Introduction
Bone cancer pain (BCP) is a major clinical
intractable problem, seriously affecting the lives of the patients
(1). Despite increasing development
of basic and clinical research for BCP, the novel clinical
analgesics and advanced analgesic strategies often provide
inadequate analgesia effect with unacceptable side-effects
(2). Thus, in-depth studies are
urgently needed for finding more effective and rational treatment
strategy for cancer pain.
Recent basic studies in chronic neuropathic and
inflammatory pain models suggests that peripheral nerve injury or
local inflammatory stimulus results in the pathology changes of the
spinal ERK and STAT3, whereas, the inhibition of both ERK and STAT3
contribute to attenuate hyperpathia (3–6).
Midazolam (MZL), a short-acting and water-soluble benzodiazepine,
is used as anesthetic for sedation, antianxiety, hypnosis of
premedication, and induction and maintenance of general anesthesia
(7). MZL is known as an adjuvant
that has many advantages in clinical practice, such as quick effect
and rapid inactivation (8).
However, the unacceptable neurotoxicity have been found at high
doses of MZL, many studies confirm that i.t. MZL produce apoptosis
of neurons, and these results restrict the wide use of MZL
(9).
Ropivacaine (Ropi), a safe amino amide local
anesthetic (LA), is widely used in local anesthesia and
postoperative pain control, because it has less impact on motor
function and fewer acceptable side-effects in a low concentration
(10). However, repeated i.t. Ropi
induces catastrophic neurotoxicity and neuronal apoptosis in a
dose-dependent manner (11,12). Hence, how to amplify its analgesic
effect and reduce the unacceptable side-effects becomes a new
research area. Thus, the combination of two different drugs with
different and/or overlapped mechanism was considered an effective
and harmless method for analgesia in the clinic.
However, it is not clear that the synergistic
analgesia of MZL and Ropi on BCP rats and those related underlying
mechanisms. Based on the following evidence, we suggested thay
combination of MZL&Ropi might induce synergistic analgesia.
First, Ropi as a block of fast voltage-gated sodium channels on
neuronal axons is used as analgesia for many kinds of pain in basic
experiments (13,14) and clinical trials (15,16).
In our previous study, Ropi c alleviated CFA-induced chronic
inflammatory pain in rats, and combination with Ropi and Dex showed
synergistic analgesia on the chronic inflammatory pain (14). Second, the increased expression of
peripheral-type benzodiazepine receptors (PBRs, also named
translocator protein, TSPO) are detected in astrocytes and
microglia in inflammatory or nerve injury models (17,18).
Ro5-4864, an agonist ligand of TSPO can attenuate pain behavior in
neuropathic pain, and MZL is also considered as a ligand for TSPO
(19,20). Besides, analgesic effect of MZL has
been confirmed by previous study in neuropathic pain (21,22).
Hence, using a model of BCP of female rats, we
designed the current experiment to test that i.t. co-delivery of
MZL and Ropi at lower dose yields synergistic analgesia, and
further explore the underlying analgesic mechanisms of MZL and
Ropi. It was found that these two clinically used drugs may be
utilized in combination to achieve pain relief in BCP patients.
Materials and methods
Animal preparation
Adult female Sprague-Dawley (SD) rats, weighing
200–220 g, were provided by Experimental Animal Center of the Xi'an
Jiaotong University. All rats were housed in controlled conditions
(standard transparent plastic cages with a 12/12-h light/dark
cycle, under 22–26°C ambient temperature with food and water ad
libitum). All rats were allowed to adapt to the housing
environment for at least 3 days before the experiments.
All experimental procedures were conducted in
accordance with the Animal Use and Care Committee for Research and
Education of the Xi'an Jiaotong University and National Institute
for Physiological Sciences Animal Care and Use Committee. All
efforts were made to minimize suffering of the animals and the
number of animals used was carefully controlled.
Cell line and drug administration
Female SD rats were administered intraperitoneal
(i.p.) inoculation of Walker 256 mammary gland carcinoma cells
(2×106 cells/ml, 1 ml). After 1 week, tumor cells were
extracted from the cancerous ascitic fluid of rats, and resuspended
in a concentration of 1×107 cells/ml in 0.01 M
phosphate-buffered saline (PBS) for inoculation.
MZL (5 mg/5 ml) and Ropi (100 mg/10 ml) were
purchased from Nhwa Pharmaceutical Co., Ltd. (Jiangsu, China) and
AstraZeneca AB (Sweden), respectively. These drugs were diluted
with artificial cerebral spinal fluid (ACSF: NaCl 124 mM, D-glucose
10 mM, NaH2PO4 1 mM, NaHCO3 25 mM,
MgSO4 1 mM, KCl 4.4 mM and
CaCl2•H2O 2 mM) prior to i.t. application.
Finally, MZL (5 mg/5 ml) was diluted to concentrations (10 µg/ml),
and the target concentrations of Ropi (100 mg/10 ml) was 1
mg/ml.
BCP model surgery
The inoculation was performed as previously
described (23). Briefly, rats were
anaesthetized with chloral hydrate (300 mg/kg, i.p.), the right
rear hindlimb was shaved in order to expose the skin over the
femoral-tibial joint. The intercondylar eminence of the right tibia
was exposed after cleaning skin 3 times with iodine tincture and
75% ethanol. A 22-gauge needle was drilled into the site as
described previously, then 20 µl microinjection syringe (Hamilton,
USA) containing a 10 µl suspension of tumor cells was used to
inject the tumor cells into the tibial cavity slowly. The drilled
hole was sealed with bone wax (Johnson & Johnson, USA) in order
to prevent tumor cells from spreading outside the bone. For the
sham group, 10 µl PBS replaced tumor cells into the tibia (5).
Intrathecal implantation
Intrathecal implantation was performed under chloral
hydrate (300 mg/kg, i.p.). Briefly, a midline incision (3 cm) was
cut on the back of the rat at the level of the thoracic vertebrae.
A pre-measured length of PE-10 tubing was passed caudally from the
T8 to the L3 level of the spinal cord, fixed at the back of rat's
ears through subcutaneous tunnel, and 2 cm of the free end was
exposed in the upper thoracic region. Rats were allowed to recover
for a period of 3–5 days before further use. The animals judged as
with no neurological deficit and that presented complete paralysis
of the tail and bilateral hind legs after administration of 2%
lidocaine (10 µl) through the intrathecal catheter were used for
the following experiments.
Behavioral test
Thermal hyperalgesia was evaluated by Hargreaves
test. In brief, rats were placed individually in crylic boxes with
wire mesh floors and allowed to adapt for 30 min. The radiant heat
stimulator was used to stimulate the plantar surface of the
hindlimb paw, and the intensity of the beam was set to produce a
basal paw withdrawal latency (PWL) of approximate 16 sec. The PWLs
were recorded according to previous studies above. To prevent
tissue thermal burn, the cut-off value was set at 20 sec. The
blinding method was implemented strictly for the whole behavioral
test.
Motor coordination was determined using a standard
rat rotarod test (Shanghai Mobiledatum Information Technology Co.,
Ltd., China). Daily training for each rat was performed at 30 min
after intrathecal administration of compound for 7 days, and
measured as following methods: rats were placed on the rotating
drums and the time was measured from the start of the acceleration
period until the rat fell off the drum. A cut-off latency was 30
sec in all rotarod assessments. The time that the animal remained
on the rotarod was recorded and expressed as a percentage of its
own baseline value.
Immunofluorescence histochemistry
staining
Rats were deeply anesthetised with overdose chloral
hydrate (10%, 0.3 ml/100 g) and transcardially perfused with 200 ml
of 0.01 M PBS (pH 7.4), followed by 500 ml of 4% paraformaldehyde
in 0.1 M phosphate buffer (PB, pH 7.4). The L4-L6 spinal cord
segments were harvested and dehydrated in 30% sucrose at 4°C.
Transverse spinal sections (30 µm thickness) were then cut on a
cryostat (CM3050S, Leica, Germany). For double immunofluorescence,
the sections were incubated with mouse anti-c-Fos (1:500, Abcam,
USA), mouse anti-GFAP (1:500, Millipore, USA) and goat anti-IBA-1
(1:500, Abcam), followed by FITC-conjugated secondary antibodies
(1:500, Invitrogen, USA) for 3 h at room temperature. The stained
sections were observed and captured with a confocal laser canning
microscope (FV1000, Olympus, Japan).
Western blot analysis
The L4-L6 spinal cord segments were rapidly
extracted from anesthetized rats. The tissues were homogenized in
lysis buffer (Bio-Rad Laboratories, USA) which contain a mixture of
protease inhibitors and phenylmethylsulfonyl fluoride (Roche
Diagnostics, Switzerland). Equivalent amounts of protein (10 µl)
were loaded and separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (10% SDS-PAGE; Bio-Rad),
transferred to PVDF membranes (Millipore). The membrane was
incubated with primary antibody overnight at 4°C, followed by
HRP-conjugated secondary antibodies for 2 h (1:5,000, CST). The
secondary antibody signal was detected with enhanced
chemiluminescence (Advansta, USA), and captured by Omega Lum G
system (Aplegen, USA). The following primary antibodies were used
in our studies: goat anti-TSPO (1:1,000, Sigma-Aldrich, USA),
rabbit anti-pERK (1:1,000, Abcam), mouse anti-ERK (1:1,000, Abcam),
rabbit anti-pSTAT3 (1:2,000, CST, USA), rabbit anti-STAT3 (1:2,000,
CST, USA), mouse anti-GFAP (1:1,000, Millipore), goat anti-IBA-1
(1:1,000, Abcam) and mouse anti-β-actin (1:10,000,
Sigma-Aldrich).
Statistical analysis
All data were expressed as mean ± standard error
mean (SEM), and analyzed by researchers with double-blind method.
One-way analysis of variance (ANOVA) with Dunnett's multiple
comparison post hoc test was used for multiple comparison between
groups for area under the curve. Two-way ANOVA with Bonferroni post
hoc test was used for measures of dose by time and rotarod.
Variable slope nonlinear regression was used for dose-response
curves by using Graph-Pad Prism 6.0 (Graph-Pad software, San Diego,
CA, USA). Isobolographic analysis was based on Tallarida and our
previous study (24). P<0.05 was
considered as statistically significant.
Results
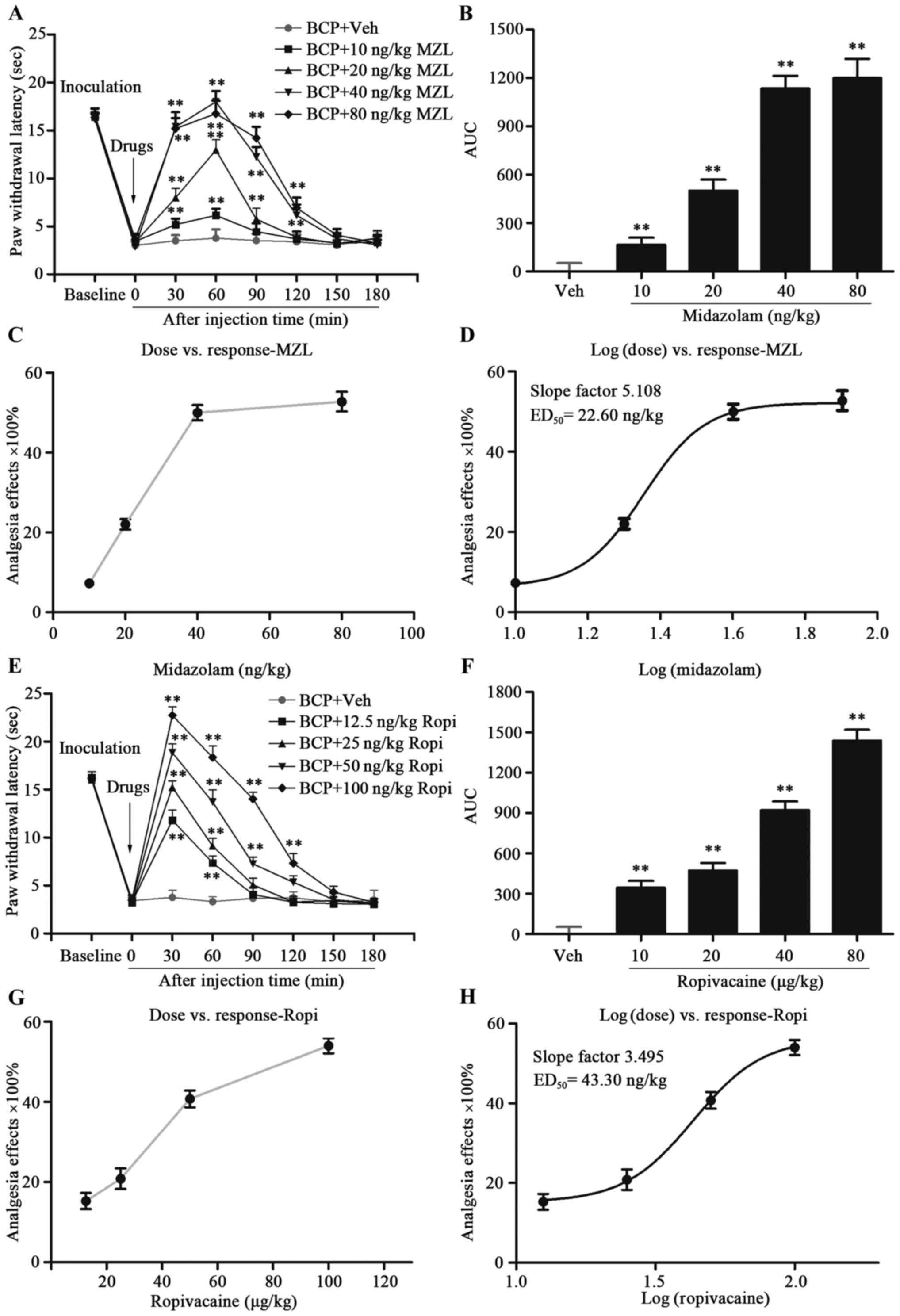
Effect of i.t. MZL on BCP
In order to identify the potential analgesic effect
of MZL in BCP, different doses of MZL were i.t. injected on day 14
after tumor cell inoculation. Based on previous studies, MZL was
given in doses of 10, 20, 40 and 80 ng and PWLs were measured at
30, 60, 90, 120, 150 and 180 min post-injection of MZL and vehicle.
Compared with BCP-Veh group, i.t. MZL significantly elevated PWLs
in a dose-dependent manner with a peak effect at dose of 80 ng/kg
at 60 min post-injection (Fig. 1A and
B; P<0.01). The ED50 for MZL was calculated as
22.6 ng/kg based on the log (dose) vs. response curve (Fig. 1C and D; P<0.01). Besides, the
rotarod test revealed 22.6 ng/kg MZL did not affect the motor
performance of rats compared to vehicle treatment at any of the
time-points tested (Fig. 2E;
P<0.01). Hence, i.t. MZL inhibited thermal hyperalgesia in BCP
rats.
Effect of i.t. Ropi on BCP
According to our previous studies (14), increasing doses of Ropi were given
in 12.5, 25, 50 and 100 µg/kg and PWLs were recorded at 30, 60, 90,
120, 150 and 180 min post-injection of Ropi and vehicle. Compared
with BCP-Veh group, i.t. Ropi also significantly elevated PWLs in a
dose-dependent manner with a peak effect at dose of 100 µg/kg at 30
min post-injection (Fig. 1E and F;
P<0.01). The ED50 for Ropi was calculated as 43.3
µg/kg based on the log (dose) vs. response curve (Fig. 1G and H; P<0.01). Similarly, the
rotarod test also revealed 43.3 µg/kg Ropi did not affect the motor
performance of rats compared to vehicle treatment at any of the
time-points tested (Fig. 2E;
P<0.01). Hence, i.t. Ropi apparently inhibits thermal
hyperalgesia in a dose-dependent manner in BCP rats.
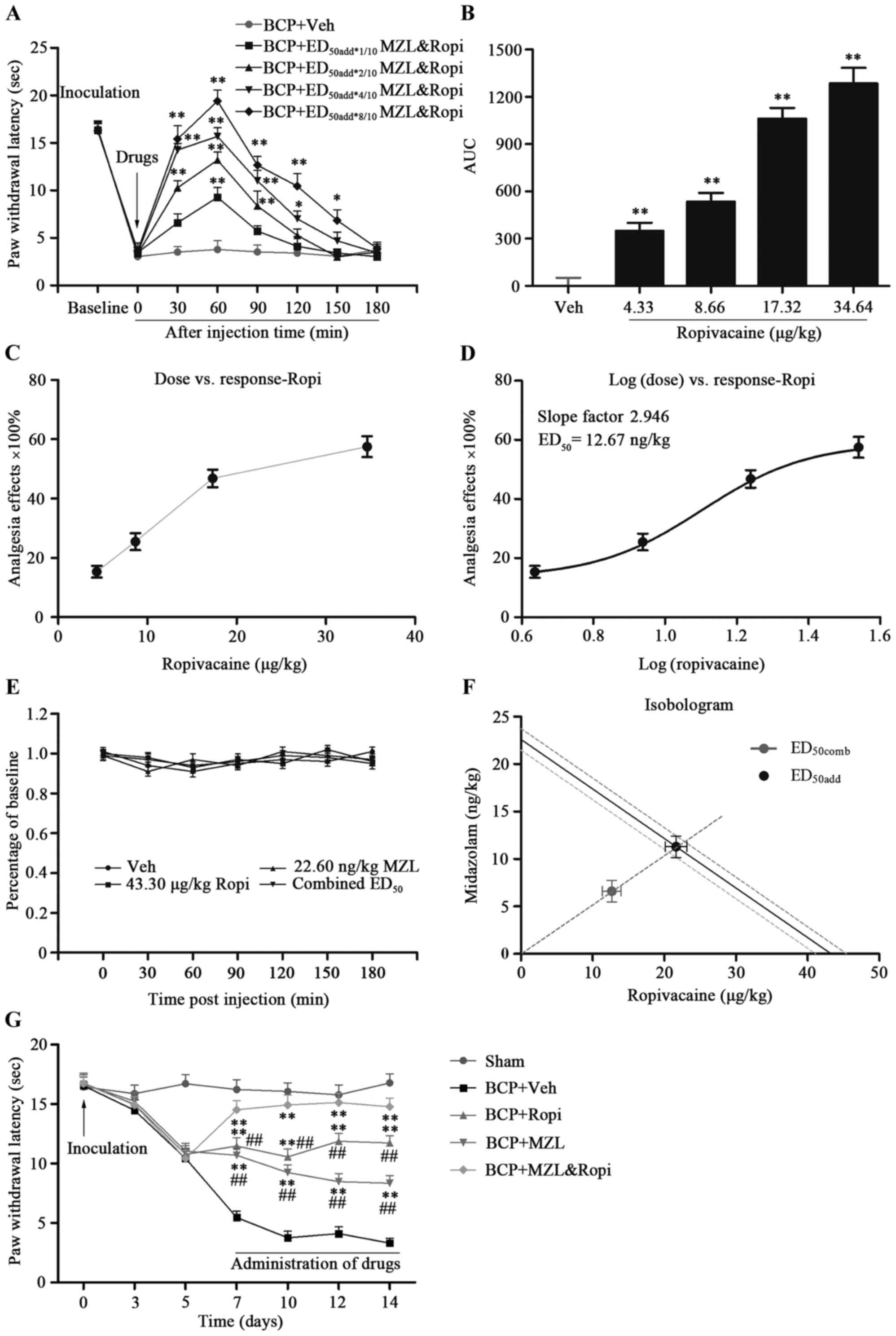
Effect of i.t. MZL and Ropi
combination on BCP
Next, we calculated synergistic analgesic effect of
MZL&Ropi on the basis of the antinociceptive effects showed by
two drugs. According to additive regression, we calculated
theoretical ED50 for a fixed-ratio (1:1) combination of
MZL and Ropi. Hence, the doses of MZL&Ropi combinations were as
follows: 2.26 Dex + 4.33 Ropi (ED50add*1/10
Dex&Ropi), 4.52 Dex + 8.66 Ropi
(ED50add*2/10Dex&Ropi), 9.04 Dex + 17.32 Ropi
(ED50add*4/10 Dex&Ropi) and 18.08 Dex + 34.64 Ropi
(ED50add*8/10 Dex&Ropi).
PWLs were measured at 30, 60, 90, 120, 150 and 180
min post-injection of MZL&Ropi and vehicle. i.t. MZL&Ropi
combination significantly elevated PWLs in a dose-dependent manner
with a peak effect at dose of 18.08 Dex and 34.64 Ropi at 60 min
post-injection (Fig. 2A and B;
P<0.01). Besides, the averaged valid analgesic duration was
significantly dose-dependently prolonged. The experimental
ED50comb at this fixed dose ratio calculated from these
dose-response curves for pain responses was 6.61 MZL + 12.67 Ropi
(Fig. 2C and D, P<0.05).
Isobolographic analysis showed that MZL&Ropi acted
synergistically to inhibit BCP in rats, because ED50comb
was smaller than the lower (95%) range of ED50add
(Fig. 2F, P<0.05). These results
revealed that the combination of MZ&Ropi facilitated to prolong
analgesic duration and enhance analgesic intensity with less dose
of MZL and Ropi. The combination of MZL&Ropi did not affect
motor coordination of the animals (Fig.
2E; P<0.01).
Continuous analgesia of i.t.
MZL&Ropi on BCP
Above the effect of i.t. MZL, and Ropi, or their
combination was described in a short-term observation. Then, their
analgesia on BCP induced chronic hyperalgesia was further explored.
According to ED50 MZL, ED50 Ropi, and
ED50comb Dex&Ropi, the dose regimens were as
follows: BCP-MZL group, 22.6 ng/kg MZL; BCP-Ropi group, 43.3 µg/kg
Ropi; and BCP-MZL&Ropi group, 6.61 ng/kg MZL and 12.67 µg/kg
Ropi.
A significant analgesic effect of all intervention
groups was observed. Compared with BCP-Vehicle group, i.t. MZL,
Ropi, or their combination significantly increased the PWLs. In
these three group, BCP-MZL&Ropi groups performed better
analgesic effect than the others, however, analgesic effect of
BCP-MZL group gradually attenuated with time. These results
suggested that i.t. MZL&Ropi combination showed better
analgesic effect in a long-term treatment in BCP rats with less
doses and longer duration than each single drug (Fig. 2F, P<0.05).
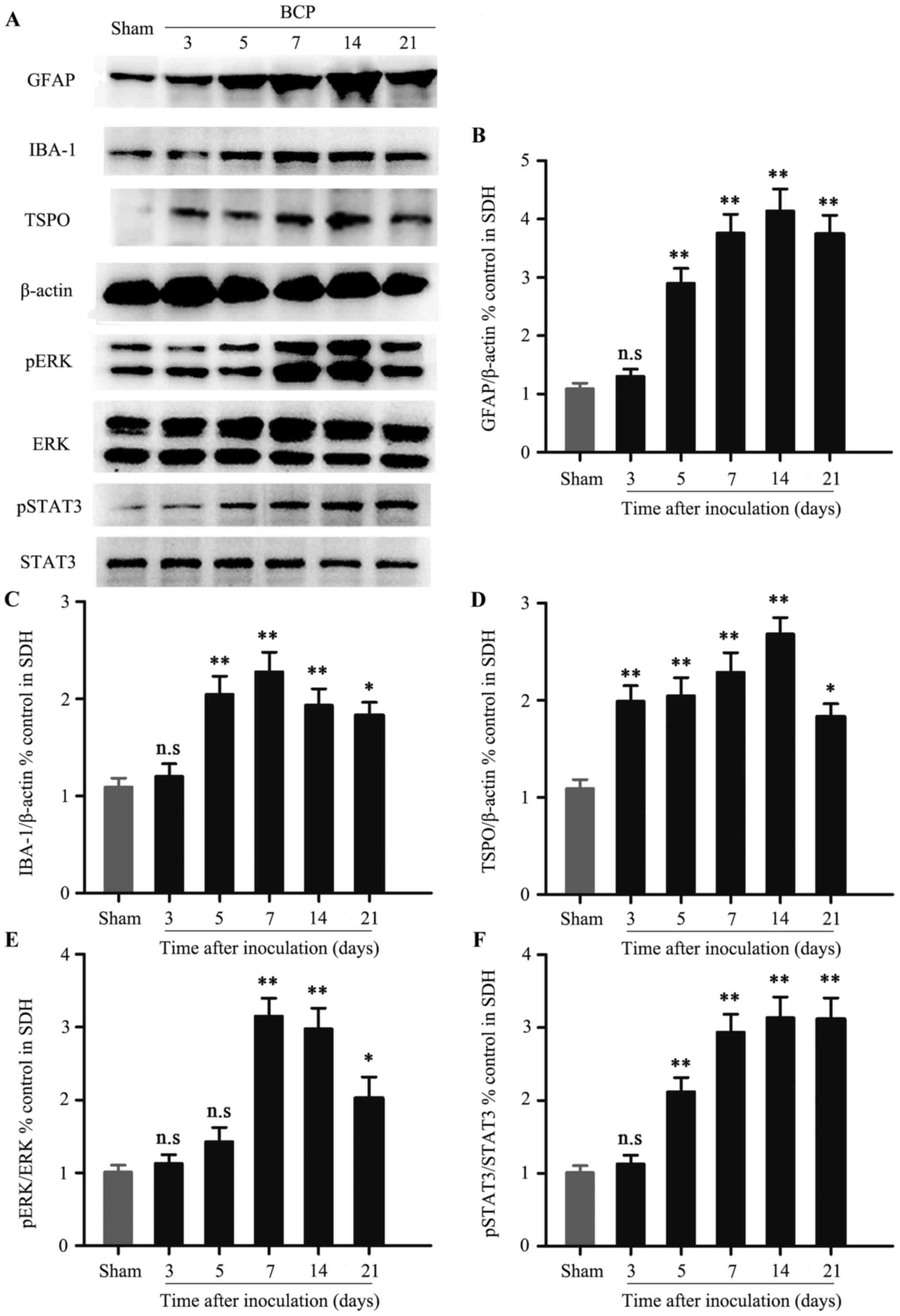
Expression of spinal TSPO, pERK and
pSTAT3 in BCP
We examined the time course of spinal GFAP, IBA-1,
TSPO, pERK and pSTAT3 expression by western blot analysis. Western
blot analysis revealed that spinal sustained elevation of GFAP
could be detected in chronological order, and was accompanied by
the development of behavioral hypersensitivity (Fig. 3A and B; P<0.05). In sharp
contrast, the significant peak value of IBA-1 was not observed on
day 14, but only on day 7 (Fig. 3A and
C; P<0.05). Similar to GFAP expression, the expression of
TSPO was significantly increased from day 3 to 14, and reached its
peak on day 14 (Fig. 3A and D;
P<0.05). Then, the significant up-regulation of spinal pERK
induced by tumor cell inculation was observed on day 7 (Fig. 3A and E, P<0.05). However, spinal
pSTAT3 expression was significantly activated on day 5 compared
with the sham group (Fig. 3A and F,
P<0.05).
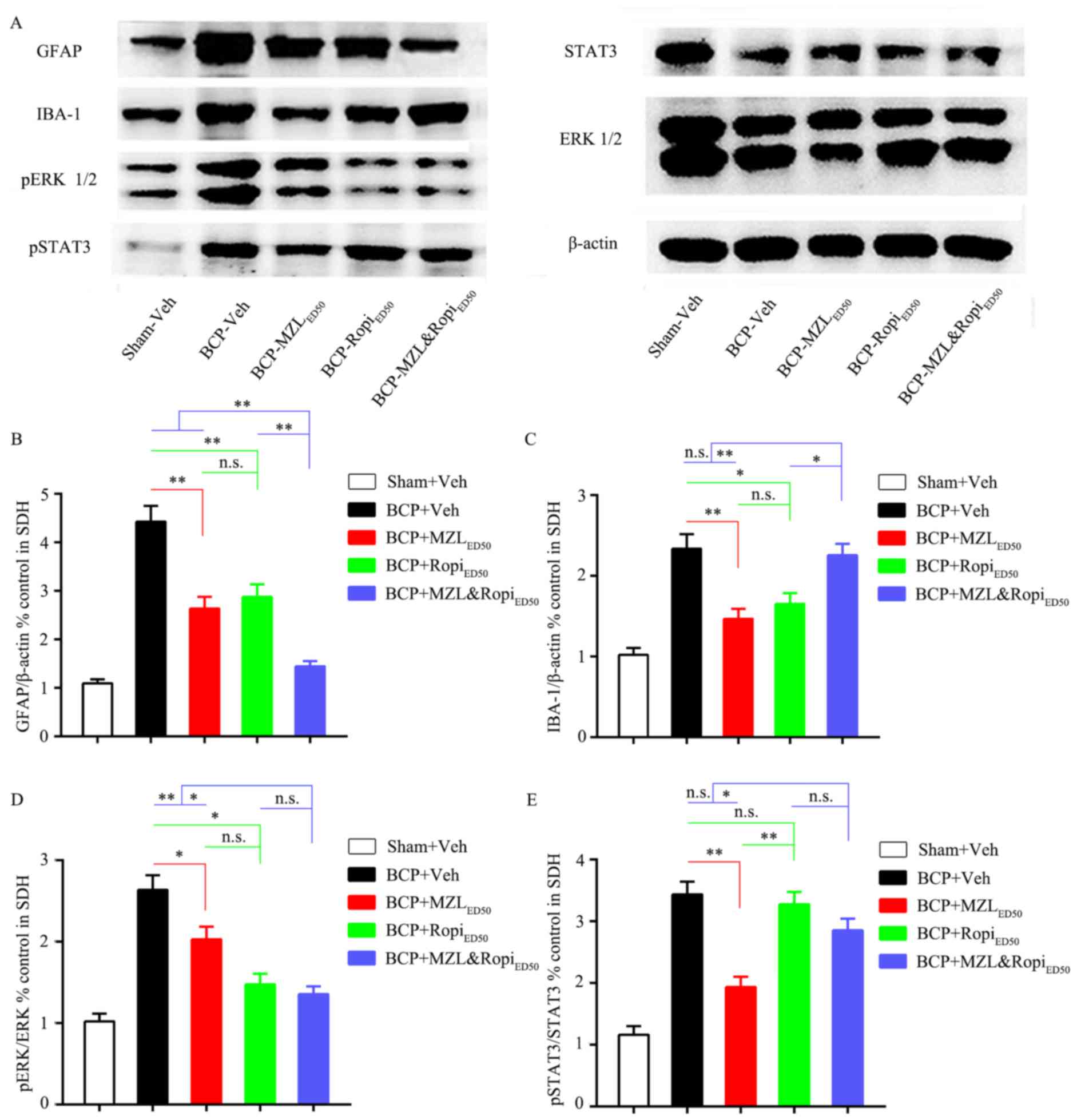
Effect of i.t. MZL, Ropi or their
combination on neuronal and glial activation
Increasing evidence has confirmed that spinal
neuron-astrocytic activation played a vital role in BCP rat models.
Hence, we further explored whether the inhibition of neurons and
neuroglia cells contributed to analgesia at doses of
ED50 MZL, ED50 Ropi, or ED50comb
MZL&Ropi which achieved equal 50% analgesia.
We administered i.t. ED50 MZL,
ED50 Ropi, or ED50comb MZL&Ropi once
daily from day 7 to 14 to observe the effects on astrocytic and
microglia activation in BCP rats on day 14. Western blot analysis
confirmed that the increased GFAP expression could be inhibited by
each intervention group compared with BCP group. However, the GFAP
expression for BCP-MZL&Ropi group was less than the other two
intervention groups, while there was no group difference between
BCP-MZL and BCP-Ropi groups (Fig. 5A
and B, P<0.05). Interestingly, the IBA-1 expression for
BCP-MZL and BCP-Ropi groups was inhibited by each intervention, but
there was no significant difference between BCP and
BCP-MZL&Ropi groups. Compared with BCP-MZL and BCP-Ropi groups,
i.t. MZ&Ropi combine did not inhibit IBA-1 expression (Fig. 5A and C, P<0.05).
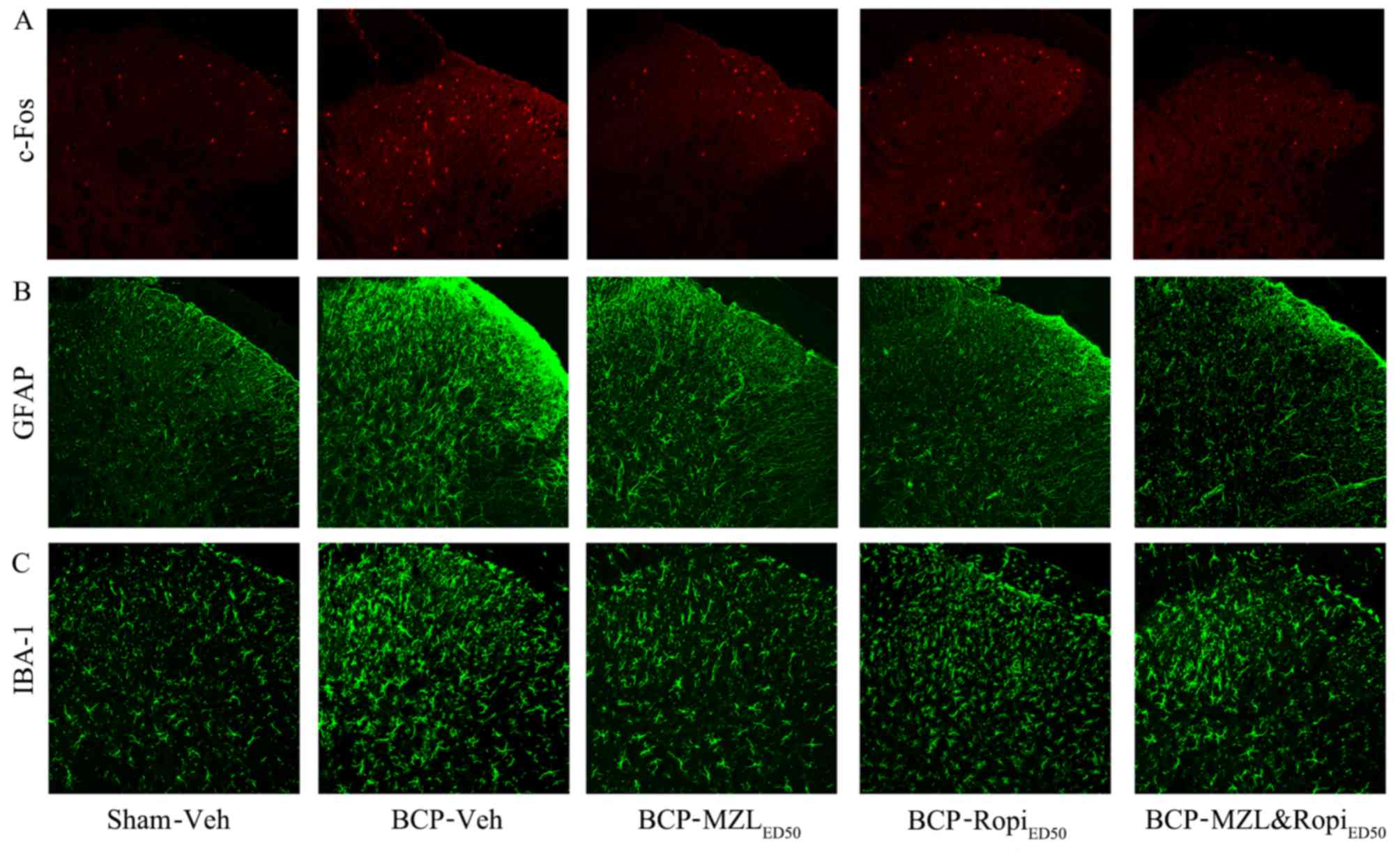
In addition, immunohistochemistry showed increased
nuclei expression of c-Fos on day 14 in BCP group. In each
intervention group, the c-Fos expression was inhibited by two drugs
respectively or combination (Fig.
4A). Immunohistochemical expression of GFAP and IBA-1 were
similar to western blot results (Fig.
4B and C). These results indicated that a synergistic effect,
which was also observed in previous behaviour tests, could be
contributed by astrocytes, but not microglia.
Effect of i.t. MZL, Ropi or their
combination on pERK and pSTAT3 expression
Next, we administered i.t. ED50 MZL,
ED50 Ropi, or ED50comb MZL&Ropi once
daily from day 7 to 14 to observe the effects on pERK and pSTAT3
activation in SDH on day 14. Analysis of the pERK expression
inhibited by each intervention group was compared with BCP group.
However, the pERK expression for BCP-Ropi and BCP-MZL&Ropi
groups were less than BCP-MZL groups, and there was no group
difference between BCP-Ropi and BCP-MZL&Ropi groups (Fig. 5A and D, P<0.05). The pSTAT3
expression inhibited only by BCP-MZL group compared with BCP group,
but there was great significant difference between BCP-Ropi and
BCP-MZL&Ropi groups. The inhibition of pSTAT3 was the strongest
in BCP-MZL group, compared with BCP-Ropi and BCP-MZL&Ropi group
(Fig. 5A and E, P<0.05). These
results indicated that i.t. MZL could inhibit pSTAT3 expression
much better than i.t. Ropi, and i.t. Ropi was just the opposite
compared with MZL, i.t. Ropi showed better inhibition of pERK than
i.t. MZL. Based on previous behavior tests on intervention groups,
we suggest that analgesia effect of MZL could be contributed to
inhibition of pSTAT3, and analgesic effect of Ropi was relevant to
inhibition of pERK, furthermore, analgesic effect of MZL and Ropi
combination could result from pERK inhibition, because it had
stronger inhibitory effect on pERK expression compared with
pSTAT3.
Discussion
Our study showed that MZL and Ropi reduced thermal
hyperalgesia in a dose-dependent manner in BCP rats, respectively.
Even more importantly, the low dose combination of MZL and Ropi
acted synergistically to reduce thermal hyperalgesia after tumor
cell inoculation. Next, we found that spinal neuron-astrocytic
activation played a vital role in BCP rats. MZL dominantly
downregulated the level of pSTAT3 while Ropi inhibited the spinal
pERK expression. Therefore, the combined use of MZL and Ropi might
be a new therapeutic approach for treatment of BCP.
Recent studies report that benzodiazepine receptor
consists of two types of receptors, one is central-type
benzodiazepine receptor (CBRs), which are coupled to type A
γ-amminobutyric acid (GABAA) receptor, and the other is
peripheral-type benzodiazepine receptors (PBRs, also named
translocator protein, TSPO), and these two types of receptors are
the targets for MZL by increasing GABA release (20). Compared with CBRs that are expressed
exclusively in the central nervous system (CNS), low expression of
TSPOs in CNS are common in normal condition. However, studies have
shown that increased expression of TSPOs in both astrocytes and
microglia are generally accompanied by neuroglia activation during
the nociceptive stimulation, such as inflammation, CNS injury and
pain (25,26). Moreover, Ro5-4864, an agonist of
TSPO, but not TSPO antagonist PK11195, can attenuate the pain
behavior in a dose-dependent manner that is contributed to suppress
the astrocytic activation (17,27,28).
In this study, similar results were confirmed: TSPO was upregulated
in the SDH on day 14 after tumor cell incubation. The single i.t.
administration of MZL dose-dependently inhibits thermal
hyperalgesia in BCP rats, indicated that spinal astrocytic
upregulation of TSPO induced by tumor cell incubation was involed
in the analgesic effect of MZL.
Ropi, one of the newest and safest LAs, is widely
used for local anesthesia in clinic. However, its relative
neurotoxicity limits the clinical use. Accumulative evidence
indicates that Ropi is considered as an effective drug in many
kinds of pain models, such as CFA-induced chronic inflammatory pain
and neuropathic pain (14,29). The analgesic effect of Ropi is
contributed to inhibition of the overexpression of spinal c-Fos and
GFAP in neuropathic pain that is consistent with previous
electrophysiological results. In our study, Ropi dose-dependently
attenuated thermal hyperalgesia in the BCP model, together with
consequent observations on suppressing spinal neurons and neuroglia
activation with the downregulation of spinal pERK.
In our study, the potential synergistically
analgesic mechanisms of MZL and Ropi might be as follows. First,
many researchers have confirmed that the TSPO upregulation was an
endogenous protective response during the process of inflammatory
stimulus and/or nerve injury, and MZL binds TSPO to reinforce this
‘protective shield’ and promotes its analgesic effect (17). TSPO mainly locates on the outer
membrane of mitochondria which are the source of reactive oxygen
species (ROS), and MZL suppresses the production of ROS and
attenuates ROS-induced nerve injury (30). Furthermore, dimethylthiourea, one of
ROS scavengers, has the ability to inhibit STAT3 activation induced
by focal cerebral ischemia-reperfusion injury in rats (31). Hence, it is possible that MZL
inhibit spinal pSTAT3 expression through suppression of ROS
production via spinal activation of TSPO. A study of rat glial cell
immune inflammation showed that MZL inhibited the release of IL-6
from rat C6 glioma cells by inhibiting astrocytic pSTAT3 activation
in TSPO dose-dependently, while increased level of IL-6 and
activation of STAT3 have been considered as trigger for initiation
and maintaining of pain (20,32).
We also found that MZL could obviously inhibit pSTAT3
activation.
Second, spinal pERK activation plays a crucially
role in the maintenance of many kinds of pain, such as nerve injury
and inflammation, and participates in regulating astrocytic
activation and proliferation (33,34).
In BCP model, spinal pERK activation was mainly expressed in
neurons, but not in microglia and astrocytes in the early phase.
However, in the late phase, spinal pERK expression was mainly
located in astrocytes (4). In our
study, we found that Ropi could downregulate spinal pERK expression
with significant inhibition of neurons and neuroglia cells
activation.
Third, based on the above evidence, we suggest that
synergistical analgesia effect of MZL and Ropi depended on
enhancing independent pathways. We showed that either MZL or Ropi
could inhibit spinal pSTAT3 and pERK expression with significant
inhibition of neurons and neuroglia cell activation in BCP rats.
However, MZL rather than Ropi showed selective inhibitory effect on
spinal pSTAT3 expression. On the contrary, Ropi predominantly
suppressed spinal pERK expression compared with MZL. In addition,
the combination of MZL and Ropi produced stronger and longer
analgesic effect with lower dose, and showed stronger inhibitory
effect on neurons and neuroglia activation.
In light of the available data above, a conservative
way to explain this synergistic effect is that Ropi blocks fast
voltage-gated sodium channels and further inhibits the spinal pERK
expression, then MZL binds to TSPO in the outer membrane of
mitochondria and the consequent production of ROS mediated by
inhibition of spinal pSTAT3 expression. However, there is a gap
between animal research and clinical application, the effective
doses of drug that works well in animals is not sure in humans. In
addition, the safety doses of drug should be confirmed with
considered judgments in different groups.
In conclusion, the major finding of our study shows
that MZL and Ropi act synergistically to inhibit BCP in rats.
Further study is needed to make sure the safe and effective doses
of i.t. MZL and Ropi in humans. We propose that the combination of
MZL and Ropi might be a novel strategy for the treatment of
BCP.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (nos. 81371112 and 81171050)
and Program for Shaanxi Province Key Research Team of Science and
Technology Innovation (2012KCT-14).
Glossary
Abbreviations
Abbreviations:
|
BCP
|
bone cancer pain
|
|
MZL
|
midazolam
|
|
Ropi
|
ropivacaine
|
|
SDH
|
spinal dorsal horn
|
|
GFAP
|
glial fibrillary acidic protein
|
|
IBA-1
|
ionized calcium binding adapter
molecule-1
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
STAT3
|
signal transducer and activator of
transcription
|
References
|
1
|
Kane CM, Hoskin P and Bennett MI: Cancer
induced bone pain. BMJ. 350:(jan29 7). h3152015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schug SA and Chandrasena C: Pain
management of the cancer patient. Expert Opin Pharmacother.
16:5–15. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guan XH, Fu QC, Shi D, Bu HL, Song ZP,
Xiong BR, Shu B, Xiang HB, Xu B, Manyande A, et al: Activation of
spinal chemokine receptor CXCR3 mediates bone cancer pain through
an Akt-ERK crosstalk pathway in rats. Exp Neurol. 263:39–49. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang LN, Yao M, Yang JP, Peng J, Peng Y,
Li CF, Zhang YB, Ji FH, Cheng H, Xu QN, et al: Cancer-induced bone
pain sequentially activates the ERK/MAPK pathway in different cell
types in the rat spinal cord. Mol Pain. 7:482011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu S, Mi WL, Li Q, Zhang MT, Han P, Hu S,
Mao-Ying QL and Wang YQ: Spinal IL-33/ST2 signaling contributes to
neuropathic pain via neuronal CaMKII-CREB and astroglial JAK2-STAT3
cascades in mice. Anesthesiology. 123:1154–1169. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang ZF, Li Q, Liu SB, Mi WL, Hu S, Zhao
J, Tian Y, Mao-Ying QL, Jiang JW, Ma HJ, et al: Aspirin-triggered
Lipoxin A4 attenuates mechanical allodynia in association with
inhibiting spinal JAK2/STAT3 signaling in neuropathic pain in rats.
Neuroscience. 273:65–78. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Olkkola KT and Ahonen J: Midazolam and
other benzodiazepines. Handb Exp Pharmacol. 182:335–360. 2008.
View Article : Google Scholar
|
|
8
|
Reddy SD and Reddy DS: Midazolam as an
anticonvulsant antidote for organophosphate intoxication - A
pharmacotherapeutic appraisal. Epilepsia. 56:813–821. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yilmaz E, Hough KA, Gebhart GF, Williams
BA and Gold MS: Mechanisms underlying midazolam-induced peripheral
nerve block and neurotoxicity. Reg Anesth Pain Med. 39:525–533.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Leone S, Di Cianni S, Casati A and Fanelli
G: Pharmacology, toxicology, and clinical use of new long acting
local anesthetics, ropivacaine and levobupivacaine. Acta
bio-medica. Atenei Parmensis. 79:92–105. 2008.PubMed/NCBI
|
|
11
|
Zink W and Graf BM: The toxicity of local
anesthetics: The place of ropivacaine and levobupivacaine. Curr
Opin Anaesthesiol. 21:645–650. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun ZH, Xu XP, Song ZB, Zhang Z, Wang N
and Guo QL: Repeated intrathecal administration of ropivacaine
causes neurotoxicity in rats. Anaesth Intensive Care. 40:825–831.
2012.PubMed/NCBI
|
|
13
|
Li TF, Fan H and Wang YX: Epidural
sustained release ropivacaine prolongs anti-allodynia and
anti-hyperalgesia in developing and established neuropathic pain.
PLoS One. 10:e01173212015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu HH, Yin JB, Zhang T, Cui YY, Dong YL,
Chen GZ and Wang W: Inhibiting spinal neuron-astrocytic activation
correlates with synergistic analgesia of dexmedetomidine and
ropivacaine. PLoS One. 9:e923742014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang X, Xu S, Qin X, Li X, Feng SW, Liu Y,
Wang W, Guo X, Shen R, Shen X, et al: Comparison between the use of
ropivacaine alone and ropivacaine with sufentanil in epidural labor
analgesia. Medicine (Baltimore). 94:e18822015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo S, Li B, Gao C and Tian Y: Epidural
analgesia with bupivacaine and fentanyl versus ropivacaine and
fentanyl for pain relief in labor: A meta-analysis. Medicine
(Baltimore). 94:e8802015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu X, Liu H, Xu S, Tang Z, Xia W, Cheng
Z, Li W and Jin Y: Spinal translocator protein alleviates chronic
neuropathic pain behavior and modulates spinal astrocyte-neuronal
function in rats with L5 spinal nerve ligation model. Pain.
157:103–116. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang DS, Tian Z, Guo YY, Guo HL, Kang WB,
Li S, Den YT, Li XB, Feng B, Feng D, et al: Anxiolytic-like effects
of translocator protein (TSPO) ligand ZBD-2 in an animal model of
chronic pain. Mol Pain. 11:162015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miao YL, Guo WZ, Shi WZ, Fang WW, Liu Y,
Liu J, Li BW, Wu W and Li YF: Midazolam ameliorates the behavior
deficits of a rat posttraumatic stress disorder model through dual
18 kDa translocator protein and central benzodiazepine receptor and
neurosteroidogenesis. PLoS One. 9:e1014502014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tanabe K, Kozawa O and Iida H: Midazolam
suppresses interleukin-1β-induced interleukin-6 release from rat
glial cells. J Neuroinflammation. 8:682011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Asiedu M, Ossipov MH, Kaila K and Price
TJ: Acetazolamide and midazolam act synergistically to inhibit
neuropathic pain. Pain. 148:302–308. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shih A, Miletic V, Miletic G and Smith LJ:
Midazolam administration reverses thermal hyperalgesia and prevents
gamma-aminobutyric acid transporter loss in a rodent model of
neuropathic pain. Anesth Analg. 106:1296–1302. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang Y, Li H, Li TT, Luo H, Gu XY, Lü N,
Ji RR and Zhang YQ: Delayed activation of spinal microglia
contributes to the maintenance of bone cancer pain in female Wistar
rats via P2X7 receptor and IL-18. J Neurosci. 35:7950–7963. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tallarida RJ: Drug synergism: its
detection and applications. J Pharmacol Exp Ther. 298:865–872.
2001.PubMed/NCBI
|
|
25
|
Li F, Liu J, Liu N, Kuhn LA, Garavito RM
and Ferguson-Miller S: Translocator protein 18 kDa (TSPO): An old
protein with new functions? Biochemistry. 55:2821–2831. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Campanella M and Turkheimer FE: TSPO:
Functions and applications of a mitochondrial stress response
pathway. Biochem Soc Trans. 43:593–594. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee JW, Kim LE, Shim HJ, Kim EK, Hwang WC,
Min S and Yu SW: A translocator protein 18 kDa ligand, Ro5-4864,
inhibits ATP-induced NLRP3 inflammasome activation. Biochem Biophys
Res Commun. 474:587–593. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wei XH, Wei X, Chen FY, Zang Y, Xin WJ,
Pang RP, Chen Y, Wang J, Li YY, Shen KF, et al: The upregulation of
translocator protein (18 kDa) promotes recovery from neuropathic
pain in rats. J Neurosci. 33:1540–1551. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Toda S, Sakai A, Ikeda Y, Sakamoto A and
Suzuki H: A local anesthetic, ropivacaine, suppresses activated
microglia via a nerve growth factor-dependent mechanism and
astrocytes via a nerve growth factor-independent mechanism in
neuropathic pain. Mol Pain. 7:22011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gatliff J and Campanella M: TSPO:
Kaleidoscopic 18-kDa amid biochemical pharmacology, control and
targeting of mitochondria. Biochem J. 473:107–121. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Batarseh A, Li J and Papadopoulos V:
Protein kinase C epsilon regulation of translocator protein (18
kDa) Tspo gene expression is mediated through a MAPK pathway
targeting STAT3 and c-Jun transcription factors. Biochemistry.
49:4766–4778. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu L, You Q, Tu Y, Tu Y, Li Q, Zheng L,
Li X, Gu J and Wang G: Midazolam inhibits the apoptosis of
astrocytes induced by oxygen glucose deprivation via targeting
JAK2-STAT3 signaling pathway. Cell Physiol Biochem. 35:126–136.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hu XD, Liu YN, Zhang ZY, Ma ZA, Suo ZW and
Yang X: Spinophilin-targeted protein phosphatase-1 alleviated
inflammatory pain by negative control of MEK/ERK signaling in
spinal cord dorsal horn of rats. J Neurosci. 35:13989–14001. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Han P, Liu S, Zhang M, Zhao J, Wang Y, Wu
G and Mi W: Inhibition of spinal interlukin-33/ST2 signaling and
downstream ERK and JNK pathways in electroacupuncture analgesia in
formalin mice. PLoS One. 10:e01295762015. View Article : Google Scholar : PubMed/NCBI
|