Introduction
Colorectal cancer (CRC) is the third most common
cancer worldwide with an estimated 1.4 million cases and 693,900
deaths in 2012 (1). Its incidence
in many developing countries has increased 2- to 4-fold over the
past two decades (2). Surgical
resection and adjuvant chemotherapy are the main CRC treatments.
However, primary or acquired resistance is a major challenge to
successful treatment (3). New
therapeutic strategies and/or new adjuvant drugs are urgently
needed.
5-Fluorouracil (5-FU), a cytostatic drug, is the
most frequently used therapy and the backbone in CRC treatment.
However, the response rates for 5-FU-based chemotherapy for
advanced CRC are only 10–15%. The combination of 5-FU with newer
chemotherapies, such as irinotecan and oxaliplatin, improved the
response rates to 40–59% (4). In
the 5-FU metabolic pathway, four enzymes, including thymidylate
synthase, methylenetetrahydrofolate reductase, dihydropyrimidine
dehydrogenase and thymidine phosphorylase, are considered important
to determining the sensitivity or resistance; however, data from
many studies have been contradictory (5). Recently, amplified insulin-like growth
factor-1 (IGF-1)/IGF-1R signaling has been reported as critical for
developing CRC, and it contributes to CRC cell survival, invasion,
metastasis and resistance to chemotherapeutic drugs by activating
survival pathways, such as the phosphatidylinositol 3-kinase
(PI3K)/Akt pathway (6–9).
Accumulating evidence suggests that microRNAs
(miRNAs) are involved in many biological pathways and
cancer-pertinent cellular processes, including cell proliferation,
apoptosis, migration, invasion, stemness and chemoresistance
(10,11). Many studies have shown that miRNAs
can modulate the sensitivity of cancer cells to anticancer drugs in
substantial ways (12–15). miRNA-302a (miR-302a) belongs to the
miR-302-367 cluster, which includes miR-302b, miR-302c, miR-302a,
miR-302d and miR-367. This cluster was first identified in human
embryonic stem cells (hESCs) and their malignant counterparts,
human embryonic carcinoma cells (hECCs), and it has been reported
to help maintain stemness and reprograming of somatic cells into
induced pluripotent stem cells (iPSCs) by suppressing several key
regulators of the G1/S transition, such as p21 and CCND (16,17).
In contrast, Lin et al reported that the subclass,
miR-302a-d, inhibited tumorigenicity and induced apoptosis in
various tumors and cancer cells, including MCF7 breast cancer
cells, HepG2 hepatocellular carcinoma cells, and Tera-2 embryonal
teratocarcinoma cells by suppressing the CCNE-CDK2 and CCND-CDK4/6
pathways (18). Zhang et al
also suggested that miR-302a inhibited prostate cancer cell
proliferation by targeting Akt (19). Liu et al suggested that
miR-302a sensitized testicular embryonal carcinoma cells to
cisplatin-induced cell death in part through downregulating p21
(20). However, the roles of
miR-302a in 5-FU treatment in CRC are unknown.
In this study, we used two human colon
cancer-derived cell lines, HCT116 and HT29, and evaluated the
influence of miR-302a on 5-FU-induced cell death and viability
inhibition. With bioinformatics tools, we speculated that IGF-1R is
a novel target of miR-302a, which was further confirmed using
luciferase reporter assays and immunoblotting. Then, we designed
siRNA against IGF-1R and observed that si-IGF-1R resembled the
influence of miR-302a on 5-FU treatment. Thus, targeting miR-302a
may offer new therapeutic interventions in CRC.
Materials and methods
Materials
5-FU was purchased from Sigma-Aldrich (St. Louis,
MO). Anti-IGF-1R, anti-caspase-3, and anti-phosphorylated Akt
antibodies were obtained from Cell Signaling Technology, Inc.
(Beverly, MA, USA). Anti-cleaved PARP was purchased from Abcam
(Cambridge, UK). Anti-Akt was from ProteinTech (Wuhan, China) and
anti-β-actin was from Santa Cruz Biotechnology, Inc. (Dallas, TX,
USA).
Cell culture
Human colon cancer cells (HCT116 and HT29) were a
gift from Professor H. Kuwano (Gunma University, Gunma, Japan). The
cells were cultured in RPMI-1640 medium (HyClone, Logan, UT, USA)
supplemented with 10% fetal bovine serum (Biological Industries,
Beit Haemek, Israel) at 37°C in a humidified atmosphere of 95% air
and 5% CO2 with medium change every 2 days. Cells in the
mid-log phase were used in this study.
Patients and tissue samples
Surgically removed paired CRC tumor tissues and
adjacent normal mucosal tissues were collected from 24 patients at
the Second Affiliated Hospital of Xi'an Jiaotong University, and
they were used for quantitative real-time polymerase chain reaction
(qRT-PCR) analysis. Surgically resected tissues were quickly frozen
in liquid nitrogen until analysis. All samples were obtained with
the informed consent of the patients. This study was approved by
the Medical Ethics Committee of the Xi'an Jiaotong University.
Plasmid construction and
oligonucleotides
Human pre-miR-302a (MI0000738) (Table I) was chemically synthesized by
Sangon Biotech (Shanghai, China) and cloned into the multiple
cloning site (MCS) of pcDNA6.2-GW-miR vector (Invitrogen, Carlsbad,
CA, USA), between EcoRI and HindIII, resulting in the
pcDNA 6.2-pre-miR-302a construct (named by us). Empty
pcDNA6.2-GW-miR vector was used as the control. pmirGLO
dual-luciferase miRNA target expression vector (pmirGLO vector;
Promega, Madison, WI, USA) was used to generate luciferase UTR
reporter constructs. Specified fragments of the 3′UTR of human
IGF-1R (Fig. 3A) were chemically
synthesized and inserted into the MCS of the pmirGLO vector between
SacI and XhoI, resulting in a plasmid that we named
pmirGLO-IGF-1R-3′UTRWT. The mutant fragment of the 3′UTR of human
IGF-1R (Fig. 3A) was also
synthesized and inserted into the pmirGLO vector, resulting in a
pmirGLO-IGF-1R-3′UTRMut plasmid. All subcloned fragments were
directly sequenced. Small interfering RNA (siRNA) against IGF-1R
(5′-CAAUGAGUACAACUACCGCUU-3′) and the scrambled siRNA, as control,
were obtained from Gene-Pharma Co. (Shanghai, China). Plasmids and
siRNAs were transfected with Lipofectamine 2000 (Invitrogen)
according to the manufacturer's instructions.
 | Table I.Primers and oligonucleotides used in
this study. |
Table I.
Primers and oligonucleotides used in
this study.
| Name | Primer sequences
(5→3) |
|---|
| pre-miR-302a
sense |
AATTCCCACCACTTAAACGTGGATGTACTTGCTTTGAAACTAAAGAAGTAAGTGCTTCCATGTTTTGGTGATGGA |
| pre-miR-302a
antisense |
AGCTTCCATCACCAAAACATGGAAGCACTTACTTCTTTAGTTTCAAAGCAAGTACATCCACGTTTAAGTGGTGGG |
| miR-302a RT |
GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACTCACCAA |
| miR-302a F |
ATCCAGTGCGTGTCGTGGA |
| miR-302a R |
AGACGTAAGTGCTTCCATGTT |
| U6 RT |
CGCTTCACGAATTTGCGTGTCAT |
| U6 F |
GCTTCGGCAGCACATATACTAAAAT |
| U6 R |
CGCTTCACGAATTTGCGTGTCAT |
| si-IGF-1R
sense |
CAAUGAGUACAACUACCGCUU |
| si-IGF-1R
antisense |
GCGGUAGUUGUACUCAUUGUU |
| si-Ctrl sense |
UUCUCCGAACGUGUCACGUTT |
| si-Ctrl
antisense |
ACGUGACACGUUCGGAGAATT |
RNA isolation and qRT-PCR
Total cellular RNA was extracted using TRIzol
(Invitrogen) and a miRNeasy mini kit (Qiagen) according to the
manufacturer's instructions; miRNA levels were measured with
qRT-PCR. Mature miRNAs were reverse transcribed into cDNA by
stem-loop reverse transcription using the PrimeScript RT reagent
kit (Takara, Otsu, Japan) and specific stem-loop primers, as shown
in Table I; qRT-PCR was performed
using FastStart Essential DNA Green Master on a light cycle 96
machine (both from Roche) according to the manufacturer's
instructions. The relative expression of miRNA to internal control
(U6 RNA) was calculated using the 2−ΔΔCt method. The
primers used are listed in Table
I.
Cell viability assay
Cell viability was determined by Trypan blue
dye-exclusion assay. Cells were seeded at a concentration of
5×105 cells/well into a 6-well plate. After treatment,
they were collected and incubated with 0.4% Trypan blue dye. The
stained and unstained cells were separately observed and counted on
a hemocytometer under a light microscope (21). Cell viability was calculated
according to the following formula: Cell viability (%) = (unstained
cell number/total cell number for control) ×100. Cell death was
calculated using the formula: Cell death (%) = (stained cell
number/total cell number) ×100.
Cell apoptosis assay
Cell apoptosis was assessed using an Annexin V-FITC
apoptosis detection kit (Invitrogen) according to the
manufacturer's instructions. Cells were harvested and stained with
Annexin V and fluorescein isothiocyanate. Then, cell apoptosis was
examined using a flow cytometer (FACSCalibur), and histograms were
analyzed using ModFit software (both from Becton-Dickinson,
Franklin Lakes, NJ, USA).
Dual luciferase assay
PmirGLO-IGF-1R-3UTRWT and pmirGLO-IGF-1R-3UTRMut
were, respectively, co-transfected with pcDNA 6.2-pre-miR-302a or
pcDNA6.2 control vector into 293 cells in a 96-well plate using
Lipofectamine 2000 (Invitrogen). At 48 h post-transfection, cells
were lysed in 100 µl of passive lysis buffer (Promega) and assayed
according to the manufacturer's instructions. For each
transfection, luciferase activity was averaged from five
replicates. Luciferase activities were expressed as the ratio of
Firefly to Renilla luciferase activity and normalized to the
control level.
Western blot analysis
Cells were harvested from culture dishes and lysed
in RIPA buffer supplemented with protease inhibitors and
phosphatase inhibitors (Invitrogen). Protein concentrations were
determined using a BCA protein assay kit (Pierce). Cell lysates (20
µg protein/lane) were separated by SDS-PAGE for PVDF membrane
blotting. The blotted membranes were blocked with 5% skim milk for
30 min and incubated with primary antibodies (1:1,000 dilutions).
The immunoreactive bands were visualized by enhanced
chemiluminescence using horseradish peroxidase-conjugated IgG
secondary antibodies. The band intensity was measured by
densitometry and then quantified using Gel Plotting Macros of NIH
image 1.62 and normalized to the indicated sample in the same
membrane.
Bioinformative analysis
RegRNA (A regulatory RNA Motifs and Elements Finder
http://regrna.mbc.nctu.edu.tw/) and
TargetScan (http://www.targetscan.org/) were used for gene-related
specified microRNA prediction. Through bioinformatics analysis, we
obtained the predicted fragment of the targeted gene (IGF-1R),
which was associated with miR-302a. DIANA-miRPath v2.0 (http://www.microrna.gr/miRPathv2) was applied to
analyze the main functions of miR-302a (22). The one-tailed Fisher's exact test
was used to identify the enriched KEGG pathways with targets of
miR-302a, and the false discovery rate (FDR) was calculated to
correct the p-value. Enrichment provides a measure of the
significance of the function; as the enrichment increases, the
corresponding function is more significant.
Statistical analysis
Experiments were independently performed with three
replicates, and data were expressed as the means ± the standard
deviation (SD). Statistical analysis was performed using Student's
t-test, and P<0.05 was considered statistically significant.
Results
miR-302a enhances 5-FU-induced
viability inhibition in HT29 and HCT116 cells
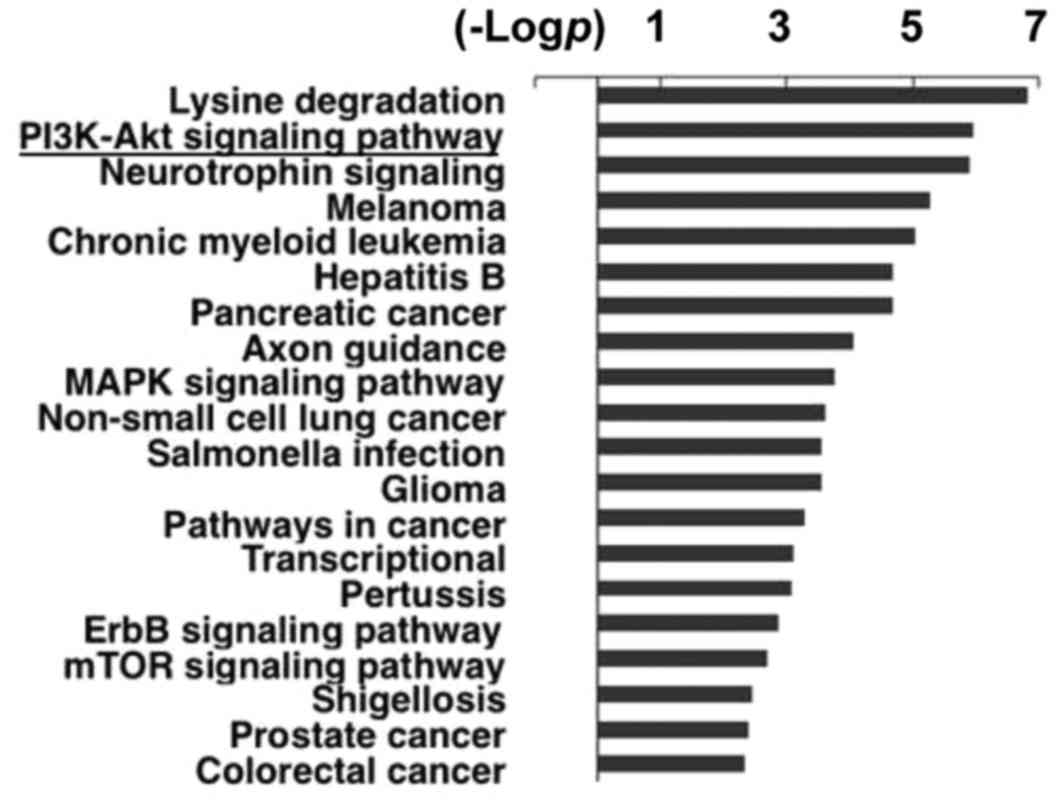
Using bioinformatics software, we found that the
function of miR-302a is significantly related to the PI3K-Akt
pathway (Fig. 1), which is closely
associated with cell survival and 5-FU resistance (23). To explore the role of miR-302a in
5-FU-induced cytotoxicity, we constructed a pre-miR-302a expression
plasmid and transfected it or the control vector (miR-Ctrl) into
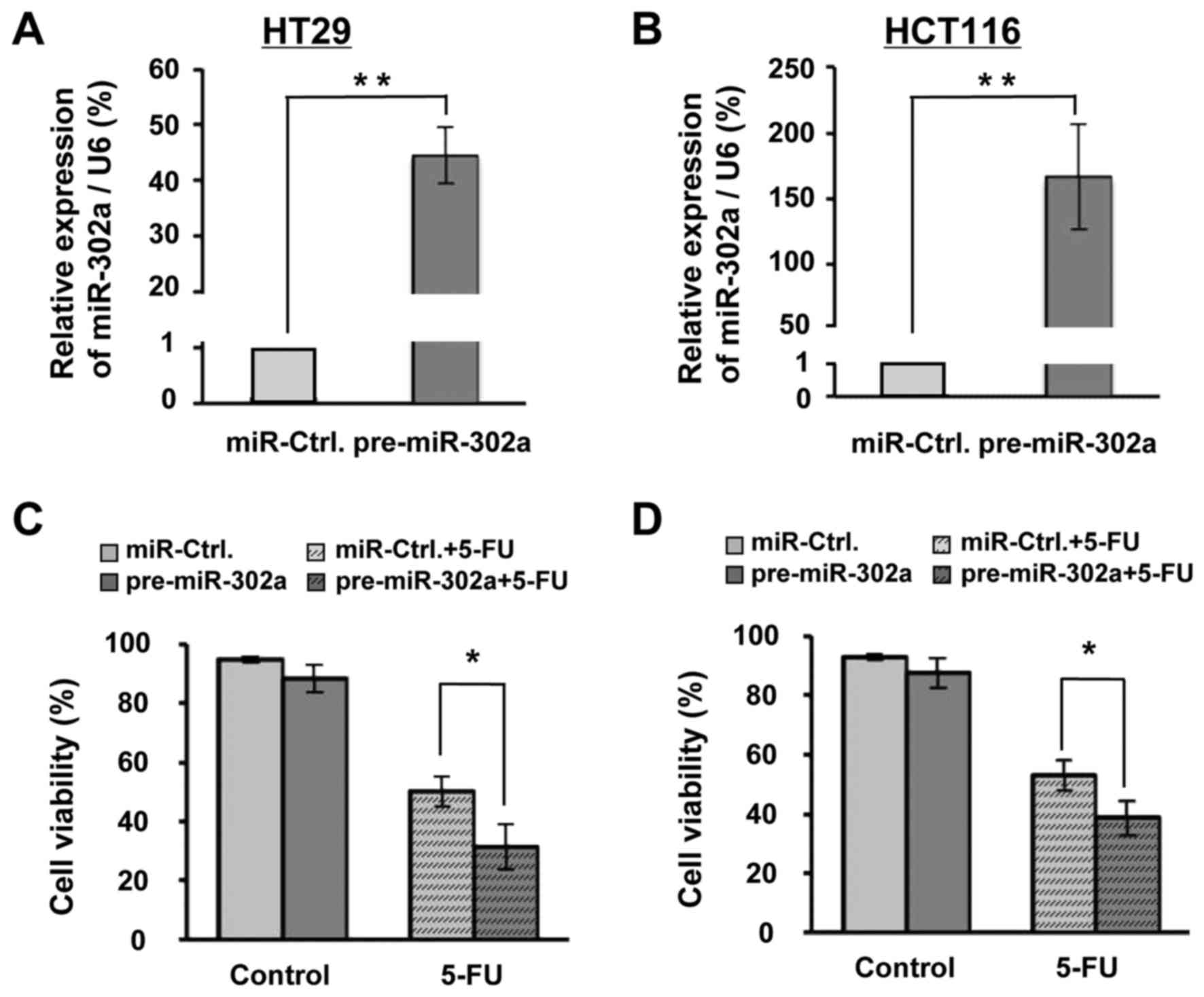
HT29 and HCT116 cells. The qRT-PCR results showed that a sizable
increase in miR-302a was achieved in both cells (Fig. 2A and B). This increase may be due to
the very low expression of miR-302a in typical HT29 and HCT116
cells such that small changes in the miR-302a expression could be
discerned after transfection of miR-302a inhibitor (data not
shown). Based on previous data (24,25),
we used 25 and 10 µM of 5-FU in HT29 and HCT116, respectively, and
observed changes at 48 h of 5-FU treatment. As the data show,
although 48 h of miR-302a overexpression had not significantly
decreased the cellular viability, it enhanced the 5-FU-induced
viability inhibition, from 50.22 to 31.44%, in HT29 cells (Fig. 2C) and from 53.00 to 38.69% in HCT116
cells (Fig. 2D). These results
suggested that miR-302a could enhance the 5-FU-induced viability
inhibition in human colon cancer cells.
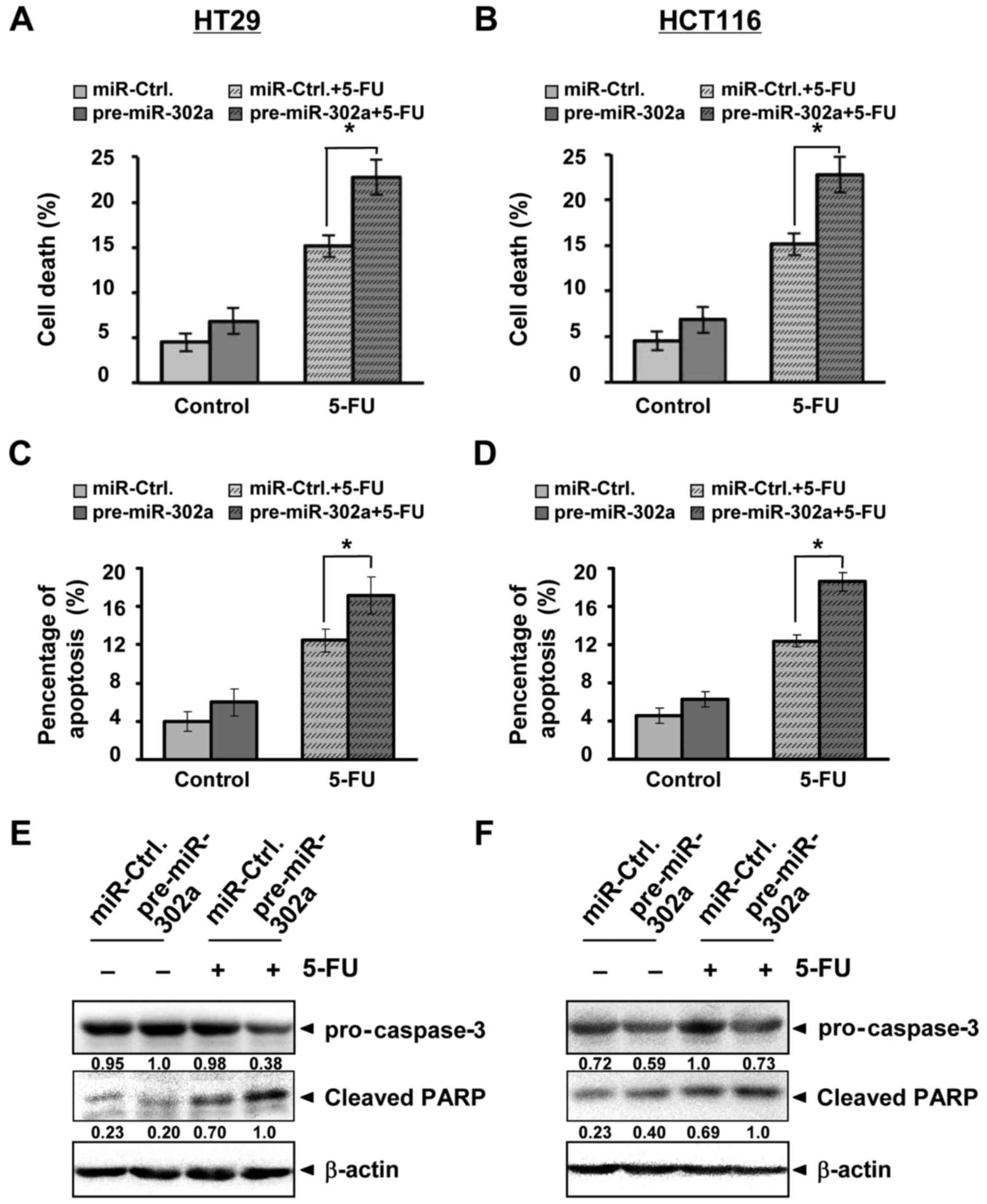
miR-302a increases 5-FU-induced cell
death
The 5-FU-induced cell death, including apoptosis, is
also a mechanism of 5-FU action (4). We measured cell death in our
experiment. As with changes in viability, miR-302a overexpression
did not significantly influence cell death in the cell lines;
however, the combination of miR-302a with 5-FU remarkably increased
cell death with 50.00% increase in HT29 and 62.72% increase in
HCT116 (Fig. 3A and B). Apoptosis
induced by 5-FU was also increased by the combination (Fig. 3C and D). We further detected
apoptotic proteins. The data showed that 5-FU affected caspase-3
and its substrate, activated PARP; miR-302a further enhanced 5-FU
activation (Fig. 3E and F). These
results indicated that miR-302a could increase 5-FU-induced cell
death, including apoptosis, in human colon cancer cells.
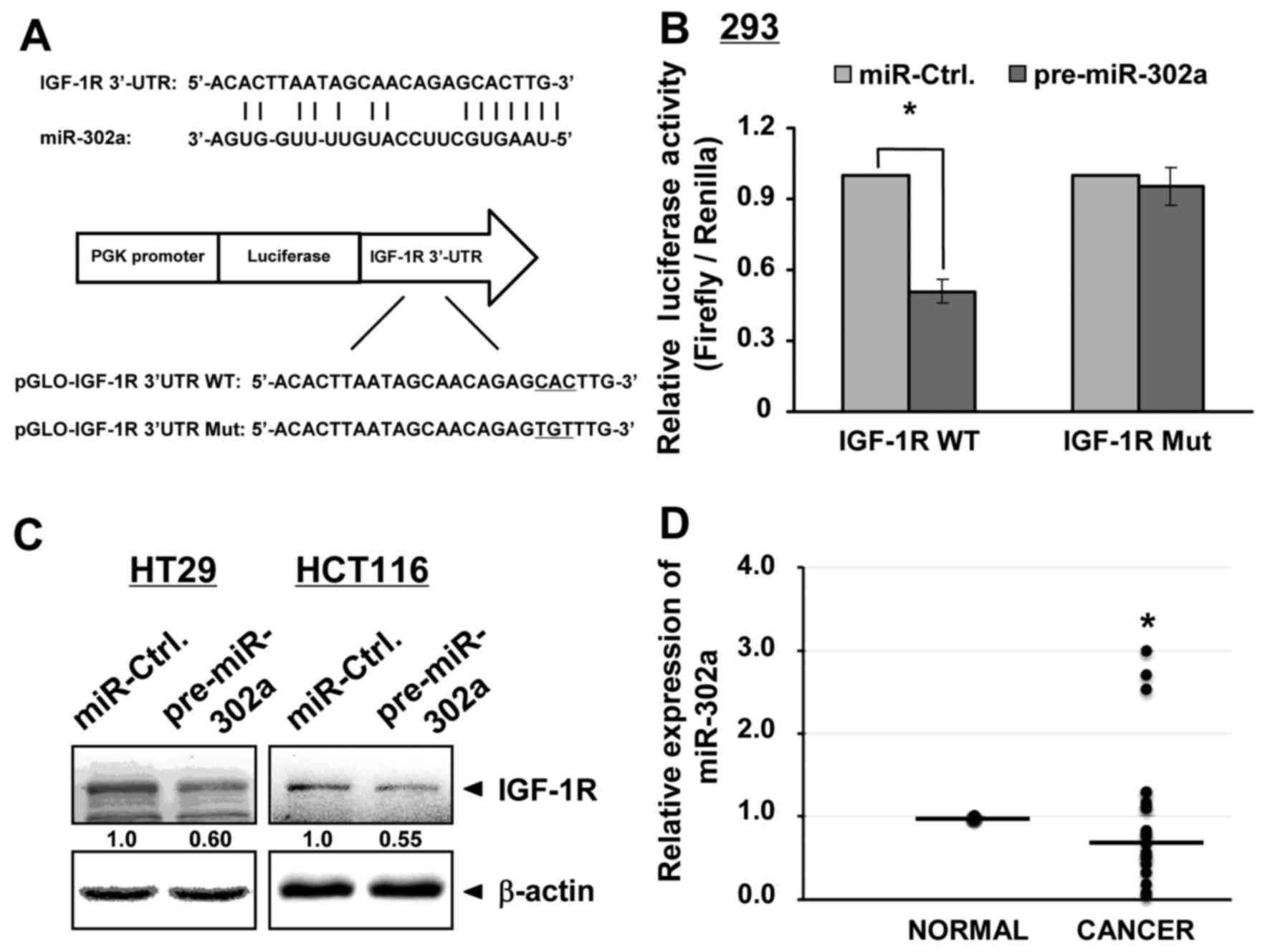
miR-302a targets IGF-1R
To search for the target gene of miR-302a involved
in 5-FU action, we utilized RegRNA 2.0 and TargetScan
bioinformative tools and found that in the 3′UTR of the IGF-1R gene
at 5404–5437 nt, there is a potential miR-302a binding site
(Fig. 4A). We then subcloned this
fragment into the luciferase pmirGLO vector. We also mutated the
binding site and inserted it into the pmirGLO vector. A dual
luciferase assay was subsequently performed. We observed a
significant decrease in the luciferase activity of IGF-1R WT in the
presence of miR-302a compared with miR-Ctrl (Fig. 4B). However, no change in the IGF-1R
Mut luciferase activity was detected between miR-302a and miR-Ctrl.
Furthermore, miR-302a targeting to IGF-1R was confirmed in HT29 and
HCT116 cells. Western blot analysis showed that ectopic miR-302a
downregulated IGF-1R expression (Fig.
4C). Beyond the data in the luciferase assay, targeting of
miR-302a to IGF-1R is also echoed by its downregulation (Fig. 4D) and the amplified IGF-1R signaling
(7) in human CRC tissues. These
data indicate that IGF-1R is a direct target of miR-302a.
Repression of IGF-1R enhanced
5-FU-induced viability inhibition and cell death
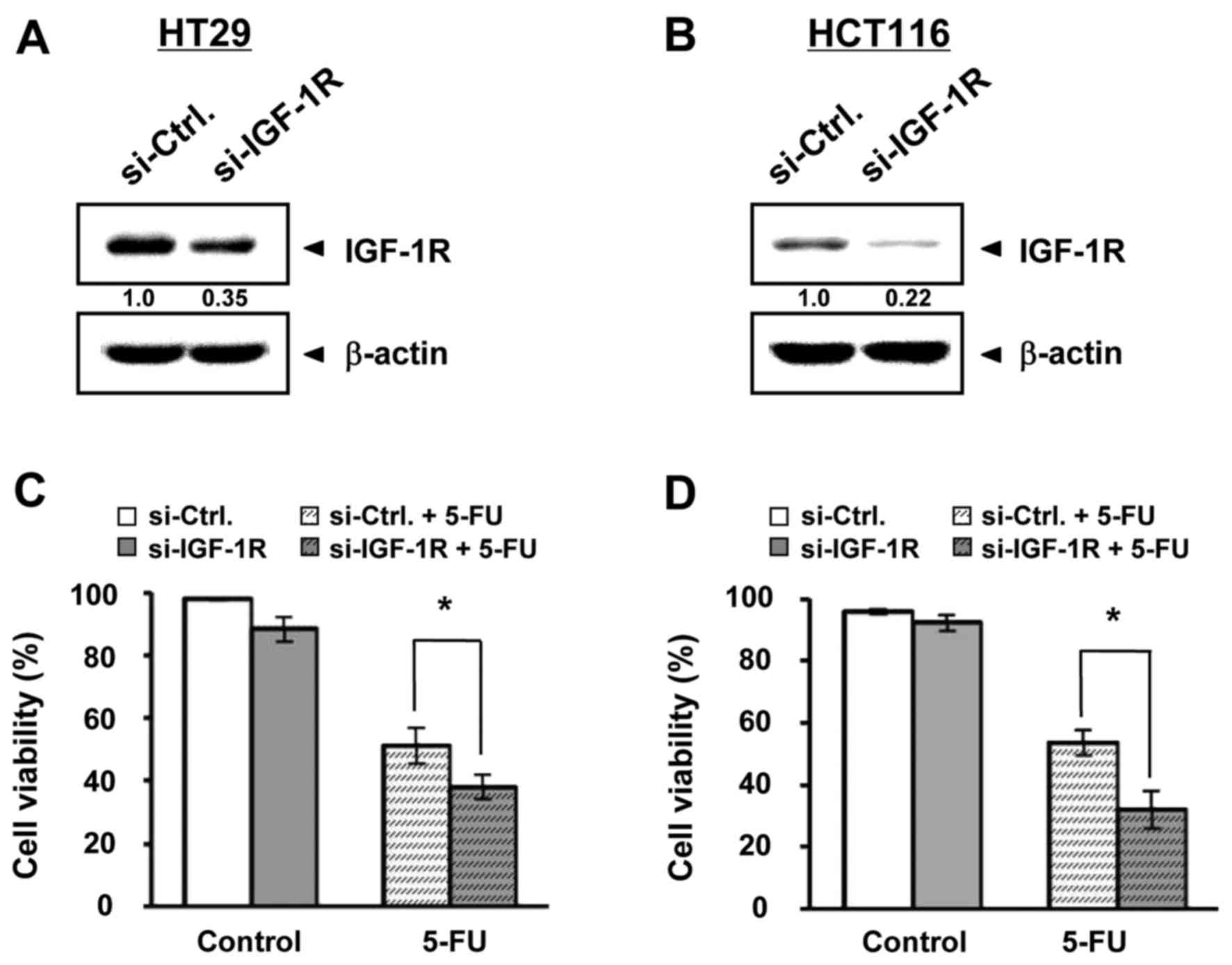
Next, we designed and synthesized si-IGF-1R and
explored whether repression of IGF-1R could influence 5-FU
treatment, such as with miR-302a. The IGF-1R expression was indeed
repressed by the transfection of si-IGF-1R compared with si-Ctrl in
HT29 (Fig. 5A) and HCT116 (Fig. 5B) cells. Additionally, the
inhibition of cell viability by 5-FU was enhanced by si-IGF-1R
transfection, from 51.21 to 37.94% in HT29 (Fig. 5C) and from 53.60 to 32.11% in HCT116
(Fig. 5D) cells, although there
were no significant changes by si-IGF-1R transfection alone.
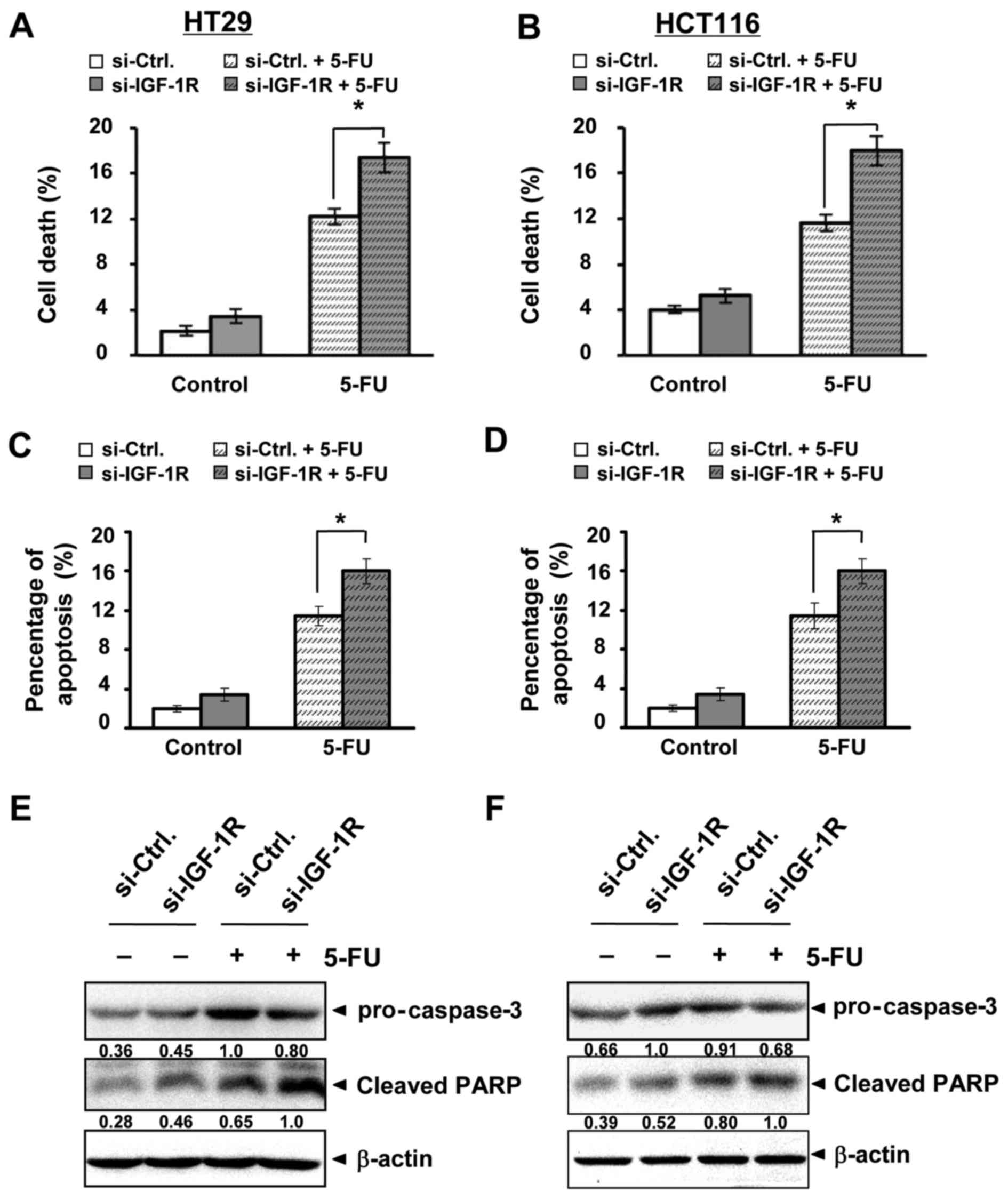
Changes in cell death were also measured. In both cell types,
IGF-1R repression enhanced 5-FU-induced cell death; there was a
42.35% increase in HT29 and 54.78% increase in HCT116 (Fig. 6A and B). Apoptosis induced by 5-FU
was also increased by the combination (Fig. 6C and D). Apoptotic proteins
supported these changes. Caspase 3 and its substrate, PARP, were
more activated by the combination of 5-FU and si-IGF-1R than by
5-FU alone in HT29 (Fig. 6E) and
HCT116 (Fig. 6F) cells. These
results suggested that IGF-1R repression could enhance 5-FU-induced
viability inhibition and cell death in human colon cancer cells,
resembling the influence of miR-302a on 5-FU treatment.
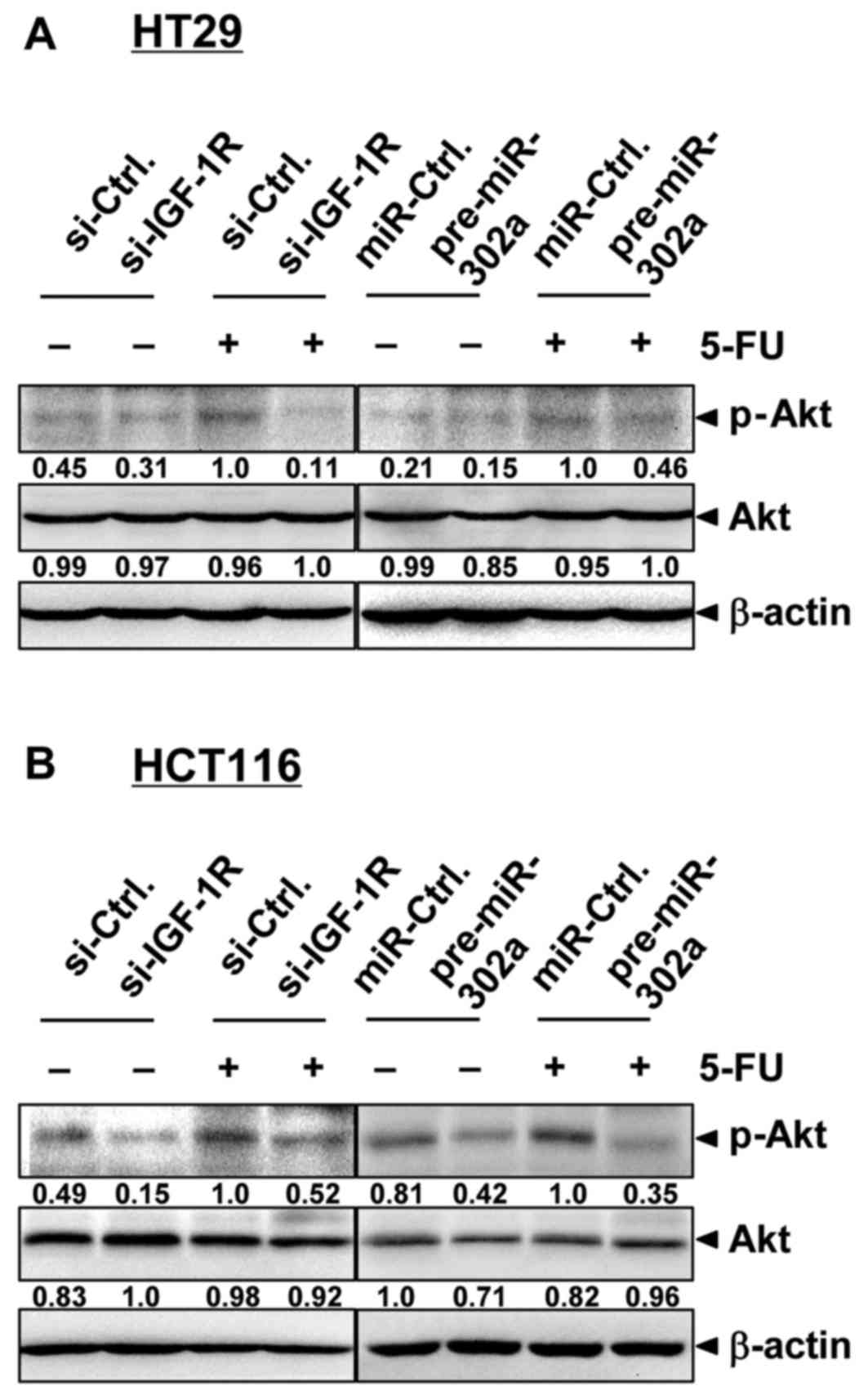
miR-302a inhibits Akt signaling
IGF-1R plays an important role in mediating the
PI3K/Akt pathway activation. We then examined whether
miR-302a-triggered IGF-1R suppression was reflected in regulation
of PI3K/Akt signaling in colon cancer cells (Fig. 7). IGF-1R repression inhibited
constitutive Akt activation, as determined by the expression of
phosphorylated Akt in HT29 and HCT116 cells. Akt is reportedly
activated by 5-FU and plays an important role in 5-FU
chemoresistance (23,26). In our experiment, 5-FU treatment
enhanced Akt activation, while the combination of 5-FU and IGF-1R
repression inhibited Akt activation, which was consistent with
changes in the cell and cell viability results. As expected,
miR-302a overexpression also caused inhibition of Akt activation,
and miR-302a combined with 5-FU significantly inhibited
5-FU-induced Akt activation. In addition, miR-302a decreased the
expression of Akt, which was in agreement with a previous study
(19). These results suggested that
miR-302a could target IGF-1R and Akt as well as inhibit Akt
signaling, and miR-302a overexpression could influence the
5-FU-induced cell death and viability.
Discussion
New therapeutic strategies and/or new adjuvant drugs
are urgently needed in CRC therapy. miRNAs are suggested to be
involved in cancer cell proliferation, apoptosis, migration,
invasion, stemness and chemoresistance. In this study, we first
revealed that miR-302a plays an important role in inhibiting IGF1-R
expression and Akt signaling. This was associated with enhanced
5-FU-induced cell death and viability inhibition by miR-302a
overexpression in human colon cancer cells.
Inappropriate activation of survival signaling
causes uncontrolled proliferation, apoptosis resistance and
increased cell motility, and it plays an important role in cancer
development, progression and resistance to treatment. In CRC, one
of the mechanisms causing increased activation of survival
signaling, especially in the PI3K/Akt pathway, is aberrant IGF-1R
expression (7). Amplified
IGF-1/IGF-1R signaling is not only associated with an increased
relative risk for developing CRC, but it also contributes to CRC
cell survival, invasion and metastasis (8). Activation of IGF-1R-dependent pathways
has also been identified as a critical step that contributes to
several mechanisms of CRC resistance to both conventional and
targeted therapeutic agents (27).
In addition, microRNAs were recently reported to participate in
post-transcriptional regulation of IGF-1R. miR-375, miR-7 and
miR-497 were reported to target IGF-1R in esophageal, tongue
squamous, and colon cancer cells, respectively (28–30).
In this study, miR-302a also targeted IGF-1R in human colon cancer
cells.
miR-302a has been reported to inhibit prostate
cancer cell proliferation by targeting Akt (19) and sensitizing testicular embryonal
carcinoma cells to cisplatin-induced cell death, partially through
the downregulation of p21 (20). In
the past, most research involving miRNA-302 has focused on its role
in hESCs. Studies analyzing miR-302 function in cancer are limited.
For the first time, we revealed that miR-302a could target IGF-1R
and enhanced the 5-FU-induced cell death in human colon cancer
cells. The targeting of miR-302a to IGF-1R was identified by a
luciferase assay and the reverse expression pattern in human CRC
tissues (Fig. 4). IGF-1R is a
direct target of miR-302a. Through targeting IGF-1R, miR-302a
inhibited downstream Akt signaling.
Akt has a major function in cell survival,
proliferation, death, invasion, and migration. Constitutive
activation of the PI3K/Akt pathway is a common event in many cancer
types, and it is associated with a poor prognosis and reduced
survival (31–33). Evidence also indicates that Akt is
frequently activated, which is implicated in cancer chemoresistance
(23,26,32,34).
Consistent with this, we found 5-FU could upregulate Akt
activation; also, overexpression of miR-302a inhibited the
5-FU-induced Akt activation and enhanced the 5-FU-induced cell
death in human colon cancer cells. Besides IGF-1R, miR-302a may
target other genes in the PI3K/Akt pathway as reported in previous
studies (17–20) and the bioinformatics analysis
(Fig. 1). miR-302a is a potentially
important tumor suppressor that merits further, extensive study. In
addition, in our experiment, the effect of only miR-302a
overexpression on cell viability and cell death was not
significant, which was inconsistent with a previous study (19). This discrepancy could be due to the
shorter period (48 h) of our system compared to that in the
previous study (96 h to 7 days).
CRC treatment via targeting IGF1-R is now in
development for clinical use. Our results suggest that
overexpression of miR-302a may be a useful alternative strategy for
inhibiting IGF1-R, inactivating Akt, and enhancing 5-FU-induced
cell death and viability inhibition in human colon cancer cells.
Targeting miR-302a may offer new therapeutic interventions in
CRC.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (nos. 81172358 and 31100969)
and the Fundamental Research Funds of Xi'an Jiaotong University
(no. xjj2016076). We thank Professor H. Kuwano and S. Torii
(Graduate School of Medicine, Gunma University, Maebashi, Japan)
for their kind assistance. We also thank Lei Ni, Aiying Wang and
Lin Yu for their technical support.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sung JJ, Lau JY, Goh KL and Leung WK: Asia
Pacific Working Group on Colorectal Cancer: Increasing incidence of
colorectal cancer in Asia: Implications for screening. Lancet
Oncol. 6:871–876. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Temraz S, Mukherji D, Alameddine R and
Shamseddine A: Methods of overcoming treatment resistance in
colorectal cancer. Crit Rev Oncol Hematol. 89:217–230. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Longley DB, Harkin DP and Johnston PG:
5-fluorouracil: Mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Scartozzi M, Maccaroni E, Giampieri R,
Pistelli M, Bittoni A, Del Prete M, Berardi R and Cascinu S:
5-Fluorouracil pharmacogenomics: Still rocking after all these
years? Pharmacogenomics. 12:251–265. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vigneri PG, Tirrò E, Pennisi MS, Massimino
M, Stella S, Romano C and Manzella L: The insulin/IGF system in
colorectal cancer development and resistance to therapy. Front
Oncol. 5:2302015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weber MM, Fottner C, Liu SB, Jung MC,
Engelhardt D and Baretton GB: Overexpression of the insulin-like
growth factor I receptor in human colon carcinomas. Cancer.
95:2086–2095. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sekharam M, Zhao H, Sun M, Fang Q, Zhang
Q, Yuan Z, Dan HC, Boulware D, Cheng JQ and Coppola D: Insulin-like
growth factor 1 receptor enhances invasion and induces resistance
to apoptosis of colon cancer cells through the Akt/Bcl-x(L)
pathway. Cancer Res. 63:7708–7716. 2003.PubMed/NCBI
|
|
9
|
Alberobello AT, D'Esposito V, Marasco D,
Doti N, Ruvo M, Bianco R, Tortora G, Esposito I, Fiory F, Miele C,
et al: Selective disruption of insulin-like growth factor-1 (IGF-1)
signaling via phosphoinositide-dependent kinase-1 prevents the
protective effect of IGF-1 on human cancer cell death. J Biol Chem.
285:6563–6572. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu WK, Law PT, Lee CW, Cho CH, Fan D, Wu
K, Yu J and Sung JJ: MicroRNA in colorectal cancer: From benchtop
to bedside. Carcinogenesis. 32:247–253. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Amirkhah R, Farazmand A, Irfan-Maqsood M,
Wolkenhauer O and Schmitz U: The role of microRNAs in the
resistance to colorectal cancer treatments. Cell Mol Biol
(Noisy-le-grand). 61:17–23. 2015.PubMed/NCBI
|
|
12
|
Karaayvaz M, Zhai H and Ju J: miR-129
promotes apoptosis and enhances chemosensitivity to 5-fluorouracil
in colorectal cancer. Cell Death Dis. 4:e6592013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Y, Zheng L, Huang J, Gao F, Lin X,
He L, Li D, Li Z, Ding Y and Chen L: MiR-124 radiosensitizes human
colorectal cancer cells by targeting PRRX1. PLoS One. 9:e939172014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou Y, Wan G, Spizzo R, Ivan C, Mathur R,
Hu X, Ye X, Lu J, Fan F, Xia L, et al: miR-203 induces oxaliplatin
resistance in colorectal cancer cells by negatively regulating ATM
kinase. Mol Oncol. 8:83–92. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Suto T, Yokobori T, Yajima R, Morita H,
Fujii T, Yamaguchi S, Altan B, Tsutsumi S, Asao T and Kuwano H:
MicroRNA-7 expression in colorectal cancer is associated with poor
prognosis and regulates cetuximab sensitivity via EGFR regulation.
Carcinogenesis. 36:338–345. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suh MR, Lee Y, Kim JY, Kim SK, Moon SH,
Lee JY, Cha KY, Chung HM, Yoon HS, Moon SY, et al: Human embryonic
stem cells express a unique set of microRNAs. Dev Biol.
270:488–498. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Baskerville S, Shenoy A, Babiarz
JE, Baehner L and Blelloch R: Embryonic stem cell-specific
microRNAs regulate the G1-S transition and promote rapid
proliferation. Nat Genet. 40:1478–1483. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin SL, Chang DC, Ying SY, Leu D and Wu
DT: MicroRNA miR-302 inhibits the tumorigenecity of human
pluripotent stem cells by coordinate suppression of the CDK2 and
CDK4/6 cell cycle pathways. Cancer Res. 70:9473–9482. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang GM, Bao CY, Wan FN, Cao DL, Qin XJ,
Zhang HL, Zhu Y, Dai B, Shi GH and Ye DW: MicroRNA-302a suppresses
tumor cell proliferation by inhibiting AKT in prostate cancer. PLoS
One. 10:e01244102015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu L, Lian J, Zhang H, Tian H, Liang M,
Yin M and Sun F: MicroRNA-302a sensitizes testicular embryonal
carcinoma cells to cisplatin-induced cell death. J Cell Physiol.
228:2294–2304. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hou N, Torii S, Saito N, Hosaka M and
Takeuchi T: Reactive oxygen species-mediated pancreatic β-cell
death is regulated by interactions between stress-activated protein
kinases, p38 and c-Jun N-terminal kinase, and mitogen-activated
protein kinase phosphatases. Endocrinology. 149:1654–1665. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hou N, Han J, Li J, Liu Y, Qin Y, Ni L,
Song T and Huang C: MicroRNA profiling in human colon cancer cells
during 5-fluorouracil-induced autophagy. PLoS One. 9:e1147792014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li B, Li J, Xu WW, Guan XY, Qin YR, Zhang
LY, Law S, Tsao SW and Cheung AL: Suppression of esophageal tumor
growth and chemoresistance by directly targeting the PI3K/AKT
pathway. Oncotarget. 5:11576–11587. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li J, Hou N, Faried A, Tsutsumi S,
Takeuchi T and Kuwano H: Inhibition of autophagy by 3-MA enhances
the effect of 5-FU-induced apoptosis in colon cancer cells. Ann
Surg Oncol. 16:761–771. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li J, Hou N, Faried A, Tsutsumi S and
Kuwano H: Inhibition of autophagy augments 5-fluorouracil
chemotherapy in human colon cancer in vitro and in vivo model. Eur
J Cancer. 46:1900–1909. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vinod BS, Antony J, Nair HH,
Puliyappadamba VT, Saikia M, Narayanan SS, Bevin A and Anto RJ:
Mechanistic evaluation of the signaling events regulating
curcumin-mediated chemosensitization of breast cancer cells to
5-fluorouracil. Cell Death Dis. 4:e5052013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dallas NA, Xia L, Fan F, Gray MJ, Gaur P,
van Buren G II, Samuel S, Kim MP, Lim SJ and Ellis LM:
Chemoresistant colorectal cancer cells, the cancer stem cell
phenotype, and increased sensitivity to insulin-like growth
factor-I receptor inhibition. Cancer Res. 69:1951–1957. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kong KL, Kwong DL, Chan TH, Law SY, Chen
L, Li Y, Qin YR and Guan XY: MicroRNA-375 inhibits tumour growth
and metastasis in oesophageal squamous cell carcinoma through
repressing insulin-like growth factor 1 receptor. Gut. 61:33–42.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jiang L, Liu X, Chen Z, Jin Y, Heidbreder
CE, Kolokythas A, Wang A, Dai Y and Zhou X: MicroRNA-7 targets
IGF1R (insulin-like growth factor 1 receptor) in tongue squamous
cell carcinoma cells. Biochem J. 432:199–205. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guo ST, Jiang CC, Wang GP, Li YP, Wang CY,
Guo XY, Yang RH, Feng Y, Wang FH, Tseng HY, et al: MicroRNA-497
targets insulin-like growth factor 1 receptor and has a tumour
suppressive role in human colorectal cancer. Oncogene.
32:1910–1920. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yoshioka A, Miyata H, Doki Y, Yasuda T,
Yamasaki M, Motoori M, Okada K, Matsuyama J, Makari Y, Sohma I, et
al: The activation of Akt during preoperative chemotherapy for
esophageal cancer correlates with poor prognosis. Oncol Rep.
19:1099–1107. 2008.PubMed/NCBI
|
|
32
|
Liu P, Cheng H, Roberts TM and Zhao JJ:
Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev
Drug Discov. 8:627–644. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Faried LS, Faried A, Kanuma T, Aoki H,
Sano T, Nakazato T, Tamura T, Kuwano H and Minegishi T: Expression
of an activated mammalian target of rapamycin in adenocarcinoma of
the cervix: A potential biomarker and molecular target therapy. Mol
Carcinog. 47:446–457. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Faried LS, Faried A, Kanuma T, Nakazato T,
Tamura T, Kuwano H and Minegishi T: Inhibition of the mammalian
target of rapamycin (mTOR) by rapamycin increases chemosensitivity
of CaSki cells to paclitaxel. Eur J Cancer. 42:934–947. 2006.
View Article : Google Scholar : PubMed/NCBI
|