Introduction
Prostate cancer (PCa) continues to be the most
frequently diagnosed cancer among males and is the cause of severe
health issues in males worldwide (1). According to 2015 statistics (2), PCa ranks second as a leading cause of
cancer-related deaths in men in the USA. Although most cases of PCa
are curable, there is still 10–20% of PCa patients with poor
prognosis who develop castration-resistant prostate cancer
(3). Generally, the risk factors
for PCa may be divided into an exogenous category, including aging,
oxidative stress and family, and an endogenous category, including
a rich fat diet and environmental agents (4,5). In
addition to the multiple risk factors, the prognosis following
traditional treatment modalities also varies greatly. The overall
survival time of patients may range from 15 years to 2 years
without a clear explanation (6,7). Thus,
it is difficult to provide accurate clinical recommendations for
PCa.
In recent years, studies regarding the dysregulation
of microRNAs in PCa have been extensively performed. Molecular
alterations associated with the miR regulation derived from these
investigations have provided various potential targets involved in
the oncogenesis and development of PCa (8–11). One
of these newly explored targets is the LIM and SH3 protein 1
(LASP-1), which encodes a membrane-bound protein of 261 amino acids
containing an N-terminal LIM domain (12,13).
Although the exact biological function of LASP-1 has yet to be
revealed, its association with multiple malignancies, including
breast, colorectal, hepatocellular and bladder cancer, is well
established (14–18). However, even with extensive interest
in the function of LASP-1 in carcinogenesis, little effort has been
made to elucidate the role of LASP-1 in PCa, not to mention
assessment of its potential as an anti-PCa therapy.
A well establishment pathway involved in the growth
of multiple types of tumor cells is nuclear transcription factor
NF-κB (19,20). This factor regulates the expression
of immediate-early and stress response genes which are mainly
implicated in acute inflammatory responses in various diseases. In
addition, regulation of the level of NF-κB has achieved
considerable outcome in the treatment of various cancer types
(21,22). Regarding PCa, the critical role of
NF-κB in the proliferation of PCa cells was also verified (23,24),
but to the best of our knowledge, no studies have given attention
to the association between LASP-1 and NF-κB in PCa. Thus, a
comprehensive study attempting to reveal the interaction between
the two indicators may promote the development of anti-PCa
therapies and the understanding of the mechanism of the
carcinogenesis of PCa.
In the present study, for the first time, the
expression level of LASP-1 in human PCa clinical samples was
investigated. Then, the expression of LASP-1 in human PCa cell
lines PC3 and DU145 was regulated by transfection of specific short
hairpin RNA (shRNA) and the influence of LASP-1 knockdown on cell
growth, cell cycle distribution, apoptosis, migration and invasion
was detected. To preliminarily reveal the mechanism through which
LASP-1 exerts its function in PCa cells, the expression levels of
NF-κB-related molecules were quantified by RT-qPCR and western blot
assay. It was expected that the function of LASP-1 in PCa and the
pathway through which LASP-1 contributes to the formation and
progression of PCa could be partially elucidated by the findings in
the present study.
Materials and methods
Chemicals and cell cultures
Antibodies against LASP-1, NF-κB subunit p65 (P65),
IκBα and GAPDH were purchased from Promega (Madison, WI, USA).
Human PCa cell lines DU145 and PC3 were obtained from the American
Type Culture Collection (ATCC; Manassas, VA, USA). DU145 cells were
cultured in Eagles minimum essential medium (EMEM)supplemented with
1.8 mM CaCl2 and PC3 cells were cultured in F-12 medium.
Both media were supplemented with 10% fetal bovine serum (FBS) and
1% penicillin/streptomycin. For experimental use, cells from three
to six passages were employed.
Patients and PCa specimen
collection
For detection of LASP-1 expression in clinical
samples, 15 pairs of PCa and para-carcinoma tissues were collected
from patients at The Second Hospital of Shandong University. All
the samples met the following criteria: i) carcinoma tissues
collected from nephrectomy were defined as primary prostatic
adenocarcinoma while para-carcinoma samples were collected from
kidney tissues of benign prostatic hyperplasia via renipuncture;
ii) detailed information was available concerning the
clinicopathological and prognostic characteristics of all the
patients enrolled in the present study (Table I). Patients who had undergone any
therapy for PCa before the surgical procedure were excluded. The
present study was approved by the Ethics Committee of the Second
Hospital of Shandong University. The Ethics Committee approved the
related screening, inspection, and data collection of the patients,
and all subjects signed a written informed consent form. All
studies were undertaken following the provisions of the Declaration
of Helsinki.
 | Table I.Clinicopathological information of the
patients employed for clinical tissue collection. |
Table I.
Clinicopathological information of the
patients employed for clinical tissue collection.
| No. | PCa or non-PCa | Age (years) | PSA (ng/ml) | Free/total PSA ratio
(f/t) | Gleason score |
|---|
| 1 | PCa | 68 | 133.2 | 0.14 | 5+4 |
| 2 | PCa | 55 | 1.25 | 0.26 | 4+5 |
| 3 | PCa | 72 | 43.0 | 0.11 | 4+4 |
| 4 | PCa | 83 | 14.0 | 0.10 | 4+3 |
| 5 | PCa | 70 | 89.6 | 0.05 | 4+4 |
| 6 | PCa | 65 | 28.6 | 0.14 | 3+4 |
| 7 | PCa | 75 | 209.6 | 0.04 | 4+4 |
| 8 | PCa | 83 | 31.3 | 0.07 | 4+4 |
| 9 | PCa | 62 | 6.29 | 0.12 | 3+3 |
| 10 | PCa | 67 | 7.5 | 0.22 | 3+3 |
| 11 | PCa | 74 | 10.7 | 0.15 | 3+3 |
| 12 | PCa | 75 | 100 | 0.56 | 4+5 |
| 13 | PCa | 83 | 575.6 | 0.06 | 4+4 |
| 14 | PCa | 78 | 152.1 | 0.06 | 4+4 |
| 15 | PCa | 76 | 9.47 | 0.06 | 2+3 |
| 1 | Non-PCa |
|
|
|
|
| 2 | Non-PCa |
|
|
|
|
| 3 | Non-PCa |
|
|
|
|
| 4 | Non-PCa |
|
|
|
|
| 5 | Non-PCa |
|
|
|
|
| 6 | Non-PCa |
|
|
|
|
| 7 | Non-PCa |
|
|
|
|
| 8 | Non-PCa |
|
|
|
|
| 9 | Non-PCa |
|
|
|
|
| 10 | Non-PCa |
|
|
|
|
| 11 | Non-PCa |
|
|
|
|
| 12 | Non-PCa |
|
|
|
|
| 13 | Non-PCa |
|
|
|
|
| 14 | Non-PCa |
|
|
|
|
| 15 | Non-PCa |
|
|
|
|
Knockdown of the LASP-1 gene in PCa
cells by shRNA
The target sequence for LASP-1-specific shRNA
(5′-GAAUCAAGAAGACCCAGGATT-3′) and the negative control (NC) shRNA
(5′-UUCUCCGAACGUGUCACGUTT-3′) were obtained from GeneChem Biotech
(Shanghai, China). Two PCa lines were grouped into two treatment
groups, respectively: cells treated with NC-shRNA and cells treated
with LASP-1-shRNA. Transfections were conducted using transfection
agents obtained from Applygen Technologies Inc. (Beijing, China)
(no. c1507) according to the manufacturer's instructions. Stable
transfected cells for further experiments were screened in medium
in the presence of G418 (0.5 µg/µl).
Real-time quantitative PCR
(RT-qPCR)
Whole RNA in the different samples was extracted
using RNAsimple Total RNA kit according to the manufacturers
instructions (no. DP419; Tiangen, Beijing, China). GAPDH was
selected as the reference gene. Then, the RNA was reversely
transcribing to cDNA templates using Super M-MLV Reverse
Transcriptase (no. RP6502; BioTeke, Beijing, China). The final
RT-qPCR reaction mixture of volume 20 µl consisted of 10 µl of
SYBR-Green Master Mix, 0.5 µl of each primer (LASP-1 forward,
5′-TTCCATTGCGAGACCTGC-3′ and reverse, 5′-TGCCACTACGCTGAAACCT-3′;
P65 forward, 5′-CTTACACTTAGCAATCATCCACCTT-3′ and reverse,
5′-GCAAATCCTCCACCACATCTT-3′; IκBα forward,
5′-CTGATGGAGTACCCTGAGGCTAT-3′ and reverse,
5′-TGTCCGCAATGGAGGAGAAGT-3′; GAPDH forward,
5′-TATGATGATATCAAGAGGGTAGT-3′ and reverse,
5′-TGTATCCAAACTCATTGTCATAC-3′), 1 µl of the cDNA template, and 8 µl
of RNase-free H2O. Thermal cycling parameters for the
amplification were set-up as follows: a denaturation step at 94°C
for 2 min, followed by 40 cycles at 94°C for 20 sec, 58°C for 20
sec and 72°C for 20 sec. Relative expression level of the targeted
gene was calculated with Exicycler™ 96 (Bioneer, Daejeon, Korea)
according to the method 2−ΔΔCt.
Western blot analysis
Protein product of different samples was extracted
using Whole Protein Extraction kit according to the manufacturers
instructions (WLA019; Wanleibio, Shenyang, China) and GAPDH was
used as a reference protein. The concentration of the extracted
protein samples was determined according to the BCA method. For
western blot assay, 40 µg of protein in 20 µl solution was subject
to a 13% sodium dodecylsulfate polyacrylamide gel electrophoresis
(SDS-PAGE), and the proteins were subsequently transferred onto
polyvinylidene difluoride (PVDF) membranes. Then, the membranes
were washed in TTBS for 5 min and incubated with 5% skim milk
powder solution for 1 h. The primary antibody against LASP-1
(1:1,500), P65 (1:2,000), IκBα (1:2,000) or GAPDH (1:1,000) was
added into the solution and incubated with the membranes at 4°C
overnight. The membranes were then washed with TTBS four times and
incubated with secondary IgG-HRP antibodies (1:20,000) for 45 min
at 37°C. After the final six washes with TTBS, the blots were
developed using Beyo ECL Plus reagent and the results were observed
using a gel imaging system. The relative expression levels of
LASP-1 in the different groups were calculated with Gel-Pro
Analyzer (Media Cybernetics, Inc., Rockville, MD, USA).
CCK-8 assay
The cell viability of the PCa cells in the different
groups was measured by Cell Counting Kit-8 (CCK-8) assay. In brief,
50 µl of exponentially growing cells (3×103 cells/ml)
were seeded into one well of a 96-well plate and cultured for 24 h.
Each group was represented by 20 replicates and the cell viability
of five randomly selected wells at 24, 48, 72 and 96 h was
determined, respectively. For each well, CCK-8 solution (10 µl) was
added and the cultures were incubated at 37°C for 1 h. The optical
density (OD) values at 450 nm in different wells were recorded
using a mircoplate reader.
Flow cytometry
Cell cycle distribution and apoptotic rates in the
different groups were determined using flow cytometry. Cells in the
different groups were collected using centrifugation at 2,000 rpm
for 5 min. Cell cycle distribution was detected according to
standard procedure. In brief, the cells were fixed with 70% alcohol
at 4°C for 2 h. Then, 500 µl propidium iodide (PI)-FITC was added
to the different samples to stain DNA in the dark at 4°C for 30
min. After a 20-min incubation at room temperature, the DNA
contents of the cells were analyzed using a flow cytometer (BD
Accuri C6; BD Biosciences, San Jose, CA, USA). Then, the cell
apoptotic rates were also measured using an Annexin V-FITC
apoptosis detection kit (WLA001c; Wanleibio) according to the
manufacturers instructions. In brief, 5 µl of Annexin V was added
to the different wells. After incubation with Annexin V for 10 min
at room temperature, the cells were resuspended with 1X binding
buffer and 5 µl PI was added. Then, the apoptotic rates were
analyzed using a FACScan flow cytometry (Accuri C6). The apoptotic
cells (UR + LR quadrants; the percentage of early and late
apoptotic cells) was equal to the sum of the late apoptotic cells
(UR, upper right quadrant; percentage of advanced stage apoptotic
cells) and the early apoptotic cells (LR, lower right quadrant;
percentage of prophase apoptotic cells).
Transwell experiment
The Transwell experiment which evaluated the
migration ability of PCa cells in the different groups was
performed. An amount of 200 µl incubation (with 1 mM
MgCl2) medium containing 1×104 cells were
seeded into the upper chamber of Transwell chambers (Corning Star,
Cambridge, MA, USA). Then, the cells were incubated at 37°C for 24
h to allow migration through the porous membrane. Upon completion
of the culture, the cells remaining on the upper surface of the
chamber were completely removed. The lower surfaces of the
membranes were fixed with 4% paraformaldehyde for 20 min and
stained in a solution containing 0.5% (w/v) crystal violet for 5
min. After being washed using ddH2O, the numbers of
cells in the different groups were determined using Image-Pro Plus
6.0 software (Nikon, Tokyo, Japan). Then, the invasion ability of
the PCa cells was measured as described above with polycarbonate
membranes being previously coated with 40 µl Matrigel (1.5 mg/ml;
BD Biosciences) at 37°C for 2 h to form a reconstituted basement
membrane.
Statistical analysis
All the data are expressed in the form of mean ± SD.
Students t-test was performed and a significant level of 0.05 was
assigned. Statistical analysis was carried out using GraphPad Prism
6 (GraphPad Software, Inc., San Diego, CA, USA).
Results
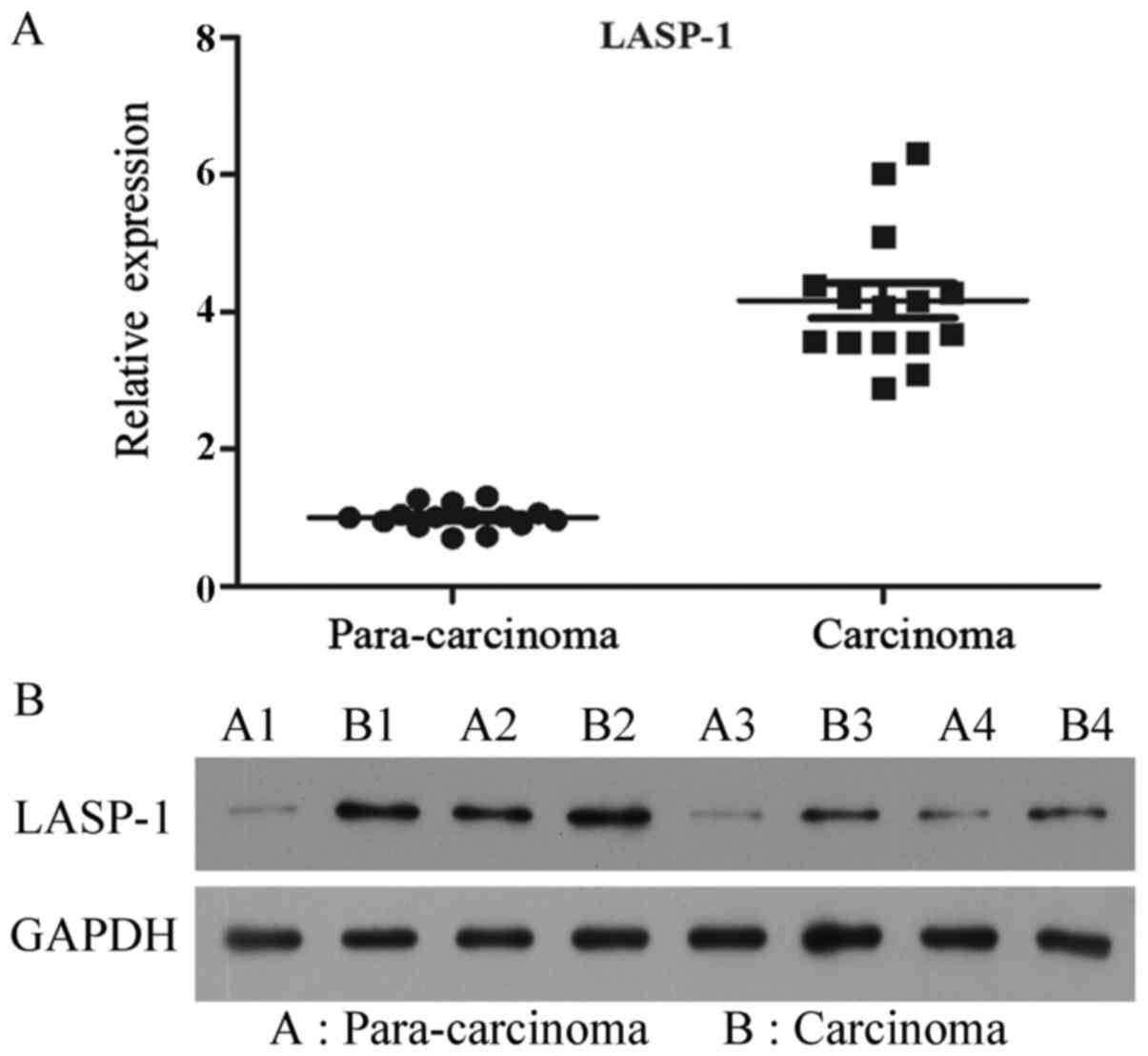
LASP-1 expression in clinical samples
is upregulated
To investigate the status of expression of LASP-1 in
clinical samples, the mRNA and protein levels in 15 pairs of PCa
specimens and corresponding para-carcinoma tissues were detected.
All the PCa samples showed upregulation of LASP-1 expression when
compared with the levels in their corresponding para-carcinoma
tissues, and the average difference between carcinoma and
para-carcinoma samples was statistically significant (P<0.05)
(Fig. 1).
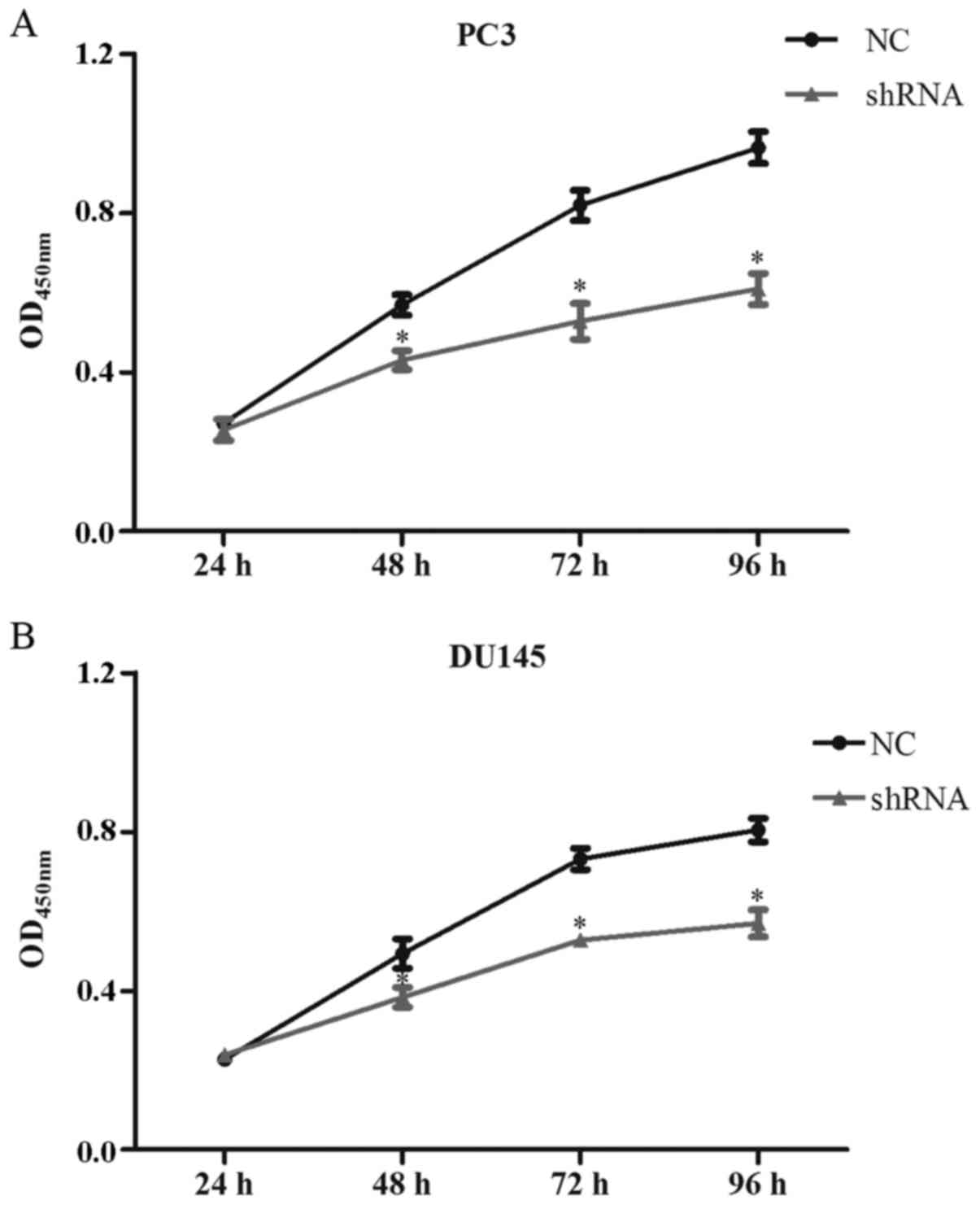
LASP-1 knockdown attenuates the
viability of the PC3 and DU145 cells
The cell viability of the cells following the
different treatments was quantified using the CCK-8 assay. As shown
in Fig. 2A, compared with control
and NC groups, transfection of LASP-1-specific shRNA decreased the
proliferation of the PC3 cells in the shRNA group at 48 h of the
assay, and the differences between NC and shRNA groups were
statistically significant for the last three time points
(P<0.05) (Fig. 2A). Similar
results were also detected for the DU145 cells. Inhibition of
LASP-1 in the PCa cells significantly decreased the cell viability
(Fig. 2B).
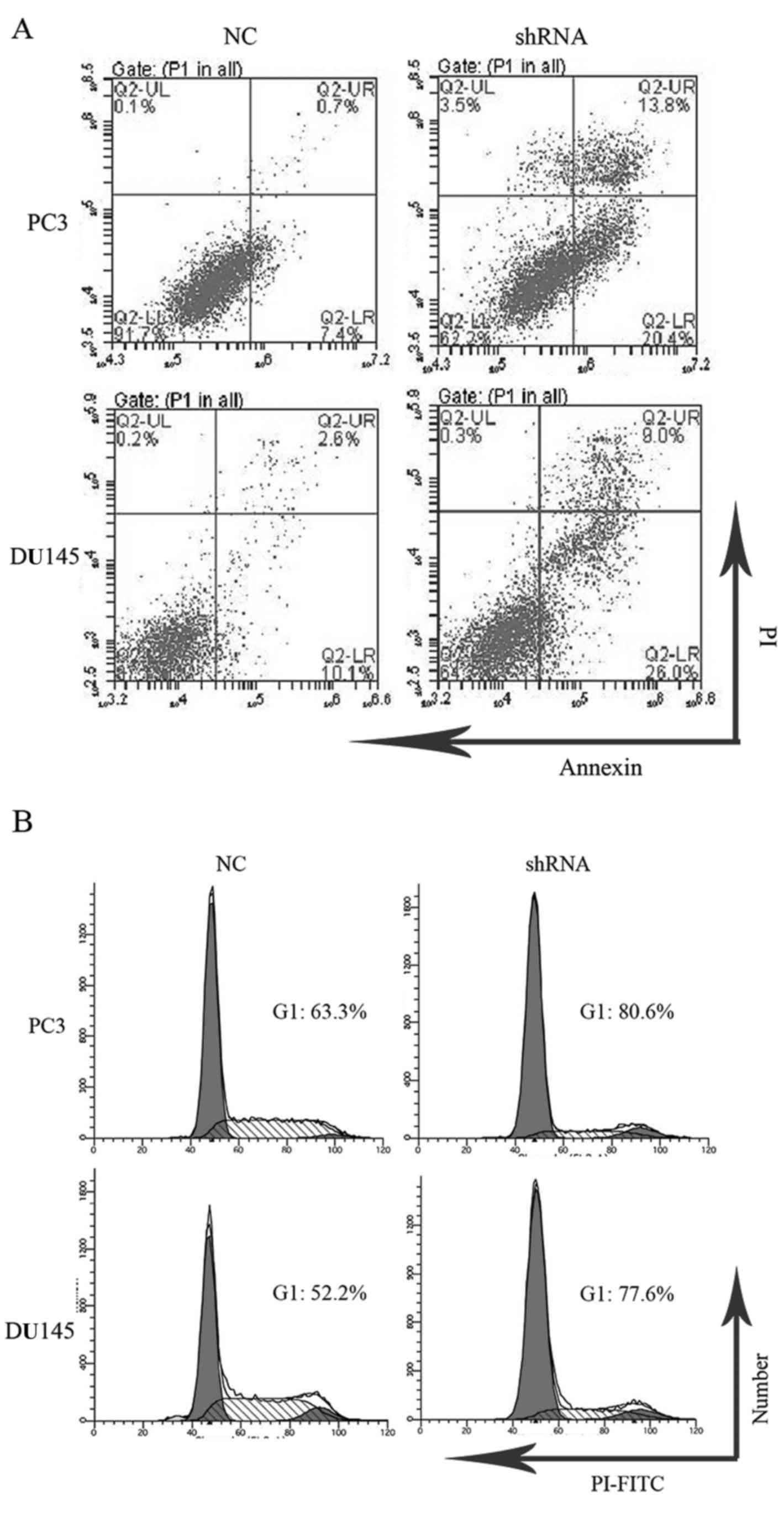
LASP-1 knockdown increases cell
apoptosis and induces cell cycle arrest in the PC3 and DU145
cells
Apoptosis and cell cycle distribution of cells in
the different groups were analyzed by flow cytometry. After
knockdown of LASP-1, late apoptotic (13.8±1.3%) and early apoptotic
rates (20.4±3.1%) of the PC3 cells transfected with LASP-1-specific
shRNA were increased compared with those in the NC groups
(0.7±0.02% for late apoptotic rate and 7.4±1.7% for early apoptotic
rate) (Fig. 3A). Similar changes
were observed for DU145 cells as well (Fig. 3A). As illustrated in Fig. 3B, PC3 cells following LASP-1
knockdown treatment showed G1 arrest by increasing the percentage
of cells in the G1 phase (80.6±2.3%), which was markedly different
from the results of the NC group (63.4±1.9%). The downregulation of
LASP-1 in the PC3 cells also resulted in a concomitant decrease in
the fraction of cells in the S phase, revealing that inhibition of
LASP-1 could halt the cell proliferation of PC3 cells via cell
cycle arrest at the G1 phase. A similar change in the pattern of
cell cycle distribution was also recorded for the DU145 cells
(Fig. 3B).
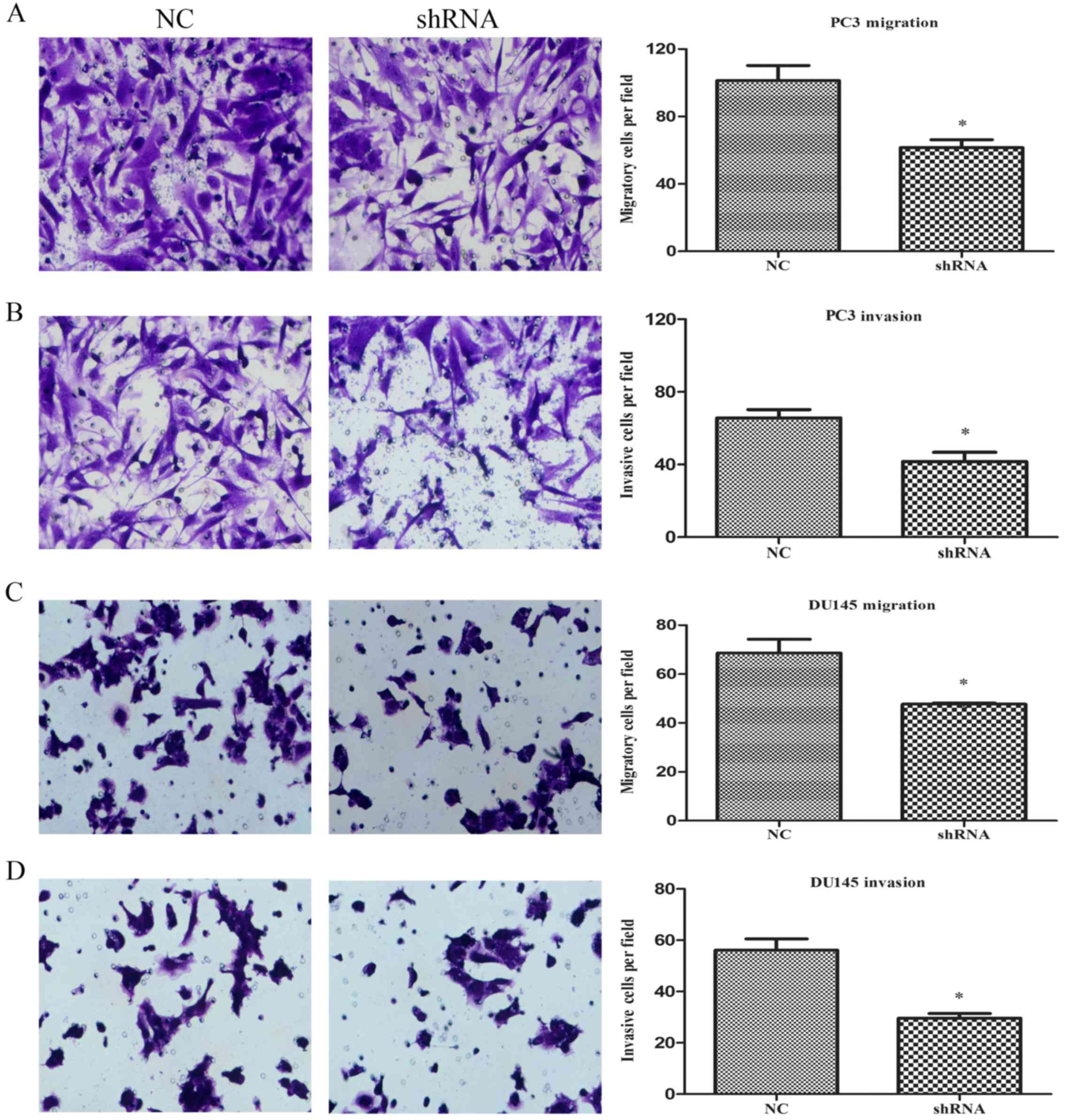
LASP-1 knockdown decreases cell
migration and invasion ability of the PC3 and DU145 cells
The effect of LASP-1 on the migration of PCa cells
was determined using Transwell assay. For measurement of the cell
invasion ability, the polycarbonate membranes were previously
coated with Matrigel to form a reconstituted basement membrane. As
shown in Fig. 4A and B, the numbers
of cells migrating through the porous membrane in the shRNA group
(62±4 for migration and 42±2 for invasion assays) were
significantly lower than those in the NC group (102±4 for migration
assay and 62±2 for invasion assay) (P<0.05), indicating an
inhibitory effect on the cell motile ability due to the knockdown
of LASP-1. Similar to the migration and invasion abilities of the
PC3 cells, knockdown of LASP-1 in DU145 cells also resulted in a
decrease in migration and invasion abilities (Fig. 4C and D).
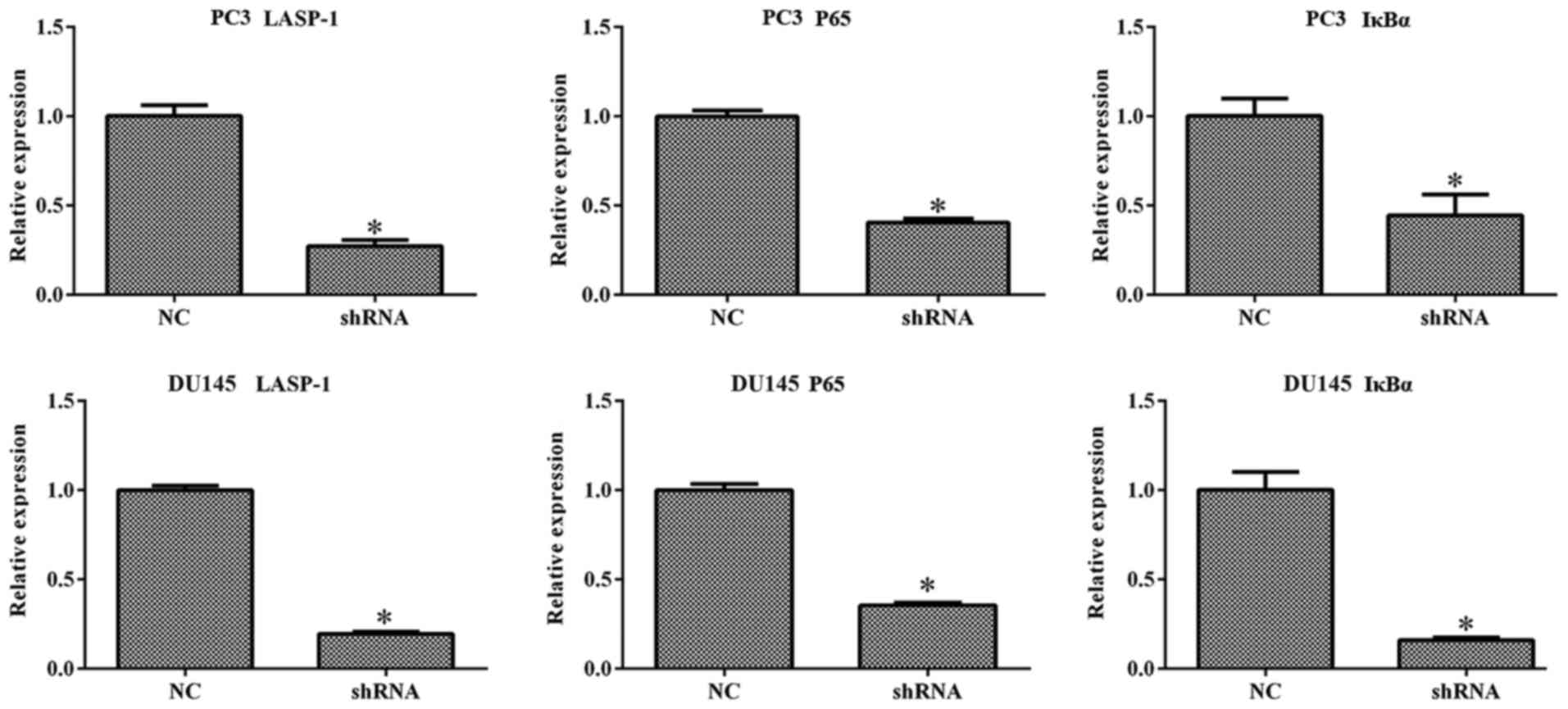
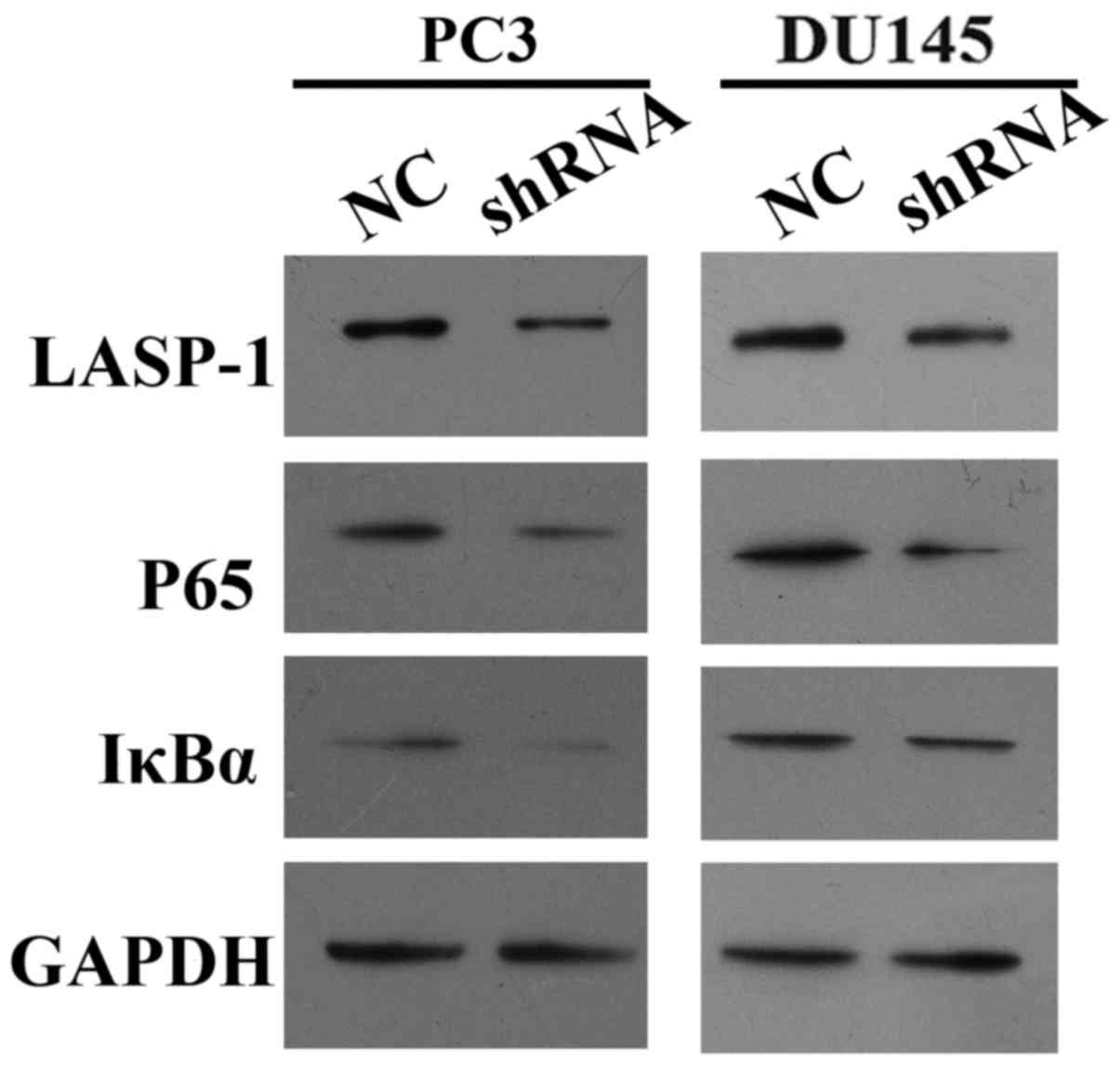
LASP-1 knockdown downregulates the
expression of NF-κB-related molecules
To explore the mechanism which drives the effect of
LASP-1 knockdown on the biological alterations in PCa cells, the
expression of LASP-1, P65 and IκBα was quantified with RT-qPCR and
western blot assay. It was found that inhibition of LASP-1
suppressed the expression of all three indicators at both the mRNA
and protein levels (Figs. 5 and
6). Based on the quantitative
analysis, the difference between shRNA and NC groups was
statistically significant (P<0.05). The result of RT-qPCR and
western blot assay demonstrated that LASP-1 may play a role in the
carcinogenesis of PCa through a NF-κB-dependent manner.
Discussion
Despite the progress in the treatment of prostate
cancer in the past few decades, PCa remains a major cause of health
issues worldwide among males. Traditional treatments including
surgery and radiation therapies seem to be less effective against
PCa cases once metastasis occurs. Thus, identification of the
biological markers related to oncogenesis and mobility of PCa cells
is crucial for the development of therapeutic strategies to improve
the outcome and survival of this malignant cancer. In the present
study, the expression of an important tumor-associated factor,
LASP-1 in clinical PCa tissues and its function in the
carcinogenesis of PCa were investigated for the first time. It was
demonstrated that both LASP-1 mRNA and protein levels were
significantly upregulated in clinical PCa tissues. Experimental
observation based on the knockdown of the LASP-1 gene in human PCa
cell lines PC3 and DU145 also clearly inferred that while growth
and movement of PCa cells were attenuated, the apoptotic process
was induced. All of these results suggest a key role of LASP-1 in
the proliferation and motility of PCa cells.
The central role of LASP-1 in the progression and
metastasis of various types of cancers was previously investigated
(14–18). Results of the present study further
support the conclusions of these studies by verifying the important
function of LASP-1 in PCa. In clinical PCa samples, the production
of LASP-1 at the mRNA and proteins levels was enhanced. Moreover,
knockdown of LASP-1 in human PCa cell lines resulted in a
deteriorating effect on cell growth. Furthermore, flow cytometric
assay also showed that the decrease in cell proliferation in
response to LASP-1-specific shRNA treatment was accompanied by the
induction of cell apoptosis and G1 phase cell cycle arrest.
However, contradiction regarding the function of LASP-1 in cancer
cell viability still exists. According to the study of Grunewald
et al (14), no significant
influence of LASP-1 knockdown on the apoptotic process in BT-20 and
MCF-7 cell lines was observed. Although the two studies focused on
distinct cancer types, this information is indicative of the
complicated mechanism of LASP-1 in regulating cancer cell
apoptosis.
LASP-1 is a well-known focal adhesion adaptor
protein and is closely associated with the modulation of
cytoskeleton and cancer cell migration and invasion (12,25–27).
The factor is capable of interacting and co-localizing with a
series of focal adhesion proteins, such as F-actin, zyxin and LPP.
Thus, silencing of LASP-1 causes cellular localization change of
its binding partners at the focal adhesion site and influences the
mobility of cells. In the present study, it was found that cells
treated with LASP-1-specific shRNA exhibited a significant decrease
in cell migration and invasion ability, which proved the critical
function of LASP-1 in the metastasis of cancer cells as well.
However, as previously reported, cells depleted of LASP-1 may still
attach to the extracellular matrix (ECM) and form focal adhesions.
It is hypothesized that the molecule may play a supportive role in
focal adhesion dynamics rather than actual formation of the related
structures (25).
To further elucidate the mechanism through which
LASP-1 exerts its function in the onset and development of PCa, the
interaction between LASP-1 and NF-κB was investigated. NF-κB is a
transcriptional factor which regulates the apoptosis and
inflammation in various diseases (28). The inactive form of NF-κB is
sequestered in the cytoplasm by binding with IκBα. Once IκBα is
phosphorylated into p-IκBα, P65 may be translocated into the
nucleus and activates transcription of the targeted genes. In the
present study, the results showed that LASP-1-specific shRNA
reduced NF-κB production and P65 translocation to the nucleus,
which would lead to the inhibition of NF-κB activity. In fact,
activation of NF-κB has been implicated in both carcinogenesis and
drug resistance in cancer cells (29). Thus, inhibition of NF-κB activation
may represent a promising opportunity for expanding therapeutic
windows in translational cancer research (30,31).
Considering the high efficiency of LASP-1 knockdown in attenuating
NF-κB activity in the present study, it was reasonable to
demonstrate that therapies based on LASP-1 interference may
antagonize PCa through a NF-κB inhibition-dependent manner.
Conclusively, the major findings outlined in the
present study elucidated that the expression of LASP-1 was
abnormally high in clinical PCa tissues, and following knockdown of
LASP-1 in human PCa cell lines, the proliferation and mobility of
cancer cells were markedly inhibited. Preliminarily, it was
hypothesized that LASP-1 may activate the carcinogenesis of PCa
through an NF-κB-dependent manner. Although a detailed explanation
of the effect of LASP-1 on PCa was not fully elucidated, the
present study highlights that LASP-1 has the potential to be a
promising target for alleviation of PCa in the clinic. To elaborate
the role of LASP-1 in PCa, more comprehensive studies need to be
conducted in the future.
Acknowledgements
The present study was supported by the Science and
Technology Development Plan Project of Medical and Health in
Shandong Province (no. 2007QW016), the Seed Fund of the Second
Hospital of Shandong University and the Key Clinical Program of the
Ministry of Health.
References
|
1
|
Howlader N, Noone AM, Krapcho M, Neyman N,
Aminou R, Waldron W, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z,
et al: SEER Cancer Statistics Review, 1975–2008. National Cancer
Institute; Bethesda, MD: 2011, http://seer.cancer.gov/csr/1975_2008/
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu Y, Yang XQ, Han CT, Dai B, Zhang HL,
Shi GH, Wang CF and Ye DW: Pathological features of localized
prostate cancer in China: A contemporary analysis of radical
prostatectomy specimens. PLoS One. 10:e01210762015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Garg M, Dalela D, Goel A, Kumar M and
Sankhwar SN: Prevention of prostate cancer with vitamins - current
perspectives. Asian Pac J Cancer Prev. 15:1897–1904. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Euling SY and Kimmel CA: Developmental
stage sensitivity and mode of action information for androgen
agonists and antagonists. Sci Total Environ. 274:103–113. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bickers B and Aukim-Hastie C: New
molecular biomarkers for the prognosis and management of prostate
cancer - the post PSA era. Anticancer Res. 29:3289–3298.
2009.PubMed/NCBI
|
|
7
|
Siddiqui E, Mumtaz FH and Gelister J:
Understanding prostate cancer. J R Soc Promot Health. 124:219–221.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hudson RS, Yi M, Esposito D, Watkins SK,
Hurwitz AA, Yfantis HG, Lee DH, Borin JF, Naslund MJ, Alexander RB,
et al: MicroRNA-1 is a candidate tumor suppressor and prognostic
marker in human prostate cancer. Nucleic Acids Res. 40:3689–3703.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hailer A, Grunewald TG, Orth M, Reiss C,
Kneitz B, Spahn M and Butt E: Loss of tumor suppressor mir-203
mediates overexpression of LIM and SH3 protein 1 (LASP1) in
high-risk prostate cancer thereby increasing cell proliferation and
migration. Oncotarget. 5:4144–4153. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nishikawa R, Goto Y, Sakamoto S, Chiyomaru
T, Enokida H, Kojima S, Kinoshita T, Yamamoto N, Nakagawa M, Naya
Y, et al: Tumor-suppressive microRNA-218 inhibits cancer cell
migration and invasion via targeting of LASP1 in prostate cancer.
Cancer Sci. 105:802–811. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Viticchiè G, Lena AM, Latina A, Formosa A,
Gregersen LH, Lund AH, Bernardini S, Mauriello A, Miano R, Spagnoli
LG, et al: MiR-203 controls proliferation, migration and invasive
potential of prostate cancer cell lines. Cell Cycle. 10:1121–1131.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chew CS, Chen X, Parente JA Jr, Tarrer S,
Okamoto C and Qin HY: Lasp-1 binds to non-muscle F-actin in vitro
and is localized within multiple sites of dynamic actin assembly in
vivo. J Cell Sci. 115:4787–4799. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakagawa H, Terasaki AG, Suzuki H, Ohashi
K and Miyamoto S: Short-term retention of actin filament binding
proteins on lamellipodial actin bundles. FEBS Lett. 580:3223–3228.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grunewald TG, Kammerer U, Schulze E,
Schindler D, Honig A, Zimmer M and Butt E: Silencing of LASP-1
influences zyxin localization, inhibits proliferation and reduces
migration in breast cancer cells. Exp Cell Res. 312:974–982. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao L, Wang H, Liu C, Liu Y, Wang X, Wang
S, Sun X, Li J, Deng Y, Jiang Y, et al: Promotion of colorectal
cancer growth and metastasis by the LIM and SH3 domain protein 1.
Gut. 59:1226–1235. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Salvi A, Bongarzone I, Miccichè F, Arici
B, Barlati S and De Petro G: Proteomic identification of LASP-1
down-regulation after RNAi urokinase silencing in human
hepatocellular carcinoma cells. Neoplasia. 11:207–219. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang H, Li W, Jin X, Cui S and Zhao L: LIM
and SH3 protein 1, a promoter of cell proliferation and migration,
is a novel independent prognostic indicator in hepatocellular
carcinoma. Eur J Cancer. 49:974–983. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chiyomaru T, Enokida H, Kawakami K,
Tatarano S, Uchida Y, Kawahara K, Nishiyama K, Seki N and Nakagawa
M: Functional role of LASP1 in cell viability and its regulation by
microRNAs in bladder cancer. Urol Oncol. 30:434–443. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miyamoto S and Verma IM: Rel/NF-kappa B/I
kappa B story. Adv Cancer Res. 66:255–292. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bargou RC, Emmerich F, Krappmann D,
Bommert K, Mapara MY, Arnold W, Royer HD, Grinstein E, Greiner A,
Scheidereit C, et al: Constitutive nuclear factor-kappaB-RelA
activation is required for proliferation and survival of Hodgkin's
disease tumor cells. J Clin Invest. 100:2961–2969. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pikarsky E, Porat RM, Stein I, Abramovitch
R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E and
Ben-Neriah Y: NF-kappaB functions as a tumour promoter in
inflammation-associated cancer. Nature. 431:461–466. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim SM, Lee SY, Yuk DY, Moon DC, Choi SS,
Kim Y, Han SB, Oh KW and Hong JT: Inhibition of NF-kappaB by
ginsenoside Rg3 enhances the susceptibility of colon cancer cells
to docetaxel. Arch Pharm Res. 32:755–765. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Suh J, Payvandi F, Edelstein LC, Amenta
PS, Zong WX, Gélinas C and Rabson AB: Mechanisms of constitutive
NF-kappaB activation in human prostate cancer cells. Prostate.
52:183–200. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Herrmann JL, Beham AW, Sarkiss M, Chiao
PJ, Rands MT, Bruckheimer EM, Brisbay S and McDonnell TJ: Bcl-2
suppresses apoptosis resulting from disruption of the NF-κB
survival pathway. Exp Cell Res. 237:101–109. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Butt E, Gambaryan S, Göttfert N, Galler A,
Marcus K and Meyer HE: Actin binding of human LIM and SH3 protein
is regulated by cGMP- and cAMP-dependent protein kinase
phosphorylation on serine 146. J Biol Chem. 278:15601–15607. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li B, Zhuang L and Trueb B: Zyxin
interacts with the SH3 domains of the cytoskeletal proteins
LIM-nebulette and Lasp-1. J Biol Chem. 279:20401–20410. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schreiber V, Moog-Lutz C, Régnier CH,
Chenard MP, Boeuf H, Vonesch JL, Tomasetto C and Rio MC: Lasp-1, a
novel type of actin-binding protein accumulating in cell membrane
extensions. Mol Med. 4:675–687. 1998.PubMed/NCBI
|
|
28
|
Mattson MP: NF-kappaB in the survival and
plasticity of neurons. Neurochem Res. 30:883–893. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Y, Ellis KL, Ali S, El-Rayes BF,
Nedeljkovic-Kurepa A, Kucuk O, Philip PA and Sarkar FH:
Apoptosis-inducing effect of chemotherapeutic agents is potentiated
by soy isoflavone genistein, a natural inhibitor of NF-kappaB in
BxPC-3 pancreatic cancer cell line. Pancreas. 28:e90–e95. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qin Z-H, Wang Y, Nakai M and Chase TN:
Nuclear factor-κ B contributes to excitotoxin-induced apoptosis in
rat striatum. Mol Pharmacol. 53:33–42. 1998.PubMed/NCBI
|
|
31
|
Beg AA and Baltimore D: An essential role
for NF-kappaB in preventing TNF-alpha-induced cell death. Science.
274:782–784. 1996. View Article : Google Scholar : PubMed/NCBI
|