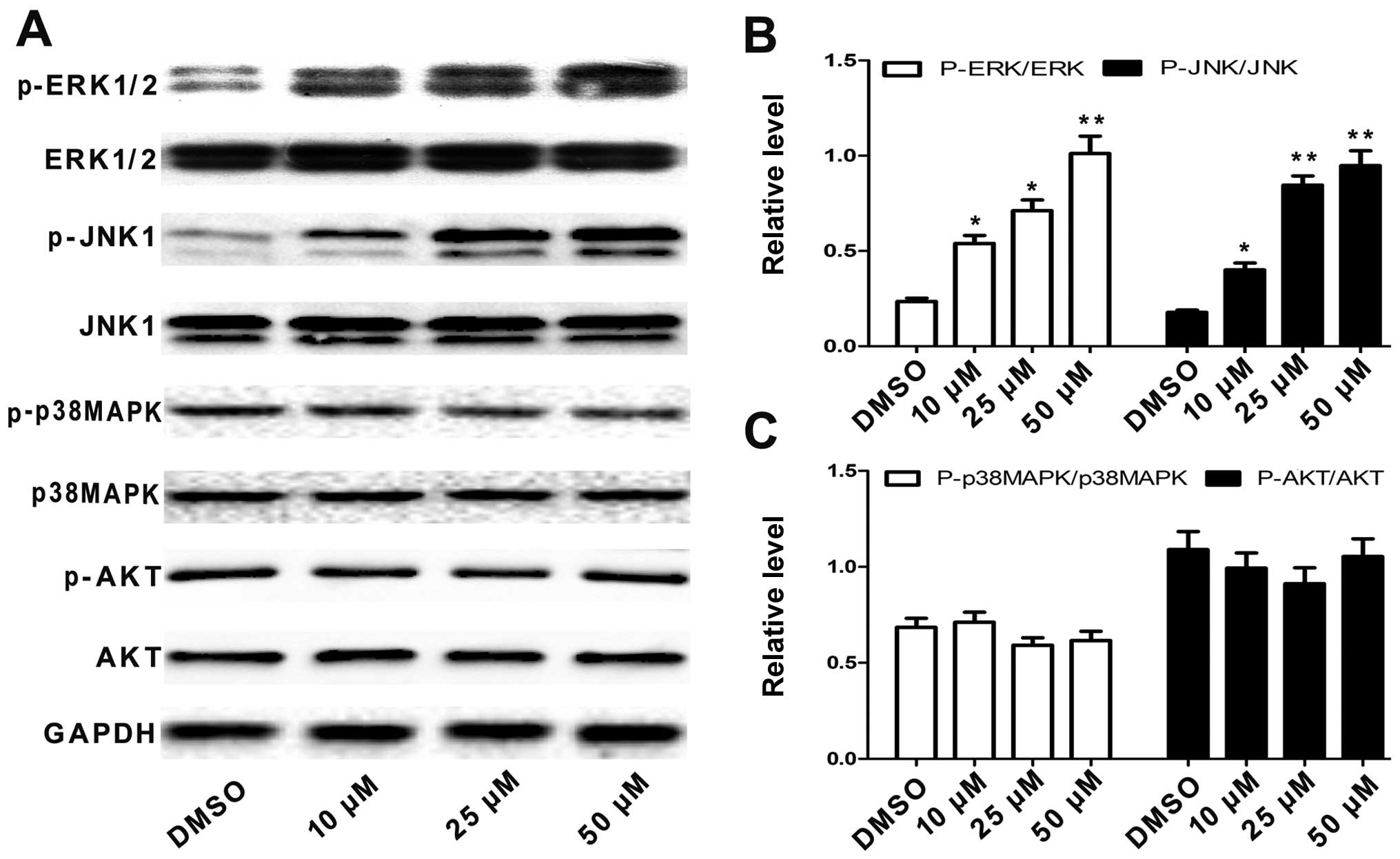
when affecting A549 cells. We first examined the activation status
of p38 MAPK, JNK, ERK and AKT by western blotting with antibodies
specific to the phosphorylated forms of these kinases. As shown in
Fig. 5A-C, treating A549 cells with
angelicin significantly increased the levels of phosphorylated JNK
and ERK compared with the total protein expression levels in a
dose-dependent manner. However, angelicin had no effect on the
phosphorylation of p38 MAPK, AKT, or the total expression levels of
these proteins. These results suggest that angelicin may activate
the JNK and ERK pathways in A549 cells.
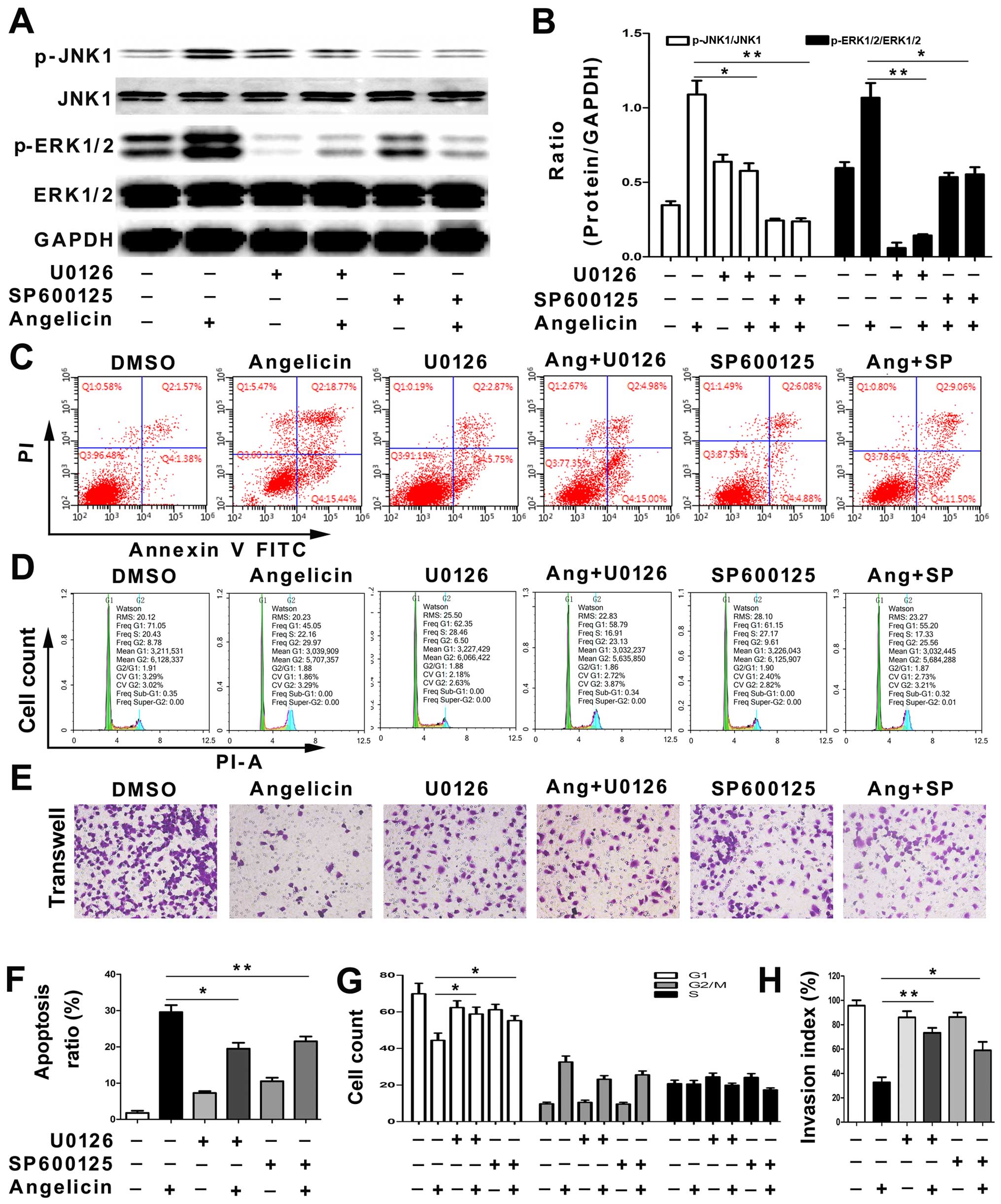
We next tested whether SP600125, a JNK inhibitor, or
U0126, an ERK inhibitor, could reverse the angelicin-induced
apoptosis, cell cycle arrest and migration inhibition in A549
cells. First, the levels of phosphorylated JNK and ERK were
analyzed by western blotting. The SP600125 and U0126 treatments
significantly attenuated angelicin-induced JNK and ERK activation
(Fig. 6A and B). The results
suggested that JNK and ERK activation may play a crucial role in
angelicin-mediated effects on A549 cells. Next, the cell cycle,
apoptosis and migration of A549 cells were examined with or without
SP600125 and U0126 pretreatments. As shown in Fig. 6C-H, the SP600125 and U0126
treatments partially alleviated the angelicin-induced apoptosis,
cell cycle arrest and migration inhibition of A549 cells. The
results indicated that angelicin inhibits A549 cell growth and
migration through both the ERK and JNK pathways.
Angelicin inhibits A549 cell growth
and metastasis in vivo
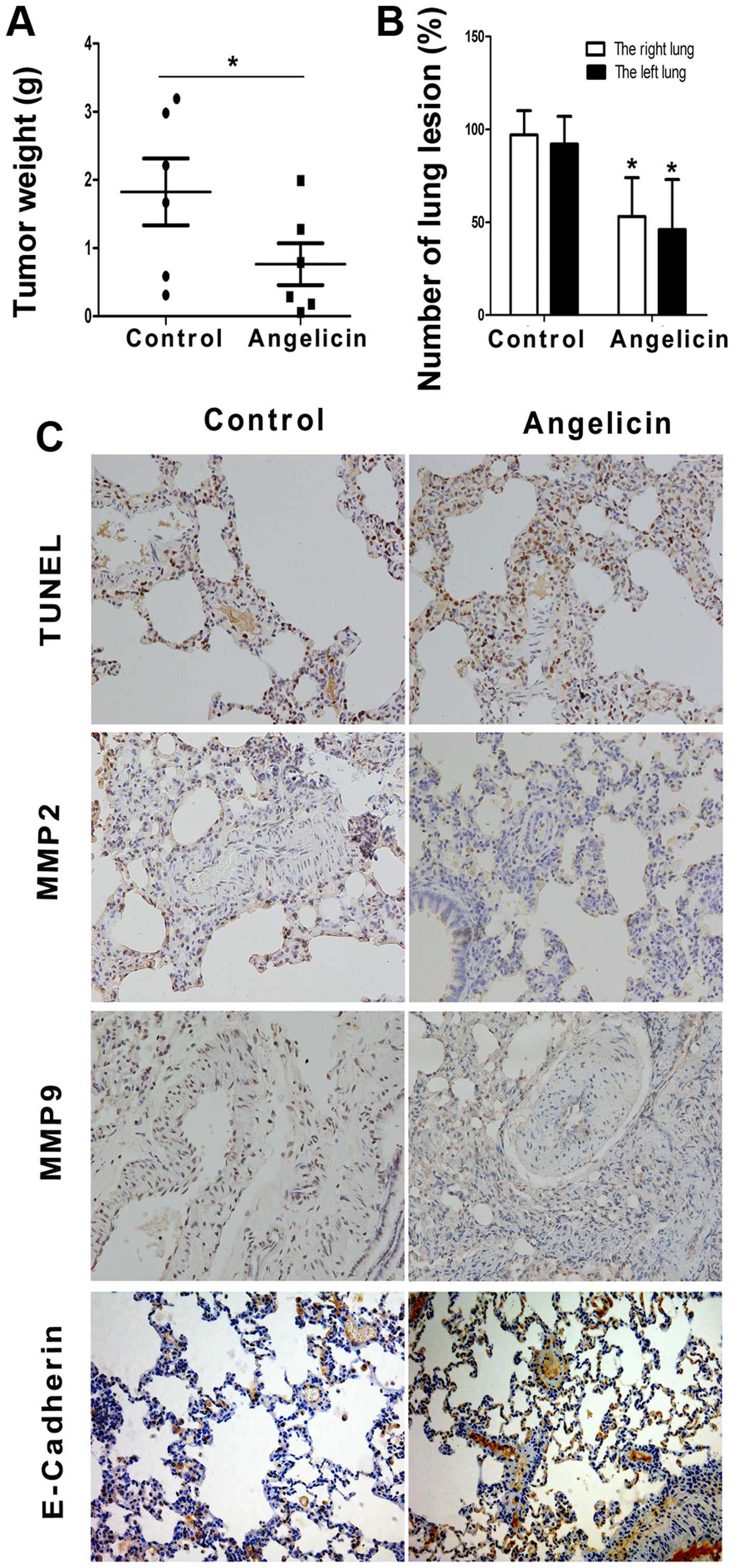
We further evaluated the antitumor effect of
angelicin in A549 cancer cells by utilizing nude mouse xenograft
models. As anticipated, tumor size and weight decreased
significantly in the angelicin group compared with the control
group (Fig. 7A). The
anti-metastatic effects of angelicin were further assessed with an
in vivo model of lung metastasis. As shown in Fig. 7B, angelicin had an inhibitory effect
on A549 tumor metastasis to the lungs compared with the control
group. TUNEL labeling and the expression levels of MMP2, MMP9 and
E-cadherin were evaluated immunohistochemically (Fig. 7C). Angelicin significantly increased
the ratio of TUNEL-positive cells, which is indicative of
apoptosis, in metastatic nodules of the lung metastasis model.
Furthermore, the angelicin treatment resulted in decreased
expression levels of MMP2 and MMP9 and increased expression of
E-cadherin, which was consistent with the in vitro study.
These results indicate that angelicin can significantly inhibit
A549 cell growth and metastasis in vivo.
Discussion
Recently, angelicin has gained much attention due to
its biological multifunctionality. In the present study, angelicin
suppressed the growth and metastasis of A549 human lung
adenocarcinoma cells both in vitro and in vivo. The
mechanisms underlying this process involve apoptosis induction,
cell cycle arrest at the G2/M phase and migration inhibition.
Additionally, angelicin was able to regulate the expression of
apoptosis-, cell cycle-, and migration-related proteins and
activate caspase activity. Moreover, the effects exerted by
angelicin in A549 cells may be regulated by ERK and JNK pathway
modulation. Angelicin was capable of inducing apoptosis, inhibiting
growth and hindering metastasis in vivo.
In this study, we observed that angelicin strongly
inhibited A549 cell growth. As shown in MTT, the IC50
values of angelicin against A549 cells was nearly 50 µmol. Further,
we found 10 µmol angelicin exhibited inhibitory effects on cell
proliferation (P<0.05). Thus, angelicin concentrations of 10, 25
and 50 µmol were selected for the subsequent assays.
The anticancer effects of therapeutics have been
observed to be linked to the process of inducing apoptosis
(19). Apoptosis is a process
leading to programmed cell death that is highly regulated by
several signaling pathways, including those of caspases and the
Bcl-2 family (20). Bax and Bcl-2
are two key proteins of the Bcl-2 family and are critical for cells
to undergo apoptosis (21). In this
study, we observed significant changes in Bax and Bcl-2 protein
expression after angelicin treatment, which occurred in a
dose-dependent manner. Specifically, the ratio of Bax to Bcl-2
markedly increased, which is considered the driving force of
apoptosis (22). The caspase family
consists of cysteine-containing proteolytic enzymes that have a
potent role in executing apoptosis (23). In the present study, angelicin was
shown to induce caspase-3 and caspase-9 activation in a
dose-dependent manner. Thus, we conclude that angelicin may induce
apoptosis by regulating these apoptosis-related proteins.
Cell cycle disorder is known to contribute to cancer
cell growth and the development of various types of cancer
(24). Thus, arresting the cell
cycle is considered a very effective method for eradicating tumor
cells. The G2/M phase is an important checkpoint in the cell cycle
that prevents the initiation of mitosis until DNA damaged during
replication is repaired (25). The
majority of the cells treated with angelicin were arrested in the
G2/M phase. Additionally, cell cycle progression is tightly related
to various cyclins and cyclin-dependent kinases (CDKs), such as
cyclin B1, cyclin E1 and Cdk2 (26). We examined the modulation of these
key proteins involved in cell cycle arrest and observed that cyclin
B1, cyclin E1 and Cdk2, master regulators of the cell cycle, were
efficiently modulated by angelicin. These data indicate that
angelicin could inhibit cell growth by regulating cyclin B1, cyclin
E1 and Cdk2 expression levels, thereby arresting the cell cycle in
the G2/M phase.
Tumor metastasis is a major cause of death in NSCLC
patients; thus, it is crucial to identify promising anti-metastatic
agents to prevent or inhibit metastasis (27). In this study, we found that
angelicin significantly inhibited A549 cell invasion and migration.
Additionally, the expression levels of several crucial metastasis
genes were assessed, including E-cadherin, MMP2 and MMP9, which
play key roles in regulating cancer cell invasion and metastasis
(28). Abnormal expression levels
of E-cadherin, MMP2 and MMP9 in tumor cells will lead to decreased
adhesion as well as enhanced migration and invasion, thus promoting
cancer progression (29). Our
results showed that angelicin increased the expression of
E-cadherin and strongly decreased the expression of MMP2 and MMP9,
which suggested that angelicin would be an effective agent against
NSCLC metastasis.
Previous studies have demonstrated that the MAPK and
AKT signaling pathways play important roles in regulating
apoptosis, the cell cycle and migration (30). The present study evaluated the
effects of angelicin on the phosphorylation of signaling molecules
in these pathways in A549 cells. The MAPK family includes
JNC/stress-activated protein kinase (SAPK), p38 MAPK and ERK
(31). In this study, we showed
that angelicin increased JNK and ERK phosphorylation in A549 cells
in a dose-dependent manner, while the total protein level remained
steady; however, angelicin had no effect on p38 MAPK and AKT
activation. Furthermore, treatment with a JNK1/2-specific inhibitor
(SP600125) or an ERK1/2 inhibitor (U0126) effectively alleviated
the angelicin-induced apoptosis, cell cycle arrest and migration
inhibition in A549 cells. These findings suggest that JNK and ERK
activation plays a critical role in angelicin-induced apoptosis,
cell cycle arrest and migration inhibition in NSCLC A549 cells.
In addition to investigating the in vitro
anticancer and anti-metastatic activity of angelicin, we assessed
the antitumor and anti-metastatic effects of angelicin in
vivo. The angelicin treatment decreased the volume and weight
of subcutaneous tumor mass in this model. Moreover, in the lung
metastasis model, we found that treatment with angelicin not only
inhibited the formation of metastatic nodules but also induced
apoptosis and decreased the expression of migration-related
proteins in the lungs.
In conclusion, angelicin exerts antitumor and
anti-metastatic activity by inducing apoptosis, arresting the cell
cycle and inhibiting migration in human lung carcinoma A549 cells.
The mechanisms underlying these effects are associated with
activation of the JNK and ERK pathways. Therefore, angelicin may be
a potential therapeutic agent for the treatment of human lung
cancer.
References
|
1
|
Pusceddu S, Lo Russo G, Macerelli M, Proto
C, Vitali M, Signorelli D, Ganzinelli M, Scanagatta P, Duranti L,
Trama A, et al: Diagnosis and management of typical and atypical
lung carcinoids. Crit Rev Oncol Hematol. 100:167–176. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Villaruz LC and Socinski MA: Is there a
role of nab-paclitaxel in the treatment of advanced non-small cell
lung cancer? The data suggest yes. Eur J Cancer. 56:162–171. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bremnes RM, Busund LT, Kilvær TL, Andersen
S, Richardsen E, Paulsen EE, Hald S, Khanehkenari MR, Cooper WA,
Kao SC, et al: The role of tumor-infiltrating lymphocytes in
development, progression, and prognosis of non-small cell lung
cancer. J Thorac Oncol. 11:789–800. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sibille A, Paulus A, Martin M, Bourhaba M,
Barthélemy N, Radermecker M, Corhay JL, Louis R and Duysinx B:
Management of non-small cell lung cancer. Rev Med Liege.
70:432–441. 2015.(In French). PubMed/NCBI
|
|
5
|
Shea M, Costa DB and Rangachari D:
Management of advanced non-small cell lung cancers with known
mutations or rearrangements: latest evidence and treatment
approaches. Ther Adv Respir Dis. 10:113–129. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sidaway P: CNS cancer: distinct subtypes
of ATRTs observed. Nat Rev Clin Oncol. 13:2642016. View Article : Google Scholar
|
|
7
|
Lee WS, Park YL, Kim N, Oh HH, Son DJ, Kim
MY, Oak CY, Chung CY, Park HC, Kim JS, et al: Myeloid cell
leukemia-1 is associated with tumor progression by inhibiting
apoptosis and enhancing angiogenesis in colorectal cancer. Am J
Cancer Res. 5:101–113. 2014.PubMed/NCBI
|
|
8
|
Li HX, Fu XJ, Yang K, Chen D, Tang H and
Zhao Q: The clock gene PER1 suppresses expression of tumor-related
genes in human oral squamous cell carcinoma. Oncotarget.
7:20574–20583. 2016.PubMed/NCBI
|
|
9
|
Jara P, Calyeca J, Romero Y, Plácido L, Yu
G, Kaminski N, Maldonado V, Cisneros J, Selman M and Pardo A:
Matrix metalloproteinase (MMP)-19-deficient fibroblasts display a
profibrotic phenotype. Am J Physiol Lung Cell Mol Physiol.
308:L511–L522. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pan B, Ren H, Ma Y, Liu D, Yu B, Ji L, Pan
L, Li J, Yang L, Lv X, et al: High-density lipoprotein of patients
with type 2 diabetes mellitus elevates the capability of promoting
migration and invasion of breast cancer cells. Int J Cancer.
131:70–82. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rahman MA, Kim NH, Yang H and Huh SO:
Angelicin induces apoptosis through intrinsic caspase-dependent
pathway in human SH-SY5Y neuroblastoma cells. Mol Cell Biochem.
369:95–104. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bordin F, Dall'Acqua F and Guiotto A:
Angelicins, angular analogs of psoralens: chemistry, photochemical,
photobiological and phototherapeutic properties. Pharmacol Ther.
52:331–363. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mosti L, Lo Presti E, Menozzi G, Marzano
C, Baccichetti F, Falcone G, Filippelli W and Piucci B: Synthesis
of angelicin heteroanalogues: preliminary photobiological and
pharmacological studies. Farmaco. 53:602–10. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lampronti I, Bianchi N, Borgatti M, Fibach
E, Prus E and Gambari R: Accumulation of gamma-globin mRNA in human
erythroid cells treated with angelicin. Eur J Haematol. 71:189–195.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jeong D, Watari K, Shirouzu T, Ono M,
Koizumi K, Saiki I, Kim YC, Tanaka C, Higuchi R and Miyamoto T:
Studies on lymphangiogenesis inhibitors from Korean and Japanese
crude drugs. Biol Pharm Bull. 36:152–157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ryu BJ, Lee H, Kim SH, Heo JN, Choi SW,
Yeon JT, Lee J, Lee J, Cho JY, Kim SH, et al: PF-3758309,
p21-activated kinase 4 inhibitor, suppresses migration and invasion
of A549 human lung cancer cells via regulation of CREB, NF-κB, and
β-catenin signalings. Mol Cell Biochem. 389:69–77. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee AY, Fan CC, Chen YA, Cheng CW, Sung
YJ, Hsu CP and Kao TY: Curcumin inhibits invasiveness and
epithelial-mesenchymal transition in oral squamous cell carcinoma
through reducing matrix metalloproteinase 2, 9 and modulating
p53-E-cadherin pathway. Integr Cancer Ther. 14:484–490. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xiang T, Fei R, Wang Z, Shen Z, Qian J and
Chen W: Nicotine enhances invasion and metastasis of human
colorectal cancer cells through the nicotinic acetylcholine
receptor downstream p38 MAPK signaling pathway. Oncol Rep.
35:205–210. 2016.PubMed/NCBI
|
|
19
|
Lee D, Kim IY, Saha S and Choi KS:
Paraptosis in the anti-cancer arsenal of natural products.
Pharmacol Ther. 162:120–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ghate NB, Hazra B, Sarkar R, Chaudhuri D
and Mandal N: Alteration of Bax/Bcl-2 ratio contributes to
Terminalia belerica-induced apoptosis in human lung and breast
carcinoma. In Vitro Cell Dev Biol Anim. 50:527–537. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saiprasad G, Chitra P, Manikandan R and
Sudhandiran G: Hesperidin induces apoptosis and triggers autophagic
markers through inhibition of Aurora-A mediated
phosphoinositide-3-kinase/Akt/mammalian target of rapamycin and
glycogen synthase kinase-3 beta signalling cascades in experimental
colon carcinogenesis. Eur J Cancer. 50:2489–2507. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu F, Jiang YJ, Zhao HJ, Yao LQ and Chen
LD: Electroacupuncture ameliorates cognitive impairment and
regulates the expression of apoptosis-related genes Bcl-2 and Bax
in rats with cerebral ischaemia-reperfusion injury. Acupunct Med.
33:478–484. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ashkenazi A and Salvesen G: Regulated cell
death: signaling and mechanisms. Annu Rev Cell Dev Biol.
30:337–356. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Carneiro BA, Meeks JJ, Kuzel TM, Scaranti
M, Abdulkadir SA and Giles FJ: Emerging therapeutic targets in
bladder cancer. Cancer Treat Rev. 41:170–178. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Asanagi M, Yamada S, Hirata N, Itagaki H,
Kotake Y, Sekino Y and Kanda Y: Tributyltin induces G2/M cell cycle
arrest via NAD(+)-dependent isocitrate dehydrogenase in human
embryonic carcinoma cells. J Toxicol Sci. 41:207–215. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Arooz T, Yam CH, Siu WY, Lau A, Li KK and
Poon RY: On the concentrations of cyclins and cyclin-dependent
kinases in extracts of cultured human cells. Biochemistry.
39:9494–9501. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhen Q, Liu J, Gao L, Liu J, Wang R, Chu
W, Zhang Y, Tan G, Zhao X and Lv B: MicroRNA-200a targets EGFR and
c-Met to inhibit migration, invasion, and gefitinib resistance in
non-small cell lung cancer. Cytogenet Genome Res. 146:1–8. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lemieux E, Bergeron S, Durand V, Asselin
C, Saucier C and Rivard N: Constitutively active MEK1 is sufficient
to induce epithelial-to-mesenchymal transition in intestinal
epithelial cells and to promote tumor invasion and metastasis. Int
J Cancer. 125:1575–1586. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qin L, Liao L, Redmond A, Young L, Yuan Y,
Chen H, O'Malley BW and Xu J: The AIB1 oncogene promotes breast
cancer metastasis by activation of PEA3-mediated matrix
metalloproteinase 2 (MMP2) and MMP9 expression. Mol Cell Biol.
28:5937–5950. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Radnai B, Antus C, Racz B, Engelmann P,
Priber JK, Tucsek Z, Veres B, Turi Z, Lorand T, Sumegi B, et al:
Protective effect of the poly(ADP-ribose) polymerase inhibitor PJ34
on mitochondrial depolarization-mediated cell death in
hepatocellular carcinoma cells involves attenuation of c-Jun
N-terminal kinase-2 and protein kinase B/Akt activation. Mol
Cancer. 11:342012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Haas N, Riedt T, Labbaf Z, Baßler K,
Gergis D, Fröhlich H, Gütgemann I, Janzen V and Schorle H: Kit
transduced signals counteract erythroid maturation by
MAPK-dependent modulation of erythropoietin signaling and apoptosis
induction in mouse fetal liver. Cell Death Differ. 22:790–800.
2015. View Article : Google Scholar : PubMed/NCBI
|